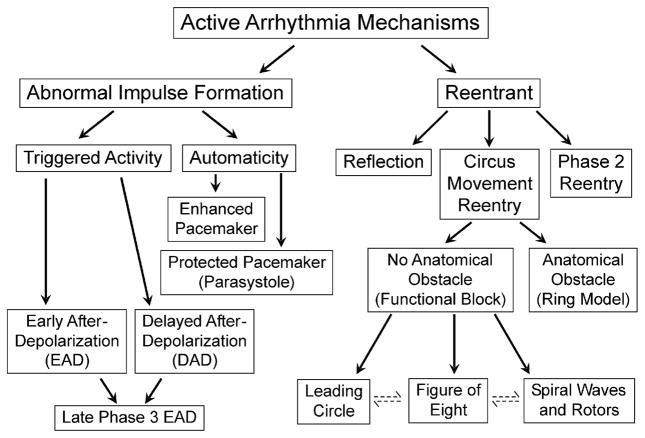
A cardiac arrhythmia simply defined is a variation from the normal heart rate and/or rhythm that is not physiologically justified. Recent years have witnessed important advances in our understanding of the electrophysiologic mechanisms underlying the development of a variety of cardiac arrhythmias. The mechanisms responsible for cardiac arrhythmias are generally divided into 2 major categories: (1) enhanced or abnormal impulse formation (ie, focal activity) and (2) conduction disturbances (ie, reentry) (Fig. 1).
Fig. 1.
Classification of active cardiac arrhythmias.
ABNORMAL IMPULSE FORMATION
Normal Automaticity
Automaticity is the property of cardiac cells to generate spontaneous action potentials. Spontaneous activity is the result of diastolic depolarization caused by a net inward current during phase 4 of the action potential, which progressively brings the membrane potential to threshold. The sinoatrial (SA) node normally displays the highest intrinsic rate. All other pacemakers are referred to as subsidiary or latent pacemakers because they take over the function of initiating excitation of the heart only when the SA node is unable to generate impulses or when these impulses fail to propagate.
The Voltage and Calcium Clocks
The terms sarcolemma voltage or Ca clocks have been used by Maltsev and colleagues1 and Lakatta2 to describe the mechanisms of SA node automaticity. The voltage clock refers to voltage-sensitive membrane currents, such as the hyperpolarization-activated pacemaker current (If).3 This current is also referred to as a “funny” current because, unlike most voltage-sensitive currents, it is activated by hyperpolarization rather than depolarization. At the end of the action potential, the If is activated and depolarizes the sarcolemmal membrane.3 If is a mixed Na-K inward current modulated by the autonomic nervous system through cAMP. The depolarization activates ICa,L, which provides Ca to activate the cardiac ryanodine receptor (RyR2). The activation of RyR2 initiates sarcoplasmic reticulum (SR) Ca release (Ca-induced Ca release), leading to contraction of the heart, a process known as EC coupling. Intracellular Ca (Cai) is then pumped back into SR by the SR Ca-ATPase (SERCA2a) and completes this Ca cycle. In addition to If, multiple time- and voltage-dependent ionic currents have been identified in cardiac pacemaker cells, which contribute to diastolic depolarization. These currents include (but are not limited to) ICa-L, ICa-T, IST, and various types of delayed rectifier K currents.2 Many of these membrane currents are known to respond to β-adrenergic stimulation. All these membrane ionic currents contribute to the regulation of SA node automaticity by altering membrane potential.
Another important ionic current capable of depolarizing the cell is the sodium-calcium exchanger current (INCx). In its forward mode, INCx exchanges 3 extracellular Na+ with 1 intracellular Ca2+, resulting in a net intracellular charge gain. This electrogenic current is active during late phase 3 and phase 4 because the Cai decline outlasts the SA node action potential duration. Recent studies showed that INCx may participate in normal pacemaker activity.4 The sequence of events includes spontaneous rhythmic SR Ca release, Cai elevation, the activation of INCx, and membrane depolarization. This process is highly regulated by cAMP and the autonomic nervous system.2 These studies suggest that sympathetic stimulation accelerates heart rate by phosphorylation of proteins that regulate Cai balance and spontaneous SR Ca cycling. These proteins include phospholamban (PLB, an SR membrane protein regulator of SERCA2a), L-type Ca channels, and RyR2. Phosphorylation of these proteins controls the phase and extent of subsarcolemmal SR Ca releases.
Subsidiary Pacemakers
In addition to the SA node, the atrioventicular (AV) node and Purkinje system are also capable of generating automatic activity. The contribution of If and IK differs in SA node/AV nodes and Purkinje fiber because of the different potential ranges of these two pacemaker types (ie, −70 to −35 mV and −90 to −65 mV, respectively). The contribution of other voltage-dependent currents can also differ among the different cardiac cell types. Whether or not the Ca clock plays a role in pacemaking of AV node and Purkinje cells remains unclear.
SA nodal cells possess the fastest intrinsic rates, making them the primary pacemakers in the normal heart. When impulse generation or conduction in the SA node is impaired, latent or subsidiary pacemakers within the atria or ventricles take control of pacing the heart. The intrinsically slower rates of these latent pacemakers generally result in bradycardia. Both atrial and AV junctional subsidiary pacemakers are under autonomic control, with the sympathetic system increasing and parasympathetic system slowing the pacing rate. Although acetylcholine produces little in the way of a direct effect, it can significantly reduce Purkinje automaticity by means of the inhibition of the sympathetic influence, a phenomenon termed accentuated antagonism.5 Simultaneous recording of cardiac sympathetic and parasympathetic activity in ambulatory dogs confirmed that sympathetic activation followed by vagal activation may be associated with significant bradycardia.6,7
AUTOMATICITY AS A MECHANISM OF CARDIAC ARRHYTHMIAS
Abnormal automaticity includes both reduced automaticity, which causes bradycardia, and increased automaticity, which causes tachycardia. Arrhythmias caused by abnormal automaticity can result from diverse mechanisms (see Fig. 1). Alterations in sinus rate can be accompanied by shifts of the origin of the dominant pacemaker within the sinus node or to subsidiary pacemaker sites elsewhere in the atria. Impulse conduction out of the SA mode can be impaired or blocked as a result of disease or increased vagal activity leading to development of bradycardia. AV junctional rhythms occur when AV junctional pacemakers located either in the AV node or in the His bundle accelerate to exceed the rate of SA node, or when the SA nodal activation rate was too slow to suppress the AV junctional pacemaker.
Bradycardia can occur in structurally normal hearts because of genetic mutations that result in abnormalities of either membrane clock or Ca clock mechanisms of automaticity. One example is the mutation of hyperpolarization-activated nucleotide-gated channel (HCN4), which is part of the channels that carry If. Mutations of the HCN4 may cause familial bradycardia as well.8,9
Secondary SA Node Dysfunction
Common diseases, such as heart failure and atrial fibrillation, may be associated with significant SA node dysfunction. Malfunction of both membrane voltage and Ca clocks might be associated with both of these common diseases. Zicha and colleagues10 reported that down-regulation of HCN4 expression contributes to heart failure-induced sinus node dysfunction. An A450 V missense loss of function mutation in HCN4 has recently been shown to underlie familial sinus bradycardia in several unrelated probands of Moroccan Jewish descent.9,11–13
Enhanced Automaticity
Atrial and ventricular myocardial cells do not display spontaneous diastolic depolarization or automaticity under normal conditions, but can develop these characteristics when depolarized, resulting in the development of repetitive impulse initiation, a phenomenon termed depolarization-induced automaticity.14 The membrane potential at which abnormal automaticity develops ranges between −70 and −30 mV. The rate of abnormal automaticity is substantially higher than that of normal automaticity and is a sensitive function of resting membrane potential (ie, the more depolarized resting potential the faster the rate). Similar to normal automaticity, abnormal automaticity is enhanced by β-adrenergic agonists and by reduction of external potassium.
Depolarization of membrane potential associated with disease states is most commonly a result of (1) an increase in extracellular potassium, which reduces the reversal potential for IK1, the outward current that largely determines the resting membrane or maximum diastolic potential; (2) a reduced number of IK1 channels; (3) a reduced ability of the IK1 channel to conduct potassium ions; or (4) electrotonic influence of neighboring cells in the depolarized zone. Because the conductance of IK1 channels is sensitive to extra-cellular potassium concentration, hypokalemia can lead to major reduction in IK1, leading to depolarization and the development of enhanced or abnormal automaticity, particularly in Purkinje pacemakers. A reduction in IK1 can also occur secondary to a mutation in KCNJ2, the gene that encodes for this channel, leading to increased automaticity and extrasystolic activity presumably arising from the Purkinje system.15,16 Loss of function KCNJ2 mutation gives rise to Andersen-Tawil syndrome, which is characterized among other things by a marked increase in extrasystolic activity.17–20
Overdrive Suppression of Automaticity
Automatic activity of most pacemakers within the heart is inhibited when they are overdrive paced,21 owing to a mechanism termed overdrive suppression. Under normal conditions, all subsidiary pacemakers are overdrive-suppressed by SA nodal activity. A possible mechanism of overdrive suppression is intracellular accumulation of Na leading to enhanced activity of the sodium pump (sodium-potassium adenosine triphosphatase [Na+-K+ ATPase]), which generates a hyperpolarizing electrogenic current that opposes phase 4 depolarization.22 The faster the overdrive rate or the longer the duration of overdrive, the greater the enhancement of sodium pump activity, so that the period of quiescence after cessation of overdrive is directly related to the rate and duration of overdrive.
Parasystole and Modulated Parasystole
Latent pacemakers throughout the heart are generally reset by the propagating wavefront initiated by the dominant pacemaker. An exception to this rule occurs when the pacemaking tissue is protected from the impulse of sinus nodal origin. A region of entrance block arises when cells exhibiting automaticity are surrounded by ischemic, infarcted, or otherwise compromised cardiac tissues that prevent the propagating wave from invading the focus, but which permit the spontaneous beat generated within the automatic focus to exit and activate the rest of the myocardium. A pacemaker region exhibiting entrance block, and exit conduction is referred to as a parasystolic focus. The ectopic activity generated by a parasystolic focus is characterized by premature ventricular complexes with variable coupling intervals, fusion beats, and inter-ectopic intervals that are multiples of a common denominator. This rhythm is relatively rare and is usually considered benign, although a premature ventricular activation of parasystolic origin can induce malignant ventricular rhythms in the ischemic myocardium or in the presence of a suitable myocardial substrate.
