Abstract
Encapsulation of within reverse micelles dissolved in low viscosity fluids offers a potential solution to the slow tumbling problem presented by large soluble macromolecules to solution NMR spectroscopy. The reduction in effective macromolecular tumbling is directly dependent upon the viscosity of the solvent. Liquid ethane is of sufficiently low viscosity at pressures below 5,000 p.s.i. to offer a significant advantage. Unfortunately, the viscosity of liquid ethane shows appreciable pressure dependence. Reverse micelle encapsulation in liquid ethane often requires significantly higher pressures, which obviates the potential advantages offered by liquid ethane over liquid propane. Addition of co-surfactants or co-solvents can be used to manipulate the minimum pressure required to obtain stable, well-behaved solutions of reverse micelles prepared in liquid ethane. A library of potential additives is examined and several candidates suitable for use with encapsulated proteins are described.
Introduction
In addition to the characterization of soluble proteins [1], the encapsulation of proteins in reverse micelles has the potential to make possible the study by solution NMR of many of the more challenging classes of macromolecules of interest to the structural biologist and biophysicist. These include unstable proteins stabilized by forced folding [2], membrane proteins of various types [3, 4] and even nucleic acids [5]. A key parameter in these studies is the effective rotational correlation time of the macromolecule of interest, which tends to follow the hydrodynamic behavior of the reverse micelle particle itself [1]. The molecular rotational correlation time largely dictates the NMR relaxation behavior of nuclei at all but the internally mobile sites. Encapsulation of macromolecules within the protective water pool of the reverse micelle introduces a large penalty in this respect by the addition of significant volume that influences the rotational correlation time significantly. Though of relatively less importance for larger proteins, the viscosity of the fluid with which the reverse micelle solution is prepared must overcome this volume penalty. This central restraint reduces to a handful the number of fluids that are of sufficiently low viscosity to be of utility: liquid or supercritical carbon dioxide [6] and xenon [7] and the liquid short chain alkanes [1]. Unfortunately, carbon dioxide and xenon are less than ideal reverse micelle solvents. The interaction of the former with the water core makes the stability of the encapsulated protein problematic while there is a limited library of surfactants available for the latter. Of the short chain alkanes, propane and ethane provide the greatest potential for reducing the effective reorientation time of dissolved reverse micelle particles through a viscosity effect [1, 8]. The preparation proteins encapsulated within reverse micelles dissolved in liquid ethane requires the application of significant pressure to maintain high quality solutions with respect to homogeneity, small size and long-term stability [8]. Unfortunately, the bulk viscosity of liquid ethane is strongly pressure dependent [9], which potentially compromises its utility.
Two surfactant systems commonly used for NMR spectroscopy of encapsulated proteins in the low viscosity short chain alkanes are bis(2-ethylhexyl)-sulfosuccinate (AOT) [1] and hexadecyltrimethylammonium bromide(CTAB) [10]. AOT will form reverse micelles by itself in liquid alkanes without the addition of co-surfactants or co-solvents. CTAB however requires a co-surfactant such as hexanol in order for reverse micelles to form. The role of hexanol in this system is not fully understood, but it is thought that hexanol penetrates the surfactant membrane to help stabilize it in the inverted form. Proteins can be successfully encapsulated in AOT and CTAB in liquid ethane and subsequently studied by high resolution NMR [8]. Ethane, being a gas at standard temperature and pressure, obviously requires liquefaction but the pressures required are modest (~610 psi or 42 bar). However, to generate a stable homogeneous solution of encapsulated proteins in AOT with a molar water to surfactant ratio of 10 (water loading or W0) required pressures on the order of 8,000 psi (550 bar) [8]. When only water is used, a pressure of 9,000 psi (620 bar) is required for the stable formation of AOT reverse micelles in ethane at equivalent water loading [11]. These results serve as a point of reference for the data contained in Table 1 and the effectiveness of a particular dielectric modifier. Unfortunately, at these pressures, the viscosity of liquid ethane is comparable to liquid propane defeating the advantage offered by the former solvent. To alleviate this limitation a co-solvent that reduces the encapsulation pressure can be added [8]. With the use of the co-solvent additive even a small encapsulated protein such as ubiquitin could be made to tumble faster than in water [8].
Table 1.
