Abstract
Stress plays a role in many psychiatric disorders that are characterized by deficits in prepulse inhibition (PPI), a form of sensorimotor gating. Corticotropin-releasing factor (CRF) is one of the most important neurotransmitters involved in behavioral components of the stress response. Central infusion of CRF reduces PPI in both rats and mice. In mice, it has been shown that CRF1 receptor activation mediates the effect of exogenous CRF on PPI. However, the roles of the two CRF receptors in a stress-induced reduction in PPI are not known. We sought to determine whether CRF1 and/or CRF2 receptor blockade attenuates a stress-induced reduction of PPI in rats. In separate experiments, we assessed PPI in Brown Norway rats after exposure to five days of 2-hour restraint, and after pretreatment with the CRF1 receptor antagonist, CP-154,526 (20.0 mg/kg), or the CRF2 receptor antagonist, antisauvagine-30 (10.0 µg). Repeated, but not acute, restraint decreased PPI and attenuated the increase in PPI caused by repeated PPI testing. Blockade of the CRF1 receptor did not attenuate the effect of repeated restraint on PPI or grooming behavior. While CRF2 receptor blockade did attenuate the effect of repeated restraint on PPI, repeated ICV infusion of the selective CRF2 receptor agonist urocortin III, did not affect PPI. These findings demonstrate the effect of stress on sensorimotor gating and suggest that the CRF2 receptor mediates this effect in rats.
Keywords: acoustic startle response; antisauvagine-30; corticotropin-releasing factor; CP-154,526; prepulse inhibition; stress
1. Introduction
The acoustic startle response (ASR) is a reflexive response to an unexpected and intense auditory stimulus and is characterized by contraction of the facial and skeletal muscles (Koch, 1999). Despite the fact that the ASR is a reflex, it can be modulated. For example, startle amplitude is reduced if a non-startling acoustic stimulus is presented shortly prior to the startling stimulus (Hoffman and Ison, 1980; Hoffman and Searle, 1968). This form of startle plasticity, referred to as prepulse inhibition (PPI), is a measure of sensorimotor gating (Braff and Geyer, 1990). Patients with a variety of psychiatric disorders, including schizophrenia (Braff et al., 1978; Braff et al., 1992), obsessive-compulsive disorder (Swerdlow et al., 1993), and post-traumatic stress disorder (Grillon et al., 1996) show less PPI than control subjects. Exposure to stressful events can trigger the onset or exacerbate symptoms of each of these disorders (Dinn et al., 1999; Keane et al., 2006; Walker and Diforio, 1997). Since PPI can be assessed in both humans and rodents under nearly identical parameters, it is a useful tool for investigating deficits in human information processing using animal models.
Corticotropin-releasing factor (CRF) is one of the most important hormones and neurotransmitters involved in endocrine, autonomic, and behavioral components of the stress response (Bale and Vale, 2004; Gray, 1993). A 41-residue peptide, CRF is synthesized in hypothalamic (Vale et al., 1981) and extra-hypothalamic brain regions including the central nucleus of the amygdala, hippocampus, and frontal cortex (Swanson et al., 1983). The peptide acts at two G-protein coupled receptors, CRF1 and CRF2 (Chang et al., 1993; Lovenberg et al., 1995), which are expressed in brain regions known to modulate PPI, including the basolateral amygdala, hippocampus, and frontal cortex (Swerdlow et al., 2001; Van Pett et al., 2000). Intracerebroventricular (ICV) infusion of CRF reduces PPI in both rats (Conti, 2005; Conti et al., 2002) and mice (Risbrough et al., 2004). Additionally, transgenic mice over-expressing CRF show reduced PPI compared to wild-type controls (Dirks et al., 2002).
The first goal of these studies was to investigate the effects of restraint stress on PPI in adult rats. It has previously been shown that PPI is reduced in adult rats, which had undergone early-life stress such as maternal deprivation and social isolation (Weiss and Feldon, 2001). However, studies on the effect of restraint stress in adulthood on PPI have produced inconsistent results (Acri, 1994; Bijlsma, et al., 2009; Faraday, 2002; Faraday et al., 1999). We have shown that repeated restraint stress attenuates the increase in PPI caused by repeated PPI testing (Sutherland et al., 2010). Here, we examined whether beginning the application of repeated restraint after PPI levels were increased by repeated testing would decrease PPI. Risbrough and colleagues (2004) have demonstrated that the CRF1 receptor mediates the reduction in PPI caused by exogenously administered CRF, while the CRF2 receptor mediates an opposing effect of CRF. However, it is not known whether the two CRF receptors similarly mediate stress-induced changes in PPI or whether the two CRF receptors act similarly in rats and mice. Thus, the second goal of these studies was to determine which CRF receptor mediates the effect of stress on PPI in rats.
In the present studies, we examined whether 2-hour restraint stress, administered once a day for five consecutive days, decreases PPI. Since PPI increases with repeated testing, we next examined whether restraint stress decreases PPI following repeated testing. We then examined whether and how the two CRF receptors mediate the effect of restraint stress on PPI. Rats were pretreated with either a selective CRF1 or CRF2 receptor antagonist (in two separate experiments) prior to restraint exposure on each of five consecutive days. Finally, we examined the effect of repeated infusion (ICV) of the selective CRF2 receptor agonist, urocortin III (Ucn III), on PPI.
We began this examination of the effects of restraint stress on PPI and the potential roles of the two CRF receptors on any such effect using Brown Norway (BN) rats. This rat strain was chosen for several reasons. First, we have extensively characterized the effects of exogenously administered CRF on PPI and startle amplitude in BN rats (Conti, 2005; Conti et al., 2006; Conti et al., 2005; Conti et al., 2002; Conti et al., 2009; Sutherland et al., 2008). Thus, comparisons can be made between the findings of this study and our previously published studies. Second, BN rats are more sensitive to the effects of ICV infusion of CRF (Conti et al., 2002; Sutherland et al., 2008) and to the effects of stress (Sutherland et al., 2010) on PPI than Wistar-Kyoto (WKY) rats, another commonly used strain in our laboratory. This sensitivity to CRF and stress suggests that the BN rat could be developed as a model of stress-induced exacerbation of psychiatric disorders in which there are deficient sensorimotor gating phenotypes.
2. Materials and Methods
2.1 Animals
A total of 163 male Brown Norway (BN) rats (Harlan Sprague-Dawley, Prattville, AL, USA) were 10 weeks old upon arrival and were maintained on a 12-hour light/dark cycle with food and water available ad libitum. Rats were group-housed for 1–2 weeks prior to undergoing surgery or restraint exposure, and single-housed thereafter. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Experimental Design
In experiment 1, rats were restrained for 2 hours, once a day for 5 consecutive days, by being placed into acrylic cylindrical restrainers designed for rodents weighing 250–500 grams (6.4 cm diameter, with adjustable length; Biomedical Research Instruments, Silver Springs, MD, USA). Control rats were handled briefly but not restrained. Rats were tested for PPI and startle amplitude 30 minutes after restraint termination on days 1, 3, and 5.
In experiment 2, PPI and startle amplitude were assessed once a day for 5 consecutive days, with no restraint being imposed. After day 5 of PPI testing, the rats were divided into two counter-balanced groups based on average percent PPI. Two days later, half of the rats underwent 2-hour restraint/day for 10 consecutive days, and the other half served as controls that were not restrained. Rats were again tested for PPI 30 minutes after restraint termination on days 1, 3, 6, and 10.
In experiment 3, rats were administered a subcutaneous (SC) injection of the non-peptide selective CRF1 receptor antagonist, CP-154,526 (20.0 mg/kg) (Schulz et al., 1996; Seymour et al., 2003), or vehicle. Forty-five minutes after injection, rats were restrained for 2 hours or were handled briefly but not restrained. Rats were subjected to injection and restraint (or brief handling) once a day for 5 consecutive days, and were tested for PPI and startle amplitude 30 minutes after restraint termination on days 1, 3, and 5. Fifteen minutes prior to PPI testing, grooming behavior was observed for 15 minutes as a secondary behavioral measure of stress (Spruijt et al., 1992).
Experiment 4 was conducted to examine the effect of CP-154,526 on a CRF-induced decrease in PPI. Rats were administered a SC injection of CP-154,526 (20.0 mg/kg) or vehicle. Two hours and 45 minutes after injection, rats received an ICV infusion of 0.3 µg CRF (in 6.0 µl saline) or 6.0 µl saline. Rats were tested for PPI and startle amplitude 30 minutes after ICV infusion. Grooming behavior was observed for 15 minutes immediately before PPI testing as a secondary behavioral measure of the effect of CRF (Dunn et al., 1987). Injection of CP-154,526 2 hours and 45 minutes before CRF infusion ensured that the time between SC injection and PPI testing was the same in all experiments in which CP-154,526 was used.
Experiment 5 was conducted to replicate previous findings that a selective CRF1 receptor antagonist blocks the CRF-induced increase in anxiety-like behavior in the elevated plus maze (Zorrilla et al., 2002). Rats were administered a SC injection of CP-154,526 (20.0 mg/kg) or vehicle. Two hours and 45 minutes after injection, rats received an ICV infusion of 1.0 µg CRF (in 5.0 µl saline) or 5.0 µl saline. Ten minutes after ICV infusion, rats were observed in the elevated plus maze for 5 minutes.
In experiment 6, rats received an ICV infusion of the selective CRF2 receptor antagonist, antisauvagine-30 (ASV-30; 10.0 µg in 5.0 µl saline) (Higelin et al., 2001), or 5.0 µl saline. Ten minutes after ICV infusion, rats were restrained for 2 hours or were handled briefly but not restrained. Rats were subjected to infusion and restraint (or brief handling) once a day for 5 consecutive days, and were tested for PPI and startle amplitude 30 minutes after restraint termination on days 1, 3, and 5. Fifteen minutes prior to PPI testing, grooming behavior was observed for 15 minutes.
Experiment 7 was conducted to examine the effect of repeated administration of a selective CRF2 receptor agonist, Ucn III, on PPI. Rats received an ICV infusion of 20.0 µg Ucn III (in 5.0 µl saline) or 5.0 µl saline once a day for 5 consecutive days. Rats were tested for PPI and startle amplitude on days 1, 3 and 5 beginning 2.5 hours after ICV infusion.