Modulated parasystole, a variant of classical parasystole, was described by Jalife and colleagues.23,24 This variant of the arrhythmia results from incomplete entrance block of the parasystolic focus. Electrotonic influences arriving early in the pacemaker cycle delayed and those arriving late in the cycle accelerated the firing of the parasystolic pacemaker, so that ventricular activity could entrain the partially protected pacemaker. As a consequence, at select heart rate, extrasystolic activity generated by the entrained parasystolic pacemaker can mimic reentry, generating extrasystolic activity with fixed coupling.23–27
AFTERDEPOLARIZATION AND TRIGGERED ACTIVITY
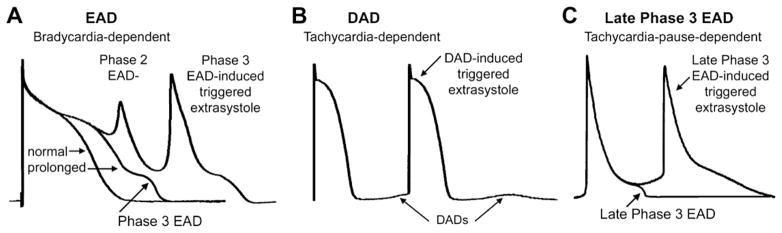
Depolarizations that attend or follow the cardiac action potential and depend on preceding transmembrane activity for their manifestation are referred to as afterdepolarizations (Fig. 2). Two subclasses are traditionally recognized: (1) early, and (2) delayed. Early afterdepolarization (EAD) interrupts or retards repolarization during phase 2 and/or phase 3 of the cardiac action potential, whereas delayed afterdepolarization (DAD) occurs after full repolarization. When EAD or DAD amplitude suffices to bring the membrane to its threshold potential, a spontaneous action potential referred to as a triggered response is the result (see Fig. 2). These triggered events give rise to extrasystoles, which can precipitate tachyarrhythmias.
Fig. 2.
Examples of early afterdepolarization (EAD) (A), delayed afterdepolarization (DAD) (B), and late phase 3 EAD (C). (Modified from Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol 2006;29:290–5; with permission.)
Early Afterdepolarizations and Triggered Activity
EADs are typically observed in cardiac tissues exposed to injury, altered electrolytes, hypoxia, acidosis, catecholamines, and pharmacologic agents, including antiarrhythmic drugs. Ventricular hypertrophy and heart failure also predispose to the development of EADs.28 EAD characteristics vary as a function of animal species, tissue or cell type, and the method by which the EAD is elicited. Although specific mechanisms of EAD induction can differ, a critical prolongation of repolarization accompanies most, but not all, EADs. Drugs that inhibit potassium currents or which augment inward currents predispose to the development of EADs.29 Phase 2 and phase 3 EADs sometimes appear in the same preparation.
EAD-induced triggered activity is sensitive to stimulation rate. Antiarrhythmic drugs with class III action generally induce EAD activity at slow stimulation rates.14 In contrast, β-adrenergic agonist–induced EADs are fast rate-dependent.30 In the presence of rapidly activating delayed rectifier current (rapid outward potassium current [IKr]) blockers, β-adrenergic agonists, and/or acceleration from an initially slow rate transiently facilitate the induction of EAD activity in ventricular M cells, but not in epicardium or endocardium and rarely in Purkinje fibers.31
Cellular Origin of Early Afterdepolarizations
EADs develop more commonly in midmyocardial M cells and Purkinje fibers than in epicardial or endocardial cells when exposed to action potential duration (APD)-prolonging agents. This is because of the presence of a weaker IKs and stronger late INa in M cells.32,33 Block of IKs with chromanol 293B permits the induction of EADs in canine epicardial and endocardial tissues in response to IKr blockers such as E-4031 or sotalol.34 The predisposition of cardiac cells to the development of EADs depends principally on the reduced availability of IKr and IKs as occurs in many forms of cardiomyopathy. Under these conditions, EADs can appear in any part of the ventricular myocardium.35
Ionic Mechanisms Responsible for the EAD
EADs develop when the balance of current active during phase 2 and/or 3 of the action potential shifts in the inward direction. If the change in current-voltage relation results in a region of net inward current during the plateau range of membrane potentials, it leads to a depolarization or EAD. Most pharmacologic interventions or pathophysiological conditions associated with EADs can be categorized as acting predominantly through 1 of 4 different mechanisms: (1) A reduction of repolarizing potassium currents (IKr, class IA and III antiarrhythmic agents; IKs, chromanol 293B or IK1); (2) an increase in the availability of calcium current (Bay K 8644, catecholamines); (3) an increase in the sodium-calcium exchange current (INCx) caused by augmentation of Cai activity or upregulation of the INCx; and (4) an increase in late sodium current (late INa) (aconitine, anthopleurin-A, and ATX-II). Combinations of these interventions (ie, calcium loading and IKr reduction) or pathophysiological states can act synergistically to facilitate the development of EADs.
Delayed Afterdepolarization-Induced Triggered Activity
DADs and DAD-induced triggered activity are observed under conditions that augment intracellular calcium, [Ca2+]i, such as after exposure to toxic levels of cardiac glycosides (digitalis)36–38 or catecholamines.30,39,40 This activity is also manifest in hypertrophied and failing hearts41,42 as well as in Purkinje fibers surviving myocardial infarction.43 In contrast to EADs, DADs are always induced at relatively rapid rates.
Role of Delayed Afterdepolarization-Induced Triggered Activity in the Development of Cardiac Arrhythmias
An example of DAD-induced arrhythmia is the catecholaminergic polymorphic ventricular tachycardia (CPVT), which may be caused by the mutation of either the type 2 ryanodine receptor (RyR2) or the calsequestrin (CSQ2).44 The principal mechanism underlying these arrhythmias is the “leaky” ryanodine receptor, which is aggravated during catecholamine stimulation. A typical clinical phenotype of CPVT is bidirectional ventricular tachycardia, which is also seen in digitalis toxicity. Wehrens and colleagues45 demonstrated that heterozygous mutation of FKBP12.6 leads to leaky RyR2 and exercise-induced VT and VF, simulating the human CPVT phenotype. RyR2 stabilization with a derivative of 1,4-benzothiazepine (JTV519) increased the affinity of calstabin2 for RyR2, which stabilized the closed state of RyR2 and prevented the Ca leak that triggers arrhythmias. Other studies indicate that delayed afterdepolarization-induced extrasystoles serve to trigger catecholamine-induced VT/VF, but that the epicardial origin of these ectopic beats increases transmural dispersion of repolarization, thus providing the substrate for the development of reentrant tachyarrhythmias, which underlie the rapid polymorphic VT/VF.46 Heart failure is associated with structural and electrophysiological remodeling, leading to tissue heterogeneity that enhances arrhythmogenesis and the propensity of sudden cardiac death.47
Late Phase 3 Early Afterdepolarizations and Their Role in the Initiation of Fibrillation
In 2003, Burashnikov and Antzelevitch48,49 described a novel mechanism giving rise to triggered activity, termed “late phase 3 EAD,” which combines properties of both EAD and DAD, but has its own unique character (see Fig. 2). Late phase 3 EAD-induced triggered extrasystoles represent a new concept of arrhythmogenesis in which abbreviated repolarization permits “normal SR calcium release” to induce an EAD-mediated closely coupled triggered response, particularly under conditions permitting intracellular calcium loading.48,49 These EADs are distinguished by the fact that they interrupt the final phase of repolarization of the action potential (late phase 3). In contrast to previously described DAD or Cai-dependent EAD, it is normal, not spontaneous SR calcium release that is responsible for the generation of the EAD. Two principal conditions are required for the appearance of late phase 3 EAD: an APD abbreviation and a strong SR calcium release.48 Such conditions may occur when both parasympathetic and sympathetic influences are combined. Simultaneous sympathovagal activation is also known to be the primary trigger of paroxysmal atrial tachycardia and AF episodes in dogs with intermittent rapid pacing.6
Late phase 3 EAD-induced extrasystoles have been shown to initiate AF in canine atria, particularly following spontaneous termination of the arrhythmia (IRAF, immediate reinduction of AF).48 The appearance of late phase 3 EAD immediately following termination of AF or rapid pacing has been reported by in the canine atria in vivo50 and pulmonary veins in vitro.51 In addition to the atrial arrhythmias, late phase 3 EAD may also be responsible for the development recurrent VF in failing hearts.52
REENTRANT ARRHYTHMIAS
Reentry is fundamentally different from automaticity or triggered activity in the mechanism by which it initiates and sustains cardiac arrhythmias. Circus movement reentry occurs when an activation wavefront propagates around an anatomic or functional obstacle or core, and reexcites the site of origin (Fig. 3). In this type of reentry, all cells take turns in recovering from excitation so that they are ready to be excited again when the next wavefront arrives. In contrast, reflection and phase 2 reentry occur in a setting in which large differences of recovery from refractoriness exist between one site and another. The site with delayed recovery serves as a virtual electrode that excites its already recovered neighbor, resulting in a reentrant reexcitation. In addition, reentry can also be classified as anatomic and functional, although there is a gray zone in which both functional and anatomic factors are important in determining the characteristics of reentrant excitation.
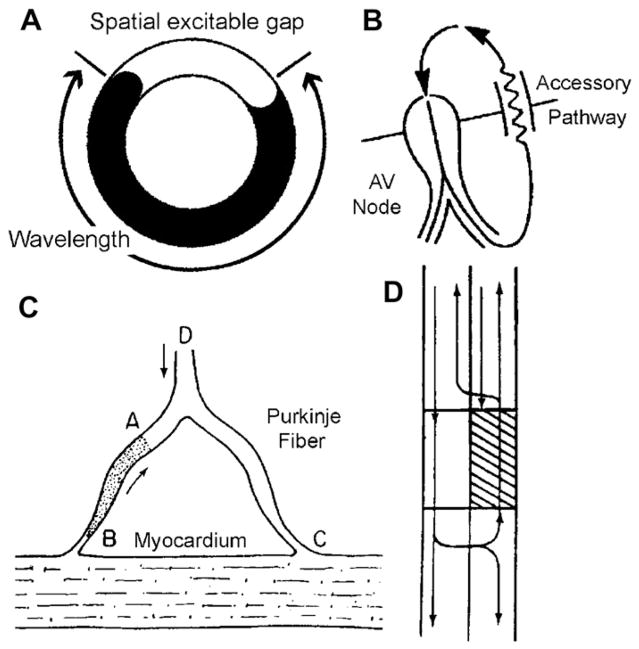
Fig. 3.
Ring models of reentry. (A) Schematic of a ring model of reentry. (B) Mechanism of reentry in the Wolf-Parkinson-White syndrome involving the AV node and an atrioventricular accessory pathway (AP). (C) A mechanism for reentry in a Purkinje-muscle loop proposed by Schmitt and Erlanger. The diagram shows a Purkinje bundle (D) that divides into 2 branches, both connected distally to ventricular muscle. Circus movement was considered possible if the stippled segment, A → B, showed unidirectional block. An impulse advancing from D would be blocked at A, but would reach and stimulate the ventricular muscle at C by way of the other terminal branch. The wavefront would then reenter the Purkinje system at B traversing the depressed region slowly so as to arrive at A following expiration of refractoriness. (D) Schematic representation of circus movement reentry in a linear bundle of tissue as proposed by Schmitt and Erlanger. The upper pathway contains a depressed zone (shaded) that serves as a site of unidirectional block and slow conduction. Anterograde conduction of the impulse is blocked in the upper pathway but succeeds along the lower pathway. Once beyond the zone of depression, the impulse crosses over through lateral connections and reenters through the upper pathway. (C and D from Schmitt FO, Erlanger J. Directional differences in the conduction of the impulse through heart muscle and their possible relation to extrasystolic and fibrillary contractions. Am J Physiol 1928;87:326–47.)
