Abstract
Macrophage colony-stimulating factor (M-CSF) promotes macrophage differentiation, increases susceptibility of macrophages to viral infection, and enhances human immunodeficiency virus (HIV) replication in infected macrophages. Given the current model of HIV neuropathogenesis, which involves monocyte trafficking into the central nervous system, immune factors linked with macrophage maturation and survival may be associated with cognitive decline (measured by neuropsychological z-score [NPZ-8] or Memorial Sloan-Kettering [MSK] score) and alterations in a marker of neuronal integrity, N-acetylaspartate (NAA). Fifty-four chronically infected HIV+ subjects underwent neuropsychological assessment, magnetic resonance spectroscopic imaging, and quantification of M-CSF in plasma and cerebrospinal fluid (CSF) at baseline. Thirty-nine of those subjects underwent further examination at 3 and 10 months after initiation of combination antiretroviral therapy (ART) regimens. Within 3 months of therapy use, CSF M-CSF and viral RNA levels were reduced, whereas NAA concentrations in many brain regions were increased. Neither baseline levels nor the change in M-CSF levels had the ability to predict changes in NAA levels observed after 10 months of combination ART use. At study entry those with the lowest M-CSF levels in the CSF had the least cognitive impairment (NPZ-8). Those who had higher baseline CSF MCSF levels and exhibited larger decreases in M-CSF after therapy, tended to have greater cognitive improvement after 10 months. Increased prevalence of M-CSF in the setting of HIV infection could contribute to neuronal injury and may be predictive of cognitive impairment.
Keywords: HAD, M-CSF, MRS, MSK, NAA, NPZ-8
Introduction
Magnetic resonance spectroscopic imaging (MRSI) allows for noninvasive, in vivo measurement of brain metabolites across many regions at once, which has proved useful in the assessment of neuronal integrity and spatial heterogeneity of several neurodegenerative diseases, including human immunodeficiency virus (HIV)-associated dementia (HAD) (Mohamed et al, 2010; Sacktor et al, 2005; Yiannoutsos et al, 2004). N-acetylaspartate (NAA) is a major metabolite measured with MRSI. NAA is generally considered to be a surrogate marker of neuronal integrity, as it is synthesized in neuronal mitochondria and predominately exists within neurons and axons in the adult brain (Barker, 2001; Moffett et al, 2007). It is speculated that NAA is a neuronal osmolyte, is coupled to energy production, and is involved in lipid, myelin, and N-acetylaspartylglutamate synthesis. NAA/creatine (NAA/Cr) ratios determined by MRS can distinguish patients with and without HAD (Chang et al, 2002, 2004b; Sacktor et al, 2005), and have been used to examine the beneficial effects of antiretroviral therapies (ARTs) in patients with HAD and acquired immunodeficiency syndrome (AIDS) (Salvan et al, 1997; Stankoff et al, 2001). However, reports are inconsistent with respect to the relationship of plasma or cerebrospinal fluid (CSF) viral levels to regional levels of NAA (Chang et al, 1999, 2002, 2003), possibly due to the influence of antiretroviral therapies, necessitating the search for better immunological correlates of neuronal injury in the context of HIV-associated cognitive impairment.
Current theories suggest that infected monocyte-derived macrophages from the periphery permeate the blood-brain barrier (BBB), bringing the virus into the brain (Buckner et al, 2006), resulting in viral expansion, infection of resident microglia, neurotoxicity, and eventually cognitive impairment (Kaul et al, 2005). Indeed, both simian immunodeficiency virus (SIV) and HIV studies indicate the best correlate of neuronal injury or HAD is the quantity of infected perivascular macrophages and microglia within brain tissue (Glass et al, 1995; Williams et al, 2005). However, activation of macrophages and microglia within the brain is not easily assessed using noninvasive methods such as imaging. Examination of cytokines involved in the activation of these cells, such as macrophage colony-stimulating factor (M-CSF), may provide insight into changes in cognition and brain metabolism induced by HIV.
