Abstract
Background
We sought to study the effects of chronic exposure to fluoxetine — a selective serotonin reuptake inhibitor (SSRI) and specific 5-HT2B receptor agonist in astrocytes — on the expression of kainate receptors (GluK1–5) in cultured astrocytes and in intact brains in mice and on GluK2 editing by adenosine deaminase acting on RNA (ADAR), as well as the ensuing effects of fluoxetine on glutamate-mediated Ca2+ influx and extracellular signal-regulated kinase (ERK)1/2 phosphorylation in astrocytes.
Methods
We performed reverse transcription–polymerase chain reaction (PCR) to assess mRNA expression. We analyzed RNA editing with amplification refractory mutation system PCR and complementary DNA sequencing. Protein expression and ERK phosphorylation were assessed using Western blots. We studied gene silencing with specific small interfering RNAs (siRNA), and we studied intracellular Ca2+ using fluorometry.
Results
All GluK subunits were present in the brain in vivo, and GluK2–5 subunits were present in cultured astrocytes. Fluoxetine upregulated GluK2 and ADAR2. Enhanced GluK2 editing by fluoxetine abolished glutamate-mediated increases in intracellular Ca2+ and ERK1/2 phosphorylation. Enhanced editing of GluK2 was prevented by siRNA against the 5-HT2B receptor or ADAR2.
Limitations
Limitations of our study include the use of an in vitro system, but our cultured cells in many respects behave like in vivo astrocytes.
Conclusion
Fluoxetine alters astrocytic glutamatergic function.
Introduction
Although the selective serotonin reuptake inhibitor (SSRI) fluoxetine is not universally effective in major depressive disorder (MDD), and in spite of a long latency period for its therapeutic action to take effect, it is widely used in the treatment of MDD. In vivo chronic treatment with fluoxetine leads to upregulation of Ca2+-dependent phospholipase A2 (cPLA2) expression in the brain.1 We have previously reproduced an increased cPLA2a expression in cultured mouse astrocytes after fluoxetine treatment for 1–4 weeks with a faster response at higher concentrations and an EC50 of 0.5 μM after 3 weeks of treatment.2 Paroxetine, citalopram, fluvoxamine and sertraline have identical effects with similar or even greater potency.3 The upregulation of cPLA2a in cultured astrocytes is secondary to the ability of acutely administered fluoxetine (and other SSRIs) to directly and specifically stimulate the serotonin (5-HT)2B receptor,2–5 whereas well differentiated cultures of astrocytes express no 5-HT transporter.5 Any 5-HT that might be present in serum added to the culturing medium will be rapidly degraded by a high activity in the cells containing monoamine oxidase (MAO).6,7
Another recent in vivo observation was that editing of the Q/R site of the kainate receptor GluK2, the product of Grik2, in the anterior portion of the rat brain was found to be slightly decreased by daily treatment with fluoxetine (10 mg/kg) for 14 days.8 Messenger RNA expression in GluK2 is edited by adenosine deaminases acting on RNA (ADAR), an enzyme family catalyzing deamination of adenosine to inosine in mRNA, thereby changing the translated protein sequence because inosine is read by the cells as guanosine.9 There are 3 members in the ADAR family, ADAR1, ADAR2 and ADAR3,10 all of which are expressed in the brain.11 The ADAR2 enzyme is found in hippocampal pyramidal neurons, cerebellar Purkinje cells and Bergmann glial cells, all of which have less expression of ADAR1 and ADAR3 than ADAR2.12 Studies of ADAR2 complementary DNA (cDNA) clones have revealed 4 different isoform mRNAs generated by self-editing and subsequent splicing at 2 different sites, 1 in the deaminase domain and 1 at the 5′-end of the coding sequence.13 The 4 isoforms have been named ADAR2Sd and ADAR2Ld, which are produced by splicing in the deaminase domain, and ADAR2Sc and ADAR2Lc, which are produced by splicing in the 5′-end of the coding sequence.14 The different splicing variants and combinations of variants result in the production of proteins with different deaminase activity.13–15 The GluK2 receptor has been demonstrated in astrocytes in the rat hippocampus16 and spinal cord,17 and S100β-positive astrocytes obtained by fluorescence-activated cell sorting from dissociated rat brain cortical tissue show GluK2 gene expression that is about half as pronounced as in the corresponding neurons.18 It is unknown whether chronic treatment with fluoxetine affects editing of GluK2 in astrocytes, let alone what effect this has on kainate receptor function.
There is now overwhelming evidence that glutamate homeostasis in the brain plays a major role in mood disorders,19,20 especially in the neuronal–glial tripartite synapse.21 Astrocytes are responsible for the supply of glutamatergic neurons with transmitter glutamate22 and for the major part of its subsequent clearance from the synaptic cleft.23 Like neurons, astrocytes are able to release glutamate in a manner stimulated by Ca2+,24,25 and they express glutamate receptors, which are involved in cross-talk with neuronal synapses and can strengthen synaptic transmission.22 Specifically regarding GluK2 and GRIK2, patients with different single nucleotide polymorphishms in the DNA of GRIK2 are at an enhanced risk for suicidal ideation during treatment with citalopram or other SSRIs.26,27 In rodents in which Grik2 is knocked out, a large number of behavioural traits have been interpreted as equivalents of clinical mania.28 Major depressive disorder has been found to correlate with decreased GluK2 expression in the entorhinal cortex,29 but mice in which Grik2 is knocked out display less despair during the forced swimming test and more social interaction,30,31 suggesting that the knockout may make them more resistant to depression.
In the present study, we examined
the effects of chronic treatment with fluoxetine on ADAR2 and GluK2 expression and editing in cultured astrocytes and intact brains in mice;
the effect of chronic treatment with fluoxetine on ADAR2 mRNA splicing in cultured astrocytes;
the effect of chronic treatment with fluoxetine on glutamate-induced extracellular signal-regulated kinase (ERK) phosphorylation and [Ca2+]i in cultured astrocytes, both of which were shown to be mediated by GluK2; and
the effect of 5-HT2B receptor downregulation with 5-HT2B receptor small interfering RNA (siRNA) or of ADAR2 knock-down with ADAR2 siRNA on chronic effects of fluoxetine in cultured astrocytes.
Methods
Animals
Male adult CD-1 mice (Charles River, Beijing, China), weighing 30–40 g, were housed in cages on a 12-hour light/dark cycle in a temperature-controlled (23–25°C) colony room with free access to food and water. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1978), and all experimental protocols were approved by the Institutional Animal Care and Use Committee of China Medical University.
Materials
We purchased chemicals for preparation of the medium and most other chemicals, including fluoxetine, NS102 and first antibody raised against β-actin, from Sigma. Santa Cruz Biotechnology supplied first antibodies raised against ERK ([K-23]:sc-94), against phosphorylated ERK (p-ERK; [E-4]:sc-7383) and against ADAR2 ([c-15]:sc-10012); the second antibody goat anti-rabbit IgG HRP conjugated; and the GluK2 siRNA (sc-37106). We purchased the first antibody raised against GluK2 from Upstate Biotechnology and the first antibody raised against the 5-HT2B receptor from BD Biosciences. The second antibody goat anti-mouse IgG HRP conjugated was from Promega, and enhanced chemiluminescence detection reagents were from Amersham Biosciences. Oligofectamine reagent for RNA interference, Opti-MEMI and fura-2 were obtained from Invitrogen.
Cell cultures
Primary cultures of mouse astrocytes were prepared as previously described,32,33 with minor modifications. The neopallia of the cerebral hemispheres were aseptically isolated, vortexed to dissociate the tissue, filtered through nylon meshes with pore sizes of 80 μm and subsequently 10 μm, diluted in culture medium and planted in Falcon Primaria culture dishes or multiwell plates. The culture medium was a Dulbecco modified Eagle’s medium (DMEM) with 7.5 mM of glucose, initially containing 20% horse serum, and the cultures were incubated at 37°C in a humidified atmosphere of CO2/air (ratio 5%:95%). The culture medium was exchanged with fresh medium of similar composition on day 3, and subsequently every 3–4 days. From day 3, the serum concentration was reduced to 10%, and after the age of 2 weeks, 0.25 mM dibutyryl cyclic adenosine monophosphate (dBcAMP) was included in the medium. Such cultures are known to be morphologically and functionally differentiated,34 to express many characteristics of astrocytes in vivo32 and to be highly enriched (> 95 purity) in glial fibrillary acidic protein (GFAP)– and glutamine synthetase–expressing astrocytes, with the main contaminating cells being macrophages (<5%) and neurons and oligodendrocytes being absent.35
Drug treatment
Chronic drug treatment
After 3 weeks of culturing, fluoxetine dissolved in a small volume of phosphate-buffered saline (PBS; 137 mM of NaCl, 2.7 mM of KCl, 10 mM of Na2HPO4, 2 mM of KH2PO4, 1 mM of CaCl2, 0.5 mM of MgCl2 and 7.5 mM of glucose, pH 7.4) was added to the cultures to a final concentration of 10 μM. A high concentration was selected to obtain a rapid effect, as previously observed with respect to cPLA2 expression;2 controls from the same batches received only PBS.
Acute drug treatment
To determine glutamate effects on ERK1/2 phosphorylation, we gently removed the culture medium, and the cells were incubated in corresponding medium without serum at 37°C for specified time periods in the absence or presence of 100 μM of L-glutamate. The reaction was stopped by washing with ice-cold PBS containing 7.5 mM of glucose, and the cells were scraped off the dishes and harvested in lysis buffer (50 mM of Hepes, 5 mM of EDTA [the Na+ salt], 50 mM of NaCl, 1% Triton-100, 10 mM of β-mercaptoethanol, 1 mM of phenylmethyl sulfonyl fluoride and 1 mM of sodium orthovanadate; pH 7.4).