Circus Movement Reentry Around an Anatomic Obstacle
The ring model is the prototypical example of reentry around an anatomic obstacle (see Fig. 3). It first emerged as a concept shortly after the turn of the last century when Mayer53 reported the results of experiments involving the subumbrella tissue of a jellyfish (Sychomedusa cassiopeia). The muscular disk did not contract until ringlike cuts were made and pressure and a stimulus applied. This caused the disc to “spring into rapid rhythmic pulsation so regular and sustained as to recall the movement of clockwork.”(p25) Mayer demonstrated similar circus movement excitation in rings cut from the ventricles of turtle hearts, but he did not consider this to be a plausible mechanism for the development of cardiac arrhythmias. His experiments proved valuable in identifying 2 fundamental conditions necessary for the initiation and maintenance of circus movement excitation: (1) unidirectional block—the impulse initiating the circulating wave must travel in one direction only; and (2) for the circus movement to continue, the circuit must be long enough to allow each site in the circuit to recover before the return of the circulating wave. G. R. Mines54 was the first to develop the concept of circus movement reentry as a mechanism responsible for cardiac arrhythmias. He confirmed Mayer’s observations and suggested that the recirculating wave could be responsible for clinical cases of tachycardia.55 The following 3 criteria developed by Mines for identification of circus movement reentry remains in use today:
An area of unidirectional block must exist.
The excitatory wave progresses along a distinct pathway, returning to its point of origin and then following the same path again.
Interruption of the reentrant circuit at any point along its path should terminate the circus movement.
It was recognized that successful reentry could occur only when the impulse was sufficiently delayed in an alternate pathway to allow for expiration of the refractory period in the tissue proximal to the site of unidirectional block. Both conduction velocity and refractoriness determine the success or failure of reentry, and the general rule is that the length of the circuit (path length) must exceed or equal that of the wavelength, the wavelength being defined as the product of the conduction velocity and the refractory period or that part of the path length occupied by the impulse and refractory to reexcitation. The theoretical minimum path length required for development of reentry was therefore dependent on both the conduction velocity and the refractory period. Reduction of conduction velocity or APD can both significantly reduce the theoretical limit of the path length required for the development or maintenance of reentry.
Circus Movement Reentry without an Anatomic Obstacle
In 1914, Garrey56 suggested that reentry could be initiated without the involvement of anatomic obstacles and that “natural rings are not essential for the maintenance of circus contractions.”(p409) Nearly 50 years later, Allessie and coworkers57 provided direct evidence in support of this hypothesis in experiments in which they induced a tachycardia in isolated preparations of rabbit left atria by applying properly timed premature extra-stimuli. Using multiple intracellular electrodes, they showed that although the basic beats elicited by stimuli applied near the center of the tissue spread normally throughout the preparation, premature impulses propagate only in the direction of shorter refractory periods. An arc of block thus develops around which the impulse is able to circulate and reexcite its site of origin. Recordings near the center of the circus movement showed only subthreshold responses. The investigators proposed the term “leading circle” to explain their observation.58 They argued that the functionally refractory region that develops at the vortex of the circulating wavefront prevents the centripetal waves from short circuiting the circus movement and thus serves to maintain the reentry. The investigators also proposed that the refractory core was maintained by centripetal wavelets that collide with each other. Because the head of the circulating wavefront usually travels on relatively refractory tissue, a fully excitable gap of tissue may not be present; unlike other forms of reentry, the leading circle model may not be readily influenced by extraneous impulses initiated in areas outside the reentrant circuit and thus may not be easily entrained. Although the leading circle reentry for a while was widely accepted as a mechanism of functional reentry, there is significant conceptual limitation to this model of reentry. For example, the centripetal wavelet was difficult to demonstrate either by experimental studies with high-resolution mapping or with computer simulation studies.
Weiner and Rosenblueth59 in 1946 introduced the concept of spiral waves (rotors) to describe reentry around an anatomic obstacle; the term spiral wave reentry was later adopted to describe circulating waves in the absence of an anatomic obstacle.60 Spiral wave theory has advanced our understanding of the mechanisms responsible for the functional form of reentry. Although leading circle and spiral wave reentry are considered by some to be similar, a number of distinctions have been suggested. The curvature of the spiral wave is the key to the formation of the core.61 The term spiral wave is usually used to describe reentrant activity in 2 dimensions. The center of the spiral wave is called the core and the distribution of the core in 3 dimensions is referred to as the filament. The 3-dimensional form of the spiral wave forms a scroll wave. In its simplest form, the scroll wave has a straight filament spanning the ventricular wall (ie, from epicardium to endocardium). Theoretical studies have described 3 major scroll wave configurations with curved filaments (L-, U-, and O-shaped), although numerous variations of these 3-dimensional filaments in space and time are assumed to exist during cardiac arrhythmias.
Spiral wave activity has been used to explain the electrocardiographic patterns observed during monomorphic and polymorphic cardiac arrhythmias as well as during fibrillation. Monomorphic VT results when the spiral wave is anchored and not able to drift within the ventricular myocardium. In contrast, a meandering or drifting spiral wave causes polymorphic VT- and VF-like activity.62 VF seems to be the most complex representation of rotating spiral waves in the heart. VF is often preceded by VT. One of the theories suggests that VF develops when a single spiral wave responsible for VT breaks up, leading to the development of multiple spirals that are continuously extinguished and re-created.63
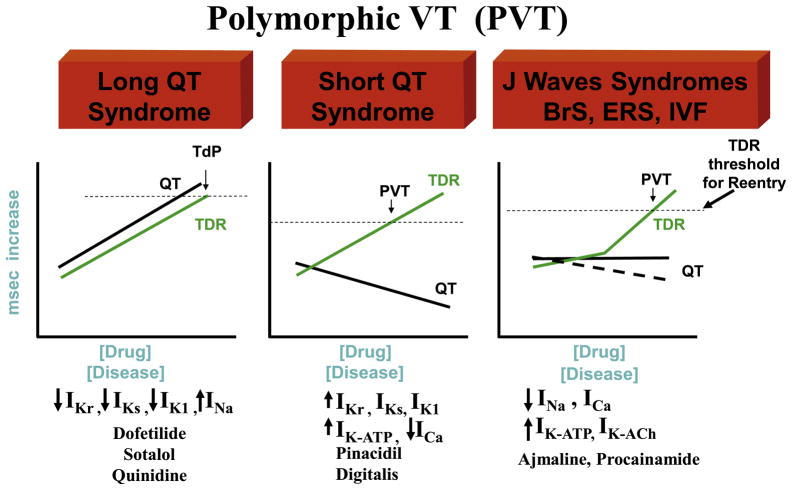
Figure 8 Reentry
Fig. 8.
The role of transmural dispersion of repolarization (TDR) in channelopathy-induced sudden death. In the long QT syndrome, QT increases as a function of disease or drug concentration. In the J wave syndromes (Brugada and early repolarization syndromes), it remains largely unchanged or is moderately abbreviated, and in the short QT syndrome, QT interval decreases as a function of disease or drug. The 3 syndromes have in common the ability to amplify TDR, which results in the development of polymorphic VT (PVT) or Torsade de Pontes (TdP) when dispersion reaches the threshold for reentry.
In the late 1980s, El-Sherif and coworkers64 delineated a figure 8 reentry in the surviving epicardial layer overlying an area of infarction produced by occlusion of the left anterior descending artery in canine hearts. The same patterns of activation can also be induced by creating artificial anatomic obstacles in the ventricles,65 or during functional reentry induced by a single premature ventricular stimulation.66 In the figure 8 model, the reentrant beat produces a wavefront that circulates in both directions around a line of conduction block rejoining on the distal side of the block. The wavefront then breaks through the arc of block to reexcite the tissue proximal to the block. The reentrant activation continues as 2 circulating wavefronts that travel in clockwise and counterclockwise directions around the 2 arcs in a pretzellike configuration.
Reflection
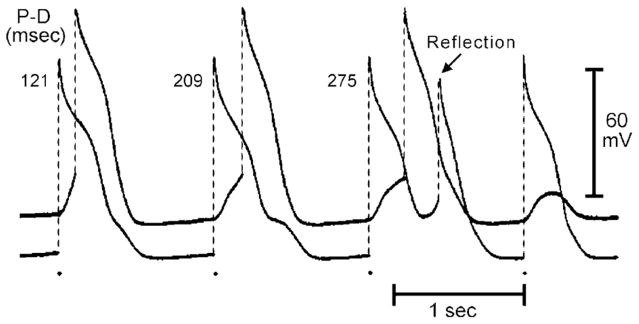
Reentry can occur without circus movement. Reflection and phase 2 reentry are 2 examples of non–circus movement reentry. The concept of reflection was first suggested by studies of the propagation characteristics of slow action potential responses in K+-depolarized Purkinje fibers.67 In strands of Purkinje fiber, Wit and coworkers67 demonstrated a phenomenon similar to that observed by Schmitt and Erlanger68 in which slow anterograde conduction of the impulse was at times followed by a retrograde wavefront that produced a “return extrasystole.” They proposed that the nonstimulated impulse was caused by circuitous reentry at the level of the syncytial interconnections, made possible by longitudinal dissociation of the bundle, as the most likely explanation for the phenomenon but also suggested the possibility of reflection. Direct evidence in support of reflection as a mechanism of arrhythmogenesis was provided by Antzelevitch and colleagues69,70 in the early 1980s. A number of models of reflection have been developed. The first of these involves use of ion-free isotonic sucrose solution to create a narrow (1.5 to 2 mm) central inexcitable zone (gap) in unbranched Purkinje fibers mounted in a 3-chamber tissue bath (Fig. 4).71 In the sucrose-gap model, stimulation of the proximal (P) segment elicits an action potential that propagates to the proximal border of the sucrose gap. Active propagation across the sucrose gap is not possible because of the ion-depleted extracellular milieu, but local circuit current continues to flow through the intercellular low-resistance pathways (an Ag/AgCl extracellular shunt pathway is provided). This local circuit or electrotonic current, very much reduced on emerging from the gap, gradually discharges the capacity of the distal (D) tissue, thus giving rise to a depolarization that manifests as a either a subthreshold response (last distal response) or a foot-potential that brings the distal excitable tissue to its threshold potential. Active impulse propagation stops and then resumes after a delay that can be as long as several hundred milliseconds. When anterograde (P to D) transmission time is sufficiently delayed to permit recovery of refractoriness at the proximal end, electrotonic transmission of the impulse in the retrograde direction is able to reexcite the proximal tissue, thus generating a closely coupled reflected reentry. Reflection therefore results from the to-and-fro electrotonically mediated transmission of the impulse across the same inexcitable segment; neither longitudinal dissociation nor circus movement need be invoked to explain the phenomenon.
Fig. 4.
Delayed transmission and reflection across an inexcitable gap created by superfusion of the central segment of a Purkinje fiber with an ion-free isotonic sucrose solution. The 2 traces were recorded from proximal (P) and distal (D) active segments. P–D conduction time (indicated in the upper portion of the figure, in ms) increased progressively with a 4:3 Wenckebach periodicity. The third stimulated proximal response was followed by a reflection. (From Antzelevitch C. Clinical applications of new concepts of parasystole, reflection, and tachycardia. Cardiol Clin 1983;1:39–50; with permission.)
