Abstract
Methionine is a highly susceptible amino acid that can be oxidized to S and R diastereomeric forms of methionine sulfoxide by many of the reactive oxygen species generated in biological systems. Methionine sulfoxide reductases (Msrs) are thioredoxin-linked enzymes involved in the enzymatic conversion of methionine sulfoxide to methionine. Although MsrA and MsrB have the same function of methionine reduction, they differ in substrate specificity, active site composition, subcellular localization, and evolution. MsrA has been localized in different ocular regions and is abundantly expressed in the retina and in retinal pigment epithelial (RPE) cells. MsrA protects cells from oxidative stress. Overexpression of MsrA increases resistance to cell death, while silencing or knocking down MsrA decreases cell survival; events that are mediated by mitochondria. MsrA participates in protein-protein interaction with several other cellular proteins. The interaction of MsrA with α-crystallins is of utmost importance given the known functions of the latter in protein folding, neuroprotection, and cell survival. Oxidation of methionine residues in α-crystallins results in loss of chaperone function and possibly its antiapoptotic properties. Recent work from our laboratory has shown that MsrA is co-localized with αA and αB crystallins in the retinal samples of patients with age-related macular degeneration. We have also found that chemically induced hypoxia regulates the expression of MsrA and MsrB2 in human RPE cells. Thus, MsrA is a critical enzyme that participates in cell and tissue protection, and its interaction with other proteins/growth factors may provide a target for therapeutic strategies to prevent degenerative diseases.
Keywords: Methionine sulfoxide reductases, Hypoxia, Protein interaction, α crystallins, Neuroprotection
INTRODUCTION
Molecular oxygen is indispensible for survival of aerobic organisms, which use oxygen for energy pathways in mitochondria and numerous other processes, but the use of oxygen is also associated with the generation of reactive oxygen species (ROS)[1]. According to free radical theory, ROS promote oxidative damage to many cellular constituents, including amino acids, lipids, and nucleic acids[2]. Oxidative damage to proteins and other biomolecules by ROS has been implicated in a variety of diseases and in aging and senescence-associated disorders[3]. The modifications caused by oxidation may or may not be reversible. Oxidized proteins may become non-functional as a result of structural changes and catalytic malfunction. The sulfur-containing amino acids methionine and cysteine are the major targets of ROS in proteins and are also the amino acids most susceptible to oxidation[4]. As a universal initiating amino acid for protein synthesis, methionine has additional importance for cellular functions. Both free methionine and protein-based methionine are readily oxidized by ROS to form methionine sulfoxide, which could alter protein structure and function[5]. To counteract ROS damage, organisms have evolved multiple defense systems, including low molecular weight compounds and antioxidant enzymes that protect against oxidative stress. The antioxidant system includes glutathione peroxidase[6,7], superoxide dismutase[8,9], catalase[10,11], thioredoxin reductase (TR)[12], methionine sulfoxide reductase (Msr)[13-17] and several other proteins, including but not limited to small heat shock proteins, particularly α-crystallins[18-21]. Msrs are prominent among these antioxidant enzymes because of their roles as repair enzymes and indirect scavengers of ROS[4,22]. Although different ROS scavenging systems/enzymes are present in the cells, in the case of methionine oxidation, a more energy-efficient mechanism involves the action of the Msr enzymes, MsrA and MsrB[23,24]. MsrA and MsrB can catalyze the reversion of the methionine S-sulfoxide and the methionine R-sulfoxide, respectively, to the reduced form of methionine within proteins[25]. Methionine can be easily oxidized into methionine sulfoxide, and its reduction by Msr could represent an efficient antioxidant system because, in proteins, the surface-exposed methionine residues can act as scavengers of a variety of oxidants[26]. Methionine oxidation denatures proteins and converts the hydrophobic properties of Met into hydrophilic properties, resulting in structural alterations[27]. Although MsrA and MsrB enzymes have the same function of methionine sulfoxide reduction, they differ, not only in substrate specificity, but also in active site composition, protein folding, subcellular localization, and evolution[28,29]. Msrs have wide tissue distribution and are present in multiple sites in the eye[14-17]. Msrs interact with other proteins and protect cells from oxidative-stress-induced cell injury[14-17]. In this review, we address specifically the role of MsrA in protecting the eye against various types of oxidative injury. Furthermore, the regulation of MsrA and MsrB is illustrated in chemically induced hypoxia in retinal pigment epithelial (RPE) cells in vitro.
Msrs: GENE STRUCTURE AND ISOFORMS
Msrs are expressed in most organisms and catalyze the thioredoxin-dependent reduction of free and protein-bound methionine sulfoxide to methionine[29-31]. Msrs, as well as glutathione and TRs, are ubiquitously expressed in cells[29,32] and, acting together with their substrates and co-factors, form repair systems that protect cells from oxidative stress and maintain cellular redox homeostasis[29,33,34]. According to current literature, there are three types of Msr: MsrA, MsrB and frMsr[10]. However, frMsr distribution is limited to unicellular organisms; multicellular organisms, including mammals, lack this protein[35]. The human MsrA gene is located on chromosome 8 and is coded by one gene regulated by two distinct promoters resulting in different isoforms: the long form and short form[36]. The long form is localized to the cytosol, mitochondria, and nucleus, while the short form is localized to the nucleus and cytosol[28,37,38]. The long form of MsrA encodes a peptide containing an N-terminal mitochondrial targeting sequence, a catalytic cysteine containing sequence, and a C-terminal thioredoxin-binding domain. The short form of MsrA lacks the mitochondrial sequence, but the cysteine-containing sequence and thioredoxin domain are active. Expression of MsrA is found in almost all human tissues with the exception of leukemia and lymphoma cell lines[39]. MsrA is specific for the reduction of free and protein-based methionine-S-sulfoxide in mammals[10,40]. MsrA can also reduce compounds such as N-acetylmethionine-S-sulfoxide, dimethyl sulfoxide, and ethionine-S-sulfoxide[22]. However, the functional differences between the long and short forms of MsrA have not yet been characterized.
