Summary
Skin-resident dendritic cells (DC) are well positioned to encounter cutaneous pathogens and are required for the initiation of adaptive immune responses. There are at least 3 subsets of skin DC — Langerhans cells (LC), Langerin+ dermal DC (dDC), and classic dDC. Whether these subsets have distinct or redundant function in vivo is poorly understood. Using a Candida albicans skin infection model, we have shown that direct presentation of antigen by LC is necessary and sufficient for the generation of antigen-specific T helper-17 (Th17) cells but not for the generation of cytotoxic lymphocytes (CTL). In contrast, Langerin+ dDC are required for the generation of antigen specific CTL and Th1 cells. Langerin+ dDC also inhibited the ability of LC and classic DC to promote Th17 responses. This work demonstrates that skin-resident DC subsets promote distinct and opposing antigen-specific responses.
Introduction
Dendritic cells (DC) in the periphery are specialized tissue-resident antigen-presenting cells that acquire exogenous antigen via multiple routes (Banchereau et al., 2000). In response to pathogen products, they become activated and migrate to regional lymph nodes where they present processed antigen acquired in the periphery to CD4+ T-helper (Th) cells and CD8+ cytotoxic T lymphocyte (CTL) cells, thereby initiating adaptive immune responses(Banchereau et al., 2000; Itano et al., 2003). Recently, it has been demonstrated that only certain DC subsets have the ability to cross-present exogenous antigen to CTL(Bedoui et al., 2009; Dudziak et al., 2007; Henri et al., 2010; Hildner et al., 2008). In contrast, most DC subsets appear capable of presenting antigen to CD4+ T cells and the Th cell-phenotype that develops is largely determined by the adjuvant to which the DC is exposed(Joffre et al., 2010; Manicassamy and Pulendran, 2009; Reis e Sousa, 2004).
In uninflamed skin, 3 subsets of resident DC can be distinguished based on anatomical location and cell surface expression of the proteins Langerin and CD103(Kaplan, 2010). Langerhans cells (Langerin+ CD103−) reside in the epidermis where they form a self-renewing population that has an ontogeny distinct from other DC(Chorro and Geissmann, 2010; Ginhoux and Merad, 2010). In the dermis, DC subsets can be segregated into the well defined Langerin+ CD103+ dermal DC (dDC) subset and a more heterogeneous Langerin− CD103− dDC subset that can be further subdivided using additional markers(Henri et al., 2010).
Contact hypersensitivity (CHS) to epicutaneous application of haptens is the classic assay to evaluate adaptive cutaneous immune responses. Examining CHS responses in mice lacking DC subsets is a commonly employed technique to determine the functional significance of skin-DC subsets but the results have been mixed(Kaplan, 2010). Murine Langerin-Diptheria Toxin Receptor (MuLangerin-DTR) mice lack LC and Langerin+ dDC shortly after administration of diphtheria toxin (DT). Langerin+ dDC repopulate the skin more quickly than LC so that 7–14 days after DT administration Langerin+ dDC are largely intact while LC remain absent(Bursch et al., 2007). CHS is reduced in mice sensitized shortly after DT administration but returns to normal when sensitization is delayed so that only LC are absent. This suggests that Langerin+ dDC but not LC are required for CHS. However, other studies using the same system with low doses of hapten or chimeric mice concluded that LC and Langerin+ dDC have redundant functions(Honda et al., 2010; Noordegraaf et al., 2010). In contrast, CHS responses to multiple haptens are increased in transgenic mice that use the human langerin promoter to express either attenuated Diphtheria toxin subunit A(huLangerin-DTA) or the primate Diptheria Toxin Receptor (huLangerin-DTR) to specifically ablate LC constitutively or inducibly(Bobr et al., 2010; Igyarto et al., 2009; Kaplan et al., 2005). Finally, Batf3−/− mice that lack Langerin+ dDC but not LC, develop normal CHS responses(Edelson et al., 2010).
LC-like cells derived in vitro from bone-marrow precursors can efficiently cross-present soluble antigen to CD8+ CTL(Klechevsky et al., 2008). Similarly, LC isolated ex vivo from human and mouse skin can also cross-present antigen when cultured in vitro(Klechevsky et al., 2008; Stoitzner et al., 2006). In contrast, Langerin+ dDC isolated ex vivo from lymph nodes of mice following herpes simplex skin infection or from mice engineered to express ovalbumin in the epidermis are the only DC subset capable of cross-presenting antigen(Bedoui et al., 2009; Henri et al., 2010). Unfortunately, studies examining cross-presentation in vivo have relied on muLangerin-DTR mice or delivery of antigen to DC with antibody-antigen conjugates that cannot distinguish Langerin+ dDC from LC(Idoyaga et al., 2008; Stoitzner et al., 2006). Hence, it is unresolved which DC subset(s) cross-presents skin-derived antigen in vivo. In addition, the contribution made to CD4+ Th cell differentiation by individual skin-resident DC subsets has not been explored. Thus, the basic question of whether skin resident DC have unique or redundant functions in vivo remains unresolved.
A major obstacle that has hampered the detailed analysis of skin-DC function in vivo is the absence of a model in which antigen can be delivered to the skin that employs a physiologic adjuvant, allows for the examination of antigen-specific responses, but does not bypass the epidermis. Antigen-specific responses are difficult to examine in CHS(Igyarto et al., 2009). In addition, haptens have poorly defined adjuvant properties and small differences in technique lead to variable results(Kaplan, 2010). Epicutaneous protein immunizations also have poorly defined adjuvants and variable results depending on the anatomic site of immunzation(Wang et al., 2008). Finally, established skin infection models require either dermal injection of pathogens(e.g., Leishmania) (Ritter et al., 2004), severe skin wounding (e.g., Staphylococcus)(Miller et al., 2006), or can infect and kill LC (e.g., HSV)(Cunningham et al., 2010).
Candida albicans is a commensal organism of mucosal sites in humans and also a pathogen that infects the vagina, oropharynx and skin. Opportunistic systemic infections in immunocompromised patients is associated with a high mortality(Gudlaugsson et al., 2003; Wisplinghoff et al., 2004). In humans, Th17 CD4+ T cells are an important component of the anti-Candida immune response. C. albicans-specific memory T cells are largely Th17 cells (Acosta-Rodriguez et al., 2007). In addition, patients with hyper IgE syndrome and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome develop severe mucocutaneous candidiasis and have defects that inhibit Th17 cell responses(Kisand et al., 2010; Ma et al., 2008). Meanwhile in mice, the secretion of interleukin-17 ( IL-17) and Th17 cells are essential for host defense against Candida(Conti et al., 2009; Kagami et al., 2010; Lin et al., 2009).
In this study, we have engineered recombinant C. albicans to express multiple well-defined T cell antigens and developed a model of C. albicans skin infection that does not bypass the epidermis. By combining these tools with mice lacking specific skin-DC subsets and examining antigen-specific responses in vivo, we have been able to delineate non-redundant functions for skin-resident DC subsets.