To determine glutamate effects on intracellular Ca2+ concentrations, we used a Tecan Infinite M200 microplate analyzer to record fluorescence intensity of fura-2 introduced in astrocyte cultures grown in 24-well plates. For fura-2 loading, we replaced the growth medium with isotonic saline solution (137 mM of NaCl, 5 mM of KCl, 0.44 mM of KH2PO4, 4 mM of NaHCO3, 1.3 mM of CaCl2, 0.8 mM of MgSO4, and 0.5 mM of MgCl2 with 10 mM of glucose) containing 5 μM of fura-2 for 30 minutes at 37°C. After washing twice with similar saline, 300 μL of preheated isotonic saline was pipetted into each well, the multiwell plate was reinserted into the microplate analyzer, and readings were made at 340-nm and 380-nm excitations and 510-nm emissions at 24-second intervals for 2 minutes (5 cycles) to establish a baseline, with the wells exposed to illumination from the bottom of the chamber at gain 120. Subsequently, we took the multiwell plate out of the analyzer and added saline solution (control) or a final concentration of 100 μM of glutamate. The multiwell plate was reintroduced into the analyzer, and readings continued for another 30 minutes (50 cycles) with similar settings.
Knock-down of the 5-HT2B receptor, GluK2 and ADAR2
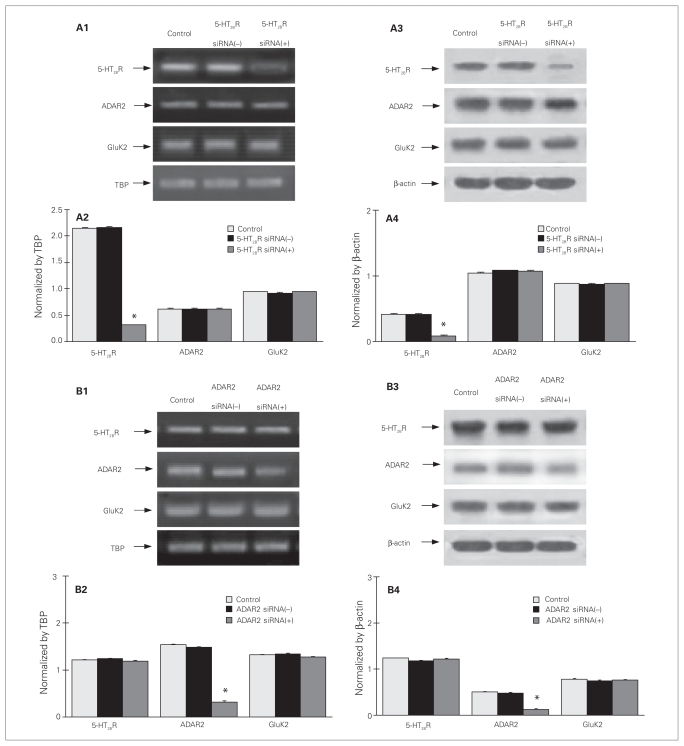
Duplexes of 5-HT2B receptor siRNA (sense 5′ gggaagcauuug-gcagguauu 3′ and antisense 5′ uaccugccaaaugcuucccuuu 3′)36 and ADAR2 siRNA (sense 5′ uacaugagugaucguggccuu 3′ and antisense 5′ ggccacgaucacucauguauu 3′)37 were synthesized by Sangon Co., Ltd. Transfection was performed as previously described.4 To allow incorporation of siRNAs into astrocytes, 3-week-old astrocytes cultured in Primaria 24-well culture plates were incubated in Dulbecco medium without serum for 24 hours on the day before transfection. Transfection solution contained 2 μL of oligofectamine and 40 μL of Opti-MEM I, and 2.5 μL of siRNA (666 ng) was added to the culture for 8 hours. In siRNA (−) control cultures, transfection solution without siRNA was added. Thereafter, 87.5 μL of DMEM with 37.5 μL serum was added to the cultures. Greatly reduced expression of mRNA and protein of the 5-HT2B receptor or ADAR2 without effect on the other genes studied was confirmed by Western blotting 3 days after transfection (Fig. 1), and the mRNA expression of ADAR2 was 100%, 70%, 20% and 20% of control samples in siRNA-treated cells after 8 hours, 1 day, 3 days and 7 days of treatment, respectively (Appendix 1, available at www.cma.ca/jpn). The corresponding effect of siRNA against GluK2 has previously been described and documented.38
Fig. 1.
Knock-down of (A) 5-HT2B receptor (5-HT2BR) and (B) ADAR2. (A) Cultured astrocytes were either untreated controls or treated with transfection solution without siRNA (siRNA (−)), siRNA specific to 5-HT2BR (siRNA (+)), (B) or specific to ADAR2 (siRNA (+)). Three days later, cells were collected for detection of mRNA and protein expression of 5-HT2BR, ADAR2 and GluK2. (A1 and B1) Southern blot from a representative experiment. Similar results were obtained from 3 independent experiments. (A2 and B2) Average mRNA expression was quantitated as ratios between 5-HT2BR, ADAR2 or GluK2, and TBP. (A3 and B3) Immunoblot from a representative experiment. Bands of 55, 80, 115 and 46 kDa represent 5-HT2BR, ADAR2, GluK2 and β-actin, used for housekeeping, respectively. Similar results were obtained from 3 independent experiments. (A4 and B4) Average protein expression was quantitated as ratios between 5-HT2BR, ADAR2 or GluK2, and β-actin. Standard error of the mean values are indicated by vertical bars. *Statistically significant (p < 0.05) difference between control and siRNA (−) groups.
Experiments in vivo
Male adult CD-1 mice were injected intraperitoneally with a daily dose of fluoxetine (10 mg/kg dissolved in PBS) or PBS for either 3 days or 1 week. They were then decapitated, and cerebral hemispheres minus olfactory bulbs and hippocampi were removed to determine mRNA and protein expression.
Western blotting for ERK, GluK2, 5-HT2B receptor and ADAR2
Samples containing 50 μg of protein were applied on slab gels of 12% polyacrylamide. After transfer to nitrocellulose membranes, the samples were blocked by 5% skimmed milk powder in tris-buffered saline with Tween 20 (TBS-T) for 2 hours, and the nitrocellulose membranes were incubated with the first antibody, specific to either p-ERK or ERK, GluK2, 5-HT2B receptor, ADAR2 or β-actin, used for house-keeping, for 1.5 hours at room temperature. After washing, specific binding was detected by goat anti-mouse or goat anti-rabbit horseradish peroxidase-conjugated secondary antibody. We determined ratios between scanned p-ERK1/2 and scanned ERK1/2, or between scanned GluK2, 5-HT2B receptor or ADAR2 protein and β-actin.
Reverse transcription–polymerase chain reaction
For determination by reverse transcription–polymerase chain reaction (RT-PCR) of mRNA expression of the 5-HT2B receptor, ADAR2, or kainate receptor subtypes, including I/V, Y/C and Q/R edited GluK2, a cell suspension was prepared by discarding the culture medium, adding Trizol to cultures on ice and scraping the cells off the culture dish. The RNA pellet was precipitated with isopropanol, washed with 70% ethanol and dissolved in 10 μL of sterile, distilled water, and an aliquot was used to determine the amount of RNA.5
Reverse transcription was initiated by a 5-minute incubation at 65°C of 1 μg of RNA extract with random hexamer at a final concentration of 12.5 ng/L and deoxyribonucleoside triphosphates (TaKaRa Biotechnology Co.) at a final concentration of 0.5 mM. The mixture was rapidly chilled on ice and briefly spun, and 4 μL of 5X first-strand buffer, 2 μL of 0.1 M dithiotreitol and 1 μL of RNaseOUT Recombinant Ribonuclease Inhibitor (40 U/μL) were added. After the mixture had been incubated at 42°C for 2 minutes, 1 μL (200 U) of SuperScript II (Life Technologies, Invitrogen) was added, and the incubation at 42°C continued for another 50 minutes. Subsequently, the reaction was inactivated by heating to 70°C for 15 minutes, and the mixture was chilled and briefly centrifuged.
Polymerase chain reaction amplification was performed in a RoboCycler thermocycler with 0.2 μM of forward and reverse nucleotide sequences (primers), as indicated in Table 1, and 0.375 units of Taq polymerase. The primer pairs used for quantification of editing at I/V, Y/C and Q/R sites by amplification refractory mutation system (ARMS)-PCR were designed so that the 3′-terminal nucleotide was specific for either adenosine (A) or guanosine (G).44 When the primer matched the template, amplification occurred normally. However, when the primer mismatched the template, the frequency of extension was very low because Taq polymerase lacks 3′ exonucleotidase activity; consequently, the effective number of sequence copies available for amplification was greatly reduced.45,46 In contrast, use of a reverse primer, which contained the appropriate complementary nucleotide sequence, allowed normal amplification. Use of the GluK2 primers allowed amplification of all forms, regardless of whether they were edited or not.
Table 1.