Three mammalian MsrBs exist[10]. MsrB1 is present in the cytosol and nucleus and exhibits the highest catalytic activity because of the presence of selenocysteine in its active site. MsrB1 occurs in two forms, 14 kDa and 5 kDa, in mouse tissues and human HEK 293 cells, and both forms are selenoproteins[10,41,42]. MsrB is encoded by three different genes and their products are: MsrB1, a selenoprotein found in the nucleus and cytosol; MsrB2, which is present in the mitochondria; and MsrB3, which encodes for two splice variants, MsrB3A and MsrB3B, localized to the endoplasmic reticulum (ER) and the mitochondria, respectively[37,43]. The MsrB enzymes are present in all eukaryotic tissues, but their level of expression varies[37]. MsrB is specific for the reduction of protein-based methionine-R-sulfoxide, and reduces free methionine-R-sulfoxide with very low efficiency[10]. Among the three MsrBs, MsrB1 has the highest catalytic activity because of the selenocysteine in its active site. MsrB2, also known as CBS1, is targeted to mitochondria with the guidance of its N-terminal signal peptide and has cysteine as the catalytic residue. It shows high activity with methionine-R-sulfoxide but is inhibited by elevated concentrations of the substrate[43-45]. The two forms of MsrB3, MsrB3A and MsrB3B, are generated by alternative first exon splicing in humans. MsrB3A is targeted to the ER with the N-terminal ER signal peptide and an ER retention signal at the C terminus, whereas MsrB3B is targeted to mitochondria by its N-terminal mitochondrial signal peptide. Interestingly, mouse MsrB3 also has the ER and mitochondrial signal peptides located consecutively at the N terminus, but it is targeted only to the ER because the mitochondrial signal is masked by the upstream ER signal[10].
CATALYTIC MECHANISM OF Msrs
Although structurally distinct, MsrA and MsrB share a common three-step catalytic mechanism[46,47]. Enzymatic studies have shown that the MsrA domain possesses two essential cysteine residues to carry out its function[48]. The catalytic mechanism of MsrA and MsrB is based on three steps. In the first step, the Msr catalytic cysteine residue interacts with the methionione sulfoxide substrate, which leads to the product release and formation of the sulfenic acid. In the second step, an intramolecular disulfide bridge is formed between the catalytic cysteine and the regenerating cysteine. In the final step, the disulfide bridge is reduced by an electron donor, the NADPH-dependent thioredoxin/TR system, leading to the regeneration of the Msr active site. The catalytic mechanism varies between different Msrs, especially in the number of recycling cysteines[49,50]. However, the selenoprotein MsrB1 is believed to use an alternative recycling cysteine residue located in a different position[28].
COMPARTMENTAL DISTRIBUTION OF MsrA IN THE EYE
The bulk of the work in the eye on MsrA has dealt with its function in the lens[51]. MsrA has been found to be highly expressed in the lens epithelium and fiber cells[14]. Its expression in the photoreceptor inner segments and in the inner nuclear layer of the retina and in the RPE has also been characterized[16,30,36,52]. In the monkey retina, MsrA gene expression is detected mostly in the macular RPE-choroid region, whereas its activity is detected mainly in the soluble fractions of neural retina and RPE-choroid[30]. MsrA protein is distributed throughout the retina but is abundant at the photoreceptor synapses and in the ganglion and Müller cells. MsrA expression is higher in macular RPE cells than in the peripheral RPE cells of the retina[30]. Our work has shown that MsrA is localized to sub-RPE macular drusen from patients with age-related macular degeneration (AMD)[16]. Our subcellular localization studies have revealed that MsrA is expressed in the cytosol and in mitochondria of human RPE cells[16].
