Abstract
Ophthalmic carbonic anhydrase inhibitors have been shown to improve retinal and optic nerve blood flow. However, the relative tissue distributions of commercially available carbonic anhydrase inhibitors to the optic nerve are not known. The objective of this study was to compare the ocular pharmacokinetics and tissue distribution profiles of dorzolamide and brinzolamide after single and multiple topical applications. Pigmented rabbits were treated with single or multiple topical administrations of 30 μl of Trusopt (dorzolamide hydrochloride ophthalmic solution, 2%) to one eye and 30 μl of Azopt (brinzolamide ophthalmic suspension, 1%) to the other eye. Rabbits were euthanized at 10 predetermined time intervals over a period of 24 h, and ocular tissues and plasma samples were collected. For multiple dosing, rabbits were dosed twice per day with an 8-h interval between two doses, groups of rabbits were euthanized at 7, 14, and 21 days at 1 h after the last dose, and ocular tissues and plasma samples were collected. Drug levels in tissue samples were measured using liquid chromatography/tandem mass spectrometry. Pharmacokinetic parameters (Cmax, Tmax, and AUC0–24) were estimated by noncompartmental analysis. After a single dose, dorzolamide delivery (AUC0–24) to the aqueous humor, anterior sclera, posterior sclera, anterior retina, posterior retina, anterior vitreous, and optic nerve was 2-, 7-, 2.6-, 1.4-, 1.9-, 1.2-, and 9-fold higher than those of brinzolamide. Cmax was 2- to 5-fold higher for dorzolamide than that of brinzolamide in all of the ocular tissue. After multiple dosing, dorzolamide levels in the aqueous humor, sclera, retina, vitreous humor, and optic nerve were higher than those of brinzolamide, but statistical significance was achieved only with aqueous humor, vitreous humor, and optic nerve. Dorzolamide levels in the aqueous humor, anterior vitreous, posterior vitreous, and optic nerve were 1.4- to 3.2-, 2.4- to 2.7-, 2.2- to 4.5-, and 2.4- to 5.2-fold higher than those of brinzolamide. Upon multiple dosing, both drugs accumulated in all of the tissues except the conjunctiva, where the drug levels were lower than those observed with single dosing. No significant differences were found in the AUC values of these two drugs in the cornea and conjunctiva after single and multiple dosing. Drug levels were significantly higher in anterior regions than posterior regions in the sclera, retina, and vitreous for both drugs.
Introduction
Glaucoma is the most prevalent eye disease and a leading cause of blindness in the Western industrialized world. Glaucoma is classified as primary when it is not associated with another disease and as secondary when it results from another disease or drug treatment (Lee and Higginbotham, 2005). Glaucoma is classified further as open angle or angle closure glaucoma on the basis of the anatomy of the anterior chamber. Primary open angle glaucoma (POAG) is the most prevalent glaucoma encountered in adults. POAG is progressive multifactorial optic neuropathy that leads to the loss of retinal ganglion cells and optic nerve damage (Siesky et al., 2008). The main etiological outcome of POAG is elevated intraocular pressure (IOP), a major risk factor for optic neuropathy.
Conventional treatment for POAG is the topical application of IOP-lowering drugs. β-Blockers and prostaglandin analogs are first-line treatments for glaucoma that reduce the IOP by decreasing aqueous humor formation and increasing aqueous humor nonconventional outflow, respectively. Second-line treatments of choice for glaucoma are carbonic anhydrase (CA) inhibitors and alpha agonists (Lee and Higginbotham, 2005). CAs are responsible for the production of bicarbonate ion, which is secreted into the posterior segment by the ciliary body along with Na+ as a counter ion (Sugrue, 1996). Inhibition of CAs results in the inhibition of bicarbonate ion production and in the reduction of IOP. Dorzolamide and brinzolamide are drugs of choice as topical CA inhibitors for glaucoma treatment.
Various preclinical as well as clinical reports suggested the involvement of reduced ocular blood flow (OBF) in the pathogenesis of POAG (Schmidt et al., 1998; Harris et al., 1999; Bathija, 2000; Zhao and Cioffi, 2000; Yoshida et al., 2010). The reduced OBF may be primarily of vascular origin or a secondary effect of elevated IOP. Grunwald et al. (1998) showed that the optic nerve blood flow is reduced by 24% in glaucoma patients. Some investigators have hypothesized that the death of retinal ganglion cells and optic nerve head may be due to ischemia as a result of elevated IOP or reduced oxygen supply because of reduced blood flow (Johnson et al., 2000; Gross et al., 2003; Kuehn et al., 2005; Rokicki et al., 2007). It has been shown that CA inhibitors increase the cerebral blood supply after systemic administration (Okazawa et al., 2001) and the ocular blood supply after topical administration (Martinez et al., 1999). To achieve an effect on OBF, topically administered CA inhibitors must reach the inner ocular tissue, such as the retina and optic nerve, in effective concentrations. Various literature reports have shown clearly the effect of dorzolamide on OBF (Harris et al., 1996; Martinez et al., 1999; Martinez and Sanchez, 2007; Martinez and Sánchez-Salorio, 2009). Topical application of dorzolamide results in significant improvement in blood flow to the retina and optic nerve head (Harris et al., 1996; Martinez and Sanchez, 2007). However, very few studies have reported the effect of brinzolamide on OBF (Barnes et al., 2000; Kaup et al., 2004; Siesky et al., 2008). Furthermore, the reports from these studies are not conclusive. Barnes et al. (2000) showed significant improvement in optic nerve head blood flow after topical application of brinzolamide in Dutch Belted rabbits, whereas Kaup et al. (2004) showed no effect of brinzolamide on OBF. A recent clinical comparison of the effects of dorzolamide and brinzolamide on OBF in a POAG patient showed an increase in retrobulbar blood flow with dorzolamide but not with brinzolamide (Martinez and Sánchez-Salorio, 2009).
In this study, we have hypothesized that the effect of dorzolamide on OBF may be due to better delivery of dorzolamide to target tissues, such as the retina and optic nerve, after topical application compared with that of brinzolamide. To evaluate this hypothesis, we have compared the ocular pharmacokinetics and tissue distribution of commercially available dorzolamide and brinzolamide formulations in Dutch Belted rabbits after single and multiple topical dosing.
Materials and Methods
Materials.