Modification of minimum encapsulation pressure for solutions of AOT reverse micelles in liquid ethanea
| Modifier | Phase at STP |
Density | Refractive Index |
Dipole | Alpha | Viscosity (µPa•s) |
Dielectric Constant |
Encapsulation Pressure (psi) 10%(v/v) added |
Predicted dielectric |
|---|---|---|---|---|---|---|---|---|---|
| Dichloromethane | liquid | 1.325 | 1.424 | 1.60 | 6.48 | 413 | 9.1 | 3500 | 1.94 |
| Chloromethane | gas | 0.991 | 1.89 | 4.72 | 3700 | ||||
| Chlorodifluoromethane | gas | 1.191b | 1.42 | 6.38 | 164b | 6.6e | 3800 | ||
| 1,1-dichloroethane | liquid | 1.176 | 1.416 | 2.06 | 8.64 | 464 | 16.7 | 4000 | 2.07 |
| 1,2-dichloroethane | liquid | 1.256 | 1.444 | 1.86 | 8 | 779 | 10.7 | 4100 | 2.00 |
| Isoflurane | liquid | 4200 | |||||||
| Chloroform | liquid | 1.492 | 1.445 | 1.04 | 9.5 | 537 | 4.8 | 4200 | 1.70 |
| Ethyl dichloroacetate | liquid | 1.28 | 1.438 | 4200 | |||||
| Bromoethane | liquid | 1.425 | 1.425 | 2.05 | 8.05 | 374 | 4.9 | 4300 | 2.15 |
| Bromoform | liquid | 2.89 | 1.595 | 0.99 | 11.8 | 1857 | 4.4 | 4800 | 1.70 |
| Dichloroacetonitrile | liquid | 1.369 | 1.44 | 4900 | |||||
| 1-chloropropane | liquid | 0.892 | 1.388 | 2.05 | 10 | 334 | 5000 | 2.04 | |
| 2-chloropropane | liquid | 0.859 | 1.378 | 2.17 | 10.2 | 303 | 5500 | 2.09 | |
| Difluoromethane | gas | 0.961b | 114b | 14.3f | 6000 | ||||
| Trichlorofluoromethane | liquid | 1.494 | 1.3821 | 421 | 2.5e | 6400 | |||
| 1,1-difluoroethane | gas | 0.899a | 163a | 7600 | |||||
| Hexanol | liquid | 0.814 | 1.418 | 1.65 | 12.8 | 4578 | 13.0 | 2500 | 1.72 |
| 1-butanol | liquid | 0.81 | 1.399 | 1.66 | 8.88 | 2544 | 17.8 | 3300 | 1.82 |
| tert-butanol | liquid | 0.775 | 1.387 | 1.64 | 8.92 | 4312 | 10.7 | 4000 | 1.81 |
| Carbon disulfide | liquid | 1.266 | 1.627 | 352 | 2.2 | 4200 | |||
| Carbon dioxide | gas | 0.711b | 57b | 1.6e | 8000 | ||||
| Benzene | liquid | 0.874 | 1.498 | 0.00 | 10.3 | 604 | 2.4 | 5200 | 1.67 |
| Pentane | liquid | 0.626 | 1.358 | 0.00 | 9.99 | 224 | 1.8 | 5200 | 1.64 |
| Cyclohexene | liquid | 0.811 | 1.446 | 0.33 | 10.7 | 625 | 2.0 | 5300 | 1.66 |
| Diethyl ether | liquid | 0.706 | 1.352 | 1.15 | 10.2 | 224 | 4.3 | 6300 | 1.70 |
| Propane | gas | 0.492b | 1.35d | 0.00 | 6.29 | 97b | 1.5 | 7100 | 1.66 |
| Xenon | gas | 1.148c | 1.34d | 0.00 | 4.04 | 53b | 1.0 | 7500 | 1.66 |
| Argon | supercritical | 0.115c | 1.34d | 0.00 | 1.64 | 24b | 1.0 | 9500 | 1.63 |
| Ethyl acetate | liquid | 0.902 | 1.37 | 1.78 | 9.7 | 423 | 6.4 | 4400 | 1.87 |
| 1-nitropropane | liquid | 0.998 | 1.401 | 3.66 | 8.5 | 798 | 4400 | 3.34 | |
| Valeronitrile | liquid | 0.795 | 1.397 | 4.12 | 10.4 | 17.7 | 4400 | 3.51 | |
| 2-hexanone | liquid | 0.812 | 1.401 | 2.80 | 10 | 583 | 14.6 | 4500 | 2.20 |
for solutions prepared with 200 mM AOT and 2 M H2O (W0 = 10). The solvent parameters and viscosity data for each fluid are for atmospheric pressure and 25 °C, except where noted. Samples were prepared in a mixing chamber described elsewhere [16]. The volume of the mixing chamber was 1.25 mL. The buffer consisted of 50 mM sodium phosphate, pH 7.0 with bromophenol blue (1 mg/mL). A light source was used to allow direct observation through high-pressure windows. Upon encapsulation the solution clarified to a dark purple color. Encapsulation pressures have a precision better than 100 psi. Liquid modifiers were added directly to the chamber and layered on top of the buffer aliquot. The compressibility of each liquid co-solvent was assumed to be insignificant. For gaseous co-solvents the syringe pump used to pressure the sample was preloaded with a 10 % (v/v) mix. This ratio was determined based on the volume of each component at the pressure where both components would liquefy. For supercritical fluids the syringe pump was loaded with components at the supercritical pressure, which was always much more than the pressure required for liquid ethane. The errors on the volume ratios are likely higher for gases than for liquids but these errors are probably not larger than one or two volume percent.
