Abstract
Humans depend on a dietary intake of lipids to maintain optimal health. Among various classes of dietary lipids, the physiological importance of carotenoids is still controversially discussed. On one hand, it is well established that carotenoids such as β,β-carotene are a major source for vitamin A that plays critical roles for vision and many aspects of cell physiology. On the other hand, large clinical trials have failed to show clear health benefits of carotenoids supplementation and even suggest adverse health effects in individuals at risk of disease. In recent years, key molecular players for carotenoid metabolism have been identified, including an evolutionarily well conserved family of carotenoid-oxygenases. Studies in knockout mouse models for these enzymes revealed that carotenoid metabolism is a highly regulated process and that this regulation already takes place at the level of intestinal absorption. These studies also provided evidence that of β,β-carotene conversion can influence retinoid-dependent processes in the mouse embryo and in adult tissues. Moreover, these analyses provide an explanation for adverse health effects of carotenoids by showing that a pathological accumulation of these compounds can induce oxidative stress in mitochondria and cell signaling pathways related to disease. Advancing knowledge about carotenoid metabolism will contribute to a better understanding of the biochemical and physiological roles of these important micronutrients in health and disease.
Keywords: Carotenoids, Retinoids, Carotenoid-oxygenases, Metabolism, Oxidative Stress
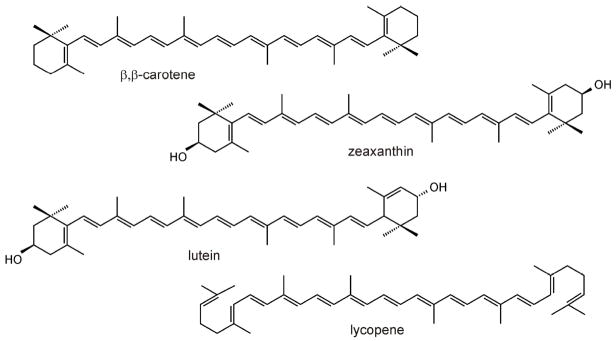
Carotenoids are tetraterpenoids (C40) containing up to 15 conjugated double bonds. According to their chemical properties these isoprenoid pigments are divided into two classes; pure hydrocarbons such as β,β-carotene and lycopene are called carotenes, whereas their oxygenated derivatives such as zeaxanthin and lutein are called xanthophylls (Figure 1). Carotenogenic organisms include all photosynthetic plants, protists and bacteria, as well as some heterotrophic bacteria and some fungi. Carotenoids are accessory pigments in the antennae of chloroplasts, where they augment the light-harvesting capacity by absorbing light in the blue-green range of the visible spectrum (450–550 nm) and transferring the energy to chlorophylls. Carotenoids are also involved in photoprotection in plants and are known antioxidants [1]. Other carotenoid roles derive from the exploitation of their intense and attractive coloration. Hence, carotenoids are important in the pigmentation of flowers and fruits to attract animals for pollination and seed dispersal. In animals, carotenoids contribute in the pigmentation of invertebrates, birds and fishes. As a coloration of male birds, carotenoids have been shown to be an important trait for sexual attractiveness [2, 3]. Human tissues also retain considerable amounts of carotenoids that may play a role in preventing oxidative damage. Additionally, the central part of the primate retina, the macula lutea, owes its yellow color to high levels of the xanthophylls, lutein and zeaxanthin. These macular pigments have been suggested to filter short-wavelength visible light, to lessen chromatic aberration and to prevent the onset of age-related macular degeneration [4, 5].
Figure 1.
Chemical structure of major carotenoids in the human blood.
Living organisms transform carotenoids to generate unique series of metabolites [6]. These cleavage products of carotenoids, apocarotenoids, are important signaling molecules and chromophores of photopigments in all kingdoms of living organisms. In higher plants, carotenoid-derived abscisic acid (ABA) and strigolactones influence processes, as diverse as seed dormancy, morphogenesis and environmental adaptation. Cleavage of carotenoids in plants also yields other derivatives (e.g., β-ionone, α-ionone and saffranal) which are important for coloration and chemoattraction (or repulsion) (for review see, [7]). Similarly, most animals metabolize carotenoids to diterpenoid molecules such as retinaldehyde, the visual chromophore [11], and all-trans-retinoic acid (RA), a hormone-like compound that regulates gene expression [12]. Retinaldehyde serves as a photosensory pigment of mammalian cone and rod visual pigments. A similar function is found in green algae [8] and it also is involved in light-dependent proton pumping in Halobacterium [9]. Additionally, asymmetric oxidative cleavage of carotenoids has been described in animals leading to the production of apocarotenoids different from retinoids [10]. Thus, carotenoids and their apocarotenoid derivatives exert critical physiological functions throughout many living organisms in the kingdoms of nature.
Out of the green wild yonder: Carotenoid cleaving enzymes
Vitamin A was recognized as an essential factor in food a century ago. Early research suggested that certain yellow plant pigments had the same activity as vitamin A. This phenomenon was explained in 1930 by Moore [11], who described a conversion of β,β-carotene into vitamin A in the small intestine of the rat, thus providing the first evidence that a plant-derived carotenoid is the direct precursor of retinoids. Karrer [12] elucidated the structure of β,β-carotene and proposed a central cleavage of the C (15,15′) double bond of β,β-carotene to form two molecules of retinaldehyde. In cell free-extracts, cleavage of β,β-carotene by a 15,15′-oxygenase to produce two molecules of RAL has been reported [13, 14]. Enzymatic oxidative cleavage of carotenoids at a specific position of the polyene chain also has been proposed in bacteria and plants as a method for the synthesis of apocarotenoids. By analyzing the molecular basis of the ABA-deficient maize mutant, vp14 (viviparous 14), the first carotenoid-cleaving enzyme was molecularly characterized [15]. These researchers proposed that enzymes related to VP14 catalyze oxidative cleavage of carotenoids in other organisms as well. Indeed, this breakthrough was followed by the molecular cloning and biochemical characterization of related carotenoid cleavage enzymes (CCEs) not only in plants but also in animals [16, 17], fungi [18], and bacteria [19]. CCEs depend on ferrous iron as a cofactor but do not contain a heme ring. Thus, CCEs belong to the family of non-heme iron oxygenases, but it is still controversial whether they act in a monooxygenase or dioxygenase fashion [20, 21]. Initially, evidence for a monooxygenase mechanism was provided for a vertebrate enzyme. However, exchange of the oxygen label of retinaldeyde with bulk water may have hampered this analysis. Studies with an eccentrically cleaving plant enzyme producing a ketone cleavage product (no exchange of the oxygen label) indicated a dioxygenase mechanism and thus incorporation of both oxygen atoms into the substrate. Studies with mammalian β,β-carotene-15,15’-monooxygenase 1 (BCMO1) suggest that carotenoid cleavage involves the formation of a carbocation intermediate and cation-π stabilization by aromatic residues in the carotenoid-binding cleft [22]. Studies with animal CCEs revealed an additional enzymatic property of these enzymes; they can isomerize the C10,C11 double bonds of their substrates [23]. This reaction is critical for the formation of 11-cis-retinal or derivatives thereof such as 11-cis-3-OH-retinal. This geometric state of the chromophore can bind to the protein moiety (opsin) to form functional visual pigments. In insects, the oxidative cleavage of carotenoids and the all-trans to 11-cis isomerization of carotenoids is catalyzed by a single protein named NinaB [24]. In mammals, carotenoid cleavage and all-trans to 11-cis isomerization is catalyzed by two distinct family members, respectively named BCMO1 and retinal pigment epithelium 65 kDa protein (RPE65). RPE65 is not an actual carotenoid-oxygenase, but the long-sought retinoid isomerase in the vertebrate visual cycle and catalyst of the conversion of retinyl esters (RE) to 11-cis-retinol [25-28]. In contrast to carotenoid-oxygenases, RPE65 does not incorporate oxygen into its substrate, but the reaction also depends on ferrous iron [29]. Mutations in NinaB and RPE65 cause chromophore deficiency and blindness [30-32], thus emphasizing the importance of this class of proteins for animal vision.