Primer sequences used for reverse transcription (RT-PCR) and amplification refractory mutation system polymerase chain reaction (ARMS-PCR)
| Method; site | Primer | Primer sequence | Size, bp | |
|---|---|---|---|---|
| RT-PCR | ||||
| GluK139 | Forward | 5′ GCTACATCCTCCCTCAGACCTC 3′ | 403 | |
| Reverse | 5′ AGATTATGCAGCTATCAGCAGGGC 3′ | |||
| GluK339 | Forward | 5′ TGGAACCCTACCGCTACTCG 3′ | 200 | |
| Reverse | 5′ TGCGACGCTCGCTGGTAGCA 3′ | |||
| GluK439 | Forward | 5′ ATGCCCCGTGTCTCTGCTCCT 3′ | 377 | |
| Reverse | 5′ TCTGGAGTTGGAACCTGACAAA 3′ | |||
| GluK539 | Forward | 5′ ATGCCGGCTGAGCTGCTGCTG 3′ | 210 | |
| Reverse | 5′ TGCAGCTCAAAGATGTC 3′ | |||
| ADAR140 | Forward | 5′ GCTCTAGAGTTCCAGTACTGTGTAGCA 3′ | 325 | |
| Reverse | 5′ ATGCGAATTCGGATCCTTGGGTTCGTGA 3′ | |||
| ADAR241 | Forward | 5′ CGCTTGCTATTTTAGTGCTGCGG 3′ | 203 | |
| Reverse | 5′ GCGGTTTTCTTTAACATCAGTGC 3′ | |||
| 5-HT2BR5 | Forward | 5′ CTCGGGGGTGAATCCTCTGA 3′ | 370 | |
| Reverse | 5′ CCTGCTCATCACCCTCTCTCA 3′ | |||
| ADAR2Lc and ADAR2Sc 42 | Forward | 5′ CCTCAAAAGTGTTTTACCATGG 3′ | 269 | |
| Reverse | 5′ TCTTCAGGCGGTACTTGGAGT 3′ | 223 | ||
| ADAR2Ld and ADAR2Sd42 | Forward | 5′ GTCCATATTTCAGAAGTCAG 3′ | 316 | |
| Reverse | 5′ GTCACTGCAGGACATGGT 3′ | 286 | ||
| TBP43 | Forward | 5′ CCACGGACAACTGCGTTGAT 3′ | 236 | |
| Reverse | 5′ GGCTCATAGCTACTGAACTG 3′ | |||
| ARMS-PCR | ||||
| GluK2 | I | Forward (I) | 5′ ATCTGGATGTATA 3′ | 246 |
| Reverse (GluK2) | 5′ TGTGAAAAACCACCAAATGC 3′ | |||
| (I/V) | V | Forward (GluK2) | 5′ GCAAGCCCAATGGTACAAAC 3′ | 83 |
| Reverse (V) | 5′ AGCCAGCAGAAC 3′ | |||
| GluK2 | Y | Forward (Y) | 5′ TCTGCTGGCTTA 3′ | 232 |
| Reverse (GluK2) | 5′ TGTGAAAAACCACCAAATGC 3′ | |||
| (Y/C) | C | Forward (GluK2) | 5′ GCAAGCCCAATGGTACAAAC 3′ | 94 |
| Reverse (C) | 5′ ACACCCAAGC 3′ | |||
| GluK2 | Q | Forward (GluK2) | 5′ GCAAGCCCAATGGTACAAAC 3′ | 246 |
| Reverse (Q) | 5′ CAGAACCTTGCT 3′ | |||
| (Q/R) | R | Forward (R) | 5′ AGCTCTCATGCG 3′ | 82 |
| Reverse (GluK2) | 5′ TGTGAAAAACCACCAAATGC 3′ | |||
| GluK2 | Forward | 5′ GCAAGCCCAATGGTACAAAC 3′ | 305 | |
| Reverse | 5′ TGTGAAAAACCACCAAATGC 3′ | |||
Initially, the template was denatured by heating to 94°C for 2 minutes. This was followed by 30 amplification cycles for GluK2, GluK3, GluK4, GluK5 and TATA-binding protein (TBP); by 40 cycles for GluK1, ADAR2Sd, ADAR2Ld, ADAR2Sc and ADAR2Lc; and by 35 cycles for I/V, Y/C and Q/R nucleotide sequences of GluK2. Each cycle consisted of 3 periods. The first period was for 45 seconds at 94°C. The second was for 45 seconds at 50°C for GluK3; at 58°C for GluK1, GluK4 and GluK5; at 62°C for GluK2 and I/V, Y/C and Q/R editing of GluK2; and at 55°C for ADAR2Sd, ADAR2Ld, ADAR2Sc, ADAR2Lc and TBP. The third was for 90 seconds at 72°C. The final step was extension at 72°C for 10 minutes. The PCR products were separated by 1% agarose gel electrophoresis and captured by FluorChem 5500 (Alpha Innotech Corporation).
Direct PCR sequencing
Polymerase chain reaction amplification was performed with forward and reverse primers for GluK2, as indicated in Table 1. Complementaty DNA sequencing was carried out by TaKaRa Biotechnology Co., Ltd., and RNA editing efficiencies at I/V, Y/C and Q/R sites were calculated by the peak heights.47
Data analysis
We analyzed the differences between multiple groups by 1-way analysis of variance (ANOVA) followed by a Fisher least significant difference multiple comparison test for unequal replications. The level of significance was set at p < 0.05. Least significant difference tests were applied to determine statistical differences in paired groups within multiple experimental groups. We also analyzed the data with a priori contrasts, an analysis without experience hypothesis, and the results were not different after post hoc comparison (results not shown). In the calibration curves, we plotted the scanned intensities as fractions of maximum intensity (ARMS-PCR) or determined ratios between edited and edited plus unedited GluK2 (direct cDNA sequencing) as a function of the ratios in the standards. Correlation analysis was used for the calibration curves of standard samples.
Results
Chronic effect of fluoxetine on mRNA expression of ADAR1 and ADAR2 in cultured astrocytes and intact brains
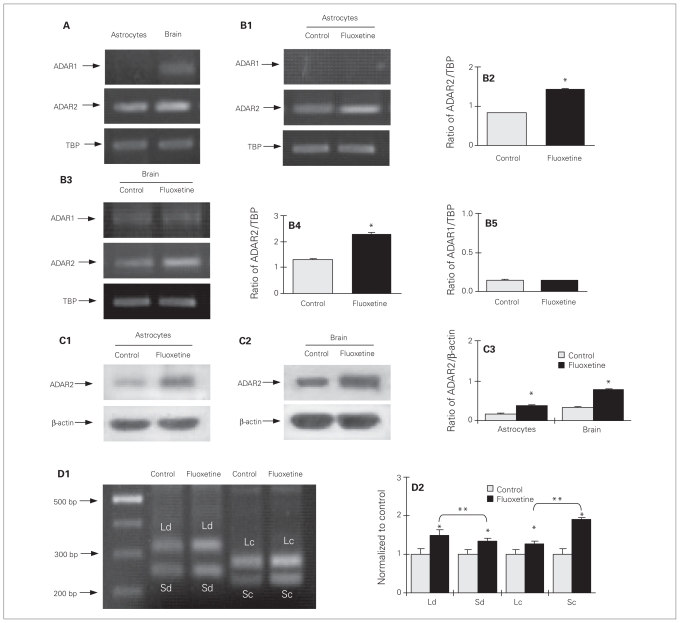
As shown in Figure 2A, astrocytes express little or no ADAR1 mRNA. The level of ADAR2 mRNA, normalized to TBP, was slightly higher in cerebral tissue than in cultured astrocytes (F1,4 = 522.2, p = 0.008 ). After 3 days of treatment with 10 μM of fluoxetine, the mRNA expression of ADAR2 was increased to 50% above that in the control group (p = 0.002) in the cultured astrocytes (F1,4 = 522.2, p = 0.001; Fig. 2B1, B2). A similar response was seen in the intact brain after 7 days of treatment (F1,4 = 203.8, p = 0.025; Fig. 2B3, B4), but there was no difference in ADAR1 expression between the treatment and control groups (F1,4 = 0.0, p = 0.89; Fig. 2B5). Protein expression of ADAR2 in fluoxetine-treated cells and in the intact brain after fluoxetine treatment was double that in the control group (F3,10 = 427.4, p = 0.001; Fig. 2C1, C2, C3). The ADAR2 splicing analysis showed that chronic treatment with fluoxetine for 3 days increased expression of all 4 isoforms in the cultured astrocytes (Fig. 2D1). When the levels of ADAR2 mRNA in treated cells were normalized to control samples (Fig. 2D2), the stimulation was higher in ADAR2Ld and ADAR2Sc than in ADAR2Sd and ADAR2Lc (F3,8 = 246.5, p = 0.001 and F3,8 = 635.6, p = 0.001, respectively).
Fig. 2.
Upregulation of mRNA and protein expression of ADAR2 by chronic treatment with fluoxetine in cultured astrocytes and intact brains of adult mice. (A) ADAR1 and ADAR2 mRNA expression in cultured astrocytes or the intact brains of adult mice with TATA-binding protein (TBP), used as a house-keeping gene. The sizes of the polymerase chain reaction (PCR) products are listed in Table 1. (A, B1) Southern blots from a representative experiment. Similar results were obtained from 3 independent experiments. (B1–2) Cells were either untreated controls or treated with 10 μM of fluoxetine for 3 days. (B1) Southern blot from a representative experiment. Similar results were obtained from 3 independent experiments. (B2) Average mRNA expression was quantitated as ratios between ADAR2 and TBP. (B3–5) Adult mice were either untreated controls or treated with fluoxetine (10 mg/kg/d) for 7 days. (B3) Southern blot from a representative experiment. Similar results were obtained from 3 independent experiments. (B4) Average mRNA expression was quantitated as ratios between ADAR2 and TBP, used for housekeeping, (B5) or between ADAR1 and TBP. (C1) Cells were either untreated controls or treated with 10 μM of fluoxetine for 3 days; (C2) adult mice were either untreated or treated with fluoxetine (10 mg/kg/d) for 7 days. (C1 and C2) Immunoblot from a representative experiment. Bands of 80 and 46 kDa represent ADAR2 and β-actin, respectively. Similar results were obtained from (C1) 3 or (C2) 4 independent experiments. (C3) Average protein expression was quantitated as ratios between ADAR2 and β-actin. (D) Messenger RNA expression of ADAR2Sd, ADAR2Ld, ADAR2Sc and ADAR2Lc in cultured astrocytes. Cells were either untreated controls or treated with 10 μM of fluoxetine for 3 days. (D1) Southern blot from a representative experiment. The sizes of the PCR product are listed in Table 1. Similar results were obtained from 3 independent experiments. Average mRNA expression was normalized in relation to the control group in the same experiment, assigned a value of 1 (D2). Standard error of the mean (SEM) values are indicated by vertical bars. *Statistically significant (p < 0.05) difference between control and treatment groups. **Statistically significant (p < 0.05) difference between 2 groups.
