Abstract
Objective:
To determine whether age-standardized brain morphometric and cognitive profiles differ in young-old (aged 60–75 years) and very-old (aged 80–91 years) patients with Alzheimer disease (AD).
Methods:
Using a case-control retrospective design, we compared hippocampal volume and cortical gray matter thickness in areas known to be affected by AD in 105 patients with AD and 125 healthy control (HC) participants divided into young-old and very-old subgroups. Brain morphometric and cognitive scores of the AD groups were standardized to their respective age-appropriate HC subgroup and then compared.
Results:
Several cognitive domains (executive function, immediate memory, and attention/processing speed) were less abnormal in the very old with AD than in the young old with AD. Similarly, the very old with AD showed less severe cortical thinning than the young old with AD in the left posterior cingulate cortex, right lateral temporal cortex, and bilateral parietal cortex and in overall cortical thickness. This effect is partially explained by an age-related decrease in cortical thickness in these brain regions in the HC participants.
Conclusions:
The typical pattern of AD-related cognitive and morphometric changes seen in the young old appear to be less salient in the very old. Thus, mild cases of AD in the very old may go undetected if one expects to see the prototypical pattern and severity of cognitive or brain changes that occur in the young old with AD. These results underscore the importance of interpreting neuropsychological test performance and morphometric brain measures in reference to the individual's age. Neurology® 2011;77:713–721
Despite the increasing prevalence of dementia with advancing age, the number of individuals diagnosed with mild dementia seems to decrease with age.1 This discrepancy may arise from the unique challenges that exist for clinical detection of early Alzheimer disease (AD) in the very old (i.e., aged ≥80 years). Normal aging is associated with cognitive decline and increasing variability in cognitive abilities,2–4 which makes the typical profile of neuropsychological deficits associated with AD less salient in the very-old patient than in the young-old (i.e., aged ≤75 years) patient.5 Age-related decline and increased interindividual variability may also attenuate the usefulness of MRI-derived measures of regional brain volumes as markers of early AD in older patients.6 A number of studies have used a regression-based approach to evaluate the influence of age on brain volume changes associated with AD, but few have directly contrasted young-old and very-old patients with AD using regional brain atrophy measures standardized to age-appropriate normative cohorts.
The current study used a sample of normally aging participants to derive standard scores for both neuropsychological and structural MRI measures to determine whether cognitive decline and regional brain atrophy profiles differ in young-old and very-old patients with early AD. We predicted that, when age-appropriate standard scores are compared, 1) very-old patients (i.e., aged ≥80 years) would have less severe cognitive impairment than young-old patients (i.e., aged ≤75 years), replicating previous findings5 and 2) very-old patients would have less abnormality of brain morphometric features than young-old patients.
METHODS
Data used were obtained from the Alzheimer Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, and private pharmaceutical companies and nonprofit organizations as a 5-year public-private partnership. The primary goal of ADNI has been to test whether serial MRI, PET, other biologic markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. Participants have been recruited from more than 50 sites across the United States and Canada (www.adni-info.org).
Standard protocol approvals, registrations, and patient consents.
This study was approved by an ethical standards committee on human experimentation at University of California, San Diego, and at each ADNI-affiliated institution. Written informed consent was obtained from all patients participating in the study or authorized representatives.
Participants.
ADNI general eligibility criteria are described at www.adni-info.org/Scientists/ADNIGrant/ProtocolSummary.aspx. Participants included in the present study were aged 60–91 years, were nondepressed, had a modified Hachinski score of 4 or less, and had a study partner able to provide an independent evaluation of functioning. Healthy control (HC) participants had a Mini-Mental State Examination (MMSE) score between 24 and 30 (inclusive), had a global Clinical Dementia Rating (CDR)7 score of 0, and did not meet the criteria for MCI.8 Participants with mild AD had MMSE scores between 20 and 26 and global CDR scores of 0.5 or 1.0 and met the National Institute of Neurological Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria for probable AD.9 A measure derived from the components of the CDR known as sum of boxes was calculated to further estimate the level of clinical impairment.