Results
Cutaneous candidiasis model
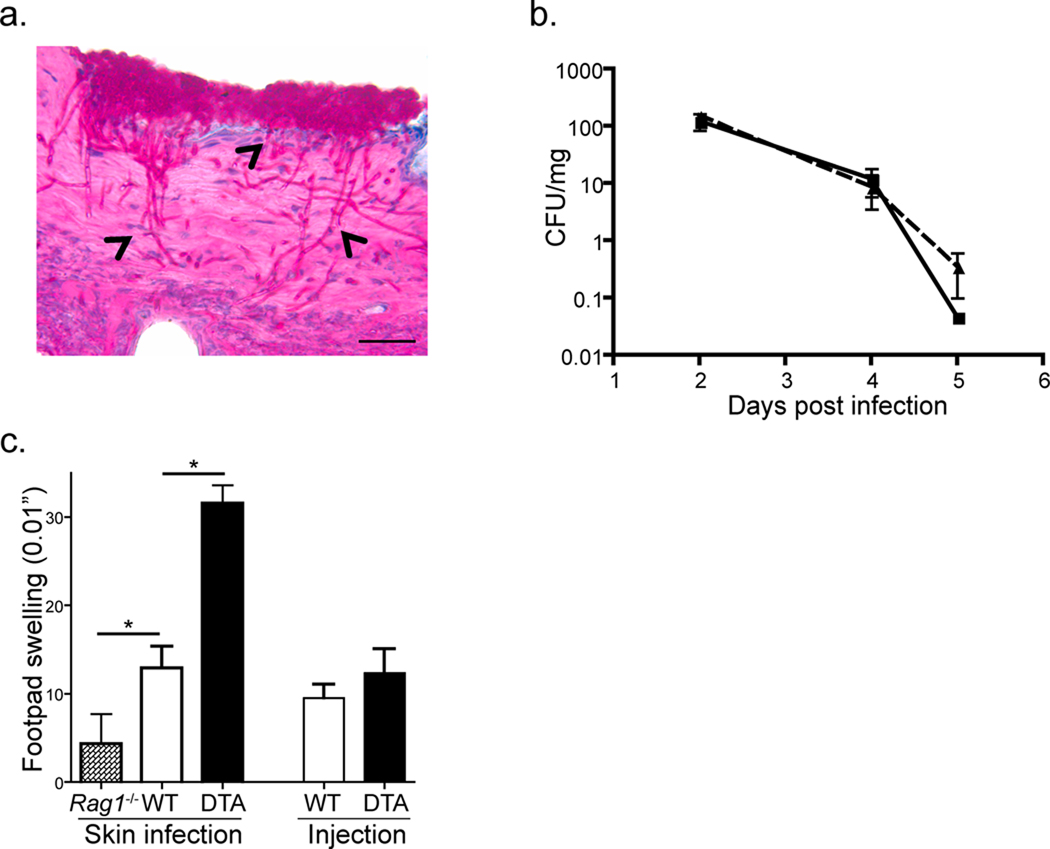
We adapted the techniques described by Sohnle et al.(Sohnle and Hahn, 1989) and Gaspari et al.(Gaspari et al., 1998) to generate a protocol to reliably infect the skin of mice with C. albicans. Back hair was removed by shaving and chemical depilation followed by disruption of the stratum corneum with sandpaper. The procedure was calibrated to remove approximately 50% of the stratum corneum while leaving the epidermis intact as assayed by hematoxylin stained sections (unpublished observation). C. albicans blastoconidia of the standard laboratory strain SC5314 (henceforth termed Calb-WT) was then inoculated onto the site. Within 48 hours, there was clear evidence of infection based on the presence of erosions, erythema and adherent crust (unpublished observation). Histologic evaluation of the skin at this time point revealed numerous foci of yeast adherent the outer epidermis with filamentous forms (arrow heads) invading through the epidermis and into the dermis (Figure 1a). Importantly, filaments were seen penetrating through intact epidermis. The lesions began to resolve by day +4 and neither yeast or filaments could be found by histologic examination of the skin by day +7 (unpublished observation).
Figure 1. C. albicans skin infection model.
Skin of wild-type mice were infected with 2 × 108 blastoconidia of the SC5314 strain of C. albicans. a) Skin from infected mice harvested on day+2 after infection was sectioned and stained with PAS to highlight fungi. Numerous yeast forms are evident on the external surface of the skin. Filamentous forms (arrow heads) can be observed penetrating through the epidermis into the dermis. Representative images are shown. Bar=50µm. b) Skin samples from cohorts of WT (solid line, n=8) and huLangerin-DTA (broken line, n=8) were cleaned with provine-iodine prior to being harvested at the indicated time after infection. C. albicans CFU is expressed as colonies per mg of tissue. c) WT, Rag1−/− and huLangerin-DTA mice were infected on the skin or by intra-dermal injection of 107 blastoconidia. DTH responses on day +7 were measured by specific footpad swelling 24 hours after injection of 107 heat killed C. albicans. Data is representative of 3 independent experiments with cohorts of at least 6 mice per group. *p<0.05.
Wild-type (WT) mice and mice with a constitutive absence of LC (huLangerin-DTA, see Table 1) developed lesions with a similar appearance and histology. We compared the number of invasive C. albicans in each strain by culturing skin from infected mice that had been cleansed with povidone-iodine to remove non-invasive C. albicans. Both strains of mice harbored similar numbers of invasive C. albicans that peaked on day +2 after infection and became undetectable on day +7 (Figure 1b). Thus, superficial infection with C. albicans produced a transient infection that was not altered by the absence of LC.
Table 1.
Comparison of the skin DC subsets ablated in DC-deficient mice.
| LC | Langerin+ dDC |
Langerin− dDC |
|
|---|---|---|---|
| WT | + | + | + |
| huLangerin- DTA |
− | + | + |
| Batf3−/− | + | − | + |
| muLangerin- DTR |
− | − | + |
To determine whether an adaptive immune response against Candida developed after infection and whether LC participated, we infected WT, Rag1−/− and huLangerin-DTA mice. Specific delayed type hypersensitivity (DTH) responses in footpads challenged with heat killed C. albicans were examined 7 days later (Figure 1c). WT mice developed a robust DTH response that was significantly greater than Rag1−/− mice in which the response was barely detectable. Langerin-DTA mice, however, developed a greatly exaggerated DTH response that was 2–3 times greater than WT mice. Importantly, dermal injection of C. albicans that bypassed the epidermis, did not produce exaggerated responses in huLangerin-DTA mice. Thus, a superficial skin infection with C. albicans generated a specific adaptive immune response that was exaggerated in the absence of LC. This was completely consistent with our prior work with CHS and demonstrated that this was an ideal system with which to compare the function of LC and dermal DC.
Generation of recombinant C. albicans
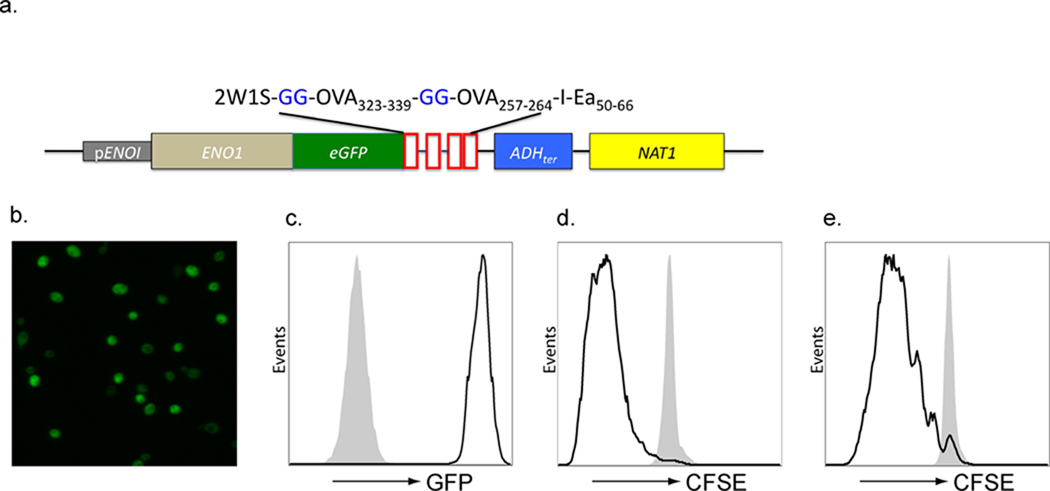
We constructed a recombinant C. albicans strain by modifying an existing plasmid (pMG2120)(Selmecki et al., 2008), which encodes green fluorescent protein (GFP), the ADH1ter transcription termination sequence and the Nat1 selectable marker. We then fused the Eno1 promoter and complete Eno1 coding sequence, in frame, to the N-terminus of GFP. Next, we inserted synthetic oligonucleotides encoding the well characterized immunodominant T cell epitopes, OVA323–339, OVA257–264, I-Eα50–66, and 2W1S(Moon et al., 2007) in frame with the C-terminus of GFP (Figure 2a) so that the ADH1ter transcription termination sequence and the Nat1 selectable marker, are 3' to the T cell epitopes (Shen et al., 2005). The fusion construct was transformed into C. albicans wild-type strain SC5314 (henceforth termed Calb-WT). A nourseothericin resistant clone, YJB11522 (Calb-Ag), was identified by GFP fluorescence (Figures 2b and c). Successful homologous recombination at the Eno1 locus was verified by PCR (unpublished observations).