The crystal structure has been resolved for three family members of carotenoid-oxygenase; a bacterial ACO (apocarotenoid-oxygenase) [33], plant VP14 [34], and vertebrate RPE65 protein [35]. The analyses of these structures revealed that the structural fold of CCEs is well conserved between family members of different kingdoms; the basic structural motif being a 7-bladed β-propeller. The iron cofactor is coordinated by four conserved histidine residues and three second shell glutamate residues. The iron is accessible through a long non-polar tunnel juxtaposing the substrate to the active center. This ground-breaking structural work will allow comparisons between CCEs for identification of functional site residues that participate in the isomerization and/or oxidative cleavage reaction at specific sites of the polyene carbon backbone of the substrates.
Mammalian genomes encode three different CCE family members
In mammals, three different members of the CCE family have been molecularly identified and biochemically characterized. RPE65 is expressed in the retinal pigment epithelium of the eyes and localizes to the endoplasmatic reticulum. The critical role of RPE65 in visual chromophore production and regeneration is well established and has been extensively reviewed (e.g., [23]). The other two family members, BCMO1 and β,β-carotene-9,10-dioxygenase 2 (BCDO2), are true carotenoid-oxygenases and catalyze the oxidative cleavage of distinct double bonds of the polyene backbone of carotenoids (Figure 2). In this reaction carotenoid-oxygenases specifically interact with one of the two ionone rings of its carotenoid substrates in a specific manner [24]. For mammalian BCMO1 this interaction clearly requires a non-substituted β-ionone ring site, explaining its specificity for a limited number of proretinoid carotenoids such as β,β-carotene, α-carotene and β-cryptoxanthin [36]. BCDO2 catalyzes the oxidative cleavage of carotenoids at the 9,10 position [10]. Initially, BCDO2 was characterized as an enzyme that converts β,β-carotene to produce apocarotenal, β-10’-apocarotenal and β-ionone [10]. Recent research revealed that BCDO2 shows much broader substrate specificity and removes even hydroxylated ionone and ε-ionone ring sites from carotenoids such as zeaxanthin and lutein [37, 38]. Interestingly, BCDO2 can remove both ring sites from its substrates by oxidative cleavage at position C9,C10 and C9’,C10’, resulting in the formation of the C14-dialdehyde rosafluene and two ionone molecules [37]. Additionally, BCDO2 can also cleave acyclic carotenoids such as lycopene [39]. There is also a marked difference in the subcellular localization of the two mammalian carotenoid-oxygenases. BCMO1 is a cytoplasmic protein [36], whereas BCDO2 localizes to mitochondria [37]. The requirement for such compartmentalization of carotenoid metabolism might be explained by the fact that BCMO1 and BCDO2 are expressed in same cell types (e.g. hepatocytes) and can metabolize the same substrate (β,β-carotene) [10]. Thus, expression in the same cellular compartment would lead to a competition between BCDO2 and BCMO1 for its common substrate β,β-carotene and may result in reduced production of retinoids. In summation, two different carotenoid metabolizing enzymes have been identified. BCMO1 is a cytosolic enzyme with narrow substrate specificity for proretinoid carotenoids and BCDO2 is a mitochondrial enzyme with broad substrate specificity for carotenoids. The identification of the genes encoding these enzymes allowed the generation of knockout mouse models by homologous recombination. These animals are versatile models to study the roles of different carotenoids in mammalian physiology.
Figure 2.
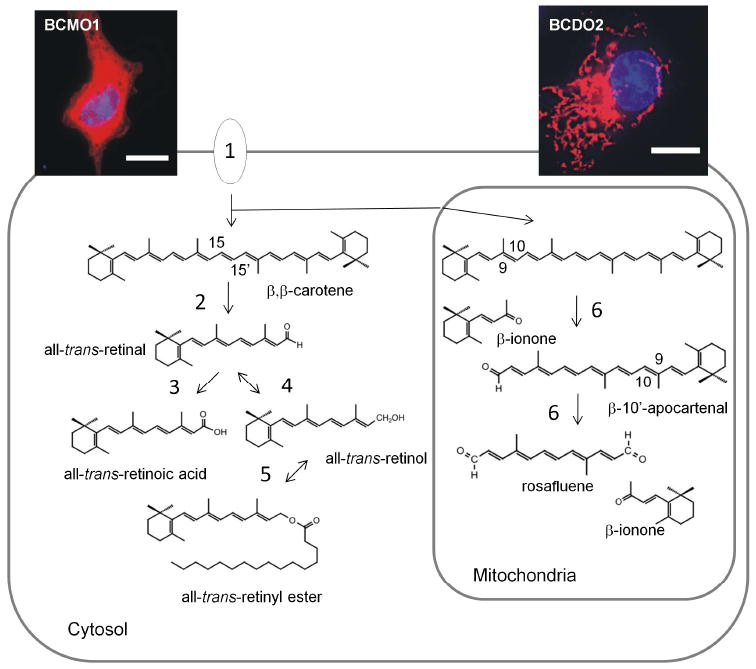
β,β-carotene metabolism in a cell that express both types of carotenoid-oxygenases. Insets at the top show images immunostained expressing recombinant V5-tagged murine BCMO1 and BCDO2. Nuclei (blue) are stained with DAPI. The scale bar gives 10 µM. Note that BCMO1 is distributed in the cytosol, whereas BCDO2 localizes to mitochondria. Enzymes and proteins involved β,β-carotene metabolism: 1, Cellular uptake of β,β-carotene is mediated by scavenger receptors such as SR-BI and CD36; 2, BCMO1 converts β,β-carotene by symmetric oxidative cleavage to all-trans-retinal; 3, all-trans-retinal can be oxidized to retinoic acid by retinal dehydrogenases; 4, all-trans-retinal also can be reduced to all-trans-retinol by alcoholdehydrogenases and short chain dehydrogenases; all-trans-retinol can be esterified by the lecithin: retinol acyl transferase and by acylCoA: retinol acyl transferases; 6, BCDO2 removes β-ionone rings from β,β-carotene by successive oxidative cleavage at 9,10 and 9’,10’ position.
BCMO1 is the key enzyme for vitamin A production
In mammals, the molecular and biochemical basis of vitamin A function has been well established. The vitamin A-derivative 11-cis-retinal serves as chromophore of cone and rod visual pigments [40]. Moreover, vitamin A is the precursor for all-trans-retinoic acid (RA), which is required for a wide range of biological processes, including embryonic and fetal development, cell differentiation and metabolic control. This hormone-like compound is the ligand of retinoic acid receptors (RARs) that require retinoid X receptors (RXRs) as partners [41]. The active receptor complex is a RAR/RXR heterodimer that binds specific DNA regulatory sequences and regulates gene transcription in response to RA binding.
β,β-Carotene is the major source for retinoids in the human diet. The formal first step in retinoid metabolism is the conversion of β,β-carotene to retinaldehyde, from which all other retinoids can be synthesized by endogenous enzymes. Studies in a BCMO1 knockout mouse model demonstrated the critical role of this enzyme for this process. On diets providing β,β-carotene as the sole source for vitamin A, these mice become retinoid-deficient and instead accumulate large amounts of β,β-carotene [25]. Recently, in human studies, a patient with a heterozygotic mutation in BCMO1 was described with both elevated plasma β,β-carotene levels and low plasma retinol levels [42]. At the protein level of BCMO1, this mutation resulted in replacement of a highly conserved threonine by a methionine residue (T170M). Biochemical characterization of the recombinant T170M BCMO1 protein variant showed that its activity was ≈90% lower than that of the wild-type protein. Moreover, single base pair polymorphisms in the human BCMO1 gene have been identified that decreased intestinal β,β-carotene conversion efficiency [43]. Studies in BCMO1-deficient mice showed that the expression of the second carotenoid-oxygenase BCDO2 is upregulated in the liver and that some β-10’-apocarotenoids are produced [44]. Increased expression of BCDO2 in BCMO1-deficient mice has been also reported in other tissues such as the lung [45]. Apocarotenoid formation indicates that β,β-carotene can be at least, in part, metabolized by BCDO2 in BCMO1-deficiency.