The commercial formulation of Trusopt was from Merck & Co., Inc. (Whitehouse Station, NJ). Trusopt contained dorzolamide hydrochloride (2% w/v) along with inactive ingredients including hydroxyethyl cellulose, mannitol, sodium citrate, sodium hydroxide, and water for injection. Benzalkonium chloride (0.0075% w/v) was present as a preservative, and the pH of the solution was approximately 5.6. The osmolarity range specified for the product was 260 to 330 mOsM. The commercial formulation of Azopt was from Alcon Laboratories, Inc. (Fort Worth, TX). Azopt contained brinzolamide (1% w/v) as a sterile ophthalmic suspension along with inactive ingredients including mannitol, carbomer 974P, tyloxapol, sodium edetate, sodium chloride, hydrochloric acid, sodium hydroxide, and water for injection. Benzalkonium chloride (0.01% w/v) was present as a preservative. The pH of the solution was approximately 7.5, and the specified osmolarity was 300 mOsM. Timolol maleate (98%) was purchased from Sigma-Aldrich (St. Louis, MO). High-performance liquid chromatography grade acetonitrile and methanol were purchased from Thermo Fisher Scientific (Waltham, MA). All of the other chemicals and reagents used in this study were of analytical reagent grade.
Methods.
Animals. Animal studies were conducted in accordance with Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and guidelines by animal care committee of the University of Colorado at Denver. A total of 39 male Dutch Belted rabbits in the weight range of 1.8 to 3 kg were used in this study. Rabbits were housed under standard conditions with access to tap water and standard dry pellet rabbit feed ad libitum.
Single Dose Ocular Pharmacokinetics.
Thirty rabbits were used for ocular pharmacokinetic comparison of Trusopt and Azopt after a single topical application. Animals were divided into 10 groups (three animals each). The rabbits were restrained in a rabbit restrainer and were allowed to stabilize for 10 min before dosing. Once the animal was stabilized in a restrainer, drug solution was applied using a positive displacement pipette (10–100 μl; Gilson, Inc., Middleton, WI) and sterile tips. Trusopt was applied randomly to one eye, and Azopt was applied to the other eye of each animal. The volume for the topical ocular dose was 30 μl per eye. To minimize the runoff of the instilled dose, the eyelids were closed gently for a few seconds after dosing. The time of the dose administered was recorded for each animal. At predetermined time intervals after dosing, blood samples were collected from the marginal ear vein. Animals were euthanized by intravenous injection of sodium pentobarbitone (150 mg/kg) into the marginal ear vein. Eyes were enucleated using surgical accessories and snap-frozen immediately in a dry ice/isopentane bath and stored at −80°C until dissection. The dry ice/isopentane bath was prepared in a stainless steel container, and a ceramic tile was placed over the container and allowed to cool for 15 min. The eyes were removed from −80°C and placed in the dry ice container pending dissection.
Multiple Dose Ocular Tissue Distribution.
Nine rabbits were used for comparison of ocular tissue distribution profiles of Trusopt and Azopt after multiple topical applications. Rabbits were divided into three groups (three animals each). Rabbits received 30 μl of Trusopt in the right eye and 30 μl of Azopt in the left eye twice per day with 8-h intervals between the doses. Group 1 received 14 doses over 7 days, group 2 received 21 doses over 14 days, and group 3 received 42 doses over 21 days. Blood samples were collected from the marginal ear vein at 1 h after the last dose. Immediately after blood collection, animals were euthanized by intravenous sodium pentobarbitone (150 mg/kg) injection into the marginal ear vein. Eyes then were enucleated using surgical accessories and snap-frozen immediately in a dry ice/isopentane bath and stored at −80°C until dissection.
Eye Dissection and Collection of Various Ocular Tissues.
Enucleated eyeballs were dissected, while frozen, to isolate various ocular tissues. All of the dissection procedures were performed on a cooled ceramic tile to avoid thawing of the eyeball during dissection. After the separation of the anterior part, the remaining posterior globe was cut into two parts, at one third of the distance from the lens and two thirds from the posterior wall, and two parts of the retina, choroid, vitreous, and sclera were separated. A new surgical blade was used for each eye. To prevent transfer of drugs between tissues of each eye, the surgical accessories were rinsed thoroughly with saline followed by methanol followed by saline and blotted dry after and between uses on each tissue. All of the samples were weighed and stored at −80°C until further processing.
Tissue Sample Processing.
Drug content in rabbit ocular tissues was estimated after the extraction of the drugs from the tissues by double liquid-liquid extraction. In brief, the ocular tissues were mixed with 500 μl of 0.1 M Tris buffer (pH 8.5) and 5 μl of 20 μg/ml timolol (internal standard) in 4-ml glass tubes, vortexed for 10 min, and then homogenized using a hand-homogenizer in an ice bath. Ethyl acetate (1.5 ml) was added to this homogenate and vortexed for 10 min on a multitube vortexer (VWR, West Chester, PA). The organic layer was separated by centrifugation at 3000g for 10 min. A total of 1.5 ml of ethyl acetate was added again to the aqueous layer to perform the double extraction and vortexed for 10 min and centrifuged at 3000g for 10 min. The separated organic layers were combined and transferred into clean glass tubes and evaporated under a nitrogen stream (Multi-Evap; Organomation, Berlin, MA) at 40°C. The residue after evaporation was reconstituted with 250 μl of acetonitrile/water (75:25 v/v) and subjected to liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis. The liquid-liquid extraction method for the extraction of dorzolamide and brinzolamide from rabbit ocular tissue was validated to determine the extraction recovery using three different concentrations (low, medium, and high) to cover the entire range of expected concentrations of dorzolamide and brinzolamide in the various tissues.
The aqueous humor and vitreous samples were analyzed directly after dilution, without liquid-liquid extraction. In brief, the aqueous humor and vitreous samples were diluted 5- and 2.5-fold, respectively, with acetonitrile containing timolol as the internal standard, vortexed for 10 min, and centrifuged at 10,000g for 5 min. The supernatant (200 μl) was transferred into LC-MS/MS vials and subjected to analysis.
Calibration standards were prepared at 10 concentrations in the appropriate blank rabbit ocular tissues by spiking known amounts of the analytes and internal standard. Quality control samples were prepared by spiking blank rabbit tissues with three different concentrations of analytes to cover the entire calibration range. Both calibration and quality control samples were processed on the same day along with study samples.
