Abstract
Fatty acid amide hydrolase (FAAH) is the primary degradative enzyme of the endocannabinoid anandamide (N-arachidonoylethanolamine), which activates cannabinoid CB1 and CB2 receptors. FAAH disruption reduces nociception in a variety of acute rodent models of inflammatory pain. The present study investigated whether these actions extend to the chronic, collagen-induced arthritis (CIA) model. We investigated the anti-arthritic and anti-hyperalgesic effects of genetic deletion or pharmacological inhibition of FAAH in the CIA model. FAAH (−/ −) mice, and FAAH-NS mice that express FAAH exclusively in nervous tissue, displayed decreased severity of CIA and associated hyperalgesia. These phenotypic anti-arthritic effects were prevented by repeated daily injections of the CB2 receptor antagonist, SR144528, but not the CB1 receptor antagonist rimonabant. Similarly, repeated administration of URB597 reduced CIA severity, and acute administration of rimonabant, but not SR144528, blocked the anti-hyperalgesic effects of prolonged FAAH inhibition, suggesting that prolonged CB2 receptor activation reduces the severity of CIA, whereas acute CB1 receptor activation reduces CIA-induced hyperalgesia. In contrast, acute administration of the FAAH inhibitor, URB597, elicited a CB1 receptor-dependent anti-hyperalgesic effect. The observed anti-arthritic and anti-hyperalgesic properties of FAAH inhibition, coupled with a lack of apparent behavioral alterations, suggest that endocannabinoid modulating enzymes offer a promising therapeutic target for the development of novel pharmacological approaches to treat rheumatoid arthritis and associated hyperalgesia.
Keywords: endocannabinoid, fatty acid amide hydrolase (FAAH), anandamide, collagen-induced arthritis, chronic inflammation, inflammatory pain
1. Introduction
Increased pain sensitivity is a common and debilitating symptom of inflammatory disorders (Dray and Bevan, 1993). The endogenous cannabinoid (endocannabinoid) system, consisting of two known cannabinoid receptors (i.e., CB1 and CB2(Gerard et al., 1991; Matsuda et al., 1990), endogenously produced ligands including N-arachidonoylethanolamine (Devane et al., 1992), and 2-arachidonoylglycerol (2-AG; (Mechoulam et al., 1995; Sugiura et al., 1995), and the enzymes regulating ligand biosynthesis and degradation (Elphick and Egertova, 2005), is believed to play a significant role in modulating physiological responses to inflammation and pain. Accordingly, the endocannabinoid system holds promise as a therapeutic target to treat peripheral and central inflammatory disorders (Booker et al., 2011; Guindon and Hohmann, 2008; Schlosburg et al., 2009b). In support of this assertion, endocannabinoids have been found in synovial fluid of patients with end stage osteoarthritis and rheumatoid arthritis, and CB1 and CB2 receptors are present in synovial tissue (Richardson et al., 2008).
Cannabis extracts and cannabinoid receptor agonists have long been known to elicit analgesic and anti-inflammatory actions in humans and laboratory animals (Kosersky et al., 1974; Reynolds, 1890; Sofia et al., 1973); however, their therapeutic utility has been greatly limited by the occurrence of psychotropic side effects. Several studies have demonstrated robust anti-inflammatory and anti-hyperalgesic phenotypes following genetic or pharmacological disruption of fatty acid amide hydrolase (FAAH) (Ahn et al., 2009; Cravatt et al., 2001; Holt et al., 2005; Karsak et al., 2007; Kinsey et al., 2011a; Lichtman et al., 2004a; Lichtman et al., 2004b; Massa et al., 2004; Naidu et al., 2010; Russo et al., 2007), the principal degradative enzyme for anandamide and other bioactive fatty acid amides (FAAs) (Cravatt et al., 1996; Maurelli et al., 1995; Ueda et al., 1995). The molecular basis for the anti-inflammatory and anti-hyperalgesic effects of FAAH disruption continues to be an area of interest (Booker et al., 2011; Kinsey et al., 2010; Rahn and Hohmann, 2009).
FAAH-disrupted rodents have been investigated in a variety of inflammatory pain models including carrageenan, lipopolysaccharide, and complete Freund’s adjuvant models (Ahn et al., 2009; Cravatt et al., 2001; Cravatt et al., 2004; Holt et al., 2005; Jayamanne et al., 2006; Lichtman et al., 2004b; Naidu et al., 2010). In the present study, we investigated the role of the endogenous cannabinoid system in the murine collagen-induced arthritis (CIA) assay, which results in a progressive inflammation of the joints, as well as degeneration of both cartilage and bone (Campbell et al., 1998; Moore, 2003). Several pathologic features found in CIA resemble symptoms that are not only found in rheumatoid arthritis patients, but are also responsive to similar pharmacological treatments (Courtenay et al., 1980; Trentham, 1982; Williams, 1998). The development of CIA occurs over a prolonged period of time, and closely models chronic inflammatory disorders in humans (Bluml et al., 2011). Of significance, repeated administration of cannabidiol, a major cannabinoid constituent of Cannabis sativa that lacks psychoactive properties or affinity for CB1 or CB2 receptors, reduces the severity of CIA (Malfait et al., 2000). However, no published studies have evaluated the role of endogenous cannabinoids in this chronic model of arthritis.