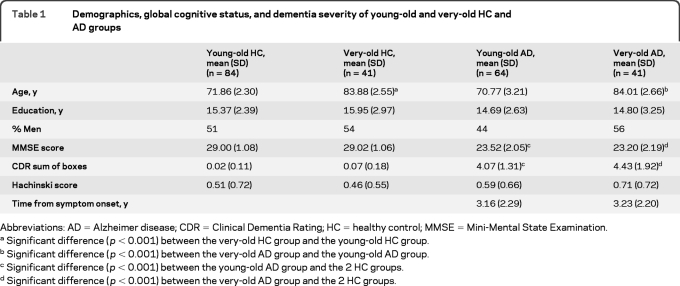
HC participants and participants with AD were divided into 2 groups on the basis of their age at testing: a young-old group (age range 60–75 years) and a very-old group (aged ≤80 years). Our initial sample included 268 participants: 89 young-old HC participants, 76 young-old patients with AD, 45 very-old HC participants, and 58 very old patients with AD. Five HC participants and 29 participants with AD were excluded based on poor quality of imaging data because they did not pass local quality control criteria for magnetic resonance images. HC participants received follow-up study visits at 6, 12, 24, and 36 months. We excluded any control subject who developed MCI or AD at any follow-up visit (3 young-old HC participants and one very-old HC participant). This method of selection and group assignment resulted in an approximately 12-year mean age difference between the young-old and very-old groups. Final group demographic characteristics are presented in table 1.
Table 1.
Demographics, global cognitive status, and dementia severity of young-old and very-old HC and AD groups
Abbreviations: AD = Alzheimer disease; CDR = Clinical Dementia Rating; HC = healthy control; MMSE = Mini-Mental State Examination.
Significant difference (p < 0.001) between the very-old HC group and the young-old HC group.
Significant difference (p < 0.001) between the very-old AD group and the young-old AD group.
Significant difference (p < 0.001) between the young-old AD group and the 2 HC groups.
Significant difference (p < 0.001) between the very-old AD group and the 2 HC groups.
Neuropsychological measures.
A standardized battery of neuropsychological tests was administered to each participant. Detailed descriptions of the tests with administration and scoring procedures have been published previously.10 The cognitive domains assessed and the specific test measures in each domain were as follows: 1) language: Boston Naming Test (30 odd-numbered items) and Category Fluency Test (animals and vegetables); 2) attention/psychomotor processing speed: Trail Making Test Part A, Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Span subtest Forward, and WAIS-R Digit Symbol subtest; 3) executive function: Trail Making Test Part B and WAIS-R Digit Span subtest Backward; 4) immediate recall: Rey Auditory Verbal Learning Test (RAVLT) Trials 1–5 Total Recall and Wechsler Memory Scale–Revised (WMS-R) Logical Memory Test: Immediate Recall; 5) delayed recall: RAVLT Long-Delay Recall and WMS-R Logical Memory Test: Delayed Recall; and 6) savings: RAVLT Long-Delay Percent Retained and WMS-R Logical Memory Test: Delayed Recall Percent Retained.
Individual test scores on each measure were converted to z scores based on the mean and SD of the respective HC group. The z scores were modified to ensure that negative scores represented poorer performance. Composite scores for each of the 6 neuropsychological domains were then calculated by averaging z scores across the tests within each domain.
Magnetic resonance scanning and brain morphometry.
