Abstract
Spinal cord injuries (SCI) often occur at the cervical level above the phrenic motor pools, which innervate the diaphragm. Unfortunately, the untoward effects of impaired breathing are a leading cause of SCI-related death, underscoring the importance of developing strategies to restore respiratory activity. Here we show that after cervical SCI, there is upregulation of the perineuronal net (PNN) associated chondroitin sulfate proteoglycans (CSPGs) around phrenic motor neurons. Digestion of these potently inhibitory extracellular matrix molecules with Chondroitinase ABC (ChABC) can, by itself, promote plasticity of spared tracts and restore limited activity to the paralyzed diaphragm. However, when combined with application of a peripheral nerve autograft, ChABC treatment results in lengthy regeneration of serotonergic axons and other bulbospinal fibers with remarkable recovery of diaphragm function. Following recovery and initial transection of the bridge, there occurs an unusual, overall increased tonic diaphragmatic EMG activity, suggesting considerable remodeling of spinal cord circuitry after regeneration. This is followed by complete elimination of the restored activity proving that regeneration is critical for the return of function. Overall, these experiments present a way to profoundly restore function of a single muscle following debilitating CNS trauma, through both plasticity of spared tracts and regeneration of essential pathways.
Keywords: C2 hemisection, phrenic nucleus, perineuronal net, chondroitin sulfate proteoglycans, chondroitinase ABC
A key component of the PNN and glial scar, CSPGs powerfully inhibit plasticity, sprouting and regeneration1-4. Degradation of these inhibitors with ChABC can restore some function through increased regeneration of severed axons, as well as enhanced sprouting and/or improved conduction of spared fibers5,6. Following lateral hemisection at the second cervical level of the spinal cord (C2), there is a rapid upregulation of CSPGs in the vicinity of the lesion but also distally at the level of the phrenic nucleus (C3-C6) (Fig. 1a, Supp. Fig. 1). An increase of PNN related proteolgycans far from a cord injury was first demonstrated in the deafferented dorsal column nuclei and we now report a similar phenomenon around denervated motor neurons4. One recently discovered mechanism governing the upregulation of CSPGs at the lesion site is the extravasation of a fibrinogen TGF-beta complex through the open blood brain barrier which triggers inhibitory matrix release by reactive astrocytes7. The function of (other than impeding plasticity) and mechanism leading to PNN/CSPG upregulation at sites distal to the lesion are unknown but are likely associated with inflammatory stimuli in deafferented nuclei.
Figure 1. 7 days following C2 hemisection there is increased expression of the perineuronal net and inhibitory proteoglycans around phrenic motor neurons.
a, Phrenic motor neurons labeled with dextran Texas red (DTR PMN; red) in uninjured animals are ensheathed by the perineuronal net, indicated by Wisteria Floribunda Agglutinin staining (WFA - green, first column), but stained poorly for CS56, a marker for CSPGs (green, second column). Following C2 hemisection and treatment with saline there is increased WFA and CSPG staining (green, second row). Seven days after ChABC treatment WFA and CS56 staining disappears (third row). Scale bar = 40 μm. b, 2B6 (green), a marker for digested CSPGs, is present following ChABC treatment. Scale bar = 40 μm. b’, A lower power montage shows the area of CSPG digestion by ChABC (the side of montage left of central canal (CC) is ipsilateral to lesion and ChABC treatment). Scale bar = 200 μm. c, Within the region of CSPG degradation there is an increase of serotonergic fibers (5HT – green) around PMNs. Scale bar = 40 μm. Pixel intensity analysis shows a doubling in the amount of 5HT in ChABC treated animals compared to controls (n = 6, inset). Error bar indicates standard error.
Treatment with ChABC (250 nl, 20 U/ml in saline) at the level of the phrenic nucleus significantly degrades this family of inhibitors and results in accumulation of the CSPG stub antigen, visualized by the 2-B-6 antibody (Fig. 1b). Immunochemistry and histology showed that at five weeks post C2 hemisection and treatment with ChABC, there was still no reappearance of the PNN allowing the potential for continued sprouting (Supp. Fig. 2a). The PNN had not reformed at twelve weeks (not shown), but mostly reappeared by five months post ChABC administration (Supp. Fig. 2b). Within one week, and persisting over time (Supp. Fig. 8) treatment with ChABC led to an increased presence of serotonergic (5HT) fibers surrounding phrenic motor neurons compared to C2 hemisected animals that only received vehicle, with pixel intensity values near double that of saline treated animals (Fig. 1c,). This is important because 5HT plays a crucial role, especially under stressful conditions, in functional respiratory plasticity by increasing the efficacy of the small contingent of contralateral glutamatergic fibers from the rostral ventral respiratory group (rVRG), which innervates the phrenic nucleus and remains after C2 hemisection, i.e. the so-called crossed phrenic pathway8-13. Indeed, electromyographic (EMG) recording showed that when inducing the crossed phrenic phenomenon by transecting the phrenic nerve contralateral to the hemisection which, although clinically irrelevant, dramatically increases respiratory drive and activates the crossed phrenic pathway, there was an augmented return of activity in the hemidiaphragm ipsilateral to the lesion in ChABC treated animals. The activity was at least twice beyond that seen in phrenicotomized, non-treated animals (Supp. Fig. 3).