NIST Reference Fluid Thermodynamic and Transport Properties, Version 7.0
NIST Chemistry workbook; taken at 1000 psi, 25°C
Estimate only
Eiseman [17]
Bararo et al. [18]
This behavior appears related to the dielectric constant of the bulk solution. For example, in order for AOT to form a reverse micelle solution with a W0 of 10, the high frequency dielectric constant of the solution must be at least 1.66 [11]. At its liquefaction pressure at 298 K, liquid ethane has a dielectric constant of approximately 1.5 [12]. To raise the dielectric constant of liquid ethane either the pressure must be increased, to increase the molecular density, or a co-solvent added to raise the dielectric of the bulk solution. It is the high frequency dielectric that is most important in this regard. This is especially true for polar compounds. For example, water is reported having a dielectric around 80 when measured in the microwave region, but measurement in the visible range place it at 1.33 [11]. Alkanes, on the other hand, have a nearly frequency independent dielectric.
The key to the studies reported here is the observation by Sen et al. [12] that the addition of 10% (v/v) ethyl acetate to hexane increases the bulk dielectric constant by about 0.2–0.3. An even more pronounced increase could be obtained using acetone. For binary mixtures comprised of nonpolar solvents the change in the dielectric is well represented by the Clausius-Mossotti relationship [12–15]:
| (1) |
where εm is the predicted dielectric of the mixture, νi is the volume fraction, ρi, αi and Mi are, respectively, the mass density, electric polarizability and molecular weight of the ith component. For mixtures where the minor component is polar, an extended version of the more complex Onsager treatment is often employed [12–15].
| (2) |
where ni is the refractive index at 589nm, and μi is the permanent electric dipole moment of the ith component, T is the temperature in Kelvin and kB is Boltzmann’s constant. All other symbols were defined previously.
Though a reverse micelle solution is a much more complex mixture than either the Clausius-Mossotti or extended Onsager relationships are designed to accommodate, they serve as a useful indicator that modulation of the dielectric of reverse micelle solutions can potentially be achieved with addition of small amounts of suitable co-solvents or co-surfactants that would maintain the integrity of the encapsulated protein. This study explores the utility of a range of additives to “standard” reverse micelle solutions based on AOT and CTAB/alcohol systems prepared in liquid ethane with the goal of discovering those that can lead to a significant reduction in the pressure required for optimal encapsulation.
Results
Various physical parameters of potential modifiers of encapsulation pressure for the anionic surfactant AOT that were examined are summarized in Table 1. Also included are the relevant parameters for the Clausius-Mossotti and Onsager relationships and the predicted dielectric constant for the corresponding simple solution, where available. Potential modifiers were generally selected based on their dielectric constant and viscosity. The latter parameter is especially important since any additive is expected to change the viscosity of the bulk solvent, usually increasing it, so the reduction in encapsulation pressure must also be sufficient to overcome this change. Most are soluble in pentane but were typically only sparingly soluble in water. The indicated encapsulation pressures are for 10% (v/v) modifier added. The viscosity of the mixture can be approximated by the summation of the viscosity scaled by the mole fraction of each component at the encapsulation pressure. It is assumed that the viscosity of the liquid additives is not dependent on pressure. This approach works well provided the additive is acting fully as a co-solvent. The encapsulation pressure is expected to increase with surfactant concentration so for these experiments the maximum practical concentration of AOT for NMR spectroscopic performance was selected as a worst-case condition.