2.3 Drugs and Peptides
CP-154,526 (N-butyl-N-[2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]-N-ethylamine) was generously donated by Pfizer, Inc (Groton, CT, USA). In a heated water bath (65°C), a stock solution was prepared by dissolving CP-154,526 in DMSO (Sigma-Aldrich, St. Louis, MO, USA) and Cremophor (Sigma-Aldrich) in the ratio 90:5:5 (100 mg/ml), and was stored at room temperature. On each testing day, stock solution was placed into a microcentrifuge tube and distilled water (heated to 60°C) was added to reach a concentration of 10 mg/ml. Rats were lightly anesthetized with isoflurane-in-oxygen (5%) for 90 seconds prior to SC injection. Rat/human CRF, kindly provided by Dr. Jean Rivier (The Salk Institute, La Jolla, CA, USA), ASV-30 (Sigma-Aldrich) and mouse Ucn III (Bachem, Torrance, CA, USA) were dissolved in 0.9% saline. Aliquots of each peptide were frozen at −80°C until needed.
2.4 Stereotaxic Surgery and ICV Infusion Procedure
Rats were anesthetized with isoflurane-in-oxygen (2.0%) and placed in a Kopf stereotaxic instrument equipped with blunt ear bars. The incisor bar was set to −3.0. A stainless steel guide cannula (22 gauge; Plastics One, Roanoke, VA, USA) was aimed at the lateral ventricle (AP −1.0 mm, ML 2.0 mm from Bregma; 4.4 mm ventral from the skull) (Paxinos and Watson, 1986). Two jewelers’ screws were placed into the skull and the assembly was held in place with dental cement. A dummy cannula was placed into the guide. To minimize pain, rats received a SC injection of buprenorphine (0.05 mg/kg) while anesthetized. Rats recovered for 5–7 days prior to testing.
For ICV infusion, a 28-gauge cannula attached to PE 20 tubing was inserted into the guide cannula and extended 0.5 mm beyond. A 10.0 µl Hamilton syringe was used to manually deliver saline, ASV-30, or Ucn III over one minute. The flow of infusate was monitored via introduction of an air bubble into the infusion line. The infusion cannula was kept in place for an additional minute following infusion.
2.5 Startle Chambers and PPI Testing
Startle amplitude and PPI were measured in two identical startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, USA) consisting of a nonrestrictive Plexiglas cylinder (9 cm diameter, 18.5 cm length) mounted on a platform located inside a sound- and vibration-attenuating cabinet equipped with a 5-watt incandescent bulb and a fan for ventilation. A piezoelectric accelerometer, mounted under each cylinder, detected whole-body startle responses. From the onset of each startle stimulus, output signals from the accelerometer were recorded once/msec for 100 msec by the computer. Signals were rectified, digitized, and stored by the SR-LAB program. Startle response sensitivities were standardized across chambers using a standard calibration tube each day. White noise stimuli were delivered through a horn tweeter controlled by the SR-LAB program.
Following a 5-minute acclimation period, stimuli were delivered over a 70 dB white noise background. The first and last six trials of the session consisted of the startle stimulus alone (120 dB, 40 msec). Remaining trials occurred in a pseudorandom order and consisted of 12 startle alone trials (used to calculate % PPI and average startle amplitude), 12 prepulse + startle trials at each of 4 prepulse intensities (76, 82, 85, 88 dB), and 8 no stimulus trials. Prepulse stimuli (20 msec) preceded startle stimuli by 100 msec. The inter-trial interval averaged 20 seconds. Testing was performed between 10 a.m. and 4 p.m.
2.6 Elevated Plus Maze
An elevated plus maze, made of white plastic (San Diego Instruments), was elevated to a height of 49.5 cm, with two open (50.2 × 10.2 cm) and two enclosed arms (50.2 × 10.2 cm, walls were 30.5 cm high). A 70-watt desk lamp was placed 0.9 meters from the maze, with the bulb angled away from the maze, to create dim lighting conditions in the small room. During observation, the experimenter always sat in the same place next to the maze. Only the rat being tested was brought into the maze room and each rat was naïve to the apparatus. Rats were placed in the center of the maze facing the same enclosed arm. During the 5-minute observation period, the amount of time spent in the open arms, as well as the total number of open and closed arm entries, were recorded. An entry required that all four limbs of the rat be in that arm.
2.7 Data and Statistical Analyses
Percent PPI was calculated for each rat at each prepulse intensity using the following equation: % PPI = 100 – (100 × [prepulse/startle]). Prepulse was the average startle amplitude on trials in which a prepulse stimulus preceded the startle stimulus. Startle was the average amplitude on trials in which the startle stimulus was presented alone, excluding the first and last 6 trials. In order to examine whether selective CRF receptor antagonists or stress altered habituation of the startle response in experiments 3 and 6, percent habituation was calculated as: 100 × (average of first 6 startle trials – average of last 6 startle trials/average of last 6 startle trials).
Data were analyzed using one-, two-, three-, or four-way analysis of variance (ANOVA), as discussed in detail in the Results section. Tukey’s post-hoc tests were performed if significant main effects were found. Independent t-tests with Bonferroni correction were used where appropriate. In all experiments assessing PPI, the average of all prepulse stimulus intensities (76, 82, 85, and 88 dB) is shown in the figures as ‘Percent Prepulse Inhibition’ to allow for easier visualization of the main statistical findings. Interactions with prepulse intensity are reported in the text and occur because experimental effects, such as restraint, are greater at the 76, 82, and 85 dB prepulse intensities than at the 88 dB intensity. Additionally, main effects of prepulse intensity, which occur because percent PPI increases with increasing prepulse intensity, are not reported since they are statistically significant in all analyses conducted.
3. Results
3.1 Experiment 1: Effect of five consecutive days of restraint stress on PPI and startle amplitude
Figure 1 shows that prepulse inhibition increased over days of testing and this increase was attenuated by repeated restraint. Acute restraint did not affect PPI. A three-way ANOVA was used to analyze PPI data from all 3 testing days, with restraint as a between-subjects factor and day and prepulse intensity as within-subjects factors (Fig. 1). Significant main effects of restraint [F(1,18) = 10.71; p = 0.005] and day [F(2,36) = 18.86; p < 0.001] on PPI were detected. There were trends toward interactions between day and restraint (p = 0.053), day and prepulse intensity (p = 0.088), and among day, prepulse intensity, and restraint (p = 0.067). In order to determine on which days restraint affected PPI, data from each day were examined separately using two-way ANOVAs.
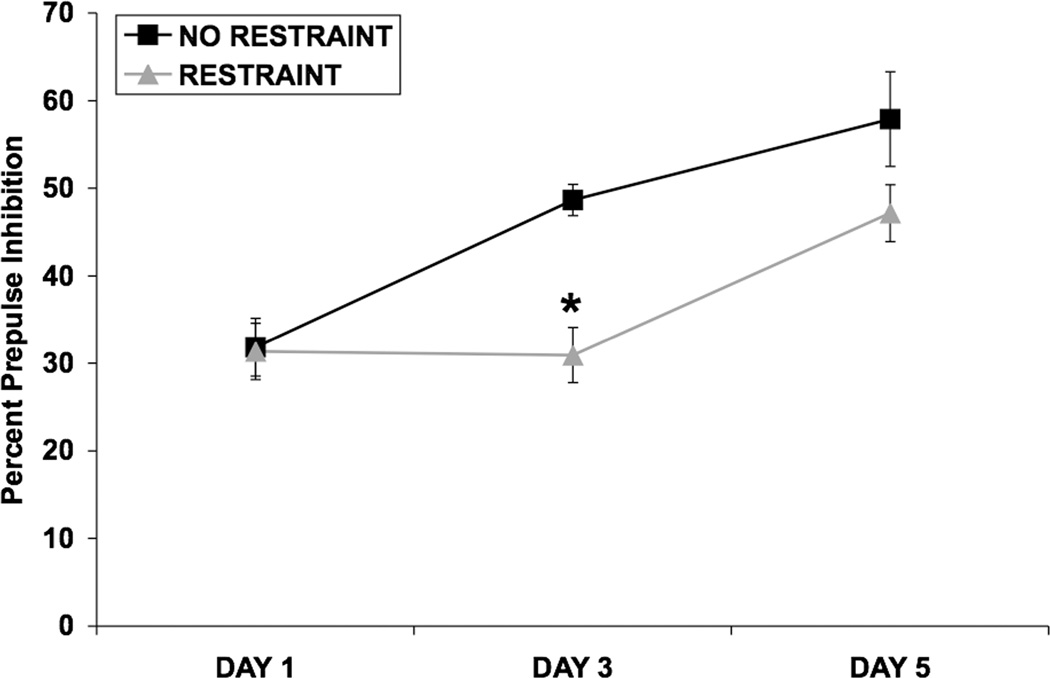
Fig. 1.
Effect of 5 consecutive days of restraint stress on PPI. Values shown are means ± SEMs. For all groups, n = 9–11. The average of all prepulse stimulus intensities (76, 82, 85, and 88 dB) is shown as Percent Prepulse Inhibition. Rats were restrained for 2 hours/day for 5 consecutive days, or were handled briefly and returned to the home cage. PPI was assessed 30 minutes after restraint termination on days 1, 3, and 5. On day 1, restraint did not alter PPI. On day 3, restraint significantly attenuated the increase in PPI caused by repeated testing (*p < 0.001 vs. No Restraint on day 3). On day 5, there was a trend for restraint to attenuate the increase in PPI caused by repeated testing.
A two-way ANOVA showed that restraint did not alter PPI on day 1 (Fig. 1). On day 3, restraint significantly attenuated the increase in PPI caused by repeated testing [F(1,18) = 21.13; p < 0.001] (Fig. 1). A significant prepulse intensity X restraint interaction [F(3,54) = 4.11; p < 0.02] indicated that the effect of restraint on PPI was more robust at lower prepulse intensities (data not shown). On day 5, there was a trend for restraint to attenuate the increase in PPI caused by repeated testing (p = 0.094) (Fig. 1). Analysis of startle amplitude data (not shown) using a two-way ANOVA showed a significant effect of day [F(2,36) = 4.24; p < 0.05], indicating that startle amplitude diminished as the days of testing progressed due to habituation. Restraint stress did not alter startle amplitude on any day.
3.2 Experiment 2: Effect of restraint stress on PPI following repeated PPI testing
In this experiment, the restraint stress sessions began after rats had been repeatedly tested for PPI on each of five consecutive days. This was done so that restraint would only begin after the repeated testing-induced increase in PPI had been achieved. Under these conditions, repeated restraint decreased PPI (Fig. 2). Thus, repeated restraint does not merely attenuate a testing-induced increase in PPI. A two-way ANOVA, with restraint as the between-subjects factor and day as the within-subjects factor, was used to analyze PPI data obtained from the first 5 days of testing, prior to restraint (Fig. 2, left side of dash). A significant main effect of day [F(4,56) = 31.0; p < 0.001] showed that PPI increased over the 5 days of testing. PPI was not different between the two groups prior to the start of restraint (Fig. 2, left side of dash). A two-way ANOVA conducted on startle amplitude data revealed a significant effect of day [F (4,56) = 19.29; p < 0.001], since startle decreased over the days of testing due to habituation (data not shown). In stress-naïve rats, average percent PPI after day 5 of testing (64.01 ± 4.02; Fig. 2, left side of dash) was comparable to that seen in experiment 1 after day 5 of testing (57.88 ± 5.40; Fig. 1).
