Abstract
The N-CoR/SMRT complex containing mSin3 and histone deacetylase (HDAC) mediates transcriptional repression by nuclear hormone receptors and Mad. The proteins encoded by the ski proto-oncogene family directly bind to N-CoR/SMRT and mSin3A, and forms a complex with HDAC. c-Ski and its related gene product Sno are required for transcriptional repression by Mad and thyroid hormone receptor (TRβ). The oncogenic form, v-Ski, which lacks the mSin3A-binding domain, acts in a dominant-negative fashion, and abrogates transcriptional repression by Mad and TRβ. In ski-deficient mouse embryos, the ornithine decarboxylase gene, whose expression is normally repressed by Mad–Max, is expressed ectopically. These results show that Ski is a component of the HDAC complex and that Ski is required for the transcriptional repression mediated by this complex. The involvement of c-Ski in the HDAC complex indicates that the function of the HDAC complex is important for oncogenesis.
Keywords: Ski, N-CoR/SMRT corepressor, mSin3, Mad, histone deacetylase complex
N-CoR (nuclear hormone receptor corepressor) was identified originally as a corepressor that binds to, and mediates transcriptional repression by, nuclear hormone receptors (Hörlein et al. 1995). Thyroid-hormone and retinoic-acid receptors (TR and RAR) of the nuclear hormone receptor family actively repress the transcription of target genes in the absence of ligand (Chambon 1994; Mangelsdorf et al. 1995). Transcriptional repression is mediated by a conserved region in the amino-terminal part of the ligand-binding domain of TR (Baniahmad et al. 1995). N-CoR binds to the ligand-binding domain, termed the Co-R box, and, thereby, mediates transcriptional repression (Hörlein et al. 1995). N-CoR is a large protein with a molecular mass of 270,000 (Mr 270K), and contains three repressor domains in its amino-terminal region (Hörlein et al. 1995). Another corepressor, SMRT, which also binds to the Co-R box, shows striking homology to N-CoR (Chen and Evans 1995). N-CoR also forms a complex with mammalian Sin3 orthologs (mSin3A and mSin3B), which bind to another repressor, Mad (Alland et al. 1997; Hassing et al. 1997; Heinzel et al. 1997; Laherty et al. 1997; Nagy et al. 1997). The basic helix–loop–helix (bHLH) proteins of the Mad family act as transcriptional repressors after heterodimerization with Max (Ayer et al. 1993). N-CoR is required for Mad-induced transcriptional repression. The same target sequence of Mad/Max, the so-called E-box, is also recognized by a heterodimer of Myc/Max that activates transcription. It is believed that transcriptional activation of a group of target genes by Myc/Max enhances cellular proliferation or transformation, whereas transcriptional repression of the same target genes by Mad/Max leads to suppression of proliferation or induction of terminal differentiation in a wide range of cell types (Ayer and Eisenman 1993; Chin et al. 1995; Roussel et al. 1996). The binding of mSins to histone deacetylase (HDAC) suggested that transcriptional repression through N-CoR involves deacetylation of nucleosomal histones (Alland et al. 1997; Hassing et al. 1997; Heinzel et al. 1997; Laherty et al. 1997; Nagy et al. 1997). Recently, a tumor suppressor gene product, Rb, was also shown to interact with HDAC (Brehm et al. 1998; Luo et al. 1998; Magnaghi-Jaulin et al. 1998). Therefore, two tumor suppressor gene products, Mad and Rb, have been linked to the HDAC complex.
The oncogene v-ski was originally identified in avian Sloan-Kettering viruses, and found to transform chicken embryo fibroblasts (Li et al. 1986). Overexpression of either c-ski or v-ski induces either transformation or muscle differentiation of quail embryo fibroblasts, depending on the growth conditions (Colmenares and Stavnezer 1989; Colmenares et al. 1991a). Furthermore, v-ski transgenic mice have increased muscle mass caused by hypertrophy of type II fast muscle fibers (Sutrave et al. 1990). The capacity of ski to induce both transformation (growth) and differentiation, which is usually associated with the cessation of growth, is an intriguing paradox. The human c-ski proto-oncogene product (c-Ski) is a 728-amino-acid nuclear protein (Nomura et al. 1989; Nagase et al. 1990). Recombinant c-Ski protein purified from Escherichia coli cannot directly bind to DNA, but c-Ski in nuclear extracts from mammalian cell cultures binds to DNA, suggesting that c-Ski binds only to DNA when associated with other proteins (Nagase et al. 1990). The amino- and carboxy-terminal regions of c-Ski possess a cysteine-rich and a coiled-coil region, respectively, and both regions contribute additionally to indirect DNA binding by c-Ski. The v-Ski protein lacks 292 amino acids from the carboxyl terminus of c-Ski, but still contains the amino-terminal cysteine-rich region (Stavnezer et al. 1989). The amino-terminal region is responsible for both the cellular transformation and myogenesis capacity of ski (Zheng et al. 1997). The ski gene family comprises two members, ski and sno (ski-related novel gene) (Nomura et al. 1989) and both have been shown to share clear homology in their amino- and carboxy-terminal regions (Nomura et al. 1989; Nagase et al. 1993). Although it was speculated that Ski/Sno proteins are involved in transcriptional repression of specific target genes (Cohen et al. 1998; Nicol and Stavnezer 1998), their function remains unknown.
Here, we demonstrate that c-Ski directly binds to N-CoR/SMRT and mSin3A, and is involved in the HDAC complex. This interaction is required for transcriptional repression by Mad and TRβ. Because the carboxy-terminal region of c-Ski is responsible for the interaction with mSin3A, v-Ski, which lacks the carboxy-terminal region of c-Ski, blocks Mad-induced transcriptional repression in a dominant-negative fashion. Furthermore, one of the Mad target genes, the ornithine decarboxylase gene, was ectopically expressed in the c-ski-deficient mouse embryo. Therefore, abrogation of Mad function appears to be critical for transformation by v-ski.
Results
Direct binding of Ski to N-CoR/SMRT
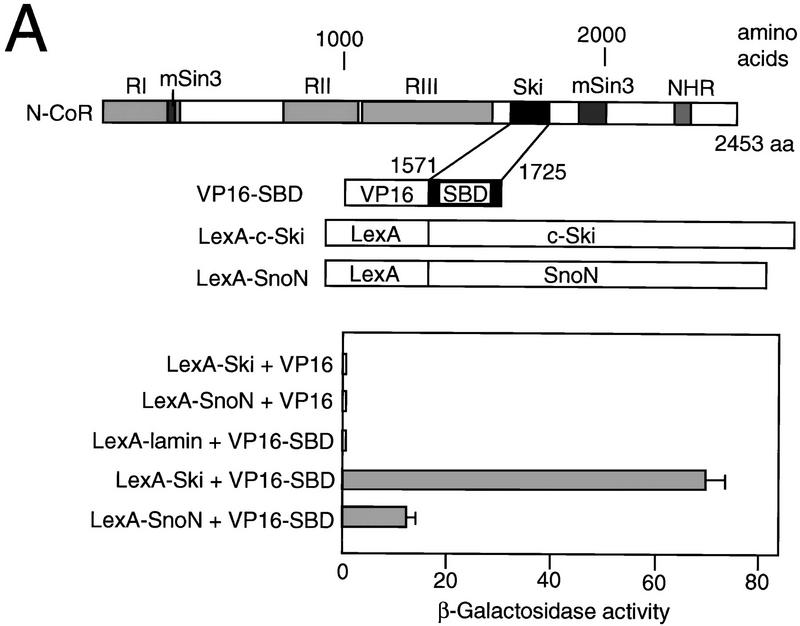
To identify the c-Ski-interacting proteins, we employed the yeast two-hybrid screen. A fusion protein consisting of the DNA-binding domain of LexA and the full-length human c-Ski protein was used as a bait to screen a mouse embryonic c-DNA library. Sixty-eight clones were isolated, and sequence analysis together with Southern blotting analyses indicated that 20 of these clones were derived from the same gene. The DNA sequences of these clones were identical to part of the N-CoR sequence, coding for a 155-amino-acid region of N-CoR (amino acids 1571–1725) (SBD: Ski-binding domain) (Fig. 1A). The interaction between c-Ski and N-CoR was further confirmed by measuring the β-galactosidase activity quantitatively in the yeast two-hybrid assay using a LexA DNA-binding domain-c-Ski fusion (Fig. 1A). In yeast, the SBD in N-CoR interacts efficiently with c-Ski, and also with SnoN encoded by the ski-related gene sno, although the interaction between Sno and N-CoR is weaker than that between Ski and N-CoR.
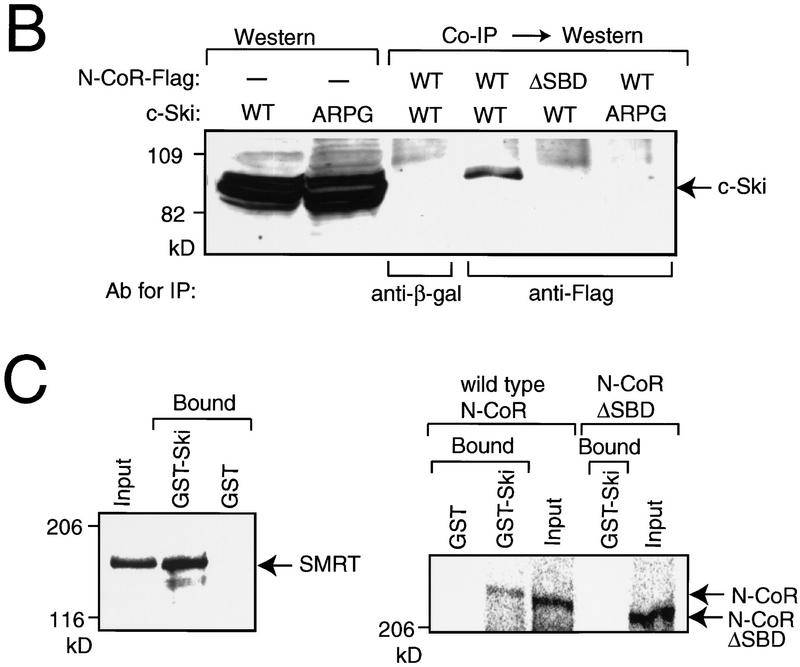
Figure 1.
