Abstract
To better understand the role of active oxygen species (AOS) in acquired resistance to increased levels of ultraviolet (UV)-B irradiation in plants, we isolated an Arabidopsis mutant that is resistant to methyl viologen, and its sensitivity to UV-B was investigated. A complementation test revealed that the obtained mutant was allelic to the ozone-sensitive radical-induced cell death1-1 (rcd1-1). Therefore, this mutant was named rcd1-2. rcd1-2 was recessive and nearly 4-fold more resistant to methyl viologen than wild type. It exhibited a higher tolerance to short-term UV-B supplementation treatments than the wild type: UV-B-induced formation of cyclobutane pyrimidine dimers was reduced by one-half after 24 h of exposure; the decrease in quantum yield of photosystem II was also diminished by 40% after 12 h of treatment. Furthermore, rcd1-2 was tolerant to freezing. Steady-state mRNA levels of plastidic Cu/Zn superoxide dismutase and stromal ascorbate peroxidase were higher in rcd1-2 than in wild type, and the mRNA level of the latter enzyme was enhanced by UV-B exposure more effectively in rcd1-2. UV-B-absorbing compounds were more accumulated in rcd1-2 than in wild type after UV-B exposure for 24 h. These findings suggest that rcd1-2 methyl viologen resistance is due to the enhanced activities of the AOS-scavenging enzymes in chloroplasts and that the acquired tolerance to the short-term UV-B exposure results from a higher accumulation of sunscreen pigments. rcd1 appears to be a mutant that constitutively shows stress responses, leading to accumulation of more pigments and AOS-scavenging enzymes without any stresses.
Plants use sunlight for photosynthesis, and as a consequence, they are exposed to the UV-B radiation present in sunlight. The genome and the photosynthetic machinery are two important targets of UV-B radiation in plants. In the cell, DNA is the primary absorbing chromophore in the UV-B region of the sunlight spectrum, and cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone photoproducts are formed upon exposure to UV-B (Mitchell and Nairn, 1989). Another primary target is the reaction center of photosystem II (PSII; Renger et al., 1986). PSII is the most sensitive protein complex of the photosynthetic electron transfer chain. Damage to this complex results in loss of photosynthesis and degradation of reaction center proteins D1 and D2. The D1 and D2 proteins constituting the PSII reaction center heterodimer are targets of UV-B radiation and can be used as an in situ sensor for UV penetration into photosynthetic tissue. Degradation of these proteins occurs under very low fluences of UV-B and is strongly accelerated in the presence of visible light (Booij-James et al., 2000). An increased H2O2 level is detected simultaneously with the inhibition of photosynthesis by UV-B irradiation (Karpinski et al., 1997). This observation suggests that the UV-B-induced oxidative burst of H2O2 is associated with the damage and degradation of PSII.
Because damage caused by UV-B can be potentially fatal, land plants have evolved a number of protection and repair mechanisms. Plants possess DNA photolyase, which repairs DNA lesions. Arabidopsis mutants defective in the repair of DNA lesions have been identified as uvr1 to 3, which are more sensitive to UV-B irradiation than wild type (Britt et al., 1993; Landry et al., 1997; Jiang et al., 1997). Another UV-hypersensitive mutant, uvh1, lacks a protein similar to a component of the endonuclease that excises dimers by nucleotide excision repair (Liu et al., 2000). Furthermore, the Arabidopsis mutant UV-B insensitive1 (uvi1), which exhibits an enhanced capacity for repair, is more resistant to UV-B (Tanaka et al., 2002). Another important adaptation to the UV-B environment is an accumulation of sunscreen pigments, mostly phenolic compounds, in epidermal tissue. An Arabidopsis UV-tolerant mutant, uvt1, and a knockout mutant for AtMYB4 accumulate flavonoid and/or sinapate esters. They display a remarkable tolerance to UV-B irradiation conditions (Jin et al., 2000; Bieza and Lois, 2001). In contrast, transparent testa 5 (tt5) mutants lacking chalcone isomerase (CHI) activity exhibit increased UV sensitivity, probably due to the absence of detectable kaempherol and reduced quantities of sinapate esters (Li et al., 1993). Induction of chalcone synthase (CHS) mRNA and protein is blocked, and the UV-B mediated induction of flavonoids is reduced in an Arabidopsis UV-B hypersensitive mutant, uvr8. This mutation is caused by disruption of a gene similar to human Regulator of Chromatin Condensation 1 (Kliebenstein et al., 2002). These findings clearly show the protective role of DNA repair and sunscreen pigments against UV-B.
During normal cellular metabolism, plants are continuously producing active oxygen species (AOS) such as superoxide, H2O2, and singlet oxygen. Under normal conditions, however, plants rapidly metabolize these AOS with the help of antioxidant enzymes and/or metabolites (Asada, 1999). Various environmental perturbations such as the UV-B radiation examined in the present study, can cause excess AOS production, overwhelming the system and necessitating additional defenses. However, at present, it is not known whether the UV-B-induced AOS play a significant role in the damage caused by UV-B radiation.
If the UV-B-induced AOS are involved in UV-B-induced damage, enhancement of AOS-scavenging activities would confer resistance to UV-B to plants. Methyl viologen is a redox-active compound that generates superoxide anions in chloroplasts. This may resemble AOS generation in chloroplasts by UV-B irradiation. Therefore, if methyl viologen-resistant mutants are available and if their UV-B sensitivity is determined, it could be investigated whether the AOS production participates in UV-B-induced injury. This argument has prompted us to screen for methyl viologen-resistant mutants of Arabidopsis and to examine their sensitivity to UV-B irradiation in the present study. An isolated mutant was not only methyl viologen resistant, but also sensitive to O3 fumigation. It was allelic to the ozone-sensitive mutant radical-induced cell death1-1 (rcd1-1; Overmyer et al., 2000). This rcd1 allele exhibited UV-B resistance when exposed to UV-B for 24 h. It contained higher levels of the AOS-scavenging enzymes but also showed an increased accumulation of sunscreen pigments.
RESULTS
Isolation of Methyl Viologen-Resistant Mutants
Methyl viologen-resistant mutants were selected in a Columbia background from 10,000 independent lines mutagenized by insertion of T-DNA harboring the hygromycin phosphotransferase gene as a plant transformation marker (Nakazawa et al., 2001). Wild-type plants were able to grow at 1.5 μm methyl viologen (paraquat) when they were sown at a high density. Therefore, mutagenized seeds were sown at about 3 mm from each other on the agar medium to avoid picking false mutants. One mutant was obtained that was able to expand true leaves on agar medium containing 1.5 μm methyl viologen.