Chronic effect of fluoxetine on gene expression of kainate receptor subunits in cultured astrocytes and intact brains
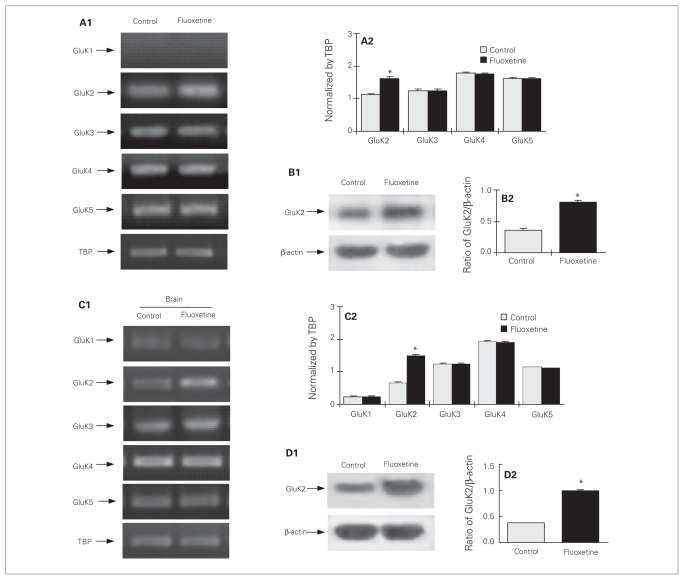
In agreement with previous observations,38 cultured astrocytes expressed GluK2, GluK3, GluK4 and GluK5 mRNA, but not GluK1 (Fig. 3A1, A2), which was also not expressed in the astrocytic cell fraction obtained by fluorescence-activated cell sorting.18 Only GluK2 mRNA was upregulated by chronic treatment with fluoxetine (F7,16 = 50.6, p = 0.005). Protein expression of GluK2 was also increased in fluoxetine-treated cells (F1,4 = 150.9, p = 0.007; Fig. 3B1, B2). The increase of GluK2 protein was more pronounced than that of GluK2 mRNA. In vivo, fluoxetine also enhanced GluK2 mRNA (F9,30 = 2148.4, p = 0.002; Fig. 3C1, C2) and protein (F1,6 = 720.2, p = 0.003; Fig. 3D1, D2) expression after 7 days of treatment, but not after 3 days of treatment (results not shown), which probably reflects that the fluoxetine concentration obtained in the brain may have been lower than that to which the cultured astrocytes were exposed.
Fig. 3.
Upregulation of mRNA and protein expression of GluK2 by chronic treatment with fluoxetine in (A–B) cultured astrocytes (C–D) and in the intact brain. (A) Cells were either untreated controls or treated with 10 μM of fluoxetine for 3 days. (A1) Southern blot from a representative experiment. Similar results were obtained from 3 independent experiments. (A2) Average mRNA expression was quantitated as ratios between GluK2, GluK3, GluK4 or GluK5 and TATA-binding protein (TBP). (B1) Immunoblot from a representative experiment. Bands of 115 and 46 kDa represent GluK2 and β-actin, respectively. Similar results were obtained from 3 independent experiments. (B2) Average protein expression was quantitated as ratios between GluK2 and β-actin. (C) Adult mice were either untreated controls or treated with fluoxetine (10 mg/kg/d) for 7 days. (C1) Southern blot from a representative experiment. Similar results were obtained from 4 independent experiments. (C2) Average mRNA expression was quantitated as ratios between GluK1, GluK2, GluK3, GluK4 or GluK5 and TBP. (D1) Immunoblot from a representative experiment. Similar results were obtained from 4 independent experiments. (D2) Average protein expression was quantitated as ratios between GluK2 and β-actin. Standard error of the mean values are indicated by vertical bars. *Statistically significant (p < 0.05) difference between treatment and control groups.
Chronic efffect of fluoxetine on glutamate-induced ERK phosphorylation and increase of intracellular Ca2+ concentration in cultured astrocytes
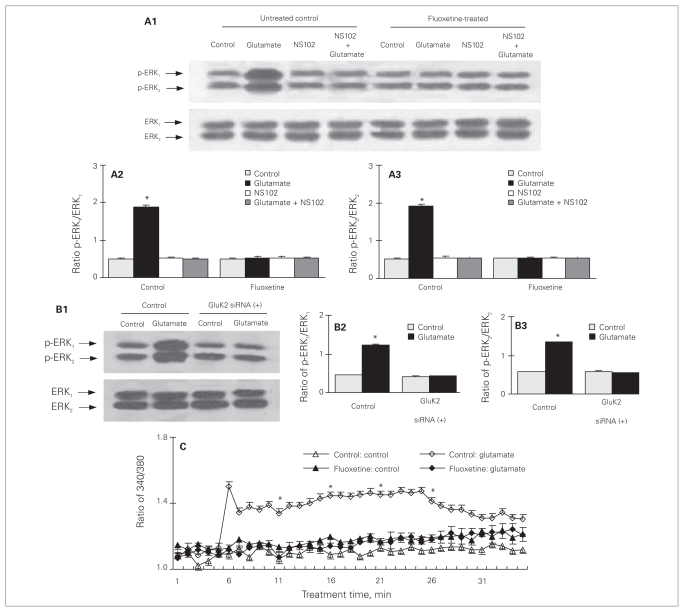
As previously reported,38 20 minutes of treatment with 100 μM of glutamate induced an increase of ERK1/2 phosphorylation in cultured astrocytes with no difference in total amount of ERK1/2 (p-ERK1: F7,16 = 189.0, p = 0.011; p-ERK2: F7,16 = 294.3, p = 0.002; Fig. 4A1, A2, A3). Chronic treatment with fluoxetine for 3 days eliminated the effect on ERK1/2 phosphorylation by glutamate (Fig. 4A1, A2, A3). The fact that the glutamate effect in untreated cultures was induced by GluK2 receptor activity was confirmed both by an inhibitory effect of NS102, a specific antagonist of GluK1 and GluK248 (Fig. 4A1, A2, A3), and by the ability of knockdown of expression of GluK2 mRNA and protein by 3 days of treatment with specific siRNA38 to abolish the effect of glutamate (p-ERK1: F3,8 = 45.0, p = 0.023; p-ERK2: F3,8 = 140.2, p = 0.002; Fig. 4B1, B2, B3). The transfection solution alone without siRNA had no effect (results not shown). As shown in Figure 4C, treatment with 100 μM of glutamate also caused a significant increase of intracellular Ca2+ concentration ([Ca2+]i), amounting to a significant 3- to 4-fold increase from the control value, lasting for at least 25 minutes. Three days of treatment with fluoxetine also abolished this effect of glutamate (5 min after drug treatment: F3,12 = 33.9, p = 0.002).
Fig. 4.
Downregulation of glutamate-induced extracellular signal-regulated kinase (ERK1/2) phosphorylation and increase in [Ca2+]i by chronic treatment with fluoxetine in cultured astrocytes. (A) Cells were untreated controls or treated with 10 μM of fluoxetine for 3 days. Thereafter, cells were incubated for 20 minutes in the absence of any drug (control) or in the presence of 100 μM of glutamate; 10 μM of NS102, an inhibitor of GluK1 and GluK2; or of glutamate plus NS 102. (A1) Immunoblot from a representative experiment. Bands of 44 and 42 kDa represent phosphorylated ERK (p-ERK1) and p-ERK2, respectively or total ERK1 and ERK2. Similar results were obtained from 3 independent experiments. (A2) Average ERK phosphorylation was quantitated as ratios between p-ERK1 and ERK1 (A3) and between p-ERK2 and ERK2. *Statistically significant (p < 0.05) difference from other groups for ERK1 and ERK2. (B) Cells were treated with transfection solution without siRNA (siRNA (−) [control]) or with siRNA specific to GluK2 receptor (siRNA (+)). Three days later, cells were incubated for 20 minutes in serum-free medium in the absence of any drug (control) or in the presence of 100 μM of glutamate. (B1) Immunoblot from a representative experiment. Similar results were obtained from 3 independent experiments. (B2) Average ERK phosphorylation was quantitated as ratios between p-ERK1 and ERK1 (B3) and between p-ERK2 and ERK2. *Statistically significant (p < 0.05) difference from all other groups for ERK1 and ERK2. (C) Downregulation of glutamate-induced increase of intracellular Ca2+ concentration by chronic treatment with fluoxetine in cultured astrocytes. Cells were untreated controls (open triangles or diamonds) or treated with 10 μM of fluoxetine (solid triangles or diamonds) for 3 days. After the cells had been loaded with fura-2, they were incubated for 2 minutes in 300 μL of saline solution in the absence of any drug. Subsequently, either 30 μL of saline solution (triangles) or 30 μL of glutamate solution (diamonds) to a final concentration of 100 μM was added to each well, and the incubation was continued for another 30 minutes. Fluorescence was recorded at 60-second intervals. Means and standard error of the means were calculated for 4 individual experiments from the fluorescence ratios 0, 5, 10, 15 and 20 minutes after addition of 30 μL of saline or glutamate solution. *Statistically significant (p < 0.05) difference from all other groups at the same recording time. Standard error of the mean values are indicated by vertical bars.