Binding of c-Ski to N-CoR/SMRT. (A) Yeast two-hybrid assay. The three repressor domains (RI, RII, and RIII) and the mSin3-, Ski-, and nuclear hormone receptor (NHR)-binding domains in N-CoR molecule are indicated on the top. The regions encoded by the clones isolated in the yeast two-hybrid screening with c-Ski as a bait are referred to as Ski-binding domains (SBD). The plasmids used for the interaction assay are shown below. Three transformants harboring the plasmids indicated on the left were isolated, and their β-galactosidase activities were measured. The data represent an average of the results obtained using three transformants and are shown with standard deviations. (B) Coimmunoprercipitation. Whole-cell lysates were prepared from 293T cells transfected with a mixture of c-Ski (wild-type or ARPG mutant) and N-CoR–Flag (wild-type or mutant lacking SBD) expression plasmids, and a samples from the lysates were directly used for Western blotting with the anti-c-Ski antibodies. Whole-cell lysates were also immuno-precipitated by anti-Flag or anti-β-galactosidase antibody as a control, and the immuno-complex was analyzed by Western blotting using anti-c-Ski antibodies. (C) GST pull-down assay. In vitro-translated SMRT (left) or N-CoR (wild-type and SBD deletion mutant) (right) bound to GST–Ski were analyzed by SDS-PAGE followed by autoradiography. In the input lanes, the amount of protein was 10% of that used for the binding assay.
To confirm the in vivo interaction between c-Ski and N-CoR in mammalian cells, coimmunoprecipitation assays were performed (Fig. 1B). The two plasmids encoding c-Ski and Flag-linked N-CoR were transfected into 293T cells, and the cell lysates were immunoprecipitated with anti-Flag. c-Ski was coimmunopreciptated with wild-type N-CoR, but not with mutant N-CoR lacking SBD. The amino-terminal region of c-Ski is responsible for the cellular transformation capacity of ski (Zheng et al. 1997) and contains two potential amphipathic helices. Disruption of the second helix by an in-frame insertion of four codons at position 145 (ARPG mutant) leads to a loss of transformation activity of v-Ski (Colmenares et al. 1991b). The ARPG mutant of Ski was not coprecipitated with wild-type N-CoR.
Another corepressor, SMRT, shows striking homology to N-CoR (Chen and Evans 1995). The amino acid sequence in SBD is significantly conserved between N-CoR and SMRT (47% identity). Therefore, we examined whether SMRT also binds to c-Ski. In vitro-translated SMRT efficiently bound to GST–Ski fusion protein (Fig. 1C, left panel). Under the same binding conditions, in vitro-translated N-CoR bound to GST–Ski, but the N-CoR mutant lacking SBD did not (Fig. 1C, right panel).
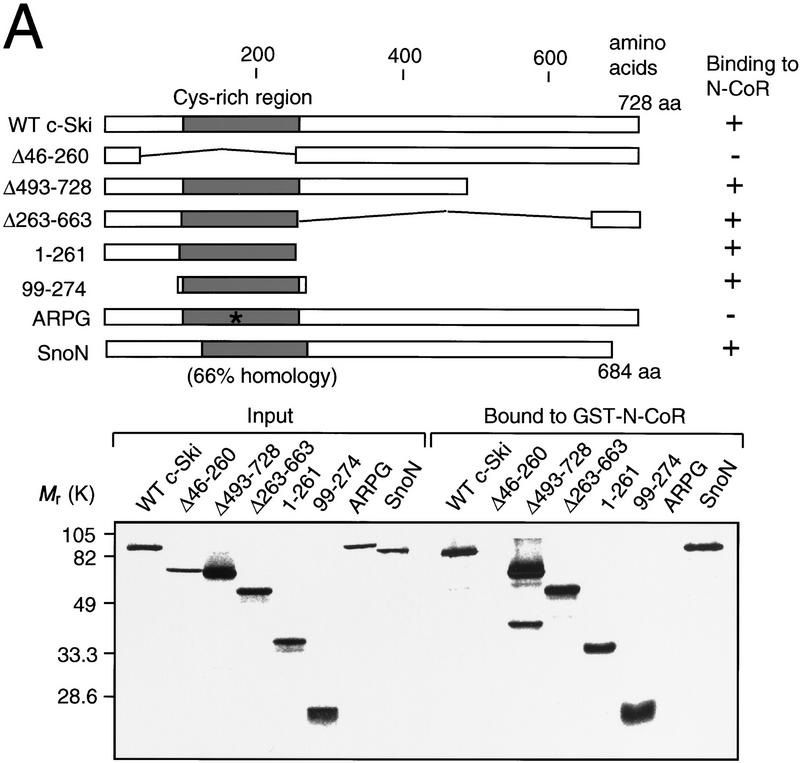
To identify the region in c-Ski that interacts with N-CoR, the GST pull-down assay was performed using the GST–SBD fusion protein resin and various forms of in vitro-translated c-Ski protein (Fig. 2A). The results indicated that the amino-terminal cysteine-rich region of c-Ski (amino acids 99–274) interacts efficiently with N-CoR. The amino-terminal region is responsible for the cellular transformation capacity of ski and snoN (Zheng et al. 1997; Cohen et al. 1998). Consistent with the coimmunoprecipitation result shown in Figure 1B, the ARPG mutant did not bind to N-CoR (Fig. 2A), suggesting that the interaction between Ski and N-CoR is important for transformation by v-ski. The high degree of homology (66%) in this cysteine-rich region between c-Ski and Sno is consistent with the result that in vitro-translated Sno also interacts with GST–N-CoR (Fig. 2A).
Figure 2.
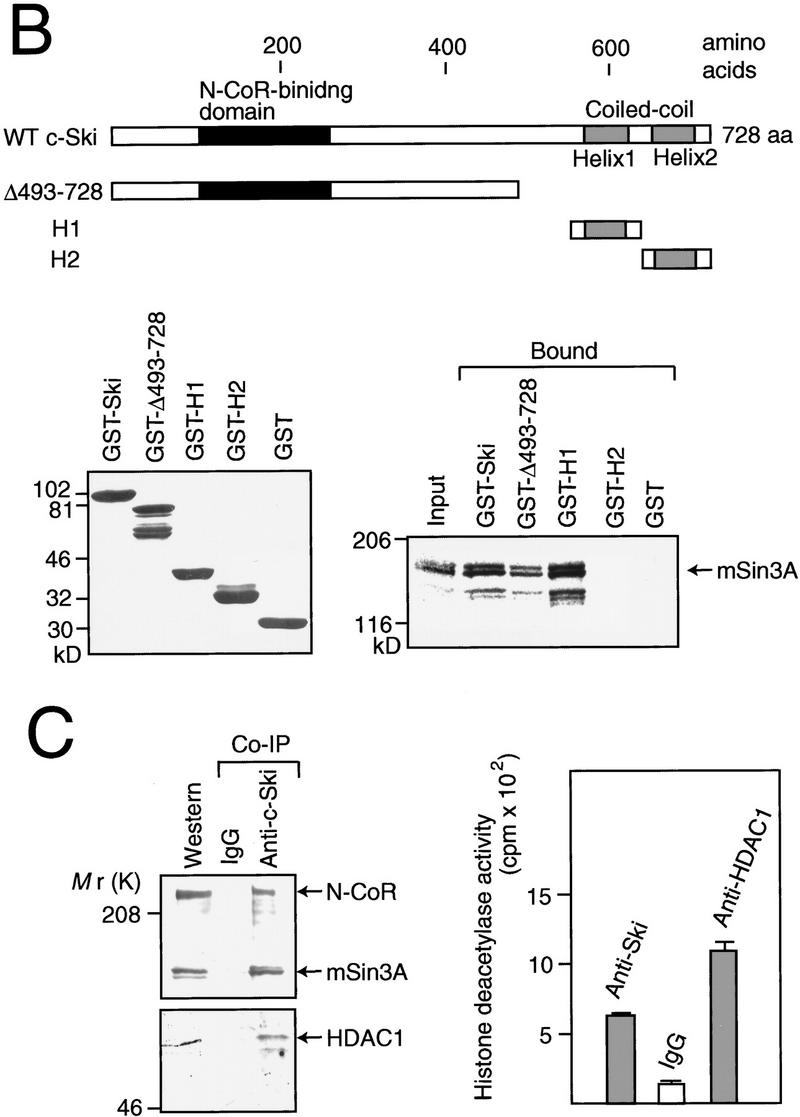
Complex formation between c-Ski, N-CoR, mSin3A, and HDAC. (A) Identification of N-CoR-binding domain in c-Ski. GST pull-down assay was performed using in vitro-translated c-Ski and GST–SBD. The various forms of c-Ski used are indicated. SnoN encoded by one of the sno mRNA species is shown below. The cysteine-rich region is indicated by a shaded box. The results of binding assays are summarized at right. (Bottom) In vitro-translated c-Ski used (input) and c-Ski bound to GST–N-CoR were analyzed by SDS-PAGE followed by autoradiography. In the input lanes, the amount of c-Ski was 10% of that used for the binding assay. (B) Binding of mSin3A to the carboxy-terminal region of c-Ski. GST pull-down assay was performed using in vitro-translated mSin3A and GST fusion proteins containing various portions of c-Ski. The various forms of c-Ski used are indicated. (Lower left) Various GST–Ski fusion proteins bound to glutathione beads were analyzed by SDS-PAGE followed by coomassie blue staining. (Lower right) In vitro-translated mSin3A used (input) and mSin3A bound to GST–Ski were analyzed by SDS-PAGE followed by autoradiography. (C) Coimmunoprecipitation of c-Ski with N-CoR, Sin3, and HDAC. (Left) Lysates from 293 cells were immunoprecipitated with anti-c-Ski antibodies or normal IgG. The immunocomplex was analyzed by Western blotting using anti-N-CoR, anti-Sin3A, and anti-HDAC1 antibodies. (Right) The immunocomplex was prepared using anti-c-Ski antibodies or IgG from 293 cells transfected with the c-Ski expression plasmid, and used for the HDAC assays. As a positive control, the immunocomplex prepared using the anti-HDAC1 antibody from 293 cells transfected with the HDAC1 expression plasmid was used.