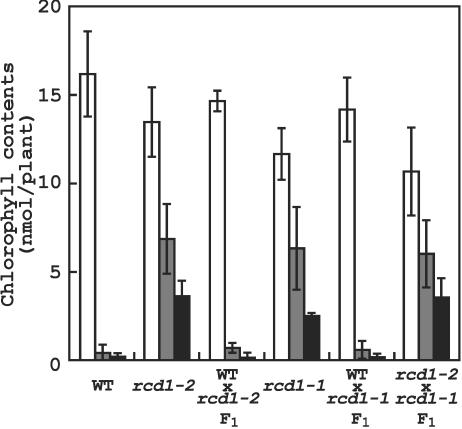
T3 progeny of the mutant that were homozygous for methyl viologen resistance were not hygromycin resistant, indicating that the mutation was not linked to the T-DNA. The mutant was found to be recessive by examining methyl viologen resistance of F1 progeny obtained by back-crossing to wild type (Fig. 1). To map the mutation, the homozygous mutant plants were crossed to wild-type plants of the Landsberg ecotype. Examination of about 250 mutants of F2 progeny for segregation of CAPS markers (Konieczny and Ausubel, 1993) revealed that the mutation was located between the GAPB and UFO markers on the upper arm of chromosome 1.
Figure 1.
Complementation test between rcd1-1 and rcd1-2 as determined by chlorophyll contents of crossed lines grown on agar medium containing 0 (white column), 0.4 (gray column), or 0.8 μm (black column) methyl viologen for 2 weeks. Values are means ± se of three independent experiments, in which 25 seedlings were used.
The mutant was more sensitive to O3 fumigation than wild type (data not shown). Because the O3-sensitive rcd1-1 has been located in the upper arm of chromosome 1 (Overmyer et al., 2000), a complementation test was carried out between the present mutant and rcd1-1. F1 plants of this cross were able to grow on 0.8 μm methyl viologen (Fig. 1), indicating that the methyl viologen-resistant mutant isolated in this study was allelic to rcd1-1. Thus, we designated the present mutant rcd1-2.
Mature rcd1-2 plants were about one-half the height of wild-type plants (Fig. 2A). Especially the leaf petioles were shorter than those of wild type, although the size of leaf blades was not much reduced. A higher content of pigments was also observed in rcd1-2 when they were young (Fig. 2B). In fact, chlorophyll contents of rcd1-2 were a little higher than wild type (Fig. 3B), and 3-week-old rcd1-2 contained about 50% more anthocyanin than wild type on the fresh weight basis (data not shown).
Figure 2.
Growth and morphology of Arabidopsis wild type (left in A, and top in B) and rcd1-2 mutant (right in A, and bottom in B). A, Six-week-old plants grown for 3 weeks on agar medium containing Murashige and Skoog salts and 1% (w/v) Suc followed by photoautotrophic growth on soil. Bar = 5 cm. B, Three-week-old plants grown on the agar medium. Bar = 5 mm.
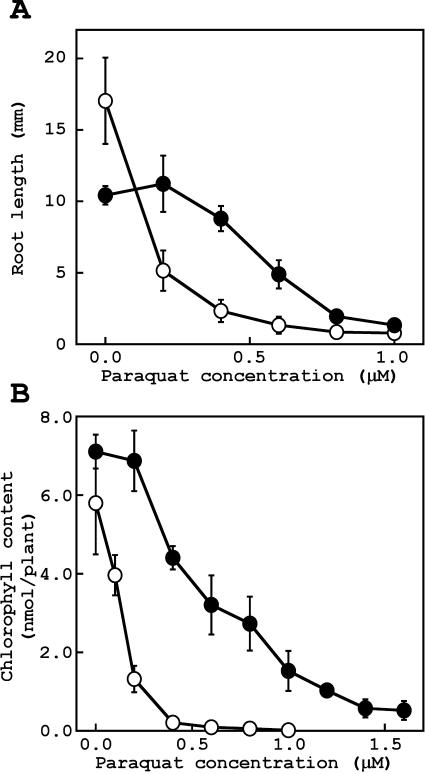
Figure 3.
Effects of methyl viologen (paraquat) on root growth (A) and chlorophyll content (B). Wild type (white circle) and rcd1-2 (black circle) were grown for 2 weeks on agar medium containing methyl viologen. A, Growth of primary roots. Values are means of eight replicates ± sd. B, Chlorophyll was extracted with methanol from aerial parts of plants. Values are means ± se of three independent experiments, in which four plants were used.
Methyl Viologen Resistance
The effects of methyl viologen on growth of Arabidopsis were examined measuring root growth (Fig. 3A) and chlorophyll content (Fig. 3B). Methyl viologen inhibited the growth of both wild type and rcd1-2 dose dependently. The concentration of methyl viologen that showed 50% inhibition of root growth and chlorophyll contents was about 0.15 μm for wild type, whereas it was about 0.55 μm for rcd1-2. These results showed that rcd1-2 was nearly 4-fold more resistant to methyl viologen than wild type.
Effects of Supplemental UV-B Irradiation on Photosynthesis
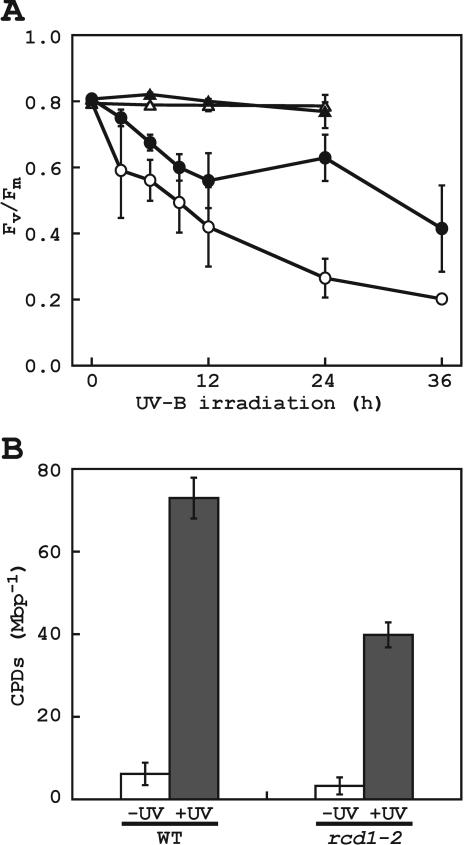
The effects of supplemental UV-B irradiation on the quantum yield of PSII were examined by measuring Fv/Fm (Fig. 4A), which reflects a decrease in the photochemical yield of open PSII reaction centers (Genty et al., 1989). Fv/Fm decreased in both wild type and rcd1-2 after UV-B exposure. After 12 h of exposure to UV-B, however, the reduction of Fv/Fm in rcd1-2 was 63% of that observed in wild type. After 24 h of irradiation, Fv/Fm was transiently recovered in rcd1-2. The results indicated that rcd1-2 was more resistant to UV-B irradiation for 24 h than wild type with respect to PSII integrity (P < 0.001 in t test).
