Abstract
Spinal cord injury (SCI), depending on the severity of injury, leads to neurological dysfunction and paralysis. Methylprednisolone, the only currently available therapy renders limited protection in SCI. Therefore, other therapeutic agents must be tested to maximize neuroprotection and functional recovery. Previous data from our laboratory indicates that estrogen (17β-estradiol) at a high dose may attenuate multiple damaging pathways involved in SCI and improve locomotor outcome. Since use of high dose estrogen may have detrimental side effects and therefore may never be used in the clinic, the current study investigated the efficacy of this steroid hormone at very low doses in SCI. In particular, we tested the impact of dosing (1 – 10 µg/kg), mode of delivery (intravenous vs. osmotic pump), and delay in estrogen application (15 min – 4 hr post-SCI) on microgliosis and neuronal death in acute SCI in rats. Treatment with 17β-estradiol (1 – 10 µg/kg) significantly reduced microglial activation and also attenuated apoptosis of neurons compared to untreated SCI animals. The attenuation of cell death and inflammation by estrogen was observed regardless of mode and time of delivery following injury. These findings suggest estrogen as a potential agent for the treatment of individuals with SCI.
Keywords: Spinal cord injury, estrogen, neurodegeneration, apoptosis, microgliosis
Introduction
Injury to the spinal cord causes deficits in neurological function, and depending on the extent of injury can lead to paralysis. Spinal cord injury (SCI) is highly debilitating, largely affecting otherwise healthy, young individuals. Although several agents have been used in clinical trials, only methylprednisolone is widely used (1, 2). Recently, methylprednisolone was found to have limited efficacy and its use in SCI remains controversial (3, 4). Therefore, new therapeutic approaches targeting secondary injury pathways occurring in the penumbra must be considered for protecting neurons and preserving spinal cord function.
Following SCI, several overlapping, and often synergistic mechanisms promote cell death and tissue destruction. Contributing factors involved in secondary SCI include oxidative stress, ischemia/reperfusion injury, phospholipase activity, Ca2+ influx, protease activation, and extracellular glutamate increase leading to excitotoxicity (5–14). Furthermore, these factors are not independent. Prostaglandins generated by the post-injury inflammatory response can lead to edema, resulting in decreased tissue blood flow and further exacerbating post-SCI ischemia and oxidative stress on reperfusion (6, 15–17). SCI also results in mitochondrial damage which may affect Na+-K+ ATPase activity and in turn may lead to increases in intracellular Ca2+ via dysfunction of the Na+-Ca2+ exchanger (18–20).
The increase in intracellular Ca2+ following SCI causes activation of Ca2+-dependent enzymes, including phospholipases and proteases, particularly the Ca2+-activated neutral protease calpain (21). Ubiquitous calpain exists in two isoforms μ-calpain and m-calpain, activated by µM and mM concentrations of Ca2+, respectively. Calpains are up-regulated in a number of neurodegenerative diseases and CNS injury. Such up-regulation promotes degradation of its specific endogenous inhibitor calpastatin, leading to unregulated calpain activity (22). Calpain cleaves many substrates, including cytoskeletal proteins, axonal and myelin proteins, and pro-apoptotic Bax causing mitochondrial cytochrome c release and activation of caspase-3 (23–26). Thus, the various actions of calpain may directly, or indirectly, be involved in apoptosis and necrosis of neurons and glial cells, suggesting that calpain may be a therapeutic target in spinal cord injury and other neurodegenerative disorders (27, 28).
Since SCI has a complex pathophysiology involving several mechanisms of cell death and axonal degeneration, therapy with a single agent may be ineffective. Instead, therapy with either multiple agents or a multi-active agent is likely necessary to prevent neuronal degeneration and loss of motor and sensory function following injury. Previous research in our laboratory and others has shown that estrogen (17β-estradiol) may be an effective neuroprotectant due to its actions as an anti-oxidant, anti-inflammatory, and anti-apoptotic steroid hormone (29–33). In addition, our recent studies indicate that estrogen attenuates Ca2+ influx via modulation of L-type Ca2+ channels and the Na+-Ca2+-exchanger (34). Estrogen has also been shown to reduce calpain expression and activity, resulting in reduced axon degeneration and neuronal apoptosis in vitro (34, 35). Most importantly, our findings indicate that pharmacological doses of estrogen (4 mg/kg) attenuate the secondary injury process and restore locomotor function in rats following chronic SCI in vivo (6 weeks post-injury) (36, 37).