Protocols are described in detail at www.loni.ucla.edu/ADNI/Research/Cores/index.shtml. Two T1-weighted volumes were acquired for each participant. These raw DICOM MRI scans were downloaded from the public ADNI site (www.loni.ucla.edu/ADNI/Data/index.shtml). Images were reviewed locally for quality, automatically corrected for spatial distortion due to gradient nonlinearity11 and B1 field inhomogeneity,12 registered, and averaged to improve the signal/noise ratio. Volumetric13,14 and cortical surface reconstruction15–17 methods based on FreeSurfer software, optimized for use on large, multisite datasets, were used. To measure thickness, the cortical surface was reconstructed15 and parcellated into distinct regions of interest (ROIs).17,18 Details of the application of these methods to the ADNI data have been described elsewhere.19 To limit the number of comparisons, only regions assumed to be involved in early AD pathology11,20–22 were included in the present analyses and several cortical thickness ROIs were combined as follows: caudal and rostral anterior cingulate regions were combined as anterior cingulate cortex (ACC); isthmus and posterior cingulate regions were combined as posterior cingulate cortex (PCC); superior, middle, and inferior temporal regions were combined as lateral temporal regions; parahippocampal and entorhinal areas were combined as medial temporal regions; rostral and caudal middle frontal and frontal pole were combined as dorsolateral prefrontal cortex (DLPFC); lateral and medial orbitofrontal areas and pars orbitalis were combined as orbitofrontal regions; precuneus, superior parietal, inferior parietal, and supramarginal regions were combined as parietal regions; and lateral occipital, cuneus, and pericalcarine areas were combined as occipital regions. All of these ROIs were also averaged to create a measure of average cortical thickness. A hippocampal ROI was based on the volume of the entire structure (not cortical thickness) and was not included in the overall cortical thickness measure.
Statistical analyses.
Group comparisons of composite z scores from each of the 6 neuropsychological domains were performed with separate independent samples t tests. When the assumption of homogeneity of variance was not met, the t value and significance of the comparison were based on unequal variances. Effect sizes for group differences on the composite scores were calculated using Cohen d, computed by dividing the mean difference between groups by the pooled SD.
To assess the group difference in MRI morphometric variables, the effect of gender was first regressed from all thickness and volumetric measures. In addition, both left and right hippocampal volumes were corrected for differences in head size by regressing the estimated total cranial vault (eTIV) volume.23 Volumetric and cortical thickness measures for the 2 AD groups were then z transformed relative to their respective HC group with negative values indicative of smaller volume or less cortical thickness. Multivariate analyses of variance (MANOVAs) and follow-up univariate analyses were performed on these z-transformed scores. Effect sizes for group differences on the z-transformed morphometric values were calculated. Results were considered significant if the effect size was 0.50 or greater, which corresponded to an α level of 0.01.
RESULTS
Cognition.
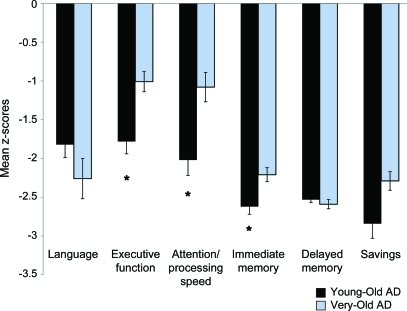
Figure 1 shows the mean composite z scores of young-old and very-old patients with AD in each cognitive domain. The young-old AD group was more impaired, relative to their age-appropriate HC group, than the very-old AD group in the domains of executive function (t93 = −3.50, p = 0.001, Cohen d = 0.75), attention/psychomotor processing speed (t98 = −3.24, p = 0.002, Cohen d = 0.68), and immediate memory (t102 = −2.77, p = 0.007, Cohen d = 0.58); savings (t84.6 = −2.30, p = 0.02, Cohen d = 0.45) approached significance. Young-old and very-old patients with AD showed comparable deficits with respect to their age-appropriate HC groups in language (t69.2 = 1.44, p = 0.15, Cohen d = 0.30) and delayed memory (t103 = 0.78, p = 0.45, Cohen d = 0.14). The raw scores of the young-old and very-old AD and HC groups for individual cognitive tests are shown in table 2. To assess group differences in cognition, independent of overall cortical atrophy, the effect of average cortical thickness (raw value) was regressed from all cognitive measures, and the above analyses were repeated. The pattern of results and significance of the findings remained unchanged; thus, only the uncorrected data are reported.
Figure 1. Mean z scores for neuropsychological domains for Alzheimer disease (AD) groups.
Mean levels of performance indicated in z scores of young-old and very-old AD groups relative to their age-respective healthy control groups on each of 6 neuropsychological domains. Error bars denote SEM. *p < 0.01.
