Abstract
Ranaviruses like Frog Virus 3 (FV3) are responsible of emerging infectious diseases spreading worldwide to fish, amphibian and reptilian species. We have developed, in Xenopus laevis, an experimental model to investigate viral transmission. We show that FV3 released in water by immunocompromised infected adults can infect adult and larval stages of Xenopus within 3 hours of exposure. Time course of virus load and viral transcription in different tissues suggests that early waterborne FV3 infection through the digestive tract leads to dissemination in the kidney. Finally, a fraction of adult macrophages becomes infected following exposure to waterborne FV3 as visualized by fluorescence microscopy using macrophage- and FV3-specific antibodies. Little cytopathicity and apoptosis were detected in infected macrophages, which is consistent with our proposition that macrophages are permissive to FV3. These data highlight the efficiency of FV3 infectivity by the water route and the ability of FV3 to adapt to its hosts.
Keywords: Iridovirus, Viral infection, viral transmission, Amphibian
Introduction
Ranaviruses (RVs; Family: Iridoviridae) are viral pathogens implicated in emerging infectious diseases that have become a major risk to captive and wild amphibians, fish, and other species worldwide (reviewed in (Chinchar et al., 2009). RVs, which frequently result in host death from hemorrhage and chronic necrosis of numerous organs, are both directly and indirectly transmitted and may survive up to several weeks outside a host (Gray, Miller, and Hoverman, 2009). Although RV outbreaks in wild and captive amphibian populations suggest an efficient transmission and dissemination, little is known about the ways RVs are transmitted and how they invade their hosts.
Since animals infected by RVs are ectothermic vertebrates mostly living in aquatic environments, water is likely a major route of viral transmission. Indeed, water and water sediment containing RVs have been shown to readily infect the salamander Ambystoma tigrinum (Brunner, Richards, and Collins, 2005; Jancovich et al., 2001). Similarly, various anuran species can be experimentally infected by immersion in water containing RVs (Harp and Petranka, 2006; Pearman et al., 2004; Robert et al., 2005). In addition to passive infection from waterborne RVs, contribution of direct contact with infected individuals by bumping and biting (Schock et al., 2008) and of cannibalism of infected individuals (Brunner, Schock, and Collins, 2007) have been reported. RV infection by ingestion has been shown to increase infection and mortality rates of tadpoles compared to water bath exposure (Hoverman, Gray, and Miller, 2010). Although it is currently unknown how RVs penetrate the cells they infect (e.g., no specific receptors identified so far), infection by ingestion suggests that RVs can target cells in the digestive tract, at least in tadpoles.
We have developed the frog Xenopus laevis as a convenient laboratory model to investigate interactions between an amphibian host and the RVs (reviewed in (Robert, 2010). Since both larval and adult X. laevis are fully aquatic, we were interested in determining how FV3 gets transmitted from infected frogs to congeners. In an attempt to mimic a natural situation where infected animals with a compromised immune system cohabit with uninfected congeners, we have previously developed a laboratory setting where a frog immunocompromised by sublethal γ-irradiation and infected by injection with the RV Frog Virus 3 (FV3) is cohoused with uninfected larval or adult congeners (Robert et al., 2005). With this system, we have shown that a single immunodeficient frog infected with FV3 was able to transmit infection to both cohoused adults and tadpoles. Using this well-suited system we have further investigated how rapidly FV3 transmission occurs in water and whether FV3 can be transmitted through the digestive tract.
Our data reveal that FV3 released in water is very efficient in cross-infecting other animals through ingestion by targeting the digestive tract and the kidneys. This study also substantiates our initial report of the permissiveness to FV3 of some adult macrophages.
Results
FV3 occurrence in adult tissues following waterborne exposure
Previously we reported that adult X. laevis immunocompromised by sub-lethal γ-irradiation and subsequently infected by intraperitoneal injection of FV3, release sufficient quantities of FV3 into the water to infect conspecifics raised in the same aquarium (Robert et al., 2005). We used the same system to determine more specifically how FV3 enters and disseminates into the host during water transmission. Specifically, a single adult frog, immunosuppressed by a sub-lethal dose of γ-irradiation (8 Gy), was infected with 1×107 PFU and placed in the water tank (2 L volume) 12 hrs before being cohoused with 9 to 12 healthy frogs. We choose a relatively low dose to only partially impair immunocompetence. Upon infection, γ-irradiated frogs got sick within a few hours (e.g., lethargy, skin redness in the abdominal region) and released detectable amount of virus (Fig. 1 and Table 1) but did not readily succumb to the infection and generally recovered within 3 weeks. Immunocompetent frogs can also release FV3 in water, albeit in significantly lesser amount, for a shorter period of time and with more variability than irradiated animals (Robert et al., 2005). The use of irradiated infected animals, therefore, was more suitable for our objective.
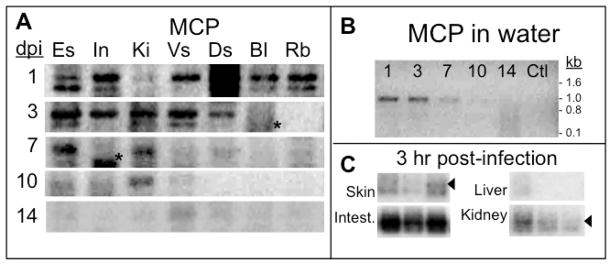
Figure 1. Detection of FV3 DNA in adult tissues during FV3 waterborne infection.