There were three objectives in the present study. First, we used complementary genetic and pharmacological approaches to investigate whether FAAH blockade reduces collagen-induced arthritis and CIA associated hyperalgesia. Second, we assessed the relative contribution of FAAH in the nervous system using neural specific transgenic FAAH mice, in which this enzyme is expressed exclusively on neural tissue. Third, we used CB1 and CB2 receptor antagonists to determine whether the anti-arthritic and anti-hyperalgesic phenotypes of FAAH-deficient mice require cannabinoid receptors.
2. Material and Methods
2.1 Subjects
Male DBA1/J mice (Jackson Laboratory, Bar Harbor, ME) were used to investigate the pharmacological effects of the FAAH inhibitor, URB597. In addition, two types of genetically altered mice were used (Center Transgenic Colony at Virginia Commonwealth University). First, male and female FAAH (−/ −) mice were used to examine the impact of FAAH deletion on CIA, as compared with their littermate FAAH (+/+) mice. Second, a transgenic mouse model was used in which the central and peripheral FAAH systems had been functionally uncoupled. Mice expressing FAAH specifically in the nervous system (FAAH-NS mice) were generated by crossing FAAH (−/ −) mice with transgenic mice that express FAAH under the neural specific enolase promoter (Cravatt et al., 2004). FAAH-NS possess wild type levels of anandamide and other FAAs in the brain and spinal cord, but significantly elevated concentrations of these lipid transmitters in peripheral tissues. FAAH-NS mice were compared to two kinds of littermate control groups, global FAAH (−/ −) mice and FAAH (+/−) mice; the latter possesses wild type levels of FAAs (Cravatt et al., 2004). Each of these three genotypes was derived from breeding pairs that included a FAAH (−/ −) mouse and a FAAH (+/−) mouse that expressed the FAAH transgene. All experiments involving genetically altered mice used male and female mice that were derived from breeding pairs that were backcrossed onto a DBA1 background for five or six generations. The genotype of each genetically altered mouse was confirmed by RT-PCR. Mice were counterbalanced across treatment groups to control for possible sex differences in the development and severity of CIA, and concomitant CIA-induced hyperalgesia.
All mice weighed 20–30 g and were housed four per cage in a temperature-controlled (20–22°C), AAALAC accredited facility. Food and water were available ad libitum. All animal protocols were approved by the VCU Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.2 Drugs
URB597 (FAAH inhibitor; Cayman Chemicals, Ann Arbor, MI), rimonabant (SR1; CB1 receptor antagonist; NIDA, Rockville, MD), and SR144528 (SR2; CB2 receptor antagonist; NIDA, Rockville, MD) were dissolved in a vehicle that consisted of 1:1:18 ethanol:alkamuls-620 (Rhone-Poulenc, Princeton, NJ):normal saline. All drugs were administered intraperitoneally (i.p.) in a volume of 10 μl/g body weight.
2.3 Collagen-induced arthritis (CIA) model
For CIA induction, DBA1/J mice were immunized with an injection of 100 μg of chicken type II collagen (Sigma) in 50 μl of 0.05 M acetic acid, emulsified in an equal volume of complete Freund’s adjuvant, distal to the fur on the sole of the left hind paw. Mice were challenged with a booster injection of an equal volume of the same collagen preparation in incomplete Freund’s adjuvant (i.p.) 21 days later. All four paws were examined twice weekly for arthritic signs as follows: 0, normal; 1, erythema and mild swelling confined to the ankle joint or toes; 2, erythema and mild swelling extending from the ankle to the midfoot or ankle joint; 3, erythema and moderate swelling extending from the ankle to the metacarpal/metatarsal joints; 4, erythema and severe swelling encompassing the ankle, foot, and digits. The scores for each of the four limbs were summed for each mouse, resulting in a composite arthritis score with a maximum of 16 total points. All arthritic mice were randomly distributed in various treatment groups and were monitored and treated daily throughout the 15-day period of heterologous CIA. Experimenter was blinded to treatment conditions.
In experiments examining the impact of repeated drug administration on CIA development, URB597 (10 mg/kg) or vehicle was administered once or twice daily from days 3–15 after the booster collagen injection. In order to determine whether the FAAH (−/ −) anti-arthritic phenotype was mediated through a cannabinoid receptor mechanism of action, mice were treated repeatedly with rimonabant (3 mg/kg), SR144528 (3 mg/kg), or vehicle. Injections were given at approximately 0900 and 1700 h each day.
At the conclusion of the experiments, mice were humanely euthanized as specified by IACUC guidelines. The hind paws of a subset of FAAH (−/ −) and (+/+) mice were dissected, fixed in 10% neutral buffered formalin, and sent to Premier Laboratories (Boulder, CO) for histology. The knees were sectioned from the limb and decalcified in 10% formic acid. The tissue was then embedded in paraffin wax blocks, sectioned to 8-μm thickness with a microtome, and stained with toluidine blue. Arthritic changes in the knee and ankle joints were assessed using a scoring system of 0–5, 0=normal; 1=minimal; 2=mild; 3= moderate; 4=marked; 5=severe for inflammation, pannus formation, cartilage damage, and bone resorption, as detailed in Table 1. The scores for both ankles and knees were summed for each mouse, resulting in a composite arthritis score with a maximum of 20 total points for each measure. In order to increase objectivity and consistency, dependent measures were assessed by a trained technician who was blinded to the treatments.