MsrA IN OXIDATIVE STRESS
Cells and tissues possess a number of antioxidant systems to prevent oxidative damage that causes protein aggregation and cell death. The enzyme MsrA plays an important role in the antioxidant response by reducing the S-stereoisomer of methionine sulfoxide (MetSO) to methionine[4]. Accordingly, cells with increased or decreased levels of MsrA are highly resistant or vulnerable to oxidative stress, respectively[14,16,17]. MsrA not only protects cells from oxidative stress by repairing proteins damaged by methionine oxidation, but it also functions by engaging in a sequence of methionine oxidation and reduction cycles that eventually results in ROS scavenging[17]. The MsrA activity is determined by the two active-site cysteine residues: 72 and 218[48]. Cysteine 72 carries out a nucleophilic attack at the sulfur atom of the methionine sulfoxide substrate, leading to the formation of a covalent intermediate, whereas cysteine 218 attacks cysteine 72 to trigger breakdown of the covalent complex[48]. Evidence that MsrA has a role in protecting cells against oxidative damage was first shown in Escherichia coli where MsrA mutants are more sensitive to H2O2[53]. Overexpression of the MsrA gene predominantly in the nervous system markedly extends the lifespan of the fruit fly Drosophila by 70%[54]. In addition, MsrA transgenic flies are more resistant to paraquat-induced oxidative stress, and the onset of senescence-induced decline in the general activity level and reproductive capacity is delayed markedly[54]. MsrA null mutants of yeast[55] and mice[56] are more sensitive to oxidative stress than wild-type organisms, and their lifespans are shortened by about 26% in yeast[57] and 40% in mice[56]. Compared with the wild type, MsrA mutant mice exhibit enhanced sensitivity under hyperoxia and have a shorter lifespan under both normal and hyperoxic conditions. Mutants also accumulate higher tissue levels of oxidized protein under oxidative stress, and ae unable to upregulate expression of TR under oxidative stress[56]. Adenovirus-mediated overexpression of MsrA significantly diminishes the hypoxia-induced increase in ROS and facilitates cell survival in neuronal cells by preserving mitochondrial membrane potential and apoptotic events[15]. MsrA is protective against hypoxia/reoxygenation stress in cardiomyocytes, suggesting that it may be an important therapeutic target for ischemic heart disease[58]. The resistance in MsrA-overexpressing human fibroblasts is accompanied by a decrease in intracellular ROS and is partially abolished when cells are cultured with suboptimal concentrations of methionine[17]. These results indicate that MsrA could play an important role in cellular defense against oxidative stress by catalytic removal of oxidant through the reduction of methionine sulfoxide and in protection against death by limiting, at least in part, the accumulation of oxidative damage to proteins.
Our laboratory examined the protective role of MsrA in human fetal RPE cells[16]. Oxidative stress from H2O2 exposure results in the generation of ROS and activation of caspase-3 in RPE cells. In addition, an increase in MsrA expression in cytosol and mitochondria was also observed. Silencing of MsrA resulted in further induction of caspase-3 and accentuated cell death from oxidative stress[16]. Similar results have been reported in ARPE-19 cells in which MsrA gene-silenced cells were susceptible to oxidative stress[30]. Kantorow et al[14] have shown that overexpression of MsrA protects lens cells against H2O2-induced oxidative stress, whereas decreased expression of MsrA results in increased sensitivity to oxidative stress and decreased lens cell viability. This is attributed to the increased lens ROS levels and loss of mitochondrial function. Furthermore, severe cytochrome c oxidation and lens cataract have been reported in hyperbaric oxygen-treated MsrA deficient mice by the same laboratory[59]. It is of interest that the isoforms of MsrB have also been shown to prevent oxidative damage to lens cells and RPE cells[60,61].
Thus, the protective effect of MsrA seems to result, at least in part, from an antioxidant mechanism, by preserving mitochondrial functions and inhibiting subsequent activation of caspases as seen during its deficiency[16]. Indeed, other studies have pointed out the protective role of MsrA against the deleterious effects of ROS in Drosophila and mammalian cells, emphasizing the important role of this enzyme in both maintenance of proteins under oxidative stress and overall redox cellular homeostasis[15,54]. Moreover, oxidized methionine may also be a critical component in redox signaling. For example, sulfiredoxin and sestrins that repair cysteine-sulfinic acid in peroxiredoxins are likely important, not only for their antioxidant function, but also in signaling pathways sensitive to peroxiredoxin hyperoxidation[62-64]. Similarly, MsrA could modulate signal transduction through the regulation of methionine oxidation/reduction within specific proteins, and MsrA modulation would expect to affect such redox-sensitive signaling pathways.
INTERACTION OF MsrA WITH OTHER PROTEINS
MsrA is involved in the repair of methionine residues in several proteins. α-crystallin and cytochrome C have been identified as major targets of MsrA in the lens[59,65]. This finding is of significance because of emerging evidence about the role of α-crystallins in ocular health. A direct link has been identified between α-crystallin methionine oxidation and age-related cataract formation[65]. α-crystallins are major proteins of the small heat shock protein family and are expressed in several tissues[66-68]. α-crystallins have been studied extensively in the lens for their chaperone function, but α-crystallins are now generally understood to have additional non-lens roles[18-21,69,70]. In addition to being a molecular chaperone, α-crystallin functions in cell death inhibition, neuroprotection, proteosomal interactions, and regulation of angiogenesis[18-21,70,71]. αB crystallin is more abundant in the RPE cells where it provides neuroprotection. α-crystallins are predominantly cytosolic proteins; however, mitochondrial and nuclear localization has also been reported[19,72,73].
Methionines of αA- and αB-crystallins are oxidized in human lenses between the ages of 45 and 65 years[74,75]. The methionine in αA-crystallin is oxidized to methionine sulfoxide in rat hereditary cataracts[76], and interestingly, substitution of methionine 68 in αB-crystallin with a less hydrophobic residue (Thr) results in loss of chaperone activity[77]. Development of cataract could be the result of protein aggregation caused by loss of α-crystallin chaperone function. Methionine oxidation damages α-crystallin chaperone activity, but MsrA can also repair oxidized methionines in α-crystallin and restore the chaperone function (Figure 1)[65]. Therefore, loss of MsrA activity upon aging or oxidative stress could result in loss of α-crystallin chaperone function and contribute to the development of cataract and other age-related oxidative stress-associated disorders, such as AMD[78], Parkinson’s disease[79], Alzheimer’s disease[80] and desmin-related myopathy[81]. α-crystallins also possess antiapoptotic properties[18-20]. Whether methionine oxidation could also affect the antiapoptotic function remains to be established.