Binding of Ski/Sno to N-CoR/SMRT suggested that Ski/Sno is a component of the complex containing N-CoR/SMRT, mSin3, and HDAC. Therefore, we examined the interaction of Ski with mSin3 or HDAC. In vitro-translated mSin3A efficiently bound to GST–Ski fusion protein (Fig. 2B). Deletion of carboxy-terminal region of c-Ski significantly but not completely decreased the binding affinity with mSin3A. In addition, the GST fusion protein containing one of the helical region in the coiled–coil region of c-Ski efficiently bound to mSin3A. These results indicate that mSin3A binds to the carboxy-terminal helical region and also the amino-terminal half of c-Ski. c-Ski did not interact with HDAC directly (data not shown).
To examine the complex formation between endogenous c-Ski, N-CoR, Sin3, and HDAC proteins, a coimmunoprecipitation assay was performed using 293 cells (Fig. 2C). The anti-c-Ski antibodies co-precipitated N-CoR, Sin3A, and HDAC1, whereas normal IgG did not (Fig. 2C, left). In addition, the immunocomplex prepared using the anti-c-Ski antibodies exhibited significant level of HDAC activity, whereas that with the normal IgG did not (Fig. 2C, right). These results indicate that c-Ski forms a complex in vivo with N-CoR, Sin3, and HDAC. N-CoR is expressed ubiquitously in many tissues (Hörlein et al. 1995). Consistent with this, both ski and sno are expressed ubiquitously like N-CoR in various tissues (T. Nomura and S. Ishii, unpubl.).
Multiple repression domains in c-Ski
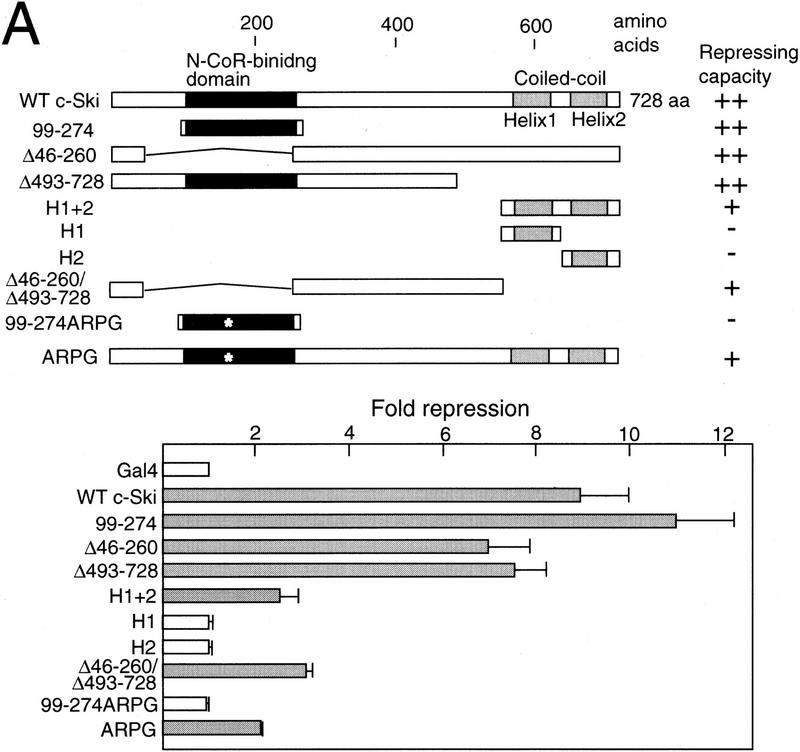
Interaction of c-Ski with N-CoR/SMRT and mSin3A suggested the presence of a repressor domain in c-Ski. Because c-Ski itself cannot directly bind to DNA, we examined the repressor function of the Gal4 DNA-binding domain–c-Ski fusion (Fig. 3A). The Gal4–Ski fusions containing the full-length c-Ski or the amino-terminal N-CoR-binding domain repressed the activity of the promoter containing the Gal4-binding sites efficiently, whereas the ARPG mutant of the N-CoR-binding domain did not, supporting the notion that c-Ski functions as a transcriptional repressor by interacting with N-CoR. However, the c-Ski mutant lacking the N-CoR-binding domain (Δ46–260) also had significant repressor activity. This is consistent with the result that mSin3A binds to the carboxy-terminal helical region of c-Ski (Fig. 2B). The Gal4–Ski fusion containing this coiled-coil region had low but significant repressor activity. In addition, the c-Ski mutant lacking both the amino-terminal N-CoR-binding domain and the carboxy-terminal coiled–coil region (Δ46–260/Δ493–728) still had low but significant repressor activity. This suggests that the region around the N-CoR-binding domain may interact with additional factors that mediate transcriptional repression.
Figure 3.
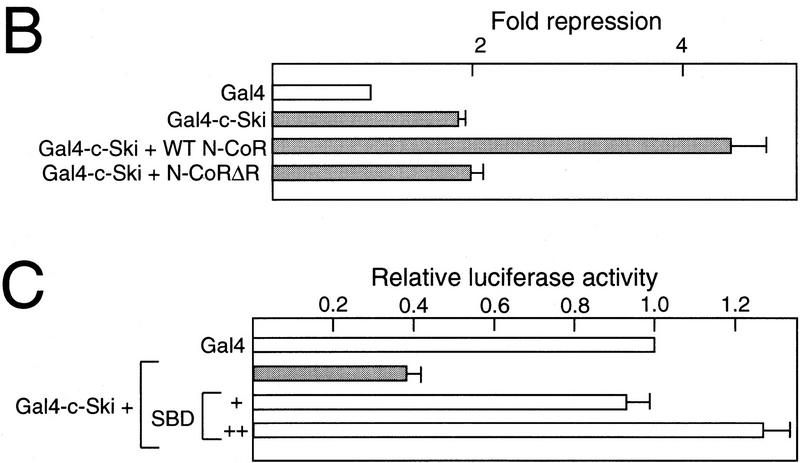
Multiple repressor domains in c-Ski. (A) Transcriptional repression by Gal4–Ski fusion proteins. (Top) The structure of Ski is schematically shown. The Gal4–Ski fusion proteins consisting of the Gal4 DNA-binding domain and the various regions of c-Ski are indicated. The transcriptional repression by various Gal4–Ski fusions was measured by cotransfection assays using the luciferase reporter containing the Gal4-binding sites, and the results are indicated below by bar graphs. The average degree of repression compared with that of the Gal4 DNA-binding domain alone is represented along with the standard deviation. The shaded bars indicate significant repressor activity. The results of the cotransfection assays are summarized at right. (++, +, −) Strong, weak, and no repressor activity, respectively. (B) Enhancement of repressor activity of Ski by N-CoR. Cotransfection experiments were done as described above using a small amount of Gal-Ski expression plasmid. The effect of wild-type or mutant N-CoR lacking the amino-proximal repressor domain (N-CoRΔR) on Gal4–Ski-induced transcriptional repression was examined by cotransfection of the N-CoR expression plasmid. (C) Inhibition of the Gal4–Ski-induced repression by SBD. The effect of SBD on Gal4–Ski-induced transcriptional repression was examined by cotransfection.
In cotransfection assays using a Gal4 site-containing reporter, the degree of repression by a small amount of Gal4–c-Ski was low (Fig. 3B). Coexpression of the wild-type but not the mutant N-CoR lacking the three repressor domains in the amino-terminal half (amino acids 1–1502) (N-CoRΔR), however, enhanced the repression by Gal4–Ski. Furthermore, the repressor activity of Gal4–full length c-Ski was abolished by coexpression of the SBD of N-CoR (Fig. 3C), supporting the idea that the repressor function of Gal4–Ski is mediated by N-CoR.
Disruption of the N-CoR dot-like nuclear structure by Ski mutants
To investigate the role of the amino- and carboxy-terminal portions of c-Ski in in vivo complex formation with N-CoR, we examined the colocalization of c-Ski and N-CoR by immunostaining (Fig. 4). 293T cells were transfected with a mixture of the c-Ski and Flag-linked N-CoR expression plasmids, and their subcellular localization was examined using anti-c-Ski and anti-Flag antibodies. As reported by another group (Söderström et al. 1997), N-CoR is localized in the dot-like nuclear structure (Fig. 4A). c-Ski-staining also displayed a punctate pattern in the nuclei (Fig. 4A), and the signals of both N-CoR and c-Ski overlapped almost completely (Fig. 4B). On the other hand, the c-Ski mutant lacking the amino-proximal N-CoR-binding domain did not exhibit the clear dot-like structure (Fig. 4A, Δ46–260 c-Ski). When N-CoR was co-expressed with this c-Ski mutant, the N-CoR punctate pattern was also disrupted (Fig. 4C, left), and the c-Ski signals that did not overlap N-CoR were detected (Fig. 4C, right). Partial overlapping of N-CoR signals with this c-Ski mutant may occur via mSin3, which binds to the carboxy-terminal region of c-Ski. The c-Ski mutant lacking the carboxy-terminal coiled–coil region were distributed uniformly in the necleoplasm (Fig. 4A, Δ493–728 c-Ski). This c-Ski mutant was able to disrupt the N-CoR dot-like structure completely, and N-CoR staining became uniform in the nucleoplasm when it was coexpressed with this mutant (Fig. 4D, left). Therefore, the c-Ski mutants led to the disruption of normal dot-like pattern of N-CoR localzation in the nucleus. These results suggest that both the N-CoR-binding domain and the coiled–coil region of the c-Ski molecule are required to form the normal N-CoR complex containing c-Ski and other components. The c-Ski mutants lacking either of these two domains appear to act in a dominant-negative fashion, and the carboxy-truncated mutant may be a stronger dominant-negative form.