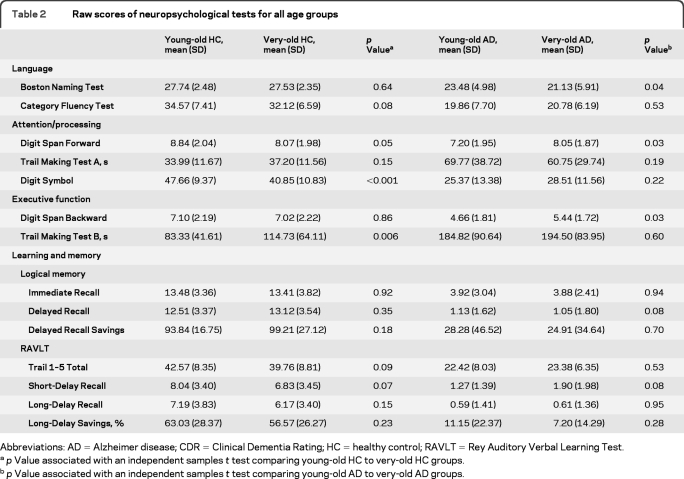
Table 2.
Raw scores of neuropsychological tests for all age groups
Abbreviations: AD = Alzheimer disease; CDR = Clinical Dementia Rating; HC = healthy control; RAVLT = Rey Auditory Verbal Learning Test.
p Value associated with an independent samples t test comparing young-old HC to very-old HC groups.
p Value associated with an independent samples t test comparing young-old AD to very-old AD groups.
Morphometry.
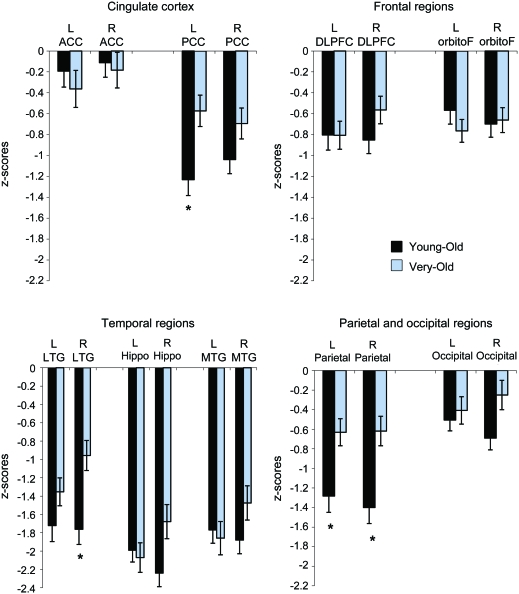
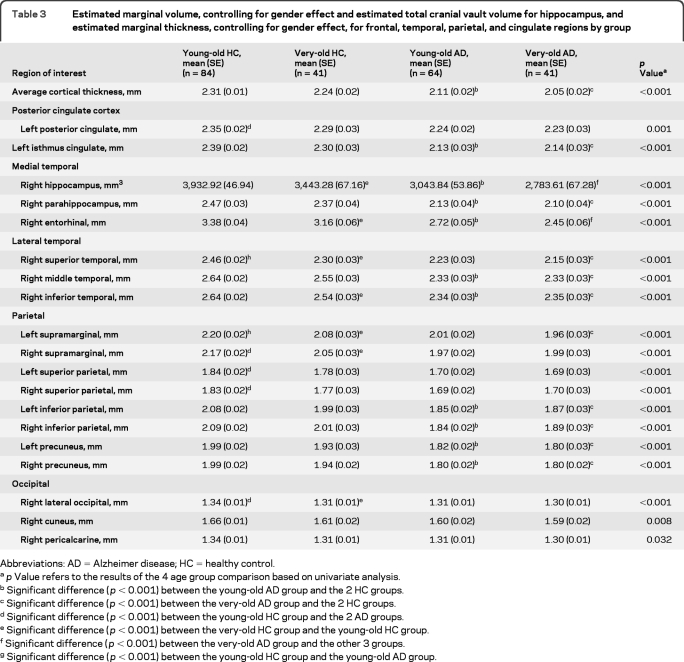
Differences between young-old and very-old AD groups on morphometric measures were assessed by comparing the z scores derived from each group's age-appropriate HC group after controlling for the effects of gender (for volumetric and thickness measures) and eTIV (for volumetric measures). The MANOVA for group effects on morphometric measures (i.e., left and right ACC, PCC, DLPFC, orbitofrontal cortex, lateral temporal cortex, hippocampus, other medial temporal cortex, parietal cortex, and occipital cortex) was significant (Wilks λ = 0.61, F18,86 = 3.05, p < 0.001, partial η2 = 0.39). Follow-up univariate analyses (figure 2) showed that abnormal cortical thinning (relative to that in their respective HC group) was greater in the young-old AD group than in the very-old AD group in the left PCC (t103 = −2.94, p = 0.004, Cohen d = 0.60), right lateral temporal cortex (t103 = −3.28, p = 0.001, Cohen d = 0.67), and parietal cortex (right t103 = −3.29, p = 0.001, Cohen d = 0.68; left t103 = −2.76, p = 0.007, Cohen d = 0.58). This difference approached significance in the right hippocampus (t103 = −2.35, p = 0.02, Cohen d = 0.47), right other medial temporal cortex (t103 = −1.69, p = 0.09, Cohen d = 0.35), and right occipital cortex (t103 = −2.26, p = 0.03, Cohen d = 0.46). The 2 AD groups did not differ in bilateral ACC, DLPFC, orbitofrontal cortex, left lateral temporal cortex, left hippocampus, left other medial temporal cortex, left occipital cortex, and right PCC (all p > 0.10; all Cohen d < 0.35). In addition, overall cortical thickness was more abnormal in the young-old AD group (mean