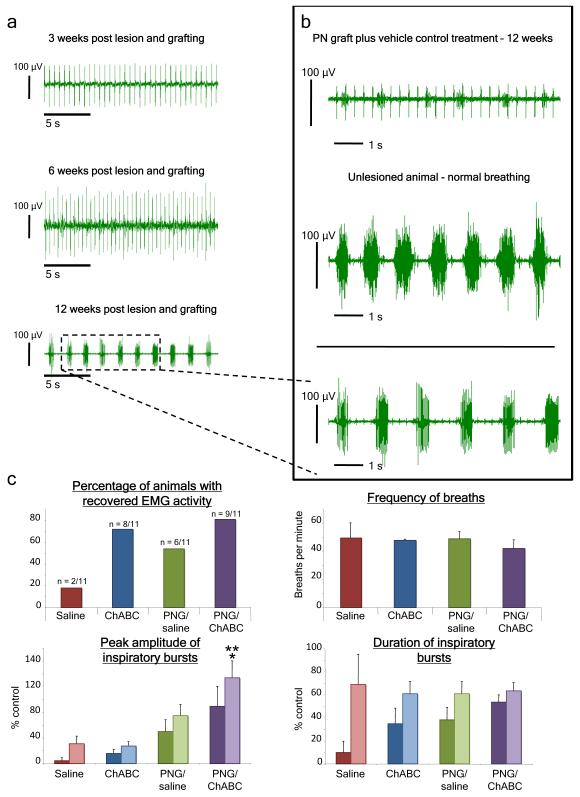
As early as one week following C2 hemisection, treatment with ChABC even without a phenicotomy could also lead to some recovery, while vehicle treated animals had no recovery at all, at least at this early time point (Supp. Fig. 4). A small minority of animals improved spontaneously, albeit minimally, over time without intervention (Fig. 2c). Thus, although recovery was more rapid (beginning at 1 wk versus 6-8 weeks) and occurred in a larger number of animals (80% versus 18%) even after 12 weeks in ChABC only treated animals, recovery was meager and did not exceed the 10-20% peak amplitude level which can be achieved infrequently in control animals (Fig. 2c).
Figure 2. After C2 hemisection and implantation of a PN graft with ChABC treatment there is an increase to near-normal levels of diaphragmatic EMG activity over time.
a, Prior to twelve weeks post C2 hemisection there is minimal activity in the hemidiaphragm ipsilateral to the lesion in animals that received ChABC treatment and application of an autologous peripheral nerve bridge. At twelve weeks there is substantial recovery of hemidiaphragmatic inspiratory activity. b, At 12 weeks recovery in animals that received a PNG and ChABC treatment was close to that of a non-lesioned animal and better than grafted animals that received only vehicle treatment. c) Animals that received the graft and ChABC had a higher percentage of recovery 12 weeks after hemisection compared to other groups. Semi-quantitative analysis showed that there was no difference in the frequency of breaths between groups. C2 hemisected animals with a PNG and ChABC treatment (purple bars) had a higher average peak amplitude of the raw inspiratory bursts compared to the other groups. Although burst duration could, on occasion, reach near normal levels (2a,2b), on balance there was no difference in the average duration of inspiratory bursts between animals that showed recovery. Darker colored bars are all animals in a group, while the lighter bars represent only animals that showed recovered activity. ** = significantly different compared to saline and ChABC treated animals, p < 0.02. * = significantly different compared to grafted animals with saline, p < 0.05. The error bar indicates standard error.