(A) PCR of FV3 MCP (40 cycles) on DNA extracted from tissues from one representative frog (out of 3 individuals) for each time point of exposure (1, 3, 7, 10 and 14 days) to waterborne FV3. Es: Esophagus; In: Intestine; Ki: kidneys; Vs: Ventral skin; Ds: Dorsal skin; Bl: blood leukocytes; Rb: Red blood cells. Note that doublets in some lane are both MCP amplified products as determined by southern blot with a labeled MCP cDNA probe, whereas the low molecular signal (*) is not MCP. (B) PCR of FV3 MCP on water samples used to infect the frogs in A. Ctl: Water sample before the addition of the infected immunocompromised frog. (C) PCR of FV3 MCP on DNA extracted from tissues of one representative frog exposed to waterborne FV3 for 3 hrs. Waterborne infection was performed by infecting a sub-lethally γ-irradiated frog by ip injection of 107 PFU of FV3 and leaving this animal 12 hrs in the tank before co-housing naïve frogs for various periods of time. The arrow indicates the MCP specific signal.
Table 1.
Summary of FV3 DNA detected in tissues of adults cohoused for different periods (from 3hr to 14 days) of time with an immunocompromised infected adult.
| PCR | 3 hrs | 6hrs | 24 hrs | 3 d | 7 d | 10 d | 14 d |
|---|---|---|---|---|---|---|---|
| Water | + | +/− | + | +/− | ns | +/− | ND |
| Skin | + (3/3) | + (3/3) | ++ (6/6) | + (3/3) | +/− (6/9) | +/− (2/4) | +/− (1/4) |
| Intestine | ++ (3/3) | + (3/3) | ++ (6/6) | ++ (3/3) | + (6/7) | +/− (3/4) | ns (4/4) |
| Liver | ns (3/3) | ns (3/3) | +/− (1/6) | nd | +/− (1/3) | nd | ns (3/3) |
| Kidney | + (3/3) | + (3/3) | + (4/4) | ++ (6/6) | ++ (8/8) | + (3/4) | ns (4/4) |
| PLs | ND | ND | + (3/3) | +/− (3/3) | ns (3/3) | ns (3/3) | ND |
| Blood | ND | ND | + (3/3) | ns (3/3) | ns (3/3) | ns (3/3) | ND |
Skeletal muscle and water control were all negative for each time point
++: strong signal; + low signal; +/− barely detectable; ns: no signal; nd: not done
In parentheses: number of individual tissues positive/total number of individual tissues
At different times following exposure to infected water, frogs were collected (usually 3 individuals per time point) separately, washed several times (to remove contaminated water), and various tissues were processed for detection of FV3 DNA by PCR. Work was done under sterile condition and special precautions were taken to minimize possible contamination from one tissue to another. PCR results of a representative of three different experiments are shown in Fig. 1; a summary of the overall results is presented in Table 1.
In a first set of experiments, a significant amount of FV3 DNA was detected 24 hrs following exposure to infected water. Amplified signal from the major capsid protein (MCP) was present in the skin (both ventral and dorsal), the esophagus and small intestine. Viral DNA was also detected in the blood (both in the leukocyte and the erythrocyte fraction), whereas only a faint signal was detectable in the kidneys. After leukocyte separation by a single ficol cushion, a residual fraction of leukocyte (20%) remained with the erythrocytes, which may explain why FV3 was also found in the erythrocyte fraction. Three days of co-housing with the infected frog resulted in a strong MCP signal in the skin and the digestive tract. Kidneys were also strongly positive at this time, whereas viral DNA became undetectable in the blood. FV3 DNA signal remained strong in kidneys after 7 days of cohousing in all animals tested, whereas it became low or undetectable in most other tissues (Table 1). The overall FV3 load markedly decreased after 10 days of co-housing, including in kidneys, and became barely detectable after 14 days of co-housing. This was presumably the result of viral clearance by the potent adult host immune response known to peak at 6 days post-infection (Morales et al., 2010; Morales and Robert, 2007). Note that a lower band is sometime amplified with the MCP primers. This band also hybridized with a MCP cDNA probe in Southern blot analysis under stringent conditions (data not shown). An attempt to clone and sequence this band was unsuccessful.
Consistent with FV3 detection in tissues of exposed frogs, we also detected FV3 in water from an infected immucompromised frog (Fig. 1B). A significant and reproducible (triplicates) MCP signal was obtained at day 1 and 3; the signal was markedly decreased at day 7 and became barely detectable at later time points. We have previously estimated that with our PCR method we can detect reproducibly (i.e. in triplicate) FV3 using 1 μl of a 1 ml dilution containing 10 PFU (Gantress et al., 2003). Since in this study we have used 5 μl of 1 ml water sample, our detection limit is in the range of 2 PFU. Our objective was to determine how rapid was the infection and where (tissues) FV3 was entering its host, so we did not attempt to concentrate viral particles from the water nor try to further quantify the amount of virus. But based on our detection limit and the intensity of signal obtained, we estimate that at least 100 PFU/ml of FV3 is present between day 1 and 3. Interestingly, the decrease of FV3 infection in tissues of co-housed animals was mirrored by the decrease of viral DNA detected in the water.
Since substantial FV3 signal was already detected in most tissues 24 hrs after exposure (Fig 1A), we decided to determine if FV3 could be detected at earlier time points in tissues of frogs co-housed with an immunocompromised and infected frog. Interestingly, a strong MCP signal was detected in the intestine as early as 3 hrs in most cohoused animals (Fig. 1C; Table 1). In contrast, only a faint MCP signal was detected in the skin and kidneys of 1 out of 3 cohoused animals. Importantly, we also detected substantial MCP signal in the water at this early time point (see Fig. 2B, right panel). The lower signal obtained in the skin compared to other tissues provides a good indication that contamination is minimal.