z = −2.14, SD = 1.41) than in the very-old AD group (mean z = −1.47, SD = 1.00; t103 = −2.64, p = 0.01, Cohen d = 0.55), despite comparable raw scores. Table 3 shows the estimated marginal mean values for morphometric measures (before collapsing across ROIs) for young-old and very-old AD and HC groups after controlling for gender (for both volumetric and thickness measures) and eTIV volumes (for the volumetric measure) for those regions with differences that were significant or approached significance (p < 0.05).
Figure 2. Mean z scores for volumetric and cortical thickness measures for Alzheimer disease (AD) groups.
Mean volumetric and thickness measures indicated in z scores of young-old and very-old AD groups relative to their age-respective healthy control groups after controlling for the effect of gender (for both volumetric and thickness measures) and estimated total cranial vault (for volumetric measure). Error bars denote SEM. *p < 0.01. ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; Hippo = hippocampus; LTG = lateral temporal gyri; MTG = other medial temporal regions including entorhinal and parahippocampal regions; orbitoF = orbitofrontal; PCC = posterior cingulate cortex.
Table 3.
Estimated marginal volume, controlling for gender effect and estimated total cranial vault volume for hippocampus, and estimated marginal thickness, controlling for gender effect, for frontal, temporal, parietal, and cingulate regions by group
Abbreviations: AD = Alzheimer disease; HC = healthy control.
p Value refers to the results of the 4 age group comparison based on univariate analysis.
Significant difference (p < 0.001) between the young-old AD group and the 2 HC groups.
Significant difference (p < 0.001) between the very-old AD group and the 2 HC groups.
Significant difference (p < 0.001) between the young-old HC group and the 2 AD groups.
Significant difference (p < 0.001) between the very-old HC group and the young-old HC group.
Significant difference (p < 0.001) between the very-old AD group and the other 3 groups.
Significant difference (p < 0.001) between the young-old HC group and the young-old AD group.