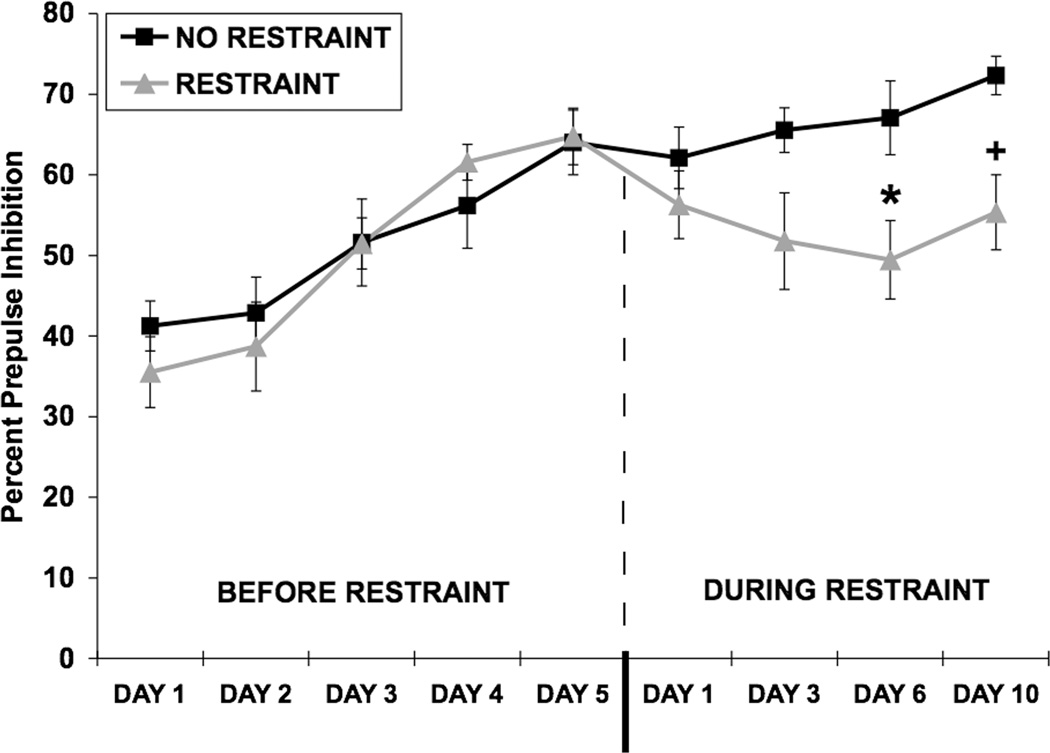
Fig. 2.
Effect of restraint stress on PPI following repeated PPI testing. Values shown are means ± SEMs. For all groups, n = 8. The average of all prepulse stimulus intensities (76, 82, 85, and 88 dB) is shown as Percent Prepulse Inhibition. PPI was assessed once a day, for 5 consecutive days, in the absence of restraint (left side of dash). After day 5 of testing, the rats were divided into two counter-balanced groups based on average percent PPI (left side of dash). After a 2-day rest period, one group of rats was exposed to 2-hour restraint/day for 10 consecutive days. Rats in the control group were handled briefly, but not restrained. Rats were tested for PPI 30 minutes after restraint termination on days 1, 3, 6, and 10. Once restraint exposure began, PPI was decreased in restrained rats compared to non-restrained rats on day 6 (*p = 0.02) and on day 10 (+p < 0.01).
After a 2-day rest period, rats were exposed to 2-hour restraint stress/day for 10 consecutive days (Restraint) or were handled briefly but not restrained (No Restraint) (Fig. 2, right side of dash). A two-way ANOVA conducted on PPI data from days 1, 3, 6, and 10 of restraint revealed a significant main effect of restraint [F(1,14) = 9.56; p < 0.01]. A three-way ANOVA conducted on PPI data from day 5 pre-restraint and days 1, 3, 6, and 10 of restraint revealed a significant main effect of restraint [F(1,14) = 6.60, p < 0.05] and a significant restraint × day interaction [F(4,42) = 2.77, p < 0.05]. The levels of PPI did not continue to increase significantly in the non-restrained group over days 1, 3 and 6, while the experimental group was undergoing restraint (p > 0.05). However, if day 10 of restraint was included in the analysis, the non-restrained group showed an increase in PPI from day 5 of pre-restraint, [F(4,28) = 3.96, p < 0.02]. Separate ANOVAs were used to determine on which days during restraint the restrained and non-restrained groups differed from each other and revealed that there was a marginal difference between the groups on day 3 (p = 0.056), a significant difference on day 6 [F(1,14) = 6.94, p = 0.02], and a significant difference on day 10 [F(1,14) = 10.56, p < 0.01], although the increase in PPI in the non-restrained group may have contributed to the difference on this day of testing. These results suggest that repeated restraint decreased PPI in addition to blunting the increase in PPI caused by repeated testing (Experiment 1). A separate two-way ANOVA showed that restraint did not alter startle amplitude on any day (data not shown).
3.3 Experiment 3: Effect of restraint stress and selective CRF1 receptor blockade on PPI, startle amplitude, and grooming behavior
Again in this experiment, there was no effect of restraint on PPI on day 1, but significant effects on days 3 and 5 (Fig. 3). The CRF1 receptor antagonist, CP-154,526, did not attenuate the effect of stress on any test day. A four-way ANOVA was used to analyze PPI data from all 3 testing days (Fig. 3), with SC injection and restraint as between-subjects factors, and day and prepulse intensity as within-subjects factors. Significant main effects of restraint [F(1,36) = 18.27; p < 0.001] and day [F(2,72) = 14.61; p < 0.001] on PPI were detected. There were significant interactions between prepulse intensity and restraint [F(3,108) = 3.76; p < 0.02] and between day and prepulse intensity [F(6,216) = 3.47; p < 0.005]. To determine on which days restraint affected PPI, data from each day were examined separately using three-way ANOVAs.
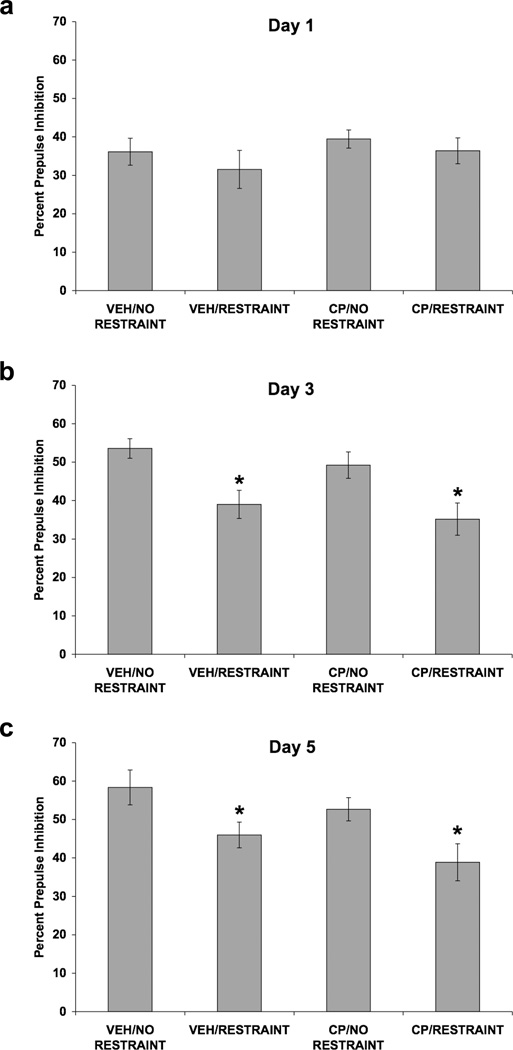
Fig. 3.
Effect of restraint stress and selective CRF1 receptor blockade on PPI. Values shown are mean ± SEMs. For all groups, n = 10. The average of all prepulse stimulus intensities (76, 82, 85, and 88 dB) is shown as Percent Prepulse Inhibition. X-axis shows Pretreatment (SC injection)/Treatment (absence or presence of restraint). Rats were administered a SC injection of CP-154,526 (20.0 mg/kg) or vehicle. Forty-five minutes later, rats were restrained for 2 hours or were handled briefly and returned to the home cage. Rats were subjected to injection and restraint (or brief handling) once a day for 5 consecutive days. Rats were tested for PPI 30 minutes after restraint termination on days 1, 3, and 5. (a) On day 1, neither pretreatment with CP-154,526 nor restraint altered PPI. (b) On day 3, restraint attenuated the decrease in PPI caused by repeated testing (*p < 0.001 vs. No Restraint, main effect). CP-154,526 pretreatment did not alter the effect of restraint on PPI. (c) On day 5, restraint attenuated the increase in PPI caused by repeated testing (*p < 0.01 vs. No Restraint, main effect). CP-154,526 pretreatment did not alter the effect of restraint on PPI.