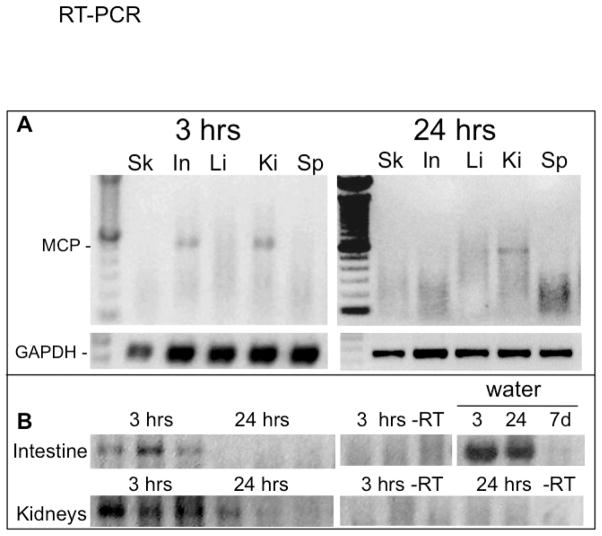
Figure 2. Detection of FV3 transcription in adult tissues during waterborne infection.
(A) Detection of FV3 MCP transcription by RT-PCR (40 cycles) on RNAs extracted from skin (Sk), Intestine (In), liver (Li), kidneys (Ki) and spleen (Sp) of one representative adult frog (out of 3 individuals) exposed to waterborne FV3 for 3 and 24 hrs. The housekeeping gene GAPDH was used to control the integrity of the RNAs and cDNAs. (B) Detection of FV3 MCP transcription by RT-PCR (40 cycles) on RNAs extracted from intestine and kidneys of 3 different frogs exposed for either 3 or 24 hrs to waterborne FV3. RT negative controls are shown for the intestine at 3 hr, and kidneys at 3 and 24 hrs post-exposure, as well as the detection of FV3 DNA in water samples at 3 hrs, 24 hrs and 7 d.
We conclude that waterborne FV3 can rapidly disseminate into tissues of adult Xenopus, and that the digestive tract appears to be the major route of waterborne infection.
Detection of viral transcription following waterborne exposure
To establish that FV3 DNA detected in tissues of frogs co-housed with an infected congener corresponds to an active infection, we monitored viral transcription by RT-PCR on DNase-treated RNA prepared from various tissues at different times following exposure to infected water. In several experiments (two shown in Fig 2, and more summarized in Table 2), we found clear evidence of active FV3 transcription in the kidney that is the preferential target tissue of FV3 in Xenopus. This active transcription was detected as early as 3 hrs following exposure to virally-infected water (Fig 2A, B) and continued to be detected after 6 hrs (Table 2) and 24 hrs of exposure to infected water. Interestingly, significant FV3 transcription was also found in the intestine of most frogs at the 3 hr time point as well as at 6 hr and 24 hr time points. In contrast, FV3 was transcribed in the skin of only a few frogs (2 of 7), and no consistent viral transcription was detected liver or spleen at these time points (3, 6 and 24 hrs). Furthermore, no signal was detected in RT negative controls, which, in addition to the treatment of the RNA samples with DNase, definitely rules out possible viral DNA contamination (Fig 2B).
Table 2.
Summary of FV3 transcription detected in tissues of adults cohoused for different periods (from 3hr to 14 days) of time with an immuno-compromised infected adult.
| RT-PCR | 3 hrs | 6 hrs | 24 hrs | 7 d |
|---|---|---|---|---|
| Skin | + (1/4) | + (1/3) | ns (4/4) | ns (3/3) |
| Intestine | + (4/6) | + (1/3) | +/− (3/6) | + (1/3) |
| Liver | ns (6/6) | ND | + (2/3) | + (1/3) |
| Kidney | + (4/6) | + (2/3) | + (4/6) | + (1/7) |
| Spleen | − (3/3) | ND | ns (3/3) | ns (3/3) |
++: strong signal; + low signal; +/− barely detectable; ns: no signal; ND: not done
In parthenthesis: number of individual tissues positive/total number of indivual tissues
RT minus control were negative
Although these results confirm the preponderant role of the kidney in FV3 infection in adult Xenopus, they clearly reveal a significant contribution of the intestine at an early stage of natural infection by the water route.
Infection of adult peritoneal leukocytes following waterborne exposure
We have previously reported that peritoneal macrophages are rapidly activated by and accumulate FV3 during infection, while at the same time some of these cells appear to be permissive to FV3 and may harbor the virus for a long time (Morales et al., 2010). In these published experiments FV3 was transmitted via by intraperitoneal (ip) injection. Since in the current studies we did detect FV3 in the blood leukocyte fraction at early stage of infection (Fig 1A), we were interested in determining whether some peritoneal leukocytes (PLs), and more specifically macrophages were also infected by waterborne FV3. To more directly visualize cells infected by FV3 we took advantage of a rabbit polyclonal antibody (Ab) kindly provided by Dr. V.G. Chinchar that recognizes p53R, a putative 54.7-kDa myristoylated viral protein that is critical for FV3 replication (Whitley et al., 2010). PLs obtained from animals exposed to waterborne FV3 for 3 days were collected, cytocentrifuged, fixed and stained for immunofluorescence microscopy. In samples from several animals from two different experiments, a consistent fraction of PLs (Average 16.7 ± 4.4% in 4 different animals) were positively stained by the anti-53R Ab, whereas no specific staining was detected in PLs from uninfected animals (Fig. 3A, B). Similar data were obtained from animals infected for 2 days (data not shown). Interestingly, as in the case of infection by ip injection, waterborne infection was accompanied by morphological changes and increase in size of PLs that are typical of activation (Fig. 3B *), whereas PLs of uninfected controls were mainly small and round. Additional evidence that FV3 was present in PLs was obtained by PCR (Fig. 4). To further determine possible changes in the relative fraction of peritoneal macrophages in animals exposed to waterborne FV3, we stained these cells with a mouse monoclonal Ab (anti-HAM56) that recognizes a macrophage antigen (HAM56) that specifically cross-reacts with Xenopus macrophages (Nishikawa et al., 1998). In animals exposed to waterborne FV3 for 3 days, fluorescence microscopy with anti-HAM56 mAb confirmed the presence of numerous HAM56+ macrophages displaying an activated monocytic morphology. By contrast, HAM56+ cells from uninfected animals were smaller and round (i.e., not activated; Fig. 3C, D). Furthermore, few (less than 25%) of the infected PLs stained with anti-53R antibody showed signs of apoptosis as determined by the staining pattern (e.g. pyknotic DNA) of the fluorescent DNA intercalator Hoechst-33258. However, although a slight increase in the relative frequency of HAM56+ cells can be observed in PLs of some infected individuals, no significant difference was found when comparing multiple animals. On average, 18±1% of HAM56+ cells identified in both uninfected (4 individuals) and 3 day post-infected frogs (4 individuals) by counting cells in 10 randomly selected areas on a slide (100 by 100 mm). This is consistent with results we have previously reported for frogs infected by injection (Morales et al., 2010). Finally, to obtain direct evidence of macrophage infection by FV3, we sequentially stained PLs infected with FV3 in vi-tro for two days with mouse anti-HAM56, PE-conjugated goat anti-mouse, rabbit anti-53R, FITC-conjugated donkey anti-rabbit Abs and the DNA dye Hoechst-33258. In several experiments (one representative shown in Fig. 5), a clear co-localization of the viral 53R antigen and the macrophage-specific Ag HAM56 was observed in a consistent fraction of PLs. In addition, few signs of apoptosis were detected in these macrophages infected in vitro.