Regulation of intestinal vitamin A production
The small intestine is responsible for absorbing dietary lipids such as carotenoids and delivering them to the organism as triglyceride-rich lipoproteins. Intestinal lipid absorption is a complex process that evidently depends on membrane receptors/transporters [46]. For carotenoids, it is now clear that scavenger receptors such as SR-B1 and CD36 facilitate their absorption [47]. Additionally, SR-BI also facilitates the intestinal absorption of tocopherols (vitamin E) [48]. Studies in a Drosophila mutant showed that SR-BI/ NinaD is critical for the absorption of both fat and soluble vitamins [49, 50].
Upon absorption, proretinoid carotenoids are readily converted to vitamin A by the action of BCMO1 in enterocytes of the intestinal mucosa [16, 51]. The primary cleavage product, retinaldehyde, is then converted to retinol and REs. These REs, along with REs produced from preformed dietary vitamin A, are packed into chylomicrons that are secreted into the blood. In humans, substantial amounts of absorbed carotenoids are not cleaved in the intestine (up to 40% of dietary intake) [52] so they, along with other lipids, become incorporated and associate with circulating lipoproteins [53].
Intervention studies in healthy volunteers have led to the description of a low-responder phenotype and a low-converter phenotype [54]. Low or poor responders are defined as individuals who show little variation in plasma β,β-carotene levels after acute or chronic supplementation. This huge inter-individual variability in conversion efficiency of β,β-carotene has been reported in several studies [54-57]. Furthermore, pharmacokinetic studies with labeled β,β-carotene indicate that low responders display a lower conversion efficiency compared to normal responders [58]. Genetic variation in the human BCMO1 gene may very well be contributing to these different phenotypes [59, 60]. Additionally, studies in animal models reported that intestinal BCMO1 activity is influenced by dietary retinoids (for the current model, see Figure 3). This regulation involves retinoic acid signaling via RARs and evidently takes place at the transcriptional level [61]. Interestingly, studies in BCMO1-deficient mice revealed that not only carotenoid conversion by BCMO1 but also carotenoid absorption is repressed by retinoids [62]. Recently, a gut-specific homeodomain transcription factor ISX was identified that controls intestinal SR-BI and BCMO1 mRNA expression [63, 64]. SR-B1 is normally found on the apical surfaces of absorptive epithelial cells, and its levels decrease from the duodenum to ileum [65-67] in contrast to the increasing duodenum-ileum gradient for ISX [63, 64]. In ISX-deficient mice, SR-BI expression is significantly enhanced and its expression extends to more distal parts of the intestine [64]. Interestingly, ISX expression is reduced in vitamin A-deficiency [64], further implicating this transcription factor as a key regulatory factor for intestinal carotenoid metabolism. The crosstalk between β,β-carotene/retinoid metabolism and ISX is mediated via a retinoic acid responsive element in the ISX promoter to which RARs can bind [62]. These findings suggest that retinoic acid produced from dietary precursors regulates the intestinal expression of SR-BI and BCMO1 by inducing ISX. Indeed, treatment of vitamin A— deficient animals with RA induced ISX expression and decreased SR-BI and BCMO1 expression. Thus, ISX acts as a retinoic acid-sensitive ‘gatekeeper’ that controls vitamin A production. This negative feedback regulation of vitamin A production may contribute to the low and high responder phenotypes in humans. The low responder phenotype may represent the vitamin A sufficient state; whereas the high responder phenotype may represent a demand for vitamin A. The intestinal regulation of vitamin A production also explains why β,β-carotene supplementation does not cause hypervitaminosis A. In contrast, preformed dietary retinoids can cause this problem because retinol is absorbed in a non-regulated manner [68]. Further studies are required to fully elucidate the role of this diet responsive regulatory network on vitamin A homeostasis. Considering the involvement of SR-BI for the absorption of other dietary isoprenoid compounds such as tocopherols (vitamin E) and xanthophylls (macula pigments), dietary preformed retinoids may more generally impact homeostasis of these important dietary lipids.
Figure 3.
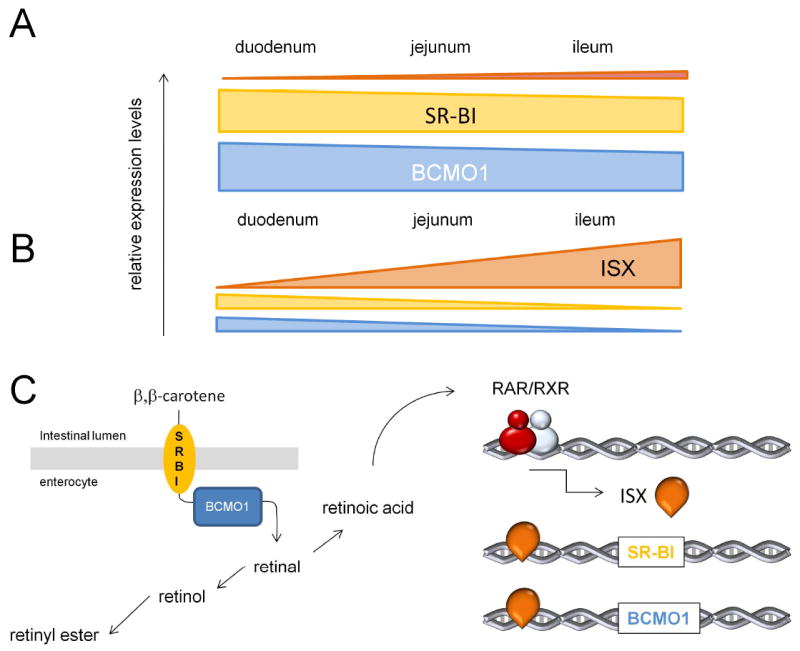
Negative feedback regulation of intestinal β,β-carotene absorption and conversion. A. In vitamin A deficiency, SR-BI and BCMO1 expression are high elevated and distally extended throughout the intestine. In contrast, ISX expression is highly reduced. Note that a similar pattern of expression of SR-BI and BCMO1 is found in ISX-deficient mice [64]. B. In vitamin A sufficiency, ISX expression is increased and proximally extended. In contrast, SR-BI and BCMO1 expression are highly reduced in a distal gradient throughout the intestine [64]. C. Schematic overview of intestinal β,β-carotene metabolism. SR-BI facilitates the absorption of dietary β,β-carotene from the intestinal lumen. BCMO1 converts absorbed β,β-carotene by symmetric oxidative cleavage to all-trans-retinal. all-trans-Retinal is reduced to all-trans-retinol and then esterified to retinyl RE. RE together with intact β,β-carotene is packed in chylomicrons and secreted into the circulation. all-trans-Retinal can be also converted to all-trans-retinoic acid. This hormone like compound activates via retinoic acid receptors (RAR) and retinoid X receptors (RXR) gene transcription of ISX. ISX in turn inhibits the expression of SR-BI and BCMO1 genes and reduces intestinal β,β-carotene absorption and conversion to retinoids.
BCMO1 expression in peripheral tissues and the embryo
In humans, substantial amounts of absorbed β-carotene are not cleaved in the intestine by BCMO1 (up to 40% of dietary intake) [52] and along with other lipids become incorporated in chylomicrons and found associated with circulating lipoproteins [53]. Circulating carotenoids in association with lipoproteins can be then taken up by the lipoprotein specific receptors. In mice, BCMO1 is expressed in both the intestine and liver but also in peripheral tissues, including the mammalian embryo [10, 69-71]. In humans, analyses of BCMO1 mRNA expression levels revealed a comparable picture [72]. Immunohistology showed that BCMO1 is expressed in the mucosal and glandular cells of stomach, small intestine, and colon, in liver, cells comprising the exocrine glands in pancreas, glandular cells in prostate, endometrium, and mammary tissue, kidney tubular cells, and keratinocytes of skin squamous epithelium [73]. Additionally, steroidogenic cells in testis, ovary, and the adrenal gland, as well as skeletal muscle cells express BCMO1 [73]. Moreover, BCMO1 is abundantly expressed in the retinal pigmented epithelium of the eyes [72, 74]. Together, these studies suggest that a local tissue specific-conversion of β,β-carotene can contribute to retinoid metabolism in peripheral tissues.