Several classes of potential co-solvents were examined. Halogenated compounds can be particularly effective in reducing the pressure required for encapsulation in AOT reverse micelles prepared in liquid ethane. Though the testing was far from exhaustive, chlorinated compounds suppress the encapsulation pressure more than their brominated and fluorinated counterparts. In addition, the fewer the number of carbons, the greater the reduction in encapsulation pressure. Di-substituted compounds are more effective than either mono- or tri-substituted analogs.
Alcohols also reduce the encapsulation pressure for AOT reverse micelles prepared in liquid ethane. The most effective compound tested for AOT reverse micelles was hexanol, which gave an encapsulation pressure of 2,500 psi. Linear alcohols appear to be more effective than branched alcohols and this presumably reflects the requirements of packing of the alcohol within the AOT surfactant shell.
Carbon disulfide and carbon dioxide show an interesting example of how similar reagents can be dramatically different in both the effectiveness and utility in reducing the encapsulation pressure. Carbon dioxide is itself a commonly used compound for preparation of reverse micelles in supercritical fluids. It has a very low viscosity as a liquid, and would seemingly be an ideal compound to use. One limitation of carbon dioxide is that as the pressure is increased the solubility of carbon dioxide in water also increases leading to a host of undesirable effects such as pH shifts and possible protein reactivity leading sample degradation (e.g. hydrolysis). The encapsulation pressure recorded is unchanged from a sample prepared without carbon dioxide. In contrast, carbon disulfide does not lead to sample degradation or protein modification and yields an encapsulation pressure that is nearly 50% less than standard conditions.
We also tested a number of non-polar compounds with dielectric constants only slightly higher than liquid ethane. Nonpolar compounds were initially attractive since the Clausius-Mossotti relation suggested significant advantages. Unfortunately, the experiment indicated that nonpolar compounds are generally less effective at reducing the encapsulation pressure. Results for two noble gases are included in Table 1. It has been shown that the low viscosity liquid xenon by itself can be used to form AOT reverse micelles[7]. Unfortunately, xenon is not even as effective as propane at reducing the encapsulation pressure of AOT reverse micelles prepared in liquid ethane, and another noble gas candidate (argon) actually raised the encapsulation pressure for AOT reverse micelles.
As a class, refrigerants are an attractive option for reduction of encapsulation pressure due to their generally low viscosity. Results with difluoromethane, 1,1-difluoroethane and trichlorofluoromethane were disappointing (Table 1). In contrast, chlorodifluoromethane led to excellent encapsulation pressure reduction but when used to encapsulate the model protein ubiquitin it appeared to interact very unfavorably with the protein causing rapid sample degradation and loss of signal (data not shown). Two refrigerants not listed in Table 1, trifluoromethane and tetrafluoromethane, failed to allow encapsulation within the pressure limits of the apparatus.
A corresponding search for modifiers of encapsulation pressure for reverse micelles based on the cationic surfactant CTAB was also carried out (Table 2). Unlike AOT, the cationic CTAB requires a co-surfactant, hexanol is commonly used, to form reverse micelles. A co-surfactant differs from a co-solvent in that the co-surfactant forms an integral part of the reverse micelle. A key observation is that CTAB reverse micelles in liquid ethane require at least 6.5% hexanol (520 mM) to make a 100 mM CTAB reverse micelle solution and required higher pressure than even an AOT reverse micelle (Table 2). Increasing the concentration of hexanol to 8% lowered the encapsulation pressure to 3,100 psi. This mixture in liquid ethane has excellent hydrodynamic performance [8] indicating that most of the hexanol is integrated within the reverse micelle wall and the slight excess of hexanol (8% compared to 6.5% [v/v] hexanol) does not contribute significantly to the bulk viscosity of the solution. This behavior makes approximating the bulk solution viscosity more difficult since it is less clear the quantity of alcohol that is acting as either the co-surfactant or dielectric modifier. Therefore care should be taken when using excess high viscosity alcohols such as hexanol the purpose of reducing the encapsulation pressure since it may still result in poor hydrodynamic performance.
Table 2.