Unfortunately, controversy still exists as to whether the multiple actions of estrogen confer functional neuroprotection in animal models of SCI (38). Despite encouraging evidence, other laboratories have failed to replicate measures of tissue sparing and improved functional recovery following estrogen therapy in SCI animals seen in other studies (39). Disparity in outcomes noted in these studies may be attributed to differences in estrogen dosing, route of administration, and delay in therapy after injury (38). Therefore, development of a more standardized therapy regime is necessary both for potential laboratory and clinical studies investigating the use of estrogen in SCI. To accommodate such therapy, we further examined the effect of differing estrogen doses (1 – 10 µg/kg), routes of administration (intravenous vs. osmotic pump), and delays in therapy application (15 min to 4 hr post-injury) on inflammatory and neurodegenerative changes following acute SCI in rats. The observations presented here may provide insight into a more appropriate and clinically relevant therapeutic regimen for the use of estrogen in CNS trauma and degeneration.
Experimental Procedure
Induction of Spinal Cord Injury
Adult male Sprague-Dawley rats weighing between 250 – 300 grams were housed in individual cages and given water and food ad libitum. All animal care, surgery, and induction of SCI was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US Department of Health and Human Services (National Institutes of Health, Bethesda, MD, USA) and was approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina. Spinal cord injury was induced by an experienced technician using the modified weight-drop method of Perot as previously described (36, 40).
Laminectomy was performed at T10, the spine was immobilized, and an impounder was gently placed onto the dura. A clinically relevant model of moderately severe SCI was induced by dropping a 5 g weight from a height of 8 cm to produce a 40 g·cm force. Sham operated animals underwent laminectomy alone. At 48 hr post-injury, rats were anesthetized and sacrificed by decapitation. Following decapitation, the original laminectomy was extended and three 1-cm segments of spinal cord were removed, representing the lesion segment, the rostral penumbra, and the caudal penumbra.
Therapeutic Estrogen Delivery
Physiologic doses of estrogen (1 µg/kg – 10 µg/kg) were delivered by subcutaneously implanted osmotic pumps to determine the lowest concentration of estrogen which produced optimal attenuation of secondary injury parameters. Following laminectomy and SCI induction, all animals received a bolus tail vein injection of 17β-estradiol (1 – 10 µg/kg) to facilitate increases in blood estrogen levels sufficient for therapeutic efficacy. Osmotic pumps were then implanted subcutaneously immediately after injury to maintain constant dosing of estrogen (1 – 10 µg/kg/day) or vehicle until 48 hr after injury.
After determining appropriate estrogen dosage, drug delivery via osmotic pump was also compared to intravenous injection to examine the effect of administration route on estrogen efficacy. Animals in the IV administration group received two doses of 17β-estradiol (10 µg/kg) or vehicle at 15 min and 24 hrs post-injury via tail-vein injection.
Finally, the effects of delay in therapy on the neuroprotective efficacy of estrogen in SCI was examined. Animals in these studies were treated via osmotic pump delivery. Pumps were implanted at 15 min, 2 hr, and 4 hr post-injury and maintained a consistent dose of 17β-estradiol (10 µg/kg/day) or vehicle until sacrifice.
Immunohistochemical Labeling for Microgliosis
Immunohistochemical staining for microgliosis in spinal cord tissue sections was performed as previously described (36, 41). Briefly, harvested spinal cord samples were immediately frozen in tissue-embedding media (HistoPrep; Fisher Scientific, Fair Lawn, NJ) at −70°C. Spinal cord tissues from the caudal penumbra were warmed to −18°C prior to sectioning at a thickness of 5 µm using a Reichart-Jung cryostat (Cryocut 1800; Leica, Wetzlar, Germany). Sections were then fixed in 95% ethanol and incubated for 1 hr in blocking buffer containing 2% horse serum in PBS (pH 7.4), followed by 3 – 4 hr incubation with primary IgG antibody for OX-42 (1:100; Biosource, Camarillo, CA). OX-42 is a macrophage/microglia marker specific for the CD11b antigen expressed on activated cells. Following incubation with the primary antibody, sections were rinsed three times in PBS for 5 mins and then incubated for 60 min with horse anti-mouse secondary IgG antibody conjugated to fluorescein isothicyanate. The slides were then mounted with one drop of anti-fade Vectashield Mounting Media (Vector Laboratories, Burlingame, CA), cover slipped, and viewed under a fluorescent microscope at 200X magnification. Images were captured using ImagePro Plus software (Media Cybernetics, Bethesda, MD).
Combined TUNEL and Neuron-Specific Fluorescent Labeling
In order to detect neuron death in spinal cord tissues, the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay was combined with neuron-specific marker labeling as previously described (41, 42). Briefly, spinal cord tissue was sectioned and fixed (see above). Sections were then post-fixed in 4% methanol-free formaldehyde (in phosphate-buffered saline) for 15 min. Slides were saturated with TdT buffer (50 µl/slide) for 5 min which was then replaced with an equal volume of TUNEL reaction mixture containing 10X polymerase chain reaction mixture with digoxigenin-11-dUTP (2.5 µl) and terminal TdT (25 units) in buffer (Promega, Madison, WI). Neuron-specific staining was performed with an anti-Neuronal Nuclei (NeuN) primary antibody (1:100; Chemicon, Temecula, CA) as described previously.