Figure 4.
Effects of supplemental UV-B radiation (10.8 kJ m-2 d-1) on wild type and rcd1-2. A, Fv/Fm was measured with the third or fourth leaf with petiole, which was excised from 3-week-old wild type (white symbol) and rcd1-2 (black symbol). The leaf explant was planted into moistened precut floral foams and was then irradiated with white light with (circle) or without (triangle) UV-B before measurements. Values are means of six replicates ± sd. B, CPD was quantified by ELISA with monoclonal antibodies specific for CPD. DNA was isolated from aerial parts of plants irradiated with white light and UV-B for 24 h. Values are means of three independent experiments ± sd.
Effects of Supplemental UV-B Irradiation on DNA
DNA lesions induced by supplemental UV-B irradiation were evaluated by measuring the production of CPD (Fig. 4B). After UV-B irradiation for 24 h, CPD accumulation was increased in both rcd1-2 and wild type. However, twice as much CPD was produced in wild type as in rcd1-2, showing that rcd1-2 was more resistant to UV-B irradiation for 24 h in terms of the formation of CPD (P = 0.0014 in t test)
Tolerance to Heat Shock and Freezing
Because methyl viologen-resistant mutants have been shown to be cross resistant to other stress conditions (Tsugane et al., 1999), we investigated tolerance of rcd1-2 to heat shock and freezing. After heat shock treatment, 2.5-d-old seedlings were grown at 23°C for 2 d, and regrowth of roots for the last 2 d was measured (Hong and Vierling, 2000). No significant differences were observed in wild type and rcd1-2 (data not shown).
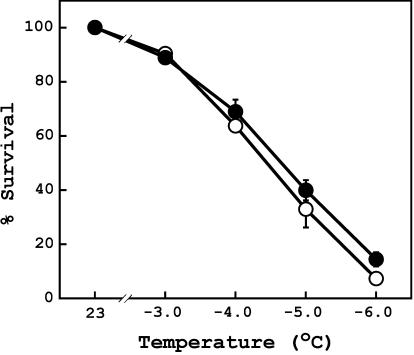
Tolerance to freezing was estimated by measuring the extent of electrolyte leakage from rosette leaves after freezing, which reflects the survival of leaf cells. After freeze treatment at -6.0°C, survivability of cells was slightly but significantly higher in rcd1-2 than in wild type (P = 0.0037 in t test; Fig. 5). This indicated that rcd1-2 was slightly more tolerant to freezing.
Figure 5.
Effects of freezing treatment on survival of leaf cell. Ion leakage from 2-week-old wild-type (white circle) and rcd1-2 (black circle) leaves were determined after freezing treatment. Values are means ± se of five independent experiments, in which three plants were used.
Gene Expression of AOS-Scavenging Enzymes and Effects of Supplemental UV-B Irradiation
Methyl viologen resistance is often associated with elevated levels of activities of AOS-scavenging enzymes, such as superoxide dismutase (SOD; Sen Gupta et al., 1993; Slooten et al., 1995) and peroxidases (Tsugane et al., 1999). Therefore, we determined the mRNA levels of SOD and peroxidases with RNA gel-blot analysis.
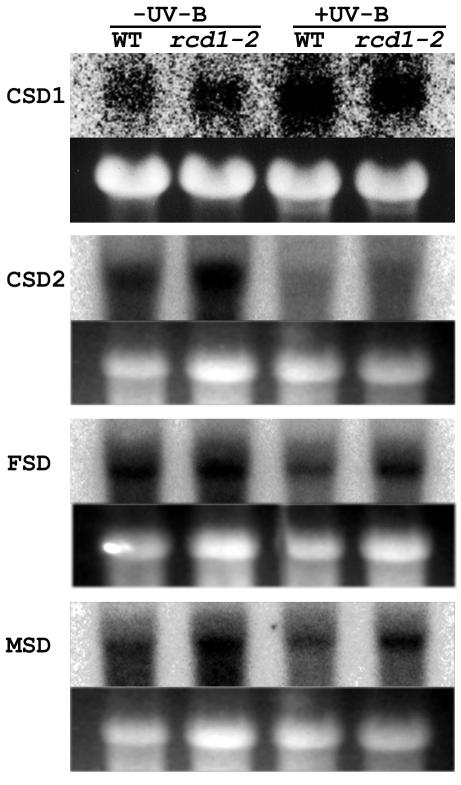
According to their metal cofactor and localization in the cell, SODs can be divided into cytosolic Cu/ZnSOD (CSD1), plastidic Cu/ZnSOD (CSD2), plastidic FeSOD (FSD) and mitochondorial MnSOD (MSD; Van Camp et al., 1990). Without UV-B exposure, no significant differences were observed in steady-state mRNA levels of CSD1 or FSD between wild type and rcd1-2 (Fig. 6). Gene expression of CSD1 was induced by UV-B exposure in both genotypes. In contrast, mRNA levels of CSD2 and MSD were higher in the mutant than in wild type. The MSD gene expression was not affected by UV-B exposure. Although the CSD2 gene expression was decreased by exposure to UV-B, higher mRNA levels were observed in the mutant than in wild type even after UV-B irradiation for 24 h. Gene expression of FSD was reduced by UV-B preferentially in wild type, resulting in higher mRNA levels in rcd1-2 after UV-B exposure. In summary, gene expression of plastidic and mitochondrial SODs was higher in rcd1-2 than in wild type before and/or after UV-B treatment.
Figure 6.
Effects of supplemental UV-B irradiation on mRNA levels of cytosolic Cu/ZnSOD (CSD1), plastidic Cu/ZnSOD (CSD2), FeSOD (FSD), and MSD genes in wild type and rcd1-2. RNA gel-blot analysis (top row) was carried out with 20 μg of total RNA prepared from aerial parts of the 3-week-old plants irradiated with (+UV-B) or without (-UV-B) supplemental UV-B for 24 h. cDNAs of the genes were used as a probe. The ethidium bromide stain of rRNA (bottom row) is shown for each lane to allow assessment of equal loading.