Figure 2. Generation of recombinant C. albicans (Calb-Ag).
a) EGFP and the model T cell antigens, 2W1S, OVA323–339, OVA257–264, and I-Eα50–66 were inserted in frame into the genome of C. albicans (SC5314) at the C-terminus of the enolase gene. Expression GFP was confirmed by (b) direct visualization fluorescence of Calb-Ag blastoconidia and by (c) flow cytometry comparing Calb-Ag (solid line) with Calb-WT (Shaded). To confirm expression and availability of the T cell epitopes, Calb-WT (shaded) and Calb-Ag (solid line) were cultured with irradiated spleen cells, and CFSE-labeled CD90.1 congenic OT-I (d) or OT-II (e) cells. The amount of CFSE on CD90.1 gated cells T cells is shown after 3 days in culture. Data are representative of 3 independent experiments.
To confirm successful expression and availability of the T cell epitopes, we cultured Calb-WT (shaded line) and Calb-Ag (solid line) with antigen presenting cells (APC) and carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD8+ T cells from OT-I mice specific for OVA257–264 and CD4+ T cells from OT-II mice specific for OVA323–339 (Figure 2d and e). The parental Calb-WT strain did not promote proliferation while Calb-Ag induced robust OT-I and OT-II proliferation. Thus, the GFP-antigen fusion protein is highly expressed and available for processing and presentation to both CD4+ and CD8+ T cells.
Cross-presentation of antigen in vivo
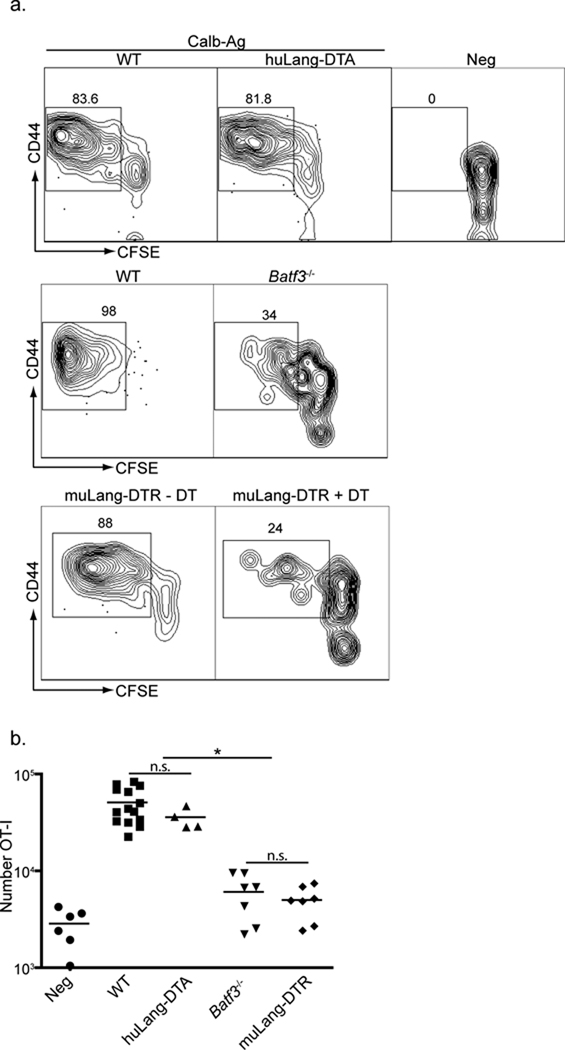
To determine whether LC participate in cross-presentation of antigen to CTL in vivo, we adoptively transferred into WT and Langerin-DTA mice CFSE-labeled CD90.1 congenic, CD44low naïve, OT-I cells isolated from OT-I TCR transgenic mice. The mice were then infected on their skin with either Calb-WT or Calb-Ag and the degree of proliferation was assessed in skin-draining lymph nodes four days later. As expected, OT-I did not proliferate in mice infected with Calb-WT (Neg) but did in mice infected with Calb-Ag (Figure 3a and b). There was, however, no difference in the degree of proliferation or total number of OT-I cells between WT and huLangerin-DTA mice. In addition, expression of granzyme B and IFN-γ, markers of CTL activation, were similar in both strains of mice (Supplemental Figure S1). Thus, LC are not required for cross-presentation of antigen in vivo.
Figure 3. LC are not required for antigen cross presentation in vivo.
a) 3×105 CFSE-labeled, CD90.1, CD44lo OT-I cells (CTL specific for OVA257–264) were adoptively transferred into WT, huLangerin-DTA (top panel), Batf3−/− (middle panel) or muLangerin-DTR (bottom panel) mice. Mice were infected on their skin with either Calb-WT (Neg) or Calb-Ag. Skin-draining LN were harvested 4 days later. CFSE and expression of CD44 are shown on CD8+, CD90.1+ gated cells. Numbers adjacent to outlined areas are the percentage of cells that have diluted CFSE. b) Total numbers of CD90.1+, OT-I cells recovered from each strain is shown. Each symbol represents an individual animal. WT and huLangerin-DTA are not significantly different. Batf3−/− and DT treated muLangerin-DTR mice are not significantly different from each other, but are significantly less than WT and huLangerin-DTA mice (p<0.0001). n.s.=not significant. See also Figure S1.
Batf3−/− mice and muLangerin-DTR mice treated with DT lack Langerin+ dDC (Table 1). As expected, OT-I proliferation in both lines was significantly reduced compared with WT mice, though a small amount of OT-I proliferation, approximately two fold greater that uninfected mice, was observed (Figure 3a and b). Although, CD8+ DC are also absent from Batf3−/− and reduced in muLangerin-DTR mice, these cells did not acquire antigen in our skin-infection model and are unlikely to participate in the response (unpublished observations). Thus, Langerin+ dDC are required for optimal cross presentation in vivo, but another cell type can inefficiently cross-present cutaneous antigen in the absence of this DC subset.