Effect of 5-HT2B receptor siRNA on chronic effects of fluoxetine in cultured astrocytes
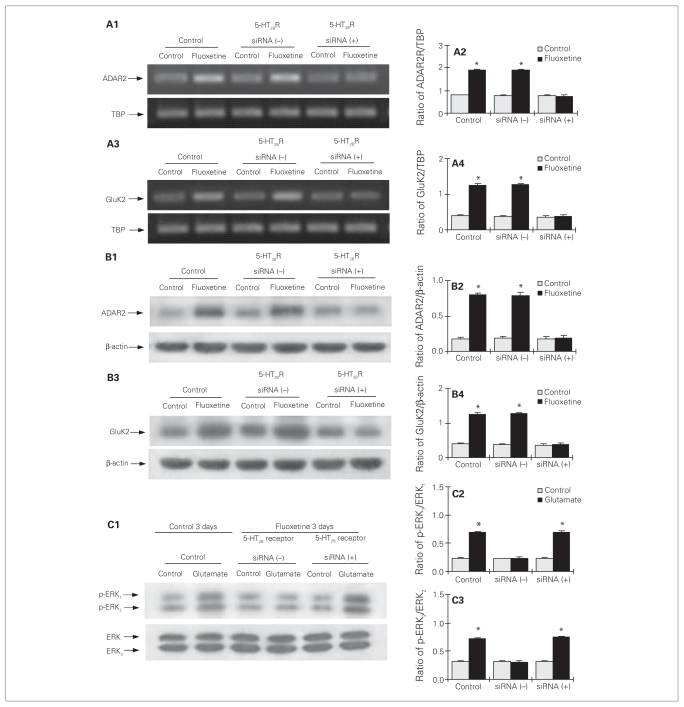
In cultured astrocytes with knock-down of the 5-HT2B receptor (Fig. 1), chronic treatment with fluoxetine for 3 days had lost its effect on mRNA (Fig. 5A1–4) and protein (Fig. 5B1–4) expression of ADAR2 (Fig. 5A1, A2, B1, B2; mRNA of ADAR2: F5,12 = 283.7, p = 0.012; protein of ADAR2: F5,12 = 217.3, p = 0.001). Similarly, treatment for 3 days also abolished its effect on mRNA and protein expression of GluK2 (Fig. 5A3, A4, B3, B4; mRNA of GluK2: F5,12 = 119.0, p = 0.003; protein of GluK2: F5,12 = 127.6, p = 0.002). Furthermore, in the cells treated with 5-HT2B receptor siRNA, the downregulation of glutamate-induced ERK1/2 phosphorylation by chronic treatment with fluoxetine was abolished (p-ERK1: F5,12 = 236.7, p = 0.003; p-ERK2: F5,12 = 237.7, p = 0.001; Fig. 5C1, C2, C3).
Fig. 5.
Upregulation of gene expression of ADAR2 and GluK2, and downregulation of glutamate-induced extracellular signal-regulated kinase (ERK) phosphorylation by chronic treatment with fluoxetine requires serotonin 2B receptor (5-HT2BR) activity in cultured astrocytes. Cells were either untreated controls or treated with transfection solution either without siRNA (siRNA (−)), or with siRNA specific to 5-HT2BR (siRNA (+)). Three days later, the cells were either untreated or treated with 10 μM of fluoxetine for 3 days. (A1 and A3) Southern blots from representative experiments. Similar results were obtained from 3 independent experiments. Average mRNA expression was quantitated as ratios between (A2) ADAR2 or (A4) GluK2 and TBP. (B1 and B3) Immunoblots from representative experiments. Similar results were obtained from 3 independent experiments. Average protein expression was quantitated as ratios between (B2) ADAR2 or (B4) GluK2 and β-actin. (C) Cells were incubated for 20 minutes in serum-free medium in the absence of any drug (control) or in the presence of 100 μM of glutamate. (C1) Immunoblot from a representative experiment. Similar results were obtained from 3 independent experiments. (C2) Average ERK phosphorylation was quantitated as ratios between phosphorylated ERK (p-ERK1) and ERK1 (C3) and between p-ERK2 and ERK2. Standard error of the mean values are indicated by vertical bars. *Statistically significant (p < 0.05) difference from all other groups for ERK1 and ERK2.
The magnitude of the response to glutamate in control cells and fluoxetine-exposed cells that had been treated with 5-HT2B receptor siRNA were virtually identical.
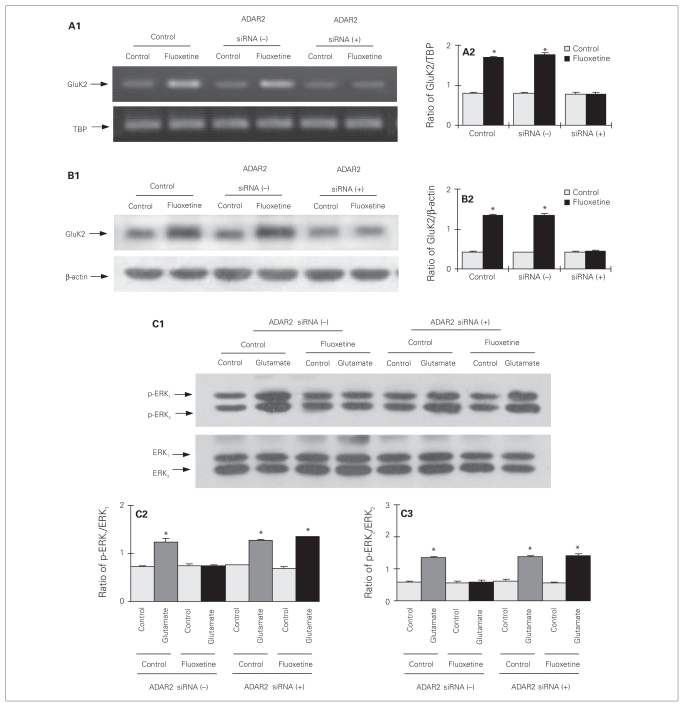
Effect of ADAR2 siRNA on chronic effects of fluoxetine in cultured astrocytes
In cultured astrocytes with ADAR2 knock-down (Fig. 1), chronic treatment with fluoxetine had lost its effect on mRNA (F5,12 = 283.7, p = 0.002; Fig. 6A1, A2) and protein (F5,12 = 119.0, p = 0.015; Fig. 6B1, B2) expression of GluK2. Furthermore, in the cells with reduced ADAR2 expression, the downregulation of glutamate-induced ERK1/2 phosphorylation by chronic treatment with fluoxetine was abolished (p-ERK1: F7,16 = 48.3, p = 0.003; p-ERK2: F7,16 = 177.6, p = 0.001; Fig. 6C1, C2, C3). The magnitude of the response to glutamate in control cells and in fluoxetine-exposed cells that had been treated with ADAR2 siRNA were similar.
Fig. 6.
Upregulation of GluK2 expression and downregulation of glutamate-induced extracellular signal-regulated kinase (ERK) phosphorylation by chronic treatment with fluoxetine require ADAR2 activity in cultured astrocytes. Cells were either untreated controls or treated with transfection solution without siRNA (siRNA (−)), or with siRNA specific to ADAR2 (siRNA (+)). Three days later, the cells were either untreated or treated with 10 μM of fluoxetine for 3 days. (A1) Southern blot from a representative experiment. Similar results were obtained from 3 independent experiments. (A2) Average mRNA expression was quantitated as ratios between GluK2 and TBP. (B1) Immunoblot from a representative experiment. Similar results were obtained from 3 independent experiments. (B2) Average protein expression was quantitated as ratios between GluK2 and β-actin. (C) Cells were incubated for 20 minutes in serum-free medium in the absence of any drug (control) or in the presence of 100 μM of glutamate. (C1) Immunoblot from a representative experiment. Similar results were obtained from 3 independent experiments. (C2) Average ERK phosphorylation was quantitated as ratios between phosphorylated ERK (p-ERK1) and ERK1 (C3) and between p-ERK2 and ERK2. Standard error of the mean values are indicated by vertical bars. *Statistically significant (p < 0.05) difference between treatment and control groups.