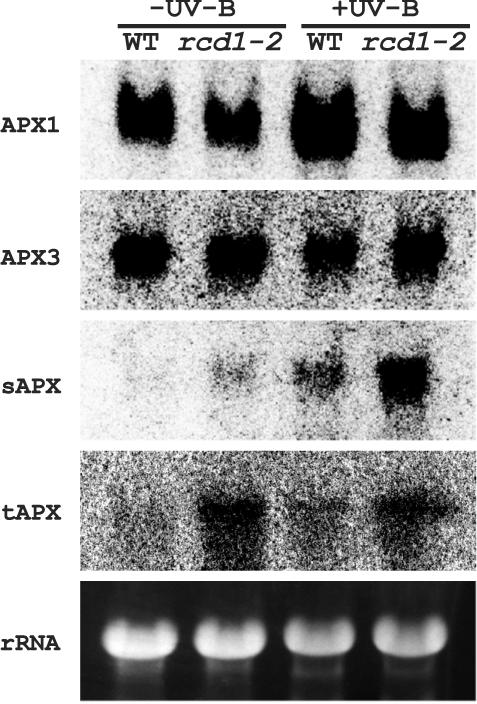
Steady-state mRNA levels of five ascorbate peroxidases (APXs; Karpinski et al., 1997; Jespersen et al., 1997) were determined next (Fig. 7). APX2 mRNA could not be detected by RNA gel-blot analysis in either wild type or rcd1-2 (data not shown). mRNA levels of cytosolic APX1 and APX3 were similar in wild type and in rcd1-2, in contrast to those of stromal APX (sAPX) and thylakoid APX (tAPX), which were higher in rcd1-2. mRNA levels of APX1 and sAPX were induced by UV-B irradiation in both genotypes; especially the latter transcripts increased dramatically. In contrast, no inductions were seen at APX3 and tAPX in either genotype after UV-B treatment. Taken together, mRNA levels of cytosolic APXs were similar in both genotypes irrespective of UV-B exposure, whereas those of plastidic APXs were higher in rcd1-2 than in wild type, especially after UV-B exposure.
Figure 7.
Effects of supplemental UV-B irradiation on mRNA levels of APX genes in wild type and rcd1-2. For more details, see the legend to Figure 6.
In Situ Detection of H2O2
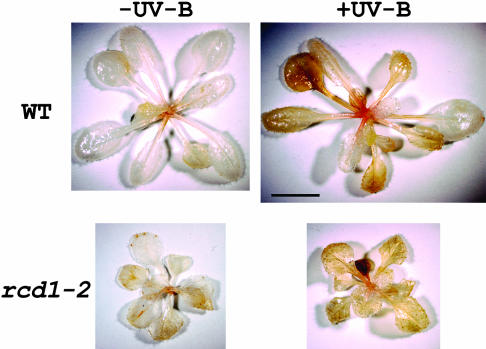
3,3′-Diaminobenzidine (DAB) polymerizes on contact with H2O2 in a reaction requiring peroxidase. Thus, H2O2 is visualized in situ as a reddish-brown precipitate (Torres et al., 2002). The contribution of rcd1-2 to AOS production was examined after supplemental UV-B irradiation for 24 h (Fig. 8). Brown precipitates were observed in UV-B-irradiated wild-type and rcd1-2 leaves, but almost no stain was detected in unirradiated plants. The number of brown precipitates was not so different between the UV-B-irradiated wild type and rcd1-2. However, they were stained in a different manner. All leaves were stained similarly along the vein in rcd1-2. In contrast, in wild type, more staining was observed in outer leaves than in inner leaves. These results suggest that oxidative burst occurs to a larger extent in older leaves than in younger ones in UV-B-irradiated wild-type plants. This may result from the AOS-scavenging system that might be highly induced in younger leaves. The age-dependent regulation of AOS-scavenging activities seems disrupted in rcd1-2.
Figure 8.
Effects of supplemental UV-B irradiation on H2O2 accumulation in wild type (top panel) and rcd1-2 (bottom panel). Plants were irradiated with white light (-UV) or white light with UV-B (+UV) for 24 h. H2O2 was detected by DAB-HCl staining. A withered cotyledon was stained dark brown in the UV-B-irradiated rcd1-2. Bar = 5 mm.
Effects of Supplemental UV-B Irradiation on Phenylpropanoid Synthesis
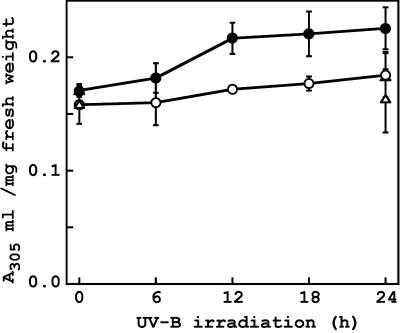
CPD accumulation was lower in rcd1-2 than in wild type (Fig. 4B), suggesting a higher accumulation of sunscreen such as colorless phenylpropanoid derivatives in the mutant. Thus, we measured contents of UV-B-absorbing compounds and the effects of supplemental UV-B irradiation on them (Fig. 9). Without the UV-B irradiation, level of UV-B-absorbing compounds was not significantly different between wild type and rcd1-2. (P = 0.324 in t test). Wild type showed no significant increase in contents of UV-B-absorbing compounds after 24 h of exposure to UV-B (P = 0.156). But rcd1-2 displayed 23% increase after the UV-B irradiation, resulting in significantly higher accumulation of the pigments in the mutant than in wild type (P = 0.028 in t test).
Figure 9.
Effects of supplemental UV-B irradiation on contents of UV-B-absorbing compounds. Contents of UV-B-absorbing compounds were measured in leaf explants prepared from the third or fourth leaf of wild type (white symbol) and rcd1-2 (black symbol) that had been irradiated with white light (triangle) or white light with UV-B (circle) for 24 h, as described in the legend to Figure 4A. Values are means ± se of three independent experiments, in which four to six replicates were taken.