BCMO1 deficiency affects embryonic retinoid metabolism
Loredana Quadro and coworkers provided evidence that BCMO1 can maintain retinoid homeostasis in embryonic tissues of VAD mice [71]. In an elegant genetic approach, they generated RBP-/- BCMO1-/- double mutant female mice. In RBP-deficiency, mice depend on a continuous dietary vitamin A supply (RE in chylomicrons) [75]. These double mutant mice were crossed with RBP-/- male mice so that dames were deficient both for RBP and BCMO1 whereas their offspring carried a functional BCMO1 allele. Pregnant dams were then subjected to dietary vitamin A restriction and received β-carotene or vehicle by IP injection. The offspring developed severe embryonic malformations when supplemented with vehicle only. In contrast, β,β-carotene could maintain retinoid homeostasis and promoted normal development of the offspring (with a functional BCMO1 allele). Thus, β,β-carotene can serve as retinoid precursor in embryonic tissue. A developmental role of BCMO1 also has been reported in lower vertebrates such as the zebrafish [76].
Additionally, the study by Quadro and coworkers provided evidence that the BCMO1 protein influences embryonic retinoid homeostasis. These researchers reported that dietary vitamin A restriction induced more severe developmental malformations in RBP-/- BCMO1-/- double mutant offspring than in RBP-/- single mutant offspring [71]. This observation was unexpected because diets essentially provided no β,β-carotene in these experiments. The authors found that embryonic retinoid metabolism was impaired in BCMO1-deficient embryos also in the absence of β,β-carotene. LRAT expression was significantly reduced as compared to embryos carrying a wild-type BCMO1 allele. Accordingly, BCMO1-deficient embryos had much lower levels of RE and RA, the developmentally active form of vitamin A. Thus, the authors established a critical role of BCMO1 protein for embryonic retinoid metabolism.
A role of BCMO1 in the regulation of body fat reserves
Among the many functions attributed to retinoids, its putative role in adipocyte biology and the regulation of body fat reserves has generated clinical and scientific interest. The vitamin A derivative RA acid has been shown to influence adipocyte differentiation [77, 78] and fat deposition [79], mitochondrial uncoupling [80, 81], oxidative metabolism [82, 83] and adipokine expression [84-87] in adipose tissues. These effects are mediated in part via the classical retinoic acid receptors (RARs), which, upon retinoid binding, regulate the expression of direct target genes [88] and interfere with the activity of other transcription factors, including early adipogenic transcription factors [77, 78]. Additionally, retinoic acid can influence peroxisome proliferator-activated receptor (PPAR)-mediated responses by activating the retinoid X receptor (RXR) moiety of permissive PPAR:RXR heterodimers [89, 90] and, possibly, by serving as an agonist, activating ligand of the PPARβ/δ isoform [91, 92]. PPARs are lipid-activated transcription factors involved in the regulating the expression of genes related to lipid and glucose metabolism [93, 94]. PPARγ, in particular, is pivotal for adipocyte differentiation and hypertrophy [95]. Additionally, the retinoic acid precursor retinaldehyde has been shown to inhibit PPARγ-induced adipogenesis both in adipocyte cell cultures and mouse models [96].
Regulation of fat reserves by dietary vitamin A could be explained by the metabolism of vitamin A to biologically active retinoid derivates, which can then impact the differentiation and function of adipose tissue. Surprisingly, obesity and obesity associated diseases are prevalent in western societies, with adequate dietary vitamin A supply. Additionally, high dose supplementation with preformed vitamin A does not affect body adiposity in mice [97]. Besides preformed vitamin A, β,β-carotene is found in the circulation and BCMO1 expression has been reported in several tissues, including the intestine, liver and adipocytes (see above). It was recently determined that, BCMO1 gene transcription is under the control of PPARγ, a key regulator of adipocyte differentiation and lipogenesis in mature adipocytes [98]. The finding of responsiveness of BCMO1 to PPAR provides evidence for a possible link between the regulation of fatty acid and β,β-carotene metabolism. Moreover, studies in BCMO1 knockout mice showed that this mouse mutant develops dyslipedemia, and is more susceptible to diet-induced obesity, and shows increased expression of PPARγ-induced genes in adipocytes, even when supplemented well with vitamin A, further indicating an important role for BCMO1 in the regulation of lipid metabolism [25].
Recently, it was demonstrated that like PPARγ, BCMO1 is induced (5-7 fold) both at the mRNA and protein levels, during differentiation of NIH 3T3-L1 pre-adipocyte cells to mature adipocytes [99]. Treatment of mature adipocytes with the BCMO1 substrate β,β-carotene resulted in a reduced staining for lipids (Oil Red O stain) and triglyceride (TG) content, while microscopic inspection showed that lipid droplets were reduced in size and number (Figure 4). Correspondingly, mRNA and protein expression levels of PPARγ and its downstream target fatty acid binding protein 4 (FABP4/aP2) were also significantly reduced in β,β-carotene treated mature adipocytes. No such observations were apparent in vitamin A treated mature adipoctes.
Figure 4.
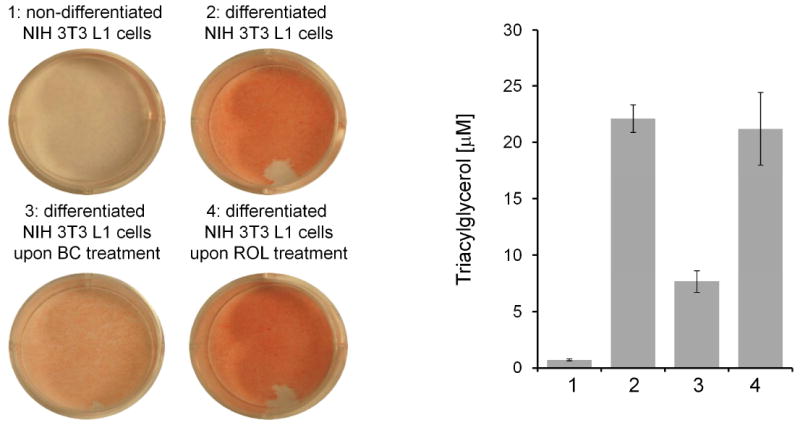
β,β-Carotene but not all-trans-retinol reduces triacylglycerol content in mature NIH 3T3 L1 adipocytes. A. Photographs of cell culture plates stained with oil-red for lipids. Non-differentiated NIH 3T3 L1 cells show weak staining, whereas mature NIH 3T3 L1 adipocytes show strong red staining for lipids. Oil red staining of mature NIH 3T3 L1 adipocytes treated with one µM β,β-carotene (BC) or all-trans-retinol (ROL). BC but not retinol reduces triacylglycerol content of these cells. For further details see text and reference [99].
To further discriminate between the effects of parent β,β-carotene and its retinoid derivatives, this study used (4-hydroxy-phenyl) retinamide ((fenritinide, (FHR)), a highly selective inhibitor of BCMO1 enzymatic activity. Although β,β-carotene treatment alone reduced TG content and decreased expression of the lipogenic marker PPARγ, this effect was largely prevented by FHR. Thus treatment of mature adipocytes with FHR selectively inhibited the anti-adipogenic effects of β,β-carotene, indicating that they are dependent on BCMO1 activity. To determine the biologically active retinoid derivative, polar and non-polar retinoids from both β,β-carotene and all-trans-retinol treated mature adipocytes were isolated and analyzed by HPLC. This analysis showed that β,β-carotene is metabolized to all-trans-retinoic acid, while all-trans-retinol was metabolized to RE, thus β,β-carotene and all-trans-retinol have different metabolic fates in mature adipocytes. In fact, endogenous all-trans-retinoic acid derived from β,β-carotene cleavage decreased the expression of key lipogenic transcription factors, namely CCAAT/ enhancer-binding protein α (C/EBPα) and PPARγ. It has been shown that the inhibitory effects of all-trans-retinoic acid on adipogenesis are mediated by RARs. In line with this concept, a 2.8 fold induction of Cyp26a1 at the transcriptional level (mRNA) was observed. Cyp26a1 is an RA hydroxylase, whose mRNA expression is induced by RA in a strictly RAR-dependent manner. Thus in agreement with RA production, β,β-carotene treatment induced the expression of this RAR-responsive gene. Taken together in vitro studies in cell culture indicated that β,β-carotene plays an important role for retinoid signaling in mature adipocytes.