Table 1.
Histopathologic scoring was performed by an independent examiner blinded to treatment condition. Individual mouse knees and ankles were given scores of 0–5 for inflammation, pannus formation, cartilage damage and bone resorption according to these criteria.
| Score | Inflammation |
|---|---|
| 0 | Normal |
| 1 | Minimal infiltration of inflammatory cells in periarticular tissue |
| 2 | Mild infiltration |
| 3 | Moderate infiltration with moderate edema |
| 4 | Marked infiltration with marked edema |
| 5 | Severe infiltration with severe edema |
| Score | Pannus Formation |
| 0 | Normal |
| 1 | Minimal infiltration of pannus in cartilage and subchondral bone |
| 2 | 2 = Mild infiltration |
| 3 | 3 = Moderate infiltration |
| 4 | 4 = Marked infiltration |
| 5 | 5 = Severe infiltration |
| Score | Cartilage Damage |
| 0 | Normal |
| 1 | Minimal to mild loss of toluidine blue staining with no obvious chondrocyte loss or collagen disruption |
| 2 | Mild loss of toluidine blue staining with focal mild (superficial) chondrocyte loss and/or collagen disruption |
| 3 | Moderate loss of toluidine blue staining with multifocal moderate (depth to middle zone) chondrocyte loss and/or collagen disruption |
| 4 | Marked loss of toluidine blue staining with multifocal marked (depth to deep zone) chondrocyte loss and/or collagen disruption |
| 5 | Severe diffuse loss of toluidine blue staining with multifocal severe (depth to tide mark) chondrocyte loss and/or collagen disruption |
| Score | Bone Resorption |
| 0 | Normal |
| 1 | Minimal: small areas of resorption, not readily apparent on low magnification, rare osteoclasts |
| 2 | Mild: more numerous areas of, not readily apparent on low magnification, osteoclasts more numerous |
| 3 | Moderate: obvious resorption of medullary trabecular and cortical bone without full thickness defects in cortex, loss of some medullary trabeculae, lesion apparent on low magnification, osteoclasts more numerous |
| 4 | Marked: Full thickness defects in cortical bone, often with distortion of profile of remaining cortical surface, marked loss of medullary bone, numerous osteoclasts |
| 5 | Severe: Full thickness defects in cortical bone, often with distortion of profile of remaining cortical surface, marked loss of medullary bone, numerous osteoclasts. |
2.4 Nociceptive tests
Nociceptive behavior was evaluated 15 days after the booster injection of collagen with the hot plate and tail immersion tests. The thermal stimulus in each assay was maintained at 52.0°C, which is below the threshold to elicit phenotypic analgesic responses in FAAH (−/ −) mice under baseline conditions (Cravatt et al., 2001). In the hot plate test, the latency to jump or lick/shake a hind paw was scored. A 30 s cutoff time was used to avoid the possibility of tissue damage. In the tail immersion test, each mouse was placed head first into a small bag fabricated from absorbent under pads (VWR Scientific Products; 4 cm diameter, 11 cm length), leaving the tail exposed. The experimenter gently held the mouse, quickly dipped the tail approximately 1 cm into the water bath, rapidly removed it, and wiped it dry with a Kimwipe, and then immersed the tail approximately 1 cm into the water bath and scored the latency for the animal to withdraw its tail from the water to the nearest 0.1 s. Because water transfers heat more quickly than the hot plate, a shorter cutoff time (10 s) was used to reduce the possibility of tissue damage.
In experiments assessing the anti-hyperalgesic effects of URB597 (10 mg/kg), nociceptive behavior was assessed before and 60 min after injection. In separate groups of animals, cannabinoid receptor mechanisms of action were evaluated by administering rimonabant (3 mg/kg) or SR144528 (3 mg/kg) 10 min before URB597 (10 mg/kg), or vehicle. Drug doses were based on pilot experiments and previous literature on anti-hyperalgesic effects of URB597 (Kinsey et al., 2009; Naidu et al., 2009; Naidu et al., 2010). The experimenter was blinded to treatment conditions.
2.5 Statistical analysis
Data were analyzed using one-way or two-way ANOVA, with the Newman-Keuls or Dunnett’s test used for post hoc analyses. Planned comparisons between genotypes were conducted using Student’s t-test. All differences were considered significant if p < 0.05.
3. Results
3.1 FAAH (−/ −) mice have an anti-arthritic phenotype
Mice lacking FAAH, which have elevated levels of anandamide as well as non-cannabinoid FAAs (Cravatt et al., 2001), displayed a striking decrease in the severity of CIA on day 15 following collagen re-exposure. Histological examination of knee and ankle joints revealed that control mice showed severe joint damage that included statistically significant increases in bone and articular cartilage erosion, pannus formation, and infiltration of inflammatory cells in the knee and ankle joints (Figure 1a). Scoring criteria for each paw are summarized in Table 1. For each measure, FAAH (−/ −) mice developed a statistically significant less severe pathological response to collagen than FAAH (+/+) mice. Macroscopic inspection of limbs revealed a significant interaction between genotype and day on arthritic index score [F(5,80) = 2.9, p < 0.05] in which FAAH (−/ −) mice showed a significant decrease in arthritis severity than FAAH (+/+) mice from days 9–15 (Figure 1b). As shown in Figure 1c, collagen led to hyperalgesic responses in FAAH (+/+), while FAAH (−/ −) mice exhibited anti-hyperalgesic phenotypes in the hot plate [F(1,16) = 13.6, p < 0.01] and tail immersion tests [F(1,16) = 15.9, p < 0.01; Figure 1c] on day 15.