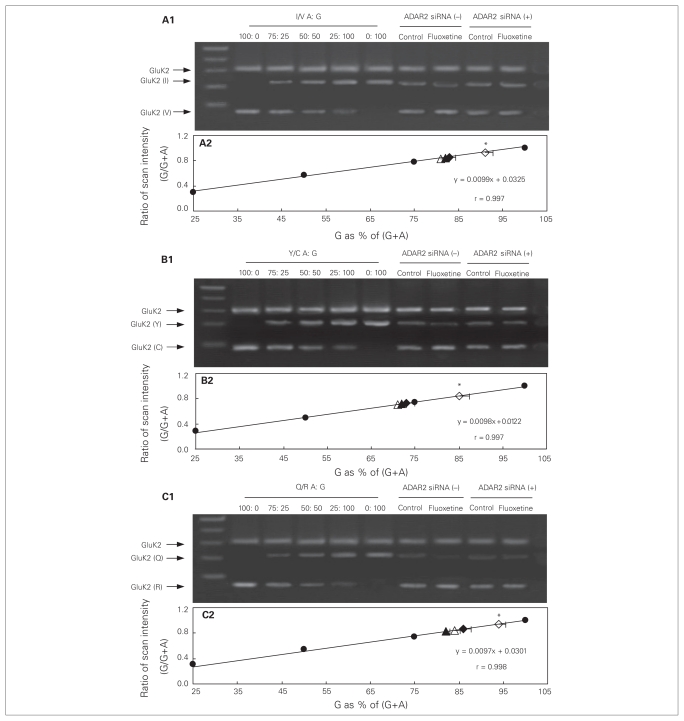
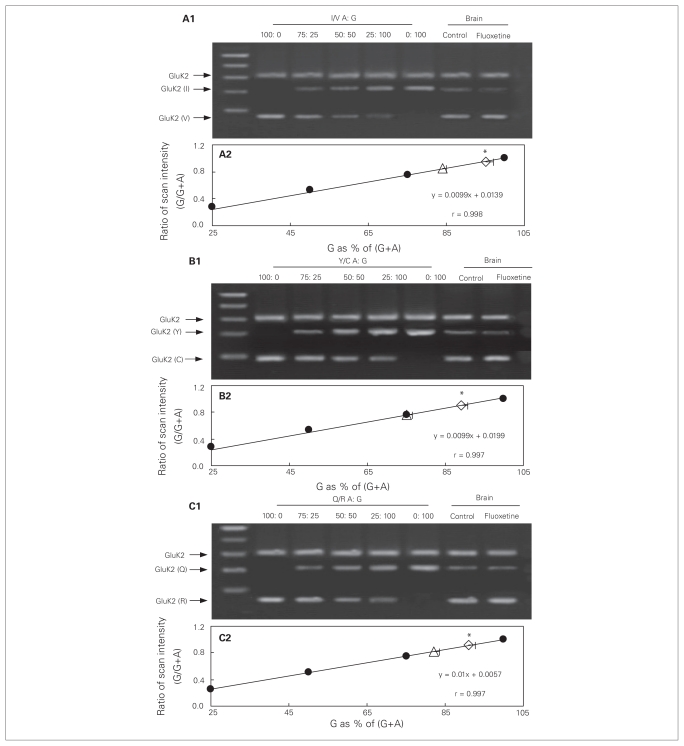
GluK2 RNA editing
Editing levels of the GluK2 receptor were quantified by comparison of the PCR products obtained from cultured astrocytes or an intact brain (right part of the gels in Figs. 7 and 8A1 [I/V], B1 [Y/C] and C1 [Q/R]) with calibration curves (solid circles in Figs. 7 and 8A2, B2 and C2). The calibration curves had been generated using PCR products of GluK2 in standard mixtures, with the following ratios between edited (G) and the sum of edited and unedited GluK2 (G + A) RNA: 100:0 (all edited), 75:25, 50:50, 25:75 and 0:100 (all unedited). We scanned the band intensities of these standard mixtures (left part of the gels in Fig. 7 and 8A1, B1 and C1) and expressed them as fractions of maximum intensity. These fractions were then plotted as functions of the ratios between edited and the sum of edited plus unedited GluK2 (Fig. 7 and 8A2, B2 and C2). The ratios between the edited, G-containing (V, C and R) and unedited, A-containing (I, Y and Q) isoforms in the PCR products obtained from the cultured astrocytes under control conditions and in fluoxetine-treated cultures (Fig. 7A2, B2 and C2, open and closed triangles and diamonds) or obtained from an intact brain (Fig. 8A2, B2 and C2, open triangle and diamond) were then read from the respective calibration curves. In control cells (open triangles), the frequency of editing was about 80% at site I/V (Fig. 7A2), > 70% at site Y/C (Fig. 7B2) and about 85% at site Q/R (Fig. 7C2). However, in cells treated chronically with fluoxetine for 3 days (open diamonds), the frequency of editing was increased to > 90% at site I/V (F3,8 = 8.4, p = 0.021), > 85% at site Y/C (F3,8 = 17.8, p = 0.018) and > 90% at site Q/R (F3,8 = 17.6, p = 0.001), indicating additional fluoxetine-induced GluK2 RNA editing. Similar results of chronic fluoxetine treatment were also seen in the intact brain (I/V: F1,8 = 80.7, p = 0.015; Y/C: F1,8 = 108.9, p = 0.007; Q/R: F1,8 = 45.0, p = 0.005; Fig. 8). Although these changes may appear minor, they implied that the amount of unedited mRNA was reduced by about half. In cultured astrocytes where ADAR2 was down-regulated, there was no difference at the 3 sites in frequency of editing between the control group (solid triangles) and cells treated chronically with fluoxetine (solid diamonds; Fig. 7A2, B2 and C2), indicating a requirement for ADAR2 in fluoxetine-induced GluK2 editing.
Fig. 7.
Chronic treatment with fluoxetine induces GluK2 editing in cultured astrocytes, which requires ADAR2 activity. Cells were treated with transfection solution without siRNA (siRNA (−); open symbols) or with siRNA specific to ADAR2 (siRNA (+); solid symbols). Three days later, cells were either not further treated (triangles) or treated with 10 μM of fluoxetine (diamonds) for 3 days. Thereafter, cells were collected for amplification refractory mutation system polymerase chain reaction (ARMS-PCR) analysis of GluK2 editing at (A) I/V, (B) Y/C and (C) Q/R sites. Representative Southern blots showing edited (guanosine; G) and unedited (adenosine; A) PCR products at (A1) I/V, (B1) Y/C or (C1) Q/R sites. Similar results were obtained from 3 independent experiments. Scans of known mixtures of G and A (n = 3) were made to prepare calibration graphs showing scanning intensity (as a fraction of maximum intensity) as a function of G/(G+A) for each mixture (A2, B2 and C2; solid circles). Based on the calibration curves and scans of G/(A+G) (n = 3) under each condition the percentages of edited (G) GluK2 at each site was determined (A2, B2 and C2). Standard error of the mean values are indicated by horizontal bars. *Statistically significant (p < 0.05) difference from all other groups.
Fig. 8.
GluK2 editing induced by chronic treatment with fluoxetine in the intact brain. Adult mice were either not treated (triangles) or treated with fluoxetine (diamonds; 10 mg/kg/d) for 7 days. Thereafter, brains were removed for amplification refractory mutation system polymerase chain reaction (ARMS-PCR) analysis of GluK2 editing at (A) I/V, (B) Y/C and (C) Q/R sites. Representative Southern blots showing edited (guanosine; G) and unedited (adenosine; A) PCR products at (A1) I/V, (B1) Y/C or (C1) Q/R sites. Similar results were obtained from 5 animals in each group. Scans of known mixtures of G and A (n = 4) were made to prepare calibration graphs showing scanning intensity as a function of G/(G+A) for each mixture (A2, B2 and C2; solid circles). Based on the calibration curves and scans of G/(A+G) (n = 4) under each condition the percentages of edited (G) GluK2 at each site was determined (A3, B3 and C3). Standard error of the mean values are indicated by horizontal bars. *Statistically significant (p < 0.05) difference between treatment and control groups.
Virtually identical results for editing under control conditions and after fluoxetine treatment were obtained by cDNA sequencing performed by TaKaRa Biotechnology Co., Ltd. (Table 2).
Table 2.
Effect of chronic treatment with fluoxetine on GluK2 editing at I/V, Y/C and Q/R sites determined by complementary DNA sequencing in cultured astrocytes or in the intact brain*
| Editing site; mean (SEM)
|
||||||
|---|---|---|---|---|---|---|
| I/V r = 0.974 |
Y/C r = 0.962 |
Q/R r = 0.989 |
||||
| Treatment | Astrocytes | Brain | Astrocytes | Brain | Astrocytes | Brain |
| Control | 83 (0.7) | 84 (0.4) | 79 (0.2) | 81 (0.2) | 81 (0.8) | 80 (0.8) |
| Fluoxetine | 90 (0.5)† | 92 (0.2)† | 90 (0.7)† | 91 (0.7)† | 91 (0.4)† | 92 (0.4)† |
SEM = standard error of the mean.
Cultured astrocytes were treated with 0 (control) or 10 μM of fluoxetine for 3 days (n = 3). Adult mice were either not treated (control) or treated with fluoxetine (10 mg/kg/d) for 7 days (n = 5).
Statistically significant (p < 0.05) difference between treatment and control groups for cultured astrocytes (I/V: F1,4 = 5.2, p = 0.002; Y/C: F1,4 = 10.0, p = 0.001; Q/R: F1,4 = 10.5, p = 0.011) and in the intact brain (I/V: F1,8 = 45.7, p = 0.005; Y/C: F1,8 = 83.3, p = 0.002; Q/R: F1,8 = 14.9, p = 0.003).
Summary of interactions between the 5-HT2B receptor, ADAR2 and GluK2 expression and editing
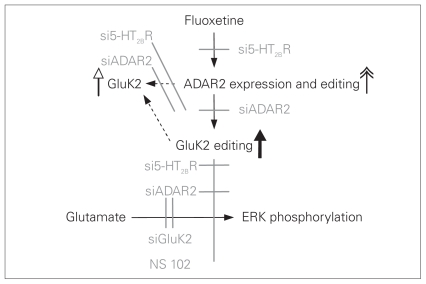
As illustrated in Figure 9, chronic administration of fluoxetine, a specific 5-HT2B agonist, resulted in upregulation of mRNA and protein expression of GluK2 in cultured and in vivo astrocytes (Fig. 9, white arrowhead). This treatment also upregulated ADAR2 (Fig. 9, double arrowhead), an adenosine deaminase that acts on RNA, catalyzing mRNA editing. In the cultured cells, the upregulation of GluK2 was prevented by siRNA directed either against the 5-HT2B receptor or ADAR2 (Fig. 9, grey lines). Under control conditions, editing of all 3 GluK2 editing sites was extensive both in cultured astrocytes and in intact brains, and editing was further enhanced by treatment with fluoxetine (Fig. 9, bold arrow). In the cultured astrocytes, the enhanced editing was abolished by 5-HT2B receptor or ADAR2 siRNA (Fig. 9, grey lines). The increased editing resulted in abolishment of glutamate-mediated increases in [Ca2+]i and phosphorylation of ERK1/2 in cultured astrocytes. The abolished effect of glutamate on ERK1/2 induced by fluoxetine and the enhanced editing were both prevented by knock-down of the 5-HT2B receptor or ADAR2 with siRNAs, suggesting important interactions between fluoxetine, astrocytic 5-HT2B and GluK2 receptors, GluK2 receptor editing and glutamatergic brain function.