To expand these observations to animals, the effects of vitamin A deficiency as well as β,β-carotene and all-trans-retinol supplementation on adipose tissues of mice was analyzed in LRAT-/- mice (which lack retinoid stores in the liver as they cannot convert all-trans-retinol to RE in most tissues) [100]. Examination of inguinal white adipose tissue from these mice showed no changes in the mRNA expression of either PPARγ or its downstream target aP2 as compared to LRAT-/- mice on vitamin A sufficient (VAS) diets. Additionally, morphometric analysis showed that adipocytes were comparable in size between the VAS and vitamin A deficient (VAD) animals. However, gavage of β,β-carotene resulted in a 3-fold decrease in PPARγ and aP2 mRNA expression and 2.1 fold increase in Cyp26a1 mRNA expression, indicating that these mice produce RA which in turn influences adipogenesis. No such results were observed in all-trans-retinol gavaged vitamin A deficient LRAT-/- mice, which further strengthens the importance of BCMO1 in influencing adipogenecity.
The role of β,β-carotene for RA metabolism might be explained by specific biochemical characteristics of adipocyte. Studies has revealed that enzymatic activity of BCMO1 is stimulated in the presence of cellular retinol-binding protein 1 (CRBP1) [101]. CRBP1 can bind RAL, the primary β,β-carotene cleavage product, and is expressed in various tissues including adipocytes [102]. In the liver, CRBP1 stimulates RE formation, the storage form of vitamin A, from CRBP1-bound retinoids in a LRAT-dependent manner [103]. Adipocytes express different acyl-transferases than LRAT for RE formation [100]. Additionally, the expression of the RAL to retinol converting enzyme ADH1 is decreased during adipogenesis [96]. Morover, β,β-carotene treatment decreased ADH1 mRNA expression, whereas it induced mRNA expression of the retinal dehydrogenase RALDH1 in iWAT of mice [99]. Thus, these biochemical characteristics may explain that β,β-carotene is predominantly metabolized into RA in adipocytes.
Cumulatively, these studies provided evidence that BCMO1 plays a role in the cross-talk between PPARγ and RAR signaling pathways and implicates β,β-carotene as an important dietary regulator of fat storage capacities of mice adipocytes. Many observational studies indicate that the consumption of food rich in plant-derived micronutrients such as carotenoids is inversely related to the incidence of obesity and associated disease. However, there are limited molecular explanations for this effect, though this assumption is widely accepted but not a strictly proven fact. Thus, the putative role of β,β-carotene for this process requires further research.
BCDO2 and apocarotenoid signaling molecules
Though the role of BCMO1 for retinoid metabolism has been well established, less is known about the second carotenoid-oxygenase, BCDO2. As described above, biochemical studies indicate that this enzyme displays broad substrate specificity and converts both carotenes and xanthophylls by oxidative cleavage at the 9’,10’ and 9,10 double bonds. Apocarotenoids such as β-14’-apocarotenal and β-13’-apocarotenal have shown to influence the activities of nuclear receptors such RXR and PPARα and γ [104, 105]. In ferrets, 10′-lycopenals are produced upon lycopene supplementation [39]. Studies in BCMO1 knockout mice, with elevated BCDO2 mRNA expression levels, show that lycopene tissue distribution is significantly altered compared to wild-type mice [106]. BCDO2-deficient mice accumulated significantly more lycopene than wild-type mice, implicating BCDO2 in the metabolism of this carotenoid [107]. In rats, lycopene supplementation results in altered expression of PPARγ in several tissues [108]. Additionally, lycopene derivative apo-10′-lycopenoic acid has been shown to transactivate RARs with relative high potency [109]. This compound also was shown to inhibit lung tumor growth in cell culture and mouse models [109]. Furthermore, lycopene supplementation inhibited hepatic carcinogenesis in rats with induced fatty livers [110]. All these studies clearly show that apo-carotenoids can influence physiological processes. However, pathways for the biochemical modifications of the primary apocarotenal cleavage product await molecular and biochemical description. It must also be considered that these compounds exist in relatively high amounts in plant food. Thus, diet-derived apocarotenoids may influence nuclear receptor activities as well. More research is needed to understand the role of BCDO2 for the production of the numerous apocarotenoid molecules that could be formed from dietary carotenoids and their putative impact on mammalian biology.
BCDO2, carotenoid homeostasis, and mitochondria
The broad substrate specificity of BCDO2 implicates this enzyme in the metabolism of both carotene and xanthophylls. A critical role of BCDO2 for xanthophyll metabolism was substantiated by findings in chickens. The yellow skin color of chickens (xanthophylls) is determined by cis-acting and tissue-specific regulatory mutation(s) that inhibit expression of BCDO2 in skin [111]. Additionally, it was shown that mutations in BCDO2 gene cause the yellow fat phenotype (xanthophyll accumulation) of sheep [112]. Moreover, the carotenoid content of cow milk and serum was demonstrated to be significantly altered by a mutation that abolished BCDO2 function [113].
To further analyze the physiological role for BCDO2 in a model organism, a BCDO2-/- knockout mouse model was established [37]. Ablation of BCDO2 expression was confirmed by protein analysis of isolated hepatic mitochondria, using a BCDO2 specific antibody. BCDO2-/- mice develop normally and were fertile on standard chow-diet. Dietary supplementation of BCDO2-/- mice with either 50 mg/ kg zeaxanthin or lutein revealed impairment in carotenoid metabolism. Gross abdominal sectioning showed an accumulation of these carotenoids, evident by strong yellow coloring of adipose tissue in only BCDO2-/- mice and not WT mice supplemented with the same diets. HPLC analysis of lipid extracts of livers revealed that only small amounts of xanthophylls from these diets were present in BCDO2-/- mice, but these mice accumulated large amounts of metabolites of these carotenoids with different retention times and absorption spectra as compared to the parent carotenoids. LC-MS/MS identified these accumulated compounds as 3-didehydro-carotenoids [37]. These compounds have been recently described as major xanthophyll derivatives in wild-type mice with very short half-life times [114]. Notably, no accumulation of apocarotenoid cleavage products was identified in WT mice, indicating that the cleavage products are rapidly metabolized/ degraded.
A consequence of carotenoid accumulation in the BCDO2-/- mice was apparent in liver histology. Histological analysis revealed that BCDO2+/- and BCDO2-/- mice developed liver steatosis with large lipid droplets in hepatocytes and a significantly increased TG content. This phenotype was not evident in age matched BCDO2-/- mice raised on a chow diet, indicating that this pathology was induced by carotenoid accumulation. Since, carotenoids also accumulated in BCDO2+/- mice, it was indicative that loss of a single allele can cause halpoinsufficiency and impairs carotenoid homeostasis. Furthermore, BCDO2 mRNA expression analysis by qRT-PCR revealed that BCDO2 expression was induced 7-fold in WT mice as compared to BCDO2+/- mice, thereby preventing accumulation of these carotenoids.
In agreement with the enzyme’s intracellular localization, carotenoids accumulated in isolated hepatic mitochondria of BCDO2-/- mice. Carotenoids are lipophilic molecules with an extended polyene chromophore that can act as an electron sink. Additionally, these rigid lipids also may disturb membrane topology. Immunoblot analysis of manganese superoxide dismutase (MnSOD), an enzyme indicative of general mitochondrial stress and dysfunction, showed a 9-fold increase in hepatic mitochondria isolated from carotenoid-supplemented BCDO2-/- mice as compared to WT mice. Detailed analysis of the mitochondrial respiration chain (ETC) revealed that ADP-dependent respiration, respiration with high ADP, and uncoupled respiration rates of mitochondria isolated from BCDO2-/- on the carotenoid diets were all significantly reduced, as compared to siblings on the similar but carotenoid-free diet.