Figure 1.
FAAH deletion reduces the severity of collagen-induced arthritis. (a,b) FAAH (−/ −) mice showed significant reductions in arthritis development and severity, as compared to wild type mice. (c). FAAH (−/ −) mice displayed phenotypic decreases in the magnitude of hot plate (left) and tail immersion (right) thermal hyperalgesic responses associated with collagen-induced arthritis. * p < 0.05; ** p < 0.05 vs. corresponding littermate FAAH (+/+) control mice treated with incomplete Freund’s adjuvant. Data depicted as means ± SEM (n = 8–10 male & female mice per group).
3.2 Deletion of FAAH in non-neuronal tissue reduces arthritis severity
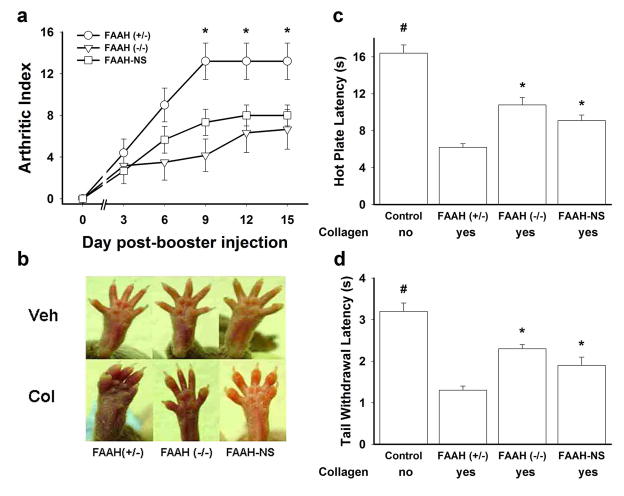
FAAH regulates anandamide and other FAAs in the nervous system and non-neuronal tissues (Cravatt et al., 2004; Elphick and Egertova, 2005), either of which could regulate arthritis development. To distinguish between the roles of neuronal and non-neuronal FAAH, we evaluated the development of CIA in transgenic mice that express FAAH exclusively in the nervous system (i.e., FAAH-NS mice). FAAH-NS mice express low, wild type levels of anandamide and other fatty acid amides in the nervous system, but have significantly elevated levels of these lipid signaling molecules in non-neuronal tissues such as liver and testes (Cravatt et al., 2004), and presumably also the ankle and knee joints. In the present study, a significant interaction was found between genotype and day for arthritic scores [Figure 2a; F(10,70) = 2.8, p < 0.001]. FAAH (−/ −) mice showed a significant attenuation in the development of arthritis (Figures 2a and 2b) as well as signification reductions in CIA-induced hyperalgesia in both the hot plate [Figure 2c; F(2,14) = 12.2, p < 0.001] and tail withdrawal assays [Figure 2d; F(2,14) = 15.0, p < 0.001], compared to their littermate FAAH (+/−) control mice. Strikingly, FAAH-NS mice retained the anti-arthritic (Figures 2a and 2b) and anti-hyperalgesic phenotypes (Figure 2c, and 2d), implicating the involvement of non-neuronal FAAs. Because FAAH (+/−), FAAH (−/ −), and FAAH-NS mice that did not receive collagen treatment displayed nearly identical nociceptive latencies in both tests, their data were collapsed in Figures 2c and 2d.
Figure 2.
Deletion of non-neuronal FAAH produces an anti-arthritis phenotype. (a) FAAH-NS mice, which express FAAH exclusively in the nervous system, showed an equivalent reduction in collagen-induced arthritis as global FAAH (−/ −) mice compared to FAAH (+/−) mice (controls). *p < 0.05 compared to FAAH (−/ −) and FAAH-NS mice. (b) Photographs of representative arthritic and non-arthritic FAAH (+/−), (−/ −), and -NS mouse hind paws. Both FAAH-NS and (−/ −) mice displayed an anti-arthritis phenotype compared to the FAAH (+/−) control mice. Vehicle was incomplete Freund’s adjuvant. (c, d) FAAH-NS and FAAH (−/ −) mice displayed antihyperalgesic phenotypes in the hot plate and tail immersion assays compared to FAAH (+/−) mice. FAAH-NS, FAAH (−/ −), and FAAH (+/−) mice were littermates. *p < 0.05 compared to FAAH (+/−) mice; #p < 0.05 compared to mice treated with collagen. Data depicted as means ± SEM (n = 6 male & female mice per group).
3.3 The FAAH (−/ −) anti-arthritic phenotype requires CB2 receptors
We next tested whether cannabinoid receptors mediate the anti-arthritic and anti-hyperalgesic FAAH (−/ −) phenotypes. Accordingly, FAAH (−/ −) mice and their littermate FAAH (+/+) control mice were given two daily i.p. injections of vehicle, the CB1 receptor antagonist, rimonabant (3 mg/kg), or the CB2 receptor antagonist, SR144528 (3 mg/kg), from days 3–15 after the booster injection of collagen. A significant interaction was found between cannabinoid receptor antagonist treatment and day in FAAH (−/ −) mice [F(10,70) = 2.7, p < 0.01]. Repeated administration of SR144528 prevented the anti-arthritic phenotype in FAAH (−/ −) mice, but rimonabant-treated FAAH (−/ −) mice did not differ from the vehicle-treated FAAH (−/ −) mice (Figure 3a). In FAAH (+/+) mice, neither cannabinoid receptor antagonist altered the development of CIA (data not shown). Representative paws of collagen-treated and control mice in each of the conditions are shown in Figure 3b.