Figure 3. Visualization of waterborne FV3 infection of peritoneal leukocytes by immunofluorescence microscopy.
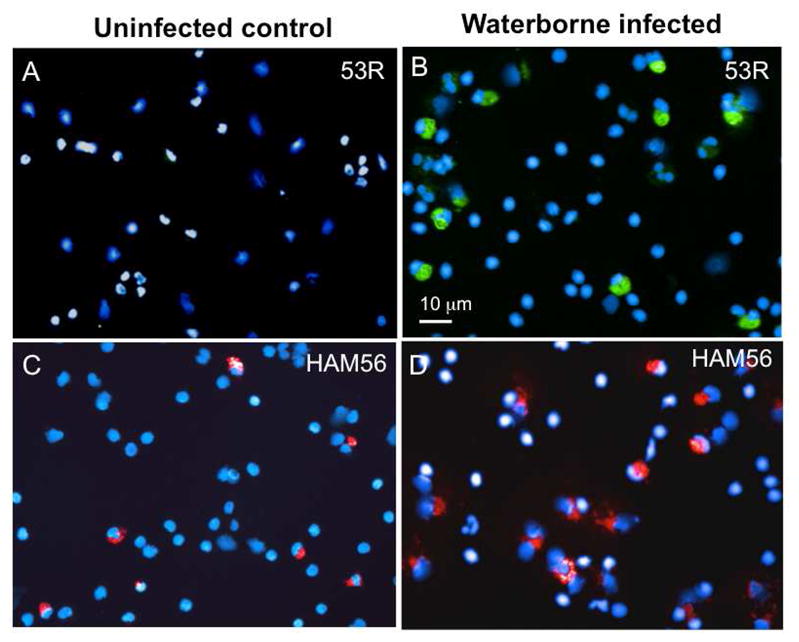
PLs were harvested from an uninfected control (A, C) or from a representative of 3 frogs cohoused for 3 days with an infected immunocompromised congener (B, D). Cells were cytocentrifuged on microscope slides, fixed with formaldehyde, permeabilized with ethanol, then stained with a rabbit anti-53R and FITC-conjugated donkey anti-rabbit Abs (Green; A, B); or a mouse anti-HAM56 and PE-conjugated goat anti-mouse Abs (Red; C, D). Cells were then stained with the DNA die Hoechst-33258 (Blue) mounted in anti-fade medium and visualized with a Leica DMIRB inverted fluorescence microscope.
Figure 4. Detection of FV3 DNA in PLs 3 days post-FV3 waterborne infection.
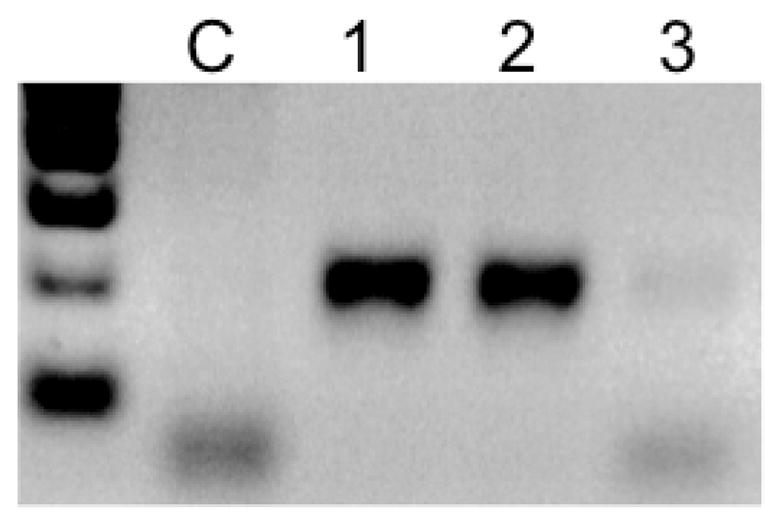
A fraction of PLs used for the experiment in Fig. 3 were used for PCR assay (40 cycles) with MCP-specific primers to confirm the presence of FV3. PLs were from (C) uninfected control; (1) γ-irradiated frog infected by ip injection; (2) frog exposed for 3 days with frog 1; (3) water sample at day 3.
Figure 5. Colocalization of FV3 53R antigen with the macrophage HAM65 marker in Xenopus.