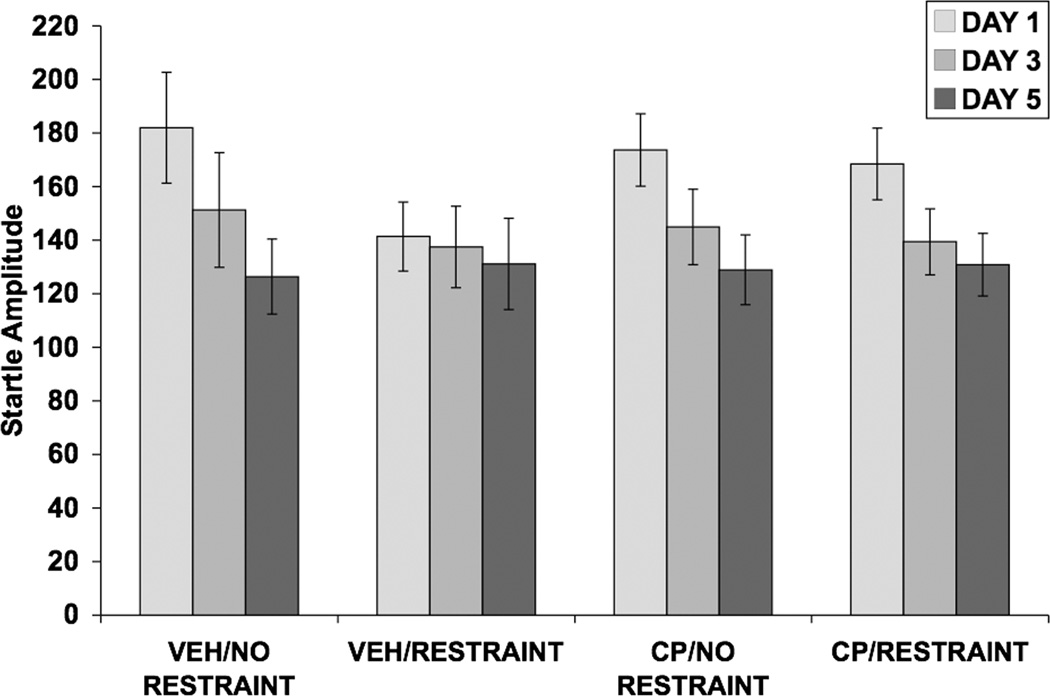
On day 1 (Fig. 3a), neither pretreatment with CP-154,526 nor restraint altered PPI, as no significant main effects or interactions were found. Restraint attenuated the increase in PPI caused by repeated testing on day 3 [F(1,36) = 16.62; p < 0.001] (Fig. 3b) and on day 5 [F(1,36) = 10.67; p = 0.002] (Fig. 3c). On day 5, a significant prepulse × restraint interaction [F(3,108) = 2.82; p < 0.05] was detected. Pretreatment with CP-154,526 did not alter the effect of restraint on PPI observed on days 3 and 5 of restraint. Analysis of startle amplitude data using a three-way ANOVA revealed a significant effect of day [F(2,72) = 18.64; p < 0.001], indicating that startle amplitude decreased over the 3 days of testing due to habituation (Fig. 4). Neither pretreatment with CP-154,526 nor restraint altered startle amplitude. Percent habituation of startle amplitude for all three days of testing is shown in Table 1. There were no significant main effects of SC injection or restraint and no interaction between the two factors.
Fig. 4.
Effect of restraint stress and selective CRF1 receptor blockade on baseline startle amplitude. Values shown are means ± SEMs and were calculated from the startle stimulus alone trials that were used to calculate percent PPI. Startle amplitude was assessed 30 minutes after restraint termination on days 1, 3, and 5. Neither pretreatment with CP-154,526 nor restraint altered startle amplitude.
Table 1.
Percent habituation of the startle response (mean±SEM) from the first block of 6 trials to the last block of 6 trials in Experiments 3 and 6. There were no effects of either CP-154,526 or ASV-30 pretreatment or restraint stress on percent habituation in either experiment.
| Experiment 3 | |||
|---|---|---|---|
| Treatment | DAY 1 | DAY 3 | DAY 5 |
| VEH/NO RESTRAINT | 99.05±23.18 | 81.00±30.12 | 111.35±38.05 |
| VEH/RESTRAINT | 124.10±16.83 | 111.33±26.17 | 88.02±20.80 |
| CP/NO RESTRAINT | 74.26±17.96 | 96.04±21.06 | 159.77±55.55 |
| CP/RESTRAINT | 80.15±16.76 | 134.82±33.89 | 91.22±32.57 |
| Experiment 6 | |||
| Treatment | DAY 1 | DAY 3 | DAY 5 |
| SAL/NO RESTRAINT | 42.63±26.42 | 96.68±22.93 | 61.45±28.17 |
| SAL/RESTRAINT | 70.10±19.97 | 55.84±7.57 | 51.77±17.32 |
| ASV-30/NO RESTRAINT | 26.54±15.39 | 76.31±48.16 | 41.28±19.73 |
| ASV-30/RESTRAINT | 68.02±11.58 | 24.34±11.54 | 49.74±23.81 |
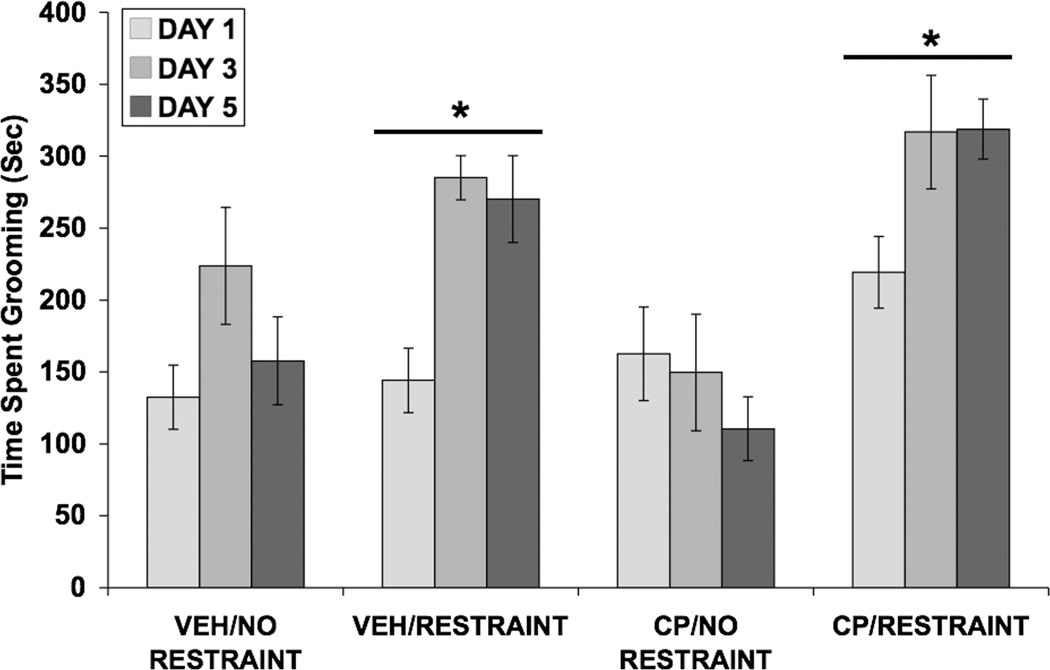
Data for time spent grooming were analyzed using a three-way ANOVA (Fig. 5). Significant main effects of restraint [F(1,36) = 28.69; p < 0.001] and day [F(2,72) = 8.40; p = 0.001], as well as significant interactions between SC injection and restraint [F(1,36) = 4.58; p < 0.05] and between day and restraint [F(2,72) = 5.35; p < 0.01], were found.
Fig. 5.
Effect of restraint stress and selective CRF1 receptor blockade on time spent grooming (in seconds). Values shown are means ± SEMs. For all groups, n = 10. Grooming was assessed for 15 minutes, beginning immediately after restraint termination on days 1, 3, and 5. Restrained groups spent more time grooming than control groups (*p < 0.001 vs. No Restraint, main effect).
3.4 Experiment 4: Effect of CRF and selective CRF1 receptor blockade on PPI, startle amplitude, and grooming behavior
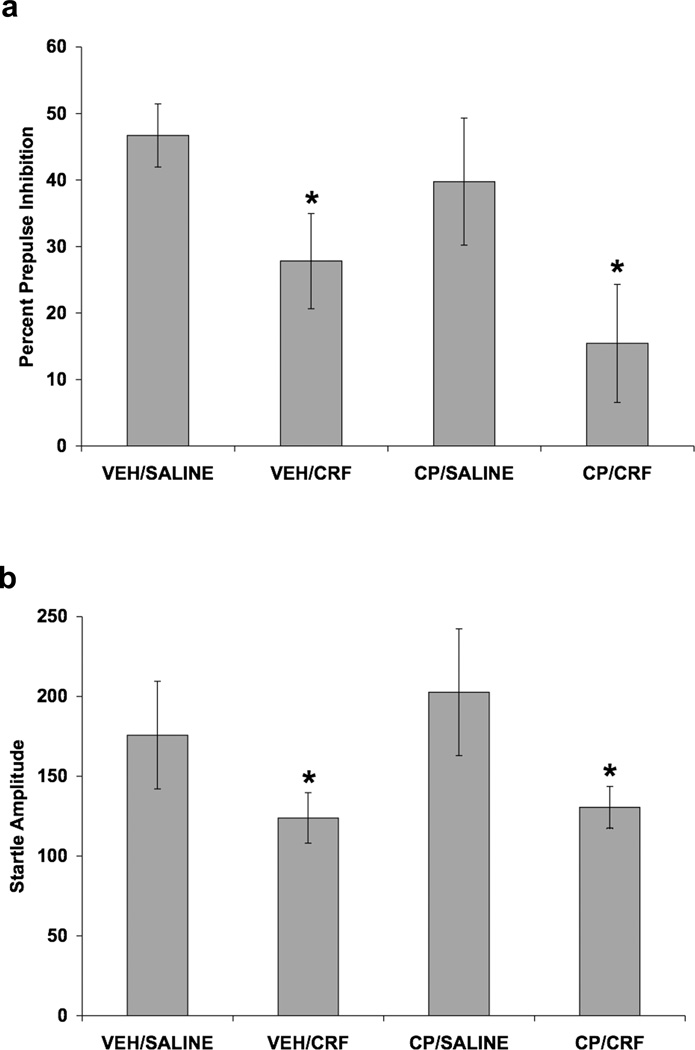
In this experiment, we examined whether the CRF1 receptor antagonist would attenuate the effect of acute exogenous CRF (ICV) administration on PPI. CRF decreased PPI and this effect was not blocked by CP-154,526 (Fig. 6). A three-way ANOVA, with SC injection and ICV infusion as between-subjects factors and prepulse intensity as a within-subjects factor, was used to analyze PPI data (Fig. 6a). ICV infusion of CRF decreased PPI [F(1,22) = 5.63; p < 0.05]. A two-way ANOVA showed that CRF infusion decreased startle amplitude [F(1,22) = 6.90; p < 0.02] (Fig.6b). A two-way ANOVA demonstrated that rats given an ICV infusion of CRF spent more time grooming than the control rats receiving ICV Saline [F(1,21) = 28.10; p < 0.001] (data not shown). Pretreatment with CP-154,526 did not alter the CRF-induced decrease in PPI or startle amplitude, or the CRF-induced increase in grooming behavior.
Fig. 6.