Fig. 9.
Scheme indicating effects of fluoxetine on expression of ADAR2 and GluK2, GluK2 and ADAR2 editing and inhibition of glutamate-induced extracellular signal-regulated kinase (ERK) phosphorylation in cultured astrocytes in mice. Stimulatory effects are indicated by the double arrowhead, and inhibitory effects are indicated in grey. Fluoxetine increases mRNA and protein expression of ADAR2 (double arrowhead), an effect that is prevented (grey lines) by small interfering RNA (siRNA) against the 5-HT2B receptor (si5-HT2BR). Fluoxetine also increases mRNA and protein expression of GluK2 (white arrowhead), an effect that is inhibited by both si5-HT2BR and siADAR2 (grey lines), indicating that it is dependent upon ADAR upregulation and perhaps also ADAR2-mediated up-regulation of GluK2 editing (bold arrow), which also is inhibited by siADAR2 (the experiments cannot distinguish between these 2 possibilities, indicated by the 2 dotted arrows). As a result of the editing of glutamate-mediated GluK2, GluK2-dependent ERK phosphorylation is inhibited, an effect that is abolished by si5-HT2BR or siADAR2, which prevent fluoxetine-induced GluK2 editing.
Discussion
The present study has shown that chronic treatment with fluoxetine upregulates the expression and editing of GluK2, and that the increased editing results in abolishment of the ability of 100 μM of glutamate to increase [Ca2+]i and phosphorylation of ERK1/2, a process known to depend on increased [Ca2+]i.4 It has been reported that activation of metabotropic glutamate receptors increases [Ca2+] in astrocytes,49,50 and it cannot be excluded that simultaneous activation of a metabotropic glutamate receptor and GluK2 is required for response. The abolishment of this response in cultured astrocytes treated with fluoxetine demonstrated in the present study does not equal receptor downregulation, since Na+ permeability is likely to be maintained and probably even enhanced owing to the upregulation of GluK2 expression.
The increased editing in in vivo brain cortical tissue observed in our study is in disagreement with the results of Barbon and colleagues,51 who found a slight decrease in editing in cortical tissue; however, these authors studied the entire anterior pole of the brain, including white matter, they used rats, and their medication protocol was different. In addition, it has been reported that editing of other receptors was modulated both by the genetic background and the behavioural state of the animals.52 The reliability of our observation of increased editing is supported by the abolishment of glutamate effects on [Ca2+]i, since editing of the Q/R site is known to reduce Ca2+ permeability of GluK2.48,53,54 Moreover, it has been reported that a mutant mouse with greatly reduced Q/R site editing showed a kainate-induced increase in [Ca2+]i in cultured hippocampal neurons, whereas there was no such effect in corresponding neurons from wild-type animals.53
The extent of GluK2 editing of the Q/R site that we observed in the cerebral tissue of control animals (84%) was consistent with previously reported values of 80%–90% in rodent brains.51,55–57 The tendency toward slightly greater editing at the Q/R site than at the 2 other sites confirms the previous results of Barbon and colleagues.51 The editing in the cultured astrocytes was much more extensive than previously reported,57–59 perhaps reflecting the differentiation induced by dBcAMP.
The abolishment of fluoxetine effects on upregulation of gene expression of ADAR2 and GluK2 in cultured astrocytes with 5-HT2B receptor knockout was consistent with our previous findings that acute exposure to fluoxetine induced no ERK phosphorylation in cultured astrocytes treated with siRNA against the 5-HT2B receptor4 and that chronic exposure to fluoxetine failed to upregulate cPLA2 expression in such cells.2 The lack of upregulation of GluK2 expression both in ADAR-reduced cells and in 5-HT2B receptor–reduced cells suggests that the upregulation was a response to the editing and its functional consequences.
The observation that cultured astrocytes expressed ADAR2, but little ADAR1, and that chronic treatment with fluoxetine caused upregulation of ADAR2 expression, but had no effect on ADAR1 in either cultured or in vivo astrocytes suggests that the increase of ADAR2 activity may be the underlying mechanism of drug action on RNA editing of GluK2. The level of expression of ADAR2 does not correlate directly with the enzyme activity alone, but also depends on isoform distribution.13,14,60 We found that fluoxetine upregulated all 4 isoforms, although it upregulated Ld to a larger extent than the Sd isoform and Sc to a larger extent than the Lc isoform. High levels of expression of both the Sd and Sc isoforms indicate an increase of editing activity, at least of the GluK2 Q/R site in hypothalamic tuberomamillary neurons.14 Thus, fluoxetine not only upregulates the expression of ADAR2 but also enhances its editing activity. The signalling events linking 5-HT2B activation with upregulation of ADAR2 are unknown, but we have previously shown that upregulation of cPLA2 was dependent on metalloproteinase-mediated release of an epidermal growth factor (EGF) receptor agonist and subsequent stimulation of the EGF receptor by this agonist, leading to ERK1/2 activation and entry of p-ERK1/2 into the nucleus.4,38
The implication of GluK2 editing for brain function is largely unknown. However, although mice deficient in GluK2 expression do not necessarily behave like animals with abolished Ca2+ response due to increased editing, it may be of interest that GluK2-deficient transgenic mice (lacking both neuronal and astrocytic GluK2) show a large and statistically significant reduction in long-term fear memory and show that synaptic potentation is reduced.61 Conversely, in mutant animals deficient in Q/R site editing, Vissel and colleagues54 found that N-methyl-d-aspartate (NMDA)–independent long-term potentiation could be induced in the medial perforant path–dentate gyrus synapse, which was not possible in wild-type animals. The mutant mice were also more sensitive to kainate-induced seizures.54 Behavioural tests showed no significant abnormalities, but nonsignificant increases in the startle response to acoustic stimulation and decreases in open field activity might suggest increased anxiety or emotionality. Accordingly, blunted GluK2 receptor function may represent a decrease in excitatory brain activity, which might at least partly be due to an effect on astrocytic receptors.
There is substantial evidence of a decrease in astrocytic glutamate transporters in the frontal cortex62,63 and locus coeruleus64 associated with MDD. The localization to the locus coeruleus is important because astrocytes constitute an important target for the adrenergic locus coeruleus signalling65 and because abnormalities were also found in several astrocyte-specific locus coeruleus genes. There is also evidence that a decrease in NMDA-mediated signalling has an antidepressant effect,66 but, to our knowledge, the present study is the first to suggest a similar effect by reduction of kainate-mediated signalling and especially astrocytic kainate-mediated signalling. However, in view of the role of astrocytic Ca2+ transients for astrocytic modulation of neuronal activity,67,68 regulation of astrocytic Ca2+ responses by down-regulation of an astrocytic ionotropic receptor may yield a more selective response.
Limitations
Limitations of the study include the use of cells developing in cultures, which often differ from in vivo astrocytes.18 However, the routine administration of dBcAMP is intended to replace differentiating noradrenergic signals, which the cultured cells do not receive because they are harvested before locus coeruleus fibres reach the neopallium, and it makes the cultured astrocytes much more similar to their in vivo counterparts.34 In the cells treated with dBcAMP, the level of expression of the 5-HT2B receptor was increased,5 but the treatment had no effect on expression of ADAR2 and GluK2 (B. Li and L. Peng, unpublished results, 2010). Nevertheless, it remains a goal to confirm key aspects of this study in vivo with subsequent fluorescence-activated cell sorting to obtain freshly isolated astrocytes and neurons.
Conclusion
Chronic administration of fluoxetine resulted in upregulation of the expression of GluK2 in cultured astrocytes and the intact brain. This treatment also upregulated ADAR2, and the upregulation of GluK2 was prevented by siRNA directed either against the 5-HT2B receptor or ADAR2. Under control conditions, editing of all 3 GluK2 editing sites was extensive both in cultured astrocytes and the intact brain, and it was further enhanced by fluoxetine treatment. In the cultured astrocytes, the enhanced editing was abolished by the siRNA treatment. The increased editing resulted in abolishment of glutamate-mediated increases in [Ca2+]i and phosphorylation of ERK1/2. In addition, ADAR2 self-edited and thereby probably enhanced its enzymatic activity.
Acknowledgements
This study was supported by Grants No. 30670651, No. 30770667 and No. 30711120572 from the National Natural Science Foundation of China (L.P.).
Footnotes
Competing interests: As declared above.
Contributors: All authors contributed to study design, data acquisition and analysis, helped write and review the article and approved its publication.