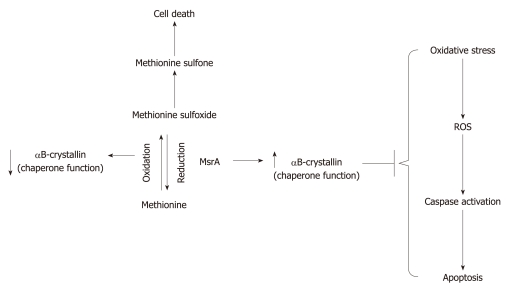
Figure 1.
Scheme depicting the role of methionine sulfoxide reductase A and α-crystallin in methionine metabolism. Note the reversible nature of oxidation-reduction of methionine and its influence on the chaperone properties of α-crystallin. The inhibition of the apoptotic events from oxidative stress by αB crystallin is also presented in the figure. ROS: Reactive oxygen species.
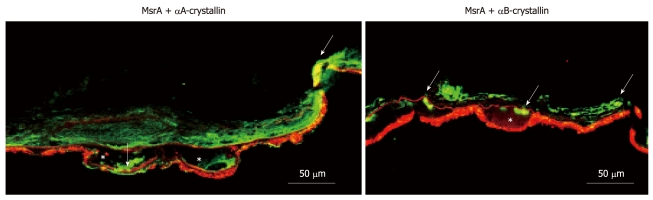
We and others have shown that α-crystallins are localized in different retinal layers and in drusen of AMD samples[20,78,82]. We have further found that MsrA is localized as a component of drusen samples[16]. Our immunofluorescence data, shown in Figure 2, indicate that both αA and αB crystallins co-localize with MsrA in the AMD retinas, especially in the drusen, suggesting potential interaction between the two types of proteins. This observation is consistent with the phenomenon described above that methionine residues of crystallins undergo oxidation and lose chaperone function with aging and oxidative stress. MsrA is involved in the repair process, so that the interaction or association of both these proteins in the AMD retina is a critical process for further investigation at the molecular level.
Figure 2.
Immunohistochemical localization of αA-crystallin (red), αB-crystallin (red) and methionine sulfoxide reductase A (green) in the retina of patients with AMD. Retinal cryosections were air-dried, fixed, and processed as described[18] using αA-crystallin and αB-crystallin rabbit polyclonal antibodies (Stressgen, Ann Arbor, MI, USA), and mouse monoclonal methionine sulfoxide reductase (Msr) A antibody (Novus Biologicals, Littleton, CO, USA). Sections were viewed under a confocal microscope (Carl Zeiss, Thornwood, NY, USA). Arrows indicate co-localization (yellow) of αA-crystallin or αB-crystallin and MsrA. AMD:Age-related macular degeneration; *: Drusen; Scale bar = 50 μm.
In summary, repair of oxidized methionines in α-crystallin is necessary for the maintenance of chaperone function. MsrA is required in eyes and other tissues for maintenance and repair of α-crystallin chaperone function. The chaperone activity is essential for a number of significant functions ranging from protein folding to cytoskeletal remodeling, apoptotic control, neuroprotection, and angiogenesis regulation[18,21,70]. Loss of α-crystallin chaperone function probably results in protein aggregation, which may account for the cataract formation found in the absence of MsrA in mice[65]. Given the role of MsrA and α-crystallin in the health and disease of retinal and other tissues, these results are probably applicable to our understanding of the oxidative-stress-associated and aging disease mechanisms, and these results may provide a basis for the development of well-designed interventions for these conditions.
Msr REGULATION UNDER HYPOXIC CONDITIONS IN RPE CELLS
Msrs are able to protect cells against oxidative damage, thus as discussed above, and because of the importance of oxidative damage in retinal tissues after hypoxia/reoxygenation[83], we wished to study the regulation of Msrs (MsrA and MsrB2) in RPE cells in hypoxia. The retina is known to be the most metabolically active tissue in the body, and it is highly sensitive to reduction in oxygen tension[84]. Therefore, the role of the oxygen microenvironment in the retina may be of importance in understanding AMD and other retinal degenerative diseases. RPE cells were subjected to chemically induced hypoxia (100 μmol/L CoCl2, 4 h) in serum-free medium[20]. CoCl2 stimulates the hypoxia responsive pathways and has been shown to induce apoptosis by mitochondrial pathways and hypoxia-inducible factor (HIF)-1α-dependent and -independent mechanisms[85,86]. CoCl2-induced hypoxia in RPE induced upregulation of HIF-1α and vascular endothelial growth factor (VEGF) (Figure 3A-C). Gene expression analysis of MsrA and MsrB2 showed initial upregulation for up to 1 h with hypoxia and a decline thereafter (Figure 3D and E), without affecting cell viability. Our findings in RPE cells are in accordance with several previous studies. For example, Msrs are reported to behave in the same manner in mouse embryonic cells under anoxia and acidosis[87]. MsrA is protective against hypoxia/reoxygenation stress in multiple cell types. Adenovirus-mediated overexpression of MsrA in primary neonatal rat cardiomyocytes subjected to hypoxia/reoxygenation reduced apoptotic cell death by > 45%[58]. Further support comes from studies using PC12 cells, in which overexpression of MsrA lowered the level of ROS[15], and in lens cells, in which depletion of MsrA using siRNA resulted in increased levels of ROS[14,16]. The lower ROS level conferred by MsrA overexpression in PC12 cells was associated with greater cell viability[15] after hypoxia/reoxygenation. As discussed above, MsrA interacts with α-crystallins, and we have shown that under prolonged hypoxic conditions, α-crystallins in RPE also showed a significant decrease, at both the transcriptional and translational levels[20]. It should be noted that retinas of αA or αB crystallin knockout mice are highly susceptible to hypoxia-induced cell death and that this could partly be due in part to decreased Msr expression. The finding that overexpression of MsrA reduces hypoxia-mediated apoptosis suggests that the endogenous antioxidant mechanism is not sufficient to counteract the oxidative stress caused by hypoxia. Thus, MsrA and MsrB, which reduce the S and R forms of methionine sulfoxide in proteins, respectively, may be viable therapeutic targets.