DISCUSSION
The present results demonstrate that certain morphometric brain abnormalities associated with AD are less salient in very-old patients than in young-old patients despite similar levels of global cognitive impairment in the 2 groups. When compared with their respective age-appropriate HC groups, very-old patients showed less severe cortical thinning than young-old patients in the left PCC, right lateral temporal cortex, and bilateral parietal cortex and in overall cortical thickness averaged across all ROIs. This effect is partially explained by an age-related decrease in cortical thickness in these brain regions in the HC participants (table 3). Although the AD groups had similar overall cortical thickness, the very-old patients' age-appropriate standard scores were less abnormal than those of the young-old patients because of reduced and more variable cortical thickness in the very-old HC participants. Thus, cortical thickness reduction that can be attributed to AD in these brain regions is more difficult to detect in very-old patients with AD than in young-old patients with AD.
The observed pattern of age-related differences in abnormalities in cortical thickness is reflected in the pattern of age-related differences in the severity of cognitive abnormalities exhibited by the AD groups. Consistent with previous findings,5,24 the present results showed that very-old patients with AD were less impaired (i.e., were less abnormal) than young-old patients with AD in the domains of immediate memory, attention/processing speed, and executive function. As with cortical thickness measures, the reduced degree of abnormality in these cognitive domains can be at least partially explained by an age-related decrease in performance in HC participants (table 2). Very-old HC participants scored significantly lower than young-old HC participants on a number of measures in these domains (e.g., Digit Symbol Substitution, Trail Making Test Part B, Digit Span–Forward). Similar age-related decrements were reported previously and were attributed, in part, to deterioration of the frontoparietal cortical systems involved in attention and response selection.25,26
Not all brain regions usually affected by AD showed differential levels of volumetric or cortical thickness abnormality in very-old and young-old patients with AD. For example, the AD groups did not significantly differ in degree of abnormality in hippocampal volume or medial temporal cortical thickness, corroborating previous results.27 Although atrophy of the hippocampus and medial temporal gyrus increased with age in the HC participants, the degree of difference between the HC and AD groups remained large. Consistent with this finding, the AD groups did not differ in the degree of abnormality on delayed recall or savings measures (although this result approached significance) that are largely mediated by the hippocampus and related medial temporal lobe (MTL) structures. These results suggest that early and severe atrophy of the hippocampus and MTL cortex due to AD greatly eclipses normal age-related changes and allows atrophy in these regions to be a salient marker of AD regardless of the elderly patient's age.
It has been proposed that imaging or other types of biomarkers are critically needed to detect AD early so that interventions can be applied before the disease significantly diminishes cognitive function.28 Previous studies have shown that MRI-derived indices of atrophy have high sensitivity and specificity for detecting disease in symptomatic patients and, therefore, have the potential to be effective biomarkers. However, our results suggest that it is critical to consider the effect of age in application of MRI-derived markers because they may be better able to distinguish patients with AD from healthy elderly individuals in the young-old than in the very-old groups. A similar loss of saliency may occur with aging for other biomarkers (e.g., tau and Aβ in CSF), given the overlap that occurs in AD and the aging process. Indeed, some evidence suggests that AD-related increases in the densities of neuritic plaques and neurofibrillary tangles that underlie some biomarkers are less profound in very-old than in young-old individuals.29,30
Some limitations of the present study should be noted. First, the cross-sectional nature of the study limits the ability to determine whether rate of change in hippocampal volume or cortical thickness is similar in very-old and young-old patients with AD and whether abnormality in rate of change is influenced by age. The results need to be replicated longitudinally to address this issue and to directly compare rate of change in regional brain volumes against rates of cognitive decline. Second, histopathologic verification of disease is not available so it is possible that some participants have a disorder other than AD or have AD with comorbid pathology that contributes to cognitive and neuroimaging presentations. The prevalence of vascular pathology in AD, for example, has been reported to be roughly 30%–60%.28,31 Although ADNI exclusionary criteria ensure a low prevalence of vascular risk factors, the impact of white matter changes on the pattern of cognitive and regional brain changes in AD across different age groups may help explain some of the observed differences in cognitive profiles. Third, impaired initial learning can lead to an inflated savings score (percentage retained) in some cases; thus, results from this measure must be interpreted with caution. For example, an individual who learns only one item on list learning and then recalls only that same item after a delay would have 100% savings, despite poor overall memory performance. To reduce the chance of significant inflating in our savings variable, we excluded any outliers (≥3 SD from the overall group mean). Fourth, other brain variables may contribute to the different cognitive deficit profiles seen in the 2 groups. For example, normal aging is associated with mild brain atrophy on structural MRI,6 decreased hemodynamic response on fMRI,32 reduced synaptic density,33 increased white matter hyperintensities,6,34,35 and a subclinical accumulation of neuritic plaques and neurofibrillary tangles in MTL regions.36 These brain changes are accompanied by age-related declines in information-processing speed, executive functions, and efficiency of learning and recall3,4,35,37 and may help explain the observed differences in severity of cognitive abnormalities in very-old and young-old patients with AD. These possibilities will be addressed in future studies.