Figure 4.
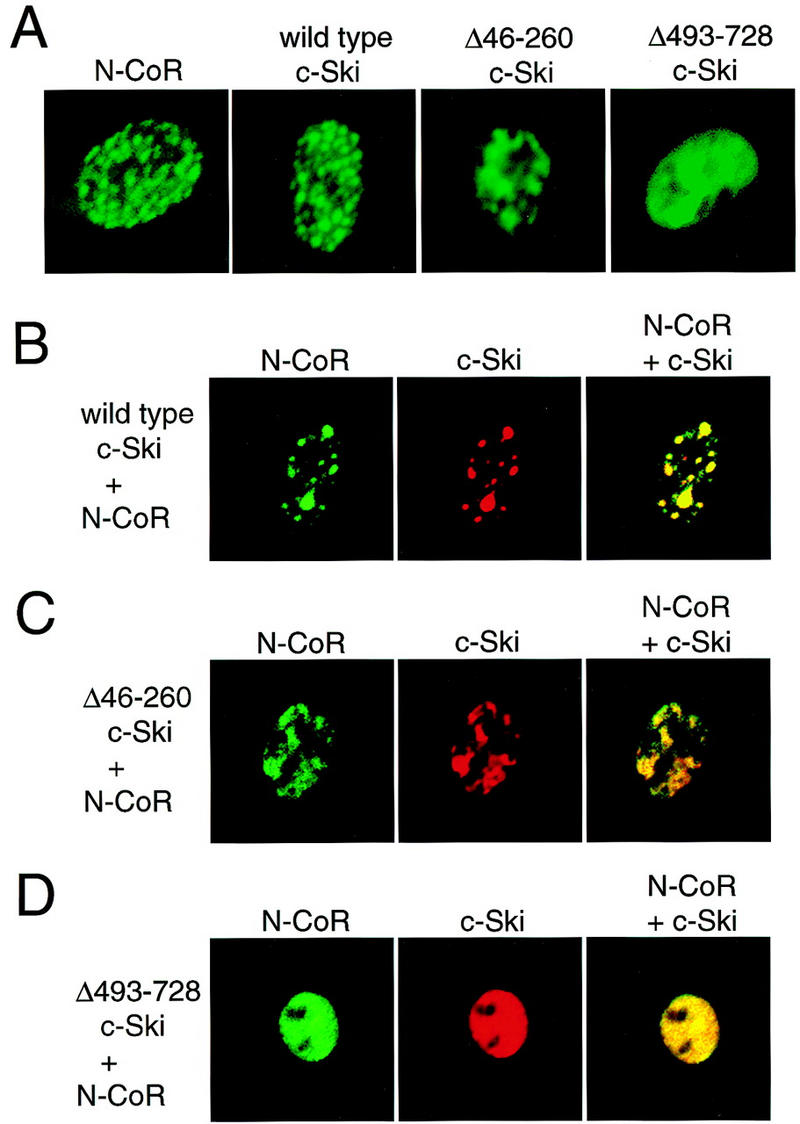
Disruption of nuclear dot-like structure of N-CoR by Ski mutants. (A) The plasmid to express wild-type N-CoR–Flag or either of three kinds of c-Ski (wild type and two mutants) was transfected into 293T cells, cells were immunostained with anti-Flag or anti-c-Ski antibodies. The stainings were visualized by FITC-conjugated secondary antibodies. (B–D) A mixture of the c-Ski [wild type (B) or a mutant lacking the amino-proximal N-CoR-binding domain (C) or a mutant lacking the carboxy-proximal coiled–coil region (D)] and the N-CoR–Flag expression plasmid was transfected into 293T cells, and cells were immunostained with anti-Flag (left) and anti-c-Ski antibodies (middle). The N-CoR- and c-Ski-stainings were visualized by FITC- and rhodamine-conjugated secondary antibodies, respectively. (Right panels) The signals for N-CoR and c-Ski are superimposed.
Abrogation of Mad- or TRβ-induced transcriptional repression by anti-Ski/Sno antibodies
Direct interaction of N-CoR with c-Ski raised the possibility that c-Ski might be essential for transcriptional repression through the N-CoR complex. To examine whether c-Ski is required for transcriptional repression by Mad or TRβ, antibody microinjection experiments were performed (Fig. 5). Injection of the reporter, in which the lacZ gene was linked to the TK promoter and the Gal4-binding sites, into Rat-1 cells gave rise to many lacZ-positive cells. Coinjecton of this lacZ reporter with the plasmid encoding the Gal4–Mad fusion protein resulted in a decrease in the number of lacZ-positive cells. This decrease in the number of lacZ-positive cells by Gal4–Mad was relieved partially by coinjection of anti-c-Ski or anti-Sno polyclonal antibodies, and significantly by coinjection of both antibodies. Recently we identified the third member of the ski gene family (M.M. Khan, T. Nomura, and S. Ishii, unpubl.), suggesting that the incomplete abrogation of the Gal4–Mad function by coinjection of both anti-Ski and anti-Sno antibodies may be attributable to the presence of other Ski-related proteins. Coinjection of the c-Ski and Sno expression plasmids significantly abrogated the effect of anti-c-Ski or anti-Sno antibodies. Similar results were also obtained with the Gal4–TRβ (Fig. 5B). Coinjection of anti-c-Ski or anti-Sno antibodies did not affect the decrease in the number of lacZ-positive cells by Gal–δEF1 (Fig. 5B), a heterogeneous repressor that is thought not to use N-CoR for its function (Sekido et al. 1997). These results indicate that the proteins encoded by the ski gene family are required for transcriptional repression by Mad or TRβ.
Figure 5.
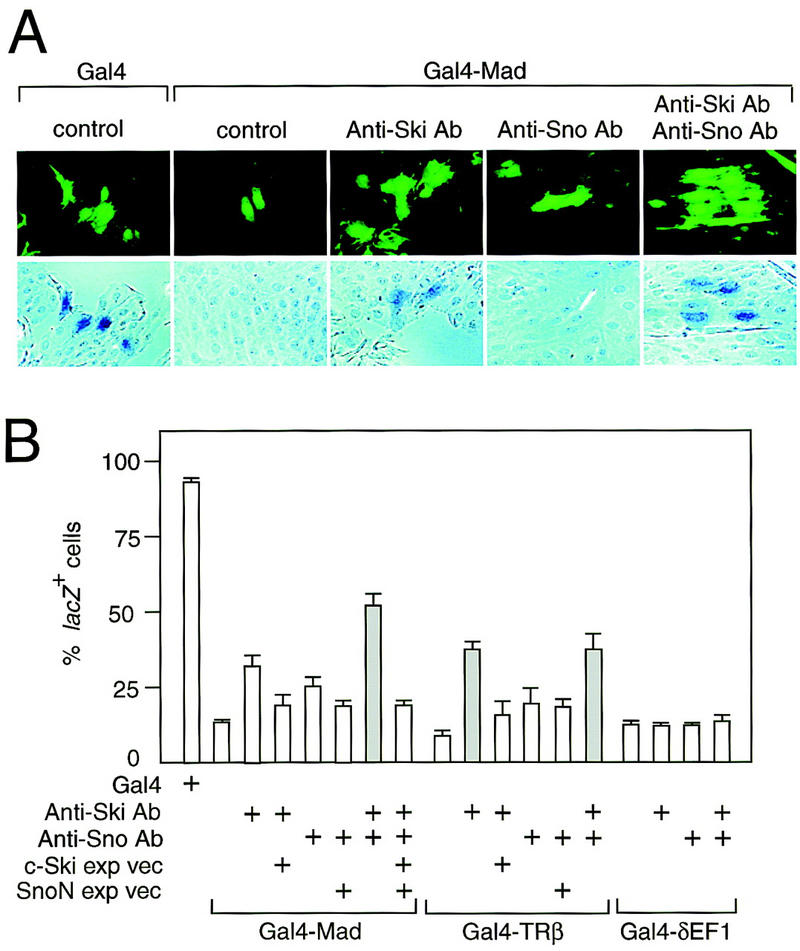
Abrogation of Mad- or TRβ-induced transcriptional repression by anti-Ski/Sno antibodies. The Gal4 site-containing TK–lacZ reporter was injected with the expression plasmid for Gal4 DNA-binding domain alone or with Gal4–Mad, Gal4–TRβ, or Gal4–δEF1 expression plasmid into the nuclei of Rat-1 cells. The GFP vector was co-injected for the identification of the injected cells. The affinity-purified anti-Ski and anti-Sno antibodies were co-injected with the Gal4–Mad, Gal4–TRβ, or Gal4–δEF1 expression plasmid. The expression of lacZ was monitored by X-gal staining. Typical photomicrographs of green fluorescence of GFP and X-gal staining for the indicated constructs and antibodies are shown (A). Experiments were repeated three (Gal4–Mad) or two times (Gal4–TRβ and Gal4–δEF1), and the number of injected cells in each experiment was 70–240. The lacZ+ cells were quantified based on the percentage of injected cells that stained blue, and the results are presented as bar graphs with the standard deviation (B). The shaded bar shows the significant abrogation of the Mad- or TRβ-induced transcriptional repression by coinjection of both anti-Ski and anti-Sno antibodies.