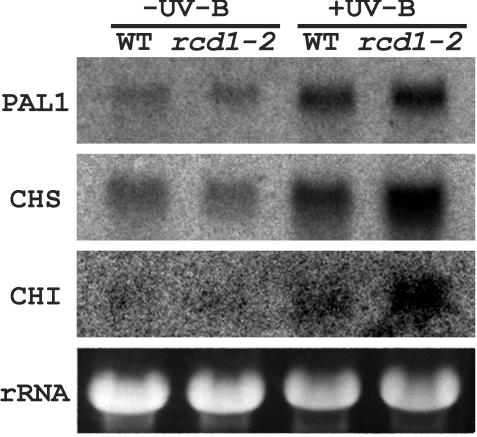
Phe ammonia-lyase (PAL), CHS, and CHI are key enzymes of phenylpropanoid pathway. Transcript levels of these enzyme genes were examined by RNA gel-blot analysis (Fig. 10). Without UV-B exposure, mRNA levels of PAL1 and CHI were similar in wild type and rcd1-2, whereas those of CHS were higher in wild type. Irradiation with supplemental UV-B increased the steady-state mRNA levels of all three genes in both genotypes. After UV-B exposure, mRNA levels of CHS and CHI were more increased in rcd1-2 than in wild type, whereas those of PAL1 were similar in both genotypes. These results indicate that the rcd1-2 responds to UV-B exposure more efficiently than wild type with respect to metabolism of phenylpropanoid derivatives.
Figure 10.
Effects of supplemental UV-B irradiation on mRNA levels of PAL1, CHS, and CHI genes in wild type and rcd1-2. cDNAs of the genes were used as a probe. For more details, see the legend to Figure 6.
DISCUSSION
We have isolated a methyl viologen-resistant, recessive mutant of Arabidopsis, which is smaller than wild type (Fig. 2) and more ozone sensitive. A complementation test showed that it is allelic to ozone-sensitive rcd1-1 (Fig. 1; Overmyer et al., 2000), thus it was named rcd1-2. rcd1-1 displays O3-induced lesions on rosette leaves more readily than wild type. In rcd1-1, ozone induces ethylene-dependent cellular accumulation of superoxide anion radicals, which is responsible for cell death, ultimately leading to lesion propagation (Overmyer et al., 2000, 2003).
Methyl Viologen Resistance
To study the molecular basis of methyl viologen resistance of rcd1-2, we have measured the steady-state mRNA levels of several isoforms of SOD and APX. The mRNA level of CSD2 localized in chloroplasts (Kliebenstein et al., 1998) was clearly increased in rcd1-2 (Fig. 6). mRNA levels of two plastidic APXs, sAPX and tAPX, were also increased in the mutant (Fig. 7). Because methyl viologen generates AOS in chloroplasts (Dodge, 1994), the higher gene expressions of the plastidic enzymes are likely to make rcd1-2 methyl viologen resistant.
To date, several mutants, ecotypes, and biotypes with methyl viologen resistance have been characterized in a few plant species. photoautotrophic salt tolerate 1 (pst1) has been identified as an Arabidopsis mutant that can grow photoautotrophically in the presence of higher concentrations of salt (Tsugane et al., 1999). pst1 seedlings are nearly 10 times more tolerant to methyl viologen than wild-type seedlings. They are also tolerant to high light intensities. There are no significant morphological differences between pst1 and wild type when they are grown without stress. Irrespective of salt stress, the activities of SOD and APX in pst1 are 1.3 and 3 times the activities of wild type, respectively. The increase of SOD and APX activities in pst1 plants may play an important role in the enhanced tolerance of the mutant to salt and high light intensities as well as to methyl viologen.
Arabidopsis ecotype Cape Verde Island (Cvi) also shows a higher resistance to methyl viologen treatment (Abarca et al., 2001). The plastidic Cu/ZnSOD activity detected in Cvi leaf extracts has been found to be encoded by two plastidic Cu/ZnSOD alleles, Csd2-1 and Csd2-2, the latter of which encodes a new isozyme, Csd2-2. Hybrid plants that contain Csd2-2 are also resistant to methyl viologen, suggesting that presence of the new allele probably explains the methyl viologen resistance of Cvi (Abarca et al., 2001). A methyl viologen-resistant biotype has also been reported in grass weed Hordeum glaucum (Lasat et al., 1997). Compartmentalization analysis indicates a greater herbicide accumulation in root vacuoles of the resistant biotype than in the susceptible biotype. In contrast, the amount of methyl viologen accumulated in the cytoplasm of the susceptible biotype was double that found in the resistant biotype. It is concluded that methyl viologen resistance in the grass weed is due to herbicide sequestration in the vacuole. A late-flowering mutant of Arabidopsis, gigantea, is resistant to methyl viologen (Kurepa et al., 1998), although the molecular basis of its methyl viologen resistance remains unknown (Huq et al., 2000).
Attempts have been made to enhance methyl viologen tolerance by generating transgenic tobacco (Nicotiana tabacum) plants that overexpress different forms of SODs and targeting them to various cellular compartments. Overexpression of MSD (Slooten et al., 1995) or Cu/ZnSOD (Sen Gupta et al., 1993) in chloroplasts enhances the oxidative stress tolerance when other antioxidant enzymes or substrates do not limit the oxygen radical-scavenging capacity.
Tolerance to Supplemental UV-B Irradiation
We have shown that rcd1-2 is more tolerant to supplemental UV-B irradiation for a relatively short period (24 h) than wild type in terms of CPD formation (Fig. 4B) and quantum yield of PSII (Fig. 4A). We did not examine whether rcd1-2 was tolerant to UV-B treatment for a longer period. The induced accumulation of UV-B-absorbing compounds as observed in rcd1-2 (Fig. 9), is particularly effective in filtering the more damaging UV-B region of the solar spectrum (Mazza et al., 2000). This protective effect is clearly revealed by a reduced accumulation of CPD in rcd1-2, which is only 50% of the accumulation in wild type (Fig. 4B). Because CPD is a product of a photochemical reaction, the level of CPD is likely to reflect a dose of UV light penetrated into the nucleus. A lower susceptibility of PSII function observed in rcd1-2 may also result from more effective shielding of UV-B by the pigments. RNA gel-blot analysis of the key enzymes of flavonoid synthesis, CHS and CHI, showed that UV-B exposure increases their mRNA levels more effectively in rcd1-2 than in wild type (Fig. 10), which may contribute to the higher accumulation of UV-B-absorbing compounds in the mutant.