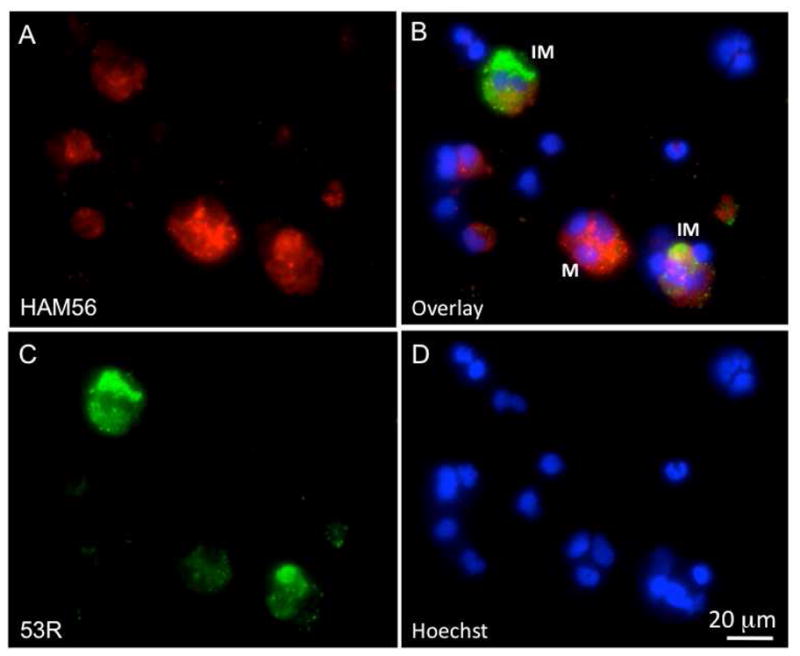
Three-color fluorescence microscopy of PLs infected for 2 days in vitro with FV3. Cells were prepared as in Fig. 3 with mouse anti-HAM56, PE-conjugated goat anti-mouse (Red; A), rabbit anti-53R and FITC-conjugated donkey anti-rabbit Abs (Green; C). Cells were then stained with the DNA dye Hoechst-33258 (Blue; D). Pictures taken with the 3 different filters were overlaid in B. M: HAM56+ macrophage; IM: FV3 infected macrophage.
In summary, these results suggest that peritoneal macrophages also become infected by FV3 during a natural waterborne infection and are permissive.
FV3 occurrence and activity in larval tissues following waterborne exposure
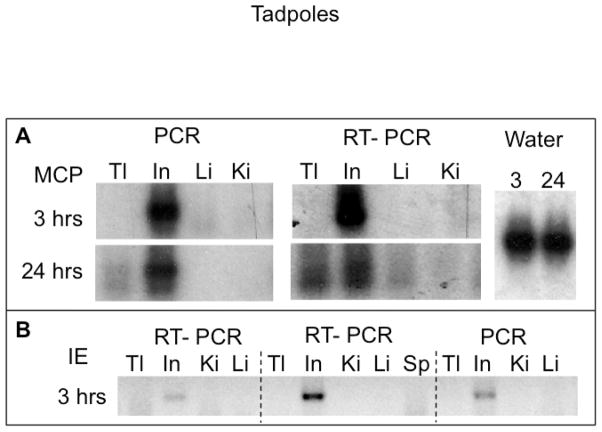
We have previously shown that Xenopus tadpoles are more susceptible to FV3 infection than adults, and can also be infected by waterborne FV3 (Gantress et al., 2003; Robert et al., 2005). Therefore, we were interested in further investigating how waterborne FV3 disseminates into tadpoles compared to Xenopus adults. Pre-metamorphic larvae were cohoused in a tank where a γ-irradiated FV3-infected adult was housed for 12 hrs beforehand. Tadpoles were physically separated from the adults by netting to prevent cannibalism. In two different experiments, we found that pre-metamorphic larvae became as rapidly infected as adults after being exposed to water containing FV3. Notably, viral DNA was detected in the larval intestine as early as 3 hrs of co-housing, as in adults (Fig. 6A). However, at this early time point no specific signal was detected in the liver or kidney, whereas there was a faint signal in 1 of 3 tadpoles' tails. Corresponding active viral transcription was found in the intestines both at 3 and 24 hrs post-exposures to waterborne FV3, whereas there was a weaker transcription in tail tissues and liver at 24 hrs. Evidence of early (3hrs) viral transcription in the intestine upon exposure to waterborne FV3 was also confirmed by another FV3 gene IE-ICP18 (Galli et al., 2006); Fig. 6B).
Figure 6. Detection of FV3 DNA and FV3 transcription in larval tissues during waterborne infection.
DNA and RNA were extracted from the tissues of pre-metamorphic tadpoles exposed for 3 or 24 hrs to waterborne FV3 and were used for PCR and RT-PCR assays respectively (40 cycles), using either MCP (A) or IE-specific (B) primers. To enhance the weak signal, the PCR products separated on an agarose gel were transferred onto a nylon membrane and hybridized with a hapten-labeled MCP cDNA probe under stringent condition (62ºC, washes at 0.2x SSC). The probe was detected with anti-hapten Ab and bioluminescence detection (see materials and methods). Tl: tail (cut after the rectum); In: intestine; Li: liver; Ki: kidneys
These data suggest that in larvae as in adults, the digestive tract constitutes a major entry for waterborne FV3.
Discussion
Although dissemination of RVs by the water route a water route has been generally assumed, so far there has been little formal investigation of this modality of transmission. In our study, we took advantage of an experimental system in which healthy Xenopus adults or larvae are co-housed in an aquarium with an infected immunocompromised adult congener that releases FV3 into the water. With this system we have been able to show that FV3 released by the infected animals can disseminate within 3 hrs by the water route into other animals. Furthermore, our data suggest that both in larvae and adult Xenopus early waterborne FV3 infection can occur through the digestive tract and lead to dissemination and propagation in the kidney.