Antigen presentation by LC is necessary for Th17 cell development
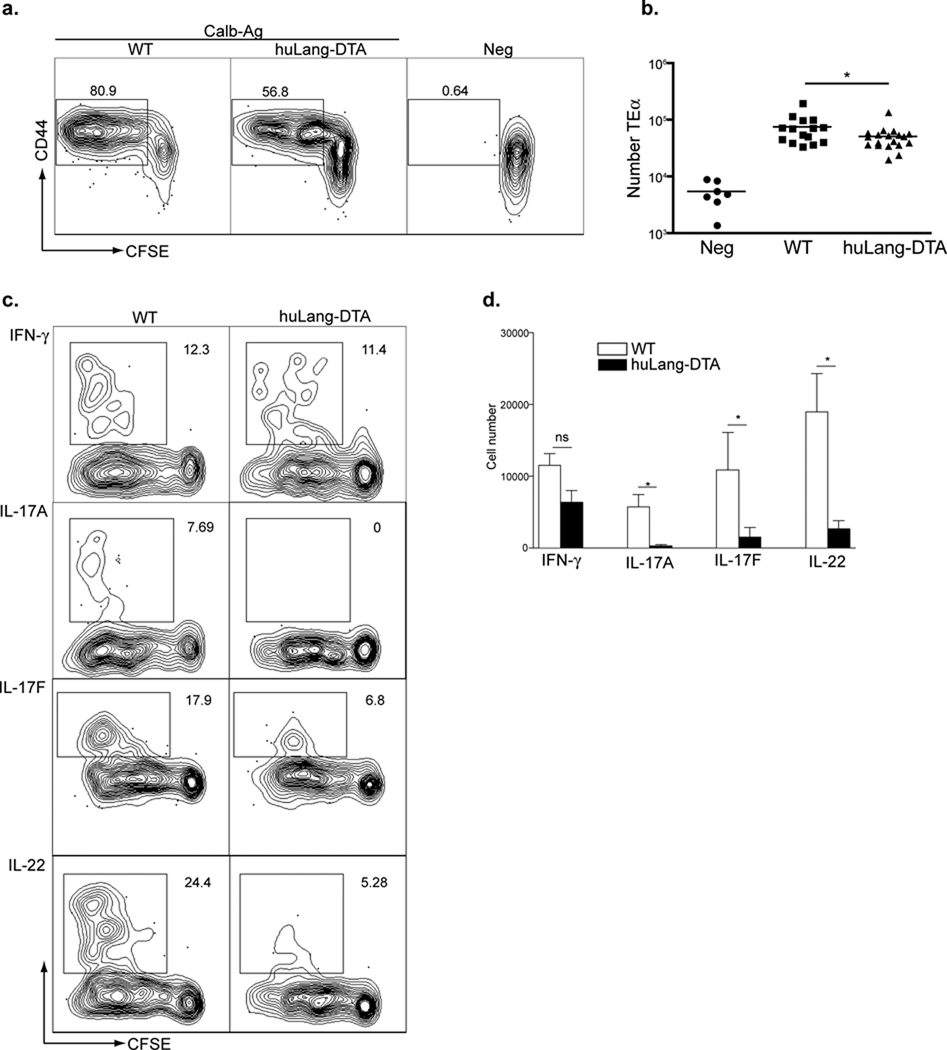
To examine whether LC can present antigen to CD4+ T cells, we adoptively transferred CFSE-labeled, CD90.1 congenic CD4+ T cells isolated from TEα transgenic mice that had been maintained on a Rag1−/− background into WT and huLangerin-DTA mice. Mice were infected and the degree of proliferation was assessed. We consistently observed slightly more CFSE dilution and greater total numbers of TEα cells in WT mice (13 fold expansion) than huLangerin-DTA mice (8 fold expansion) (Figure 4a and b). Experiments using CD4+ T cells isolated from OT-II mice produced similar results (unpublished observations).
Figure 4. LC are necessary for Th17 cell development.
3×105 CFSE-labeled, CD90.1 TEα cells (CD4+ T cells specific for I-Eα50–66) were transferred into WT and huLangerin-DTA mice. a) Mice were infected on their skin with either Calb-WT (Neg) or Calb-Ag. Skin-draining LN were harvested 4 days later. CFSE and expression of CD44 are shown on CD4+, CD90.1+ gated cells. b) Total numbers of CD90.1+, TEα cells recovered from each strain is shown. Each symbol represents an individual animal. c) As in (a) except that cells were re-stimulated in vitro with PMA-ionomycin prior to intracellular staining with the indicated cytokine antibody. Representative plots are shown. d) Total numbers of cytokine producing TEα cells from each strain is shown. Data have been pooled from 3 independent experiments (ns, not significant; * p<0.05). See also Figure S2.
We next examined the cytokine profile of TEα cells that expanded during infection. Calb-Ag infection produced a mix of Th1 cells producing IFN-γ and Th17 cells producing IL-17A, IL-17F, and IL-22 in WT mice. (Figure 4c and 4d). All Th17 cells co-expressed IL-22 but not IFN-γ (Supplemental Figure S2). Surprisingly, the number of cells producing IL-17A, IL-17F and IL-22 was dramatically reduced in the absence of LC (Figure 4d). In contrast, the number of cells producing IFN-γ was only slightly reduced in huLangerin-DTA mice and did not achieve statistical significance. We did not observe any expression of IL-4, IL-10, IL-13 or Foxp3 (unpublished observations). Thus, the absence of LC has a profound effect on the development of Th17 cells.
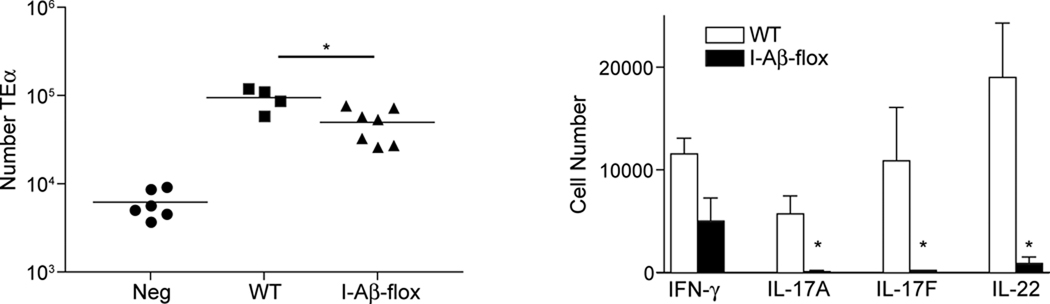
To determine whether direct presentation by LC is required for Th17 development, we performed similar experiments in huLangerin-Cre x I-Aβ-flox mice. Cre recombinase is constitutively expressed by LC in Langerin-Cre mice(Kaplan et al., 2007). Like the huLangerin-DTA mice, cells other than LC are not affected. Thus, huLangerin-Cre on an I-Aβ-flox background have a constitutive and durable absence of major histocompatibility complex class II (MHC-II) limited to LC(Igyarto et al, 2009). As was observed with huLangerin-DTA mice, the absence of MHC-II on LC led to reduced proliferation of TEα cells and a near-complete absence of Th17 cells (Figure 5). Thus, direct presentation of antigen by LC is required for the development of a Th17 but not a Th1 cell response.
Figure 5. Direct antigen presentation by LC is required for Th17 cell development.
As in figure 4, TEα cells were adoptively transferred into WT and huLangerin-Cre x I-Aβ-flox mice prior to skin infection. a) The expansion of TEα cells in Calb-WT (neg) infected WT mice Calb-Ag infected WT and huLangerin-Cre x I-Aβ-flox is shown. Each symbol represents an individual animal. b) Total numbers of cytokine producing TEα cells from each strain is shown. Data have been pooled from 2 independent experiments (ns, not significant; * p<0.05).
Antigen presentation by LC is sufficient for Th17 cell development
In order to limit antigen presentation to LC, we sought to target antigen to LC using Langerin antibodies that have been associated with antigen. Published efforts using this technique have used antibodies to mouse Langerin that target several DC subsets in addition to LC(Idoyaga et al., 2008). To overcome this problem, we generated a monoclonal antibody (2G3) specific for the ectodomain of human Langerin that does not cross react with murine Langerin (Supplemental Figure S3). Thus, introduction of 2G3-antigen complexes into transgenic mice expressing huLangerin will selectively target antigen to LC.