A second model of reflection involved the creation of an inexcitable zone permitting delayed conduction by superfusion of a central segment of a Purkinje bundle with a solution designed to mimic the extracellular milieu at a site of ischemia.70 The gap was shown to be largely composed of an inexcitable cable across which conduction of impulses was electrotonically mediated. Reflected reentry has been demonstrated in isolated atrial and ventricular myocardial tissues as well.72–74 Reflection has also been demonstrated in Purkinje fibers in which a functionally in-excitable zone is created by focal depolarization of the preparation with long duration constant current pulses.75 Reflection is also observed in isolated canine Purkinje fibers homogeneously depressed with high K+ solution as well as in branched preparations of normal Purkinje fibers.76
Phase 2 Reentry
Another reentrant mechanism that does not depend on circus movement and can appear to be of focal origin is Phase 2 reentry.77–79 Phase 2 reentry occurs when the dome of the action potential, most commonly epicardial, propagates from sites at which it is maintained to sites at which it is abolished, causing local reexcitation of the epicardium and the generation of a closely coupled extra-systole. Severe spatial dispersion of repolarization is needed for phase 2 reentry to occur.
Phase 2 reentry has been proposed as the mechanism responsible for the closely coupled extrasystole that precipitates ventricular tachycardia/ventricular fibrillation (VT/VF) associated with Brugada and early repolarization syndromes.80,81
Spatial Dispersion of Repolarization
Studies conducted over the past 20 years have established that ventricular myocardium is electrically heterogeneous and composed of at least 3 electrophysiologically and functionally distinct cell types: epicardial, M, and endocardial cells.82,83 These 3 principal ventricular myocardial cell types differ with respect to phase 1 and phase 3 repolarization characteristics (Fig. 5). Ventricular epicardial and M, but not endocardial, cells generally display a prominent phase 1, because of a large 4-aminopyridine (4-AP)-sensitive transient outward current (Ito), giving the action potential a spike and dome or notched configuration. These regional differences in Ito, first suggested on the basis of action potential data,84 have now been directly demonstrated in ventricular myocytes from a wide variety of species including canine,85 feline,86 guinea pig,87 swine,88 rabbit,89 and humans.90,91 Differences in the magnitude of the action potential notch and corresponding differences in Ito have also been described between right and left ventricular (LV) epicardium.92 Similar inter-ventricular differences in Ito have also been described for canine ventricular M cells.93 This distinction is thought to form the basis for why the Brugada syndrome is a right ventricular disease.
Fig. 5.
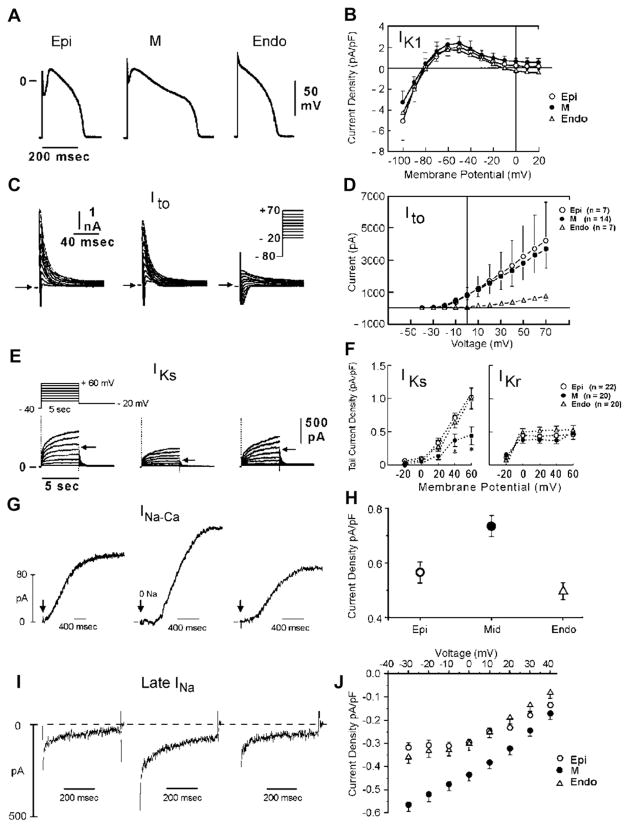
(A) Ionic distinctions among epicardial, M, and endocardial cells. Action potentials recorded from myocytes isolated from the epicardial, endocardial, and M regions of the canine left ventricle. (B) I-V relations for IK1 in epicardial, endocardial, and M region myocytes. Values are mean ± SD. (C) Transient outward current (Ito) recorded from the 3 cell types (current traces recorded during depolarizing steps from a holding potential of −80 mV to test potentials ranging between −20 and +70 mV). (D) The average peak current-voltage relationship for Ito for each of the 3 cell types. Values are mean ± SD. (E) Voltage-dependent activation of the slowly activating component of the delayed rectifier K+ current (IKs) (currents were elicited by the voltage pulse protocol shown in the inset; Na+-, K+-, and Ca2+- free solution). (F) Voltage dependence of IKs (current remaining after exposure to E-4031) and IKr (E-4031-sensitive current). Values are mean ± SE. *P < .05 compared with Epi or Endo. (G) Reverse-mode sodium-calcium exchange currents recorded in potassium- and chloride-free solutions at a voltage of −80 mV. INa-Ca was maximally activated by switching to sodium-free external solution at the time indicated by the arrow. (H) Midmyocardial sodium-calcium exchanger density is 30% greater than endocardial density, calculated as the peak outward INa-Ca normalized by cell capacitance. Endocardial and epicardial densities were not significantly different. (I) TTX-sensitive late sodium current. Cells were held at −80 mV and briefly pulsed to −45 mV to inactivate fast sodium current before stepping to −10 mV. (J) Normalized late sodium current measured 300 msec into the test pulse was plotted as a function of test pulse potential. (Data from Zygmunt AC, Goodrow RJ, Antzelevitch C. INaCa contributes to electrical heterogeneity within the canine ventricle. Am J Physiol Heart Circ Physiol 2000;278:H1671;8; and Refs.32,84,97)
Myocytes isolated from the epicardial region of the LV wall of the rabbit show a higher density of cAMP-activated chloride current when compared with endocardial myocytes.94 Ito2, initially ascribed to a K+ current, is now thought to be largely composed of a calcium-activated chloride current (ICl(Ca)) that contributes to the action potential notch, but it is not known whether this current differs among the 3 ventricular myocardial cell types.95
Between the surface epicardial and endocardial layers are transitional cells and M cells. M cells are distinguished by the ability of their action potential to prolong disproportionately relative to the action potential of other ventricular myocardial cells in response to a slowing of rate and/or in response to APD-prolonging agents.82,96,97 In the dog, the ionic basis for these features of the M cell includes the presence of a smaller slowly activating delayed rectifier current (IKs),32 a larger late sodium current (late INa),33 and a larger Na-Ca exchange current (INCx).98 In the canine heart, the rapidly activating delayed rectifier (IKr) and inward rectifier (IK1) currents are similar in the 3 transmural cell types. Transmural and apical-basal differences in the density of IKr channels have been described in the ferret heart.99 Amplification of transmural heterogeneities normally present in the early and late phases of the action potential can lead to the development of a variety of arrhythmias, including Brugada, long QT, and short QT syndromes, as well as catecholaminergic VT. The genetic mutations associated with these inherited channelopathies are listed in Table 1. The resulting gain or loss of function underlies the development of the arrhythmogenic substrate and triggers.
Table 1.
Genetic disorders causing cardiac arrhythmias in the absence of structural heart disease (Primary Electrical Disease)
| Rhythm | Inheritance | Locus | Ion Channel | Gene | ||
|---|---|---|---|---|---|---|
| LQTS | (RW) | TdP | AD | |||
| LQT1 | (Andersen-Tawil Syndrome) (Timothy Syndrome) | 11p15 | IKs | KCNQ1, KvLQT1 | ||
| LQT2 | 7q35 | IKr | KCNH2, HERG | |||
| LQT3 | 3p21 | INa | SCN5A, Nav1.5 | |||
| LQT4 | 4q25 | ANKB, ANK2 | ||||
| LQT5 | 21q22 | IKs | KCNE1, minK | |||
| LQT6 | 21q22 | IKr | KCNE2, MiRP1 | |||
| LQT7 | 17q23 | IK1 | KCNJ2, Kir 2.1 | |||
| LQT8 | 6q8A | ICa | CACNA1C, Cav1.2 | |||
| LQT9 | 3p25 | INa | CAV3, Caveolin-3 | |||
| LQT10 | 11q23.3 | INa | SCN4B. Navb4 | |||
| LQT11 | 7q21-q22 | IKs | AKAP9, Yotiao | |||
| LQT12 | 20q11.2 | INa | SNTA1, α–1 Syntrophin | |||
| LQT13 | 11q24 | IK-ACh | KCNJ5, Kir3.4 | |||
| LQTS | (JLN) | TdP | AR | 11p15 | IKs | KCNQ1, KvLQT1 |
| 21q22 | IKs | KCNE1, minK | ||||
| BrS | BrS1 | PVT | AD | 3p21 | INa | SCN5A, Nav1.5 |
| BrS2 | PVT | AD | 3p24 | INa | GPD1L | |
| BrS3 | PVT | AD | 12p13.3 | ICa | CACNA1C, CaV1.2 | |
| BrS4 | PVT | AD | 10p12.33 | ICa | CACNB2b, Cavβ2b | |
| BrS5 | PVT | AD | 19q13.1 | INa | SCN1B, Navβ1 | |
| BrS6 | PVT | AD | 11q13–14 | ICa | KCNE3. MiRP2 | |
| BrS7 | PVT | AD | 11q23.3 | INa | SCN3B, Navb3 | |
| BrS8 | PVT | AD | 7q21.11 | ICa | CACNA2D1, Cavα2δ | |
| ERS | ERS1 | PVT | AD | 12p11.23 | IK-ATP | KCNJ8, Kir6.1 |
| ERS2 | PVT | AD | 12p13.3 | ICa | CACNA1C, CaV1.2 | |
| ERS3 | PVT | AD | 10p12.33 | ICa | CACNB2b, Cavβ2b | |
| ERS4 | PVT | AD | 7q21.11 | ICa | CACNA2D1, Cavα2δ | |
| SQTS | SQT1 | VT/VF | AD | 7q35 | IKr | KCNH2, HERG |
| SQT2 | 11p15 | IKs | KCNQ1, KvLQT1 | |||
| SQT3 | AD | 17q23.1–24.2 | IK1 | KCNJ2, Kir2.1 | ||
| SQT4 | 12p13.3 | ICa | CACNA1C, CaV1.2 | |||
| SQT5 | AD | 10p12.33 | ICa | CACNB2b, Cavβ2b | ||
| Catecholaminergic Polymorphic VT | ||||||
| CPVT1 | VT | AD | 1q42–43 | RyR2 | ||
| CPVT2 | VT | AR | 1p13–21 | CASQ2 | ||
Abbreviations: AD, autosomal dominant; AR, autosomal recessive; BrS, Brugada syndrome; ERS, early repolarization syndrome; JLN, Jervell and Lange –Nielsen; LQTS, long QT syndrome; RW, Romano-Ward; SQTS, short QT syndrome; TdP, Torsade de Pointes; VF, ventricular fibrillation; VT, ventricular tachycardia.
MECHANISMS UNDERLYING CHANNELOPATHIES
In the following sections we briefly discuss how the reentrant and triggered mechanisms described previously contribute to development of VT/VF associated with the long QT, short QT, and J wave syndromes.