Western Blot Analysis
Spinal cord tissue segments were harvested and frozen at −70°C. Each tissue segment was then homogenized using a Polytron batch homogenizer (Kinematica, Cincinnati, OH) in buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM phenylmethylsulfonyl fluoride (PMSF; Bethesda Research Laboratories, Gaithersburg, MD), and 5 mM EGTA (Sigma Aldrich, St. Louis, MO). Protein concentration in each sample was then measured using Coomassie Plus Protein Assay Reagent (Pierce, Rockford, IL) by spectrophotometric analysis at 595 nm (Spectronic, Rochester, NY). Protein samples were then mixed 1:1 with sample buffer (62.5 mM Tris-HCl (pH 6.8), 2% SDS, 5 mM β-mercaptoethanol, and 10% glycerol), boiled for 5 min, and stored at −20°C. Samples were then diluted to 1 mg/ml protein and equal volumes of samples (10 µl) were loaded on 4–20% gradient gels. Gels were electrophoresed for 30 min at 200 V and resolved proteins were then transferred to nylon membranes (Millipore, Billerica, MA) in a Genie transfer apparatus (Idea Scientific, Minneapolis, MN). Membranes were then blocked in 5% nonfat milk in Tris/Tween buffer (20 mM Tris-HCl (pH 7.6), 137 mM NaCl, and 0.1% Tween 20) for 1 hr. Monocolonal primary IgG antibodies used were Bax and Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA). Bax (Bcl-2 associated X protein) is a pro-apoptotic member of the Bcl-2 family of proteins. Bcl-2 is an anti-apoptotic protein. In addition, monoclonal β-actin primary IgG antibody (clone AC-15, Sigma Aldrich) was used to standardize protein loading in Western Blots. Following overnight incubation with primary antibody, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody (ICN Biomedicals, Aurora, OH) for 1 hr. Between all steps, membranes were washed three times with Tris/Tween buffer. Membranes were then incubated with enhanced chemiluminescent (ECL) reagent (Amersham, Piscataway, NJ) and exposed to X-OMAT AR films (Kodak, Rochester, NY). Autoradiograms were scanned with a UMAX PowerLook Scanner using Photoshop software (Adobe Systems, Seattle, WA). The optical density (OD) of each band was determined using Quantity One software (Bio-Rad, Hercules, CA).
Statistical Analysis
Data was expressed as mean ± S.E.M. of separate experiments (n ≥ 3) and compared by one way analysis of variance (ANOVA) followed by Fisher’s post hoc testing. A significant difference between groups (p < 0.05) was determined by ANOVA using StatView software (Abacus Concepts, Berkeley, CA).
Results
Physiologic Doses of Estrogen Prevented Microgliosis Following Acute SCI
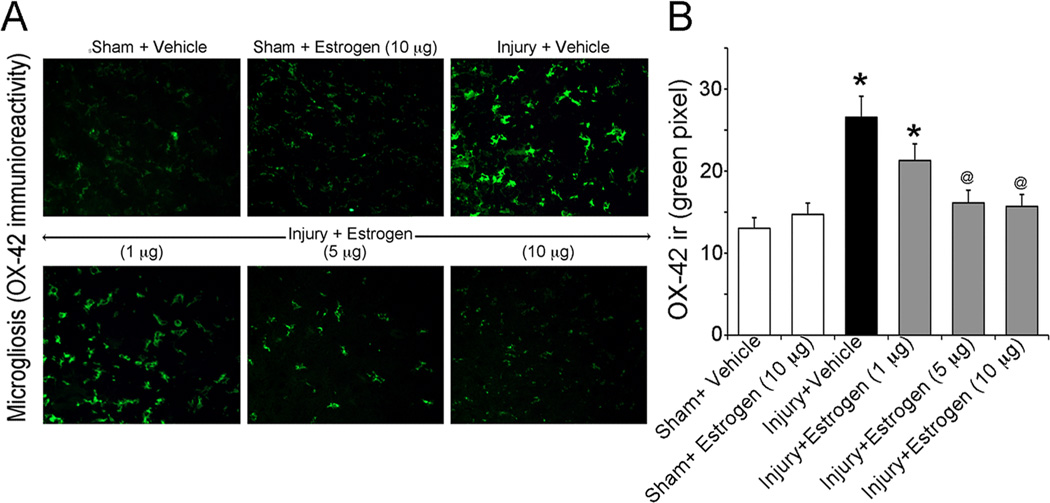
One of the main aims of this investigation was to determine the lowest dose of estrogen required to attenuate secondary pathophysiology following acute SCI. Since inflammatory events play a major role in the degenerative process associated with secondary injury, activation of microglia was examined in the injured cord. In particular, the efficacy of estrogen (delivered via osmotic pump) in ameliorating microgliosis was examined via immunofluorescent labeling of OX-42 (a marker specific to the CD11b antigen on activated macrophages/microglia) in the caudal penumbra of injured animals. Our findings indicated that reactive microgliosis was significantly enhanced following acute SCI (Figure 1). The 1 µg/kg/day dose of 17β-estradiol showed a negligible effect on microglial activation whereas 5 µg/kg/day significantly ameliorated increases in reactivity of OX-42 positive cells compared to untreated SCI. However, the 10 µg/kg/day dose of estrogen appeared to be the most effective in reducing microgliosis following injury (Figure 1). Interestingly, this dose showed greater efficacy in reducing post-injury changes in microglial reactivity than even higher doses (100 – 200 µg/kg) (43). Based on these data, all subsequent experiments were conducted using only the 10 µg/kg/day dose.