Because recovery was ultimately disappointing following ChABC treatment alone, we sought to increase it by implanting an autologous peripheral nerve graft (PNG), whose resident Schwann cells provide trophic/tropic support, re-myelination, and guided, long distance regeneration bypassing the lesion directly to the vicinity of the denervated phrenic nucleus (Supp. Fig. 5)14-18. The addition of ChABC allows for local sprouting as well as enhanced entrance and exodus of axons into and from the graft (Supp. Fig. 5)19,20. Three, six, nine, and twelve weeks after C2 hemisection, the animals were assessed for return of diaphragmatic muscle activity by bilateral EMG recordings (Fig. 2a). At around 10 weeks (not shown) and finally at about twelve weeks post C2 hemisection and grafting, animals that received ChABC and application of an autologous peripheral nerve bridge displayed the highest percentage of recovery compared to lesioned animals that received ChABC or saline alone or PNG application with vehicle treatment (Fig. 2b, 2c). Furthermore, in recovered animals, the peak inspiratory amplitude of the raw EMG trace was nearly equivalent to and often even surpassed that of the normal, non-injured side, as well as a non-injured animal (Fig. 2b, 2c). While, on occasion, the duration of inspiratory bursting could approach normal levels (Fig. 2a, 2b), on average, bursting duration remained comparably less than the non-injured side.
Although recording from the diaphragm can give semi-quantitative indications of muscle activity and neuromuscular junction effectiveness, phrenic nerve recordings under standardized conditions allow for more quantitative measurements of total phrenic nucleus motor output. When recording phrenic nerve activity chronically after C2 hemisection and treatment, the peak amplitude followed trends similar to those revealed with EMG analyses, with ChABC treated grafted animals having the highest values (Supp. Fig. 6). Although peak amplitude was high, the reduction in burst duration for both EMG and neurogram activity suggests either that an increased phrenic motor neuron threshold develops following injury and/or regeneration, or that the number of re-innervated motor neurons may be sub-optimal. Inducing expression of neurotrophins to lure regenerating axons towards the phrenic motor pool or transiently diminishing the PTEN gene might further augment connectivity and correct this deficit21-23. Interestingly, at 6 and 9 weeks post C2 hemisection and grafting, the emerging hemidiaphragmatic activity, although mostly patterned with the contralateral side, was neither uniform nor consistent. Instead, specific motor unit activity occurred intermittently with spikes of differing amplitudes appearing and disappearing throughout the recording session (Supp. Fig. 7). In preliminary studies of C2 hemi-lesioned animals at 6 months following the various treatments (saline, ChABC, PNG+saline, PNG+ChABC; n = 3 per group), recovery in all groups remained at levels equivalent to that at 3 months (data not shown).
Immunohistochemistry and tracing experiments at the twelve-week time point suggested robust regeneration into the PNG. An abundance of TAU, a marker for microtubule-associated proteins normally found in axons, indicated that there was significant regeneration of axons into the pre-degenerated graft (Fig. 3a). Similar to a normal dorsal root entry zone, at the ChABC treated, graft/CNS interface, GFAP positive astrocytes extended a short distance into the graft and were aligned with the regenerated axons (Fig. 3a)24. However, in saline treated animals GFAP positive astrocytes were haphazardly organized in a geometry that could potentially obstruct exit of regenerated axons at the interface (Fig. 3a).
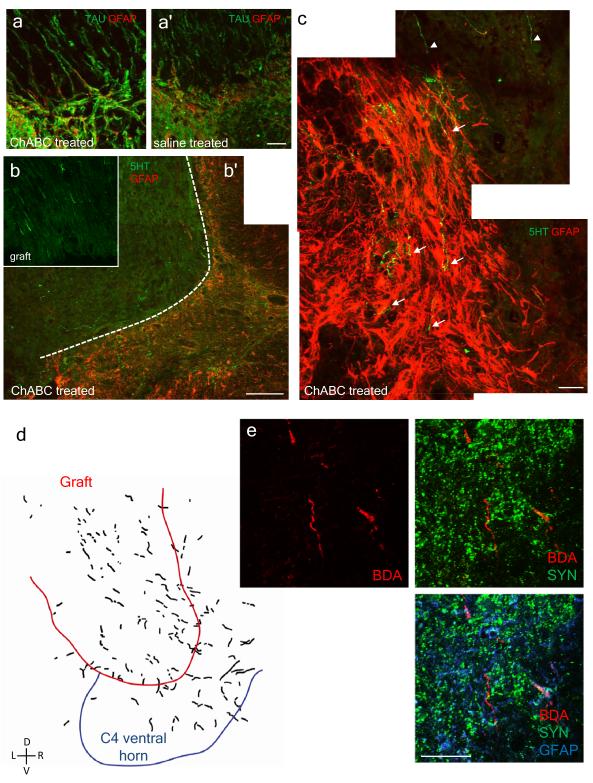
Figure 3. There is significant regeneration of axonal fibers in the PN graft and back into the CNS with ChABC treatment.
