Considerable evidence suggests that the S-nitrosation of cysteine residues, thought to regulate the activities of many proteins, is involved in the uptake and intracellular trafficking of NO.1 Indeed, to absorb the neutral NO with thiols, a redox reaction is needed to form S-nitrosothiols (RSNOs).1-3
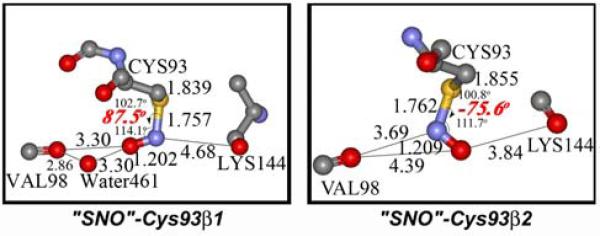
A structure of S-nitrosocysteine in hemoglobin proteins was first reported by Arnone and coworkers in 1998.4a Interestingly, the resulting protein structure obtained under non-biological conditions (NO pressure, anaerobic) exhibits unexpected C-S-N-O dihedral angles of 88° and -76° for Cys93 of β1 and β2 subunits. The crystallographic results contradict the planar geometries of all RSNOs in small molecule crystallographic data,5 and from quantum mechanical calculations.6 Recently, Arnone et al. refined the X-ray data again and reported that the electron density of the SNO-Cys98(F9)β could not be fit with a planar syn or an anti S-nitrosothiol model. They found C-S-N-O dihedral angles between 70° and 90° for SNO-Cys93(F9)β1 and SNO-Cys93(F9)β2.(Fig. 1)4b To explain this, they proposed that a N-centered radical, Cys-S-N·-OH, was present and stabilized by hydrogen bonding with Val98. Such a radical was previously proposed as an intermediate in reactions of NO with thiols.7 However, the SNO moiety in the Cys93(F9)β2 subunit is unlikely to be stabilized in this way, because the shortest O---O distance between the oxygen atom of the studied SNO species and the nearest oxygen of Lys144 is about 3.84 Å, a distance too long for significant OH---O hydrogen bonding interactions.
Figure 1.
The crystallographic C-S-N-O structures in purported S-nitroso-hemoglobin (distances in angstrom, unexpected dihedral angles in red).
By contrast, Montfort and coworkers have reported that the nitric oxide transport protein, nitrophorin cNP,8 couples reduction and oxidation of a heme moiety with reversible S-nitrosation of a cysteine residue (PDB: 1Y21). This S-nitrosothiol species has a C-S-N-O dihedral angle of 0°, coinciding with a planar CSNO geometry in small molecule crystallographic data. The cysteine sulfide coordinating to ferric heme is the key to the S-nitrosothiol formation in this protein. Unlike nitrophorins, the NO-bound cysteine in hemoglobin is 10 Å (Fe---S) from the closest heme.
To determine the identity of the ~90° CSNO moiety in the purported S-nitrosohemoglobin, we have explored all four types of SNO species (RSNO, RS-N·-OH, RS-NH-O·, and RS-NH-OH) that could be candidates for the observed SNO species, using simple models (R = Me) that can be computed at a high level. By comparison of the calculated structures to the experimental crystallographic data, we have discovered that the thionitroxide radical (Cys-S-NH-O·) is consistent with the observed SNO structures in both β1 and β2 subunits.