Figure 1.
Assessment of microgliosis in acute SCI animals. Spinal cord injured animals were treated with low doses of estrogen (1, 5, 10 µg/kg/day via osmotic pump) or vehicle and compared to sham surgery alone. Microgliosis was measured by OX-42 immunoreactivty in thin (5 µm) frozen sections. (A) Representative images (200X) are shown from the ventral horn of caudal penumbra spinal cord tissue. (B) Bar diagram quantifying OX-42 immunoreactivity. Fluorescence intensity was measured in a per field manner with 3 fields per animal. *P < 0.05 compared to sham + vehicle; @P < 0.05 compared to injury + vehicle (n ≥ 3 animals).
Low Dose Estrogen Protected Neurons and Reduced Microglial Reactivity Regardless of Route of Administration in Acute SCI
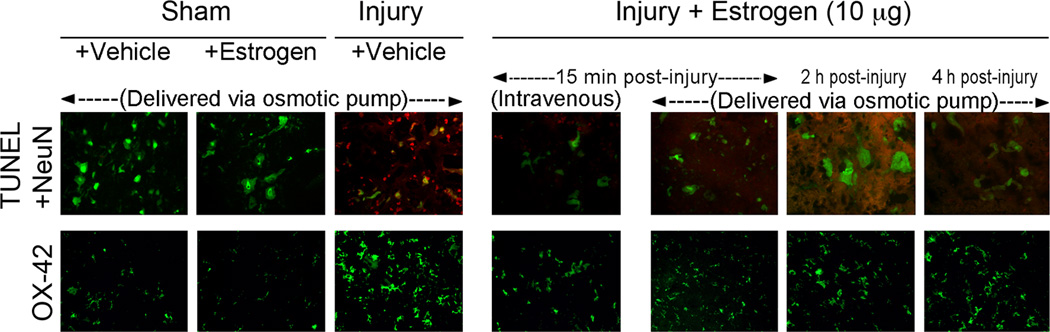
Once the lowest effective dose was determined, we also aimed to examine whether alternative modes of delivery alter the neuroprotective and anti-inflammatory effects of estrogen. For these studies, similar doses of 17β-estradiol were delivered via osmotic pump (10 µg/kg) or intravenous injection (10 µg/kg) beginning at 15 min post-injury. In order to determine the extent of cellular damage in the caudal penumbra, neuronal apoptosis was measured using a cell-specific marker for neurons (NeuN – green) and TUNEL (red) staining for DNA fragmentation. A significant increase in TUNEL-positive neurons (merged image – yellow) was noted in injury-vehicle animals when compared to sham (Figure 2). However, 17β-estradiol markedly reduced TUNEL-labeling of spinal cord neurons. No apparent qualitative differences were seen in attenuation of NeuN/TUNEL co-labeling when comparing osmotic pump to intravenous delivery (Figure 2). These findings indicate that low, physiologic doses of estrogen may protect against neuronal apoptosis following acute SCI regardless of route of administration.
Figure 2.
Effect of route of administration and therapy delay on estrogen treatment in acute SCI animals. SCI animals were treated with low dose estrogen (10 µg/kg) via osmotic pump (p) or intravenous injection (iv) at 15 min (15), 2 hr (2), and 4 hr (4) post-injury. Neuron-specific apoptosis was assessed by double fluorescent labeling of NeuN and TUNEL (top panel). Microgliosis was measured by OX-42 staining (bottom panel). Representative images (200X) are shown from frozen sections (5 µm) in the ventral horn of caudal penumbra spinal cord tissue (n ≥ 3 animals).
To further confirm that the protective effects of estrogen in SCI were independent of delivery mode, reactive microgliosis was measured by immunofluorescent labeling of OX-42. As previously described, microglial activation was substantially increased in the caudal penumbra of injured animals receiving vehicle alone compared to sham (Figure 2). However, estrogen therapy (10 µg/kg) greatly reduced the number of OX-42 positive cells in acute SCI rats. Similar to our data concerning neuronal death, the extent of estrogen-mediated attenuation of reactive microgliosis was comparable in both osmotic pump and intravenous injection groups.