LC-MS/MS Analysis.
Tissue levels of dorzolamide and brinzolamide in ocular tissue samples were measured by means of LC-MS/MS. An API-3000 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) coupled with a Series 200 liquid chromatography system (PerkinElmer Life and Analytical Sciences, Waltham, MA) was used for the analysis. Analytes were separated on Zorbax (Agilent Technologies, Santa Clara, CA) extended C18 column (2.1 × 50 mm, 5 μm) using 5 mM ammonium formate in water (A) and acetonitrile (B) as the mobile phase. A linear gradient elution at a flow rate of 0.3 ml/min with a total run time of 6 min was as follows: 60% A (0–0.8 min), 10% A (2.0–4.0 min), and 60% A (5.0–6.0 min). Dorzolamide, brinzolamide, and timolol (internal standard) were analyzed in positive ionization mode with the following multiple reaction monitoring transitions: 325 → 199 (dorzolamide), 384 → 281 (brinzolamide), and 317 → 261 (timolol).
Pharmacokinetic and Statistical Analysis.
Pharmacokinetic analysis was performed by noncompartmental analysis using WinNonlin software (version 1.5; Scientific Consulting, Inc., Cary, NC). Statistical comparisons between two experimental groups were performed using an independent samples Student's t test. The comparison of the means between multiple ocular tissues of same group was performed using one-way analysis of variance followed by Tukey's post hoc analysis (SPSS, version 11.5; SPSS, Inc., Chicago, IL). The results were considered statistically significant at P < 0.05.
Results
LC-MS/MS Method for Dorzolamide and Brinzolamide.
A simple, selective, and sensitive LC-MS/MS method was developed for the simultaneous analysis of brinzolamide and dorzolamide in rabbit ocular tissues. Timolol was chosen as an internal standard because its log P and pKa values are close to those of brinzolamide and dorzolamide. A representative chromatogram of the analytes along with the internal standard is shown in Supplemental Fig. 1. A simple liquid-liquid extraction method was developed for the extraction of analytes from the rabbit ocular tissue. Both the analytes and the internal standard have basic pKa values; therefore, a basic pH was used during the extraction to keep the analytes in the un-ionized form. Ethyl acetate was used as the organic solvent for the extraction because both analytes have good solubility in ethyl acetate. The method was validated for the extraction recovery at three different concentrations (low, medium, and high quality control samples) (i.e., 25, 250, and 1250 ng/ml). The extraction recovery of the internal standard was estimated at the medium concentration. The mean extraction recoveries (mean ± S.D., n = 3) in the rabbit eye tissues are shown in Table 1.
TABLE 1.
Mean extraction recovery of dorzolamide and brinzolamide in rabbit ocular tissues
Data are expressed as mean ± S.D. for n = 3. Extraction recovery was calculated as the ratio of the analyte peak area spiked before extraction to the analyte peak area of the unextracted standard multiplied by 100.
| Tissue Name | % Extraction Recovery of Dorzolamide |
% Extraction Recovery of Brinzolamide |
||||
|---|---|---|---|---|---|---|
| Low QC | Medium QC | High QC | Low QC | Medium QC | High QC | |
| 25 ng/ml | 250 ng/ml | 1250 ng/ml | 25 ng/ml | 250 ng/ml | 1250 ng/m | |
| Cornea | 72.0 ± 3.1 | 68.28 ± 9.33 | 68.97 ± 3.95 | 63.9 ± 4.75 | 67.32 ± 9.16 | 73.11 ± 3.67 |
| Aqueous humor | 76.1 ± 5.84 | 81.7 ± 6.06 | 71.69 ± 4.05 | 73.05 ± 6.55 | 81.43 ± 7.96 | 79.96 ± 5.12 |
| Conjunctiva | 69.19 ± 6.91 | 70.02 ± 5.03 | 70.83 ± 1.19 | 66.06 ± 4.27 | 70.83 ± 6.64 | 74.96 ± 2.75 |
| Vitreous | 74.28 ± 11.28 | 67.24 ± 4.31 | 77.39 ± 8.27 | 70.7 ± 6.57 | 68.23 ± 7.09 | 75.48 ± 6.82 |
| Retina | 63.6 ± 3.38 | 70.67 ± 7.11 | 69.37 ± 2.54 | 62.57 ± 7.6 | 73.08 ± 3.75 | 75.68 ± 3.61 |
| Optic nerve | 75.29 ± 10.47 | 68.64 ± 11.76 | 67.8 ± 3.60 | 65.22 ± 5.07 | 71.71 ± 3.69 | 78.68 ± 4.66 |
| Sclera | 65.97 ± 8.03 | 78.89 ± 6.27 | 73.02 ± 9.45 | 66.33 ± 6.97 | 80.97 ± 5.90 | 73.96 ± 7.45 |
Single Dose Ocular Pharmacokinetics.