Disturbances in the mitochondrial ETC generally result in the production of excessive reactive oxygen species (ROS). Indeed, in vitro studies in human liver HepG2 cells showed that zeaxanthin, lutein or their 3-dehyro derivatives, the latter isolated from livers of BCDO2-/- mice, all could induce ROS production, in a dose dependent manner. Additionally, treatment of these cells with β,β-carotene evidently resulted in ROS. Conversely, overexpressing recombinant murine BCDO2 in HepG2 cells significantly reduced ROS production, further demonstrating the importance of this carotenoid-oxygenase in preventing oxidative stress. Accordingly, determination of the mitochondrial membrane potential using the well established JC-1 dye technique yielded similar outcomes. Again, overexpressing recombinant murine BCDO2 in HepG2 cells significantly ameliorated membrane depolarization in carotenoid-treated cells.
Additionally, various components of the oxidative-stress regulated signaling pathways were analyzed by immunoblot analysis of hepatic and cardiac tissues of BCDO2-/- and WT animals maintained on supplemented carotenoid diets. Analysis of the hypoxia inducible factor 1α (HIF1α), phospho-AKT and phospho-MAPK kinase pathways all showed a 5-7 fold induction at the protein levels in hepatocytes and cardiac tissues isolated from BCDO2 mice. Similar observations were also noted in BCDO2 heterozygous mice, but no such induction was observed in WT mice on similar carotenoid diets. The induction of these cell signaling pathways can at least in part counteract carotenoid-induced impairment in BCDO2-deficent mice. However, a overstimulation of these pathways also is associated with disease, including cardiovascular and neurodegenerative disease, type 2 diabetes and cancer.
For BCDO2, a single base pair polymorphism in intron 2 has been identified and correlates with altered blood levels of interleukin 18, a pro-inflammatory cytokine associated with type 2 diabetes and cardiovascular disease [115]. A relationship between BCDO2 and cardiovascular disease has also been observed in mice [116]. An induction of cell signaling pathways related to oxidative stress and cell proliferation has been previously described in rats and ferrets supplemented with supraphysiological doses of β,β-carotene [117, 118]. Large clinical trials revealed that high dose β,β-carotene supplementation can harm individuals at risk of disease, such as smokers and asbestos workers (for review, see [119, 120]). Studies in BCDO2 knockout mice implicate mutation in the BCDO2 gene as a genetic risk factor and may provide a mechanistic explanation for adverse health effects of carotenoids.
Concluding remarks
A ubiquitous family of non-heme iron oxygenases has been identified that modify double bonds of carotenoids and their apocarotenoid derivatives by trans-to-cis isomerization and oxidative cleavage. Mammalian genomes encode three distinct family members. RPE65 is the isomerase in the visual cycle, whereas BCMO1 and BCDO2 catalyze the oxidative cleavage of carotenoids. BCMO1 cleaves at the C15,C15’ double bond and has a limited substrate specificity for proretinoid carotenoids such as β,β-carotene. The second enzyme cleaves carotenoids at the C9,C10 double bond and displays broad substrate specificity. Studies in knockout mouse models for these enzymes identified BCMO1 as the key enzyme for retinoid production. Analysis in this mouse model also helped to unravel a diet-responsive regulatory network that controls intestinal vitamin A production. In this process, intestine-specific homeodomain transcription factor ISX acts as a RA-sensitive gatekeeper that controls carotenoid absorption and conversion to vitamin A. Increasing evidence has been acquired that a tissue-specific BCMO1 also plays an important role in a tissue-specific context. BCMO1-deficient embryo show altered vitamin A homeostasis. Furthermore, β-carotene conversion to vitamin A can maintain normal development in the embryo under conditions of limited maternal vitamin A supply. A tissue-specific conversion of β-carotene to retinoids can also influence retinoid-dependent processes in adult mammals. In adipocytes, a reciprocal influence between the β,β-carotene metabolite retinoic acid and PPARγ activity has been demonstrated. Importantly, preformed dietary vitamin A does not show this activity. These findings suggest that a tissue-specific conversion of β,β-carotene influence nuclear receptor activities that help regulate energy balance. Genetic studies in chickens, cows, and sheep showed that mutations in the BCDO2 gene can indeed alter carotenoid metabolism. A critical role of BCDO2 for carotenoid homeostasis has been confirmed in a mouse model. In accordance with the mitochondrial localization of this enzyme, carotenoids accumulated in these organelles and impaired respiration. This impairment caused oxidative stress and induced cell signaling pathways related to cell survival and proliferation. This action of carotenoids may explain adverse health effects of these compounds reported in clinical studies and identify BCDO2 as a key player for their prevention. It also shows that carotenoid homeostasis – as it is already a well known fact for other isoprenoid lipids such as cholesterol – must be tightly regulated to avoid disease. In humans, genetic polymorphisms exist that alter carotenoid and retinoid homeostasis. In the future, this genetic variability as well as regulatory aspects must be considered when effects of carotenoids on health and disease are evaluated. Advancing knowledge about carotenoid metabolism will contribute to the understanding of the biochemical, physiological, developmental, and medical roles of carotenoids and their numerous derivatives.
Acknowledgments
The authors would like to thank Drs. Ouliana Ziouzenkova and Earl Harrison for the invitation to contribute this article to this special issue of BBA. This work was supported by the National Institute of Health grant EY019641. Darwin Babino was supported by a visual science training grant (NIH T32-EY07157).
Abbreviations
- C/EBPα
CCAAT/enhancer-binding protein α
- BCMO1
β,β-carotene-15,15’-monooxygenase 1
- BCDO2
β,β-carotene-9,10-dioxygenase 2
- CCE
carotenoid cleaving enzyme
- CD36 (SCARB3)
Cluster of Differentiation 36
- FABP4 (aP2)
fatty acid-binding protein 4
- FHR
fenretinide
- HPLC
high-performance liquid chromatography
- iWAT
inguinal white adipose tissue
- PPAR
peroxisome proliferator-activated receptor
- SR-BI
scavenger receptor class B type I
- RAL
all-trans-retinal
- RA
all-trans-retinoic acid
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- RetSat
retinol saturase
- RPE65
retinal pigment epithelium 65 kDa protein
- RE
retinyl esters
- ROS
reactive oxygen species
- TG
triacylglycerol
- VAD
vitamin A-deficient diet
- VAS
vitamin A-sufficient diet
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demmig-Adams B, Adams WW., 3rd Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2149–2153. doi: 10.1126/science.1078002. [DOI] [PubMed] [Google Scholar]
- 2.Faivre B, Gregoire A, Preault M, Cezilly F, Sorci G. Immune activation rapidly mirrored in a secondary sexual trait. Science (New York, NY. 2003;300:103. doi: 10.1126/science.1081802. [DOI] [PubMed] [Google Scholar]
- 3.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science (New York, NY. 2003;300:125–127. doi: 10.1126/science.1082142. [DOI] [PubMed] [Google Scholar]
- 4.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 5.Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Investigative ophthalmology & visual science. 2001;42:439–446. [PubMed] [Google Scholar]
- 6.Krinsky NI. The Biological Activity of Carotenoid Metabolites, SIGHT AND LIFE. Newsletter. 2005;2005:10–15. [Google Scholar]
- 7.Moise AR, von Lintig J, Palczewski K. Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids. Trends Plant Sci. 2005;10:178–186. doi: 10.1016/j.tplants.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Sineshchekov OA, Govorunova EV. Rhodopsin-mediated photosensing in green flagellated algae. Trends Plant Sci. 1999;4:201. doi: 10.1016/s1360-1385(99)01409-0. [DOI] [PubMed] [Google Scholar]
- 9.Jung KH. The distinct signaling mechanisms of microbial sensory rhodopsins in Archaea, Eubacteria and Eukarya. Photochem Photobiol. 2007;83:63–69. doi: 10.1562/2006-03-20-IR-853. [DOI] [PubMed] [Google Scholar]
- 10.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 11.Moore T. Vitamin A and carotene. VI. The conversion of carotene to vitamin A in vivo. Biochem J. 1930;24:692–702. doi: 10.1042/bj0240692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karrer P, Helfenstein A, Wehrli H, Wettstein A. Über die Konstitution des Lycopins und Carotins. Helv Chim Acta. 1930;13:1084. [Google Scholar]