Figure 3.
CB2 receptors mediate the FAAH (−/ −) anti-arthritis phenotype. (a) Chronic treatment (days 3 to 15 following the booster collagen injection) of the CB2 receptor antagonist SR144528 (SR2; 3 mg/kg, i.p., twice daily), but not the CB1 receptor antagonist rimonabant (Rim; 3 mg/kg, i.p., twice daily), prevented the FAAH anti-arthritis phenotype in collagen-treated mice. (b) Photographs of representative mouse hind paws showing FAAH (+/+) and (−/ −) non-arthritic (Veh+Veh) and arthritic mice that were given two daily injections of vehicle, rimonabant (Rim, 3 mg/kg), or SR144528 (SR2, 3 mg/kg) for 12 days. Chronic SR144528 attenuated the anti-arthritis phenotype in FAAH (−/ −) mice, whereas chronic rimonabant treatment did not significantly alter this response. *p < 0.05 as compared to corresponding littermate FAAH (+/+) control mice (planned comparisons). #p < 0.05 as compared to vehicle-treated FAAH (−/ −) mice (Dunnett’s test). Data depicted as means ± SEM (n = 5–6 male & female FAAH (−/ −) mice per group and 4 vehicle-treated FAAH (+/+) male & female mice).
3.4 Pharmacological inhibition of FAAH reduces CIA
In the final set of experiments, we evaluated whether pharmacologic inhibition of FAAH reduces the development of CIA and attenuates CIA-induced hyperalgesia. Repeated administration of URB597 (10 mg/kg), beginning on the third day and continuing through the 15th day after the collagen booster injection, significantly reduced CIA progression [Figure 4a; F(10,95) = 3.3, p < 0.001 for interaction between URB597 treatment and day]. Representative paws in each of the conditions are depicted in Figure 4b. Acute injection of URB597 significantly attenuated CIA-induced hyperalgesia in the hot plate test [Figure 4c; F(4,28) = 11.3, p < 0.001 for interaction between antagonist and URB597] and the tail immersion test [Figure 4d; F(4,28) = 11.8, p < 0.001 for interaction between antagonist and URB597]. Rimonabant, but not SR144528, blocked the anti-hyperalgesic effects of URB597, implicating the involvement of CB1 receptors. In the absence of URB597, neither antagonist altered nociceptive latencies in arthritic control mice (data not shown).
Figure 4.
Chronic and acute effects of the FAAH inhibitor URB597 (URB) on collagen-induced arthritis. (a) URB (10 mg/kg, i.p.) administered twice daily from day 3–15 post-booster injection, significantly diminished arthritis severity, while URB administered once daily only partially blocked these effects. *p < 0.05 vs. URB-2X/day mice; #p < 0.05 vs. URB-1X/day mice (Newman-Keuls). (b) Photographs of representative mouse hind paws showing chronic URB administration (two injections per day) reduced arthritis severity compared to arthritic mice given daily injections of vehicle. Acute administration of the FAAH inhibitor URB597 (URB; 10 mg/kg) reduces thermal hyperalgesia in arthritic mice through a CB1 receptor mechanism of action. An acute injection of URB (10 mg/kg, i.p.) reduced the hyperalgesic responses of arthritic wild type mice in (c) the hot plate and (d) tail immersion tests. Pretreatment with rimonabant (Rim; 3 mg/kg, i.p), but not SR144528 (SR2; 3 mg/kg, i.p) completely blocked both antihyperalgesic effects of URB597. Mice were evaluated for nociceptive latencies in both tests before injections and again 1 h after injections. #p < 0.05 vs. vehicle-treated arthritic and URB+Rim groups; **p < 0.01; ***p < 0.001 vs. corresponding pretreatment condition (planned comparison). Data depicted as means ± SEM (n = 6–9 male DBA1/J mice per group).
4. Discussion
Rheumatoid arthritis is a debilitating autoimmune disease that affects approximately 1% of the human population and is characterized by inflammation of multiple joints, synovial thickening, ankylosis, joint swelling, and joint pain (Lee and Weinblatt, 2001). The chronic nature of this disease results in destruction of joint cartilage, erosion, loss of function, and reduced quality of life. First-line pharmacological treatments for rheumatoid arthritis include steroids and nonsteroidal anti-inflammatory drugs (NSAIDs), both of which are associated with serious side effects. Similarly, disease modifying anti-rheumatic drugs, such as methotrexate, gold salts, and anti-TNF antibodies also elicit considerable toxic effects (Bongartz et al., 2006; Lee and Weinblatt, 2001). Thus, there is a substantial need for new pharmacological approaches that curtail the progression of the disease and also reduce the pain, without producing serious side effects associated with existing treatments.