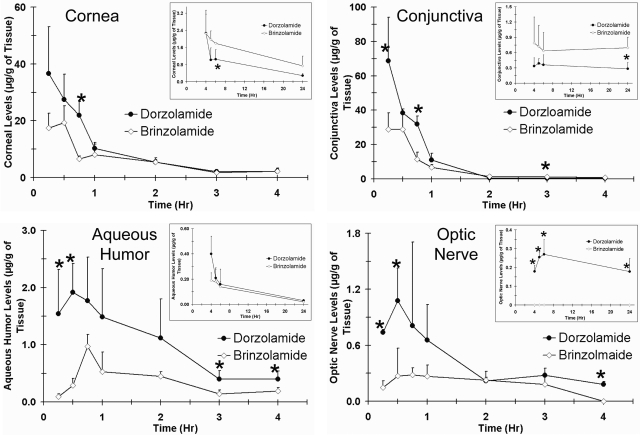
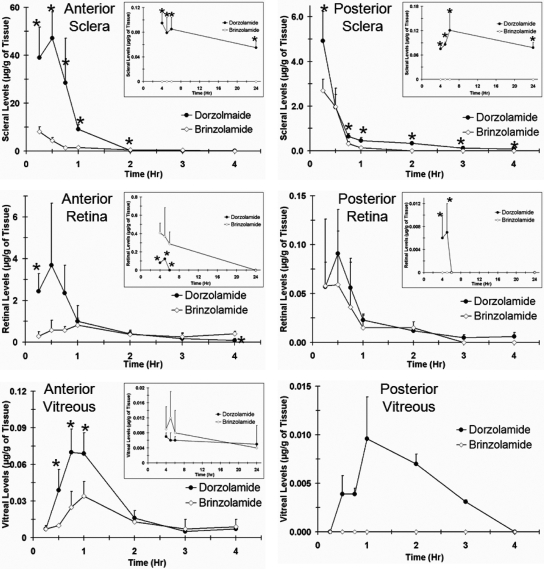
The concentration-time profiles of dorzolamide and brinzolamide after a single topical administration in cornea, conjunctiva, and aqueous humor are shown in Fig. 1. Data for the posterior segment tissues are shown in Fig. 2. Dorzolamide showed significantly higher drug levels in the aqueous humor, sclera, retina, vitreous, and optic nerve than those of brinzolamide for the initial few hours. Both dorzolamide and brinzolamide rapidly distributed to the inner ocular tissue after topical application. The maximum concentration of dorzolamide in the aqueous humor occurred within 30 min, whereas that of brinzolamide occurred after 45 min. Aqueous humor levels of dorzolamide at the peak times were 1.8-fold higher than those of brinzolamide. Both dorzolamide and brinzolamide showed regioselective distribution in the sclera, retina, and vitreous. Drug levels in the anterior part of the sclera, retina, and vitreous were significantly higher than those in the posterior parts. Measurable drug levels were observed in the anterior vitreous and retina for both drugs over a duration of 24 h. No drug levels were detected in the posterior vitreous for brinzolamide at any time point, whereas dorzolamide was detected in the posterior vitreous up to 3 h after dosing. Drug levels were not detected in the plasma for both dorzolamide and brinzolamide. A summary of pharmacokinetic parameters estimated by noncompartmental analysis is shown in Table 2. The area under the curve [AUC0–24 (μg h/ml or g)] of dorzolamide was 2-fold higher in the aqueous humor than that of brinzolamide, whereas there was no significant difference for AUC0–24 in the cornea and conjunctiva. The AUC0–24 of dorzolamide was significantly higher than that of brinzolamide for all of the posterior segment tissues. The AUC0–t value of dorzolamide was 7-fold higher in the anterior sclera, 3.5-fold higher in the posterior sclera, 1.5-fold higher in the anterior retina, 1.9-fold higher in the posterior retina, 4-fold higher in the anterior vitreous, and 9-fold higher in the optic nerve compared with those of brinzolamide. The peak concentration (Cmax) was 2- to 5-fold higher for dorzolamide than that for brinzolamide in all of the ocular tissue. After a single dose topical administration, dorzolamide showed significantly enhanced delivery to the posterior segment ocular tissue compared with that of brinzolamide.
Fig. 1.
Dorzolamide showed better delivery to the cornea, conjunctiva, aqueous humor, and optic nerve than that of brinzolamide after single topical application in pigmented rabbits. Plots show concentration versus time profiles in the cornea, conjunctiva, aqueous humor, and optic nerve of pigmented rabbits after single topical ocular administration of Trusopt (dorzolamide hydrochloride, 2.0%) or Azopt (brinzolamide suspension, 1.0%). Data represent mean ± S.D. for n = 3. *, P ≤ 0.05, significantly different from brinzolamide.
Fig. 2.
Dorzolamide showed better delivery to the sclera, retina, and vitreous than that of brinzolamide after single topical application in pigmented rabbits. Plots show concentration versus time profiles in the sclera, retina, and vitreous of pigmented rabbits after single topical ocular administration of Trusopt (dorzolamide hydrochloride, 2.0%) or Azopt (brinzolamide suspension, 1.0%). Data represent mean ± S.D. for n = 3. *, P ≤ 0.05, significantly different from brinzolamide.
TABLE 2.
Pharmacokinetic parameters estimated for dorzolamide and brinzolamide after single topical ocular administration of Trusopt (dorzolamide hydrochloride solution, 2%) and Azopt (brinzolamide suspension, 1%) to pigmented rabbit
Parameters were estimated using noncompartmental analysis (WinNonlin, version 1.5; Pharsight, Inc.). For brinzolamide, no drug levels were detected in the posterior vitreous.
| Tissue | Dorzolamide |
Brinzolamide |
||||||
|---|---|---|---|---|---|---|---|---|
| Cmax | Tmax | T1/2 | AUC0–24 | Cmax | Tmax | T1/2 | AUC0–24 | |
| μg/g | h | h | μg h/ml or g | μg/g | h | h | μg h/ml or g | |
| Cornea | 36.61 | 0.25 | 1.08 | 51.21 | 19.27 | 0.50 | 1.71 | 50.88 |
| Conjunctiva | 68.74 | 0.25 | 0.720 | 49.55 | 28.80 | 0.25 | 1.01 | 37.94 |
| Aqueous humor | 1.92 | 0.50 | 7.06 | 6.159 | 0.97 | 0.75 | 6.16 | 3.14 |
| Sclera (anterior) | 47.18 | 0.50 | 0.559 | 36.13 | 8.17 | 0.25 | 0.929 | 5.22 |
| Sclera (posterior) | 4.92 | 0.25 | 1.09 | 3.46 | 2.70 | 0.25 | 0.134 | 1.34 |
| Retina (anterior) | 3.68 | 0.50 | 0.87 | 3.46 | 0.82 | 1.0 | 3.94 | 2.42 |
| Retina (posterior) | 0.091 | 0.50 | 1.23 | 0.095 | 0.059 | 0.50 | 0.812 | 0.051 |
| Vitreous (anterior) | 0.07 | 0.75 | 1.99 | 0.208 | 0.034 | 1.0 | 8.07 | 0.185 |
| Vitreous (posterior) | 0.0096 | 1.0 | 1.23 | 0.018 | – | – | – | – |
| Optic nerve | 1.08 | 0.50 | 2.77 | 6.16 | 0.28 | 0.75 | 3.52 | 0.66 |
Cmax, the maximum observed drug concentration in a particular tissue; Tmax, the time at which Cmax occurs; T1/2, the half-life of the drug in tissue; AUC0–24, the area under the curve obtained by plotting the concentration-time data, where t is the last time point at which drug concentration was measured.
–, Pharmacokinetic parameters are not available due to undetected drug levels.