J Wave Syndromes
Because they share a common arrhythmic platform related to amplification of Ito-mediated J waves, and because of similarities in ECG characteristics, clinical outcomes and risk factors, congenital and acquired forms of Brugada syndrome (BrS) and early repolarization syndrome (ERS) have been grouped together under the heading of J wave syndromes.80
Brugada syndrome
In 1992, Pedro and Josep Brugada100 reported a new syndrome associated with ST elevation in ECG leads V1-V3, right bundle branch appearance during sinus rhythm, and a high incidence of VF and sudden cardiac death. BrS has been associated with mutations in 7 different genes. Mutations in SCN5A (Nav1.5, BrS1) have been reported in 11% to 28% of BrS probands, CACNA1C (Cav1.2, BrS3) in 6.7%, CACNB2b (Cavβ2b, BrS4) in 4.8%, and mutations in Glycerol-3-phophate dehydrogenase 1–like enzyme gene (GPD1L, BrS2), SCN1B (β1-subunit of sodium channel, BrS5), KCNE3 (MiRP2; BrS6), and SCN3B (β3-subunit of sodium channel, BrS7) are much more rare.101–105 The newest gene associated with BrS is CACNA2D1 (Cavα2δ, BrS8).106
The mechanisms of arrhythmogenesis in BrS can be explained by the heterogeneous shortening of the APD on the right ventricular epicardium (Fig. 6).81
Fig. 6.
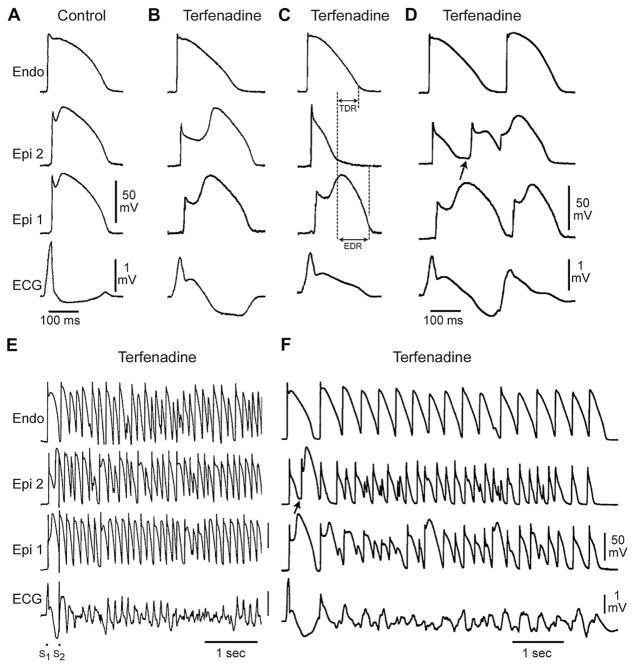
Cellular basis for electrocardiographic and arrhythmic manifestation of BrS. Each panel shows transmembrane action potentials from 1 endocardial (top) and 2 epicardial sites together with a transmural ECG recorded from a canine coronary-perfused right ventricular wedge preparation. (A) Control (basic cycle length (BCL) 400 msec). (B) Combined sodium and calcium channel block with terfenadine (5 μM) accentuates the epicardial action potential notch creating a transmural voltage gradient that manifests as an ST segment elevation or exaggerated J wave in the ECG. (C) Continued exposure to terfenadine results in all-or-none repolarization at the end of phase 1 at some epicardial sites but not others, creating a local epicardial dispersion of repolarization (EDR) as well as a transmural dispersion of repolarization (TDR). (D) Phase 2 reentry occurs when the epicardial action potential dome propagates from a site where it is maintained to regions where it has been lost giving rise to a closely coupled extrasystole. (E) Extrastimulus (S1–S2 = 250 msec) applied to epicardium triggers a polymorphic VT. (F) Phase 2 reentrant extrasystole triggers a brief episode of polymorphic VT. (Modified from Fish JM, Antzele-vitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm 2004;1:210–17; with permission.)
In regions of the myocardium exhibiting a prominent Ito, such as the right ventricular outflow tract epicardium, accentuation of the action potential notch secondary to a reduction of calcium or sodium channel current or an increase in outward current, results in a transmural voltage gradient that leads to coved ST segment elevation, which is the only form of ST segment elevation diagnostic of BrS (see Fig. 6B). Under these conditions, there is little in the way of an arrhythmogenic substrate. However, a further outward shift of the currents active during the early phase of the action potential can lead to loss of the action potential dome, thus creating a dispersion of repolarization between epicardium and endocardium as well as within epicardium, between regions at which the dome is maintained and regions where it is lost (see Fig. 6C). The extent to which the action potential notch is accentuated leading to loss of the dome depends on the initial level of Ito.107–109 When Ito is prominent, as it is in the right ventricular epicardium,92,107,109 an outward shift of current causes phase 1 of the action potential to progress to more negative potentials at which the L-type calcium current (ICa,L) fails to activate, leading to an all-or-none repolarization and loss of the dome (see Fig. 6C). Because loss of the action potential dome is usually heterogeneous, the result is a marked abbreviation of action potential at some sites but not others. The epicardial action potential dome can then propagate from regions where it is maintained to regions where it is lost, giving rise to a very closely coupled extrasystole via phase 2 reentry (see Fig. 6D).77 The extrasystole produced via phase 2 reentry often occurs on the preceding T wave resulting in an R-on-T phenomenon. This in turn can initiate polymorphic VT or VF (see Fig. 6E, F).
Potent sodium channel blockers like procainamide, pilsicainide, propafenone, and flecainide can be used to induce or unmask ST segment elevation in patients with concealed J-wave syndromes because they facilitate an outward shift of currents active in the early phases of the action potential.110–112 Sodium channel blockers like quinidine, which also inhibits Ito, reduce the magnitude of the J wave and normalize ST segment elevation.107,113
Recent studies point to a prominent role of depolarization impairment resulting in local conduction delay in the RV114; however, the role of conduction delay in the RV in the electrocardiographic and arrhythmic manifestations of BrS remains a matter of debate.115
Early repolarization syndrome
An early repolarization (ER) pattern, consisting of a J point elevation, a notch or slur on the QRS (J wave), and tall/symmetric T waves, is commonly found in healthy young males and has traditionally been regarded as totally benign.116,117 A report in 2000 that an ER pattern in the coronary-perfused wedge preparation can easily convert to one in which phase 2 reentry gives rise to polymorphic VT/VF, prompted the suggestion that ER may in some cases predispose to malignant arrhythmias in the clinic.80,118 Many case reports and experimental studies have long suggested a critical role for the J wave in the pathogenesis of idiopathic ventricular fibrillation (IVF).119–127 Several recent studies have provided a definitive association between ER and IVF.128–132
The high prevalence of ER in the general population suggests that it is not a sensitive marker for sudden cardiac death (SCD), but that it is a marker of a genetic predisposition for the development of VT/VF via an ERS. Thus, when observed in patients with syncope or malignant family history of sudden cardiac death, ER may be prognostic of risk. We recently proposed a classification scheme for ERS based on the available data pointing to an association of risk with spatial localization of the ER pattern.80 In this scheme, Type 1 is associated with ER pattern predominantly in the lateral precordial leads; this form is very prevalent among healthy male athletes and is thought to be largely benign. Type 2, displaying an ER pattern predominantly in the inferior or inferolateral leads, is associated with a moderate level of risk and Type 3, displaying an ER pattern globally in the inferior, lateral, and right precordial leads, appears to be associated with the highest level of risk and is often associated with electrical storms.80 Of note, BrS represents a fourth variant in which ER is limited to the right precordial leads.
In ERS, as in BrS, the dynamic nature of J wave manifestation is well recognized. The amplitude of J waves, which may be barely noticeable during sinus rhythm, may become progressively accentuated with increased vagal tone and bradycardia and still further accentuated following successive extrasystoles and compensatory pauses giving rise to short long short sequences that precipitate VT/VF.80,129,133
Studies examining the genetic and molecular basis for ERS are few and data are very limited (see Table 1). Haissaguerre and colleagues134 were the first to associate KCNJ8 with ERS. Functional expression of the S422L missense mutation in KCNJ8 was not available at the time but was recently reported by Medeiros-Domingo and colleagues.135 The investigators genetically screened 101 probands with BrS and ERS and found one BrS and one ERS proband with an S422L-KCNJ8 (Kir6.1) mutation; the variation was absent in 600 controls. The investigators co-expressed the KCNJ8 mutation with ATP regulatory subunit SUR2A in COS-1 cells and measured IK-ATP using whole cell patch clamp techniques. A significantly larger IK-ATP was recorded for the mutant versus wild type in response to a high concentration of pinacidil (100 μM). The presumption is that the S422L-KCNJ8 mutant channels fail to close properly at normal intracellular ATP concentrations, thus resulting in a gain of function. The prospect of a gain of function in IK-ATP as the basis for ERS is supported by the observation that pinacidil, an IK-ATP opener, has been shown to induce both the electrocardiographic and arrhythmic manifestation of ERS in LV wedge preparations.80
Recent studies from our group have identified 4 probands in whom mutations in highly conserved residues of CACNA1C, CACNB2, and CACNA2D1 were found to be associated with ERS.106 Preliminary studies involving heterologous expression of these genes in HEK293 cells indicate that these mutations are associated with a loss of function of ICa, supporting the thesis that all 3 are ERS-susceptibility genes (Barajas, unpublished observation, 2010).
The ECG and arrhythmic manifestations of ERS are thought to be attributable to mechanisms similar to those operative in BrS. In ERS, the outward shift of current may extend beyond the action potential notch, thus leading to an elevation of the ST segment akin to early repolarization. Activation of the ATP-sensitive potassium current (IK-ATP) or depression of inward calcium channel current (ICa) can effect such a change.106 Transmural gradients generated in response to ICa loss of function or IK-ATP gain of function could manifest in the ECG as a diversity of ER patterns including J point elevation, slurring of the terminal part of the QRS, and mild ST segment elevation. The ER pattern could facilitate loss of the dome because of other factors and thus lead to the development of ST segment elevation, phase 2 reentry, and VT/VF.
The Long QT Syndrome
The long QT syndromes (LQTS) are phenotypically and genotypically diverse, but have in common the appearance of long QT interval in the ECG, an atypical polymorphic ventricular tachycardia known as Torsade de Pointes (TdP), and, in many but not all cases, a relatively high risk for sudden cardiac death.136–138 Congenital LQTS has been associated with 13 genes in at least 7 different ion genes and a structural anchoring protein located on chromosomes 3, 4, 6, 7, 11, 17, 20, and 21 (see Table 1).139–146 Timothy syndrome, also referred to as LQT8, is a rare congenital disorder characterized by multiorgan dysfunction including prolongation of the QT interval, lethal arrhythmias, webbing of fingers and toes, congenital heart disease, immune deficiency, intermittent hypoglycemia, cognitive abnormalities, and autism. Timothy syndrome has been linked to loss of voltage-dependent inactivation owing to mutations in Cav1.2, the gene that encodes for an α subunit of the calcium channel.147 The most recent gene associated with LQTS is KCNJ5, which encodes Kir3.4 protein, the protein that encodes the α subunit of the IK-ACh channel. Mutations in this gene produce a loss of function that produces an LQT phenotype via a mechanism that is not clearly understood.148
Two patterns of inheritance have been identified in LQTS: (1) a rare autosomal recessive disease associated with deafness (Jervell and Lange-Nielsen), caused by 2 genes that encode for the slowly activating delayed rectifier potassium channel (KCNQ1 and KCNE1); and (2) a much more common autosomal dominant form known a the Romano Ward syndrome, caused by mutations in 13 different genes (see Table 1).