Modification of minimum encapsulation pressure for solutions of CTAB reverse micelles in liquid ethanea
| Co-surfactant (v/v) | Encapsulation Pressure (psi) |
|---|---|
| 12% octanol | 2100 |
| 12% decanol | 2300 |
| 12% hexanol | 2400 |
| 6.5% octanol, 3.5% decanol | 2500 |
| 10% octanol | 2700 |
| 10% hexanol | 2700 |
| 8% octanol | 2900 |
| 8% hexanol | 3100 |
| 8% 3-cyclopentyl-1-propanol | 6100 |
| 8% pentanol | 9500 |
| 6.5% hexanol | >14000 |
For solutions prepared with 100 mM CTAB and 1.25 M H2O (W0 = 12.5). Samples prepared and tested as described in Table 1.
With these results in mind several other alcohols and combinations of alcohols were examined. Pentanol (9,500psi) does not perform well while octanol is moderately superior to hexanol and gave the lowest encapsulation pressure of 2,100 psi (12% (v/v) octanol). It is not known if the latter condition led to improved hydrodynamic performance over the equivalent sample made with hexanol. Using longer chain alcohols does not appear to offer further decreases in encapsulation pressure. At 12% (v/v) decanol, the encapsulation pressure is 200 psi higher than for octanol. One other alcohol, 3-cyclopentyl-1-propanol was also tested to see if a bulkier chain could improve the performance by requiring less of the reagent. In pentane, only 5.6% (v/v) 3-cyclopentyl-1-propanol was required to form 100mM CTAB reverse micelles compared to 6.5% (v/v) for hexanol. However, in ethane, even at 8% (v/v) concentration, the encapsulation pressure was elevated to 6,100 psi.
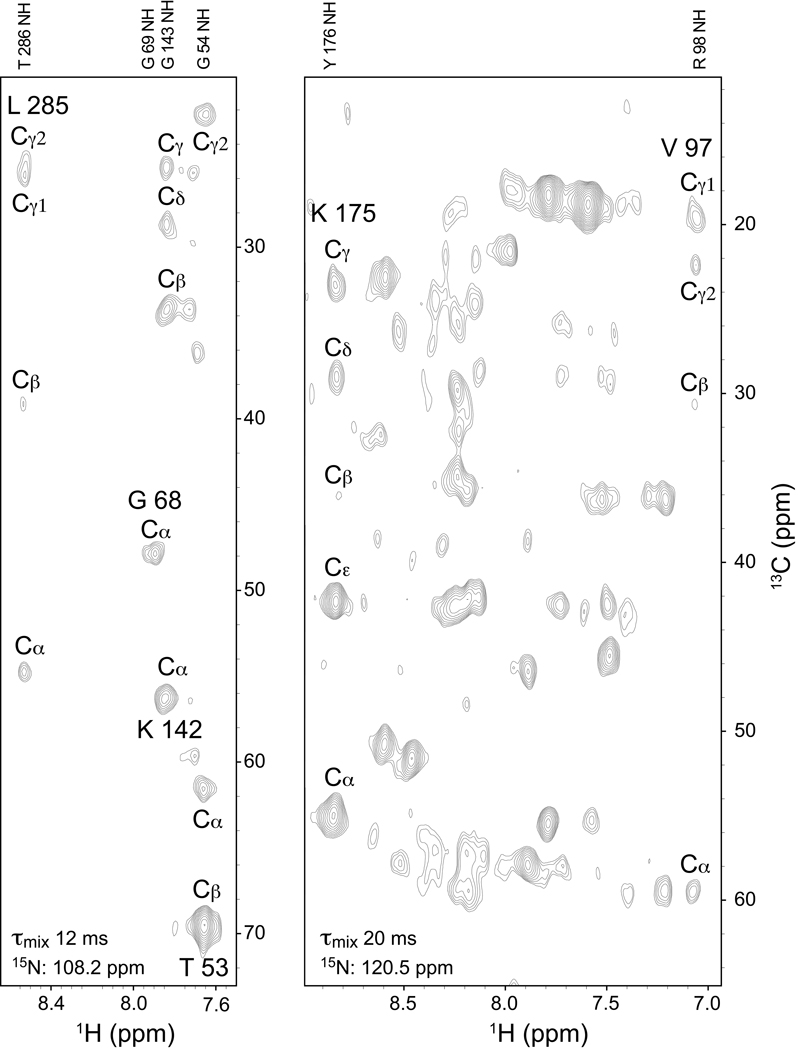
The advantages of optimal downward adjustment of the pressure through use of co-surfactant additives used in preparations of encapsulated proteins dissolved in liquid ethane are illustrated in Figure 1. The 42 kDa monomeric maltose binding protein is optimally encapsulated in CTAB (75 mM) and hexanol (5.6 % v/v; 450 mM) with a suitable encapsulation pressure of 4500 p.s.i (175 bar) in liquid ethane. The protein effectively tumbles sufficiently fast to allow high quality long-range side chain carbon TOCSY correlations resolved on the adjacent amide NH to be obtained [16, 17]. Typically, this experiment is only suitable for small proteins or larger proteins if perdeuterated. The extensive correlations evident in the 15N slices shown in Figure 1 illustrate that the favorable relaxation properties provided by the faster effective molecular reorientation allow experiments typically associated with small protein triple resonance spectroscopy to be carried out on proteins of significant size without the benefit and limitations imposed by use of the TROSY-effect or extensive deuteration.