- 13.Olson JA, Hayaishi O. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proceedings of the National Academy of Sciences of the United States of America. 1965;54:1364–1370. doi: 10.1073/pnas.54.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman DS, Huang HS. Biosynthesis of Vitamin a with Rat Intestinal Enzymes. Science (New York, NY. 1965;149:879–880. doi: 10.1126/science.149.3686.879. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science (New York, NY. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 16.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 17.Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W. Cloning and expression of beta,beta-carotene 15,15′-dioxygenase. Biochem Biophys Res Commun. 2000;271:334–336. doi: 10.1006/bbrc.2000.2619. [DOI] [PubMed] [Google Scholar]
- 18.Prado-Cabrero A, Scherzinger D, Avalos J, Al-Babili S. Retinal biosynthesis in fungi: characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryotic cell. 2007;6:650–657. doi: 10.1128/EC.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruch S, Beyer P, Ernst H, Al-Babili S. Retinal biosynthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Mol Microbiol. 2005;55:1015–1024. doi: 10.1111/j.1365-2958.2004.04460.x. [DOI] [PubMed] [Google Scholar]
- 20.Leuenberger MG, Engeloch-Jarret C, Woggon WD. The Reaction Mechanism of the Enzyme-Catalyzed Central Cleavage of beta-Carotene to Retinal. Angew Chem Int Ed Engl. 2001;40:2613–2617. doi: 10.1002/1521-3773(20010716)40:14<2613::AID-ANIE2613>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt H, Kurtzer R, Eisenreich W, Schwab W. The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J Biol Chem. 2006;281:9845–9851. doi: 10.1074/jbc.M511668200. [DOI] [PubMed] [Google Scholar]
- 22.Poliakov E, Gentleman S, Chander P, Cunningham FX, Jr, Grigorenko BL, Nemuhin AV, Redmond TM. Biochemical evidence for the tyrosine involvement in cationic intermediate stabilization in mouse beta-carotene 15, 15′-monooxygenase. BMC Biochem. 2009;10:31. doi: 10.1186/1471-2091-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010;35:400–410. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci U S A. 2008;105:19000–19005. doi: 10.1073/pnas.0807805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 26.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moiseyev G, Takahashi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, Ma JX. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem. 2006;281:2835–2840. doi: 10.1074/jbc.M508903200. [DOI] [PubMed] [Google Scholar]
- 30.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 31.Samardzija M, von Lintig J, Tanimoto N, Oberhauser V, Thiersch M, Reme CE, Seeliger M, Grimm C, Wenzel A. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Human molecular genetics. 2008;17:281–292. doi: 10.1093/hmg/ddm304. [DOI] [PubMed] [Google Scholar]
- 32.von Lintig J, Dreher A, Kiefer C, Wernet MF, Vogt K. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation invivo. Proc Natl Acad Sci U S A. 2001;98:1130–1135. doi: 10.1073/pnas.031576398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science. 2005;308:267–269. doi: 10.1126/science.1108965. [DOI] [PubMed] [Google Scholar]
- 34.Messing SA, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzel LM. Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell. 22:2970–2980. doi: 10.1105/tpc.110.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009;106:17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J Biol Chem. 2002;277:23942–23948. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 37.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch Biochem Biophys. 2010;506:109–121. doi: 10.1016/j.abb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- 42.Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr. 2007;137:2346–2350. doi: 10.1093/jn/137.11.2346. [DOI] [PubMed] [Google Scholar]
- 43.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding {beta}-carotene 15,15′-monoxygenase alter {beta}-carotene metabolism in female volunteers. Faseb J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 44.Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, Blaner WS. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys. 2010;504:3–10. doi: 10.1016/j.abb.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Helden YG, Heil SG, van Schooten FJ, Kramer E, Hessel S, Amengual J, Ribot J, Teerds K, Wyss A, Lietz G, Bonet ML, von Lintig J, Godschalk RW, Keijer J. Knockout of the Bcmo1 gene results in an inflammatory response in female lung, which is suppressed by dietary beta-carotene. Cell Mol Life Sci. 2010;67:2039–2056. doi: 10.1007/s00018-010-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 47.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–4525. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- 48.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem. 2006;281:4739–4745. doi: 10.1074/jbc.M509042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–13437. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- 51.von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta. 2005;1740:122–131. doi: 10.1016/j.bbadis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Castenmiller JJ, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998;18:19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- 53.Johnson EJ, Russell RM. Distribution of orally administered beta-carotene among lipoproteins in healthy men. Am J Clin Nutr. 1992;56:128–135. doi: 10.1093/ajcn/56.1.128. [DOI] [PubMed] [Google Scholar]
- 54.Borel P, Grolier P, Mekki N, Boirie Y, Rochette Y, Le Roy B, Alexandre-Gouabau MC, Lairon D, Azais-Braesco V. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J Lipid Res. 1998;39:2250–2260. [PubMed] [Google Scholar]
- 55.Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability of the conversion of beta-carotene to vitamin A in women measured by using a double-tracer study design. Am J Clin Nutr. 2000;71:1545–1554. doi: 10.1093/ajcn/71.6.1545. [DOI] [PubMed] [Google Scholar]
- 56.Edwards AJ, You CS, Swanson JE, Parker RS. A novel extrinsic reference method for assessing the vitamin A value of plant foods. Am J Clin Nutr. 2001;74:348–355. doi: 10.1093/ajcn/74.3.348. [DOI] [PubMed] [Google Scholar]
- 57.Hickenbottom SJ, Lemke SL, Dueker SR, Lin Y, Follett JR, Carkeet C, Buchholz BA, Vogel JS, Clifford AJ. Dual isotope test for assessing beta-carotene cleavage to vitamin A in humans. European journal of nutrition. 2002;41:141–147. doi: 10.1007/s00394-002-0368-0. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Yin S, Zhao X, Russell RM, Tang G. beta-Carotene-vitamin A equivalence in Chinese adults assessed by an isotope dilution technique. The British journal of nutrition. 2004;91:121–131. doi: 10.1079/bjn20031030. [DOI] [PubMed] [Google Scholar]
- 59.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding {beta}-carotene 15,15′-monoxygenase alter {beta}-carotene metabolism in female volunteers. Faseb J. 2008 doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 60.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bachmann H, Desbarats A, Pattison P, Sedgewick M, Riss G, Wyss A, Cardinault N, Duszka C, Goralczyk R, Grolier P. Feedback regulation of beta,beta-carotene 15,15′-monooxygenase by retinoic acid in rats and chickens. J Nutr. 2002;132:3616–3622. doi: 10.1093/jn/132.12.3616. [DOI] [PubMed] [Google Scholar]
- 62.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray PA, Shivdasani RA. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–4129. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- 64.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–4911. doi: 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- 65.Cai SF, Kirby RJ, Howles PN, Hui DY. Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J Lipid Res. 2001;42:902–909. [PubMed] [Google Scholar]
- 66.Hauser H, Dyer JH, Nandy A, Vega MA, Werder M, Bieliauskaite E, Weber FE, Compassi S, Gemperli A, Boffelli D, Wehrli E, Schulthess G, Phillips MC. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry. 1998;37:17843–17850. doi: 10.1021/bi982404y. [DOI] [PubMed] [Google Scholar]
- 67.Voshol PJ, Schwarz M, Rigotti A, Krieger M, Groen AK, Kuipers F. Down-regulation of intestinal scavenger receptor class B, type I (SR-BI) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem J. 2001;356:317–325. doi: 10.1042/0264-6021:3560317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43:1086–1095. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- 69.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem. 2001;276:32160–32168. doi: 10.1074/jbc.M010086200. [DOI] [PubMed] [Google Scholar]