Abrogation of Mad- and TRβ-induced transcriptional repression by v-Ski
The carboxy-terminal region of c-Ski binds to mSin3A (Fig. 2B), and the carboxy-truncated c-Ski mutant led to the disruption of normal dot-like pattern of N-CoR localization in the nucleus (Fig. 4). This raised the possibility that v-Ski may inhibit the Mad-mediated transcriptional repression in a dominant-negative fashion. To investigate whether v-Ski abrogates the Mad function, we examined the effect of overexpression of the four forms of Ski on Mad-induced transcriptional repression (Fig. 6A,B). The Gal4–Mad fusion, which consists of the Gal4 DNA-binding domain and the mSin3-interacting domain of Mad (Ayer et al. 1996), strongly repressed transcription from the Gal4 site-containing reporter. This Gal4–Mad-induced repression was abrogated by the three forms of Ski, the amino- or carboxy-proximal deleted forms (Δ46–260 and Δ493–728) and v-Ski in a dose-dependent manner (Fig. 6B). Because the major difference between c-Ski and v-Ski is a truncation of the carboxy-terminal region (Stavnezer et al. 1989; Zheng et al. 1997), the similar results obtained with v-Ski and the carboxy-truncated form (Δ493–728) appear reasonable. Furthermore, wild-type c-Ski partly abrogated Mad-induced transcriptional repression. It was reported that transcriptional repression by RAR can be either positively or negatively regulated by changes in the levels of N-CoR expression, probably attributable to the relatively strict stoichiometric relationship between N-CoR and other components of the N-CoR complex (Söderström et al. 1997). Therefore, overexpression of wild-type c-Ski may also lead to an imbalance between the components of the corepressor complex, and may abrogate transcriptional repression, rather than potentiating transcriptional repression. In fact, we observed that the punctate pattern of N-CoR was disrupted by coexpression of high amount of c-Ski relative to that of N-CoR (data not shown). This is consistent with the fact that overexpression of normal c-Ski also leads to cellular transformation of chicken embryonic fibroblasts (Colmenares et al. 1991a). Similarly, the four forms of Ski also abrogated Gal4–TRβ-induced transcriptional repression (Fig. 6C). By performing Western blotting, we have confirmed that the level of Gal4–Mad and Gal4–TRβ was not decreased (data not shown), indicating that the abrogation of the Gal4–Mad and Gal4–TRβ-mediated transcriptional repression by Ski is not attributable to a decrease in the level of repressor proteins. Furthermore, the four forms of c-Ski did not abrogate the repression mediated by Gal4–δEF1 (Sekido et al. 1997) (Fig. 6D).
Figure 6.
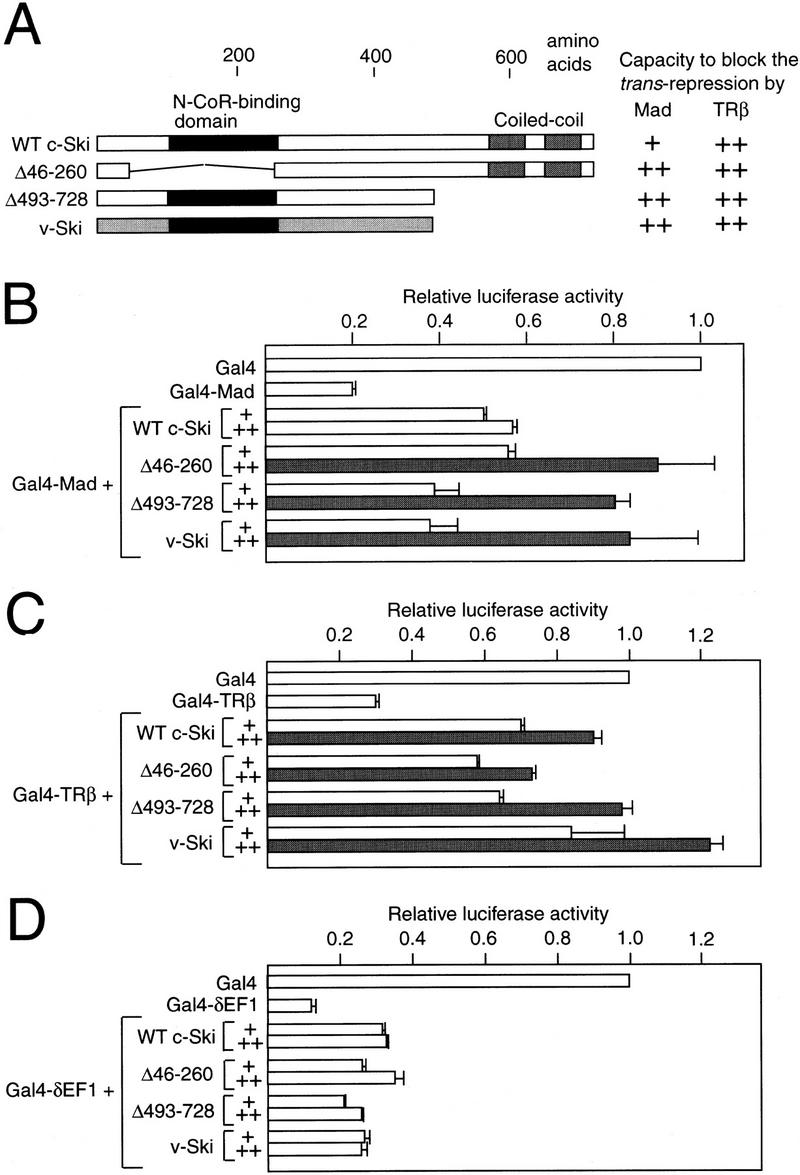
Inhibition of the Mad- or TRβ-induced transcriptional repression by v-Ski. (a) Summary of the cotransfection assays. The structures of the various forms of Ski, whose capacity to abrogate the transcriptional repression by Gal–Mad, Gal–TRβ, or Gal4–δEF1 was examined, are shown. The results of the cotransfection assays shown in B and C are summarized as almost complete abrogation of transcriptional repression (++) and partial abrogation of transcriptional repression (+). (B–D) Cotransfection assays. A mixture of Gal4 site-containing luciferase reporter, the Gal4–Mad (B), Gal4–TRβ (C), Gal4–δEF1 (D) or Gal4 expression plasmid, and the effector plasmid encoding various forms of Ski was transfected into CV-1 cells, and the luciferase activity was measured. The amounts of effector plasmid were 4 (+) or 8 μg (++). The data shown are an average of two experiments with standard deviations. The shaded bar indicates the relative luciferase activity >0.7.
Ectopic ODC expression in ski-deficient mouse embryo
So far, several gene targets of Myc, including cdc25A (Galaktionov et al. 1996), ornithine decarboxylase (ODC) (Bello-Ferdenandez et al. 1993), p53 (Hermerking and Eick 1994), eIF-4E, and eIF-2α (Rosenwald et al. 1993) genes have been identified. Because the Myc–Max and Mad–Max complex activates and represses, respectively, the same target genes through the E-box transcription of these target genes may be repressed by Mad–Max. Myc regulates not only cellular proliferation, but also apoptosis (Evan et al. 1992). Interestingly, the c-ski-deficient mutant mice show excessive apoptosis in the cranial neuroepithelium, and the timing of apoptosis coincides with a failure of neural tube closure during neurulation (Berk et al. 1997) (Fig. 7a,b). To investigate whether this excessive apoptosis is caused by the ectopic expression of Myc target genes resulting from a decrease in Mad activity, we examined the expression of ODC and p53, which are known to induce apoptosis (Hermerking and Eick 1994; Packham and Cleveland 1994), in c-ski-deficient homozygous and heterozygous embryos. In situ hybridization and immuno-staining indicated that ODC was ectopically expressed in the cranial neuroepithelium of homozygous mutant (Fig. 7). Ectopic expression of p53 was not observed (data not shown). These results suggest that c-ski deficiency causes the loss of Mad-dependent transcriptional repression of ODC, leading to ectopic expression of ODC followed by excessive apoptosis.
Figure 7.