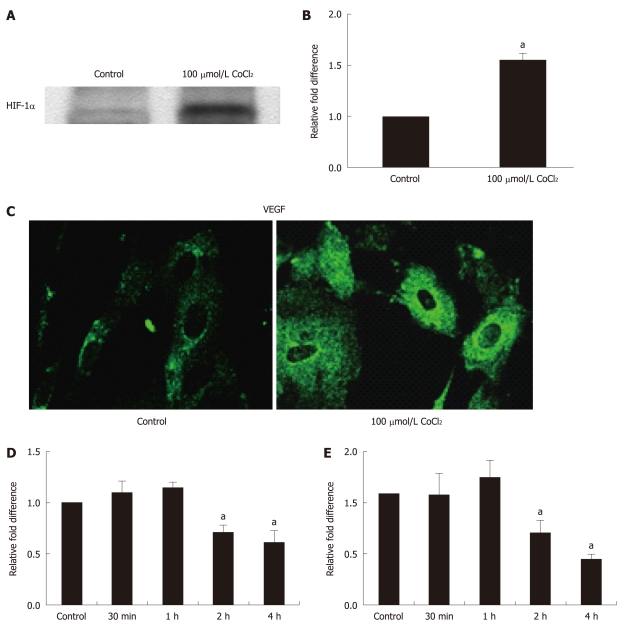
Figure 3.
Regulation of methionine sulfoxide reductase A and B2 in retinal pigment epithelial subjected to chemically induced hypoxia. Hypoxia-inducible factor (HIF)-1α (A) and vascular endothelial growth factor (VEGF) (B and C) were used to validate hypoxia from CoCl2. Confluent human fetal retinal pigment epithelial (RPE) cells were serum starved overnight and treated with 100 μmol/L CoCl2 for 4 h. Western blot analysis shows HIF-1α (mouse monoclonal, Novus Biologicals) upregulation in nuclear extracts of CoCl2-treated RPE cells (A). VEGF expression was significantly upregulated both at the mRNA (B) and protein levels (C). Real-time polymerase chain reaction was performed as described[19] in a light cycler (Roche, IN, USA) using β-actin as normalizing gene. A polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for the detection of VEGF. Methionine sulfoxide reductase (Msr) A and MsrB showed initial upregulation but, at 4 h, showed significant downregulation (D and E). Data presented are mean ± SE. aP < 0.05 vs control.
FUTURE PERSPECTIVES
A great deal of attention to MsrA has been directed towards its functional properties in the lens. Detailed studies on the action and importance of MsrA in the retina are scarce. As an important reductant, MsrA is likely to have a beneficial role in retinal diseases such as AMD, diabetic retinopathy and oxygen-induced retinopathy, which are associated with oxidative stress. The emerging role of other proteins such as the chaperone αB crystallin in retinal as well as choroidal angiogenesis[21] suggests that interaction of MsrA with such proteins may prove to be a critical area of investigation. α-crystallins are crucial molecules with multiple functions, and whether MsrA has a direct role in protecting their chaperone function will be worthy of study in vivo. Both MsrA and α-crystallins share antiapoptotic properties and both are present or translocated to the mitochondria during stress, therefore, it is hypothesized that their interaction occurs in mitochondria which is the major source of endogenous ROS. Recent findings indicate that, in AMD, both MsrA and α-crystallin accumulate in the drusen, and the nature of their physical and molecular interactions under pathological conditions is not known. Further, α-crystallins are neuroprotective in multiple neurodegenerative diseases[71,88-92], and given the fact that oxidation of the methionine residues inhibits the chaperone function of crystallins, it is plausible that modulation (by overexpression) of the Msr activity may be beneficial in the treatment of these diseases. In addition, aging contributes to increased oxidation of methionine in proteins so that MsrA alone or in combination therapy could offer significant and long-term protection.