Finally, our study shows that a fraction of adult peritoneal macrophages also becomes infected by waterborne FV3. Since these macrophages do not show typical FV3-induced cytopathicity or apoptosis, they appear to be permissive to FV3. Overall, this study underscores the high potential of FV3 to spread via a totally aquatic environment, although it does not exclude other transmission modes such as cannibalism and animal-to-animal contact.
The experimental system we have developed allows us to mimic, in a laboratory setting, the contribution of animals carrying FV3 and releasing it in the water. Sublethal γ-irradiation of Xenopus depletes thymocytes and to impairs T cell responses to skin allografts (Hsu, Julius, and Du Pasquier, 1983; Russ and Horton, 1987) and transplanted tumors (Rau, Cohen, and Robert, 2001; Robert and Cohen, 1998; Robert, Guiet, and Du Pasquier, 1995). The convenience (easy to dose) and already existing information related to its effect on immunity, allowed us to use γ-irradiation to explore the importance of environmentally-associated immunomodulation. We have used a relatively low dose of γ-irradiation (8 G) that did not completely abrogate the host immune defense since in most cases irradiated animals did not succumb to infection from injected FV3 and indeed, recovered within 3–4 weeks. However, these frogs released sufficient infectious FV3 particles in the water as determined by PCR of water samples and monitoring of FV3 transmission in co-housed animals. As already mentioned, we have shown that non-irradiated immunocompe-tent adults infected by ip injection also release virus particles in the water, albeit in smaller amount and for a shorter period of time than irradiated frogs (Robert et al., 2005). This lower level of FV3 released can nevertheless transmit disease to immunocompromised adult or susceptible larval Xenopus.
Rapid transmission of waterborne FV3
The rapidity and effectiveness of FV3 transmission into the host is remarkable since active transcription of MCP, a late gene in RVs, can occur within 3 hrs after exposure to infected water in a large faction of both larval and adult Xenopus. The timing corresponds to what can be obtained in a typical cell-virus in vitro system (e.g., fathead minnow cells infected at a multiplicity of infection of 10 PFU/cell), where viral macromolecular synthesis is already detected by 2 hrs post-infection (Goorha and Granoff, 1974). More recently, expression of several FV3 transcripts including MCP were detected by RT-PCR 2 hrs after infection (Majji et al., 2009). Given the sensitivity of PCR and RT-PCR assays, it is quite possible that this initial infection is localized and may affect only a limited number of cells or areas in the intestine and the kidney. In a previous study we have shown by immunohistology that FV3 infection is localized in discrete area of the kidneys (Robert 2005). It will be interesting to determine if infection in the intestine is also localized and what types of cells are infected.
In contrast, the absence of detectable viral transcription in the skin, strongly suggests that despite the presence of FV3 on the skin surface (as determined by PCR), little or no productive infection is initiated at this level at an ealry stage of infection. Besides a mechanical barrier that may prove to be impenetrable for FV3, the Xenopus skin glands secretes potent antimicrobial peptides that may have anti-viral activity. Several antimicrobial peptides have been shown to inactivate FV3 and other RVs in vitro (Chinchar et al., 2004; Chinchar et al., 2001). These defenses are likely to be effective even in the case of small wounds. The potency of skin antimicrobial peptides is usually invoked to explain the ease of performing surgery in Xenopus with limited precaution taken for sterile procedures. Although some antimicrobial peptides are also produced in the intestinal tract and by macrophages it is likely that their concentration and repertoire is different from that of the skin. As such it is possible that this route of infection is “easier” for FV3 provided that it can resist the acidity in the stomach. This issue remains to be investigated.
Although, the intestine appears to contribute significantly to FV3 transmission by the water route our results suggest that the kidney is the main site of infection, a finding that is in agreement to our previous reports (Gantress et al., 2003; Robert et al., 2005). Both the viral transcription estimated by RT-PCR, as well as the viral load, estimated by PCR, are more prominent in the kidney.
In fact our kinetics (Table 1) suggest oral infection within 24 hours and then greater replication in the kidneys from days 3–10. Therefore, early infection through the digestive system leads to dissemination and propagation in the kidney.
Contribution of macrophages to FV3 transmission
We have proposed that FV3 is capable of covert infection as are certain other iridoviruses, and can remain in asymptomatic infected animals for a long period of time (Morales et al., 2010; Robert, 2010; Robert et al., 2007). We postulated that macrophages are critically involved in this process by being permissive to FV3. In support of this view, we have shown that in Xenopus some peritoneal macrophages can survive FV3 infection and harbor the virus after viral clearance (Morales, Abramowitz et al. 2010). Here, we have obtained further evidence that during a natural infection through the water route, a fraction of peritoneal macrophages identified by macrophage markers are indeed infected, although this infection is not accompanied by typical cytopathicity and apoptosis. These observations are consistent with a higher permissivity of macrophages to FV3. Macrophages are cells known to migrate in an organism and to be long lived. As such they are often targeted by pathogens for dissemination of infection and to persist in the host (reviewed in (Jarvis and Nelson, 2002). FV3 appears to have adapted similarly.
A possible quiescence phase of RVs such as FV3 should increase our concern about the threat of these pathogens for biodiversity and population conservation, since host individuals from resistant species or populations, despite clearing most of the infection, may still harbor quiescent virus. These viruses can be reactivated and released upon various stimuli such as a stressor that can affect the hosts’ immune status. Considering the combination of the low host specificity of RVs (e.g., FV3 and Bohle virus can infect various amphibian and fish species; Chinchar, 2002; Daszak et al., 1999), their ability to survive for long periods of time at the bottom of ponds (e.g., in nature, ATV survives during the winter and in the laboratory and it remains infectious at 25ºC in water for up to 2 weeks; Jancovich et al., 1997) and the high transmission capacity through the water route of RVs (e.g., the present study with FV3), the contribution of asymptomatic carriers would needs to be carefully evaluated.