Distribution of Dorzolamide and Brinzolamide in the Ocular Tissue after Multiple Topical Dosing.
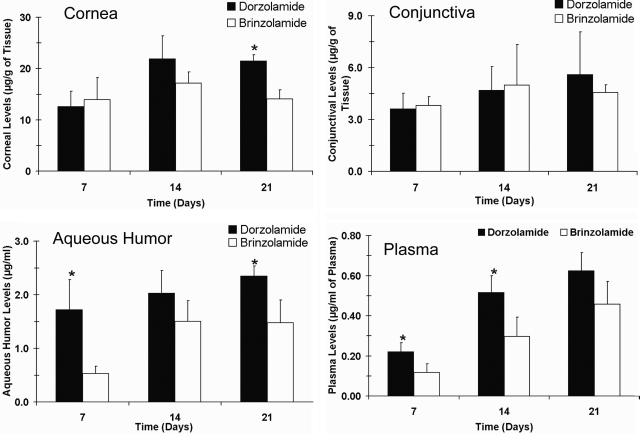
The tissue distribution of dorzolamide and brinzolamide after multiple topical applications in the anterior segment ocular tissues and plasma on days 7, 14, and 21 are shown in Fig. 3. Drug levels in the conjunctiva and cornea were similar for both drugs at all three time points. Aqueous humor levels of dorzolamide were 1.4- to 3.2-fold higher than those of brinzolamide, with the levels being statistically different on days 7 and 21. Plasma dorzolamide levels were 1.3- to 1.9-fold higher than those of brinzolamide, with the levels being significantly different on days 7 and 14.
Fig. 3.
Dorzolamide showed better delivery to the aqueous humor and plasma than that of brinzolamide after multiple topical dosing in pigmented rabbits. Plots show tissue levels of dorzolamide and brinzolamide in the cornea, conjunctiva, aqueous humor, and plasma after multiple topical ocular administration of Trusopt (dorzolamide hydrochloride, 2.0%) or Azopt (brinzolamide suspension, 1.0%). Animals were sacrificed at 1 h after the last dose. Data represent mean ± S.D. for n = 3. *, P ≤ 0.05, significantly different from brinzolamide.
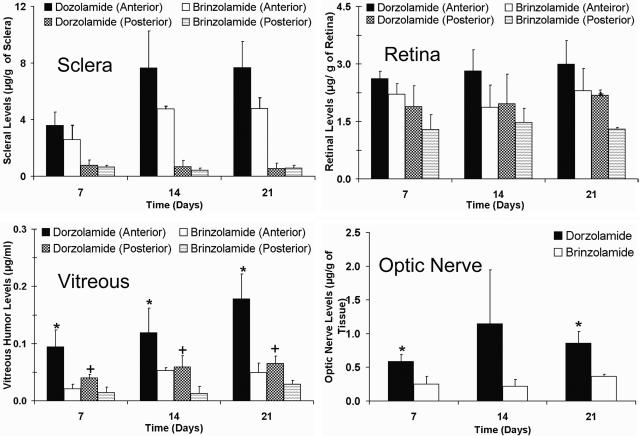
Distribution of dorzolamide and brinzolamide to the sclera, retina, vitreous, and optic nerve after multiple topical dosing is shown in Fig. 4. Both drugs exhibited regioselective distribution in the sclera, retina, and vitreous, with the drug levels in the anterior sclera, retina, and vitreous being significantly higher than those in the posterior sclera, retina, and vitreous, respectively. Between the two drugs, dorzolamide levels in the anterior sclera and retina were higher than those of brinzolamide at all three time points, but the differences were not statistically significant. Dorzolamide levels were 1.4- to 1.6-fold higher in the anterior sclera and 1.2- to 1.5-fold higher in the anterior retina compared with those of brinzolamide. Dorzolamide levels were 1.1- to 1.6-fold higher in the posterior sclera and 1.3- to 1.8-fold higher in the posterior retina compared with those of brinzolamide. The dorzolamide concentrations in both the anterior and the posterior vitreous and optic nerve were significantly higher than those of brinzolamide. Concentrations of dorzolamide were 2.4- to 2.7-fold higher in the anterior vitreous and 2.2- to 4.5-fold higher in the posterior vitreous compared with those of brinzolamide. Furthermore, dorzolamide levels in the optic nerve were 2.4- to 5.2-fold higher compared with those of brinzolamide.
Fig. 4.
Dorzolamide showed better delivery to the posterior segment eye tissues than that of brinzolamide after multiple topical dosing in pigmented rabbits. Plots show tissue levels of dorzolamide and brinzolamide in the sclera, retina, vitreous, and optic nerve after multiple topical ocular administration of Trusopt (dorzolamide hydrochloride, 2.0%) or Azopt (brinzolamide suspension, 1.0%). Animals were sacrificed at 1 h after the last dose. Data represent mean ± S.D. for n = 3. *, P ≤ 0.05, significantly different from brinzolamide (anterior); †, P ≤ 0.05, significantly different from brinzolamide (posterior).
Discussion
Our studies with commercially available formulations of dorzolamide and brinzolamide in pigmented rabbits indicated that dorzolamide delivery to the aqueous humor, vitreous humor, and optic nerve is significantly higher than that of brinzolamide after both single and multiple topical applications. Both drugs exhibited regioselective distribution in the sclera, retina, and vitreous, with drug levels in the anterior regions of these tissues being higher compared with those in the posterior regions.
Topically applied CA inhibitors enhance the retinal and optic nerve blood flow, in addition to reducing IOP (Martinez et al., 1999; Martinez and Sánchez-Salorio, 2009). In humans, dorzolamide treatment resulted in 3.8-fold higher peak systolic velocity and 6.7-fold higher end diastolic velocity in the central retinal artery than brinzolamide treatment (Martinez and Sánchez-Salorio, 2009). Furthermore, the central retinal artery resistance index was reduced by dorzolamide, whereas brinzolamide did not have any effect on this index. To achieve their effects on OBF, topically applied CA inhibitors must reach the retina and optic nerve at therapeutically effective concentrations after topical application. The premise of this study was that the above differences between dorzolamide and brinzolamide may be explained on the basis of delivery differences to target tissues such as the retina and optic nerve.