Effects of Delay in Low Dose Estrogen Therapy on Neuronal Death and Inflammatory Response in Acute SCI
In order to further determine the most appropriate window of therapy following acute SCI, estrogen administration via osmotic pump was also delayed to multiple time points post-injury (15 min, 2 hr, and 4 hr). The effects of therapy delay on secondary pathophysiology were determined by analysis of neuron-specific apoptosis (NeuN/TUNEL co-labeling) and reactive microgliosis (OX-42 immunofluorescence) (Figure 2). Low dose of 17β-estradiol (10 µg/kg/day) attenuated neuronal death associated with acute SCI at all time points tested. Estrogen-mediated protection of spinal cord neurons was qualitatively greatest at 15 min post-injury. However, beneficial anti-apopotic effects of estrogen were also seen when therapy was delayed to 2 hr and 4 hr post-injury.
Similar results were also noted when examining the impact of therapy delay on microgliosis in acute SCI (Figure 2). Optimal reduction in microglial reactivity was observed in animals receiving low doses of estrogen at 15 min post-injury. However, OX-42 immunoreactivity was also substantially reduced at later delays in therapy onset (2 hr and 4 hr post-injury).
Low Dose Estrogen Reverses Post-Injury Increases in the Bax:Bcl-2 Ratio in Caudal Penumbra Tissue
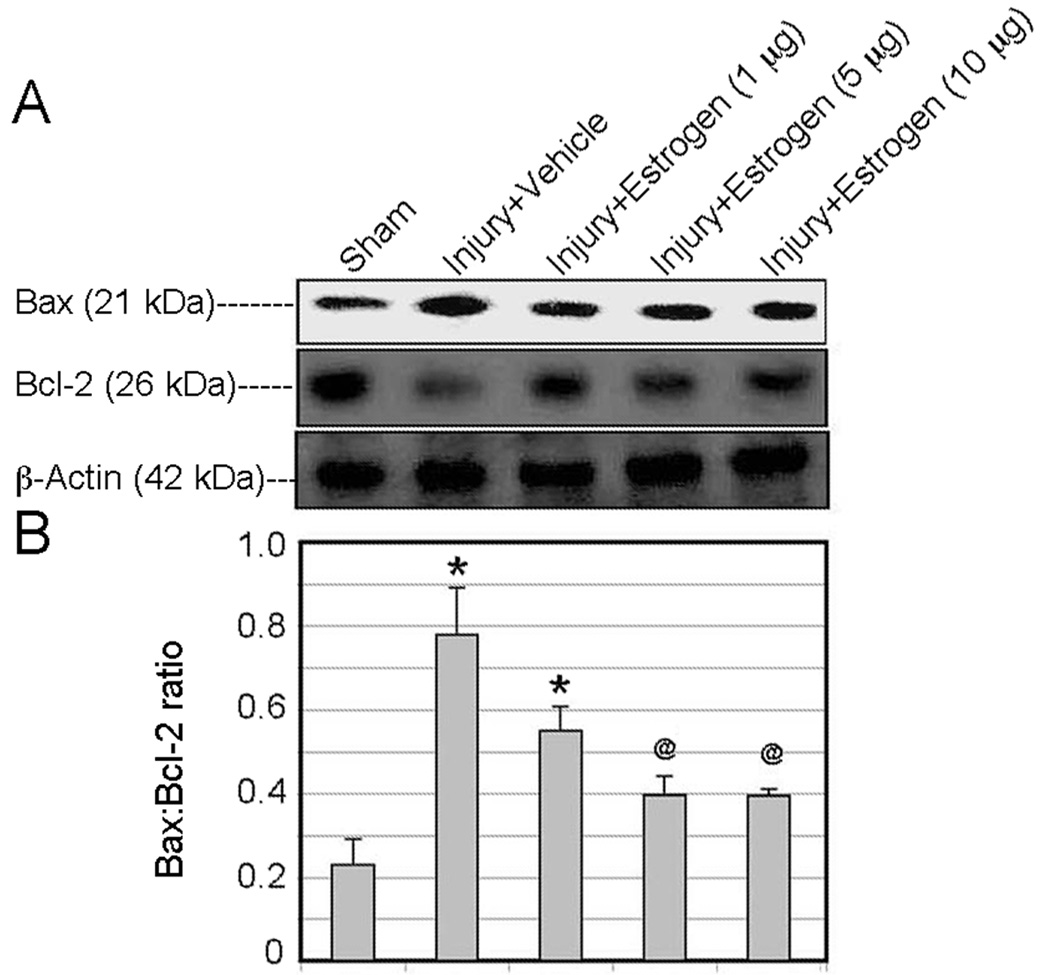
In order to further quantitatively assess the effects of estrogen on activation of apoptotic pathways, we measured changes in the Bax:Bcl-2 ratio following post-injury administration of low doses (1, 5, and 10 µg/kg via osmotic pump) by Western blot analysis. Bax is a pro-apoptotic member of the Bcl-2 family of proteins whereas Bcl-2 is anti-apoptotic. A significant increase in the Bax:Bcl-2 ratio was observed in acute SCI animals receiving vehicle alone. All doses of estrogen tested attenuated increase in the Bax:Bcl-2 ratio, with the 10 µg/kg dose demonstrating the greatest degree of reduction (Figure 3).