a, a’, In animals that received ChABC there is alignment of astroglial processes from the spinal cord (SC), identified by GFAP labeling (red), with the TAU positive (green) axons at the graft/SC interface that have regenerated back into the CNS. In saline treated animals, it appears that the astrocytes form a barrier-like structure at the interface. Scale bar = 40 μm. b, Only a small portion of the regenerated axons in the graft are serotonergic (green). Scale bar = 200 μm. b, b’, c, In ChABC treated animals, serotonergic fibers (arrows) penetrated deep into the CNS (identified by GFAP, red) from the graft (arrowheads). In c, scale bar = 40 μm. d, Anterograde tracing from the medulla with dextran Texas red shows regenerated fibers in the graft and back into the gray matter of the spinal cord. e, BDA labeling and immunocytochemistry show close proximity of regenerated fibers with synapsin puncta. Scale bar = 40 μm.
At the distal interface between the graft and the SC in ChABC/PNG animals, there was a suggestion of deep penetration of both TAU and 5HT positive axons into CNS tissue (the CNS compartment is demarcated by the presence of glial fibrillary acidic protein (GFAP) (Fig. 3a, 3b, 3c). In a group of animals where the anterograde tracers dextran Texas red or biotinylated dextran amine (BDA) were injected into the medulla to label bulbospinal tracts, reconstructions of multiple sections confirmed substantial regeneration into the graft and further into the cervical spinal cord (Fig. 3d). The regenerated axons tended to remain within their segment of re-entrance. Furthermore, labeled axons were associated with synapsin puncta, suggesting the possibility of functional re-connection (Fig. 3e). At twelve weeks, quantification of 5HT immunoreactivity in the ventral horns near C4 motor neurons showed that serotonergic fibers were significantly increased in PNG+ChABC grafted animals versus PNG+saline animals (Supp. Fig. 8). Interestingly, even though the extent of recovery between PNG+ChABC and ChABC-only treated animals was markedly different, their 5HT fiber densities in the vicinity of the phrenic motor pools were comparable (Fig. 2c, Supp. Fig. 8). Taken together these results suggest that the raphe-spinal system is not acting alone in fostering restoration of hemidiaphragmatic function. Injection of dextran Texas red directly into the graft to allow a glimpse of the entire population of neurons that projected axons through the graft indicated that there was a small portion of retrogradely labeled neurons within the raphe and, more importantly, the rVRG - a nucleus critical for projecting respiratory rhythm and drive caudally (Supp. Fig. 9). However, there were also a significant number of projections from the medial medullary reticular formation (Supp. Fig. 9). Additionally, there were a few labeled cell bodies in the dorso-lateral medulla (Supp. Fig. 9).
In animals with restored hemidiaphragmatic function, the all-important transection of the graft led to complete elimination of inspiratory hemidiaphragmatic activity ipsilaterally (along with a compensatory increase in frequency and amplitude on the contralateral side) although this occurred in a manner distinctly different from that following acute hemisection in an intact spinal cord (see below). The dramatic reduction of EMG activity upon bridge transection and compensatory changes in the contralateral side strongly suggest that recovery of diaphragm motor function was primarily mediated through regeneration of respiratory-related axons and played a critical role in ventilation of the animal (Fig. 4a, 4b). Surprisingly, in addition to abolishing inspiratory activity eventually, transection of the PNG initially led to an unusual increase in overall tonic EMG activity (Fig. 4g), which varied in frequency between animals, and which never occurs normally during or after a C2 hemisection (Supp. Fig. 10). Thus, tonic activity in the motor neurons themselves only results when the regenerated fibers re-innervating the cord are eliminated. The pattern of this activity was reminiscent of extracellular recordings of interneurons normally found deep within spinal respiratory circuitry, where during times of inspiration (identified by breaths on the contralateral side) there were repeated, transient increases in their tonic spiking frequency (Fig. 4b, 4d, 4f)25. At acute stages after graft lesion, any remnants of patterned inspiratory activity buried within the tonic bursting episode could be attributed to these interneurons that, in turn, might receive inputs from the crossed phrenic pathway11,26. The increased tonic activity after graft lesion subsided at two hours and completely disappeared when assessed twenty-four hours later (Fig. 4e, 4g). These results suggest that following SCI, regenerated axons may incorporate the activity of interneurons and/or propriospinal neurons that innervate motor neurons, leading to proper firing and restoration of function. Other potential mechanisms behind this observation include disinhibition of phrenic motor neurons directly resulting from removal of regenerated supraspinal inputs or alterations in a phenomenon known as homeostatic plasticity where denervated neurons can re-acquire stable, repetitive firing characteristics27.