In our study, dorzolamide showed enhanced delivery to the aqueous humor, vitreous humor, and optic nerve compared with that of brinzolamide, in both single and multiple topical dosing regimens (Figs. 1–4), possibly due to differences in drug physicochemical properties such as lipophilicity and solubility. The amount of the drug that crosses biological barriers to reach its target site depends on drug physicochemical properties including lipophilicity, water solubility, and molecular size. Because dorzolamide (324 Da) and brinzolamide (383 Da) have similar molecular masses, differences in lipophilicity and water solubility might explain the enhanced drug delivery observed with dorzolamide.
The log D values of brinzolamide and dorzolamide at pH 7.4 are 6.6 and 1.72, respectively (DeSantis, 2000). Schoenwald and colleagues showed that the optimum log D value for corneal permeability was between 2.0 and 3.0, with a further increase in lipophilicity resulting in lower corneal permeability (Schoenwald and Ward, 1978; Schoenwald and Huang, 1983). Thus, an increase in drug lipophilicity beyond a certain point may not be beneficial for corneal transport, possibly due to a trade-off in drug solubility. The higher lipophilicity of brinzolamide (log P = 6.6) may hinder its corneal permeability and entry into the aqueous humor. Dorzolamide with a log D value of 1.72 is within the optimum range of pH for corneal transport. Indeed, the apparent in vitro permeability of dorzolamide (10 μM dorzolamide solution in a balanced salt solution) across the excised rabbit cornea was 7.5-fold higher (1.5 × 10−6 cm/s) (Xiang et al., 2009) compared with that of the highly lipophilic brinzolamide (Azopt 1% suspension) (0.20 × 10−6 cm/s) (Palma et al., 2009). However, normalization of brinzolamide in vitro permeability with soluble concentration (0.05%) indicated that the actual permeability of brinzolamide is 4.0 × 10−6 cm/s, which is 2.66-fold higher than that of dorzolamide. The dosing strength of the dorzolamide drop was 2%, which is 2-fold higher than that of the brinzolamide eye drop (1%), and the soluble concentration for dorzolamide in the eye drop was 40-fold higher. Furthermore, at physiological pH (7.4), the aqueous solubility of dorzolamide is 0.67%, which is 13.4-fold higher than the aqueous solubility of brinzolamide (0.05%) (DeSantis, 2000). Thus, the major reason for the higher delivery of dorzolamide to the aqueous humor appears to be its higher concentration-dependent flux across the cornea than that of brinzolamide. Consistent with these differences in dosing strength and solubility, after single dose topical application, the Cmax and AUC0–t values of dorzolamide in the aqueous humor were 2-fold higher than those of brinzolamide (Table 2).
Although the Cmax levels in the cornea and conjunctiva in the single dose study were 1.9- to 2.4-fold higher for dorzolamide than those for brinzolamide, there was no significant difference in the exposure (AUC0–t) of dorzolamide and brinzolamide to these surface tissues. Brinzolamide was administered as a suspension, which was potentially retained on the ocular surface for a longer duration compared with a solution. Indeed, Gupta et al. (2010) showed a significant reduction in precorneal drainage rate for a sparfloxacin nanosuspension compared with that for a sparfloxacin solution after topical application in a rabbit model. Furthermore, brinzolamide, due its highly lipophilic nature, may have entered the cornea and conjunctiva well but did not partition well out of the surface tissues into the underlying fluid compartments.
After the administration of nipradilol by topical, intracameral, and subtenon routes, it was shown using autoradiography that topically applied nipradilol reaches the choroid-retina via the conjunctival and transscleral pathway (Mizuno et al., 2009). Higher delivery of the drug from the conjunctiva and sclera into the posterior segment ocular tissues (retina, vitreous, and optic nerve) occurs when the drug is present at a higher concentration in the periocular space. In our single dose study, the Cmax values of dorzolamide were 1.9-fold higher in the conjunctiva and 5.8-fold higher in the anterior sclera than those of brinzolamide. This is consistent with the concentration-dependent flux of dorzolamide to the posterior segment tissues via the conjunctival and transscleral pathway. In our single dose study, dorzolamide showed significantly higher delivery (both AUC and Cmax) to the vitreous and optic nerve than that of brinzolamide. Optic nerve levels of dorzolamide and brinzolamide were significantly higher than those of the vitreous and posterior retina, which is consistent with a previously published report for betaxolol (Holló et al., 2006). Maurice (2002) suggested that drug entry into the retrobulbar space via the conjunctival pathway contributes toward optic nerve drug delivery.
The multiple dosing study was undertaken to compare the effects of repeated instillation on tissue accumulation and distribution profiles of dorzolamide and brinzolamide. A comparison of drug levels in the ocular tissue after multiple and single dose studies showed that the drug levels in the cornea, aqueous humor, retina, and vitreous were 1.6- to 90-fold higher after multiple dosing than single dosing (Table 3). After multiple dosing, dorzolamide exhibited greater drug levels in all of the tissues except the posterior sclera, where the levels were equal for both drugs. Multiple dosing was particularly beneficial in enhancing the delivery of both drugs to the posterior regions of the back of the eye tissues including the sclera, retina, and vitreous humor. However, multiple dosing resulted in a decline in the drug levels in the conjunctiva compared with those of single dosing, possibly due to enhanced drug clearance. We speculate that both dorzolamide and brinzolamide might enhance conjunctival blood flow with repeated topical dosing. Optic nerve, however, did not experience such a reduction in delivery upon multiple dosing. It should be noted that the optic nerve also may receive the drug from systemic circulation and that the drug levels in the plasma were higher with multiple dosing. Drug accumulation was also evident in the cornea and aqueous humor for both drugs after multiple dosing. Although the drug accumulation indices were comparable between both drugs in the anterior retina, posterior retina, and optic nerve, the accumulation indices in the anterior as well as posterior sclera were greater for brinzolamide. On the basis of our dosing regimens and the half-lives extracted from the terminal phases in single dose studies, the estimated accumulation index with multiple dosing in various tissues is close to 1. In spite of this, nearly all of the tissues except the conjunctiva and optic nerve exhibited significant drug accumulation upon multiple dosing. The reasons for such accumulation are unclear at this stage. Either enhanced drug uptake or reduced clearance in several tissues upon multiple dosing might contribute to this effect.
TABLE 3.