References
- 1.Rao JS, Ertley RN, Lee HJ, et al. Chronic fluoxetine up-regulates activity, protein and mRNA levels of cytosolic phospholipase A2 in rat frontal cortex. Pharmacogenomics J. 2006;6:413–20. doi: 10.1038/sj.tpj.6500391. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Zhang S, Li M, et al. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA(2) expression secondary to 5-HT (2B)-induced, transactivation-mediated ERK (1/2) phosphorylation. Psychopharmacology (Berl) 2009a;207:1–12. doi: 10.1007/s00213-009-1631-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Li B, Lovatt D, et al. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol. 2010;6:113–25. doi: 10.1017/S1740925X10000141. [DOI] [PubMed] [Google Scholar]
- 4.Li B, Zhang S, Zhang H, et al. Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology (Berl) 2008;201:443–58. doi: 10.1007/s00213-008-1306-5. [DOI] [PubMed] [Google Scholar]
- 5.Kong EKC, Peng L, Chen Y, et al. Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem Res. 2002;27:113–20. doi: 10.1023/a:1014862808126. [DOI] [PubMed] [Google Scholar]
- 6.Yu PH, Hertz L. Differential expression of type A and type B monoamine oxidase of mouse astrocytes in primary cultures. J Neurochem. 1982;39:1492–5. doi: 10.1111/j.1471-4159.1982.tb12598.x. [DOI] [PubMed] [Google Scholar]
- 7.Yu PH, Hertz L. Type A and B monoamine oxidase in glial cells in long term culture. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:687–90. doi: 10.1016/0278-5846(83)90046-5. [DOI] [PubMed] [Google Scholar]
- 8.Barbon A, Popoli M, La Via L, et al. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–20. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–46. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valente L, Nishikura K. ADAR gene family and A-to-I RNA editing: diverse roles in posttranscriptional gene regulation. Prog Nucleic Acid Res Mol Biol. 2005;79:299–338. doi: 10.1016/S0079-6603(04)79006-6. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara Y, Ito K, Sun H, et al. Regulation of glutamate receptor RNA editing and ADAR mRNA expression in developing human normal and Down’s syndrome brains. Dev Brain Res. 2004;148:151–5. doi: 10.1016/j.devbrainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Köhr G, Melcher T, Seeburg PH. Candidate editases for GluR channels in single neurons of rat hippocampus and cerebellum. Neuropharmacology. 1998;37:1411–7. doi: 10.1016/s0028-3908(98)00149-x. [DOI] [PubMed] [Google Scholar]
- 13.Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- 14.Sergeeva OA, Amberger BT, Haas HL. Editing of AMPA and serotonin 2C receptors in individual central neurons, controlling wake-fulness. Cell Mol Neurobiol. 2007;27:669–80. doi: 10.1007/s10571-007-9153-1. [DOI] [PubMed] [Google Scholar]
- 15.Maas S, Patt S, Schrey M, et al. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci U S A. 2001;98:14687–92. doi: 10.1073/pnas.251531398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strutz-Seebohm N, Seebohm G, Shumilina E, et al. Glucocorticoid adrenal steroids and glucocorticoid-inducible kinase isoforms in the regulation of GluR6 expression. J Physiol. 2005;565:391–401. doi: 10.1113/jphysiol.2004.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brand-Schieber E, Lowery SL, Werner P. Select ionotropic glutamate AMPA/kainate receptors are expressed at the astrocyte-vessel interface. Brain Res. 2004;1007:178–82. doi: 10.1016/j.brainres.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarate CA, Jr, Payne JL, Quiroz J, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–4. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 20.Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 21.Machado-Vieira R, Salvadore G, Diazgranados N, et al. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–50. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–43. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 24.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–55. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. J Cancer Surviv. 2010;4:33–44. doi: 10.1007/s11764-009-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–8. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 27.Menke A, Lucae S, Kloiber S, et al. Genetic markers within glutamate receptors associated with antidepressant treatment-emergent suicidal ideation. Am J Psychiatry. 2008;165:917–8. doi: 10.1176/appi.ajp.2008.08020274. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Guo JJ, Steinbuch M, et al. Risk of neuroleptic malignant syndrome in patients with bipolar disorder: a retrospective, population-based case-control study. Int J Psychiatry Med. 2009;39:439–50. doi: 10.2190/PM.39.4.h. [DOI] [PubMed] [Google Scholar]
- 29.Beneyto M, Kristiansen LV, Oni-Orisan A, et al. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 30.Shaltiel G, Maeng S, Malkesman O, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–72. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malkesman O, Austin DR, Chen G, et al. Reverse translational strategies for developing animal models of bipolar disorder. Dis Model Mech. 2009;2:238–45. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertz L, Schousboe A, Boechler N, et al. Kinetic characteristics of the glutamate uptake into normal astrocytes in cultures. Neurochem Res. 1978;3:1–14. doi: 10.1007/BF00964356. [DOI] [PubMed] [Google Scholar]
- 33.Hertz L, Peng L, Lai JC. Functional studies in cultured astrocytes. Methods. 1998;16:293–310. doi: 10.1006/meth.1998.0686. [DOI] [PubMed] [Google Scholar]
- 34.Meier E, Hertz L, Schousboe A. Neurotransmitters as developmental signals. Neurochem Int. 1991;19:1–15. [Google Scholar]
- 35.Hertz L, Juurlink BHJ, Szuchet S. Cell cultures. In: Lajtha A, editor. Handbook of neurochemistry. 2nd ed. New York (NY): Plenum; 1985. [Google Scholar]
- 36.Liang YJ, Lai LP, Wang BW, et al. Mechanical stress enhances serotonin 2B receptor modulating brain natriuretic peptide through nuclear factor-kappaB in cardiomyocytes. Cardiovasc Res. 2006;72:303–12. doi: 10.1016/j.cardiores.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Wong SK, Lazinski DW. Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc Natl Acad Sci U S A. 2002;99:15118–23. doi: 10.1073/pnas.232416799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Zhang S, Li M, et al. Down-regulation of GluK2 kainate receptor expression by chronic treatment with mood-stabilizing anticonvulsants or lithium in cultured astrocytes and brain, but not in neurons. Neuropharmacology. 2009;57:375–85. doi: 10.1016/j.neuropharm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Bureau I, Dieudonne S, Coussen F, et al. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci U S A. 2000;97:6838–43. doi: 10.1073/pnas.97.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong J, Qian Z, Shen S, et al. High doses of siRNAs induce eri-1 and adar-1 gene expression and reduce the efficiency of RNA interference in the mouse. Biochem J. 2005;390:675–9. doi: 10.1042/BJ20050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Y, Sansam CL, Singh M, et al. Altered RNA editing in mice lacking ADAR2 autoregulation. Mol Cell Biol. 2006;26:480–8. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sergeeva OA, Amberger BT, Haas HL. Editing of AMPA and serotonin 2C receptors in individual central neurons, controlling wake-fulness. Cell Mol Neurobiol. 2007;27:669–80. doi: 10.1007/s10571-007-9153-1. [DOI] [PubMed] [Google Scholar]
- 43.el-Marjou A, Delouvée A, Thiery JP, et al. Involvement of epidermal growth factor receptor in chemically induced mouse bladder tumour progression. Carcinogenesis. 2000;21:2211–8. doi: 10.1093/carcin/21.12.2211. [DOI] [PubMed] [Google Scholar]
- 44.Ye S, Dhillon S, Ke X, et al. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:E88. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton CR, Graham A, Heptinstall LE, et al. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503–16. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Punia P, Saunders NA. The Quantitative amplification refractory mutation system. In: Logan J, Edwards K, Saunders N, editors. Real-time PCR: current technology and applications. Norfolk (VA): Horizon Scientific Press, Inc.; 2009. [Google Scholar]
- 47.Iwamoto K, Bundo M, Kato T. Estimating RNA editing efficiency of five editing sites in the serotonin 2C receptor by pyrosequencing. RNA. 2005;11:1596–603. doi: 10.1261/rna.2114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Kim WT, Rioult MG, Cornell-Bell AH. Glutamate-induced calcium signaling in astrocytes. Glia. 1994;11:173–84. doi: 10.1002/glia.440110211. [DOI] [PubMed] [Google Scholar]
- 50.Shelton MK, McCarthy KD. Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia. 1999;26:1–11. doi: 10.1002/(sici)1098-1136(199903)26:1<1::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 51.Barbon A, Popoli M, La Via L, et al. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–20. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Englander MT, Dulawa SC, Bhansali P, et al. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–51. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Köhler M, Burnashev N, Sakmann B, et al. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- 54.Vissel B, Royle GA, Christie BR, et al. The role of RNA editing of kainate receptors in synaptic plasticity and seizures. Neuron. 2001;29:217–27. doi: 10.1016/s0896-6273(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 55.Sommer B, Köhler M, Sprengel R, et al. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–9. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 56.Schmitt J, Dux E, Gissel C, et al. Regional analysis of developmental changes in the extent of GluR6 mRNA editing in rat brain. Brain Res Dev Brain Res. 1996;91:153–7. doi: 10.1016/0165-3806(95)00175-1. [DOI] [PubMed] [Google Scholar]
- 57.Paschen W, Schmitt J, Gissel C, et al. Developmental changes of RNA editing of glutamate receptor subunits GluR5 and GluR6: in vivo versus in vitro. Dev Brain Res. 1997;98:271–80. doi: 10.1016/s0165-3806(96)00193-9. [DOI] [PubMed] [Google Scholar]
- 58.Lowe DL, Jahn K, Smith DO. Glutamate receptor editing in the mammalian hippocampus and avian neurons. Brain Res Mol Brain Res. 1997;48:37–44. doi: 10.1016/s0169-328x(97)00072-7. [DOI] [PubMed] [Google Scholar]
- 59.Bernard A, Ferhat L, Dessi F, et al. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–16. doi: 10.1046/j.1460-9568.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 60.Lai F, Chen CX, Carter KC, et al. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol Cell Biol. 1997;17:2413–24. doi: 10.1128/mcb.17.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko S, Zhao MG, Toyoda H, et al. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J Neurosci. 2005;25:977–84. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–8. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miguel-Hidalgo JJ, Waltzer R, Whittom AA, et al. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010 Jun 24; doi: 10.1016/j.jad.2010.06.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernard R, Kerman IA, Thompson RC, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2010 Apr 13; doi: 10.1038/mp.2010.44. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–95. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell ND, Baker GB. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr Scand. 2010;122:192–210. doi: 10.1111/j.1600-0447.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- 67.Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–52. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 68.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–55. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]