Figure 1. (H)C(CC)(CO)NH TOCSY spectra of encapsulated 1H,13C,15N-maltose binding protein (MBP) complexed with β-cyclodextrin dissolved in liquid ethane.
Long range correlations of side chain carbons resolved on the adjacent (i+1) amide N-H. Not all peaks shown are centered at the indicated 15N slice. Spectra obtained at 600 MHz (1H) on a Bruker Avance III spectrometer equipped with a cryoprobe. Left Panel: 200 µM MBP in 75 mM CTAB with 450 mM hexanol at a water loading (W0) of 15. The pressure was 4,500 psi. (H)C(CC)(CO) TOCSY spectrum obtained with 64 transients per FID and 32 and 40 complex points in the 15N and 13C increment time domains respectively. A 12 ms DIPSI mixing sequence was used. Right Panel: 150 µM MBP in 75 mM CTAB with 450 mM hexanol at a W0 of 15. (H)C(CC)(CO) TOCSY spectrum obtained with 176 transients per FID and 32 and 40 complex points in the 15N and 13C increment time domains respectively. The pressure was 4,500 psi. A 20 ms DIPSI mixing sequence was used.
Discussion
We have shown that various small molecular additives can be used to significantly reduce the pressure required for the formation of reverse micelles in liquid ethane. This is desirable owing to the large pressure dependence of the bulk viscosity of liquid ethane. More optimal NMR performance is achieved at lower viscosities due to the corresponding decrease in rotational correlation time and concomitant increase in characteristic spin-spin relaxation time constants [1]. Though not quantitatively predictive in this context, commonly used theoretical estimates of the change in dielectric constant due to the introduction of such modifiers in simple solutions can be used to rationally guide the choice of additive. Several useful modifiers have been identified and thereby provide significant flexibility in the preparation and optimization of solutions of encapsulated proteins in liquid ethane. Many potential modifiers have been found to be ineffective. Hopefully, this nascent database will also assist in a search for other additives with the ability to significantly reduce the encapsulation pressure necessary to optimize the NMR performance of reverse micelles prepared in the ultra-low viscosity liquid ethane.
The encapsulation pressures listed in Tables 1 & 2 were obtained using aqueous buffer without protein, and are meant to serve only as a reference point for assessing dielectric modifiers. We have already noted an example where the addition of the protein ubiquitin resulted in an encapsulation pressure in AOT below that for water only. When ubiquitin is encapsulated in AOT in ethane with 10% carbon disulfide (v/v) present the encapsultion pressure is reported to be 3,900 psi [8]. In Table 1 the encapsulation pressure is noted as 4,200 psi. The difference is more pronounced since for technical reasons the ubiquitin sample was transferred to the NMR cell at 300 psi above the encapsulation pressure resulting in a net decrease in 600 psi over simple buffer results in Table 1. For the protein flavodoxin the encapsulation shift is opposite that for AOT and ubiquitin. Flavodoxin is reported to encapsulate in 100 mM CTAB, 8% hexanol and W0 = 12.5 at 4,100 psi [8]. This sample was also transferred at 300 psi above the encapsulation pressure. However, the reported encapsulation pressure in Table 2 for the same conditions was 3,100 psi, for a net increase in encapsulation pressure of 700 psi presumably due to the presence of flavodoxin. It should be expected that other protein and buffer systems may also influence the encapsulation pressure.
Highlights.