One promising target to treat inflammatory diseases is FAAH, the enzyme responsible for the degradation of the endocannabinoid anandamide, as well as an array of other lipid signaling molecules. Indeed, FAAH (−/ −) mice and rodents treated with FAAH inhibitors show anti-inflammatory and analgesic effects in a variety of models of inflammation and inflammatory pain (Ahn et al., 2009; Cravatt et al., 2001; Ezzili et al., 2011; Holt et al., 2005; Jayamanne et al., 2006; Karsak et al., 2007; Lichtman et al., 2004a; Lichtman et al., 2004b; Naidu et al., 2010; Wise et al., 2008). The present study is the first of its kind demonstrating that genetic deletion or pharmacological inhibition of FAAH possess efficacy in a chronic murine arthritis model.
FAAH inhibition or deletion attenuated both inflammation and inflammation-induced thermal hyperalgesia. Prolonged pharmacological inhibition of the CB2 receptor, but not the CB1 receptor, blocked the anti-arthritic FAAH (−/ −) phenotype. In contrast, the anti-hyperalgesic phenotypes of FAAH-disrupted mice required acute activation of CB1, but not CB2, receptors. These effects are consistent with previous observations showing that CB1 receptors are located at multiple sites throughout the nervous system (Herkenham et al., 1991), including peripheral terminals of nociceptors (Agarwal et al., 2007), the dorsal horn of the spinal cord, and key brain areas associated with pain (Lichtman et al., 1996; Martin et al., 1995; Meng et al., 1998; Yaksh, 1981), which play a role in cannabinoid-induced antinociception.
The observation that FAAH-NS mice, which express FAAH exclusively in nervous tissue, that is in cells containing a neural-specific enolase (Cravatt et al., 2004), exhibited reductions in the development of arthritis equivalent in magnitude to those observed in global FAAH (−/ −) mice suggests that non-neuronal FAAs mediate the anti-arthritic phenotype. Accordingly, the presence or absence of FAAH expressed in peripheral nerves that innervate the knee or ankle joints, as well as the metacarpals/metatarsals, would influence the peripheral breakdown of anandamide in these regions. These findings are consistent with a recent report showing that the peripheral FAAH inhibitor URB937 possesses anti-hyperalgesic, anti-allodynic, and anti-inflammatory properties (Clapper et al., 2010).
Reports examining the tonic involvement of endocannabinoids in pain have been mixed, with some research showing that rimonabant produces hyperalgesia in wild type animals (Calignano et al., 1998; Richardson et al., 1997), and work finding no changes from basal nociceptive behavior (Beaulieu et al., 2000). On the other hand, exposure to prolonged foot shock elicits an endocannabinoid-mediated stress-induced analgesia that is further augmented by inactivation of FAAH or monoacylglycerol lipase, the primary enzyme responsible for 2-AG metabolism (Hohmann et al., 2005). Accordingly, it is plausible that the endogenous cannabinoid system is typically quiescent, but becomes active in response to stressors. In the present study, there was no apparent influence of endocannabinoid signaling over nociception or inflammation in the absence of FAAH disruption. Also, FAAH (+/+) mice as well as FAAH (+/−) mice did not differ from DBA1/J mice in terms of CIA development or CIA-induced hyperalgesia. Thus, FAAH may normally curtail endocannabinoid dampening of inflammatory responses, making it an attractive target to treat pain and inflammation.
The observations that prolonged antagonism of CB2 receptors prevents the FAAH (−/ −) anti-arthritic phenotype, and that acute CB1 receptor antagonism reverses the anti-hyperalgesic phenotype of prolonged FAAH inhibition in mice via repeated administration of URB597, indicate multiple cannabinoid receptors are involved. Moreover, these data support the hypothesis that increased anandamide levels play a predominant role in dampening the severity of CIA, because it is the only known FAAH substrate that binds to cannabinoid receptors. However, the apparent involvement of anandamide does not preclude the possibility that other substrates of FAAH may also have beneficial effects in the CIA model. In addition to anandamide, FAAH metabolizes a variety of lipid signaling molecules, including N-palmitoylethanolamide (PEA), N-oleoylethanolamide (OEA), oleamide, and the N-acyl taurines (Cravatt et al., 2001; Saghatelian et al., 2006). Genetic deletion or pharmacological inhibition of FAAH increases levels of each of these lipids in vivo. For example FAAH (−/ −) mice possess elevated levels of PEA, and OEA in brain, spinal cord, testis, liver and kidney, as compared with wild type mice. FAAH-NS mice, on the other hand, have elevated PEA and OEA in non-neuronal tissues, but normal levels in nervous tissue (e.g., brain and spinal cord) (Cravatt et al., 2004). PEA has long been known to possess anti-inflammatory actions (Coburn et al., 1954). More recently, the anti-inflammatory and anti-hyperalgesic effects of PEA and OEA in the carrageenan model of inflammatory pain have been shown to be mediated through a PPARα receptor mechanism of action (D’Agostino et al., 2009; D’Agostino et al., 2007; Genovese et al., 2008; Lo Verme et al., 2005, 2004; LoVerme et al., 2006). These findings raise the possibility that, in addition to the cannabinoid effects reported herein, non-cannabinoid FAAs may elicit anti-inflammatory and anti-hyperalgesic effects on their own and may also augment the actions of anandamide. Similarly, elevated FAAs may bind to other targets to attenuate CIA-induced inflammation and thermal hyperalgesia. At high concentrations, anandamide is a ligand for TRPV1 receptors (Di Marzo and De Petrocellis, 2010; Schlosburg et al., 2009b). For example, the TRPV1 receptor antagonist capsazepine blocks the antiedematous and anti-hyperalgesic effects of anandamide in the carageenan model (Horvath et al., 2008).