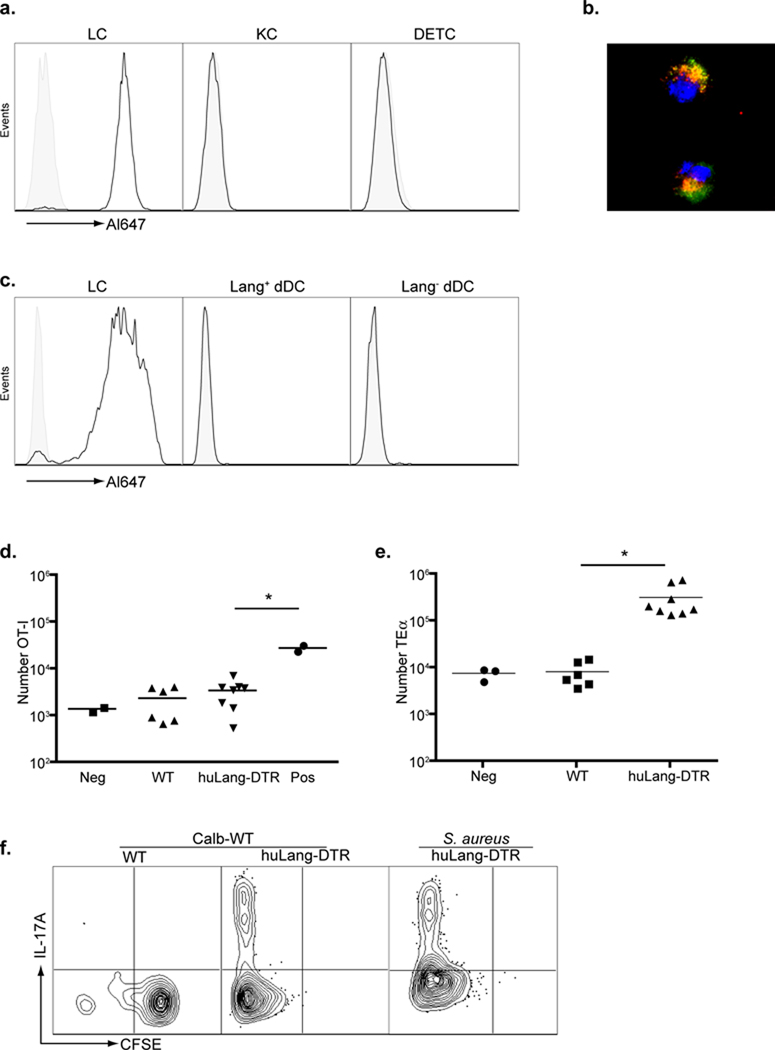
HuLangerin-DTR transgenic mice express human Langerin and the diphtheria toxin receptor exclusively on LC(Bobr et al., 2010). HuLangerin-DTR mice that had not been treated with DT, were injected intraperitoneally (i.p.) with anti-huLangerin (2G3) conjugated to Alexa-647. As expected, LC isolated from the epidermis had efficiently acquired the antibody (Figure 6a). Keratinocytes, dendritic epidermal T cells (DETC) as well as LC from WT mice did not acquire the antibody. Similarly, in lymph node, 2G3 could only be detected in LC from huLangerin-DTR mice and not in LC from WT mice, Langerin+ dDC or Langerin-dDC (Figure 6c). All other cells, including B cells and macrophages, in both lymph node and spleen did not acquire 2G3 (unpublished observation). Immunofluorescence of LC isolated from the epidermis showed that 2G3 (red) co-localized with the endogenous mouse Langerin (green) (Figure 6b). Thus, 2G3 efficiently binds to huLangerin LC in vivo and localizes to the same subcellular compartments as the endogenous muLangerin suggesting that it should enter the endocytic processing pathway.
Figure 6. Antigen presentation by LC is sufficient for Th17 development.
a) WT (shaded) and huLangerin-DTR (solid line) mice were injected i.p. with 10 µg anti-huLangerin (2G3) conjugated to Alexa-647. The amount of Alexa-647 16 hours after injection is shown in epidermal cells gated on LC (CD45+, MHC-II+), keratinocytes (KC, CD45-, MHC-II-) and dendritic epidermal T cells (DETC, CD45+, MHC-II−). b) As in (a) except that LC isolated from the epidermis were identified by expression of endogenous mouse Langerin (green). The cellular location of 2G3-Alexa-647 (red) and colocalization with muLangerin (yellow) was visualized by immunofluorescence. c) As in (a), cells were isolated from skin-draining LN. The amount of 2G3-Alexa647 on LC (MHC-IIhi, CD11 c+, CD8−, Langerin+, CD103−), Langerin+ dDC (MHC-IIhi, CD11c+, CD8−, Langerin+, CD103+), and Langerin− dDC (MHC-IIhi, CD11c+, CD8−, Langerin−) is shown. d) WT or huLangerin-DTR mice were adoptively transferred with 3×105 naïve OT-I cells 24 hours prior to i.p injection of 1.0 ug 2G3-OVA257–264 or isotype control (neg). Mice were then infected with Calb-WT on their skin. As a positive control, WT mice were immunized with 20 ug 2G3-OVA257–264 in CFA (pos). Skin-draining LN were harvested on day+4 and the total number of OT-I cells is shown. e) As in (d) except that TEα cells were transferred and mice were immunized with 2G3-Eα. f) CFSE and expression of IL-17A from TEα isolated from WT and huLangerin-DTR mice infected with Calb-WT or S. aureus are shown. Data are representative of 3 individual experiments. See also Figure S3 and S4.
We generated recombinant 2G3 with the peptide sequences for OVA257–264, I-Eα50–66 or 2W1S (for which an efficient MHC-II tetramer is available) fused to the C-terminus(Moon et al., 2007). To test the ability of LC to cross-present antigen during infection with C. albicans, OT-I adoptively transferred mice were injected i.p. with 2G3-OVA257–264. Mice were then infected with Calb-WT to activate LC. The only source of antigen was 2G3-OVA257–264. OT-I did not proliferate in WT or huLangerin-DTR mice (Figure 6d). However, OT-I did proliferate in response to sub cutaneous (s.c.) immunization with 2G3-OVA257–264 in complete Freund’s adjuvant (CFA) thereby demonstrating OVA257–264 was present. In contrast to OT-I, analogous experiments with TEα cells and 2G3-I-Eα50–66 showed a robust CD4+ T cell expansion in C. albicans-infected mice (Figure 6e). Activation of LC with either C. albicans or the common skin pathogen, Staphylococcus aureus, resulted in expression of IL-17A (Figure 6f). We also observed that endogenous 2W1 S-specific CD4+ T cells expanded and expressed IL-17A in C. albicans infected huLangerin-DTR mice injected with 2G3-2W1S (Supplemental Figure S4). Thus, antigen presentation by LC during skin infection with C. albicans or S. aureus is sufficient to drive proliferation of CD4 T cells and differentiation of Th17 cells but not for cross-priming of CTL.