Effect of CRF and selective CRF1 receptor blockade on PPI and baseline startle amplitude. Values shown are mean ± SEMs. For all groups, n = 4–9. X-axis shows Pretreatment (SC injection)/Treatment (ICV infusion). Rats received a SC injection of CP-154,526 (20.0 mg/kg) or vehicle. Two hours and forty-five minutes later, rats received an ICV infusion of 0.3 µg CRF (in 6.0 µl saline) or 6.0 µl saline. Rats were tested for PPI and startle amplitude 30 minutes after ICV infusion. (a) The average of all prepulse stimulus intensities (76, 82, 85, and 88 dB) is shown as Percent Prepulse Inhibition. ICV infusion of CRF decreased PPI (*p < 0.05 vs. Saline, main effect). CP-154,526 pretreatment did not alter the CRF-induced decrease in PPI. (b) Startle amplitude on startle stimulus alone trials that were used to calculate percent PPI. ICV infusion of CRF decreased startle amplitude (*p < 0.05 vs. Saline, main effect). CP-154,526 pretreatment did not alter the CRF-induced decrease in startle amplitude.
3.5 Experiment 5: Effect of CRF and selective CRF1 receptor blockade in the elevated plus maze
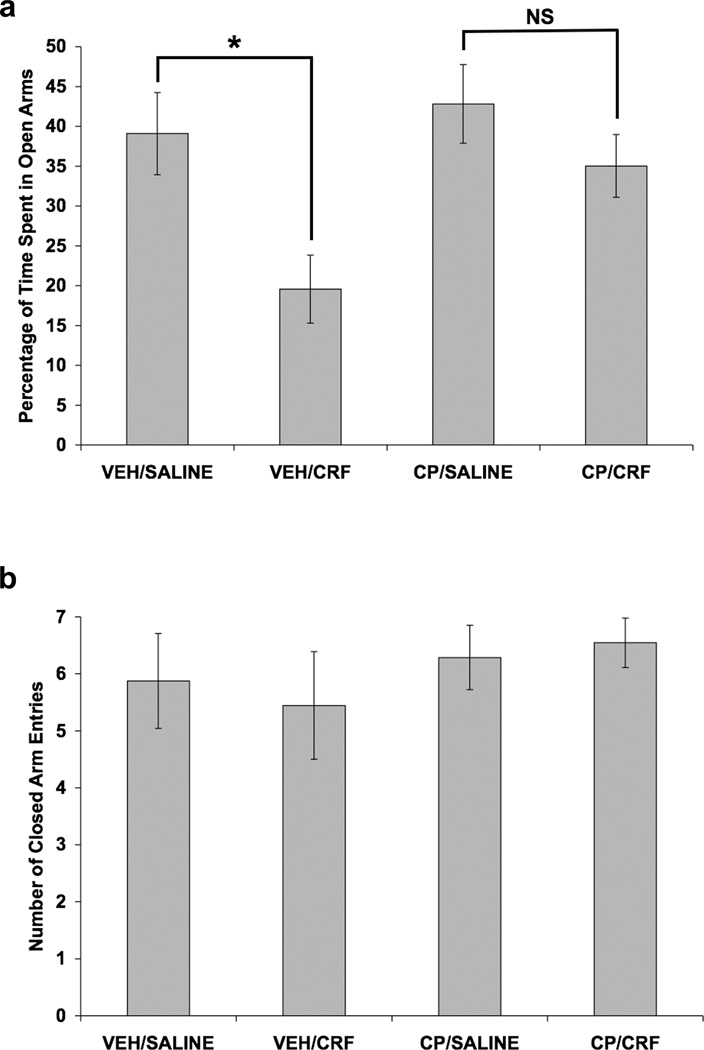
The effect of CP-154,526 on a CRF-induced increase in anxiety-like behavior in the elevated plus maze was also examined. Alone, CRF significantly reduced the percentage of time that rats spent in the open arms of the maze, and this effect was blocked by the CRF1 receptor antagonist (Fig. 7). Data for percentage of time spent in the open arms of the elevated plus maze (Fig. 7a) were analyzed using a two-way ANOVA, with SC injection and ICV infusion as between-subjects factors. Significant main effects of SC injection [F(1,31) = 4.40; p < 0.05] and ICV infusion [F(1,31) = 8.90; p < 0.01] were shown. CRF infusion decreased the percentage of time spent in the open arms of the maze only in vehicle-pretreated rats [t(15) = 2.95; p = 0.01], as shown by a separate independent t-test with Bonferroni correction comparing VEH/SALINE to VEH/CRF. Since the CP/SALINE and CP/CRF groups did not differ significantly by a separate independent t-test, CP-154,526 pretreatment attenuated the CRF-induced increase in anxiety-like behavior in the elevated plus maze. A two-way ANOVA showed that neither CP-154,526 injection nor CRF infusion altered the number of closed arm entries in the elevated plus maze (Fig. 7b).
Fig. 7.
Effect of CRF and selective CRF1 receptor blockade on anxiety-like behavior in the elevated plus maze. Values shown are mean ± SEMs. For all groups, n = 7–11. X-axis shows Pretreatment (SC injection)/Treatment (ICV infusion). Rats received a SC injection of CP-154,526 (20.0 mg/kg) or vehicle. Two hours and forty-five minutes later, rats received an ICV infusion of 1.0 µg CRF (in 5.0 µl saline) or 5.0 µl saline. Ten minutes after ICV infusion, rats were observed in the elevated plus maze for 5 minutes. (a) CRF infusion decreased the percentage of time spent in the open arms of the maze only in vehicle-pretreated rats (*p = 0.010, independent t-test comparing VEH/SALINE to VEH/CRF). Since CP/SALINE and CP/CRF groups did not differ significantly (NS), CP-154,526 pretreatment blocked the CRF-induced increase in anxiety-like behavior in the elevated plus maze. (b) Neither CP-154,526 injection nor CRF infusion altered the number of closed arm entries.
3.6 Experiment 6: Effect of restraint stress and selective CRF2 receptor blockade on PPI, startle amplitude, and grooming behavior
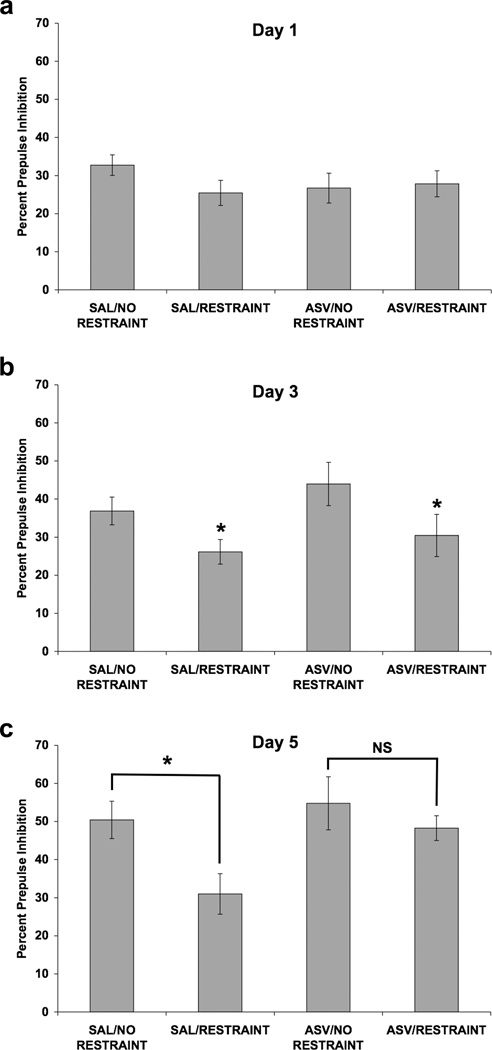
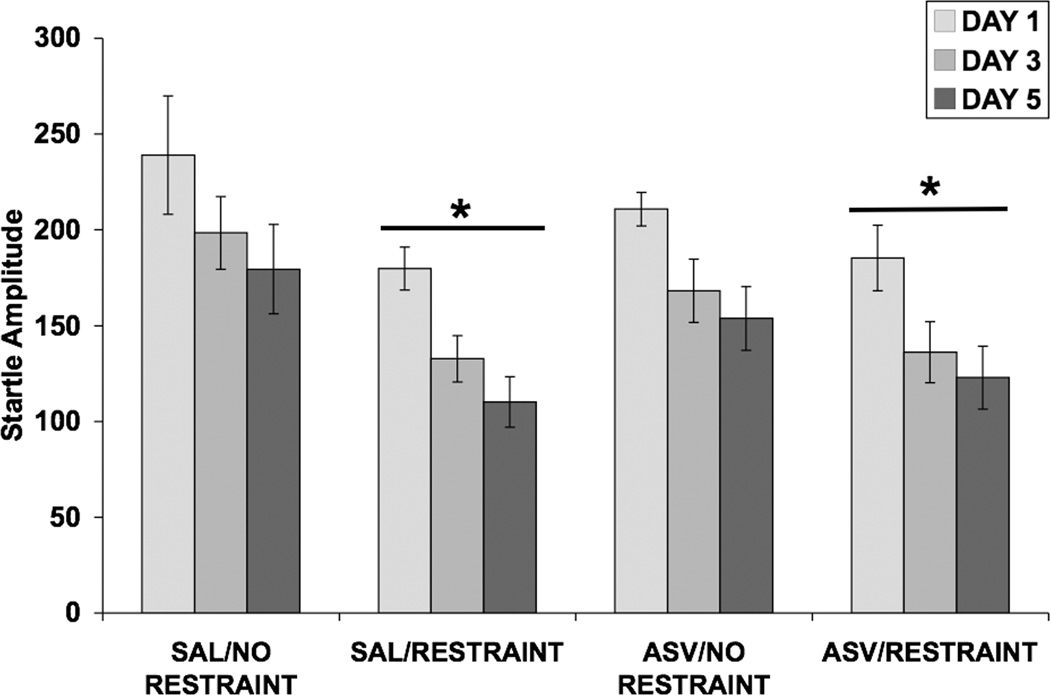
In this experiment we examined whether the selective CRF2 receptor antagonist, ASV-30, would attenuate the effect of repeated restraint on PPI. Once again, Figure 8 shows that repeated, but not acute, restraint had a significant effect on PPI and that ASV-30 blocked this effect on day 5, but not on day 3, of restraint. A four-way ANOVA was used to analyze PPI data from all 3 days of testing (Fig. 8), with ICV infusion and restraint as between-subjects factors, and day and prepulse intensity as within-subjects factors. Significant main effects of restraint [F(1,30) = 7.37; p < 0.02] and day [F(2,60) = 27.47; p < 0.001] on PPI were detected. Additionally, there were significant interactions between day and ICV infusion [F(2,60) = 3.34; p < 0.05], between prepulse intensity and restraint [F(3,90) = 5.90; p = 0.001], between day and prepulse intensity [F(6,180) = 4.56; p < 0.001], and among day, prepulse intensity, and restraint [F(6,180) = 3.65; p = 0.002]. In order to understand this wide array of interactions, PPI data from each day were examined separately using three-way ANOVAs.
Fig. 8.