Quantum mechanical calculations were carried out with GAUSSIAN 03,9 using the (U)B3LYP density functional theory10 and the 6-31+G* basis set. The lowest energy minima were verified by harmonic vibrational frequency analysis. Geometries and energies were re-evaluated using the CBS-QB3 and G3 methods,11,12 expected to predict experimental energies to 1 kcal/mol. The solvation energies in water were calculated with the B3LYP (CPCM) /6-31+G* method with Pauling (Merz-Kollman) atomic radii to mimic a polar environment.13
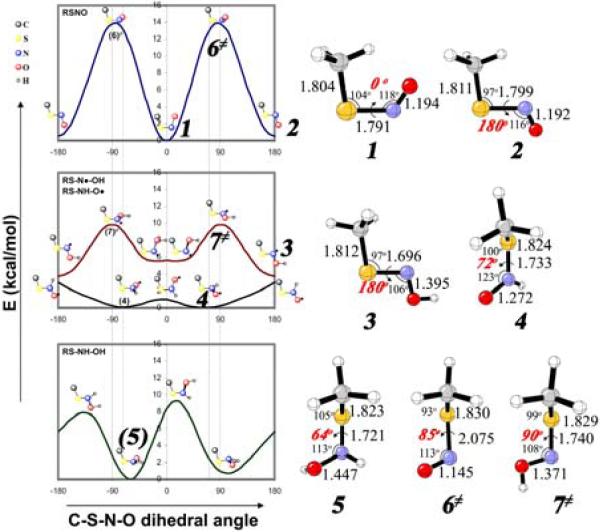
The global minima of these species (1-5) are given in Fig. 2. Relative energies and selected geometrical parameters of energy minima and transition states, with CBS-QB3 and G3, are collected in Supporting Information.
Figure 2.
Rotational energy profiles of RSNO, RS-NH-O·, RS-N·-OH, and RS-NH-OH (R = Me) with the B3LYP/6-31+G* method (two dashed lines stand for the dihedral angles of the observed SNO species in the proteins), and the CBS-QB3 calculated global minima of cis-RSNO (1), trans-RSNO (2), RS-N·-OH (3), RS-NH-O· (4), and RS-NH-OH (5), and rotational transition states for RSNO (6≠) and RS-N·-OH (7≠).
The S-nitrosothiol, MeSNO, has two planar energy minima on the potential surface, 1 and 2. The cis-isomer is more stable, but bulky alkyl groups make the trans-isomer more stable.5,6 Partial S-N double-bond character is manifested by a bond length of 1.79-1.80 Å and an S-N rotational barrier of 12 kcal/mol (6).
The global minimum of RS-N·-OH, 3, is a planar trans, trans-geometry. There are two other near-planar energy local minima, higher in energy by a few kcal/mol (Supporting Information).
The global minimum of the thionitroxide radical, 4, has a twisted 72° C-S-N-O geometry. The O-centered radical is more stable by 3 kcal/mol (B3LYP) in the gas phase than the N-centered radical, 3. Higher level calculations (CBS-QB3 and G3) result in very similar stabilities for the two radicals; 4 is stabilized more than 3 by 0.4 kcal/mol in aqueous solution.
For the S-hydroxylamine species, RS-NH-OH (5), with a 62° dihedral angle is the global minimum among four local minima.
RS-N·-OH and RS-NH-O· are less stable than the stable radical, TEMPO· (2,2,6,6-tetramethyl-1-piperidyloxyl). Using the bond dissociation energy (BDE) of the O-H bond in TEMPOH (69.8 kcal/mol),14 the BDEs of O-H and N-H in RS-NH-OH are predicted to be 77.9 and 77.5 kcal/mol (CBS-QB3), respectively.
According to the energy profiles in Fig. 2, the S-N rotational barrier of MeSNO is the highest among the four SNO species (12 kcal/mol). The nitrogen inversion of RS-N·-OH is facile with a small barrier height (<2 kcal/mol), but the S-N rotation requires 8-9 kcal/mol (7). The thionitroxide radical, RS-NH-O·, has a shallow C-S-N-O potential energy surface. The RS-NH-OH has S-N rotational barrier of 7 - 8 kcal/mol in the gas phase and water.
The S-nitrosothiol itself, RSNO, is the least probable for the crystallographic SNO species, not only due to the large barrier of 12 kcal/mol needed to distort into the observed geometry, but also due to the long S-N distance (2.08 Å, 6) that deviates substantially from the observed S-N bond length (1.76 Å). Similarly, the geometry of the nitrogen radical RS-N·-OH that corresponds to the crystallographically determined geometry is an energy maximum, 7 kcal/mol above the planar minimum, and with a N-O bond length of 1.37 Å.