- 70.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15′-dioxygenase. J Biol Chem. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 71.Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. {beta}-Carotene and its cleavage enzyme {beta}-carotene-15,15′-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. doi: 10.1096/fj.10-175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan W, Jang GF, Haeseleer F, Esumi N, Chang J, Kerrigan M, Campochiaro M, Campochiaro P, Palczewski K, Zack DJ. Cloning and characterization of a human beta,beta-carotene-15,15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics. 2001;72:193–202. doi: 10.1006/geno.2000.6476. [DOI] [PubMed] [Google Scholar]
- 73.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 74.Chichili GR, Nohr D, Schaffer M, von Lintig J, Biesalski HK. beta-Carotene conversion into vitamin A in human retinal pigment epithelial cells. Investigative ophthalmology & visual science. 2005;46:3562–3569. doi: 10.1167/iovs.05-0089. [DOI] [PubMed] [Google Scholar]
- 75.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, Von Lintig J. Provitamin A conversion to retinal via the beta,beta-carotene-15,15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development. 2003;130:2173–2186. doi: 10.1242/dev.00437. [DOI] [PubMed] [Google Scholar]
- 77.Kuri-Harcuch W. Differentiation of 3T3 F442A cells into adipocytes is inhibited by retinoic acid. Differentiation. 1982;23:164–169. doi: 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 78.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribot J, Felipe F, Bonet ML, Palou A. Changes of adiposity in response to vitamin A status correlate with changes of PPAR gamma 2 expression. Obes Res. 2001;9:500–509. doi: 10.1038/oby.2001.65. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez R, de Andres J, Yubero P, Vinas O, Mampel T, Iglesias R, Giralt M, Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem. 1995;270:5666–5673. doi: 10.1074/jbc.270.10.5666. [DOI] [PubMed] [Google Scholar]
- 81.Puigserver P, Vázquez F, Bonet ML, Picó C, Palou A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem J. 1996;317:827–833. doi: 10.1042/bj3170827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mercader J, Ribot J, Murano I, Felipe F, Cinti S, Bonet ML, Palou A. Remodeling of white adipose tissue after retinoic Acid administration in mice. Endocrinology. 2006;147:5325–5332. doi: 10.1210/en.2006-0760. [DOI] [PubMed] [Google Scholar]
- 83.Mercader J, Madsen L, Felipe F, Palou A, Kristiansen K, Bonet ML. All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell Physiol Biochem. 2007;20:1061–1072. doi: 10.1159/000110717. [DOI] [PubMed] [Google Scholar]
- 84.Hollung K, Rise CP, Drevon CA, Reseland JE. Tissue-specific regulation of leptin expression and secretion by all-trans retinoic acid. J Cell Biochem. 2004;92:307–315. doi: 10.1002/jcb.20047. [DOI] [PubMed] [Google Scholar]
- 85.Felipe F, Bonet ML, Ribot J, Palou A. Modulation of resistin expression by retinoic acid and vitamin A status. Diabetes. 2004;53:882–889. doi: 10.2337/diabetes.53.4.882. [DOI] [PubMed] [Google Scholar]
- 86.Felipe F, Mercader J, Ribot J, Palou A, Bonet ML. Effects of retinoic acid administration and dietary vitamin A supplementation on leptin expression in mice: lack of correlation with changes of adipose tissue mass and food intake. Biochim Biophys Acta. 2005;1740:258–265. doi: 10.1016/j.bbadis.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 87.Mercader J, Granados N, Bonet ML, Palou A. All-trans retinoic acid decreases murine adipose retinol binding protein 4 production. Cell Physiol Biochem. 2008;22:363–372. doi: 10.1159/000149815. [DOI] [PubMed] [Google Scholar]
- 88.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 89.Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 90.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 91.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 92.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O’Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 94.Grimaldi PA. Peroxisome proliferator-activated receptors as sensors of fatty acids and derivatives. Cell Mol Life Sci. 2007;64:2459–2464. doi: 10.1007/s00018-007-7278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005;73:31–34. doi: 10.1016/j.plefa.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Felipe F, Bonet ML, Ribot J, Palou A. Up-regulation of muscle uncoupling protein 3 gene expression in mice following high fat diet, dietary vitamin A supplementation and acute retinoic acid-treatment. Int J Obes Relat Metab Disord. 2003;27:60–69. doi: 10.1038/sj.ijo.0802188. [DOI] [PubMed] [Google Scholar]
- 98.Boulanger A, McLemore P, Copeland NG, Gilbert DJ, Jenkins NA, Yu SS, Gentleman S, Redmond TM. Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. Faseb J. 2003;17:1304–1306. doi: 10.1096/fj.02-0690fje. [DOI] [PubMed] [Google Scholar]
- 99.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, Palczewski K, von Lintig J. {beta},{beta}-Carotene Decreases Peroxisome Proliferator Receptor {gamma} Activity and Reduces Lipid Storage Capacity of Adipocytes in a {beta},{beta}-Carotene Oxygenase 1-dependent Manner. J Biol Chem. 2010;285:27891–27899. doi: 10.1074/jbc.M110.132571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fierce Y, de Morais Vieira M, Piantedosi R, Wyss A, Blaner WS, Paik J. In vitro and in vivo characterization of retinoid synthesis from beta-carotene. Arch Biochem Biophys. 2008;472:126–138. doi: 10.1016/j.abb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zovich DC, Orologa A, Okuno M, Kong LW, Talmage DA, Piantedosi R, Goodman DS, Blaner WS. Differentiation-dependent expression of retinoid-binding proteins in BFC-1 beta adipocytes. J Biol Chem. 1992;267:13884–13889. [PubMed] [Google Scholar]
- 103.Molotkov A, Ghyselinck NB, Chambon P, Duester G. Opposing actions of cellular retinol-binding protein and alcohol dehydrogenase control the balance between retinol storage and degradation. Biochem J. 2004;383:295–302. doi: 10.1042/BJ20040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol. 2007;21:77–88. doi: 10.1210/me.2006-0225. [DOI] [PubMed] [Google Scholar]
- 105.Eroglu A, Hruszkewycz DP, Curley RW, Jr, Harrison EH. The eccentric cleavage product of beta-carotene, beta-apo-13-carotenone, functions as an antagonist of RXRalpha. Arch Biochem Biophys. 504:11–16. doi: 10.1016/j.abb.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, Erdman JW., Jr Lycopene biodistribution is altered in 15,15′-carotenoid monooxygenase knockout mice. J Nutr. 2008;138:2367–2371. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr. 2010;140:2134–2138. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zaripheh S, Nara TY, Nakamura MT, Erdman JW., Jr Dietary lycopene downregulates carotenoid 15,15′-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136:932–938. doi: 10.1093/jn/136.4.932. [DOI] [PubMed] [Google Scholar]
- 109.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer. 2009 doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eriksson J, Larson G, Gunnarsson U, Bed’hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008;4:e1000010. doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vage DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries) BMC genetics. 11:10. doi: 10.1186/1471-2156-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, van der Does Y, Macgibbon AH, Spelman RJ, Lehnert K, Snell RG. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics. 2009;182:923–926. doi: 10.1534/genetics.109.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yonekura L, Kobayashi M, Terasaki M, Nagao A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J Nutr. 140:1824–1831. doi: 10.3945/jn.110.126466. [DOI] [PubMed] [Google Scholar]
- 115.He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ, Hu FB, Qi L. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010;30:885–890. doi: 10.1161/ATVBAHA.109.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang SS, Schadt EE, Wang H, Wang X, Ingram-Drake L, Shi W, Drake TA, Lusis AJ. Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ Res. 2007;101:e11–30. doi: 10.1161/CIRCRESAHA.107.152975. [DOI] [PubMed] [Google Scholar]
- 117.Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF, Cantelli-Forti G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis. 2001;22:1483–1495. doi: 10.1093/carcin/22.9.1483. [DOI] [PubMed] [Google Scholar]
- 118.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological beta-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis. 2000;21:2245–2253. doi: 10.1093/carcin/21.12.2245. [DOI] [PubMed] [Google Scholar]
- 119.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, Shiels M, Hammond E, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–383. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 120.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]