Figure 3.
Effect of estrogen treatment on changes in the Bax:Bcl-2 ratio in spinal cord injured animals. SCI animals were treated with low doses of estrogen (1, 5, and 10 µg/kg) via osmotic pump at 15 min following injury. Changes in the Bax:Bcl-2 were assessed by Western blot analysis. (A) Representative Western blot showing changes in Bax and Bcl-2 expression in caudal penumbra tissue. (B) Bar graph quanitifying densitometry of Western blots. *P < 0.05 compared to sham + vehicle; @P < 0.05 compared to injury + vehicle (n = 3).
Discussion
Given the severity of SCI and the lack of effective treatments, development of novel therapies for this devastating condition is essential. Estrogen (17β-estradiol) has received particular attention as a potential therapy because it targets many of the pathways active in secondary injury including oxidative stress, inflammation, apoptosis, and ischemia (29, 30). A number of reports indicate that estrogen may have neuroprotective effects in experimental models of CNS trauma and neurodegenerative disorder including traumatic brain injury (TBI), stroke, and Parkinson disease (PD) (44–46). Previous studies in our laboratory have demonstrated that pharmacologic doses of estrogen (4 mg/kg) may attenuate a number of secondary injury parameters and restore locomotor function in an animal model of SCI (36, 37, 47). However, chronic administration of such high doses of estrogen may elicit a number of unwanted side effects in SCI patients including increased risk of fatal deep venous thrombosis (DVT), breast/endometrial cancer, and feminization in males. In addition, debate remains about the use of estrogen in SCI because at least one pre-clinical study failed to show beneficial neuroprotective effects (39). However, the differential therapeutic outcomes in animal models of SCI are hypothesized to be attributable to differences in estrogen dosing, route of administration, and delay in therapy initiation (38). In contrast to other findings, Swartz et al. utilized silastic capsules in low and high doses to maintain plasma estrogen levels at 25 ng/ml and 75 ng/ml respectively and failed to show tissue sparing or improvement in locomotor function (39). In addition, various studies have estrogen efficacy in doses ranging from 100 µg/kg to 4 mg/kg which are non-physiologic (36, 43). We therefore tested the effects of low dose estrogen, administration mode, and delay on neuroprotective efficacy in vivo in an effort to further standardize therapy regimens for pre-clinical studies.
To our knowledge, the lowest dose of estrogen used in previous studies that demonstrated efficacy in attenuating multiple secondary injury mechanisms was 100 µg/kg (48). We have shown here that 17β-estradiol doses as low as 10 µg/kg may act via anti-inflammatory and anti-apoptotic mechanisms to attenuate damage associated with secondary SCI. In particular, we found that low dose estrogen (5 – 10 µg/kg) reduced both microglial activation and neuronal apoptosis in the caudal penumbra of spinal cord injured animals. Our findings indicated that lower doses of 17β-estradiol than previously used may be beneficial in ameliorating inflammatory and neurodegenerative pathways in acute SCI. These data further demonstrate promise for estrogen as a neuroprotectant because smaller dosages may avoid potentially harmful side effects. However, it is important to note, these studies were in an acute SCI model, hence they did not focus on behavioral outcomes following spinal cord trauma. Future studies aimed at determining whether low dose estrogen confers restoration of locomotor function in chronic SCI are necessary and ongoing.
This study also investigated the effects of delivery mode on estrogen treatment of SCI. We specifically compared microgliosis and neuronal death in animals receiving estrogen administration via osmotic pump or intravenous injection at similar dosage levels (10 µg/kg). As previously noted, 17β-estradiol greatly reduced microglial reactivity following acute SCI and no apparent differences were noted between osmotic pump delivery and intravenous injection. Measurement of neuron-specific apoptosis showed a similar result, indicating little difference between the two routes of administration. These findings are similar to those of other laboratories using a variety of administration routes to determine neuroprotective efficacy of estrogen at higher doses (48–50). Together, our results indicate that low dose estrogen has anti-apoptotic and anti-inflammatory actions in the setting of acute SCI independent of mode of delivery.
The final aim of this study was to investigate effects of therapy delay on the efficacy of estrogen in acute SCI. Outcomes were again based on attenuation of microgliosis and neuronal death after injury. As expected, estrogen was most effective in reversing microglial reactivity and neuronal apoptosis when therapy was initiated at 15 min post-injury. However, even when started at 4 hr after initial insult, 17β-estradiol was capable of ameliorating SCI-associated increases in activation of microglia and apoptotic death. These findings indicate that physiologic doses of 17β-estradiol may have neuroprotective and anti-inflammatory effects when therapy begins as late as 4 hr after SCI in rats. This is an important contribution to current knowledge about the use of estrogen in SCI and other CNS traumas because most studies have initiated its use either prior to injury or very shortly afterwards with substantially higher doses (0.1 – 4.0 mg/kg) (38). However, it is important to note that therapy will likely be delayed up to 48–72 hr in human cases to allow for stabilization of neurologic and medical condition (51). Debate also remains about whether biochemical alterations occur over the same time period when comparing rats and humans (52). Further work is necessary to determine the maximal in vivo delay which still results in estrogen-mediated improvement in functional outcome.