Figure 4. Transection of the PN graft after recovery leads to increased tonic EMG activity of the diaphragm.
a, 12 weeks after C2 hemisection and graft+ChABC, there is recovery ipsilateral to the lesion that is rhythmic and synchronous with the contralateral side. b, Immediately after transection of the graft there is a reduction in the recovered activity but an increase in tonic activity and compensatory changes on the contralateral side. c-e, In another recovered animal where the PNG was transected the tonic activity declined after one hour. f, During times of inspiration (indicated by upper trace of the right hemidiaphragm) there is an increase in the spiking frequency in the resulting tonic activity of the left hemidiaphragm. g, In a third animal, the tonic activity is gone 24 hours after graft transection. h and i, In two examples of animals with graft and ChABC treatment, there is an abundance of activity in the graft that can be augmented during and after respiratory challenge, suggesting regeneration of respiratory related axons. j, Phrenic nerve activity after similar challenge mirrors the patterned increased activity in the graft (h and i).
When recording from the implanted graft itself, while there was a continuous barrage of firing during full artificial ventilation, there occurred a strong and persisting increase in activity in response to respiratory challenge (i.e. by temporarily shutting off the ventilator) (Fig. 4h, 4i). Such enhanced activity clearly suggests that among the many axons in the graft, there was also a contingent of respiratory related axons, confirming earlier work15,17. Furthermore, and rather remarkably, the regular firing pattern of the sub-group of axons in the graft with augmented activity closely mirrored the pattern of enhanced activity that developed in the ipsilateral phrenic nerve (Fig. 4j). Importantly, restored output activity in the phrenic nerve was never contaminated with non-respiratory related input activity that was present in the graft (Fig. 4j).
Taken together, our experiments show that in adult rats, robust activation of a critical muscle paralyzed by SCI can be returned through long distance regeneration of axons to the vicinity of the denervated motor neurons. EMG recordings showed that recovery takes place very slowly but is remarkably strong when finally manifested. Importantly, ChABC treatment on its own, while sufficient to initiate rapid functional recovery in most C2 hemisected animals was ultimately quite limited. Perhaps inducing respiratory drive through intermittent hypoxia, theophylline administration, or ChR2/light stimulation in combination with ChABC would result in more rapid or higher levels of recovery without or even with grafting10, 28-31. In addition to returning function to a paralyzed muscle and the clinical implications of restoring the ability to breathe after SCI, our results also provide new insights into the role of adult CNS plasticity and reorganization after injury and regeneration. Indeed, the increased tonic activity of the diaphragm resulting from transecting the PNG suggests widespread alterations in spinal circuitry after injury, and in particular, an important role for interneurons whose activity can be recruited by regenerating axons. However, it has not yet been proven that such interneurons are, in fact, the anatomical substrate for recovery. It is also possible that they are extraneous and being inhibited, at least when the graft is intact. In addition, the adult CNS, even after injury, has the remarkable ability to organize a composite of regenerating axons and their diverse signals into meaningful synaptic connections, as well as eliminate or silence abnormal and potentially abhorrent connections which could result in misfiring muscle activity. Perhaps these endogenous mechanisms in the CNS determine the time course of recovery. These processes could include pruning or inhibition of unwanted synaptic connections (non-respiratory related), re-myelination of necessary (as well as unnecessary) regenerated fibers, or competition with spared tracts for a finite number of post-synaptic sites. Undoubtedly, these findings provide many new avenues of research regarding CNS regeneration and promotion of recovery after SCI.
Methods Summary
Sprague Dawley female rats (240-300 g) received a C2 hemisection and at the same time received either saline or ChABC at the level of the phrenic nucleus, ipsilateral to the lesion. In animals where a pre-degenerated autologous peripheral tibial nerve bridge was applied, ChABC was administered at the C2 lesion site where the proximal end of the graft was inserted to enhance axonal entry. One week later, the SC was re-exposed and the distal end of the graft was inserted into a pocket at C4 along with ChABC injection.
For the electromyographic recordings, two bipolar electrodes connected to amplifiers and a data acquisition system, were inserted into the left and right hemidiaphragms. For phrenic neurogram recordings, animals were vagotomized, ventilated, and the femoral blood vessels cannulated to monitor blood pressure and administer drugs. The left phrenic nerve, ipsilateral to the lesion, was isolated, transected desheathed, placed on bipolar silver electrodes, covered with mineral oil, and activity recorded under standardized conditions. Preparation of the animal to record from the graft itself was similar to the above procedures but the spinal cord was re-exposed and the graft isolated. From there, the freed graft was placed on the electrodes and activity recorded.