In contrast to the evident contribution of sunscreen pigments to rcd1-2 tolerance to short-period UV-B treatment, the role of enhanced expression of AOS-scavenging enzyme genes in rcd1-2 chloroplasts is obscure. In rcd1-2, mRNA levels of CSD2 and FSD are higher than in wild type both before and after UV-B treatment (Fig. 6). Gene expression of sAPX is much induced by UV-B irradiation, resulting in higher accumulation of its mRNA in rcd1-2 (Fig. 7). Although these findings raise the possibility that the induced AOS-scavenging enzymes in chloroplasts effectively detoxify AOS that are generated by UV-B irradiation, we cannot evaluate the significance of the enzymes in the observed tolerance of rcd1-2 to UV-B at present.
Besides the tolerance to short-period UV-B exposure, rcd1-2 is also more tolerant to freezing damage than wild type (Fig. 5). Kendall and McKersie (1989) found a close relationship between the incidence of lethal freezing damage and increased superoxide accumulation. McKersie et al. (1993) could decrease the accumulation of free oxygen radicals after freezing stress and simultaneously increase freezing tolerance in transgenic alfalfa (Medicago sativa) by overexpressing MSD in chloroplasts. The enhanced freezing tolerance of rcd1-2, therefore, may result from the increased gene expression of the AOS-scavenging enzymes described above.
Ozone Sensitivity and Methyl Viologen Resistance
rcd1 is methyl viologen resistant (Figs. 1 and 3) but ozone sensitive (Overmyer et al., 2000). The effect of ozone on biological organisms is attributed to its ability to spontaneously dismutate or react with cellular constituents to generate excess AOS (Rao et al., 2000). It is suggested that ozone does not penetrate deep into intercellular spaces but rather decomposes at the cell wall and plasma membrane (Sandermann, 1996). This has also been shown to be the case for the ozone-induced necrosis in rcd1 by applying AOS or AOS-scavenging enzyme to the apoplast (Overmyer et al., 2000). Ozone fumigation activates membrane-localized oxidases such as NADPH-oxidase, which are capable of generating AOS, and thus introducing cell death (Rao and Davis, 1999). Because the primary reaction of methyl viologen resistance in rcd1 is likely to occur in chloroplasts, a direct relationship between methyl viologen resistance and ozone sensitivity seems unlikely. It is especially noteworthy, however, that Arabidopsis ecotype Cvi, which exhibits methyl viologen resistance, is also sensitive to ozone fumigation (Rao and Davis, 1999). The observations that Cvi maintains the glutathione redox state and that the induction of antioxidant defenses is much greater in magnitude compared with ecotype Columbia, which does not develop lesions in response to standard ozone treatment (0.3 μL L-1), suggest that the ozone sensitivity of Cvi is not due to its inability to perceive and respond to ozone. However, Cvi rapidly exhibits characteristics associated with programmed cell death, such as DNA fragmentation and chromatin condensation, before ozone-induced lesion formation, as is the case in rcd1 (Overmyer et al., 2000). The finding that rcd1 and ecotype Cvi have both methyl viologen resistance and ozone susceptibility in common may suggest the presence of a causal relationship between the two seemingly unrelated properties.
CONCLUSIONS
The present study shows that a methyl viologen-resistant mutant, rcd1-2, exhibits tolerance to supplemental UV-B irradiation for 24 h and to freezing. It is also ozone susceptible as has been reported by Overmyer et al. (2000). rcd1 displays pleiotropic defects in stress responses. It probably responds abnormally to many kinds of stress, although heat shock response is not affected. We examined the distribution of H2O2 in leaves after UV-B exposure. Although wild-type plants exhibited an age-dependent distribution of H2O2, a uniform distribution was seen in rcd1-2 (Fig. 8). rcd1 mutation may disrupt the tempo-spatial regulation of gene expression of AOS-scavenging enzymes. Taken together, rcd1 appears to be a mutant that shows a constitutive stress response. Consequently, it accumulates more pigments and AOS-scavenging enzymes without any stresses and displays necrosis even after the removal of ozone (Overmyer et al., 2000). The RCD1 gene might negatively regulate a wide range of stress-related downstream genes, which is also postulated for the PST1 gene (Tsugane et al., 1999). Possessing such a property, the RCD1 gene could be an important genetic resource to improve plant function against various stresses including UV-B radiation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis ecotype Columbia was used as wild type. Methyl viologen-resistant mutants were isolated from T2 seeds of an activation tagging library generated as described by Nakazawa et al. (2001). The rcd1-2 mutant was back-crossed twice before characterization.
Seeds were surface sterilized with 1.5% (v/v) sodium hypochlorite and 0.02% (v/v) Triton X-100 for 5 min with vigorous shaking and then washed three times with sterile water. Seeds chilled at 4°C for 2 to 3 d in 0.02%(w/v) agarose were sown on 1% (w/v) agar plates containing one-half-strength Murashige and Skoog medium (Murashige and Skoog, 1962) supplemented with 1% (w/v) Suc. They were grown at 23°C under continuous white fluorescent light. The photosynthetically active radiation of the white light was about 40 μmol m-2 s-1 at plant height. When plants were grown in soil or on rock wool, they were watered with 1,000-fold diluted Hyponex fertilizer (Hyponex Japan, Osaka).
UV-B Treatments
UV-B irradiation was carried out using two fluorescent sunlamps (FL 20SE, Toshiba, Tokyo) suspended 0.5 m above the plants. To absorb all UV light with a wavelength shorter than 290 nm, UV-transmitting filters (UV-28, Hoya Co. Ltd., Tokyo) were used (Fujibe et al., 2000). The spectral UV flux density was measured using a double monochromator spectroradiometer (PGD-25C, Japan Spectroscopic Co. Ltd., Tokyo). The weighted effective irradiances (biologically effective UV-B, UV-BBE) were calculated by referencing to the generalized plant spectrum of Caldwell (1971) and normalized at 300 nm. UV-BBE after passage through the UV-28 filters was 10.8 kJ m-1 d-1 at plant height.
Other Stress Treatments
O3 fumigation was performed as described by Nakajima et al. (2002). Three-week-old seedlings were exposed to 0.2 μL L-1 O3 in a growth chamber at 25°C and 70% relative humidity under light from metal halide lamps with a photosynthetic photon flux density of 300 μmol m-2 s-1. Ozone was generated by an O3 generator (Sumitomo Seika Chemicals Co., Tokyo). After treatment, plants were grown at 25°C.
Heat shock treatment was carried out as described by Hong and Vierling (2000). Seedlings were grown on 1% (w/v) agar medium placed in a vertical position at 23°C for 2.5 d in the dark. They were treated at 38°C for 90 min followed by 2 h at 23°C and then 2 h at 45°C. Immediately following treatment, the position of the roots was marked on the bottom of the dishes. After incubation at 23°C for additional 2 d in the dark, root elongation during the last 2 d was measured.