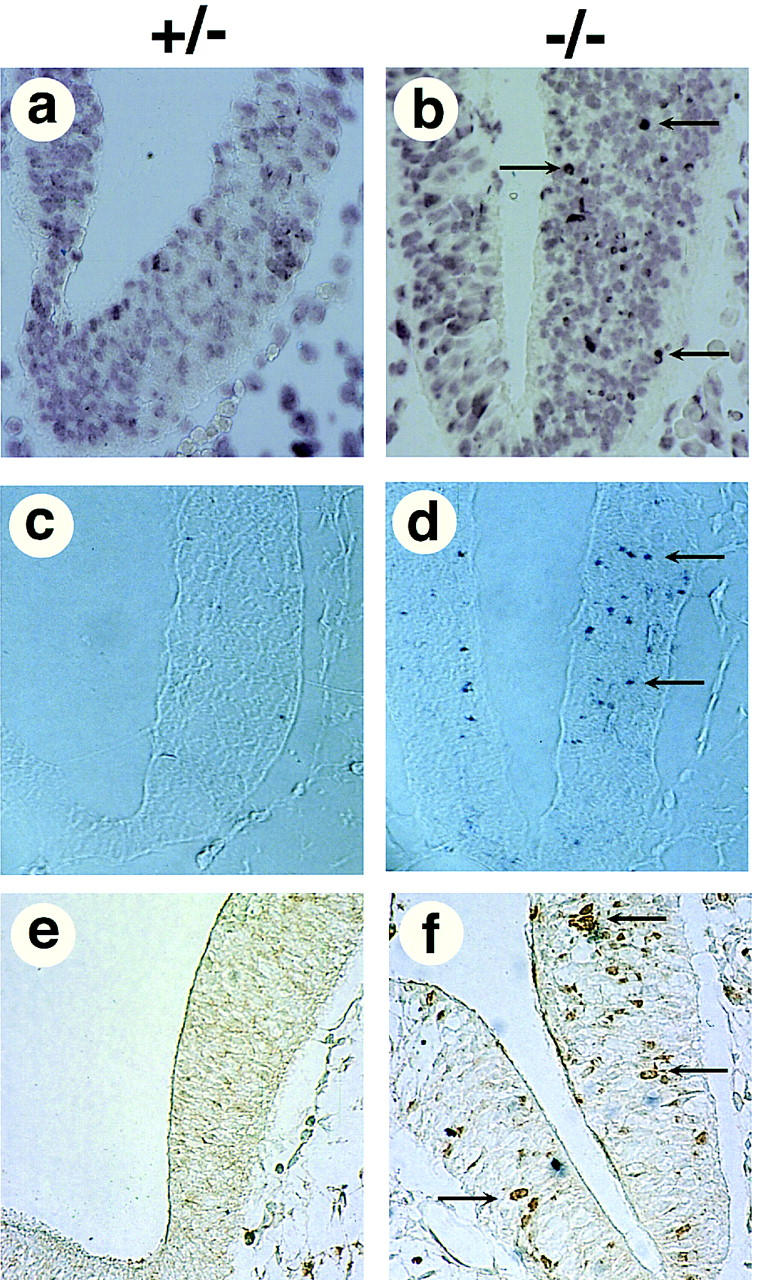
Ectopic expression of ODC in ski-deficient embryos. Frontal sections were prepared from c-ski heterozygous and homozygous mutant embryos at 9.5 dpc, and analyzed with the TUNEL assay (a,b). Apoptotic cells in the neuroepithelium that had incorporated labeled dUTP are indicated by arrows. The signals of in situ hybridization (c,d) or immunostaining for ODC (e,f) are shown. Cells that overexpressed ODC are indicated by arrows.
Discussion
Our results indicate that c-Ski is a component of the complex containing N-CoR/SMRT, mSin3, and HDAC, which is required for transcriptional repression by Mad and TRβ. During preparation of this paper, it was shown that Ski can modulate the transcriptional regulation mediated by RARα (Dahl et al. 1998a). Therefore, c-Ski may be needed for the transcriptional regulation mediated by other nuclear hormone receptors that use N-CoR/SMRT. HDAC is involved in the N-CoR/SMRT complex via its interaction with mSin3 (Fig. 8). Ski binds to N-CoR/SMRT via the amino-terminal cysteine-rich region (Fig. 2A), whereas it also binds to mSin3A through the carboxy-terminal helical region (Fig. 2B). Our results dealing with the repressor domains of the c-Ski molecule indicated the presence of at least another repressor domain in addition to the N-CoR-binding domain and the carboxy-proximal coiled–coil region. Therefore, at least another Ski-interacting factor may be involved in the Ski–HDAC complex and have a role in transcriptional repression. One candidate is the Skip (Ski-interacting protein) protein that was recently found to interact with the amino-terminal region of c-Ski (Dahl et al. 1998b) (Fig. 8). Skip has a striking homology with the Drosophila protein Bx42 that is associated with chromatin in transcriptionally active puffs of salivary glands (Wieland et al. 1992). The complex containing Sin3 consists of multiple proteins such as SAP30 and the histone-binding proteins RbAp46 and RbAp48 (Zhang et al. 1997). Interestingly, SAP30 is required for the transcriptional repression mediated by the estrogen receptor, but not by TR or RAR (Laherty et al. 1998). Therefore, it will be needed to investigate whether c-Ski is required for all the transcriptional repressors that use N-CoR/SMRT. The results described here are consistent with our previous observation that c-Ski indirectly interacts with DNA via other factors (Nagase et al. 1990). Multiple transcriptional repressors including nuclear hormone receptors and Mad may recruit the Ski complex to specific DNA sequences. This is consistent with our unpublished data showing that various DNA sequences can serve for c-Ski complex DNA-binding. The Gal4 fusions of two mutants (Δ46–260 and Δ493–728) repressed transcription as well as wild-type c-Ski (Fig. 3), although they did not form a nuclear dot-like structure (Fig. 4). These results, however, merely indicate that these two Gal4 fusions can recruit N-CoR/SMRT and mSin3A, respectively, even though they do not form a nuclear dot-like structure. These two c-Ski mutants inhibited the transcriptional repression mediated by Mad or TRβ when they did not fuse with the Gal4 DNA-binding domain (Fig. 6). These results suggest that the subcellular localization of c-Ski in a nuclear dot-like structure is important to mediate the transcriptional repression.
Figure 8.
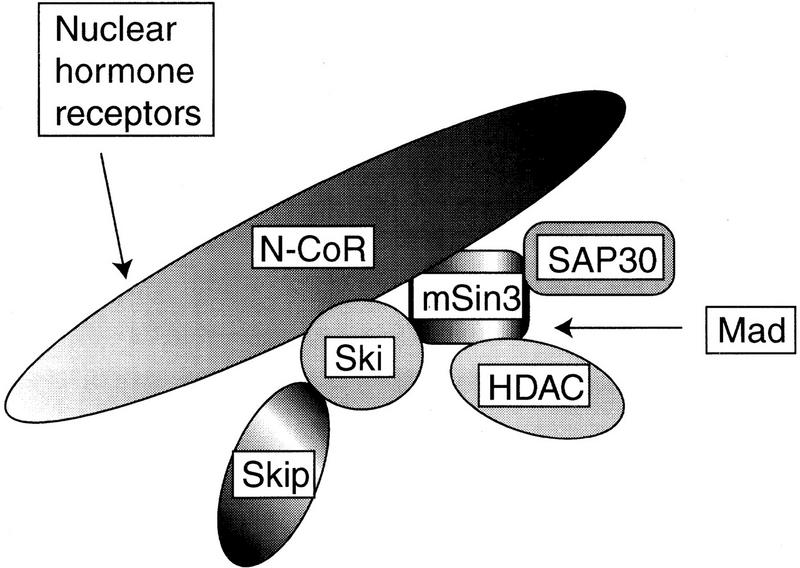
Schematic model of the HDAC complex. N-CoR/SMRT directly binds to mSin3, which interacts with HDAC. The amino-terminal region of c-Ski binds to N-CoR through the carboxy-terminal region of N-CoR. Skip also binds to the amino-terminal region of c-Ski. The carboxy-terminal region of c-Ski binds to mSin3A. The nuclear hormone receptor is linked to the HDAC complex by direct binding to N-CoR, whereas a transcriptional repressor Mad is linked to the HDAC complex via mSin3.
Study of the transformation capacity of various forms of c-Ski indicated that the amino-terminal cysteine-rich region is responsible for cellular transformation, however, the mechanism of transformation has remained obscure. Our results indicate that the amino-terminal region, which is needed for cellular transformation, is responsible for interaction with N-CoR/SMRT. Furthermore, v-Ski and the carboxy-truncated form of c-Ski lack the carboxy-terminal mSin3A-binding domain, and abrogate transcriptional repression by Mad by functioning in a dominant-negative fashion. Transcriptional activation by Myc causes cell proliferation, whereas transcriptional repression by Mad inhibits cell proliferation (Ayer et al. 1993; Ayer and Eisenman 1993; Chin et al. 1995; Roussel et al. 1996). Therefore, Mad is thought to act as a tumor suppressor, and in fact, one of the mad-related genes, mxi1, was recently demonstrated to act as a tumor suppressor using mutant mice (Schreiber-Agus et al. 1998). Therefore, abrogation of Mad-induced transcriptional repression by v-Ski may lead to induction of Myc target genes and cellular transformation. Another tumor suppressor gene product Rb was also recently shown to interact HDAC (Brehm et al. 1998; Luo et al. 1998; Magnaghi-Jaulin et al. 1998), although it remains unknown whether c-Ski and N-CoR/SMRT interacts with Rb. Therefore, it is possible that v-Ski also transform cells by inhibiting transcriptional repression by Rb. Overexpression of normal c-Ski was reported to transform chicken embryonic fibroblasts (Colmenares et al. 1991a). Consistent with this, we observed that overexpression of c-Ski partly abrogates Mad-induced transcriptional repression, possibly by creating an imbalance between the components of the N-CoR complex. Because our results indicate that c-Ski is required for transcriptional repression by Mad, c-ski may be a negative regulator of cellular proliferation, although it has been held to be a proto-oncogene. This is consistent with the fact that v-Ski inhibits the function of normal c-Ski in a dominant-negative fashion. This situation is reminiscent of the case of PML. The PML gene was originally identified as a PML–RAR fusion oncogene causing leukemia; however, later studies showed that it to be a tumor suppressor gene (Doucas and Evans 1996).
Ski can transform chicken embryonic fibroblasts, whereas it also paradoxically induces muscle differentiation in quail embryo cells through enhanced myoD and myogenin expression (Colmenares et al. 1991b). Retinoic acid and thyroid hormone induce muscle cell differentiation by inducing myoD and myogenin expression (Arnold et al. 1992; Muscat et al. 1995). Therefore, v-Ski could inhibit transcriptional repression by the nuclear hormone receptors, or overexpression of c-Ski could lead to induction of target genes of the nuclear hormone receptors including myoD and myogenin. Therefore, the paradoxical nature of ski might be explained by Ski modulation of the activities of different types of transcriptional repressors such as Mad and nuclear hormone receptors. It is possible that many other transcriptional repressors in addition to Mad and nuclear hormone receptors may bind to the Ski–HDAC complex. Therefore, we cannot exclude the possibility that Ski induces myoD and myogenin expression by modulating the activity of such uncharacterized repressors.
The results described here establish Ski as a component of the HDAC complex. This contributes to our understanding of the molecular mechanism of cellular transformation by Ski and also the mechanism of transcriptional repression through the HDAC complex.