Langerin+ dDC promote Th1 and inhibit Th17 cell differentiation
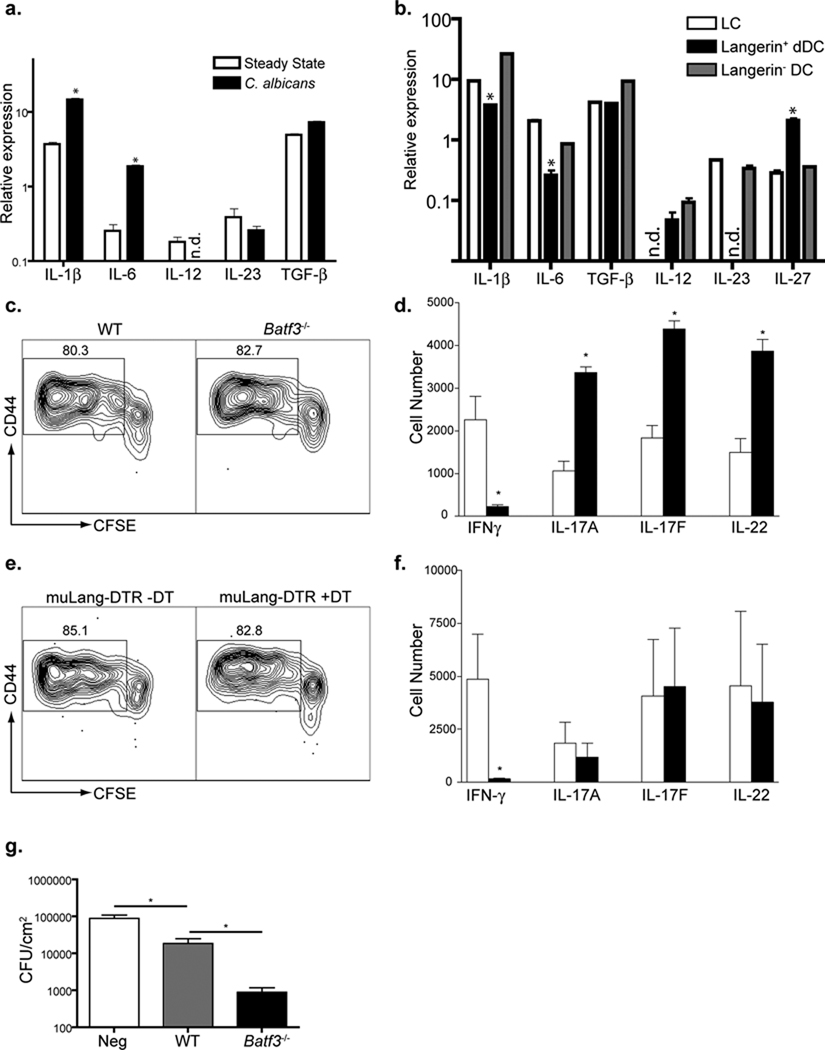
IL-1β, IL-6 and transforming growth factor β (TGF-β) participate in the initial development of Th17 cells and IL-23 maintains their phenotype(Korn et al., 2009). To examine the mechanism of LC-mediated induction of Th17 cells, we compared cytokine expression in LC sorted from lymph nodes of naïve and infected mice (Figure 7a and Supplemental Figure S5a). LC increased expression of IL-1β (~6 fold) and IL-6 (~8 fold) in response to infection, but TGF-β and IL-23 remained unchanged. IL-12 could not be reliably detected. We next compared expression of IL-1β, IL-6, TGF-β, and the regulated chains of the IL-12 family members IL-12(p35), IL-23(p19), and IL-27(p28) between LC, Langerin+ dDC and Langerin- dDC sorted from lymph node (LN) after skin infection with C. albicans. Langerin+ dDC produced significantly less IL-1β and IL-6 than LC (Figure 7b and Supplemental Figure S5a). We also observed that Langerin+ dDC but not LC expressed message for IL-12. Interestingly, Langerin+ dDC expressed higher amounts of IL-27 which has been demonstrated to suppress Th17 cell development(Stumhofer et al., 2010). Similar patterns of expression were observed after skin infection with S. aureus (Supplemental Figure S5b). This pattern of cytokine expression is certainly consistent with our observations that LC promote Th17 cells. However, it also raises the possiblity that Langerin+ dDC could promote Th1 and simultaneously inhibit Th17 cell responses.
Figure 7. Langerin+ dDC promote Th1 and inhibit Th17 cell differentiation.
a) LC were sorted by flow cytometry on day+4 from skin-draining LN from sham (open bars) or Calb-WT infected (black bars) muLangerin-EGFP reporter mice. Relative expression of mRNA of the indicated cytokines are shown. b) LC (open bars), Langerin+ dDC (black bars) and Langerin− dDC (gray bars) were sorted by flow cytometry from Calb-WT infected muLangerin-EGFP mice. Relative expression of mRNA of the indicated cytokines are shown. c and d) As in Figure 4, WT (open bars) and Batf3−/− (black bars) mice were infected with Calb-Ag. Proliferation (c) and cytokine expression (d) are shown. e and f) As in (c) except that muLangerin-DTR mice that had been injected with DT(black bars) or PBS (open bars) were used. Data are representative of 3 individual experiments. g) Cohorts of 4–8 WT and Batf3−/− mice were skin infected with Calb-WT or sham(“Neg”). Mice were then re-infected by intradermal injection of 5×106 Calb-WT on day +9. Three days later, skin was harvested and CFU obtained. (* p<0.05). Data are pooled from 2 independent experiments. See also Figure S5 and S6.
To test this hypothesis, we infected WT and Batf3−/− mice (Table 1) with Calb-Ag and analyzed the phenotype of TEα cells. The absence of Langerin+ dDC did not alter proliferation based on CFSE dilution (Figure 7c) or total numbers of TEα cells (Supplemental Figure S6a). IFN-γ producing cells did not develop in Batf3−/− mice and the numbers of cells expressing Th17-associated cytokines was increased approximately two fold (Figure 7d). Thus, Langerin+ dDC are required for the development of Th1 and inhibit the development of Th17 cells.
We repeated these experiments in muLangerin-DTR mice that lack both LC and Langerin+ dDC (Table 1). As in the Batf3−/− mice, TEα cells in muLangerin-DTR mice showed normal proliferation and a near complete absence of IFN-γ-producing cells (Figures 7e, 7f and supplemental Figure S6b). Unexpectedly, the absence of both LC and Langerin+ dDC resulted in normal numbers of Th17 cells (Figure 7f), indicating that Langerin- dDC were sufficient for Th17 cell induction. Interestingly, the cytokines expressed by Langerin- dDC were similar to LC (Figure 7b and supplemental 5b). Thus, like LC, Langerin- dDC can promote Th17 cell differentiation but only in the absence of inhibition from Langerin+ dDC.
To confirm that altered cytokine expression in TEα cells correlated with functional outcome, we next examined whether exaggerated Th17 and reduced Th1 cell responses in Batf3−/− mice affected rechallenge with C. albicans. To this end, naïve and previously infected WT and Batf3−/− mice were challenged by intradermal injection of 5 × 106 C. albicans blastoconidia. Three days later, the skin was harvested and colony forming units (CFU) determined. As expected, WT mice harbored significantly fewer C. albicans than naïve (“Neg”) mice (Figure 7g). Importantly, the number of CFU in Batf3−/− mice was reduced by approximately 20 fold compared to WT. Thus, the Th cell skewing observed in Batf3−/− mice in response to skin infection with C. albicans was associated with an increased resistance to C. albicans infection.
Discussion
Herein we have demonstrated that LC are neither necessary nor sufficient for cross-presentation of C. albicans antigen to CD8+ CTL in vivo. Instead, dermal Langerin+ DC were required for efficient cross-presentation. We also found that LC were necessary and sufficient for the development of Th17 cell responses. In contrast, Langerin+ dDC were required for the generation of Th1 cell responses. Moreover, the absence of Langerin+ dDC enhanced Th17 cell responses suggesting that these cells also inhibited the development of Th17 cells. Thus, skin-resident DC subsets have specific, non-redundant functions and promote distinct and opposing antigen-specific responses in vivo.
By comparing C. albicans-infected mice in which antigen cannot be presented by LC or can be presented only by LC, we have demonstrated that LC are necessary and sufficient for Th17 cell development. We observed that in response to infection with C. albicans or S. aureus, LC produced elevated amounts of IL-6, IL-1β, and IL-23 which both promote and stabilize Th17 cell differentiation(Korn et al., 2009). Thus, we propose that presentation of cutaneous antigen to naïve CD4+ T cells in the presence of these cytokines is responsible for the ability of LC to promote Th17 cells which is consistent with in vitro data (Aliahmadi et al., 2009; Mathers et al., 2009). Activated LC expressed low amounts of IL-27 and do not express IL-12 which are both key cytokine for Th1 cell differentiation(Szabo et al., 2003). In addition, C. albicans infected Langerin-DTA mice had an intact Th1 cell response which suggested that LC did not participate in Th1 cell development. Batf3−/− mice lack Langerin+ dDC and did not develop Th1 cells. Langerin+ dDC expressed higher amounts of IL-12 and IL-27 than LC which likely explains the requirement of this DC subset for Th1 cell development. Langerin+ dDC also had lower IL-1 β and IL-6 expression as well as the absence of IL-23 which makes them poor promoters of Th17 differentiation. Interestingly, IL-12 and IL-27 as well as IFN-γ from Th1 cells have all been reported to inhibit Th17 cell formation and proliferation(McGeachy and Cua, 2008; Stumhofer et al., 2006; Stumhofer et al., 2010). The absence of these inhibitory cytokines in Batf3−/− mice likely explains the observed exaggerated Th17 cell responses. Thus, presentation of the same antigen by LC and Langerin+ dDC promotes opposing effects that together allowed for the development of both Th1 and appropriately balanced Th17 cell responses.