Freezing tolerance of the seedlings was evaluated by the electrolyte-leakage method as described by Zhou et al. (1994) with several modifications. Leaves were cut into halves along the mid-vein. Three pieces of leaf sections were put into each test tube, and 20 μL of water was added. The samples were frozen at -1°C with ice seeding for 1 h in a programmable freezer and cooled at a rate of 0.04°C min-1 to designated temperatures. One mL of water was added into each test tube after thawing at 4°C in air overnight and vacuum-infiltrated for 5 min, and then tubes were rocked at room temperature for 30 min in the dark. After electric conductivity of aqueous solution was measured, samples were heated in boiling water for 10 min and rocked at room temperature for 30 min, and electric conductivity was measured again. Relative electric conductivity was calculated as the proportion of the electric conductivity before heating to that after heating. Values of relative electric conductivity from leaf samples frozen in liquid nitrogen and those kept at 4°C were taken as having 0% and 100% cell survival, respectively.
Determination of Root Growth
To measure root growth, eight seedlings were fixed with 5% (v/v) formaldehyde and 10% (v/v) acetic acid. An image of the fixed seedlings, put on an agar plate, was recorded by a color-image scanner (GT-9000, Epson, Tokyo). Root length was determined using image analyzing software (NIH Image, National Institutes of Health, Bethesda, MD).
Determination of Chlorophyll Fluorescence
The third or fourth leaf with petiole was excised from 3-week-old plants and planted into moistened precut floral foams (OASIS Floral Foams Bricks LC-1, Smithers-Oasis, Kent, OH). It was then irradiated with white light with or without UV-B for 1 to 24 h. After dark adaptation for 5 min, in situ measurements of chlorophyll fluorescence were done on adaxial leaf surfaces using a pulse amplitude modulated fluorometer (PAM-2000, Heinz Walz, Effeltrich, Germany). The initial (F0), maximal (Fm) and variable (Fv = Fm - F0) fluorescence were determined. The maximal quantum yield of PSII photochemistry (Fv/Fm) was calculated according to Genty et al. (1989).
Pigment Analyses
Pigments were extracted from the UV-B-treated leaf explants prepared as described above. UV-B-absorbing compounds were extracted with 1% (v/v) HCl-methanol for 48 h at -20°C. Absorbance of the extracts was read at 305 nm for determinations of total UV-absorbing compounds (Mazza et al., 2000). Chlorophylls were extracted with methanol, and their content was estimated according to Porra et al. (1989). Both extractions were performed under dark conditions.
Determination of CPD
Immediately after UV-B irradiation, 0.2 g of aerial tissue was collected and frozen in liquid nitrogen. DNA was extracted according to the method of Doyle and Doyle (1990) using hexadecyltrimethylammoniumbromide. The CPD was quantified by ELISA according to Mori et al. (1991) with monoclonal antibodies (XP-01, Kyowa Medex Co., Tokyo). CPD levels were evaluated using 50 μg mL-1 λDNA (Takara, Kyoto) irradiated with 1 J m-2 UV-C (λmax = 260 nm) as a standard, which contains 1.5 CPDs per 1 kb of DNA (Mitchell, 1988).
In Situ Detection of H2O2
To visualize H2O2 in situ, DAB staining was performed as described by Torres et al. (2002). After UV-B irradiation for 24 h, aerial parts of Arabidopsis were vacuum-infiltrated with 1 mg mL-1 DAB-HCl, pH 3.8. The aerial parts were placed in a plastic box under high humidity for 6 h, when a brown precipitate was observed. They were then fixed with a solution of 3:1:1 ethanol:lactic acid:glycerol.
RNA Isolation and RNA Gel-Blot Analysis
RNA isolation and hybridization were carried out essentially as described by Yamamoto et al. (1992). The probe was prepared by using a multiprime labeling system (Amersham Biosciences, Tokyo) and expressed sequence tag (EST) clones obtained from the Arabidopsis Biological Resource Center. ESTs used were 247O9T7 for CSD1, 178G17T7 for CSD2 (Kliebenstein et al., 1998), 91O7T7 for APX1, 2109F7T7 for APX3 (Karpinski et al., 1997), 63G9T7 for sAPX, 251M21T7 for tAPX (Jespersen et al., 1997), 82B7T7 for PAL1 (Ohl et al., 1990), 177N23T7 for CHS, and 177A20T7 for CHI. cDNAs of FSD (At4g25100; Van Camp et al., 1990), MSD (At3g10920; Kliebenstein et al., 1998), and APX2 were amplified by reverse transcriptase-PCR using primers designated as FSD-forward (5′-ATTCGCACTGGATGCTTTGG-3′), FSD-reverse (5′-TCGGTTCTGGAAGTCAAGGT-3′), MSD-forward (5′-CGGAGGTCATGTCAACCATTC-3′), and MSD-reverse (5′-CATTGTAAAACAACGTACCACACA-3′). APX2 primers used were described by Santos et al. (1996). Inserts of the EST clones were prepared by digesting with NotI and SalI and then purifying with QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) after agarose gel electrophoresis. RNA gel-blot analysis was carried out at least three times, and similar results were obtained in each experiment.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We thank Profs. Ayumi Tanaka and Takashi Koyama (Hokkaido University) for the use of a pulse amplitude modulated fluorometer and Prof. Jaakko Kangasjärvi (University of Helsinki, Finland) for his generous gift of rcd1 seeds and his invaluable discussions. The Arabidopsis Biological Resource Center, Ohio University, is also acknowledged for EST clones of Arabidopsis. This study was partly carried out in Laboratory of Genetic Research, Center for Advanced Science and Technology, Hokkaido University.
This work was supported in part by Grants-in-Aid and the Special Coordination Fund from the Ministry of Education, Culture, Sports, Science and Technology (to K.T.Y. and H.S., respectively) and by the Research for the Future Program of the Japan Society for the Promotion of Science (to K.A., Y.T., and K.T.Y.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.033480.