Effect of restraint stress and selective CRF2 receptor blockade on PPI. Values shown are mean ± SEMs. For all groups, n = 8–9. The average of all prepulse stimulus intensities (76, 82, 85, and 88 dB) is shown as Percent Prepulse Inhibition. X-axis shows Pretreatment (ICV infusion)/Treatment (absence or presence of restraint). Rats received an ICV infusion of 10.0 µg ASV-30 (in 5.0 µl saline) or 5.0 µl saline. Ten minutes later, rats were restrained for 2 hours or were handled briefly and returned to the home cage. Rats were subjected to infusion and restraint (or brief handling) once a day for 5 consecutive days. Rats were tested for PPI 30 minutes after restraint termination on days 1, 3, and 5. (a) On day 1, neither pretreatment with ASV-30 nor restraint altered PPI. (b) On day 3, restraint attenuated the decrease in PPI caused by repeated testing (*p < 0.05 vs. No Restraint, main effect). ASV-30 pretreatment did not alter the effect of restraint on PPI on day 3. (c) On day 5, restraint decreased PPI only in rats that received ICV saline, as a significant difference was present between the SAL/NO RESTRAINT and SAL/RESTRAINT groups (*p = 0.018) and was absent (NS) between the ASV/NO RESTRAINT and ASV/RESTRAINT groups (separate two-way ANOVAs). Thus, ASV-30 pretreatment blocked the restraint-induced decrease in PPI on day 5 of restraint.
On day 1 (Fig. 8a), there was no effect of ASV-30 infusion or restraint and no interaction between these two factors. On day 3 (Fig. 8b), restraint attenuated the increase in PPI due to repeated testing [F(1,30) = 6.75; p < 0.02]. A significant prepulse × restraint interaction [F(3,90) = 3.92; p = 0.011] indicated that the effect of restraint on PPI was more robust at the lower prepulse intensities. On day 5 (Fig. 8c), there were significant main effects of ICV infusion [F(1,30) = 4.33; p < 0.05] and restraint [F(1,30) = 6.25; p < 0.02], and a significant prepulse × restraint interaction [F(3,90) = 8.56; p < 0.001]. Separate two-way ANOVAs were conducted to determine whether the effect of restraint was present only in saline-pretreated rats. Indeed, restraint decreased PPI only in rats that received ICV saline, as a significant difference was present between the SAL/NO RESTRAINT and SAL/RESTRAINT groups [F(1,15) = 7.10; p < 0.02] and was absent between the ASV/NO RESTRAINT and ASV/RESTRAINT groups. Thus, ASV-30 pretreatment blocked the restraint-induced decrease in PPI on day 5 of restraint.
Analysis of startle amplitude data (Fig. 9) using a three-way ANOVA demonstrated that restraint decreased startle amplitude [F(1,30) = 10.54; p < 0.01]. A significant effect of day [F(2,60) = 30.12; p < 0.001] showed that startle amplitude decreased as the days of testing progressed due to habituation. ASV-30 pretreatment did not alter the restraint-induced decrease in startle amplitude. Percent within-session habituation of startle amplitude for all three days of testing is shown in Table 1. There were no significant main effects of ICV infusion or restraint and no interaction between the two factors. There was a significant day × restraint interaction [F(2,60) = 3.298; p < 0.05]. Separate two-way ANOVAs conducted on data from days 1, 3, and 5 revealed that this interaction was due to a trend for restraint to increase habituation on day 1 (p = 0.078) and a trend for restraint to decrease habituation on day 3 (p = 0.086).
Fig. 9.
Effect of restraint stress and selective CRF2 receptor blockade on baseline startle amplitude. Values shown are mean ± SEMs and were calculated from the startle stimulus alone trials that were used to calculate percent PPI. X-axis shows Pretreatment (ICV infusion)/Treatment (absence or presence of restraint). Startle amplitude was assessed 30 minutes after restraint termination on days 1, 3, and 5. Restraint decreased startle amplitude (*p < 0.01 vs. No Restraint, main effect). ASV-30 pretreatment did not alter the restraint-induced decrease in startle amplitude.
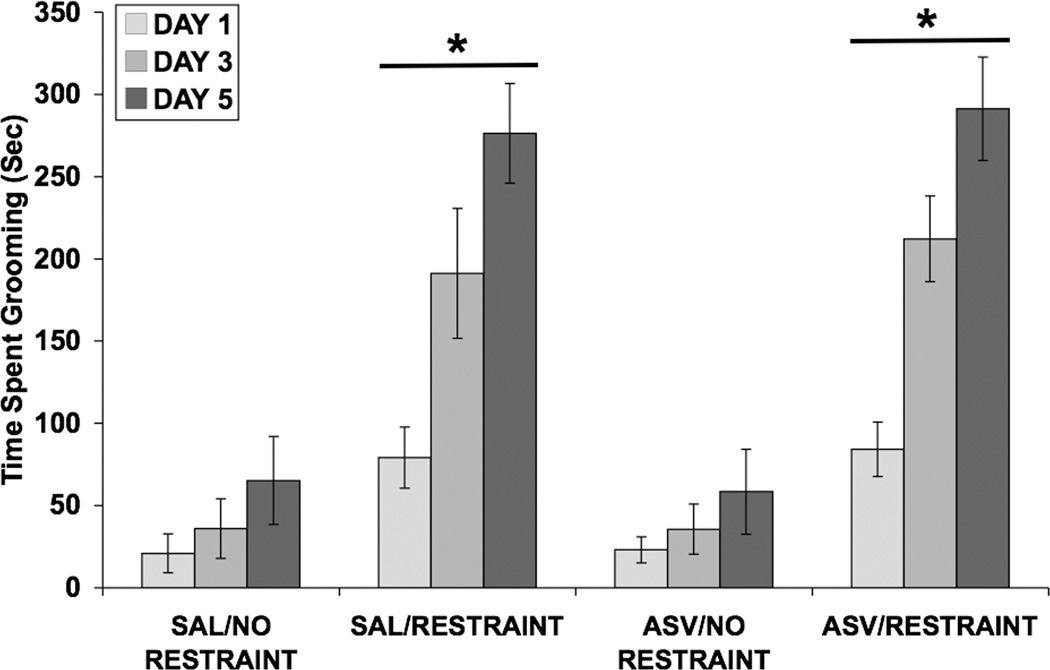
Figure 10 shows the effect of restraint with and without ASV-30 pretreatment on time spent grooming. A three-way ANOVA demonstrated that the restrained groups spent more time grooming than the control groups [F(1,30) = 62.88; p < 0.001]. A significant effect of day [F(2,60) = 38.53; p < 0.001] indicated that time spent grooming increased over the three observation periods and a significant day × restraint interaction [F(2,60) = 17.82; p < 0.001] showed that restraint increased grooming to a greater extent as the days progressed. ASV-30 pretreatment did not alter the restraint-induced increase in grooming behavior.
Fig. 10.
Effect of restraint stress and selective CRF2 receptor blockade on time spent grooming (in seconds). Values shown are means ± SEMs. For all groups, n = 8–9. Grooming was assessed for 15 minutes, beginning immediately after restraint termination on days 1, 3, and 5. Restrained rats spent more time grooming than control rats (*p < 0.001 vs. No Restraint, main effect). Administration of ASV-30 prior to restraint did not alter the restraint-induced increase in grooming behavior.
3.7 Experiment 7: Effect of repeated Ucn III infusion on PPI and startle amplitude
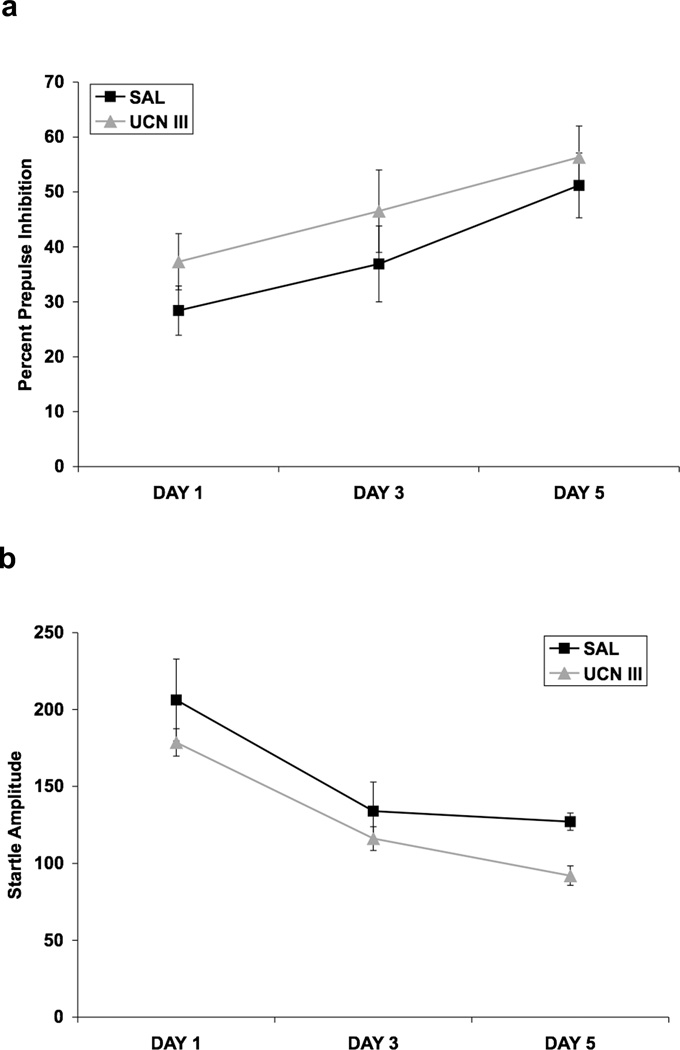
Figure 11a shows that repeated ICV infusion of Ucn III did not alter PPI on any test day. Ucn III was infused once a day for five consecutive days, and PPI was assessed on days 1, 3, and 5. ANOVA with ICV infusion (saline vs. Ucn III) as a between-subjects factor showed no effect of Ucn III (p > 0.05), and there were no significant interactions involving the ICV infusion factor. Figure 11b shows the effect of Ucn III on baseline startle amplitude. Here, there was a significant effect of Ucn III [F(1,10) = 8.26, p < 0.02], and a significant effect of day [F (2,20) = 15.40, p < 0.001], but no interaction with day.
Fig. 11.