Material and Methods
Animals and FV3
Outbred adults and premetamorphic (stage 56–58) tadpoles were obtained from our Xe-nopus laevis Research Resource for Immunology at the University of Rochester (http://www.urmc.rochester.edu/smd/mbi/xenopus/index.htm). All animals were handled under strict laboratory and UCAR regulations (Approval number 100577 / 2003–151), minimizing discomfort at all times. Absence of RV contamination in our frog colony is determined by frequently testing kidneys samples from randomly chosen animal by PCR.
FV3 stock and infection: FV3 was grown by a single passage on baby hamster kidney (BHK-21) cells, purified using on a 30% sucrose cushion by ultracentrifugation and quantified by plaque assay on BHK-21 monolayer under an overlay of 1% methylcellulose (Morales et al., 2010).
Sublethal irradiation
Adult frogs (2-years-old) were sublethally irradiated (8 Gy) with a cobalt source one day prior to infection with FV3 (1×106 TCID50) by intraperitoneal injection.
FV3 PCR assay
Detection of FV3 DNA in water was performed on sample of 1 ml taken at different time following exposure of animals in water containing a γ-irradiated infected frog. The PCR (40 cycles) was performed using 5 ml of each water sample. Animals were euthanized by immersion in 5% tricaine methane sulfonate (MS-222) buffered with bicabonate. For both DNA and RNA extraction, liver and kidneys, 5 mm pieces of of tissues were homogenized. For the gut, contents were extensively flushed out with APBS before they were dissociated. The skin (about 1 cm square) was washed 3 times in APBS before homogenization. DNA was extracted from different tissues with DNAzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. For 50 ng of genomic DNA was amplified by 40 cycles (45 sec denaturation at 95ºC, 45 sec annealing at 52ºC, and 45 sec extension at 72ºC) and analyzed on a 1% agarose gel stained with ethidium bromide. Primers specific for the FV3 MCP are: forward primer 5′-GTCTCTGGAGAAGAAGAA-3′; and reverse primer 5′-GAC TTGGCCACTTATGAC-3′ (Mao et al., 1996). Primers for FV3 IE-ICP18 gene are: forward 5′-ATG ATC-CAAGCCTACCTGTGC-3′; and reverse 5′-AAATGTCCTAATCTATACACC-3′. GAPDH primers: forward 5′-ACCCCTTCATCGACTTGGAC-3′; reverse 5′-GGAGCCAGACAGTTGTAGTC-3′ (Galli et al., 2006). RNA was extracted from cells and tissues using Trizol reagent following the manufacturer’s protocol (Invitrogen). 0.5 to 1.0 μg of total RNA in 20 μl was used to synthesize cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). RT negative controls were included for every reaction. A water-only control was also included in each reaction. Sizes of the products were determined using a standardized marker of 1kb+ from Invitrogen (Carlsbad, CA).
In some cases, when the PCR signal was week, the gel was transferred onto a nylon membrane. After UV-cross-linking the membrane was incubated with heat-denatured MCP cDNA probe labeled using the BrightStar Psolaren-Biotin kit from Ambion (Austin, TX). Detection with Alkalin phosphatase-conjugated streptavidin by chemolumisnescence was performed following to the manufacturer instructions.
Cytospin and Staining
200,000 cells (200 μl volume) were cytocentrifuged using a Shandon Southern cytospin centrifuge (600 rpm, 5 min.), fixed with 3.7% formalin for 1 min., permeabilized with 100% cold methanol (−20ºC) and briefly washed with APBS. After blocking with 1%BSA in APBS for 1 hr, the cells were incubated overnight with the following primary Abs: rabbit anti-FV3 53R serum, HAM56-macrophage-specific mAb, or normal rabbit serum as negative control. After washing, cells were incubated with DyLight 488-conjugated F(ab’)2 Donkey Anti-Rabbit IgG (H+L) (Jackson ImmunoReaserch, PA), DyLight 594-conjugated F(ab’)2 Donkey Anti-Mouse IgG (H+L) (Jackson ImmunoResearch, PA) and a fluorescent DNA intercalator (Hoechst-33258). Preparations were mounted in anti-fade medium (Molecular Probes, Oregon) and visualized with a Leica DMIRB inverted fluorescence microscope with a cooled charge-couple device (Cooke) controlled by Image-Pro software (Media Cybernetics).
Acknowledgments
The expert animal husbandry provided by Tina Martin and David Albright is gratefully appreciated. We also thank Jennifer Gantress for her technical contribution, as well as Hristina Nedel-kovska, Nikesha Haynes and Dr. Nicholas Cohen for their critical review of the manuscript. This research was supported by grants R24-AI-059830-1 from the NIH and NSF IOS-09-23772 and IOS-07-42711.