Dissolution of encapsulated proteins in low viscosity fluids reduces the tumbling time
The tumbling of the reverse micelle particle is governed by solvent viscosity
Liquid ethane is a promising solvent but its viscosity is pressure dependent
Additive molecules that reduce the encapsulation pressure are identified
Acknowledgements
Supported by NIH grant GM 085120, NSF grant MCB 0842814, a grant from the Mathers Foundation and NIH postdoctoral fellowship GM087099 to N.V.N. R.W.P. and A.J.W. acknowledge a competing financial interest as Members of Daedalus Innovations, a manufacturer of reverse micelle and high pressure NMR apparatus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wand AJ, Ehrhardt MR, Flynn PF. High-resolution NMR of encapsulated proteins dissolved in low-viscosity fluids. Proc. Nat. Acad. Sci. USA. 1998;95:15299–15302. doi: 10.1073/pnas.95.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson RW, Anbalagan K, Tommos C, Wand AJ. Forced folding and structural analysis of metastable proteins. J. Am. Chem. Soc. 2004;126:9498–9499. doi: 10.1021/ja047900q. [DOI] [PubMed] [Google Scholar]
- 3.Kielec JM, Valentine KG, Babu CR, Wand AJ. Reverse micelles in integral membrane protein structural biology by solution NMR spectroscopy. Structure. 2009;17:345–351. doi: 10.1016/j.str.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentine KG, Peterson RW, Saad JS, Summers MF, Xu X, Ames JB, Wand AJ. Reverse micelle encapsulation of membrane-anchored proteins for solution NMR studies. Structure. 2010;18:9–16. doi: 10.1016/j.str.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workman H, Flynn PF. Stabilization of RNA oligomers through reverse micelle encapsulation. J. Am. Chem. Soc. 2005;131:3806–3807. doi: 10.1021/ja8084753. [DOI] [PubMed] [Google Scholar]
- 6.Gaemers S, Elsevier CJ, Bax A. NMR of biomolecules in low viscosity, liquid CO2. Chem. Phys. Lett. 1999;301:138–144. [Google Scholar]
- 7.Meier M, Fink A, Brunner E. Reverse micelles dissolved in supercritical xenon: An NMR spectroscopic study. J. Phys. Chem. B. 2005;109:3494–3498. doi: 10.1021/jp044863g. [DOI] [PubMed] [Google Scholar]
- 8.Peterson RW, Lefebvre BG, Wand AJ. High-resolution NMR studies of encapsulated proteins in liquid ethane. J. Am. Chem. Soc. 2005;127:10176–10177. doi: 10.1021/ja0526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younglove BA, Ely JF. Thermophysical properties of fluids. II. Methane, ethane, propane, isobutane, and normal butane. J. Phys.Chem. Ref. Data. 1987;16:577–798. [Google Scholar]
- 10.Lefebvre BG, Liu W, Peterson RW, Valentine KG, Wand AJ. NMR spectroscopy of proteins encapsulated in a positively charged surfactant. J. Magn. Reson. 2005;175:158–162. doi: 10.1016/j.jmr.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Tingey JM, Fulton JL, Smith RD. Interdroplet attractive forces in AOT water-in-oil microemulsions formed in subcritical and supercritical solvents. J. Phys. Chem. 1990;94:1997–2004. [Google Scholar]
- 12.Sen AD, Anicich VG, Arakelian T. Dielectric constant of liquid alkanes and hydrocarbon mixutres. J. Physics. 1992;D25:516–521. doi: 10.1088/0022-3727/25/3/027. [DOI] [PubMed] [Google Scholar]
- 13.Bottcher CJF. Theory of Electric Polarisation. Houston: Elsevier; 1952. [Google Scholar]
- 14.Bottcher CJF. Theory of Electric Polarization. Vol. 1. New York: Dielectrics in Static Fields, Elsevier; 1973. [Google Scholar]
- 15.Hill NE, Vaughan WE, Price AH, Davies M. Dielectric Properties and Molecular Behavior (The Van Nostrand Series in Physical Chemistry) New York: Van Nostrand Reinhold; 1969. [Google Scholar]
- 16.Grzesiek S, Anglister J, Bax A. Correlation of backbone amide and aliphatic side-chain resonances in C-13/N-15 enriched proteins by isotropic mixing of C-13 magnetization. J. Magn. Reson. Ser B. 1993;101:114–119. [Google Scholar]
- 17.Montelione GT, Lyons BA, Emerson SD, Tashiro M. An efficient triple resonance experiment using C-13 isotropic mixing for determining sequence-specific resonance assignments of isotropically-enriched proteins. J. Am. Chem. Soc. 1992;114:10974–10975. [Google Scholar]