Footnotes
Supported by Grants from NIH (EY01545, EY03040); The Arnold and Mabel Beckman Foundation (to Hinton DR) and a grant to the Department of Ophthalmology by Research to Prevent Blindness
Peer reviewer: Sung H Kim, Professor, College of Oriental Medicine, Kyunghee University, 1 Hoegidong Dongdaemungu, Seoul 130-701, South Korea
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Moskovitz J, Stadtman ER. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50:301–307. doi: 10.1080/713803735. [DOI] [PubMed] [Google Scholar]
- 5.Brot N, Weissbach H. Biochemistry of methionine sulfoxide residues in proteins. Biofactors. 1991;3:91–96. [PubMed] [Google Scholar]
- 6.Spector A, Kuszak JR, Ma W, Wang RR. The effect of aging on glutathione peroxidase-i knockout mice-resistance of the lens to oxidative stress. Exp Eye Res. 2001;72:533–545. doi: 10.1006/exer.2001.0980. [DOI] [PubMed] [Google Scholar]
- 7.Barkats M, Millecamps S, Abrioux P, Geoffroy MC, Mallet J. Overexpression of glutathione peroxidase increases the resistance of neuronal cells to Abeta-mediated neurotoxicity. J Neurochem. 2000;75:1438–1446. doi: 10.1046/j.1471-4159.2000.0751438.x. [DOI] [PubMed] [Google Scholar]
- 8.Warner HR. Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med. 1994;17:249–258. doi: 10.1016/0891-5849(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 9.Orr WC, Sohal RS. Effects of Cu-Zn superoxide dismutase overexpression of life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch Biochem Biophys. 1993;301:34–40. doi: 10.1006/abbi.1993.1111. [DOI] [PubMed] [Google Scholar]
- 10.Lee BC, Dikiy A, Kim HY, Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790:1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr WC, Sohal RS. The effects of catalase gene overexpression on life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch Biochem Biophys. 1992;297:35–41. doi: 10.1016/0003-9861(92)90637-c. [DOI] [PubMed] [Google Scholar]
- 12.Arnér ES, Holmgren A. The thioredoxin system in cancer-introduction to a thematic volume of Seminars in Cancer Biology. Semin Cancer Biol. 2006;16:419. doi: 10.1016/j.semcancer.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman ER, Moskovitz J, Berlett BS, Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem. 2002;234-235:3–9. [PubMed] [Google Scholar]
- 14.Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro GO, Reddy VN, Hejtmancik JF. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci USA. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci USA. 2004;101:1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sreekumar PG, Kannan R, Yaung J, Spee CK, Ryan SJ, Hinton DR. Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochem Biophys Res Commun. 2005;334:245–253. doi: 10.1016/j.bbrc.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 17.Picot CR, Petropoulos I, Perichon M, Moreau M, Nizard C, Friguet B. Overexpression of MsrA protects WI-38 SV40 human fibroblasts against H2O2-mediated oxidative stress. Free Radic Biol Med. 2005;39:1332–1341. doi: 10.1016/j.freeradbiomed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaung J, Jin M, Barron E, Spee C, Wawrousek EF, Kannan R, Hinton DR. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis. 2007;13:566–577. [PMC free article] [PubMed] [Google Scholar]
- 20.Yaung J, Kannan R, Wawrousek EF, Spee C, Sreekumar PG, Hinton DR. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp Eye Res. 2008;86:355–365. doi: 10.1016/j.exer.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Brot N, Weissbach L, Werth J, Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci USA. 1981;78:2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mary J, Vougier S, Picot CR, Perichon M, Petropoulos I, Friguet B. Enzymatic reactions involved in the repair of oxidized proteins. Exp Gerontol. 2004;39:1117–1123. doi: 10.1016/j.exger.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Grimaud R, Ezraty B, Mitchell JK, Lafitte D, Briand C, Derrick PJ, Barras F. Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J Biol Chem. 2001;276:48915–48920. doi: 10.1074/jbc.M105509200. [DOI] [PubMed] [Google Scholar]
- 26.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadtman ER, Moskovitz J, Levine RL. Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal. 2003;5:577–582. doi: 10.1089/152308603770310239. [DOI] [PubMed] [Google Scholar]
- 28.Kim HY, Gladyshev VN. Different catalytic mechanisms in mammalian selenocysteine- and cysteine-containing methionine-R-sulfoxide reductases. PLoS Biol. 2005;3:e375. doi: 10.1371/journal.pbio.0030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HY, Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J. 2007;407:321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- 30.Lee JW, Gordiyenko NV, Marchetti M, Tserentsoodol N, Sagher D, Alam S, Weissbach H, Kantorow M, Rodriguez IR. Gene structure, localization and role in oxidative stress of methionine sulfoxide reductase A (MSRA) in the monkey retina. Exp Eye Res. 2006;82:816–827. doi: 10.1016/j.exer.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brot N, Weissbach H. Peptide methionine sulfoxide reductase: biochemistry and physiological role. Biopolymers. 2000;55:288–296. doi: 10.1002/1097-0282(2000)55:4<288::AID-BIP1002>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 33.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 35.Lin Z, Johnson LC, Weissbach H, Brot N, Lively MO, Lowther WT. Free methionine-(R)-sulfoxide reductase from Escherichia coli reveals a new GAF domain function. Proc Natl Acad Sci USA. 2007;104:9597–9602. doi: 10.1073/pnas.0703774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascual I, Larrayoz IM, Rodriguez IR. Retinoic acid regulates the human methionine sulfoxide reductase A (MSRA) gene via two distinct promoters. Genomics. 2009;93:62–71. doi: 10.1016/j.ygeno.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansel A, Kuschel L, Hehl S, Lemke C, Agricola HJ, Hoshi T, Heinemann SH. Mitochondrial targeting of the human peptide methionine sulfoxide reductase (MSRA), an enzyme involved in the repair of oxidized proteins. FASEB J. 2002;16:911–913. doi: 10.1096/fj.01-0737fje. [DOI] [PubMed] [Google Scholar]