Materials and methods
Yeast two-hybrid screening
The yeast two-hybrid screening was performed using a modified version of the originally developed system (Vojtek et al. 1993) and the mouse embryonic cDNA library. The LexA–c-Ski fusion protein containing the full-length human c-Ski was used as a bait. The β-galactosidase activity was measured as described (Dai et al. 1996).
In vitro binding assay
To express the GST fusion protein containing various portions of c-Ski or SBD of N-CoR in E. coli, the plasmid was constructed by the PCR-based method using the pGEX-2T vector (Pharmacia). Preparation of the GST–Ski or GST–SBD fusion protein, in vitro translation of various forms of c-Ski, mSin3A, N-CoR, or SMRT, and binding assays were done essentially as described (Dai et al. 1996). The buffer used for the binding assay between Ski and GST–SBD consisted of 20 mm HEPES (pH 7.7), 75 mm KCl, 2.5 mm MgCl2, 0.1 mm EDTA, 1 mm DTT, 0.05% NP-40, and 0.02% skim milk, and PBS for washing. The buffer consisting of 10 mm HEPES (pH 7.7), 50 mm KCl, 2.5 mm MgCl2, 0.5 mm DTT, 0.025% NP-40, and 0.01% bovine serum albumin was used for binding assays between GST–Ski and mSin3A, N-CoR, or SMRT.
Coimmunoprecipitation
The plasmid to express various forms of c-Ski was made by the PCR-based method using the vector containing the chicken cytoplasmic β-actin promoter. The human N-CoR cDNA was isolated by the PCR-based method, and the plasmid encoding wild-type or mutant N-CoR, which was linked to two tandem repeats of the Flag tag at their carboxyl termini, was constructed using the pcDNA3 vector (Invitrogen). A mixture of 4 μg of the wild-type or mutant c-Ski expression plasmid, pact–c-Ski, and 6 μg of the Flag-linked N-CoR expression plasmid, pCMV–N-CoR–Flag, was transfected into 293T cells. Forty hours after transfection, cells were lysed in the lysis buffer (50 mm HEPES at pH 7.5, 250 mm NaCl, 0.2 mm EDTA, 10 μm NaF, 0.5% NP-40), and whole-cell lysates were prepared. Lysates were immunoprecipitated using anti-Flag antibodies (KODAK), and the immune complex was analyzed by Western blotting using the anti-Ski monoclonal antibodies, which were made by using the bacterially made full-length human c-Ski as an antigen, and ECL detection reagents (Amersham). In coimmunoprecipitation assay with endogenous proteins, 293 cells were lysed by mild sonication in IP buffer (1× PBS, 10% glycerol, 0.1% NP-40), and immunoprecipitation was performed using the anti-Ski monoclonal antibodies. The anti-N-CoR antibody raised against SBD, and the anti-Sin3A and anti-HDAC1 antibodies (Santa Cruz) were used for Western blotting.
HDAC assay
Assays for HDAC activity were performed essentially as described (Yoshida et al. 1990). Lysates were prepared from 293 cells transfected with 2 μg of the c-Ski or HDAC1 expression plasmid by lysis in NET-N buffer (20 mm Tris-HCl at pH 8.0, 150 mm NaCl, 1 mm EDTA, 0.5% NP-40), and immunoprecipitated with anti-Ski, anti-HDAC1 antibodies, or normal IgG. Immunocomplexes were incubated for 5 hr at 37°C with ∼10 μg of acid-soluble 3H-labeled histones. The pcDNA3 vector (Invitrogen) was used for the HDAC1 expression plasmid.
Analysis of repressor domains in c-Ski
The plasmids to express Gal4–Ski fusion proteins, which consist of the Gal4 DNA-binding domain (amino acids 1–147) and various portions of c-Ski, were constructed by the PCR-based method using the CMV promoter-containing vector. To analyze the repressor domain of c-Ski in the experiments of Figure 3A, a mixture of 3 μg of the luciferase reporter, in which six tandem repeats of the Gal4-binding site were linked to the TK promoter, 0.33 μg of the Gal4–Ski or Gal4 expression plasmid, and 1 μg of the internal control plasmid pRL-TK (Promega), in which the sea-pansey luciferase gene is linked to the TK promoter, was transfected into CV-1 cells. The luciferase assays were performed using the dual-luciferase assay system (Promega). An average of two experiments ±s.e.m. is shown as a result. To examine the enhancement of Gal–Ski-induced transcriptional repression by N-CoR in the experiments of Figure 3B, a mixture of 3 μg of the Gal4 sites-containing luciferase reporter, 0.02 μg of the Gal4–Ski or Gal4 expression plasmid, 8 μg of the wild-type or amino-truncated mutant N-CoR expression plasmid, and 1 μg of pRL-TK was transfected into CV-1 cells. The amino-truncated form of N-CoR lacked the amino-terminal 1502 amino acids containing the repressor domains. To examine the effect of SBD on the Gal4–Ski-mediated transcriptional repression in the experiments of Figure 3C, a mixture of 3 μg of the Gal4 sites-containing reporter, 0.02 μg of the Gal4–Ski expression plasmid, 4 or 8 μg of the SBD expression plasmid, and 1 μg of pRL-TK was transfected. The pCMV/myc/nuc vector (Invitrogen) was used to construct the plasmid to express SBD linked to the nuclear localization signal of the SV40 large T antigen.
Subcellular localization of Ski and N-CoR
Subcellular localization of Ski and N-CoR was essentially examined as described (Nakagoshi et al. 1993). A mixture of 3.5 μg of the N-CoR–Flag expression plasmid and 3 μg of the plasmid encoding various forms of c-Ski was transfected into 293T cells. Forty hours after transfection, cells were fixed, and stained with anti-Ski monoclonal antibodies and the anti-Flag rabbit polyclonal antibody (Santa Cruz). The Ski and N-CoR signals were visualized by rhodamine- and FITC-conjugated secondary antibodies, respectively, and analyzed by confocal microscopy.
Single-cell microinjection assay
The rabbit polyclonal antibodies were prepared using the bacterially made full-length c-Ski and Sno as antigens. The antibodies were purified using the antigen column. Single-cell microinjection assays were performed essentially as described by Heinzel et al. (1997) except for the use of the GFP–vector plasmid as a marker. Rat-1 fibroblasts were seeded on glass coverslips at subconfluent density and grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. Before the injection, the cells were rendered quiescent by incubation in serum-free medium for 24 hr. A mixture of the lacZ reporter plasmid, in which three tandem repeats of the Gal4-binding site were linked to the TK promoter, the Gal4–Mad or Gal4–TRβ expression plasmid described above, and appropriate antibody (control IgG, anti-Ski, or anti-Sno antibodies) were injected into the nuclei of cells. The concentration of DNA and antibody was 100 μg/ml and 1 mg/ml, respectively. Microinjection was done using an Eppendorf semiautomated microinjection system mounted on an inverted Zeiss microscope. The GFP-vector plasmid was coinjected for the identification of the injected cells. After overnight incubation, the cells were fixed with 4% formaldehyde, permeabilized with PBS containing 0.1% Triton X-100 for 5 min on ice, washed three times with PBS, and then stained with FITC-conjugated secondary antibodies to detect injected IgG and β-galactosidase expression using the β-gal staining kit (Boehringer). GFP-vector cotransfected cells could be visualized by green fluorescence of the protein. Cells were viewed and the results were analyzed under a Zeiss microscope. All cells showing any trace of blue staining were scored as positive for expression.
Effect of Ski on the Mad- or TRβ-induced transcriptional repression
The v-Ski expression plasmid was constructed by replacing the 1.2-kb SacI fragment of pactSkiΔ493–728 by the SacI fragment encoding the chicken v-Ski. The plasmid used to express the Gal4–Mad containing the mSin3-binding domain (amino acids 1–35) of Mad (Ayer et al. 1996) or Gal4–TRβ containing the ligand-biding domain (amino acids 173–456) of the human TRβ (Chen and Evans 1995), or Gal4–δEF1NR containing the NR repressor domain of δEF1 (Sekido et al. 1997) has already been described. A mixture of 3 μg of the Gal site-containing luciferase reporter described above, 0.02 μg of the Gal4–Mad, Gal4–TRβ, Gal4–δEF1, or Gal4 expression plasmid, and 4 or 8 μg of the plasmid to express various forms of Ski, and 1 μg of internal control plasmid pRL-TK was transfected into CV-1 cells as described. The total amount of plasmid DNA was adjusted to 13 μg by addition of the control plasmid DNA lacking the cDNA. The luciferase assays were performed as described above. An average of two experiments ±s.e.m. is shown as a result.
Analysis of c-ski-deficient mouse embryos
Embryos generated by mating between the c-ski-deficient heterozygotes were prepared at 9.5 days postcoitum (dpc), and paraffin-embedded tissue sections were prepared. Sections were permeabilized with proteinase K after treatment with xylene and ethanol, and incubated in TUNEL assay mix (Boehringer-Mannheim) prepared according to the manufacturer’s instructions. ODC immunostaining and in situ hybridization were performed as described (Koibuchi et al. 1993; Rex et al. 1997) using the polyclonal antibody raised against highly purified ODC from mouse kidney and EcoRI–HindIII 0.9-kb digoxygenin-labeled mouse ODC RNA, respectively. The mice were maintained by the Division of Experimental Animal Research, RIKEN.