Acquired LQTS refers to a syndrome similar to the congenital form but caused by exposure to drugs that prolong the duration of the ventricular action potential149 or QT prolongation secondary to cardiomyopathies, such as dilated or hypertrophic cardiomyopathy, as well as to abnormal QT prolongation associated with bradycardia or electrolyte imbalance.150–154 The acquired form of the disease is far more prevalent than the congenital form, and in some cases may have a genetic predisposition.
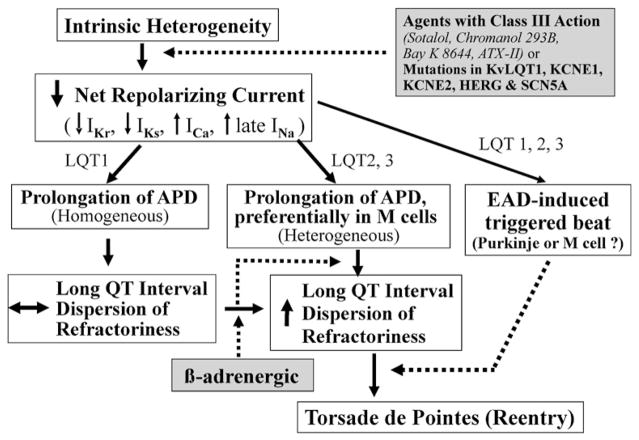
Amplification of spatial dispersion of repolarization within the ventricular myocardium has been identified as the principal arrhythmogenic substrate in both acquired and congenital LQTS. The accentuation of spatial dispersion, typically secondary to an increase of transmural, trans-septal, or apico-basal dispersion of repolarization, and the development of early afterdepolarization (EAD)-induced triggered activity underlie the substrate and trigger for the development of TdP arrhythmias observed under LQTS conditions.155,156 Models of the LQT1, LQT2, and LQT3, and LQT7 forms of the long QT syndrome have been developed using the canine arterially perfused left ventricular wedge preparation (Fig. 7).16,157,158 Data from these studies suggest that in LQTS, preferential prolongation of the M cell APD leads to an increase in the QT interval as well as an increase in transmural dispersion of repolarization (TDR), which contributes to the development of spontaneous as well as stimulation-induced TdP.159–161 The unique characteristics of the M cells, ie, the ability of their action potential to prolong more than that of epicardium or endocardium in response to a slowing of rate,96,162,163 is at the heart of this mechanism.-Fig. 7 presents our working hypothesis for our understanding of the mechanisms underlying LQTS-related TdP based on available data. The hypothesis presumes the presence of electrical heterogeneity in the form of transmural dispersion of repolarization under baseline conditions and the amplification of TDR by agents that reduce net repolarizing current via a reduction in IKr or IKs or augmentation of ICa or late INa. Conditions leading to a reduction in IKr or augmentation of late INa lead to a preferential prolongation of the M cell action potential. As a consequence, the QT interval prolongs and is accompanied by a dramatic increase in transmural dispersion of repolarization, thus creating a vulnerable window for the development of reentry. The reduction in net repolarizing current also predisposes to the development of EAD-induced triggered activity in M and Purkinje cells, which provide the extrasystole that triggers TdP when it falls within the vulnerable period. β adrenergic agonists further amplify transmural heterogeneity (transiently) in the case of IKr block, but reduce it in the case of INa agonists.161,164
Fig. 7.
Proposed cellular and ionic mechanisms for the long QT syndrome.
Short QT Syndrome
The short QT syndrome (SQTS), first proposed as a clinical entity by Gussak and colleagues165 in 2000, is an inherited syndrome characterized by a QTc of 360 msec or less and high incidence of VT/VF in infants, children, and young adults.166,167 The familial nature of this sudden death syndrome was highlighted by Gaita and colleagues168 in 2003. Mutations in 5 genes have been associated with SQTS: KCNH2, KCNJ2, KCNQ1, CACNA1c, and CACNB2b.102,169–171 Mutations in these genes cause either a gain of function in outward potassium channel currents (IKr, IKs and IK1) or a loss of function in inward calcium channel current (ICa).
Experimental studies suggest that the abbreviation of the action potential in SQTS is heterogeneous with preferential abbreviation of either ventricular epicardium or endocardium, giving rise to an increase in TDR.172,173 In the atria, the IKr agonist PD118057 causes a much greater abbreviation of the action potential in epicardium when compared with cristae terminalis, thus creating a marked dispersion of repolarization in the right atrium.174 Dispersion of repolarization and refractoriness serve as substrates for reentry by promoting unidirectional block. The marked abbreviation of wavelength (product of refractory period and conduction velocity) is an additional factor promoting the maintenance of reentry. Tpeak-Tend interval and Tpeak-Tend /QT ratio, an electrocardiographic index of spatial dispersion of ventricular repolarization, and perhaps TDR, have been reported to be significantly augmented in cases of SQTS.175,176 Interestingly, this ratio is more amplified in patients who are symptomatic.177
Evidence supporting the role of augmented TDR in atrial and ventricular arrhythmogenesis in SQTS derives from experimental studies involving the canine left ventricular wedge and atrial preparations.172–174,178
The Role of Spatial Dispersion of Repolarization in Channelopathy-Mediated Sudden Death
The inherited and acquired sudden death syndromes discussed previously differ with respect to the behavior of the QT interval (Fig. 8). In the long QT syndrome, QT increases as a function of disease or drug concentration. In the Brugada and early repolarization syndromes, it remains largely unchanged or is abbreviated, and in the short QT syndrome, QT interval decreases as a function of disease or drug. What these syndromes have in common is an amplification of TDR, which results in the development of polymorphic VT when TDR reaches the threshold for reentry. In the setting of a prolonged QT, we refer to it as TdP. It is noteworthy that the threshold for reentry decreases as APD and refractoriness are reduced, thus requiring a shorter path length for reentry, making it easier to induce.
Acknowledgments
Financial support: Supported by grant HL47678 from the National Heart, Lung, and Blood Institute (CA) and NYS and Florida Masons.
Footnotes
Conflict of interest: None.
References
- 1.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–69. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG. A paradigm shift for the heart’s pacemaker. Heart Rhythm. 2010;7:559–64. doi: 10.1016/j.hrthm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiFrancesco D. The pacemaker current If plays an important role in regulating SA node pacemaker activity. Cardiovasc Res. 1995;30:307–8. [PubMed] [Google Scholar]
- 4.Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524(Pt 2):415–22. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–45. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- 6.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–25. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–43. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 8.Schulze-Bahr E, Neu A, Friederich P, et al. Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest. 2003;111:1537–45. doi: 10.1172/JCI16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nof E, Luria D, Brass D, et al. Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation. 2007;116:463–70. doi: 10.1161/CIRCULATIONAHA.107.706887. [DOI] [PubMed] [Google Scholar]
- 10.Zicha S, Fernandez-Velasco M, Lonardo G, et al. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res. 2005;66:472–81. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Laish-Farkash A, Marek D, Brass D, et al. A novel mutation in the HCN4 gene causes familial sinus bradycardia in two unrelated Moroccan families [abstract] Heart Rhythm. 2008;5S:S275. [Google Scholar]
- 12.Laish-Farkash A, Glikson M, Brass D, et al. A novel mutation in the HCN4 gene causes symptomatic sinus bradycardia in Moroccan Jews. J Cardiovasc Electrophysiol. doi: 10.1111/j.1540-8167.2010.01844.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nof E, Antzelevitch C, Glickson M. The contribution of HCN4 to normal sinus nose function in humans and animal models. Pacing Clin Electrophysiol. 2010;33:100–6. doi: 10.1111/j.1540-8159.2009.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wit AL, Rosen MR. Afterdepolarizations and triggered activity: distinction from automaticity as an arrhythmogenic mechanism. In: Fozzard HA, Haber E, Jenning RB, et al., editors. The heart and cardiovascular system. New York: Raven Press; 1992. pp. 2113–64. [Google Scholar]
- 15.Zhang L, Benson DW, Tristani-Firouzi M, et al. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation. 2005;111:2720–6. doi: 10.1161/CIRCULATIONAHA.104.472498. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboi M, Antzelevitch C. Cellular basis for electrocardiographic and arrhythmic manifestations of Andersen-Tawil syndrome (LQT7) Heart Rhythm. 2006;3:328–35. doi: 10.1016/j.hrthm.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barajas-Martínez H, Hu D, Ontiverod G, et al. Biophysical characterization of a novel KCNJ2 mutation associated with Andersen-Tawil syndrome and CPVT mimicry [abstract] Biophys J. 2009;96:260a. [Google Scholar]
- 18.Tristani-Firouzi M. Andersen-Tawil syndrome: an ever-expanding phenotype? Heart Rhythm. 2006;3:1351–2. doi: 10.1016/j.hrthm.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Tristani-Firouzi M, Etheridge SP. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch. doi: 10.1007/s00424-010-0820-6. in press. [DOI] [PubMed] [Google Scholar]
- 20.Tristani-Firouzi M, Jensen JL, Donaldson MR, et al. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome) J Clin Invest. 2002;110:381–8. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassalle M. The relationship among cardiac pacemakers. Overdrive suppression. Circ Res. 1977;41:269–77. doi: 10.1161/01.res.41.3.269. [DOI] [PubMed] [Google Scholar]
- 22.Gadsby DC, Cranefield PF. Electrogenic sodium extrusion in cardiac Purkinje fibers. J Gen Physiol. 1979;73:819–37. doi: 10.1085/jgp.73.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalife J, Moe GK. A biological model of parasystole. Am J Cardiol. 1979;43:761–72. doi: 10.1016/0002-9149(79)90076-6. [DOI] [PubMed] [Google Scholar]
- 24.Jalife J, Antzelevitch C, Moe GK. The case for modulated parasystole. Pacing Clin Electrophysiol. 1982;5:911–26. doi: 10.1111/j.1540-8159.1982.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 25.Nau GJ, Aldariz AE, Acunzo RS, et al. Modulation of parasystolic activity by nonparasystolic beats. Circulation. 1982;66:462–9. doi: 10.1161/01.cir.66.2.462. [DOI] [PubMed] [Google Scholar]
- 26.Antzelevitch C, Bernstein MJ, Feldman HN, et al. Parasystole, reentry, and tachycardia: a canine preparation of cardiac arrhythmias occurring across inexcitable segments of tissue. Circulation. 1983;68:1101–15. doi: 10.1161/01.cir.68.5.1101. [DOI] [PubMed] [Google Scholar]
- 27.Jalife J, Moe GK. Effect of electrotonic potentials on pacemaker activity of canine Purkinje fibers in relation to parasystole. Circ Res. 1976;39:801–8. doi: 10.1161/01.res.39.6.801. [DOI] [PubMed] [Google Scholar]
- 28.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 29.Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 30.Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am J Physiol. 1990;258:H1796–805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 31.Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–48. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res. 1995;76:351–65. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 33.Zygmunt AC, Eddlestone GT, Thomas GP, et al. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol. 2001;281:H689–97. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- 34.Burashnikov A, Antzelevitch C. Prominent IKs in epicardium and endocardium contributes to development of transmural dispersion of repolarization but protects against development of early afterdepolarizations. J Cardiovasc Electrophysiol. 2002;13:172–7. doi: 10.1046/j.1540-8167.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- 35.Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrier GR, Saunders JH, Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973;32:600–9. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- 37.Rosen MR, Gelband H, Merker C, et al. Mechanisms of digitalis toxicity—effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973;47:681–9. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- 38.Saunders JH, Ferrier GR, Moe GK. Conduction block associated with transient depolarizations induced by acetylstrophanthidin in isolated canine Purkinje fibers. Circ Res. 1973;32:610–7. doi: 10.1161/01.res.32.5.610. [DOI] [PubMed] [Google Scholar]