- 38.Vougier S, Mary J, Friguet B. Subcellular localization of methionine sulphoxide reductase A (MsrA): evidence for mitochondrial and cytosolic isoforms in rat liver cells. Biochem J. 2003;373:531–537. doi: 10.1042/BJ20030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuschel L, Hansel A, Schönherr R, Weissbach H, Brot N, Hoshi T, Heinemann SH. Molecular cloning and functional expression of a human peptide methionine sulfoxide reductase (hMsrA) FEBS Lett. 1999;456:17–21. doi: 10.1016/s0014-5793(99)00917-5. [DOI] [PubMed] [Google Scholar]
- 40.Boschi-Muller S, Gand A, Branlant G. The methionine sulfoxide reductases: Catalysis and substrate specificities. Arch Biochem Biophys. 2008;474:266–273. doi: 10.1016/j.abb.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Kryukov GV, Kryukov VM, Gladyshev VN. New mammalian selenocysteine-containing proteins identified with an algorithm that searches for selenocysteine insertion sequence elements. J Biol Chem. 1999;274:33888–33897. doi: 10.1074/jbc.274.48.33888. [DOI] [PubMed] [Google Scholar]
- 42.Fomenko DE, Novoselov SV, Natarajan SK, Lee BC, Koc A, Carlson BA, Lee TH, Kim HY, Hatfield DL, Gladyshev VN. MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J Biol Chem. 2009;284:5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scalmati A, Lipkin M. Intermediate biomarkers of increased risk for colorectal cancer: comparison of different methods of analysis and modifications by chemopreventive interventions. J Cell Biochem Suppl. 1992;16G:65–71. doi: 10.1002/jcb.240501113. [DOI] [PubMed] [Google Scholar]
- 44.Huang W, Escribano J, Sarfarazi M, Coca-Prados M. Identification, expression and chromosome localization of a human gene encoding a novel protein with similarity to the pilB family of transcriptional factors (pilin) and to bacterial peptide methionine sulfoxide reductases. Gene. 1999;233:233–240. doi: 10.1016/s0378-1119(99)00131-6. [DOI] [PubMed] [Google Scholar]
- 45.Cabreiro F, Picot CR, Perichon M, Castel J, Friguet B, Petropoulos I. Overexpression of mitochondrial methionine sulfoxide reductase B2 protects leukemia cells from oxidative stress-induced cell death and protein damage. J Biol Chem. 2008;283:16673–16681. doi: 10.1074/jbc.M708580200. [DOI] [PubMed] [Google Scholar]
- 46.Olry A, Boschi-Muller S, Marraud M, Sanglier-Cianferani S, Van Dorsselear A, Branlant G. Characterization of the methionine sulfoxide reductase activities of PILB, a probable virulence factor from Neisseria meningitidis. J Biol Chem. 2002;277:12016–12022. doi: 10.1074/jbc.M112350200. [DOI] [PubMed] [Google Scholar]
- 47.Neiers F, Kriznik A, Boschi-Muller S, Branlant G. Evidence for a new sub-class of methionine sulfoxide reductases B with an alternative thioredoxin recognition signature. J Biol Chem. 2004;279:42462–42468. doi: 10.1074/jbc.M407464200. [DOI] [PubMed] [Google Scholar]
- 48.Lowther WT, Brot N, Weissbach H, Honek JF, Matthews BW. Thiol-disulfide exchange is involved in the catalytic mechanism of peptide methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2000;97:6463–6468. doi: 10.1073/pnas.97.12.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranaivoson FM, Antoine M, Kauffmann B, Boschi-Muller S, Aubry A, Branlant G, Favier F. A structural analysis of the catalytic mechanism of methionine sulfoxide reductase A from Neisseria meningitidis. J Mol Biol. 2008;377:268–280. doi: 10.1016/j.jmb.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Ugarte N, Petropoulos I, Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 51.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moskovitz J, Jenkins NA, Gilbert DJ, Copeland NG, Jursky F, Weissbach H, Brot N. Chromosomal localization of the mammalian peptide-methionine sulfoxide reductase gene and its differential expression in various tissues. Proc Natl Acad Sci USA. 1996;93:3205–3208. doi: 10.1073/pnas.93.8.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, Brot N, Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kryukov GV, Kumar RA, Koc A, Sun Z, Gladyshev VN. Selenoprotein R is a zinc-containing stereo-specific methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2002;99:4245–4250. doi: 10.1073/pnas.072603099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koc A, Gasch AP, Rutherford JC, Kim HY, Gladyshev VN. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc Natl Acad Sci USA. 2004;101:7999–8004. doi: 10.1073/pnas.0307929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun. 2008;366:775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 59.Brennan LA, Lee W, Cowell T, Giblin F, Kantorow M. Deletion of mouse MsrA results in HBO-induced cataract: MsrA repairs mitochondrial cytochrome c. Mol Vis. 2009;15:985–999. [PMC free article] [PubMed] [Google Scholar]