In the immunocytochemistry experiments, animals were perfused with phosphate buffered saline and 4% paraformaldehyde. The medulla and SC was harvested and sectioned on a cryostat at 20 μm thickness, and then processed for detection of the relevant molecules/proteins. For anatomical tracing studies, biotinylated dextran amine and/or dextran Texas red was injected into: 1) the diaphragm to retrogradely label phrenic motor neurons; 2) into the graft to retrogradely label the population of regenerating medullary cells; or 3) into the medulla to label regenerated axons in the graft and back into the SC.
Methods
Surgical procedures
C2 Hemisection and Grafting
Adult female Sprague Dawley rats (retired breeders, 240-300 g) were anesthetized with a ketamine (70mg/kg) and xylazine (7mg/kg) solution administered intraperitoneally (i.p.). After the animals reached a surgical plane of anesthesia, the animals were prepared for surgery by shaving the dorsal surface of the neck area and scrubbing with betadine and 70% ethyl alcohol. A midline incision of approximately four cm was made and the paravertebral muscles retracted. A multi-level laminectomy was performed to expose the upper cervical segments of the spinal cord. The dura was cut, and with a microblade a hemisection was made on the animal’s left spinal cord caudal to the C2 dorsal roots starting at the midline and extending to the lateral most extent of the spinal cord. Sham hemisected animals received all procedures but the lesion.
In animals that received a peripheral nerve graft, the animal was anesthetized as above and the left tibial nerve was uncovered and transected one week before the C2 hemisection for pre-degeneration. At the time of C2 hemisection, the tibial nerve was dissected out (1-1.5 cm) and placed in the C2 hemisection along with ChABC. One week later the SC was again revealed and the distal end of the graft was placed into a slit made at the C4 spinal cord with ChABC. At both proximal and distal ends of the graft, the epineurium and dura were sutured together for stability with 9-0 polypropylene suture. Please see Houle et al. (2009) to see a visual demonstration of the grafting technique32.
Chondroitinase ABC Injections
For animals that received either ChABC (20 U/ml) only or saline vehicle control only, a pulled pipet attached to a Drummond Nanoject II (Broomall, PA) was stereotaxically placed 1.1 mm left of the spinal cord midline and 1.6 mm ventral from the dorsal surface of the spinal cord, in close proximity to the phrenic nucleus. After placement, approximately 250 nl of drug or vehicle was administered per injection. For each 250 nl injection, the ChABC spread effect was approximately 20.5 mm3. Three injections were made at the C3 to C5 spinal cord, ~1mm apart.
In animals that received a peripheral nerve graft, 500 nl of 20 U/ml ChABC was injected into the C2 lesion area with the Nanoject prior to implantation of the proximal end. One week later for the distal end implantation, 250 nl of 20 U/ml was injected into a slit made tangentially to the plane of the cord at a depth of about 1 mm at the C4 cervical level prior to insertion.
Following this, the muscle layers were drawn back together with 3-0 vicryl and the skin stapled together with wound clips. The animals received marcaine and buprenorphine for analgesic purposes. 5-10 ml of saline was administered subcutaneously post-operatively and if animals appeared to be dehydrated. All animals were housed in groups of 2 to 3 and exposed to a normal dark and light schedule with free access to food (normal rodent chow) and water. All animal procedures were approved by Case Western Reserve University’s Institutional Animal Care and Use Committee.
Physiological Recordings
Electromyographic and Neurogram Recordings
For diaphragmatic EMG recordings, animals were anesthetized with ketamine and xylazine and an eight cm incision at the base of the ribcage was made to expose the abdominal surface of the diaphragm. Following exposure of the diaphragm, bi-polar electrodes, connected to an amplifier and data acquisition system and program (CED Power 1401/Spike 2, Cambridge Electronic Designs, Cambridge, UK), were inserted bilaterally into the crural area of the diaphragm and EMG muscle activity recorded. The EMG signal was band passed filtered between 30 and 3000 Hz (Grass Instruments Amplifier Model P511, West Warwick, RI). If animals were to survive, the abdominal muscles were sutured together with 3-0 vicryl and the skin closed with wound clips. For induction of the crossed phrenic phenomenon, the above was performed after isolation of the phrenic nerve contralateral to the lesion (from a ventral approach) and then transected. For transection of the graft during EMG recording, the SC was re-exposed and the graft was isolated and cut. The graft in the living animal is not tightly apposed to the dura and can still be manipulated, lifted, and cut without touching or damaging the dorsal surface of the SC.