- 39.Rozanski GJ, Lipsius SL. Electrophysiology of functional subsidiary pacemakers in canine right atrium. Am J Physiol. 1985;249:H594–603. doi: 10.1152/ajpheart.1985.249.3.H594. [DOI] [PubMed] [Google Scholar]
- 40.Wit AL, Cranefield PF. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977;41:435–45. doi: 10.1161/01.res.41.4.434. [DOI] [PubMed] [Google Scholar]
- 41.Aronson RS. Afterpotentials and triggered activity in hypertrophied myocardium from rats with renal-hypertension. Circ Res. 1981;48:720–7. doi: 10.1161/01.res.48.5.720. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen JT, McGuire MA, Opthof T, et al. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc Res. 1994;28:1547–54. doi: 10.1093/cvr/28.10.1547. [DOI] [PubMed] [Google Scholar]
- 43.Lazzara R, El-Sherif N, Scherlag BJ. Electrophysiological properties of canine Purkinje cells in one-day-old myocardial infarction. Circ Res. 1973;33:722–34. doi: 10.1161/01.res.33.6.722. [DOI] [PubMed] [Google Scholar]
- 44.Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 45.Wehrens XH, Lehnart SE, Reiken SR, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 46.Nam GB, Burashnikov A, Antzelevitch C. Cellular mechanisms underlying the development of catecholaminergic ventricular tachycardia. Circulation. 2005;111:2727–33. doi: 10.1161/CIRCULATIONAHA.104.479295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–63. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 48.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–60. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 49.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:290–5. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe I, Okumura Y, Ohkubo K, et al. Steady-state and nonsteady-state action potentials in fibrillating canine atrium: alternans of action potential and late phase 3 early afterdepolarization as a precursor of atrial fibrillation [abstract] Heart Rhythm. 2005;2:S259. [Google Scholar]
- 51.Patterson E, Po SS, Scherlag BJ, et al. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa M, Morita N, Tang L, et al. Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm. 2009;6:784–92. doi: 10.1016/j.hrthm.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayer AG. Rhythmical pulsations is scyphomedusae. Washington, DC: Publication 47 of the Carnegie Institute; 1906. pp. 1–62. [Google Scholar]
- 54.Mines GR. On circulating excitations in heart muscles and their possible relation to tachycardia and fibrillation. Trans R Soc Can. 1914;8:43–52. [Google Scholar]
- 55.Mines GR. On dynamic equilibrium in the heart. J Physiol. 1913;46:350–83. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garrey WE. The nature of fibrillatory contraction of the heart—its relation to tissue mass and form. Am J Physiol. 1914;33:397–414. [Google Scholar]
- 57.Allessie MA, Bonke FIM, Schopman JG. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Circ Res. 1973;33:54–62. [PubMed] [Google Scholar]
- 58.Allessie MA, Bonke FIM, Schopman JG. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 59.Weiner N, Rosenblueth A. The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle. Arch Inst Cardiol Mex. 1946;16:205–65. [PubMed] [Google Scholar]
- 60.Davidenko JM, Cohen L, Goodrow RJ, et al. Quinidine-induced action potential prolongation, early afterdepolarizations, and triggered activity in canine Purkinje fibers. Effects of stimulation rate, potassium, and magnesium. Circulation. 1989;79:674–86. doi: 10.1161/01.cir.79.3.674. [DOI] [PubMed] [Google Scholar]
- 61.Jalife J, Delmar M, Davidenko JM, et al. Basic cardiac electrophysiology for the clinician. Armonk (NY): Futura Publishing; 1999. [Google Scholar]
- 62.Gray RA, Jalife J, Panfilov AV, et al. Mechanisms of cardiac fibrillation. Science. 1995;270:1222–3. [PubMed] [Google Scholar]
- 63.Garfinkel A, Kim YH, Voroshilovsky O, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000;97:6061–6. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Sherif N, Smith RA, Evans K. Canine ventricular arrhythmias in the late myocardial infarction period. 8. Epicardial mapping of reentrant circuits. Circ Res. 1981;49:255–65. doi: 10.1161/01.res.49.1.255. [DOI] [PubMed] [Google Scholar]
- 65.Valderrabano M, Kim YH, Yashima M, et al. Obstacle-induced transition from ventricular fibrillation to tachycardia in isolated swine right ventricles: insights into the transition dynamics and implications for the critical mass. J Am Coll Cardiol. 2000;36:2000–8. doi: 10.1016/s0735-1097(00)00941-4. [DOI] [PubMed] [Google Scholar]
- 66.Chen PS, Wolf PD, Dixon EG, et al. Mechanism of ventricular vulnerability to single premature stimuli in open-chest dogs. Circ Res. 1988;62:1191–209. doi: 10.1161/01.res.62.6.1191. [DOI] [PubMed] [Google Scholar]
- 67.Wit AL, Cranefield PF, Hoffman BF. Slow conduction and reentry in the ventricular conducting system. II. Single and sustained circus movement in networks of canine and bovine Purkinje fibers. Circ Res. 1972;30:11–22. doi: 10.1161/01.res.30.1.11. [DOI] [PubMed] [Google Scholar]
- 68.Schmitt FO, Erlanger J. Directional differences in the conduction of the impulse through heart muscle and their possible relation to extrasystolic and fibrillary contractions. Am J Physiol. 1928;87:326–47. [Google Scholar]
- 69.Antzelevitch C, Jalife J, Moe GK. Characteristics of reflection as a mechanism of reentrant arrhythmias and its relationship to parasystole. Circulation. 1980;61:182–91. doi: 10.1161/01.cir.61.1.182. [DOI] [PubMed] [Google Scholar]
- 70.Antzelevitch C, Moe GK. Electrotonically-mediated delayed conduction and reentry in relation to “slow responses” in mammalian ventricular conducting tissue. Circ Res. 1981;49:1129–39. doi: 10.1161/01.res.49.5.1129. [DOI] [PubMed] [Google Scholar]
- 71.Antzelevitch C. Clinical applications of new concepts of parasystole, reflection, and tachycardia. Cardiol Clin. 1983;1:39–50. [PubMed] [Google Scholar]
- 72.Rozanski GJ, Jalife J, Moe GK. Reflected reentry in nonhomogeneous ventricular muscle as a mechanism of cardiac arrhythmias. Circulation. 1984;69:163–73. doi: 10.1161/01.cir.69.1.163. [DOI] [PubMed] [Google Scholar]
- 73.Lukas A, Antzelevitch C. Reflected reentry, delayed conduction, and electrotonic inhibition in segmentally depressed atrial tissues. Can J Physiol Pharmacol. 1989;67:757–64. doi: 10.1139/y89-121. [DOI] [PubMed] [Google Scholar]
- 74.Davidenko JM, Antzelevitch C. The effects of milrinone on action potential characteristics, conduction, automaticity, and reflected reentry in isolated myocardial fibers. J Cardiovasc Pharmacol. 1985;7:341–9. doi: 10.1097/00005344-198503000-00021. [DOI] [PubMed] [Google Scholar]
- 75.Rosenthal JE, Ferrier GR. Contribution of variable entrance and exit block in protected foci to arrhythmogenesis in isolated ventricular tissues. Circulation. 1983;67:1–8. doi: 10.1161/01.cir.67.1.1. [DOI] [PubMed] [Google Scholar]
- 76.Antzelevitch C, Lukas A. Reflection and circus movement reentry in isolated atrial and ventricular tissues. In: Dangman KH, Miura DS, editors. Electrophysiology and pharmacology of the heart. A clinical guide. New York: Marcel Dekker; 1991. pp. 251–75. [Google Scholar]
- 77.Krishnan SC, Antzelevitch C. Flecainide-induced arrhythmia in canine ventricular epicardium. Phase 2 reentry? Circulation. 1993;87:562–72. doi: 10.1161/01.cir.87.2.562. [DOI] [PubMed] [Google Scholar]
- 78.Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc Res. 1996;32:593–603. [PubMed] [Google Scholar]
- 79.Di Diego JM, Antzelevitch C. Pinacidil-induced electrical heterogeneity and extrasystolic activity in canine ventricular tissues. Does activation of ATP-regulated potassium current promote phase 2 reentry? Circulation. 1993;88:1177–89. doi: 10.1161/01.cir.88.3.1177. [DOI] [PubMed] [Google Scholar]
- 80.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–58. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–59. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antzelevitch C, Sicouri S, Litovsky SH, et al. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–49. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 83.Antzelevitch C, Sicouri S, Lukas A, et al. Clinical implications of electrical heterogeneity in the heart: the electrophysiology and pharmacology of epicardial, M, and endocardial cells. In: Podrid PJ, Kowey PR, editors. Cardiac arrhythmia: mechanism, diagnosis and management. Baltimore (MD): William & Wilkins; 1995. pp. 88–107. [Google Scholar]
- 84.Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988;62:116–26. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- 85.Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res. 1993;72:671–87. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- 86.Furukawa T, Myerburg RJ, Furukawa N, et al. Differences in transient outward currents of feline endocardial and epicardial myocytes. Circ Res. 1990;67:1287–91. doi: 10.1161/01.res.67.5.1287. [DOI] [PubMed] [Google Scholar]
- 87.Sicouri S, Quist M, Antzelevitch C. Evidence for the presence of M cells in the guinea pig ventricle. J Cardiovasc Electrophysiol. 1996;7:503–11. doi: 10.1111/j.1540-8167.1996.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 88.Stankovicova T, Szilard M, De Scheerder I, et al. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc Res. 2000;45:952–60. doi: 10.1016/s0008-6363(99)00418-6. [DOI] [PubMed] [Google Scholar]
- 89.McIntosh MA, Cobbe SM, Smith GL. Heterogeneous changes in action potential and intracellular Ca2+in left ventricular myocyte sub-types from rabbits with heart failure. Cardiovasc Res. 2000;45:397–409. doi: 10.1016/s0008-6363(99)00360-0. [DOI] [PubMed] [Google Scholar]
- 90.Wettwer E, Amos GJ, Posival H, et al. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–82. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- 91.Nabauer M, Beuckelmann DJ, Uberfuhr P, et al. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–77. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- 92.Di Diego JM, Sun ZQ, Antzelevitch C. Ito and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548–61. doi: 10.1152/ajpheart.1996.271.2.H548. [DOI] [PubMed] [Google Scholar]
- 93.Volders PG, Sipido KR, Carmeliet E, et al. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99:206–10. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- 94.Takano M, Noma A. Distribution of the isoprenaline-induced chloride current in rabbit heart. Pflugers Arch. 1992;420:223–6. doi: 10.1007/BF00374995. [DOI] [PubMed] [Google Scholar]
- 95.Zygmunt AC. Intracellular calcium activates chloride current in canine ventricular myocytes. Am J Physiol. 1994;267:H1984–95. doi: 10.1152/ajpheart.1994.267.5.H1984. [DOI] [PubMed] [Google Scholar]
- 96.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729–41. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 97.Anyukhovsky EP, Sosunov EA, Rosen MR. Regional differences in electrophysiologic properties of epicardium, midmyocardium and endocardium: in vitro and in vivo correlations. Circulation. 1996;94:1981–8. doi: 10.1161/01.cir.94.8.1981. [DOI] [PubMed] [Google Scholar]
- 98.Zygmunt AC, Goodrow RJ, Antzelevitch C. INaCa contributes to electrical heterogeneity within the canine ventricle. Am J Physiol Heart Circ Physiol. 2000;278:H1671–8. doi: 10.1152/ajpheart.2000.278.5.H1671. [DOI] [PubMed] [Google Scholar]
- 99.Brahmajothi MV, Morales MJ, Rasmusson RL, et al. Heterogeneity in K+ channel transcript expression detected in isolated ferret cardiac myocytes. Pacing Clin Electrophysiol. 1997;20:388–96. doi: 10.1111/j.1540-8159.1997.tb06198.x. [DOI] [PubMed] [Google Scholar]
- 100.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 101.Schulze-Bahr E, Eckardt L, Breithardt G, et al. Sodium channel gene (SCN5A) mutations in 44 index patients with Brugada syndrome: different incidences in familial and sporadic disease. Hum Mutat. 2003;21:651–2. doi: 10.1002/humu.9144. [DOI] [PubMed] [Google Scholar]
- 102.Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.London B, Michalec M, Mehdi H, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–8. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watanabe H, Koopmann TT, Le Scouarnec S, et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–8. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu D, Barajas-Martinez H, Burashnikov E, et al. A mutation in the β3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–8. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burashnikov E, Pfeifer R, Barajas-Martinez H, et al. Mutations in the cardiac L-type calcium channel associated J wave syndrome and sudden cardiac death. Heart Rhythm. doi: 10.1016/j.hrthm.2010.08.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 108.Antzelevitch C, Shimizu W, Yan GX. Electrical heterogeneity and the development of arrhythmias. In: Olsson SB, Yuan S, Amlie JP, editors. Dispersion of ventricular repolarization: state of the art. Armonk (NY): Futura Publishing Company, Inc; 2000. pp. 3–21. [Google Scholar]