- 60.Pascual I, Larrayoz IM, Campos MM, Rodriguez IR. Methionine sulfoxide reductase B2 is highly expressed in the retina and protects retinal pigmented epithelium cells from oxidative damage. Exp Eye Res. 2010;90:420–428. doi: 10.1016/j.exer.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marchetti MA, Pizarro GO, Sagher D, Deamicis C, Brot N, Hejtmancik JF, Weissbach H, Kantorow M. Methionine sulfoxide reductases B1, B2, and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Invest Ophthalmol Vis Sci. 2005;46:2107–2112. doi: 10.1167/iovs.05-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 63.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 64.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 65.Brennan LA, Lee W, Giblin FJ, David LL, Kantorow M. Methionine sulfoxide reductase A (MsrA) restores alpha-crystallin chaperone activity lost upon methionine oxidation. Biochim Biophys Acta. 2009;1790:1665–1672. doi: 10.1016/j.bbagen.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivasan AN, Nagineni CN, Bhat SP. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- 68.Sreekumar PG, Kannan R, Hinton DR. There are three major families of crystallins: misnaming of alphaB crystallin. Acta Physiol (Oxf) 2009;195:503; author reply 503. doi: 10.1111/j.1748-1716.2009.01976_1.x. [DOI] [PubMed] [Google Scholar]
- 69.Bhat SP. Crystallins, genes and cataract. Prog Drug Res. 2003;60:205–262. doi: 10.1007/978-3-0348-8012-1_7. [DOI] [PubMed] [Google Scholar]
- 70.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 72.van Rijk AE, Stege GJ, Bennink EJ, May A, Bloemendal H. Nuclear staining for the small heat shock protein alphaB-crystallin colocalizes with splicing factor SC35. Eur J Cell Biol. 2003;82:361–368. doi: 10.1078/0171-9335-00321. [DOI] [PubMed] [Google Scholar]
- 73.Jin JK, Whittaker R, Glassy MS, Barlow SB, Gottlieb RA, Glembotski CC. Localization of phosphorylated alphaB-crystallin to heart mitochondria during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H337–H344. doi: 10.1152/ajpheart.00881.2007. [DOI] [PubMed] [Google Scholar]
- 74.Lund AL, Smith JB, Smith DL. Modifications of the water-insoluble human lens alpha-crystallins. Exp Eye Res. 1996;63:661–672. doi: 10.1006/exer.1996.0160. [DOI] [PubMed] [Google Scholar]
- 75.Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 76.Fujii N, Takeuchi N, Fujii N, Tezuka T, Kuge K, Takata T, Kamei A, Saito T. Comparison of post-translational modifications of alpha A-crystallin from normal and hereditary cataract rats. Amino Acids. 2004;26:147–152. doi: 10.1007/s00726-003-0050-8. [DOI] [PubMed] [Google Scholar]
- 77.Shroff NP, Bera S, Cherian-Shaw M, Abraham EC. Substituted hydrophobic and hydrophilic residues at methionine-68 influence the chaperone-like function of alphaB-crystallin. Mol Cell Biochem. 2001;220:127–133. doi: 10.1023/a:1010834107809. [DOI] [PubMed] [Google Scholar]
- 78.Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch’s membrane-choroid complex from AMD and age-matched donor eyes. Exp Eye Res. 2005;80:821–826. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- 80.Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of alpha B-crystallin in Alzheimer’s disease. Acta Neuropathol. 1994;87:155–160. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- 81.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De S, Rabin DM, Salero E, Lederman PL, Temple S, Stern JH. Human retinal pigment epithelium cell changes and expression of alphaB-crystallin: a biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch Ophthalmol. 2007;125:641–645. doi: 10.1001/archopht.125.5.641. [DOI] [PubMed] [Google Scholar]
- 83.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 84.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 85.Badr GA, Zhang JZ, Tang J, Kern TS, Ismail-Beigi F. Glut1 and glut3 expression, but not capillary density, is increased by cobalt chloride in rat cerebrum and retina. Brain Res Mol Brain Res. 1999;64:24–33. doi: 10.1016/s0169-328x(98)00301-5. [DOI] [PubMed] [Google Scholar]
- 86.Guo M, Song LP, Jiang Y, Liu W, Yu Y, Chen GQ. Hypoxia-mimetic agents desferrioxamine and cobalt chloride induce leukemic cell apoptosis through different hypoxia-inducible factor-1alpha independent mechanisms. Apoptosis. 2006;11:67–77. doi: 10.1007/s10495-005-3085-3. [DOI] [PubMed] [Google Scholar]
- 87.Zhang C, Jia P, Jia Y, Li Y, Webster KA, Huang X, Achary M, Lemanski SL, Lemanski LF. Anoxia, acidosis, and intergenic interactions selectively regulate methionine sulfoxide reductase transcriptions in mouse embryonic stem cells. J Cell Biochem. 2011;112:98–106. doi: 10.1002/jcb.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masilamoni JG, Vignesh S, Kirubagaran R, Jesudason EP, Jayakumar R. The neuroprotective efficacy of alpha-crystallin against acute inflammation in mice. Brain Res Bull. 2005;67:235–241. doi: 10.1016/j.brainresbull.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 89.Masilamoni JG, Jesudason EP, Baben B, Jebaraj CE, Dhandayuthapani S, Jayakumar R. Molecular chaperone alpha-crystallin prevents detrimental effects of neuroinflammation. Biochim Biophys Acta. 2006;1762:284–293. doi: 10.1016/j.bbadis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 90.Wilhelmus MM, Boelens WC, Otte-Höller I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 91.Ying X, Zhang J, Wang Y, Wu N, Wang Y, Yew DT. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci. 2008;35:253–258. doi: 10.1007/s12031-007-9010-1. [DOI] [PubMed] [Google Scholar]
- 92.Fort PE, Lampi KJ. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp Eye Res. 2011;92:98–103. doi: 10.1016/j.exer.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]