Abbreviations
- APBS
amphibian phosphate buffered saline
- FV3
frog virus 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brunner JL, Richards K, Collins JP. Dose and host characteristics influence virulence of ranavirus infections. Oecologia. 2005;144(3):399–406. doi: 10.1007/s00442-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Brunner JL, Schock DM, Collins JP. Transmission dynamics of the amphibian ranavirus Ambystoma tigrinum virus. Dis Aquat Organ. 2007;77(2):87–95. doi: 10.3354/dao01845. [DOI] [PubMed] [Google Scholar]
- Chinchar VG, Bryan L, Silphadaung U, Noga E, Wade D, Rollins-Smith L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology. 2004;323(2):268–75. doi: 10.1016/j.virol.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Chinchar VG, Hyatt A, Miyazaki T, Williams T. Family Iridoviridae: poor viral relations no longer. Curr Top Microbiol Immunol. 2009;328:123–70. doi: 10.1007/978-3-540-68618-7_4. [DOI] [PubMed] [Google Scholar]
- Chinchar VG, Wang J, Murti G, Carey C, Rollins-Smith L. Inactivation of frog virus 3 and channel catfish virus by esculentin-2P and ranatuerin-2P, two antimicrobial peptides isolated from frog skin. Virology. 2001;288(2):351–7. doi: 10.1006/viro.2001.1080. [DOI] [PubMed] [Google Scholar]
- Galli L, Pereira A, Marquez A, Mazzoni R. Ranavirus detection by PCR in cultured tadpoles (Rana catesbeiana) from South America. Aquaculture. 2006;257:78–82. [Google Scholar]
- Gantress J, Maniero GD, Cohen N, Robert J. Development and characterization of a model system to study amphibian immune responses to iridoviruses. Virology. 2003;311(2):254–62. doi: 10.1016/s0042-6822(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Goorha R, Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974;60(1):237–50. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Miller DL, Hoverman JT. Ecology and pathology of amphibian ranaviruses. Dis Aquat Organ. 2009;87(3):243–66. doi: 10.3354/dao02138. [DOI] [PubMed] [Google Scholar]
- Harp EM, Petranka JW. Ranavirus in wood frogs (Rana sylvatica): potential sources of transmission within and between ponds. J Wildl Dis. 2006;42(2):307–18. doi: 10.7589/0090-3558-42.2.307. [DOI] [PubMed] [Google Scholar]
- Hoverman JT, Gray MJ, Miller DL. Anuran susceptibilities to ranaviruses: role of species identity, exposure route, and a novel virus isolate. Dis Aquat Organ. 2010;89(2):97–107. doi: 10.3354/dao02200. [DOI] [PubMed] [Google Scholar]
- Hsu E, Julius MH, Du Pasquier L. Effector and regulator functions of splenic and thymic lymphocytes in the clawed toad Xenopus. Ann Immunol (Paris) 1983;134D(3):277–92. doi: 10.1016/s0769-2625(83)80022-1. [DOI] [PubMed] [Google Scholar]
- Jancovich JK, Davids EW, Seiler A, Jacobs BL, Collins JP. Transmission of the Ambystoma tigrinum virus to alternative hosts. Dis Aquat Organ. 2001;46(3):159–63. doi: 10.3354/dao046159. [DOI] [PubMed] [Google Scholar]
- Jarvis MA, Nelson JA. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr Opin Microbiol. 2002;5(4):403–7. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- Majji S, Thodima V, Sample R, Whitley D, Deng Y, Mao J, Chinchar VG. Transcriptome analysis of Frog virus 3, the type species of the genus Ranavirus, family Iridoviridae. Virology. 2009;391(2):293–303. doi: 10.1016/j.virol.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales HD, Abramowitz L, Gertz J, Sowa J, Vogel A, Robert J. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J Virol. 2010;84(10):4912–22. doi: 10.1128/JVI.02486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales HD, Robert J. Characterization of primary and memory CD8 T-cell responses against ranavirus (FV3) in Xenopus laevis. J Virol. 2007;81(5):2240–8. doi: 10.1128/JVI.01104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa A, Murata E, Akita M, Kaneko K, Moriya O, Tomita M, Hayashi H. Roles of macrophages in programmed cell death and remodeling of tail and body muscle of Xenopus laevis during metamorphosis. Histochem Cell Biol. 1998;109(1):11–7. doi: 10.1007/s004180050197. [DOI] [PubMed] [Google Scholar]
- Pearman PB, Garner TW, Straub M, Greber UF. Response of the Italian agile frog (Rana latastei) to a Ranavirus, frog virus 3: a model for viral emergence in naive populations. J Wildl Dis. 2004;40(4):660–9. doi: 10.7589/0090-3558-40.4.660. [DOI] [PubMed] [Google Scholar]
- Rau L, Cohen N, Robert J. MHC-restricted and -unrestricted CD8 T cells: an evolutionary perspective. Transplantation. 2001;72(11):1830–5. doi: 10.1097/00007890-200112150-00020. [DOI] [PubMed] [Google Scholar]
- Robert J. Emerging ranaviral infectious diseases and amphibian decline. Diversity. 2010;2 (Special Issue: “Amphibian Conservation”):314–333. [Google Scholar]
- Robert J, Abramowitz L, Gantress J, Morales HD. Xenopus laevis: a possible vector of Ranavirus infection? J Wildl Dis. 2007;43(4):645–52. doi: 10.7589/0090-3558-43.4.645. [DOI] [PubMed] [Google Scholar]
- Robert J, Cohen N. Evolution of immune surveillance and tumor immunity: studies in Xenopus. Immunol Rev. 1998;166:231–43. doi: 10.1111/j.1600-065x.1998.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Guiet C, Du Pasquier L. Ontogeny of the alloimmune response against a transplanted tumor in Xenopus laevis. Differentiation. 1995;59(3):135–44. doi: 10.1046/j.1432-0436.1995.5930135.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Morales H, Buck W, Cohen N, Marr S, Gantress J. Adaptive immunity and histopathology in frog virus 3-infected Xenopus. Virology. 2005;332(2):667–75. doi: 10.1016/j.virol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Russ JH, Horton JD. Cytoarchitecture of the Xenopus thymus following gamma-irradiation. Development. 1987;100(1):95–105. doi: 10.1242/dev.100.1.95. [DOI] [PubMed] [Google Scholar]
- Schock D, Bollinger T, Chinchar V, Jancovich J, Collins J. Experimental Evidence that Amphibian Ranaviruses Are Multi-Host Pathogens. Copeia. 2008;1:133–143. [Google Scholar]
- Whitley DS, Yu K, Sample RC, Sinning A, Henegar J, Norcross E, Chinchar VG. Frog virus 3 ORF 53R, a putative myristoylated membrane protein, is essential for virus replication in vitro. Virology. 2010;405(2):448–56. doi: 10.1016/j.virol.2010.06.034. [DOI] [PubMed] [Google Scholar]