For phrenic nerve recordings, the animals were first anesthetized with urethane (1.6 g/kg, i.p.). The femoral vein and artery were then cannulated with PE-50 to administer fluids and drugs and monitor blood pressure. Following this, PE-240 tubing connected to a ventilator (Harvard Apparatus, Holliston, MA) was inserted into an opening made into the trachea to ventilate the animal, with a 1:1 mixture of oxygen to air. Both vagus nerves were transected to abolish mechanoreceptor feedback and entrainment. During the whole recording procedure, the animal was paralyzed with vancuronium, the end-tidal CO2 was monitored (Kent Scientific, Torrington, CT) and the animal placed on a heated circulating water pad. From the dorsal approach the left phrenic nerve was dissected, transected far distal from the CNS, and desheathed. The dissected nerve was then placed on bipolar silver electrodes and covered with mineral oil. Under standardized recording conditions, phrenic nerve activity was amplified (20,000x). band-pass filtered (30-1000Hz) Grass Instruments, West Warwick, RI), and activity was monitored both visually and through an audio monitor. The signal was recorded and analyzed using the CED/Spike 2 data acquisition system. For recording activity of the surgically applied peripheral nerve graft, the above procedures were performed but instead of dissecting the phrenic nerve, the SC was re-exposed and the graft isolated. Following this, the still proximally attached graft was placed on the silver bipolar electrodes and activity was recorded.
Quantification and Statistical Analysis
During recording, raw diaphragmatic EMG signal or phrenic nerve activity was rectified and integrated using Spike2 software. Frequency was determined by counting total number of inspiratory bursts for five minutes. Peak amplitude and burst duration of inspiratory bursts were measured through Spike2, for at least 10 breaths per animal for each group. Peak amplitude values were standardized to either a 10 or 100 μV calibration pulse and contralateral hemidiaphragm or homolateral phrenic nerve activity. Values were averaged and statistical analysis was performed using one-way ANOVA and Tukey’s post hoc analysis using Minitab 15 (Minitab Inc., State College, PA). All values with a p value less than 0.05 were considered significant. All error bars indicate standard errors.
Immunocytochemistry and Tracing
Perfusion and Sectioning
At the time of perfusion animals were anesthetized with ketamine and xylazine and perfused first with 50 mls of PBS and then 250 mls of 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS). The spinal cord was harvested and post fixed in 4% PFA overnight and cryoprotected in 30% sucrose in PBS until sectioning. Immediately prior to sectioning, a pinhole was made on the side contralateral to the lesion to denote laterality. The spinal cord was sectioned on a Hacker cryostat at a thickness of 20 μm and mounted on Superfrost/coated slides.
Immunocytochemistry
Mounted sections were washed three times with PBS followed by blocking in 5% normal goat serum (NGS), 0.1% bovine serum albumin (BSA) in PBS. 0.1% Triton X-100 was added to the blocking buffer depending on antigen used. Following blocking, sections were incubated in primary antibody diluted in blocking buffer for two nights at 4° C. Primary antibodies used were mouse anti-chondroitin sulfate (1:200, Sigma), anti-2-B-6 (1:200, Sigma) antibodies, anti-NeuN (1:500, Chemicon), anti-synapsin (1:1000, Chemicon), anti-GFAP (1:500, Sigma) and rabbit anti-5-HT (1:15,000, Immunostar, Hudson, WI), and anti-TAU (1:1500, Abcam, Cambridge, MA). Furthermore, the PNN and glycosaminoglycan chains were detected with Wisteria Floribunda (WFA) lectin conjugated to biotin (1:50, Sigma). The next day, the sections were washed extensively with PBS and incubated in the appropriate secondary antibody or avidin substrate conjugated to Alexafluor 488, 594, or 633 (1:500, Molecular Probes, Eugene, OR) for two days. After extensive washing, the sections were mounted, coverslipped, and viewed with a confocal microscope (Zeiss, Germany). Pixel intensity was measured on images taken on a standard fluorescent microscope (Leica, Germany) with a standard exposure setting and analyzed using MetaMorph Image Program (Molecular Devices, Downingtown, PA)
Tracing
For all tracing studies, tracers were injected one week before animal perfusion. To retrogradely label phrenic motor neurons, 10 μl of 0.4% 3000 MW/Dalton dextran Texas red (Molecular Probes) in PBS was injected five times into the hemidiaphragm ipsilateral to the hemisection with a Hamilton syringe. To label regenerating medullary axons in the graft and back into the SC, the medulla was exposed and 500 nl of 10% 10,000 MW/Dalton dextran Texas red or biotinylated dextran amine (Molecular Probes) was injected bilaterally, ~ 2.5-3 mm lateral of the obex with a Nanoject. Labeled axons from five sections were traced on a light box onto a composite. To retrogradely label the medullary cell bodies projecting axons into the graft, the spinal cord/graft area was re-exposed and 125 nl of 0.4% 3000 MW/Dalton dextran Texas red was injected into the graft with a Nanoject. To identify the rostro-caudal position of the sections, the transition from the central canal to the fourth ventricle was identified and the labeled cell bodies positioned onto the coronal sections of the Paxinos and Watson rat brain atlas33.