Cross-presentation by LC has been clearly demonstrated using cells derived in vitro from human bone-marrow precursors or isolated ex vivo from human skin explants when cultured with soluble antigen in vitro(Klechevsky et al., 2008). LC isolated ex vivo from murine skin behave similarly(Flacher et al., 2010; Stoitzner et al., 2006). In contrast, LC isolated ex vivo from LN of mice in which antigen is only available to LC in vivo either during steady-state or via herpes virus infection, cannot cross-present antigen(Bedoui et al., 2009; Henri et al., 2010). Our data from C. albicans-infected mice clearly demonstrates that LC are not necessary for cross-presentation. Similarly, presentation by LC was not sufficient to activate OT-I when antigen was selectively targeted to LC using anti-huLangerin-Ag complexes. Thus, it appears that in vivo, LC do not have the capacity for cross-presentation. The ability of LC isolated from skin to cross-present in vitro may be specific to LC while in the epidermis or could be acquired during in vitro culture. Our experiments with Batf3−/− and muLangerin-DTR mice demonstrated that Langerin+ dDC were required for efficient cross-presentation of skin-derived antigen. This is consistent with the data examining DC populations in vivo(Wang et al., 2008) and those isolated from LN ex vivo(Bedoui et al., 2009; Henri et al., 2010). Targeting antigen to LC in vivo using muLangerin antibodies (clone L31) has been reported to result in efficient cross-presentation(Idoyaga et al., 2008). Since both Langerin+ dDC and LC express muLangerin, we suggest that Langerin+ dDC, not LC are responsible for the observed effect.
Throughout this report we have divided DC into LC, Langerin+ dDC and Langerin- dDC for the sake of expediency. Although the first two are well defined and can be manipulated in vivo, Langerin− dDC represent a heterogeneous population that include “classic” CD11b+ dDC as well as a more recently defined CD11 b− population(Henri et al., 2010). The function of these DC has been poorly explored, though, intriguingly, CD103− dDC have the unique ability to express retinoic acid and may be important for the generated of Treg cell during steady-state(Guilliams et al., 2010). We cannot directly manipulate these Langerin− dermal DC subsets with our present tools but they are the only skin-resident subset that remains in DT-treated muLangerin-DTR mice. In the absence of LC and Langerin+ dDC, we observed a modest amount of cross-presentation of C. albicans-derived antigen. Thus, at least one of the subsets contained within the Langerin− dDC population has the capacity for cross presentation. Moreover, this population has a cytokine profile similar to LC (high IL1β, IL-6, IL-23 and low IL-12, and IL-27) and is sufficient to promote Th17 cell differentiation when inhibition from Langerin+ dDC is absent (Figure 7). Recently, yet another DC subset derived from monocytes in response to LPS via TLR4 stimulation has been reported(Cheong et al., 2010). Although LPS is absent from our system, we cannot exclude that a similar inflammatory-type DC was present within the Langerin- dDC subset.
In conclusion, we have demonstrated in vivo, that skin-resident DC subsets can promote unique and opposite T cell responses directed against the same antigen. This has important implications for vaccination strategies that selectively target DC populations(Palucka et al., 2010). In addition, the requirement for LC in the development of Th17 cells suggests these cells may participate in the early pathogenesis of Th17 cell-mediated skin diseases such as psoriasis. At present, we have explored the response to C. albicans and S. aureus which both promote Th17 cell-type responses. An important question for future investigation is whether Langerin+ dDC and LC also promote opposite T cell responses during the steady-state and in response to pathogens or adjuvants that do not induce Th17 type responses.
Experimental Procedures
Mice
HuLangerin-DTA(Kaplan et al., 2005), huLangerin-Cre I-Aβ-flox(Igyarto et al., 2009), huLangerin-DTR(Bobr et al., 2010), muLangerin-DTR(Kissenpfennig et al., 2005) and muLangerin-EGFP(Kissenpfennig et al., 2005) mice have been previously described. Batf3−/− mice(Hildner et al., 2008) that had been back-crossed 10 generations onto the C57BL/6 background were a generous gift from Dr. K. Murphy (Washington University, St Louis, MO). B6 Rag-1−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). OT-I.PL Cd8 T cell receptor (TCR) transgenic specific for OVA257–264(Hogquist et al., 1994), OT-II Rag1−/− Cd4 TCR transgenic recognizing OVA323–339(Barnden et al., 1998) and TEα Rag1−/− Cd4 TCR-transgenic to I-Eα50–66(Grubin et al., 1997) mice on CD90.1 C57BL/6 backgrounds were also used. All experiments were performed with 6- to 10 week-old and sex-matched mice. Mice were housed in microisolator cages and fed irradiated food and acidified water. The University of Minnesota institutional care and use committee approved all mouse protocols.
DC-depletion with diphtheria toxin
MuLangerin-DTR mice were i.p. injected with 1 µg of diphtheria toxin (List Biological Laboratories Inc., Campbell, CA) 2 days before infection, as previously described(Bobr et al., 2010; Kissenpfennig et al., 2005).
Antibodies
Fluorochrome-conjugated antibodies to CD4, CD8, CD11b, CD11c, CD90.1, CD103, MHC class II, IFN-γ, and IL-17A were purchased from Biolegend (San Diego, CA). Anti-mouse Langerin (L31), IL-17F and IL-22 were acquired from eBioscience (San Diego, CA).
Adoptive T cell transfer
Skin-draining lymph nodes and mesenteric lymph node of OT-II and TEα mice were disrupted through a cell strainer and washed with sterile HBSS. Naive (CD44low) OT-I T cells were purified from lymph nodes of OT-I.PL mice as described(Bursch et al., 2009). Cell purity (>95%) was determined by flow cytometry before adoptive transfer. The cells were labeled with CFSE (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions, and resuspended in sterile PBS at a concentration of 1 × 106 cell/ml. Three hundred µl (3 × 105 cells) was injected intravenously. Transferred cells were detected in recipient mice based on expression of congenic CD90.1.
Flow cytometry
Single-cell lymph node suspensions were obtained and stained as previously described(Kaplan et al., 2005). To evaluate cytokine expression, cells were incubated for 5 hours in complete RPMI 1640 supplemented with PMA (50 ng/ml) and ionomycin (1.5 µM; Sigma-Aldrich, St. Louis, MO), with GolgiStop (BD Pharmingen, San Jose, CA) added for the final 4 hours. The intracellular cytokine staining was performed using BD Bioscience Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) according to the manufacturer’s directions. Samples were analyzed on LSR-II flow cytometers (BD Biosciences). Detection of 2W1S specific CD4 T cells was performed as published using I-Ab-2W1S tetramer (a kind gift of Dr. Marc Jenkins)(Moon et al., 2007). Data were analyzed with FlowJo software (TreeStar, Ashland, OR) . Isolation of DC subsets by flow cytometric cell sorting and analysis of mRNA expression is described in supplemental methods.
Skin infection model
Mice were first anesthetized with a mixture of ketamine and xylazine (100/10 µg/kg body weight), shaved on back with electric clipper and chemically depilated with Nair hair remover (Church & Dwight, Princeton, NJ) per the manufacturer’s directions. The stratum corneum was removed using 15 strokes with 220 grit sandpaper (3M, St Paul, Mn). C. albicans (WT or recombinant) was grown in YPAD medium (Sherman, 1991) at 30°C until the OD600 reached 1.5–2.0. After washing with sterile PBS, 2 × 108 C. albicans in 50 µl of sterile PBS was applied on to the skin. In some experiments, Staphylococcus aureus (ALC2906) grown in LB media was applied onto shaved mouse skin at a concentration of 2 × 108 bacteria in 50ul of PBS.