M-CSF is expressed by many cell types, but its receptor resides on macrophages, microglia, and osteoclasts. M-CSF inhibits apoptotic pathways of monocytes and promotes their preferential differentiation into long-lived macrophages (Stanley et al, 1978). Beyond promoting macrophage maturation, M-CSF greatly increases HIV-1 viral production within macrophages (a role that may be unique to HIV) and the expression of cell surface receptors, such as CD4, CCR5, and αvβ3, allowing for improved infection efficiency (Haine et al, 2006). HIV-infected macrophages produce M-CSF, resulting in the activation of more microglia and monocytes that could become infected (Canque et al, 1996; Kutza et al, 2000; Si et al, 2002). Those and many other events ultimately contribute to neuronal dysfunction and apoptosis if not prevented with the use of ART (Kaul et al, 2005). With all of these potentially deleterious roles, it is important to evaluate the relationship of M-CSF with respect to neuronal function and HIV-associated cognitive impairment. Accordingly, the purpose of this study was to explore the relationship of M-CSF to both NAA levels and cognitive measures (neuropsychological z-score [NPZ-8] and Memorial Sloan-Kettering [MSK] score), and to determine if the use of combination antiretroviral therapies disrupts these relationships.
Results
Differences in immunologic and virologic assays
As shown in Table 1, plasma viral load and CD4+ lymphocyte levels were found to be similar between subjects with and without dementia, and a high percentage in both groups had AIDS. Viral RNA in the CSF was detectable in all subjects, indicating a lack of viral control in the CNS and M-CSF concentrations of the CSF were elevated in those with dementia (P < .02). The 39 subjects who underwent further examination responded positively to their combination ART regimens (Table 2). Increased plasma CD4+ T lymphocytes between baseline measures and those after 3 (t test: P = .003) and 10 (t test: P = .01) months of therapy were observed. Similarly, after 3 months of therapy, viral RNA levels in both the plasma and CSF were reduced (P < 3 × 10−8, P < 5 × 10−6, respectively) and remained lower than baseline after 10 months (P = .0001, P = .01, respectively). However, plasma viral loads rebounded slightly between 3 and 10 months (P < .04), indicating that therapy was not completely effective at controlling viral production (Table 2). M-CSF levels in the CSF were reduced within 3 months of therapy (P < .007) but not so in plasma. Plasma and CSF concentrations of M-CSF were significantly decreased after 10 months of therapy (P = .008, P < .02, respectively).
Table 1.
Cohort demographics and baseline characteristicsa
| HAD− (N = 30) | HAD+ (N = 24) | P valueb | |
|---|---|---|---|
| Gender | |||
| Male | 22 (73%) | 15 (63%) | .39 |
| Female | 8 (27%) | 9 (38%) | |
| Race | |||
| Black | 25 (83%) | 24 (100%) | <.04 |
| White | 5 (17%) | ||
| Age (years) | 40.2 ± 5.9 | 41.4 ± 7.6 | .70 |
| Education (years) | 12.8 ± 2.3 | 11.6 ± 1.5 | .02 |
| MSK | 0.42 ± 0.19 | 1.33 ± 0.48 | <10−10 |
| NPZ-8 (z scores) | −0.79 ± 0.70 | −2.37 ± 0.94 | <10−7 |
| Mode of infection | |||
| IV-drug use | 6 (20%) | 9 (38%) | .18 |
| Sexual contact | 19 (63%) | 8 (33%) | |
| Sexual contact/IV-drug use | 1 (3%) | 2 (8%) | |
| Transfusion | 1 (3%) | 0 (0%) | |
| Unknown | 3 (10%) | 5 (21%) | |
| CD4+ T cells (cells/µl) | 179 ± 166 | 162 ± 136 | .77 |
| AIDS (CD4 <200) | |||
| Yes | 19 (68%) | 15 (65%) | .84 |
| No | 9 (32%) | 8 (35%) | |
| Viral load (log10 copies/ml) | |||
| Plasma | 4.86 ± 0.55 | 4.82 ± 0.71 | .96 |
| CSF | 3.69 ± 0.92 | 3.22 ± 0.82 | <.07 |
| M-CSF (ng/ml) | |||
| Plasma | 2.02 ± 1.78 | 2.08 ± 1.02 | .17 |
| CSF | 1.20 ± 1.43 | 2.05 ± 1.66 | <.02 |
| Therapy regimenc | |||
| At least 2 NRTIs | |||
| Yes | 20 (95%) | 18 (100%) | .75 |
| No | 1 (5%) | ||
| At least 1 protease inhibitor | |||
| Yes | 9 (43%) | 7 (39%) | .80 |
| No | 12 (57%) | 11 (61%) | |
| Highly active ART | |||
| Yes | 20 (95%) | 18 (100%) | .35 |
| No | 1 (5%) |
Values represent mean ± SD or N (%).