Overall, the present results indicate that overlap between normal and AD-related MRI-based morphometric changes is greater in the very old than in the young old. Thus, the typical pattern of AD-related morphometric changes seen in the young old is less salient in the very old. A similar loss of saliency in cognitive profiles is evident because of greater overlap in normal and AD-related decline in executive functions, attention/psychomotor processing, and immediate memory in very-old than in young-old individuals. These results highlight the possibility that mild cases of AD in the very old may go undetected and underscore the importance of interpreting neuropsychological test performance and morphometric changes (i.e., atrophy) in reference to the individual's age. A clarification of how the presentation of AD changes with age may enhance our ability to detect early AD in the very old, one of the fastest growing segments of the population.38 Indeed, enhanced detection is crucial for early application of interventions that may slow the disease process, thus preserving cognitive status, functional independence, and quality of life.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Alain Koyama, Robin G. Jennings, Michele Perry, Chris Pung, and Elaine Wu for downloading and preprocessing the ADNI MRI data.
GLOSSARY
- ACC
anterior cingulate cortex
- AD
Alzheimer disease
- ADNI
Alzheimer Disease Neuroimaging Initiative
- CDR
Clinical Dementia Rating
- DLPFC
dorsolateral prefrontal cortex
- eTIV
estimated total cranial vault
- HC
healthy control
- MANOVA
multivariate analyses of variance
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MTL
medial temporal lobe
- PCC
posterior cingulate cortex
- RAVLT
Rey Auditory Verbal Learning Test
- ROI
region of interest
- WAIS-R
Wechsler Adult Intelligence Scale–Revised
- WMS-R
Wechsler Memory Scale–Revised
Footnotes
Editorial, page 706
Supplemental data at www.neurology.org
The Alzheimer's Disease Neuroimaging Initiative Coinvestigators are listed in appendix e-1 on the Neurology® Web site at www.neurology.org.
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu⧹ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
AUTHOR CONTRIBUTIONS
Dr. Stricker: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and study supervision. Dr. Chang: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, and study supervision. Dr. Fennema-Notestine: drafting/revising the manuscript, study concept or design, and analysis or interpretation of data. Dr. Delano-Wood: drafting/revising the manuscript, study concept or design, acquisition of data, and study supervision. Dr. Salmon: drafting/revising the manuscript, study concept or design, and analysis or interpretation of data. Dr. Bondi: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and study supervision. Dr. Dale: study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, and obtaining funding.
STUDY FUNDING
This research was also supported by the NIH (NIA R01-AG012674, NIA R01-AG031224, K24-AG026431, P50-AG05131, P30-AG010129, and K01-AG030514) and the Dana Foundation. Data collection and sharing for this project was funded by the ADNI (NIH grant U01-AG024904). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as nonprofit partners, the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles.
DISCLOSURE
Dr. Stricker receives/has received research support from The Rosalind and Arthur Gilbert Foundation/American Federation for Aging Research (AFAR) and the NIH/NIMH. Dr. Chang receives research support from the Alzheimer's Association and the Stein Institute for Research on Aging, University of California, San Diego. Dr. Fennema-Notestine receives/has received research support from the NIH, the US Department of Veterans Affairs, and the Alzheimer's Association. Dr. Delano-Wood receives research support from the Alzheimer's Association. Dr. Salmon serves as a consultant for CHDI Foundation, Novartis, and Bristol-Meyers Squibb and receives/has received research support from the NIH and the State of California Department of Health Services. Dr. Bondi serves as an Associate Editor for the Journal of the International Neuropsychological Society and receives research support from the Alzheimer's Association and the NIH/NIA. Dr. Dale receives research support from the NIH; receives funding to his laboratory from GE Heathcare as part of a Master Research Agreement with UCSD; and is a founder of, holds equity in, and serves on the scientific advisory board for CorTechs Labs, Inc. The terms of this arrangement have been reviewed and approved by UCSD in accordance with its conflict of interest policies.