In summary, we have expanded on previous studies in our laboratory and others to demonstrate that low or physiologic doses of estrogen reverse secondary pathophysiology in an animal model of spinal cord injury by both anti-inflammatory and anti-apoptotic actions. We have also demonstrated that these effects are similar regardless of mode of delivery (osmotic pump vs. intravenous injection) and are maintained even with therapy delay. Whether the use of low dose estrogen in SCI promotes improvement in locomotor recovery is still unknown. Work in our laboratory is underway to address this concern. However, the findings presented herein further strengthen the notion that estrogen may be a potentially viable clinical alternative in the treatment of SCI.
Acknowledgements
Completion of this project was made possible by funding from the National Institutes of Health (NIH) and National Institute of Neurological Disorders and Stroke (NINDS): (NS-31622, NS-38146, and NS-41088) and the State of South Carolina Spinal Cord Injury Research Fund (SCSCIRF).
References
- 1.Dumont RJ, Verma S, Okonkwo DO, et al. Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol. 2001;24(5):265–279. doi: 10.1097/00002826-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Peter Vellman W, Hawkes AP, Lammertse DP. Administration of corticosteroids for acute spinal cord injury: the current practice of trauma medical directors and emergency medical system physician advisors. Spine (Phila Pa 1976) 2003;28(9):941–947. doi: 10.1097/01.BRS.0000058708.46933.3D. discussion 947. [DOI] [PubMed] [Google Scholar]
- 3.Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251(1):45–52. [PubMed] [Google Scholar]
- 4.Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(1 Suppl):1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic Biol Med. 1999;27(3–4):478–482. doi: 10.1016/s0891-5849(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai M, Nagata T, Abe K, et al. Survival and death-promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg. 2003;125(2):370–377. doi: 10.1067/mtc.2003.112. [DOI] [PubMed] [Google Scholar]
- 7.Young W. The role of calcium in spinal cord injury. Cent Nerv Syst Trauma. 1985;2(2):109–114. doi: 10.1089/cns.1985.2.109. [DOI] [PubMed] [Google Scholar]
- 8.Happel RD, Smith KP, Banik NL, et al. Ca2+-accumulation in experimental spinal cord trauma. Brain Res. 1981;211(2):476–479. doi: 10.1016/0006-8993(81)90976-8. [DOI] [PubMed] [Google Scholar]
- 9.Stokes BT, Fox P, Hollinden G. Extracellular calcium activity in the injured spinal cord. Exp Neurol. 1983;80(3):561–572. doi: 10.1016/0014-4886(83)90307-2. [DOI] [PubMed] [Google Scholar]
- 10.Ray SK, Matzelle DD, Wilford GG, et al. Increased calpain expression is associated with apoptosis in rat spinal cord injury: calpain inhibitor provides neuroprotection. Neurochem Res. 2000;25(9–10):1191–1198. doi: 10.1023/a:1007631826160. [DOI] [PubMed] [Google Scholar]
- 11.Springer JE, Azbill RD, Kennedy SE, et al. Rapid calpain I activation and cytoskeletal protein degradation following traumatic spinal cord injury: attenuation with riluzole pretreatment. J Neurochem. 1997;69(4):1592–1600. doi: 10.1046/j.1471-4159.1997.69041592.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17(10):871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 13.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54(4):369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 14.Mills CD, Xu GY, Johnson KM, et al. AIDA reduces glutamate release and attenuates mechanical allodynia after spinal cord injury. Neuroreport. 2000;11(14):3067–3070. doi: 10.1097/00001756-200009280-00007. [DOI] [PubMed] [Google Scholar]
- 15.Carlson SL, Parrish ME, Springer JE, et al. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 16.Sharma HS, Olsson Y, Nyberg F, et al. Prostaglandins modulate alterations of microvascular permeability, blood flow, edema and serotonin levels following spinal cord injury: an experimental study in the rat. Neuroscience. 1993;57(2):443–449. doi: 10.1016/0306-4522(93)90076-r. [DOI] [PubMed] [Google Scholar]
- 17.Barut S, Canbolat A, Bilge T, et al. Lipid peroxidation in experimental spinal cord injury: time-level relationship. Neurosurg Rev. 1993;16(1):53–59. doi: 10.1007/BF00308614. [DOI] [PubMed] [Google Scholar]
- 18.Wingrave JM, Schaecher KE, Sribnick EA, et al. Early induction of secondary injury factors causing activation of calpain and mitochondria-mediated neuronal apoptosis following spinal cord injury in rats. J Neurosci Res. 2003;73(1):95–104. doi: 10.1002/jnr.10607. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal SK, Nashmi R, Fehlings MG. Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience. 2000;99(1):179–188. doi: 10.1016/s0306-4522(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Jiang Q, Stys PK. Important role of reverse Na(+)-Ca(2+) exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84(2):1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- 21.Sribnick EA, Matzelle DD, Banik NL, et al. Direct evidence for calpain involvement in apoptotic death of neurons in spinal cord injury in rats and neuroprotection with calpain inhibitor. Neurochem Res. 2007;32(12):2210–2216. doi: 10.1007/s11064-007-9433-7. [DOI] [PubMed] [Google Scholar]