An attractive feature of FAAH inhibitors is their apparent lack of untoward side effects. URB597 does not produce THC-like effects in the drug discrimination paradigm (Gobbi et al., 2005) and lacks the cannabimimetic activity of cannabinoid receptor agonists (Compton et al., 1992). Unlike Δ9-tetrahydrocannabinol (THC), the primary active constituent of marijuana (Justinova et al., 2003; Tanda et al., 2000), URB597 is not self-administered by nonhuman primates (Justinova et al., 2008) and does not support conditioned place preferences in rodents (Gobbi et al., 2005; Scherma et al., 2008). Similarly, unlike THC, FAAH inhibition does not cause locomotor suppression (Kinsey et al., 2011b). The lack of reinforcing effects supports the idea that FAAH inhibition lacks abuse potential. Also, unlike the consequences of repeated administration of cannabinoid agonists such as WIN55, 212-2 or THC (Aceto et al., 1996; Aceto et al., 2001; Tsou et al., 1995), repeated administration of URB597 does not lead to physical dependence (Schlosburg et al., 2009a). Finally, unlike nonsteroidal anti-inflammatory drugs (NSAIDs), which are well known to cause gastrointestinal ulcers, URB597 produces gastroprotective effects against NSAID-induced ulcers (Naidu et al., 2009).
The present results demonstrate that FAAH inhibition or genetic deletion functions via multiple cannabinoid receptor levels to dampen the development of collagen-induced arthritis and concomitant hyperalgesia. The observation that these phenotypes are maintained in FAAH-NS mice, in which FAAH is exclusively expressed in the nervous system, suggests that targeting non-neuronal FAAH is sufficient to reduce CIA. These results are consistent with the recent finding that the peripherally restricted FAAH inhibitor, URB937, decreases nociceptive behavior in sciatic nerve ligation, carrageenan, and formalin models of pain (Clapper et al., 2010). Moreover, URB937 reduced the development of carrageenan-induced paw edema. Thus, blocking endocannabinoid metabolism could have the added advantage in the treatment of rheumatoid arthritis by producing dual anti-inflammation and anti-hyperalgesia, without the rewarding effects and abuse potential of direct cannabinoid receptor agonists.
5. Conclusions
Prolonged FAAH blockade in non-neuronal tissue reduces the severity of CIA through a CB2 receptor mechanism of action. In contrast, CB1 receptors mediate the reduction of CIA-induced thermal hyperalgesia caused by acute inhibition of FAAH. These findings demonstrate that simultaneous elevations in neuronal and non-neuronal endocannabinoid signaling are possible through inhibition of a single enzymatic target (FAAH), thereby offering a potentially powerful strategy to treat chronic inflammatory pain syndromes that operate at multiple levels of anatomical integration.
Research highlights.
Chronic inhibition or genetic deletion of the endocannabinoid regulatory enzyme fatty acid amide hydrolase (FAAH) also reduced collagen-induced arthritis (CIA) severity and CIA-induced hyperalgesia in mice.
The anti-inflammatory and anti-hyperalgesic phenotypes of FAAH deletion were mediated by non-neuronal cells.
Phenotypic anti-inflammatory effects of FAAH deletion were prevented by the CB2 receptor antagonist, SR144528, but not the CB1 receptor antagonist rimonabant. Acute administration of rimonabant, but not SR144528, blocked the antihyperalgesic phenotype, suggesting that prolonged CB2 receptor activation reduces the severity of CIA, whereas acute CB1 receptor activation reduces CIA-induced hyperalgesia.
Acknowledgments
The authors are grateful for the contributions of the late Dr. Billy R. Martin for his vision and support of this project. This work was supported by the National Institute on Drug Abuse (P01DA017259, P01DA009789, P50DA005274, R01DA15197, and T32DA007027). The authors declare that they have no competing interests.