Supplementary Material
Acknowledgements
This project was funded by The Christopher and Dana Reeve Foundation (WA), the International Spinal Research Trust (WA), NINDS NS25713 (JS), NS060767 (JS), and NHLBI HL080318 (TED). We also offer special thanks to the Brumagin Memorial Fund (JS) and the Ellen Becker Neuroscience Regenerative Medicine Research Fund (JS). The authors would also like to acknowledge and thank Drs. John D. Houle and Veronica J. Tom of Drexel University College of Medicine for teaching the peripheral nerve grafting technique.
Footnotes
Supplementary Information is included with this manuscript.
Author Contributions W.J.A. performed the surgeries, recorded and analyzed the electophysiological data, and completed the immunocytochemical, tracing, and histological experiments. K.P.H. helped with the immunocytochemical detection and quantification. H.H. assisted with the animal care, surgeries, and data quantification and performed all the tissue processing. T.E.D. gave technical guidance and helped discuss results. W.J.A. and J.S. designed all studies, analyzed the data, and wrote the paper. All authors discussed and helped prepare the manuscript.
Author Information The authors declare no competing financial interests.
References
- 1.Davies SJ, et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 3.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 4.Massey JM, et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neuroscience. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nature Neuroscience. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 6.Hunanyan AS, et al. Role of chondroitin sulfate proteoglycans in axonal conduction in Mammalian spinal cord. J Neuroscience. 2010;30:7761–7769. doi: 10.1523/JNEUROSCI.4659-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schachtrup C, et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neuroscience. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi H, et al. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neuroscience. 2005;25:4550–4559. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller DD, et al. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. Journal of Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- 10.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neuroscience. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Applied Physiology. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- 12.Hadley SD, Walker PD, Goshgarian HG. Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Experimental Neurology. 1999;160:479–488. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- 13.Hodges MR, et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neuroscience. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science (New York, N.Y. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 15.Munz M, et al. Functional activity of rat brainstem neurons regenerating axons along peripheral nerve grafts. Brain Research. 1985;340:115–125. doi: 10.1016/0006-8993(85)90780-2. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier P, Rasminsky M. Activity of medullary respiratory neurons regenerating axons into peripheral nerve grafts in the adult rat. Brain Research. 1988;438:225–236. doi: 10.1016/0006-8993(88)91341-8. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier P, et al. Functional reconnections established by central respiratory neruons regenerating axons into a nerve graft bridging the respiratory centers to the cervical spinal cord. Journal of Neuroscience Research. 2002;70:65–81. doi: 10.1002/jnr.10379. [DOI] [PubMed] [Google Scholar]
- 18.Fouad K, et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neuroscience. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houle JD, et al. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neuroscience. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang LJ, et al. Sialidase enhances spinal axon outgrowth in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11057–11062. doi: 10.1073/pnas.0604613103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Baumgartner BJ, Hill-Felberg SJ, McGowen LR, Shine HD. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J Neuroscience. 2003;23:1424–1431. doi: 10.1523/JNEUROSCI.23-04-01424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alto LT, et al. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nature Neuroscience. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature Neuroscience. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPhail LT, Plunet WT, Das P, Ramer MS. The astrocytic barrier to axonal regeneration at the dorsal root entry zone is induced by rhizotomy. The European Journal of Neuroscience. 2005;21:267–270. doi: 10.1111/j.1460-9568.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Applied Physiology. 2003;94:1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- 26.Lane MA, et al. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. The Journal of Comparative Neurology. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature Reviews. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 28.Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Experimental Neurology. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 29.Fuller DD, Johnson SM, Olson EB, Jr., Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neuroscience. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng YD, et al. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neuroscience. 2003;23:4182–4189. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alilain WJ, et al. Light-induced rescue of breathing after spinal cord injury. J Neuroscience. 2008;28:11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houle JD, et al. Combining peripheral nerve grafting and matrix modulation to repair the injured rat spinal cord. J Visual Experience. 2009;29:14881–14890. doi: 10.3791/1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.