- 22.Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2(3):173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- 23.Pike BR, Zhao X, Newcomb JK, et al. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. Neuroreport. 1998;9(11):2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- 24.Banik NL, Chou CH, Deibler GE, et al. Peptide bond specificity of calpain: proteolysis of human myelin basic protein. J Neurosci Res. 1994;37(4):489–496. doi: 10.1002/jnr.490370408. [DOI] [PubMed] [Google Scholar]
- 25.Gao G, Dou QP. N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem. 2000;80(1):53–72. doi: 10.1002/1097-4644(20010101)80:1<53::aid-jcb60>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Blomgren K, Zhu C, Wang X, et al. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of "pathological apoptosis"? J Biol Chem. 2001;276(13):10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- 27.Pang Z, Bondada V, Sengoku T, et al. Calpain facilitates the neuron death induced by 3-nitropropionic acid and contributes to the necrotic morphology. J Neuropathol Exp Neurol. 2003;62(6):633–643. doi: 10.1093/jnen/62.6.633. [DOI] [PubMed] [Google Scholar]
- 28.Nath R, Raser KJ, Stafford D, et al. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319(Pt 3):683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sribnick EA, Wingrave JM, Matzelle DD, et al. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. discussion 159-60. [DOI] [PubMed] [Google Scholar]
- 30.Sribnick EA, Ray SK, Banik NL. Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res. 2004;29(11):2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- 31.Samantaray S, Sribnick EA, Das A, et al. Neuroprotective efficacy of estrogen in experimental spinal cord injury in rats. Ann N Y Acad Sci. 2010;1199:90–94. doi: 10.1111/j.1749-6632.2009.05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999;96(16):8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimayuga FO, Reed JL, Carnero GA, et al. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161(1–2):123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Sribnick EA, Del Re AM, Ray SK, et al. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sribnick EA, Ray SK, Nowak MW, et al. 17beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res. 2004;76(5):688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- 36.Sribnick EA, Samantaray S, Das A, et al. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88(8):1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sribnick EA, Matzelle DD, Ray SK, et al. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84(5):1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- 38.Kwon BK, Okon E, Hillyer J, et al. A Systematic Review of Non-Invasive Pharmacologic Neuroprotective Treatments for Acute Spinal Cord Injury. J Neurotrauma. 2010 doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swartz KR, Fee DB, Joy KM, et al. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. 2007;24(3):473–480. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- 40.Perot PL, Jr, Lee WA, Hsu CY, et al. Therapeutic model for experimental spinal cord injury in the rat: I. Mortality and motor deficit. Cent Nerv Syst Trauma. 1987;4(3):149–159. doi: 10.1089/cns.1987.4.149. [DOI] [PubMed] [Google Scholar]
- 41.Samantaray S, Sribnick EA, Das A, et al. Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J Pineal Res. 2008;44(4):348–357. doi: 10.1111/j.1600-079X.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray SK, Schaecher KE, Shields DC, et al. Combined TUNEL and double immunofluorescent labeling for detection of apoptotic mononuclear phagocytes in autoimmune demyelinating disease. Brain Res Brain Res Protoc. 2000;5(3):305–311. doi: 10.1016/s1385-299x(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 43.Samantaray S, Matzelle DD, Ray SK, et al. Physiological low dose of estrogen-protected neurons in experimental spinal cord injury. Ann N Y Acad Sci. 2010;1199:86–89. doi: 10.1111/j.1749-6632.2009.05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown CM, Suzuki S, Jelks KA, et al. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med. 2009;27(3):240–249. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30(2):142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Sribnick EA, Wingrave JM, Matzelle DD, et al. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82(2):283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- 48.Yune TY, Kim SJ, Lee SM, et al. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21(3):293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- 49.Chaovipoch P, Jelks KA, Gerhold LM, et al. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23(6):830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- 50.Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- 51.Rahimi-Movaghar V, Saadat S, Vaccaro AR, et al. The efficacy of surgical decompression before 24 hours versus 24 to 72 hours in patients with spinal cord injury from T1 to L1--with specific consideration on ethics: a randomized controlled trial. Trials. 2009;10:77. doi: 10.1186/1745-6215-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21(6):775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]