Nonstandard Abbreviations
- AEA
anandamide, N-arachidonoylethanolamine
- 2-AG
2-arachidonoylglycerol
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CIA
Collagen-induced arthritis
- FAAH
fatty acid amide hydrolase
- NSAID
Nonsteroidal anti-inflammatory drug OEA, N-oleoylethanolamide
- OEA
N-oleoylethanolamide
- PEA
N-palmitoylethanolamide
- Rim
Rimonabant, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl
- SR2
SR144528, N-[(1S)-endo-1,3,3,-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- THC
Δ9-tetrahydrocannabinol
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
- URB597
cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto M, Scates S, Lowe J, Martin B. Dependence on D9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther. 1996;278:1290–1295. [PubMed] [Google Scholar]
- Aceto MD, Scates SM, Martin BB. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur J Pharmacol. 2001;416:75–81. doi: 10.1016/s0014-2999(01)00873-1. [DOI] [PubMed] [Google Scholar]
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu P, Bisogno T, Punwar S, Farquhar-Smith WP, Ambrosino G, Di Marzo V, Rice AS. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur J Pharmacol. 2000;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Bluml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K. Essential role for micro-RNA 155 in the pathogenesis of autoimmune arthritis. Arthritis Rheum. 2011 doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J Am Med Assoc. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, Boger DL, Cravatt BF, Lichtman AH. The FAAH Inhibitor PF-3845 Acts in the Nervous System to Reverse Lipopolysaccharide-induced Tactile Allodynia in Mice. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010 doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn AF, Graham CE, Haninger J. The effect of egg yolk in diets on anaphylactic arthritis (passive Arthus phenomenon) in the guinea pig. J Exp Med. 1954;100:425–435. doi: 10.1084/jem.100.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: Cannabimimetic activity of a class of compounds structurally distinct from D9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Mattace Raso G, Cuzzocrea S, Loverme J, Piomelli D, Meli R, Calignano A. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur J Pharmacol. 2009;613:54–59. doi: 10.1016/j.ejphar.2009.04.022. [DOI] [PubMed] [Google Scholar]
- D’Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D, Meli R, Calignano A. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-alpha agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther. 2007;322:1137–1143. doi: 10.1124/jpet.107.123265. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Sci. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Dray A, Bevan S. Inflammation and hyperalgesia: highlighting the team effort. Trends Pharmacol Sci. 1993;14:287–290. doi: 10.1016/0165-6147(93)90041-H. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb Exp Pharmacol. 2005:283–297. doi: 10.1007/3-540-26573-2_9. [DOI] [PubMed] [Google Scholar]
- Ezzili C, Mileni M, McGlinchey N, Long JZ, Kinsey SG, Hochstatter DG, Stevens RC, Lichtman AH, Cravatt BF, Bilsky EJ, Boger DL. Reversible Competitive alpha-Ketoheterocycle Inhibitors of Fatty Acid Amide Hydrolase Containing Additional Conformational Constraints in the Acyl Side Chain: Orally Active, Long-Acting Analgesics. J Med Chem. 2011;54:2805–2822. doi: 10.1021/jm101597x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Bramanti P, Piomelli D, Calignano A, Cuzzocrea S. Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J Pharmacol Exp Ther. 2008;326:12–23. doi: 10.1124/jpet.108.136903. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279 ( Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tuting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Sci. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase Inhibitors Produce Anti-Allodynic Effects in Mice Through Distinct Cannabinoid Receptor Mechanisms. J Pain. 2010 doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, Selley DE, Imad Damaj M, Lichtman AH. The CB(2) cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011a;60:244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011b;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosersky DS, McMillan DE, Harris LS. D9-tetrahydrocannabinol and 11-hydroxy-D9-tetrahydrocannabinol: Behavioral effects and tolerance development. J Pharm Exp Ther. 1974;159:61–65. [PubMed] [Google Scholar]
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid -induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton C, Saghatelian A, Hardouin C, Boger D, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004a doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004b;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor PPAR-{alpha} mediates the antiinflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2004 doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti- arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Maurelli S, Bisogno T, De Petrocellis L, Di Luccia A, Marino G, Di Marzo V. Two novel classes of neuroactive fatty acid amides are substrates for mouse neuroblastoma ‘anandamide amidohydrolase’. FEBS Lett. 1995;377:82–86. doi: 10.1016/0014-5793(95)01311-3. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski N, Schatz A, Gopher A, Almog S, Martin B, Compton D, Pertwee R, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Moore AR. Collagen-induced arthritis. Methods Mol Biol. 2003;225:175–179. doi: 10.1385/1-59259-374-7:175. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR. On the therapeutical uses and toxic effects of cannabis indica. Lancet. 1890 March 22;:637–638. [Google Scholar]
- Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10:R43. doi: 10.1186/ar2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Aanonsen L, Hargreaves KM. SR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur J Pharmacol. 1997;319:R3–R4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, Piomelli D. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinova Z, Mikics E, Haller J, Medalie J, Stroik J, Barnes C, Yasar S, Tanda G, Piomelli D, Fratta W, Goldberg SR. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008;327:482–490. doi: 10.1124/jpet.108.142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009a;11:342–352. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH. Targeting Fatty Acid Amide Hydrolase (FAAH) to Treat Pain and Inflammation. Aaps J. 2009b doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Nalepa SD, Harakal JJ, Vassar HB. Anti-edema and analgesic properties of delta9-tetrahydrocannabinol (THC) J Pharmacol Exp Ther. 1973;186:646–655. [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoyglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Comm. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Trentham DE. Collagen arthritis as a relevant model for rheumatoid arthritis. Arthritis Rheum. 1982;25:911–916. doi: 10.1002/art.1780250801. [DOI] [PubMed] [Google Scholar]
- Tsou K, Patrick S, Walker JM. Physical withdrawal in rats tolerant to D9-tetrahydrocannabinol precipated by a cannabinoid receptor antagonist. Eur J Pharmacol. 1995;280:R13–R15. doi: 10.1016/0014-2999(95)00360-w. [DOI] [PubMed] [Google Scholar]
- Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. Partial purifaction and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- Williams RO. Rodent models of arthritis: relevance for human disease. Clin Exp Immunol. 1998;114:330–332. doi: 10.1046/j.1365-2249.1998.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2008;54:181–188. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. The antinociceptive effects of intrathecally-administered levonantradol and desacetyllevonantradol in the rat. J Clin Pharmacol. 1981;21:3345–3405. doi: 10.1002/j.1552-4604.1981.tb02612.x. [DOI] [PubMed] [Google Scholar]