Delayed-type hypersensitivity
Eight days after skin infection, mice were challenged with footpad injection of 107 heat killed (60°C for an hour) Candida albicans yeast cells. The specific DTH was determined based on the degree of footpad swelling 24 hours after challenge in infected mice minus the degree of swelling in sham infected control mice.
Mouse skin CFU
The mice infected with Candida albicans were sacrificed prior to infection and on day 2, 4, 5 and 7 after infection. The infected area was cleansed using povidone-iodine which was removed once dry and a 2.0 cm2 section of skin was homogenized in sterile PBS containing penicillin and streptomycin prior to plating on YPAD plates. In some experiments, previously infected mice were re-challenged by intradermal injection of 5 × 106 C. albicans. Three days later, 1.0 cm2 of skin surrounding the injection cite was harvested, homogenized in sterile PBS containing penicillin and streptomycin prior to plating on YPAD plates. Colony counts were obtained 24–48 hrs later.
Construction of recombinant C. albicans
All Candida albicans strains used in this study were derived from SC5314 (Calb-WT)(Fonzi and Irwin, 1993). Construction of recombination vectors and generation of recombinant C. albicans is described in supplemental methods.
In vitro T cell stimulation
OT-I and OT-II cells were labeled with CFSE and used as responders. For stimulators, Rag1−/− CD90.1 spleen cells were harvested and irradiated with cesium-irradiator with 25 Gy. 4×105 responders were co-cultured with 2×105 stimulators in 200 µl complete RPMI 1640 in CO2 incubator for 3 days supplemented with 105 heat killed Calb-WT or Calb-Ag yeast. Cells were harvested and analyzed by flow cytometry.
Monoclonal antibody generation
The anti-human Langerin 2G3 hybridoma (ATCC PTA 9853) was derived from mice immunized with human Langerin ectodomain fused to human IgFc as previously described(Klechevsky et al., 2010; Ni et al., 2010). Recombinant antibody fusion protein production is described in supplementary methods.
LC targeting using anti-huLangerin
WT and huLangerin-DTR, mice were injected with 10 µg of 2G3-AL647. The mice were sacrificed 16 hours later and the presence of the AL647 signal was analyzed in epidermal- and lymph node cells by flow cytometry as described(Kaplan et al., 2005). In separate experiments 3×105 TEα or OT-I cells were transferred into WT and huLangerin-DTR mice. A day later, the mice were immunized by intra-peritoneal injection of 1.0 µg of 2G3-Eα, 2G3-OVA257–264 or 2G3-2W1S in 100µl of sterile PBS. Six hours later, the mice were infected with Calb-WT on the dorsal skin. The skin draining lymph nodes were harvested 4 days later and analyzed by flow cytometry.
Immunofluorescence staining
Immunofluorescence was performed as previously described(Kaplan et al., 2005). Images were captured using a microscope (DM5500; Leica) with digital system and LAS AF software (version 1.5.1).
Histology
Skin samples were fixed overnight in 10% formalin, dehydrated and embedded in paraffin. The 7µm microtome sections were stained with Periodic Acid-Schiff stain according to the manufacturer’s directions (Sigma-Aldrich).
Statistics
Significant differences were calculated using the Student’s unpaired, two-tailed, t-test.
Supplementary Material
Acknowledgements
The authors have no conflicting financial interests, except that S.Z and G.Z. are inventors on patent applications for LC-targeting vaccines. We would like to acknowledge Drs Kristin Hogquist and Marc Jenkins for their insightful input and critique of the manuscript. We thank the University of Minnesota Research Animal Resources staff for animal care. Paul Champoux and the Flow Cytometry Core Facility at the Center for Immunology (University of Minnesota) assisted with sorting and flow-cytometry experiments. This work was supported by a grant from the NIH (R01-AR056632) to DHK, a Dermatology Foundation research award (BZI) and an American Skin Association research award (BZI). DHK is also supported by the Al Zelickson endowed Professorship. The 2G3 antibody reagents were developed with the support of National Institutes of Health Grant U19 AI057234 and the Baylor Health Care System Foundation. B.T.E. is the recipient of a Burroughs Wellcome Fund Career Award for Medical Scientists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009;39:1221–1230. doi: 10.1002/eji.200838742. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Benton T, Chen T, McEntee M, Fox B, King D, Crombie R, Thomas TC, Bebbington C. The use of UCOE vectors in combination with a preadapted serum free, suspension cell line allows for rapid production of large quantities of protein. Cytotechnology. 2002;38:43–46. doi: 10.1023/A:1021141712344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch LS, Rich BE, Hogquist KA. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J Immunol. 2009;182:4657–4664. doi: 10.4049/jimmunol.0803656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care RS, Trevethick J, Binley KM, Sudbery PE. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol Microbiol. 1999;34:792–798. doi: 10.1046/j.1365-2958.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorro L, Geissmann F. Development and homeostasis of 'resident' myeloid cells: the case of the Langerhans cell. Trends Immunol. 2010 doi: 10.1016/j.it.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Abendroth A, Jones C, Nasr N, Turville S. Viruses and Langerhans cells. Immunol Cell Biol. 2010;88:416–423. doi: 10.1038/icb.2010.42. [DOI] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B, Koch F, Park CG, Steinman RM, Idoyaga J, Romani N. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2010;130:755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari AA, Burns R, Nasir A, Ramirez D, Barth RK, Haidaris CG. CD86 (B7-2), but not CD80 (B7-1), expression in the epidermis of transgenic mice enhances the immunogenicity of primary cutaneous Candida albicans infections. Infect Immun. 1998;66:4440–4449. doi: 10.1128/iai.66.9.4440-4449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerami-Nejad M, Berman J, Gale CA. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast. 2001;18:859–864. doi: 10.1002/yea.738. [DOI] [PubMed] [Google Scholar]
- Gerami-Nejad M, Hausauer D, McClellan M, Berman J, Gale C. Cassettes for the PCR-mediated construction of regulatable alleles in Candida albicans. Yeast. 2004;21:429–436. doi: 10.1002/yea.1080. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol Cell Biol. 2010;88:387–392. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Honda T, Nakajima S, Egawa G, Ogasawara K, Malissen B, Miyachi Y, Kabashima K. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol. 2010;125:1154–1156. doi: 10.1016/j.jaci.2009.12.005. e1152. [DOI] [PubMed] [Google Scholar]
- Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- Igyarto BZ, Jenison MC, Dudda JC, Roers A, Muller W, Koni PA, Campbell DJ, Shlomchik MJ, Kaplan DH. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol. 2010;40:1255–1265. doi: 10.1002/eji.201040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010 doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGF{beta}1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and Function of Langerhans Cells In Vivo Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers AR, Janelsins BM, Rubin JP, Tkacheva OA, Shufesky WJ, Watkins SC, Morelli AE, Larregina AT. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol. 2009;182:921–933. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, O'Bar A, Clayton S, Palucka AK, Zurawski G, et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordegraaf M, Flacher V, Stoitzner P, Clausen BE. Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. J Invest Dermatol. 2010;130:2752–2759. doi: 10.1038/jid.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–641. doi: 10.1111/j.1365-2958.2008.06176.x. [DOI] [PubMed] [Google Scholar]
- Shen J, Guo W, Kohler JR. CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect Immun. 2005;73:1239–1242. doi: 10.1128/IAI.73.2.1239-1242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sohnle PG, Hahn BL. Effect of immunosuppression on epidermal defenses in a murine model of cutaneous candidiasis. J Lab Clin Med. 1989;113:700–707. [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, Fielding CA, O'Hara AC, Chen Y, Jones ML, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin Expressing Cells Promote Skin Immune Responses under Defined Conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.