Comparison of mean drug levels in ocular tissues after single and multiple topical dosing (42 doses)
Drug levels were assessed at 1 h after the last dose. To assess drug accumulation, ratios of the drug levels between multiple and single dose regimens were calculated. Brinzolamide was not detected in the posterior vitreous after single dose administration.
| Tissue | Dorzolamide |
Brinzolamide |
||||
|---|---|---|---|---|---|---|
| Single Dose | Multiple Dose | Multiple Dose/Single Dose Ratio | Single Dose | Multiple Dose | Multiple Dose/Single Dose Ratio | |
| μg/g | μg/g | μg/g | μg/g | |||
| Cornea | 10.31 | 21.4 | 2.08 | 7.99 | 14.1 | 1.76 |
| Conjunctiva | 10.10 | 5.89 | 0.60 | 6.77 | 4.57 | 0.68 |
| Aqueous humor | 1.49 | 2.35 | 1.58 | 0.530 | 1.48 | 2.80 |
| Sclera (anterior) | 9.12 | 7.67 | 0.85 | 1.57 | 4.20 | 2.60 |
| Sclera (posterior) | 0.460 | 0.56 | 1.21 | 0.140 | 0.57 | 4.07 |
| Retina (anterior) | 1.02 | 3.00 | 2.94 | 0.821 | 2.30 | 2.80 |
| Retina (posterior) | 0.023 | 2.10 | 90.13 | 0.015 | 1.30 | 86.7 |
| Vitreous (anterior) | 0.07 | 0.17 | 2.43 | 0.034 | 0.05 | 1.47 |
| Vitreous (posterior) | 0.0096 | 0.065 | 6.80 | – | 0.029 | – |
| Optic nerve | 0.657 | 0.86 | 1.31 | 0.269 | 0.370 | 1.38 |
–, Pharmacokinetic parameters are not available due to undetected drug levels.
Both dorzolamide and brinzolamide showed regioselective distribution patterns in the sclera, retina, and vitreous after single as well as multiple topical dosing (Figs. 2 and 4), with the drug levels in the anterior parts of these tissues being significantly higher than those in the posterior parts. However, the extent of drug accumulation in the posterior segment was higher for dorzolamide than that for brinzolamide. Using autoradiography in albino rabbits, Mizuno et al. (2009) showed regioselective distribution of nipradilol in the posterior segment after topical application. Nipradilol levels in equatorial choroid-retina were 11.7-fold higher than those in posterior choroid-retina. Furthermore, the nipradilol levels in the periocular tissue around the equator region were 8.6-fold higher than those in the periocular tissue near the optic nerve. Furthermore, Holló et al. (2006) also showed that after topical application of betaxolol in Cynomolgus monkey, drug levels in the anterior part were significantly higher than those in the posterior part of the back of the eye. Such regional differences in the tissue distribution after topical administration might be because the conjunctiva covers only the anterior part of the globe and makes an intimate contact with the sclera in this region, allowing ready exposure of the drug to the sclera and underlying tissues in the anterior part of the globe. In addition, differential binding of brinzolamide and dorzolamide to the pigment in the choroid-retinal pigmented epithelium also may contribute to relative differences in their delivery. A single dose did not result in detectable drug levels in the plasma for both drugs, whereas multiple dosing resulted in significant drug levels in the plasma for both drugs. CA inhibitors preferentially bind to CA in erythrocytes (Iester, 2008). After single topical application, a significant amount of drug binds to the CA in erythrocytes, potentially leaving the concentration of the plasma below the quantification limits. With multiple dosing, CA in erythrocytes might become saturated, resulting in significant drug levels in the plasma.
In summary, dorzolamide showed higher delivery to the aqueous humor, vitreous, and optic nerve than brinzolamide after single and multiple topical administrations in pigmented rabbits. Higher tissue levels of dorzolamide in the vitreous and optic nerve might be the reason for its greater effects on OBF in the retina and optic nerve compared with those of brinzolamide. Furthermore, the drug distribution within the ocular tissues sclera, retina, and vitreous is regioselective for both brinzolamide and dorzolamide. Although the drug levels were greater with dorzolamide with multiple dosing, the drug accumulation index was >80 for both drugs in the posterior retina, and there was significant accumulation in the posterior vitreous.
Supplementary Material
Acknowledgments
We thank Puneet Tyagi and Vidhya Rao for assistance during rabbit studies and Dr. Michael Wempe of the University of Colorado at Denver LC-MS/MS core facility for the analysis of some of the samples with ultra-low drug quantities.
This work was supported in part by the National Institutes of Health National Eye Institute [Grants R01-EY018940, R01-EY017533]; and a research grant from Merck and Co. Inc.
This work was presented in part as follows: Jadhav GR, Kadam RS, Tyagi P, Ogidigben MJ, and Kompella UB (2011) Comparison of ocular pharmacokinetics of dorzolamide and brinzolamide in pigmented rabbit after single and multiple topical administrations. ARVO 2011 Annual Meeting; 2011 May 1–5; Fort Lauderdale, FL. Association for Research in Vision and Ophthalmology, Rockville, MD.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.040055.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- POAG
- primary open angle glaucoma
- IOP
- intraocular pressure
- CA
- carbonic anhydrase
- OBF
- ocular blood flow
- LC-MS/MS
- liquid chromatography/tandem mass spectrometry
- AUC
- area under the curve
- Cmax
- maximum concentration
- Tmax
- time at maximum concentration.
Authorship Contributions
Participated in research design: Kadam, Ogidigben, and Kompella.
Conducted experiments: Kadam and Jadhav.
Contributed new reagents or analytic tools: Kadam and Jadhav.
Performed data analysis: Kadam and Kompella.
Wrote or contributed to the writing of the manuscript: Kadam, Ogidigben, and Kompella.