Effect of repeated ICV infusion of Ucn III on PPI (a) and startle amplitude (b). Values shown are means ± SEMs. For all groups, n = 6. The average of all prepulse intensities is shown as Percent Prepulse Inhibition. The average startle amplitude across all trials on which the startling stimulus was presented alone is shown as Startle Amplitude. Rats received an ICV infusion of either saline (5.0 µl) or mouse Ucn III (20.0 µg) once/day on each of 5 consecutive days. The rats were tested for PPI 2.5 hours after the ICV infusion on days 1, 3, and 5. (a) Ucn III did not affect PPI on any of the test days. (b) There was an overall decrease in startle amplitude due to Ucn III, which was clearly due to effects on day 5.
4. Discussion
The present studies were designed to examine the effects of acute and repeated restraint stress on PPI in BN rats, and to determine whether the effect of restraint stress on PPI could be attenuated by a CRF1 and/or a CRF2 receptor antagonist. The major findings of the present studies were that 1) repeated, but not acute, restraint stress attenuated the increase in PPI caused by repeated PPI testing, and PPI was reduced by restraint following repeated PPI testing, 2) CRF2 receptor blockade attenuated the effect of repeated restraint stress on PPI, but had no effect on restraint stress-induced changes in startle amplitude or on grooming behavior, 3) repeated ICV infusion of the selective CRF2 receptor agonist, Ucn III, did not attenuate PPI on any test day, and 4) CRF1 receptor blockade did not alter the effect of repeated restraint (or exogenous CRF) on PPI, startle amplitude, or grooming behavior, but did attenuate the anxiogenic effect of CRF. Although repeated restraint decreased PPI, restraint did not have acute effects on PPI, as exogenous CRF administration does.
In the first experiment, stress-naïve rats exhibited an increase in PPI over the 3 testing sessions of the 5-day experiment. This increase was significantly blunted in repeatedly restrained rats on day 3 and was marginally blunted on day 5. The second experiment showed that restraint stress decreased PPI in rats exhibiting elevated levels of PPI due to repeated PPI testing prior to restraint exposure. Thus, restraint stress decreased PPI in addition to blunting the increase in PPI caused by repeated testing. This suggests that stress may alter sensorimotor gating in adult animals in two ways. Existing literature shows that early-life stress, particularly maternal separation and isolation-rearing, results in diminished PPI in adulthood (Weiss and Feldon, 2001). The effect of restraint stress on PPI in adult rodents has been examined in only a few studies to date and these findings are inconsistent. In one experiment, exposure to 1 or 11 days of 15–20 minute restraint stress did not alter PPI (Acri, 1994; Faraday et al., 1999). In another experiment, four days of 2-hour restraint exposure did not alter PPI, when PPI was assessed 3 weeks after restraint ended (Bijlsma et al., 2009). Exposure to 20-minutes of restraint for 3 weeks has been shown to decrease PPI on day 20 in male Long-Evans rats but not in male Sprague-Dawley rats (Faraday, 2002). Perhaps increasing the length of each restraint session beyond 20 minutes, or examining PPI soon after restraint termination, would have resulted in decreased PPI in the studies in which no effect of restraint was observed. It is evident that rat strain, number of days of restraint exposure, duration of each restraint session, and the time at which PPI is assessed post-restraint are important factors in the effects of restraint on PPI.
We used ASV-30 to examine whether CRF2 receptor activation mediates the effect of restraint on PPI. As in our other experiments, one day of restraint did not affect PPI. On day 3 of restraint, there was an attenuation of the increase in PPI due to repeated testing and ASV-30 pretreatment did not alter this effect of restraint. However, on day 5 of stress, ASV-30 pretreatment blocked the effect of restraint on PPI. Although it is unclear why ASV-30 blocked the effect of restraint on PPI on day 5 but not on day 3, an intriguing possibility involves changes in CRF receptor localization following stress exposure. A recent study demonstrated that, in unstressed rats, CRF1 receptors were primarily localized to the plasma membrane while CRF2 receptors were mainly in the cytoplasm of dorsal raphe nucleus neurons (Waselus et al., 2009). Interestingly, 24 hours after swim stress, this localization changed and CRF1 receptors became more apparent in the cytoplasm while CRF2 receptors were localized to the plasma membrane (Waselus et al., 2009). In locus coeruleus neurons, swim stress caused CRF1 receptor internalization which was apparent as early as one hour after swim stress, with a greater effect at 24 hours, highlighting the persistence of the internalization (Reyes et al., 2008). In another study, CRF1 receptors were down-regulated in the cortex and hippocampus (CA1 and CA3) beginning 2 hours, and lasting up to 24 hours, after the termination of one 30-minute restraint period (Greetfeld et al., 2009). In the study by Greetfeld and colleagues, reductions in CRF1 receptor binding were evident for up to 7 days post-stress, while CRF2 receptors were up-regulated in the cortex at 4 and 24 hours post-stress and in the hippocampal dentate gyrus beginning 24 hours after stress. Thus, in our studies, it is possible that CRF2 receptors were mainly localized to the cytoplasm during the initial days of restraint stress. As the days of restraint continued, CRF2 receptors may have become present at the plasma membrane in sufficient numbers that ASV-30 binding could modulate behavior and attenuate the effect of restraint stress on PPI. Thus, dynamic stress-induced changes in CRF1 and CRF2 receptor localization at the plasma membrane may explain why repeated restraint affects PPI while acute restraint has no effect, and why several days of restraint are required for CRF2 receptor blockade to affect behavior.
The fact that ASV-30 attenuated the effect of restraint on PPI suggests the possibility that Ucn II or Ucn III, peptides that have a selective affinity for the CRF2 receptor, rather than CRF, may have mediated the effect of repeated restraint on PPI. In light of this possibility, we examined the effect of repeated ICV infusion of Ucn III on PPI. We found that infusion of this peptide had no effect on PPI on any test day. These results suggest that activation of the CRF2 receptor alone is not sufficient to produce effects on PPI that mimic those produced by CRF or stress. A second possibility is that Ucn III effects at the CRF2 receptor are mediated by different signal transduction pathways than the effects of either CRF or stress (Brar et al., 2004; Hauger et al., 2006). It is possible that Ucn III would have decreased PPI had the rats undergone repeated restraint prior to infusion. This possibility will be tested in future experiments.
It is also possible that ASV-30 blocked the effect of restraint on PPI on day 5, but not on day 3, because neurotransmitters other than CRF may have initially mediated the effect of restraint on PPI. Since norepinephrine (NE) is released in response to restraint (Carrasco and Van de Kar, 2003), decreases PPI (Alsene et al., 2006; Carasso et al., 1998), and stimulates the release of CRF (Plotsky, 1987; Szafarczyk et al., 1987), it is possible that NE mediated the effect of restraint on PPI on day 3. As the days of restraint continued, a NE-induced release of CRF (Berridge and Dunn, 1989) may have allowed ASV-30 to attenuate the effect of restraint on PPI on day 5. It may also be that serotonin (5-HT) played a role in the delayed effect of ASV-30 on restraint stress in our study. In rats, acute restraint stress increases 5-HT release in the central nucleus of the amygdala and this effect is blocked by pretreatment with a non-selective CRF receptor antagonist, suggesting that stress-induced 5-HT release is mediated by central CRF receptor activation (Mo et al., 2008). Additionally, CRF2 receptors are up-regulated in the dorsal raphe nucleus, a primary site of forebrain-projecting serotonergic neurons (Jacobs and Azmitia, 1992), in response to both acute (Waselus et al., 2009) and chronic (Lukkes et al., 2009) stress exposure. Anatomically, the central nucleus of the amygdala is one of the major sources of CRF innervation to the dorsal raphe nucleus (Gray, 1993) and the dorsal raphe nucleus provides 5-HT innervation to the central nucleus of the amygdala (Petrov et al., 1994). Thus, it is possible that repeated restraint stress increased 5-HT release in the central nucleus of the amygdala, which caused sufficient up-regulation of CRF2 receptors in the dorsal raphe nucleus by day five of restraint such that ASV-30 was able to effectively antagonize the CRF2 receptors on this day. However, prior to day five of restraint, 5-HT itself may have been responsible for the restraint-induced decrease in PPI, as drugs that cause 5-HT release have been shown to reduce PPI (Kehne et al., 1996; Mansbach et al., 1989; Martinez and Geyer, 1997).
To our knowledge, these are the first studies to examine whether and which CRF receptor mediates the effect of restraint stress on PPI in rats. We demonstrated that CRF2 receptor activation appeared to mediate the effect of repeated restraint stress on PPI in BN rats under the parameters used in this study, while CRF1 receptor activation did not mediate this effect of stress. In 2004, Risbrough et al. showed that the CRF1 receptor mediated a CRF-induced decrease in PPI in mice. It has also been shown that pretreatment with a selective CRF1 receptor antagonist significantly increases PPI in CRF over-expressing mice (Groenink et al., 2008). In addition to the fact that mice were used in this study, it is possible that chronic over-expression of the transgene caused a developmental abnormality or adaptation of the CRF receptor system. In our studies, the CRF1 receptor antagonist did not attenuate the effect of exogenously administered CRF in rats. More studies are needed to determine whether the differences between the results of our studies and those of others are due to species effects (mice vs. rats), or are peculiar to the BN rats used here. Future studies will examine how pretreatment with selective CRF receptor antagonists affect the restraint-induced decrease in PPI in other rat strains such as the WKY rats, which are less sensitive to the effects of both exogenous CRF and stress than BN rats (Conti, 2005; Conti et al., 2002; Conti et al., 2009; Sutherland et al., 2010; Sutherland et al., 2008). These studies will reveal whether our findings generalize to other rat strains or whether our findings are unique to BN rats, which could perhaps make the BN rat a model for dysfunctional behavioral responses upon stress-induced activation of the CRF system. Overall, examining additional rat strains would greatly improve our understanding of how stress affects sensorimotor gating via the endogenous CRF system. Since 2.5 hours elapsed between the onset of restraint stress and PPI testing in our experiment, a second possibility for the lack of an effect of CP-154,526 is that we were assessing PPI at a time-point when CRF1 receptors were down-regulated and/or internalized such that CP-154,526 could not have altered behavior.
Although CP-154,526 did not attenuate the effect of CRF on PPI in our study it did attenuate the anxiogenic effect of CRF in the elevated plus maze, as previously shown by Zorrilla et al. (2002). Thus, CP-154,526 functioned effectively as a CRF1 receptor antagonist in our hands and the lack of an effect of the drug in the PPI experiments was not due to improper preparation or injection of the compound, ineffective dosing, or choosing a sub-optimal pretreatment period. It is possible that repeated stress altered CRF1 receptor dynamics such that the antagonist was ineffective in the PPI experiments. Nevertheless, in these experiments, the CRF1 receptor antagonist also did not attenuate the effect of acute CRF even though the antagonist was administered prior to the agonist.