Acknowledgments
We are grateful to T. Nagase and N. Nomura for earlier contributions to this work; S. Hollenberg for the yeast two-hybrid system; R. Eisenman and D. Ayer for the mSin3A and Gal4-Mad cDNAs; K. Umesosno and R. Evans for the Gal4–TRβ and SMRT cDNAs; D. Rose and M. Rosenfeld for the TK–lacZ reporter containing the Gal4 sites; S.L. Schreiber for the HDAC1 cDNA; S. Matsufuji for the anti-ODC antibody; K. Igarashi for the mouse ODC cDNA; H. Kondoh for the Gal4–δEF1 construct; M. Harbers for assistance for Ski–mSin3 interaction assay; H. Akimaru for assistance with confocal microscopy; and T. Yamamoto for encouragement. This work was supported in part by National Institutes of Health grant HD 30728 to C.C.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL sishii@rtc.riken.go.jp; FAX 81-298-36-9030.
References
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Screiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- Arnold HH, Gerhartz CD, Gabbert HE, Salminen A. Retinoic acid induces myogenin synthesis and myogenic differentiation in the rat rhabdmyosarcoma cell line BA-Han1c. J Cell Biol. 1992;118:877–887. doi: 10.1083/jcb.118.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer DE, Eisenman RN. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes & Dev. 1993;7:2110–2119. doi: 10.1101/gad.7.11.2110. [DOI] [PubMed] [Google Scholar]
- Ayer DE, Kretzner L, Eisenman RN. Mad: A heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993;72:211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]
- Ayer DE, Laherty CD, Lawrence QA, Armstrong AP, Eisenman RN. Mad proteins contain a dominant transcription repression domain. Mol Cell Biol. 1996;16:5772–5781. doi: 10.1128/mcb.16.10.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A, Leng X, Burris TP, Tsai SY, Tsai M-J, O’Malley BW. The t4 activation domain of the thyroid hormone receptor is required for release of a putative corepressors necessary for transcriptional silencing. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Ferdenandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci. 1993;90:7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial patterning, and skeletal muscle development. Genes & Dev. 1997;11:2029–2039. doi: 10.1101/gad.11.16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: Molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM. A transcriptional corepressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- Chin L, Schreiber-Agus N, Pellicer I, Chen K, Lee H-W, Dudast M, Cordon-Cardo C, Depinho RA. Contrasting roles for Myc and Mad proteins in cellular growth and differentiation. Proc Natl Acad Sci. 1995;92:8488–8492. doi: 10.1073/pnas.92.18.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Nicol R, Stavnezer E. A domain necessary for the transforming activity of SnoN is required for specific DNA binding, transcriptional repression and interaction. Oncogene. 1998;17:2505–2531. doi: 10.1038/sj.onc.1202177. [DOI] [PubMed] [Google Scholar]
- Colmenares C, Stavnezer E. The ski oncogene induces muscle differentiation in quail embryo cells. Cell. 1989;59:293–303. doi: 10.1016/0092-8674(89)90291-2. [DOI] [PubMed] [Google Scholar]
- Colmenares C, Sutrave P, Hughes SH, Stavnezer E. Activation of the c-ski oncogene by overexpression. J Virol. 1991a;65:4929–4935. doi: 10.1128/jvi.65.9.4929-4935.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares C, Teumer JK, Stavnezer E. Transformation-defective v-ski induces myoD and myogenin expression but not myotube formation. Mol Cell Biol. 1991b;11:1167–1170. doi: 10.1128/mcb.11.2.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, Kieslinger M, Beug H, Hayman MJ. Transformation of hematopoietic cells by the Ski oncoprotein involves repression of retonoic acid receptor signaling. Proc Natl Acad Sci. 1998a;95:11187–11192. doi: 10.1073/pnas.95.19.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, Wani B, Hayman MJ. The Ski oncoprotein interacts with Skip, the human homolog of Drosophila Bx42. Oncogene. 1998b;16:1579–1585. doi: 10.1038/sj.onc.1201687. [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Hou D-X, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional co-activator of c-Myb. Genes & Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- Doucas V, Evans RM. The PML nuclear compartment and cancer. Biochim Biophys Acta. 1996;1288:25–29. doi: 10.1016/s0304-419x(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- Hassing CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen T-M, Söderström M, Laherty CD, Torchia J, Yang W-M, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- Hermerking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK, Rosenfeld MG. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor corepressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Matsuzaki S, Ma H-T, Sakai M, Yamaoka S. Induction of ornithine decarboxylase immunoreactivity in the male mouse kidney following teststerone treatment: An axial heterogeneity in the proximal tubule. J Endocrinol. 1993;136:85–89. doi: 10.1677/joe.0.1360085. [DOI] [PubMed] [Google Scholar]
- Laherty CD, Yang W-M, Sun J-M, Davie JR, Seto E, Eisenman RN. Histone deacetylase associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Laherty CD, Billin AN, Lavinsky RM, Yochum GS, Bush AC, Sun J-M, Mullen T-M, Davie JR, Rose DW, Glass CK, Rosenfeld MG, Ayer DE, Eisenman RN. SAP30, a component of the mSin3 corepressor complex involved in N-CoR-mediated repression by specific transcription factors. Mol Cell. 1998;2:33–42. doi: 10.1016/s1097-2765(00)80111-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Turck CM, Teumer JK, Stavnezer E. Unique sequence, ski, in Sloan-Kettering avian retroviruses with properties of a new cell-derived oncogene. J Virol. 1986;57:1065–1072. doi: 10.1128/jvi.57.3.1065-1072.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat GE, Downes M, Dowhan DH. Regulation of vertebrate muscle differentiation by thyroid hormone: the role of the myoD gene family. BioEssays. 1995;17:211–218. doi: 10.1002/bies.950170307. [DOI] [PubMed] [Google Scholar]
- Nagase T, Mizuguchi G, Nomura N, Ishizaki R, Ueno Y, Ishii S. Requirement of protein co-factor for the DNA-binding function of the human ski proto-oncogene product. Nucleic Acids Res. 1990;18:337–343. doi: 10.1093/nar/18.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Nomura N, Ishii S. Complex formation between proteins encoded by the ski gene family. J Biol Chem. 1993;268:13710–13716. [PubMed] [Google Scholar]
- Nagy L, Kao H-Y, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Nakagoshi H, Takemoto Y, Ishii S. Functional domains of the human B-myb gene product. J Biol Chem. 1993;268:14161–14167. [PubMed] [Google Scholar]
- Nicol R, Stavnezer E. Transcriptional repression by v-Ski and c-Ski mediated by a specific DNA binding site. J Biol Chem. 1998;273:3588–3597. doi: 10.1074/jbc.273.6.3588. [DOI] [PubMed] [Google Scholar]
- Nomura N, Sasamoto S, Ishii S, Date T, Matsui M, Ishizaki R. Isolation of human cDNA clones of ski and ski-related gene sno. Nucleic Acids Res. 1989;17:5489–5500. doi: 10.1093/nar/17.14.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham G, Cleveland JL. Ornithine decarboxylase is a mediator of c-myc-induced apoptosis. Mol Cell Biol. 1994;14:5741–5747. doi: 10.1128/mcb.14.9.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M, Uwanogho DA, Orme A, Scotting PJ, Sharpe PT. cSox21 exhibits a complex and dynamic pattern of transcription during embryonic development of the chick central nervous system. Mech Dev. 1997;66:39–53. doi: 10.1016/s0925-4773(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Rosenwald IB, Rhoads DB, Callnan LD, Isselbacher KJ, Schmidt EV. Increased expression of eukaryotic translation initiation factors eIF-4E and eIF-2a in response to growth induction by c-myc. Proc Natl Acad Sci. 1993;90:6175–6178. doi: 10.1073/pnas.90.13.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel MF, Ashmun RA, Sher CJ, Eisenman RN, Ayer DE. Inhibition of cell proliferation by the Mad1 transcriptional repressor. Mol Cell Biol. 1996;16:2796–2801. doi: 10.1128/mcb.16.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber-Agus N, Meng Y, Hoang T, Hou H, Jr, Chen K, Greenberg R, Cordon-Cardo C, Lee H-W, DePinho RA. Role of Mxi1 in aging organ systems and the regulation of normal and neoplastic growth. Nature. 1998;393:483–487. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]
- Sekido R, Murai K, Kamachi Y, Kondoh H. Two mechanisms in the action of repressor δEF1: Binding site competition with an activator and active repression. Genes Cells. 1997;2:771–783. doi: 10.1046/j.1365-2443.1997.1570355.x. [DOI] [PubMed] [Google Scholar]
- Söderström M, Vo A, Heinzel T, Lavinisky RM, Yang W-M, Seto E, Peterson DA, Rosenfeld MG, Glass CK. Differential effects of nuclear receptor corepressor (N-CoR) expression levels on retinoic acid receptor-mediated repression support the existence of dynamically regulated corepressor complexes. Mol Endocrinol. 1997;11:682–692. doi: 10.1210/mend.11.6.0018. [DOI] [PubMed] [Google Scholar]
- Stavnezer E, Brodeur D, Brennan LA. The v-ski oncogene encodes a truncated set of c-ski coding exons with limited sequence and structural relatedness to v-myc. Mol Cell Biol. 1989;9:4038–4045. doi: 10.1128/mcb.9.9.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrave P, Kelly AM, Hughes SH. Ski can cause selective growth of skeletal muscle in transgenic mice. Genes & Dev. 1990;4:1462–1472. doi: 10.1101/gad.4.9.1462. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wieland C, Mann S, von Besser H, Saumweber H. The Drosophila nuclear protein Bx42, which is found in many puffs on polytene chromosomes, is highly charged. Chromosoma. 1992;101:517–525. doi: 10.1007/BF00352475. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Cell. 1997;89:357–364. doi: 10.1016/s1097-2765(00)80102-1. [DOI] [PubMed] [Google Scholar]
- Zheng G, Teumer J, Colmenares C, Richmond C, Stavnezer E. Identification of a core functional and structural domain of the v-Ski oncoprotein responsible for both transformation and myogenesis. Oncogene. 1997;15:459–471. doi: 10.1038/sj.onc.1201205. [DOI] [PubMed] [Google Scholar]