REFERENCES
- 1. von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol 1999;56:587–592 [DOI] [PubMed] [Google Scholar]
- 2. Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging 2005;26:1245–1260 [DOI] [PubMed] [Google Scholar]
- 3. Christensen H, Mackinnon AJ, Korten AE, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychol Aging 1999;14:365–379 [DOI] [PubMed] [Google Scholar]
- 4. Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193 [PubMed] [Google Scholar]
- 5. Bondi MW, Houston WS, Salmon DP, et al. Neuropsychological deficits associated with Alzheimer's disease in the very-old: discrepancies in raw vs. standardized scores. J Int Neuropsychol Soc 2003;9:783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 2001;22:581–594 [DOI] [PubMed] [Google Scholar]
- 7. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572 [DOI] [PubMed] [Google Scholar]
- 8. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992 [DOI] [PubMed] [Google Scholar]
- 9. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 10. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006;30:436–443 [DOI] [PubMed] [Google Scholar]
- 12. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97 [DOI] [PubMed] [Google Scholar]
- 13. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 14. Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23(suppl 1):S69–S84 [DOI] [PubMed] [Google Scholar]
- 15. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194 [DOI] [PubMed] [Google Scholar]
- 16. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207 [DOI] [PubMed] [Google Scholar]
- 17. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22 [DOI] [PubMed] [Google Scholar]
- 18. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980 [DOI] [PubMed] [Google Scholar]
- 19. Fennema-Notestine C, Hagler DJ, Jr, McEvoy LK, et al. Structural MRI biomarkers for preclinical and mild Alzheimer's disease. Hum Brain Mapp 2009;30:3238–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fennema-Notestine C, Ozyurt IB, Clark CP, et al. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: effects of diagnosis, bias correction, and slice location. Hum Brain Mapp 2006;27:99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006;32:180–194 [DOI] [PubMed] [Google Scholar]
- 22. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004;23:724–738 [DOI] [PubMed] [Google Scholar]
- 24. Reid W, Broe G, Creasey H, et al. Age at onset and pattern of neuropsychological impairment in mild early-stage Alzheimer disease: a study of a community-based population. Arch Neurol 1996;53:1056–1061 [DOI] [PubMed] [Google Scholar]
- 25. Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci 2010;65:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bledowski C, Rahm B, Rowe JB. What “works” in working memory? Separate systems for selection and updating of critical information. J Neurosci 2009;29:13735–13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van de Pol LA, Hensel A, Barkhof F, Gertz HJ, Scheltens P, van der Flier WM. Hippocampal atrophy in Alzheimer disease: age matters. Neurology 2006;66:236–238 [DOI] [PubMed] [Google Scholar]
- 28. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1143–1153 [DOI] [PubMed] [Google Scholar]
- 29. Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol 2008;65:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology 2006;66:49–55 [DOI] [PubMed] [Google Scholar]
- 31. Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol 1986;19:253–262 [DOI] [PubMed] [Google Scholar]
- 32. D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 1999;10:6–14 [DOI] [PubMed] [Google Scholar]
- 33. Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 1993;43:192–197 [DOI] [PubMed] [Google Scholar]
- 34. de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: the Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 2000;14:224–232 [DOI] [PubMed] [Google Scholar]
- 36. Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology 1999;52:1158–1165 [DOI] [PubMed] [Google Scholar]
- 37. Park HL, O'Connell JE, Thomson RG. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry 2003;18:1121–1134 [DOI] [PubMed] [Google Scholar]
- 38. Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60:1119–1122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.