References
- Abarca D, Roldán M, Martín M, Sabater B (2001) Arabidopsis thaliana ecotype Cvi shows an increased tolerance to photo-oxidative stress and contains a new chloroplastic copper/zinc superoxide dismutase isoenzyme. J Exp Bot 360: 1417-1425 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601-639 [DOI] [PubMed] [Google Scholar]
- Bieza K, Lois R (2001) An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiol 126: 1105-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij-James IS, Dube SK, Jansen MAK, Edelman M, Mattoo AK (2000) Ultraviolet-B radiation impacts light-mediated turnover of the photosystem II reaction center heterodimer in Arabidopsis mutants altered in phenolic metabolism. Plant Physiol 124: 1275-1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt AB, Chen JJ, Wykoff D, Mitchell D (1993) A UV-sensitive mutant of Arabidopsis defective in the repair of pyrimidine-pyrimidinone (6-4) dimers. Science 261: 1571-1574 [DOI] [PubMed] [Google Scholar]
- Caldwell MM (1971) Solar UV irradiation and the growth and development of higher plants. In AC Giese, ed, Photophyshiology, Vol 6. Academic Press, New York, pp 131-177 [Google Scholar]
- Dodge A (1994) Herbicide action and effects on detoxification processes. In CH Foyer, PM Mullineaux, eds, Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, FL, pp 219-236
- Doyle JF, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13-15 [Google Scholar]
- Fujibe T, Watanabe K, Nakajima N, Ohashi Y, Mitsuhara I, Yamamoto KT, Takeuchi Y (2000) Accumulation of pathogenesis-related protein in tobacco leaves irradiated with UV-B. J Plant Res 113: 387-394 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87-92 [Google Scholar]
- Hong S-W, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392-4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Tepperman JM, Quail PH (2000) GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc Natl Acad Sci USA 97: 9789-9794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen HM, Kjfrsgerd IV, Oestergaard L, Welinder KG (1997) From sequence analysis of three novel ascorbate peroxidases from Arabidopsis thaliana to structure, function and evolution of seven types of ascorbate peroxidase. Biochem J 326: 305-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CZ, Yee J, Mitchell DL, Britt AB (1997) Photorepair mutants of Arabidopsis. Proc Natl Acad Sci USA 94: 7441-7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C (2000) Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 19: 6150-6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinski B, Creissen G, Mullineaux P (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall EJ, McKersie BD (1989) Free radical and freezing injury to cell membranes of winter wheat. Physiol Plant 76: 86-94 [Google Scholar]
- Kliebenstein DJ, Lim JE, Landry LG, Last RL (2002) Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human Regulator of Chromatin Condensation 1. Plant Physiol 130: 234-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Monde R-A, Last RL (1998) Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol 118: 637-650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403-410 [DOI] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Van Montagu M, Inzé D (1998) Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J 14: 759-764 [DOI] [PubMed] [Google Scholar]
- Landry LG, Stapleton AE, Lim J, Hoffman P, Hays JB, Walbot V, Last RL (1997) An Arabidopsis photolyase mutant is hypersensitive to ultraviolet-B radiation. Proc Natl Acad Sci USA 94: 328-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasat MM, DiTomaso JM, Hart JJ, Kochian LV (1997) Evidence for vacuolar sequestration of paraquat in roots of a paraquat-resistant Hordeum glaucum biotype. Physiol Plant 99: 255-263 [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 39: 769-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hossain GS, Islas-Osuna MA, Mitchell DL, Mount DW (2000) Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1. Plant J 21: 519-528 [DOI] [PubMed] [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL (2000) Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol 122: 117-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inzé D, D'Halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol 103: 1155-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL (1988) The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol 48: 51-57 [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Nairn RS (1989) The biology of the (6-4) photoproduct. Photochem Photobiol 49: 805-819 [DOI] [PubMed] [Google Scholar]
- Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O (1991) Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimmer or (6-4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol 54: 225-232 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assay with tobacco tissue cultures. Physiol Plant 15: 473-497 [Google Scholar]
- Nakajima N, Itoh I, Takikawa S, Asai N, Tamaoki M, Aono M, Kubo A, Azumi Y, Kamada H, Saji H (2002) Improvement in ozone tolerance of tobacco plants with an antisense DNA for 1-aminocyclopropane-1-carboxylate synthase. Plant Cell Environ 25: 727-735 [Google Scholar]
- Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M (2001) DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J 25: 213-221 [DOI] [PubMed] [Google Scholar]
- Ohl S, Hedrick SA, Chory J, Lamb CL (1990) Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell 2: 837-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Kangasjärvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335-342 [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H Jr, Kangasjärvi J (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384-394 [Google Scholar]
- Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17: 603-614 [DOI] [PubMed] [Google Scholar]
- Rao MV, Koch JR, Davis KR (2000) Ozone: a tool for probing programmed cell death in plant. Plant Mol Biol 44: 345-358 [DOI] [PubMed] [Google Scholar]
- Renger G, Voss M, Gräber P, Schulz A (1986) Effect of UV irradiation on different partial reactions of the primary processes of photosynthesis. In RC Worrest, MM Caldwell, eds, Stratospheric Ozone Reduction, Solar Ultraviolet Radiation and Plant Life. Springer-Verlag, Berlin, pp 171-184
- Sandermann H (1996) Ozone and plant health. Annu Rev Phytopathol 34: 347-366 [DOI] [PubMed] [Google Scholar]
- Santos M, Gousseau H, Lister C, Foyer C, Creissen G, Mullineaux P (1996) Cytosolic ascorbate peroxidase from Arabidopsis thaliana L. is encoded by a small multigene family. Planta 198: 64-69 [DOI] [PubMed] [Google Scholar]
- Sen Gupta A, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90: 1629-1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooten L, Capiau C, Van Camp W, Van Montagu M, Sybesma C, Inzé D (1995) Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts. Plant Physiol 107: 737-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Sakamoto A, Ishigaki Y, Nikaido O, Sun G, Hase Y, Shikazono N, Tano S, Watanabe H (2002) An ultraviolet-B-resistant mutant with enhanced DNA repair in Arabidopsis. Plant Physiol 129: 64-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11: 1195-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W, Bowler C, Villarroel R, Tsang EWT, Van Montagu M, Inzé D (1990) Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci USA 87: 9903-9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KT, Mori H, Imaseki H (1992) Novel mRNA sequences induced by indole-3-acetic acid in sections of elongating hypocotyls of mung bean (Vigna radiata). Plant Cell Physiol 33: 13-20 [Google Scholar]
- Zhou BL, Arakawa K, Fujikawa S, Yoshida S (1994) Cold-induced alterations in plasma membrane proteins that are specifically related to the development of freezing tolerance in cold-hardy winter wheat. Plant Cell Physiol 35: 175-182 [Google Scholar]