The observed twisted SNO species is most likely to be a thionitroxide radical, RS-NH-O·. Geometrical parameters, like the N-O bond length (ca. 1.27 Å), the S-N bond length (ca. 1.73 Å), the C-S bond length (ca. 1.82 Å), C-S-N bending angle (ca. 100°), and C-S-N-O dihedral angle (ca. 72°), are all in good agreement with the observed SNO structures (N-O: 1.21 Å; S-N: 1.76 Å; C-S: 1.84-1.86 Å; C-S-N: 101-103°; C-S-N-O: -76, 88°).4 Furthermore, the shallow potential surface of thionitroxide radical is consistent with the considerable difference between two observed C-S-N-O dihedral angles. The fully reduced species, RS-NH-OH, also has a dihedral angle matching that of the crystal structure, but with a longer N-O bond length of 1.45 Å.
Persistent nitroxide radicals, such as TEMPO, have been used as spin-labels for biophysical probes15 and as stable intermediates in living radical polymerizations.16 Numerous compounds containing the nitroxide radical have been synthesized and characterized by EPR, including thionitroxides generated from reactions of nitroso-compounds and thiyl radical.17 These N-aryl or alkyl, N-thioalkyl nitroxides undergo bimolecular decompositions, a pathway not available in protein-bound species. Examples of thionitroxides with a proton on nitrogen have not been isolated, but probably already detected by McMahon et al.17c
The thionitroxide could be responsible for some of the biological effects attributed to S-nitrosothiols.18 In particular, when NO· concentration increases, thionitroxide formation will be enhanced; thionitroxides can be readily reversible carriers of NO·. MeSH + NO· → MeS-NH-O· is endothermic by about 10 kcal/mol in the gas phase, but thermoneutral in aqueous solution or potentially in the hydrogen-bonding environment of a protein such as hemoglobin. Furthermore, the reaction MeS-NH-O· + NO· → MeSNO + HNO, is feasible;19 thionitroxide could be a precursor of S-nitrosothiol when excess NO is present.
The conclusion that thionitroxides are formed from proteinic cysteines when NO is present at appreciable pressure suggests a variety of ways that thionitroxides might be involved in the already remarkably diverse chemistry of NO.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the National Institute of General Medical Sciences, National Institutes of Health, for financial support of this research. The computations were performed in part on the National Science Foundation Terascale Computing System at the Pittsburgh Supercomputing Center. We thank Eric Toone for suggesting this study, and Jonathan Stamler, David Singel, and Arthur Arnone for extensive discussions.
Footnotes
Supporting Information Available: Complete reference 9, the NO-modified hemoglobin, and the detailed structures and energetics of stationary points on potential energy surfaces of the four SNO species. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Jia L, Bonaventura C, Bonaventura J, Stamler JS. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]; b Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]; c Gow AJ, Stamler JS. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]; d Minning DM, Gow AJ, Bonaventura J, Braun R, Dewhirst M, Goldberg DE, Stamler JS. Nature. 1999;401:497–502. doi: 10.1038/46822. and references therein. [DOI] [PubMed] [Google Scholar]
- 2.Fukuto JM, Cho JY, Switzer CH. In: Nitric Oxide Biology and Pathobiology. Ignarro LJ, editor. Academic; 2000. pp. 23–40. [Google Scholar]
- 3.a Zhang YH, Hogg N. PNAS. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. PNAS. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pezacki JP, Ship NJ, Kluger R. J. Am. Chem.Soc. 2001;123:4615–4616. doi: 10.1021/ja015716o. [DOI] [PubMed] [Google Scholar]