In these experiments, neither ASV-30 nor CP-154,526 affected baseline startle or the CRF- or stress-induced decrease in startle amplitude. Since we have previously shown that repeated restraint stress decreases startle amplitude in some recombinant inbred rat strains with a BN and a Spontaneously Hypertensive Rat progenitor (Conti and Printz, 2003) and that ICV infusion of CRF decreases startle amplitude in BN rats (Sutherland et al., 2008), the effect of both restraint stress and CRF infusion on startle amplitude in BN rats is not unique to these experiments. Typically, however, CRF infusion increases startle amplitude in WKY and Sprague-Dawley rats (Conti et al., 2002; Jones et al., 1998; Liang et al., 1992; Schulz et al., 1996; Swerdlow et al., 1986; Walker et al., 2009), and this effect is blocked by pretreatment with a selective CRF1 receptor antagonist (Schulz et al., 1996; Walker et al., 2009). In mice, pretreatment with either a CRF1 or CRF2 receptor antagonist attenuates the CRF-induced increase in startle amplitude, demonstrating that the two CRF receptors act in concert to mediate this effect of CRF (Risbrough et al., 2003). However, transgenic mice over-expressing CRF show decreased startle amplitude compared to wild-type controls (Dirks et al., 2002). Thus, stress-induced (Conti and Printz, 2003) and CRF over-expression-induced (Dirks et al., 2002) reductions in startle amplitude have been previously observed and BN rats are not unique in this feature. It is possible that CRF1 receptors mediate stress- and CRF-induced increases in startle amplitude but not stress- and CRF-induced decreases in startle amplitude.
In rats, both stressful stimuli (Dunn et al., 1987; Spruijt et al., 1992) and ICV infusion of CRF (Dunn et al., 1987; Jones et al., 1998; Lazosky and Britton, 1991) have been shown to increase grooming behavior. In our studies, the restraint stress-induced increase in grooming behavior was not affected by either CP-154,526 or ASV-30 pretreatment, indicating that this effect of restraint was not mediated by activation of either CRF receptor type alone in BN rats or that blockade of both receptors is required to reduce the effect of stress on grooming. We also showed that CP-154,526 pretreatment did not alter the CRF-induced increase in grooming behavior, even though it attenuated a CRF-induced increase in anxiety-like behavior in the elevated plus maze. Our findings are in contrast to previous findings that a CRF1 receptor antagonist attenuates stress-induced (Hotta et al., 1999) and CRF-induced increases in grooming behavior (Howard et al., 2008). Although we did not examine the effect of CRF2 receptor blockade on the CRF-induced increase in grooming, it is possible that CRF2 receptors mediate this effect of CRF in BN rats. However, treatment with a CRF2 receptor agonist does not increase grooming behavior in Sprague-Dawley rats (Howard et al., 2008). Since we observed grooming after a 2-hour restraint period, it is possible that changes in CRF receptor localization have already occurred, such that CRF1 receptors have become internalized while CRF2 receptors are localized to the plasma membrane (Waselus et al., 2009). Thus, we may have assessed grooming behavior at a time when the main CRF receptor that is present at the plasma membrane is the CRF receptor that does not appear to mediate grooming behavior (Howard et al., 2008).
In conclusion, our studies demonstrate that restraint stress decreases PPI, in addition to blunting the increase in PPI caused by repeated testing, in BN rats. CRF1 receptor activation does not appear to mediate the effect of repeated restraint (or exogenous CRF) on PPI, startle amplitude, or grooming behavior under the experimental parameters used here. CRF2 receptor activation did, however, mediate the effect of repeated restraint stress on PPI in these experiments. It would be interesting to examine whether CRF1 and CRF2 receptors mediate stress and/or CRF effects on PPI at different times post-treatment, given the dynamic effects of stress and CRF on CRF receptor localization. These findings highlight the effect that stress can have on adult animals’ information processing abilities, and elucidate a possible mechanism for the effect of restraint stress on PPI. Our studies, along with future studies aimed at understanding the mechanisms by which stress affects sensorimotor gating in more detail, may have important clinical applications for informing new treatments for people afflicted with psychiatric disorders in which information processing deficits are a hallmark.
Research highlights.
Repeated, but not acute, restraint stress decreases prepulse inhibition in rats.
CRF2 receptor blockade attenuates the effect of repeated restraint stress on PPI.
CRF1 receptor blockade does not alter the effect of repeated restraint stress on PPI.
The restraint stress-induced decrease in PPI is mediated by CRF2 receptors in rats.
Acknowledgments
Research was supported by MH065467, AA017367, and 5T32NS041224-08. The funding sources had no role in the conduct of this research or in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that, except for income received from our primary employers, no financial support or compensation has been received for research and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Acri JB. Nicotine modulates effects of stress on acoustic startle reflexes in rats: dependence on dose, stressor and initial reactivity. Psychopharmacology. 1994;116:255–265. doi: 10.1007/BF02245326. [DOI] [PubMed] [Google Scholar]
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacology. 2006;31:2150–2161. doi: 10.1038/sj.npp.1300989. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Dunn AJ. Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J Neurosci. 1989;9:3513–3521. doi: 10.1523/JNEUROSCI.09-10-03513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma EY, Oosting RS, Olivier B, Groenink L. Disrupted startle modulation in animal models for affective disorders. Behav Brain Res. 2009;208:383–390. doi: 10.1016/j.bbr.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- Carasso BS, Bakshi VP, Geyer MA. Disruption in prepulse inhibition after alpha-1 adrenoceptor stimulation in rats. Neuropharmacology. 1998;37:401–404. doi: 10.1016/s0028-3908(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, 2nd, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Conti LH. Characterization of the effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in Brown Norway and Wistar-Kyoto rats. Eur J Pharmacol. 2005;507:125–134. doi: 10.1016/j.ejphar.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Conti LH, Berridge CW, Tayler JE. Both corticotropin-releasing factor and apomorphine reduce prepulse inhibition following repeated central infusion of corticotropin-releasing factor. Pharmacol Biochem Behav. 2006;85:261–272. doi: 10.1016/j.pbb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Conti LH, Costill JE, Flynn S, Tayler JE. Effects of a typical and an atypical antipsychotic on the disruption of prepulse inhibition caused by corticotropin-releasing factor and by rat strain. Behav Neurosci. 2005;119:1052–1060. doi: 10.1037/0735-7044.119.4.1052. [DOI] [PubMed] [Google Scholar]
- Conti LH, Murry JD, Ruiz MA, Printz MP. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology. 2002;161:296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- Conti LH, Printz MP. Rat strain-dependent effects of repeated stress on the acoustic startle response. Behav Brain Res. 2003;144:11–18. doi: 10.1016/s0166-4328(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Conti LH, Sutherland JE, Muhlhauser CM. Interaction between the effects of corticotropin-releasing factor and prepulse parameters on prepulse inhibition in two inbred rat strains and the F1 generation of a cross between them. Behav Brain Res. 2009;200:165–172. doi: 10.1016/j.bbr.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, Raynard RC. Posttraumatic obsessive-compulsive disorder: a three-factor model. Psychiatry. 1999;62:313–324. doi: 10.1080/00332747.1999.11024877. [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, Olivier B. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol Psychiatry. 2002;51:583–590. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW, Lai YI, Yachabach TL. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Faraday MM. Rat sex and strain differences in responses to stress. Physiol Behav. 2002;75:507–522. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Faraday MM, O'Donoghue VA, Grunberg NE. Effects of nicotine and stress on startle amplitude and sensory gating depend on rat strain and sex. Pharmacol Biochem Behav. 1999;62:273–284. doi: 10.1016/s0091-3057(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Greetfeld M, Schmidt MV, Ganea K, Sterlemann V, Liebl C, Muller MB. A single episode of restraint stress regulates central CRH receptor expression and binding in specific areas of the mouse brain. J Neuroendocrinol. 2009 doi: 10.1111/j.1365-2826.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64:169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, de Graaff M, Peeters BW, Millan MJ, Olivier B. CRF1 not glucocorticoid receptors mediate prepulse inhibition deficits in mice overexpressing CRF. Biol Psychiatry. 2008;63:360–368. doi: 10.1016/j.biopsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higelin J, Py-Lang G, Paternoster C, Ellis GJ, Patel A, Dautzenberg FM. 125I-Antisauvagine-30: a novel and specific high-affinity radioligand for the characterization of corticotropin-releasing factor type 2 receptors. Neuropharmacology. 2001;40:114–122. doi: 10.1016/s0028-3908(00)00105-2. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic and temporal factors in the evocation of startle. J Acoust Soc Am. 1968;43:269–282. doi: 10.1121/1.1910776. [DOI] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Arai K, Demura H. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res. 1999;823:221–225. doi: 10.1016/s0006-8993(99)01177-4. [DOI] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology. 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology. 1998;138:124–132. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu Rev Clin Psychol. 2006;2:161–197. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Padich RA, McCloskey TC, Taylor VL, Schmidt CJ. 5-HT modulation of auditory and visual sensorimotor gating: I. Effects of 5-HT releasers on sound and light prepulse inhibition in Wistar rats. Psychopharmacology. 1996;124:95–106. doi: 10.1007/BF02245609. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Lazosky AJ, Britton DR. Effects of 5-HT-1A receptor agonists on CRF-induced behavior. Psychopharmacology. 1991;104:132–136. doi: 10.1007/BF02244567. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Braff DL, Geyer MA. Prepulse inhibition of the acoustic startle response is disrupted by N-ethyl-3,4-methylenedioxyamphetamine (MDEA) in the rat. Eur J Pharmacol. 1989;167:49–55. doi: 10.1016/0014-2999(89)90746-2. [DOI] [PubMed] [Google Scholar]
- Martinez DL, Geyer MA. Characterization of the disruptions of prepulse inhibition and habituation of startle induced by alpha-ethyltryptamine. Neuropsychopharmacology. 1997;16:246–255. doi: 10.1016/S0893-133X(96)00240-0. [DOI] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res Bull. 2008;76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego; 1986. [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Sutherland JE, Burian LC, Covault J, Conti LH. The effect of restraint stress on prepulse inhibition and on corticotropin-releasing factor (CRF) and CRF receptor gene expression in Wistar-Kyoto and Brown Norway rats. Pharmacol Biochem Behav. 2010;97:227–238. doi: 10.1016/j.pbb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Page ME, Conti LH. The effect of corticotropin-releasing factor on prepulse inhibition is independent of serotonin in Brown Norway and Wistar-Kyoto rats. Pharmacol Biochem Behav. 2008;89:324–337. doi: 10.1016/j.pbb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology. 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Malaval F, Laurent A, Gibaud R, Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinology. 1987;121:883–892. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34:1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology. 2001;156:305–326. doi: 10.1007/s002130100800. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]