References
- Barnes GE, Li B, Dean T, Chandler ML. (2000) Increased optic nerve head blood flow after 1 week of twice daily topical brinzolamide treatment in Dutch-belted rabbits. Surv Ophthalmol 44:S131–S140 [DOI] [PubMed] [Google Scholar]
- Bathija R. (2000) Optic nerve blood flow in glaucoma. Clin Exp Optom 83:180–184 [DOI] [PubMed] [Google Scholar]
- DeSantis L. (2000) Preclinical overview of brinzolamide. Surv Ophthalmol 44:S119–S129 [DOI] [PubMed] [Google Scholar]
- Gross RL, Ji J, Chang P, Pennesi ME, Yang Z, Zhang J, Wu SM. (2003) A mouse model of elevated intraocular pressure: retina and optic nerve findings. Trans Am Ophthalmol Soc 101:163–169; discussion 169–171 [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Piltz J, Hariprasad SM, DuPont J. (1998) Optic nerve and choroidal circulation in glaucoma. Invest Ophthalmol Vis Sci 39:2329–2336 [PubMed] [Google Scholar]
- Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. (2010) Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 6:324–333 [DOI] [PubMed] [Google Scholar]
- Harris A, Arend O, Arend S, Martin B. (1996) Effects of topical dorzolamide on retinal and retrobulbar hemodynamics. Acta Ophthalmol Scand 74:569–572 [DOI] [PubMed] [Google Scholar]
- Harris A, Chung HS, Ciulla TA, Kagemann L. (1999) Progress in measurement of ocular blood flow and relevance to our understanding of glaucoma and age-related macular degeneration. Prog Retin Eye Res 18:669–687 [DOI] [PubMed] [Google Scholar]
- Holló G, Whitson JT, Faulkner R, McCue B, Curtis M, Wieland H, Chastain J, Sanders M, DeSantis L, Przydryga J, et al. (2006) Concentrations of betaxolol in ocular tissues of patients with glaucoma and normal monkeys after 1 month of topical ocular administration. Invest Ophthalmol Vis Sci 47:235–240 [DOI] [PubMed] [Google Scholar]
- Iester M. (2008) Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol 2:517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. (2000) Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci 41:431–442 [PubMed] [Google Scholar]
- Kaup M, Plange N, Niegel M, Remky A, Arend O. (2004) Effects of brinzolamide on ocular haemodynamics in healthy volunteers. Br J Ophthalmol 88:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MH, Fingert JH, Kwon YH. (2005) Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin North Am 18:383–395, vi [DOI] [PubMed] [Google Scholar]
- Lee DA, Higginbotham EJ. (2005) Glaucoma and its treatment: a review. Am J Health Syst Pharm 62:691–699 [DOI] [PubMed] [Google Scholar]
- Martinez A, Gonzalez F, Capeans C, Perez R, Sanchez-Salorio M. (1999) Dorzolamide effect on ocular blood flow. Invest Ophthalmol Vis Sci 40:1270–1275 [PubMed] [Google Scholar]
- Martinez A, Sanchez M. (2007) Retrobulbar haemodynamic effects of the latanoprost/timolol and the dorzolamide/timolol fixed combinations in newly diagnosed glaucoma patients. Int J Clin Pract 61:815–825 [DOI] [PubMed] [Google Scholar]
- Martínez A, Sánchez-Salorio M. (2009) A comparison of the long-term effects of dorzolamide 2% and brinzolamide 1%, each added to timolol 0.5%, on retrobulbar hemodynamics and intraocular pressure in open-angle glaucoma patients. J Ocul Pharmacol Ther 25:239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DM. (2002) Drug delivery to the posterior segment from drops. Surv Ophthalmol 47:S41–S52 [DOI] [PubMed] [Google Scholar]
- Mizuno K, Koide T, Shimada S, Mori J, Sawanobori K, Araie M. (2009) Route of penetration of topically instilled nipradilol into the ipsilateral posterior retina. Invest Ophthalmol Vis Sci 50:2839–2847 [DOI] [PubMed] [Google Scholar]
- Okazawa H, Yamauchi H, Sugimoto K, Toyoda H, Kishibe Y, Takahashi M. (2001) Effects of acetazolamide on cerebral blood flow, blood volume, and oxygen metabolism: a positron emission tomography study with healthy volunteers. J Cereb Blood Flow Metab 21:1472–1479 [DOI] [PubMed] [Google Scholar]
- Palma SD, Tartara LI, Quinteros D, Allemandi DA, Longhi MR, Granero GE. (2009) An efficient ternary complex of acetazolamide with HP-ss-CD and TEA for topical ocular administration. J Control Release 138:24–31 [DOI] [PubMed] [Google Scholar]
- Rokicki W, Dorecka M, Romaniuk W. (2007) [Retinal ganglion cells death in glaucoma–mechanism and potential treatment. Part I]. Klin Oczna 109:349–352 [PubMed] [Google Scholar]
- Schmidt KG, von Rückmann A, Pillunat LE. (1998) Topical carbonic anhydrase inhibition increases ocular pulse amplitude in high tension primary open angle glaucoma. Br J Ophthalmol 82:758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwald RD, Huang HS. (1983) Corneal penetration behavior of beta-blocking agents I: Physiochemical factors. J Pharm Sci 72:1266–1272 [DOI] [PubMed] [Google Scholar]
- Schoenwald RD, Ward RL. (1978) Relationship between steroid permeability across excised rabbit cornea and octanol-water partition coefficients. J Pharm Sci 67:786–788 [DOI] [PubMed] [Google Scholar]
- Siesky B, Harris A, Cantor LB, Kagemann L, Weitzman Y, McCranor L, Marques C, Werne A, Stefansson E. (2008) A comparative study of the effects of brinzolamide and dorzolamide on retinal oxygen saturation and ocular microcirculation in patients with primary open-angle glaucoma. Br J Ophthalmol 92:500–504 [DOI] [PubMed] [Google Scholar]
- Sugrue MF. (1996) The preclinical pharmacology of dorzolamide hydrochloride, a topical carbonic anhydrase inhibitor. J Ocul Pharmacol Ther 12:363–376 [DOI] [PubMed] [Google Scholar]
- Xiang CD, Batugo M, Gale DC, Zhang T, Ye J, Li C, Zhou S, Wu EY, Zhang EY. (2009) Characterization of human corneal epithelial cell model as a surrogate for corneal permeability assessment: metabolism and transport. Drug Metab Dispos 37:992–998 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Sugiyama T, Utsunomiya K, Ogura Y, Ikeda T. (2010) A pilot study for the effects of donepezil therapy on cerebral and optic nerve head blood flow, visual field defect in normal-tension glaucoma. J Ocul Pharmacol Ther 26:187–192 [DOI] [PubMed] [Google Scholar]
- Zhao DY, Cioffi GA. (2000) Anterior optic nerve microvascular changes in human glaucomatous optic neuropathy. Eye 14:445–449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.