- 4.a Chan NL, Rogers PH, Arnone A. Biochemistry. 1998;37:16459–16464. doi: 10.1021/bi9816711. [DOI] [PubMed] [Google Scholar]; b Chan NL, Kavanaugh JS, Rogers PH, Arnone A. Biochemistry. 2004;43:118–132. doi: 10.1021/bi030172j. [DOI] [PubMed] [Google Scholar]
- 5.a Field L, Dilts RV, Ravichandran R, Lenhert PG, Carnahan GE. Chem. Comm. 1978:249–250. [Google Scholar]; b Arulsamy N, Bohle DS, Butt JA, Irvine GJ, Jordan PA, Sagan E. J. Am. Chem. Soc. 1999;121:7115–7123. [Google Scholar]; c Carnahan GE, Lenhert PG, Ravichandran R. Acta Cryst. B. 1978;34:2645–2648. [Google Scholar]; d Lee J, Yi GB, Powell DR, Khan MA, Richter-Addo GB. Canadian Journal of Chemistry. 2001;79:830–840. [Google Scholar]
- 6.a Bartberger MD, Houk KN, Powell SC, Mannion JD, Lo KY, Stamler JS, Toone EJ. J. Am. Chem. Soc. 2000;122:5889–5890. doi: 10.1021/ja0109390. [DOI] [PubMed] [Google Scholar]; b Baciu C, Gauld JW. J. Phys. Chem.A. 2003;107:9946–9952. [Google Scholar]
- 7.a Gow AJ, Buerk DG, Ischiropoulos H. J. Bio. Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]; b Demaster EG, Quast BJ, Redfern B, Nagasawa HT. Biochemistry. 1995;34:11494–11499. doi: 10.1021/bi00036a023. [DOI] [PubMed] [Google Scholar]; c Pryor WA, Church DF, Govindan CK, Crank G. J. Org. Chem. 1982;47:156–159. [Google Scholar]; d Hogg N, Singh RJ, Kalyanaraman B. FEBS Lett. 1996;382:223–228. doi: 10.1016/0014-5793(96)00086-5. [DOI] [PubMed] [Google Scholar]
- 8.Weichsel A, Maes EM, Andersen JF, Valenzuela JG, Shokhireva T, Walker FA, Montfort WR. PNAS. 2005;102:594–599. doi: 10.1073/pnas.0406549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisch MJ, et al. Gaussian, Inc.; Pittsburgh PA: 2003. [Google Scholar]
- 10.a Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; b Lee CT, Yang WT, Parr RG. Physical Review B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA. J Chem. Phys. 1999;110:2822–2827. [Google Scholar]
- 12.Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JA. J. Chem. Phys. 1998;109:7764–7776. [Google Scholar]
- 13.Toubin C, Yeung DYH, English AM, Peslherbe GH. J. Am. Chem. Soc. 2002;124:14816–14817. doi: 10.1021/ja027386t. [DOI] [PubMed] [Google Scholar]
- 14.a Koshino N, Saha B, Espenson JH. J. Org. Chem. 2003;68:9364–9370. doi: 10.1021/jo0348017. [DOI] [PubMed] [Google Scholar]; b Ciriano MV, Korth HG, van Scheppingen WB, Mulder P. J. Am. Chem. Soc. 1999;121:6375–6381. (references therein) [Google Scholar]
- 15.a Axel FS. Biophysics of Structure and Mechanism. 1976;2:181–218. doi: 10.1007/BF00535367. [DOI] [PubMed] [Google Scholar]; b Humphries GMK, McConnell HM. Methods of Experimental Physics. 1982;20:53–122. [Google Scholar]; c Kroll C, Schwarz KH, Surmann P, Borchert HH. Bioelectrochemistry &Bioenergetics. 1999;48:233–236. doi: 10.1016/s0302-4598(98)00219-0. [DOI] [PubMed] [Google Scholar]
- 16.a Georges MK, Veregin RPN, Kazmaier PM, Hamer GK. Macromolecules. 1993;26:2987–2988. [Google Scholar]; b Greszta D, Matyjaszewski K. Macromolecules. 1996;29:7661–7670. and references therein. [Google Scholar]
- 17.a Terabe S, Kuruma K, Konaka R. Perkin Transactions 2. 1973;9:1252–1258. [Google Scholar]; b Ito O, Matsuda M. J. Am. Chem. Soc. 1983;105:1937–1940. [Google Scholar]; c McMahon T, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA;, Stamler JS. Nature Medicine. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 18.Baciu C, Cho K-B, Gauld JW. J. Phys. Chem. B. 2005;109:1334–1336. doi: 10.1021/jp0443759. (See Figure S1 in Supporting Information of the reference).
- 19.Zhao Y-L, Bartberger MD, Goto K, Shimada K, Kawashima T, Houk KN. J. Am. Chem. Soc. 2005;127:7964–7965. doi: 10.1021/ja042247s. In the reference, An S-nitrosothiol was formed experimentally with the hindered thiol (TrmSH) plus NO.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.