- 109.Yan GX, Lankipalli RS, Burke JF, et al. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401–9. doi: 10.1016/s0735-1097(03)00713-7. [DOI] [PubMed] [Google Scholar]
- 110.Shimizu W, Antzelevitch C, Suyama K, et al. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320–9. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 111.Brugada R, Brugada J, Antzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–5. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 112.Morita H, Morita ST, Nagase S, et al. Ventricular arrhythmia induced by sodium channel blocker in patients with Brugada syndrome. J Am Coll Cardiol. 2003;42:1624–31. doi: 10.1016/j.jacc.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 113.Gussak I, Antzelevitch C, Bjerregaard P, et al. The Brugada syndrome: clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol. 1999;33:5–15. doi: 10.1016/s0735-1097(98)00528-2. [DOI] [PubMed] [Google Scholar]
- 114.Postema PG, van Dessel PF, Kors JA, et al. Local depolarization abnormalities are the dominant pathophysiologic mechanism for type 1 electrocardiogram in Brugada syndrome: a study of electrocardiograms, vectorcardiograms, and body surface potential maps during ajmaline provocation. J Am Coll Cardiol. 2010;55:789–97. doi: 10.1016/j.jacc.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 115.Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–53. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wasserburger RH, Alt WJ. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–92. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 117.Mehta MC, Jain AC. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–11. doi: 10.1097/00000441-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 118.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 119.Bjerregaard P, Gussak I, Kotar SL, Gessler JE. Recurrent syncope in a patient with prominent J-wave. Am Heart J. 1994;127:1426–30. doi: 10.1016/0002-8703(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 120.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–9. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 121.Geller JC, Reek S, Goette A, et al. Spontaneous episode of polymorphic ventricular tachycardia in a patient with intermittent Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:1094. doi: 10.1046/j.1540-8167.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 122.Daimon M, Inagaki M, Morooka S, et al. Brugada syndrome characterized by the appearance of J waves. Pacing Clin Electrophysiol. 2000;23:405–6. doi: 10.1111/j.1540-8159.2000.tb06770.x. [DOI] [PubMed] [Google Scholar]
- 123.Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–8. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 124.Komiya N, Imanishi R, Kawano H, et al. Ventricular fibrillation in a patient with prominent J wave in the inferior and lateral electrocardiographic leads after gastrostomy. Pacing Clin Electrophysiol. 2006;29:1022–4. doi: 10.1111/j.1540-8159.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 125.Shinohara T, Takahashi N, Saikawa T, et al. Characterization of J wave in a patient with idiopathic ventricular fibrillation. Heart Rhythm. 2006;3:1082–4. doi: 10.1016/j.hrthm.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Riera AR, Ferreira C, Schapachnik E, et al. Brugada syndrome with atypical ECG: downsloping ST-segment elevation in inferior leads. J Electrocardiol. 2004;37:101–4. doi: 10.1016/j.jelectrocard.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Shu J, Zhu T, Yang L, et al. ST-segment elevation in the early repolarization syndrome, idiopathic ventricular fibrillation, and the Brugada syndrome: cellular and clinical linkage. J Electrocardiol. 2005;38:26–32. doi: 10.1016/j.jelectrocard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–23. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 129.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–9. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rosso R, Kogan E, Belhassen B, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–8. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 131.Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–37. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 132.Sinner MF, Reinhard W, Muller M, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7:e1000314. doi: 10.1371/journal.pmed.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nam GB, Ko KH, Kim J, et al. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–9. doi: 10.1093/eurheartj/ehp423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haissaguerre M, Chatel S, Sacher F, et al. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–8. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 135.Medeiros-Domingo A, Tan BH, Crotti L, et al. Gain-of-function mutation, S422L, in the KCNJ8-encoded cardiac K ATP channel kir6.1 as a pathogenic substrate for J wave syndromes. Heart Rhythm. 2010;7(10):1466–71. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schwartz PJ. The idiopathic long QT syndrome: progress and questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 137.Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome: prospective longitudinal study of 328 families. Circulation. 1991;84:1136–44. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 138.Zipes DP. The long QT interval syndrome. A rosetta stone for sympathetic related ventricular tachyarrhythmias. Circulation. 1991;84:1414–9. doi: 10.1161/01.cir.84.3.1414. [DOI] [PubMed] [Google Scholar]
- 139.Plaster NM, Tawil R, Tristani-Firouzi M, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–9. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 140.Wang Q, Shen J, Splawski I, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–11. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 141.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 142.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 143.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 144.Splawski I, Tristani-Firouzi M, Lehmann MH, et al. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–40. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 145.Ye B, Tester DJ, Vatta M, et al. Molecular and functional characterization of novel cav3-encoded cav-eolin-3 mutations in congenital long QT syndrome [abstract] Heart Rhythm. 2006;3:S1. [Google Scholar]
- 146.Domingo AM, Kaku T, Tester DJ, et al. Sodium channel β4 subunit mutation causes congenital long QT syndrome. Heart Rhythm. 2006;3:S34. [Google Scholar]
- 147.Splawski I, Timothy KW, Sharpe LM, et al. Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 148.Yang Y, Yang Y, Liang B, et al. Identification of a Kir3.4 mutation in congenital long QT syndrome. Am J Hum Genet. 2010;86:872–80. doi: 10.1016/j.ajhg.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis. 2001;43:1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 150.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–83. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 151.Sipido KR, Volders PG, De Groot SH, et al. Enhanced Ca2+ release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation. 2000;102:2137–44. doi: 10.1161/01.cir.102.17.2137. [DOI] [PubMed] [Google Scholar]
- 152.Volders PG, Sipido KR, Vos MA, et al. Downregulation of delayed rectifier K(+) currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100:2455–61. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- 153.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maltsev VA, Sabbah HN, Higgins RS, et al. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–52. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 155.Belardinelli L, Antzelevitch C, Vos MA. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol Sci. 2003;24:619–25. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 156.Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol. 2002;17:43–51. doi: 10.1097/00001573-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 157.Shimizu W, Antzelevitch C. Effects of a K+ channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation. 2000;102:706–12. doi: 10.1161/01.cir.102.6.706. [DOI] [PubMed] [Google Scholar]
- 158.Antzelevitch C. Heterogeneity of cellular repolarization in LQTS: the role of M cells. Eur Heart J Suppl. 2001;3:K2–16. [Google Scholar]
- 159.Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long QT syndrome: effects of β-adrenergic agonists and antagonists and sodium channel blockers on trans-mural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–22. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- 160.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade de pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–47. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 161.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–86. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 162.Antzelevitch C, Shimizu W, Yan GX, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–52. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 163.Anyukhovsky EP, Sosunov EA, Gainullin RZ, et al. The controversial M cell. J Cardiovasc Electrophysiol. 1999;10:244–60. doi: 10.1111/j.1540-8167.1999.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 164.Li GR, Feng J, Yue L, et al. Transmural heterogeneity of action potentials and Ito1 in myocytes isolated from the human right ventricle. Am J Physiol. 1998;275:H369–77. doi: 10.1152/ajpheart.1998.275.2.H369. [DOI] [PubMed] [Google Scholar]
- 165.Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 166.Gussak I, Brugada P, Brugada J, et al. ECG phenomenon of idiopathic and paradoxical short QT intervals. Card Electrophysiol Rev. 2002;6:49–53. doi: 10.1023/a:1017931020747. [DOI] [PubMed] [Google Scholar]
- 167.Patel C, Yan GX, Antzelevitch C. Short QT syndrome: from bench to bedside. Circ Arrhythm Electrophysiol. 2010;3:401–8. doi: 10.1161/CIRCEP.109.921056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–70. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 169.Bellocq C, Van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394–7. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 170.Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–5. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 171.Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–7. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 172.Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short QT syndrome. Circulation. 2004;110:3661–6. doi: 10.1161/01.CIR.0000143078.48699.0C. [DOI] [PubMed] [Google Scholar]
- 173.Patel C, Antzelevitch C. Cellular basis for arrhythmogenesis in an experimental model of the SQT1 form of the short QT syndrome. Heart Rhythm. 2008;5:585–90. doi: 10.1016/j.hrthm.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nof E, Burashnikov A, Antzelevitch C. Cellular basis for atrial fibrillation in an experimental model of short QT1: implications for a pharmacological approach to therapy. Heart Rhythm. 2010;7:251–7. doi: 10.1016/j.hrthm.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anttonen O, Vaananen H, Junttila J, et al. Electrocardiographic transmural dispersion of repolarization in patients with inherited short QT syndrome. Ann Noninvasive Electrocardiol. 2008;13:295–300. doi: 10.1111/j.1542-474X.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gupta P, Patel C, Patel H, et al. Tp-e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–74. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 177.Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm. 2009;6:267–71. doi: 10.1016/j.hrthm.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 178.Milberg P, Tegelkamp R, Osada N, et al. Reduction of dispersion of repolarization and prolongation of postrepolarization refractoriness explain the antiarrhythmic effects of quinidine in a model of short QT syndrome. J Cardiovasc Electrophysiol. 2007;18:658–64. doi: 10.1111/j.1540-8167.2007.00813.x. [DOI] [PubMed] [Google Scholar]