All categorical variables were compared between dementia groups using a Pearson chi-squared test. All continuous variables were compared between dementia groups using a nonparametric Wilcoxon rank sum test.
Description of therapy regimens initiated by the 39 subjects followed for 10 months.
Table 2.
Cognitive and immunologic measures pre- and post-therapya
| Number of subjects | Baseline | 3 months | 10 months | P valueb | |
|---|---|---|---|---|---|
| MSK | 39/39 | 0.81 ± 0.56 | 0.79 ± 0.57 | 0.73 ± 0.55 | .59 |
| NPZ-8 | 32/39 | −1.63 ± 1.18 | −1.74 ± 1.24 | −1.38 ± 0.97 | <.08 |
| CD4+ T cells | 35/39 | 184 ± 164 | 265 ± 220* | 256 ± 270* | .007 |
| Log10 viral RNA | |||||
| Plasma | 26/39 | 4.74 ± 0.61 | 3.01 ± 0.83* | 3.64 ± 1.29* | <10−6 |
| CSF | 26/39 | 3.30 ± 0.82 | 2.40 ± 0.29* | 2.66 ± 0.82* | .00005 |
| M-CSF (ng/ml) | |||||
| Plasma | 23/39 | 2.22 ± 1.47 | 3.01 ± 2.76 | 1.21 ± 0.89* | <.002 |
| CSF | 23/39 | 1.82 ± 1.88 | 0.73 ± 0.46* | 0.73 ± 0.89* | .002 |
Means ± SD for the 39 subjects who underwent repeat testing after initiation of combination ART.
RM ANOVA measures performed on data across all three time points.
Significant changes (P ≤ .01) occurred between baseline measures and later time points.
Neuronal metabolism measured by MRSI
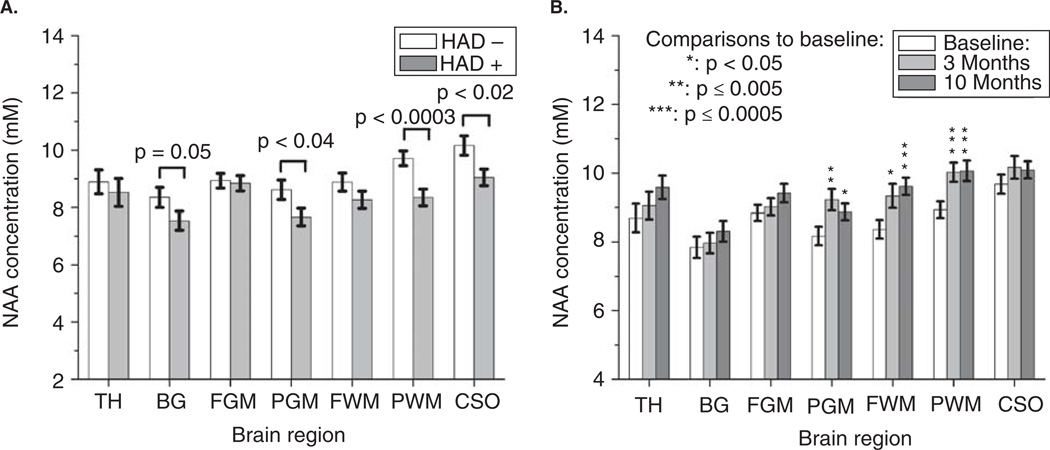
NAA concentrations were measured within seven brain regions (Figures 1 and 2A) in all 54 subjects at study entry. As expected, NAA levels were typically lower in subjects with dementia (Figure 2A), but only significantly so within the centrum semiovale (CSO; P < .02), basal ganglia (BG; P = .05), parietal gray matter (PGM; P < .04), and parietal white matter (PWM; P < .0003). The 39 subjects who were followed after use of combination antiretroviral therapies had increased NAA concentrations in frontal white matter (FWM), PGM, and PWM (repeated measure analysis of variance [RM ANOVA]: P = .001, P = .004, P = .0002, respectively) indicating improved neuroaxonal metabolism within these regions. Matched pairs t test indicated NAA levels were elevated in these regions after 3 months of therapy use and remained so (Figure 2B).
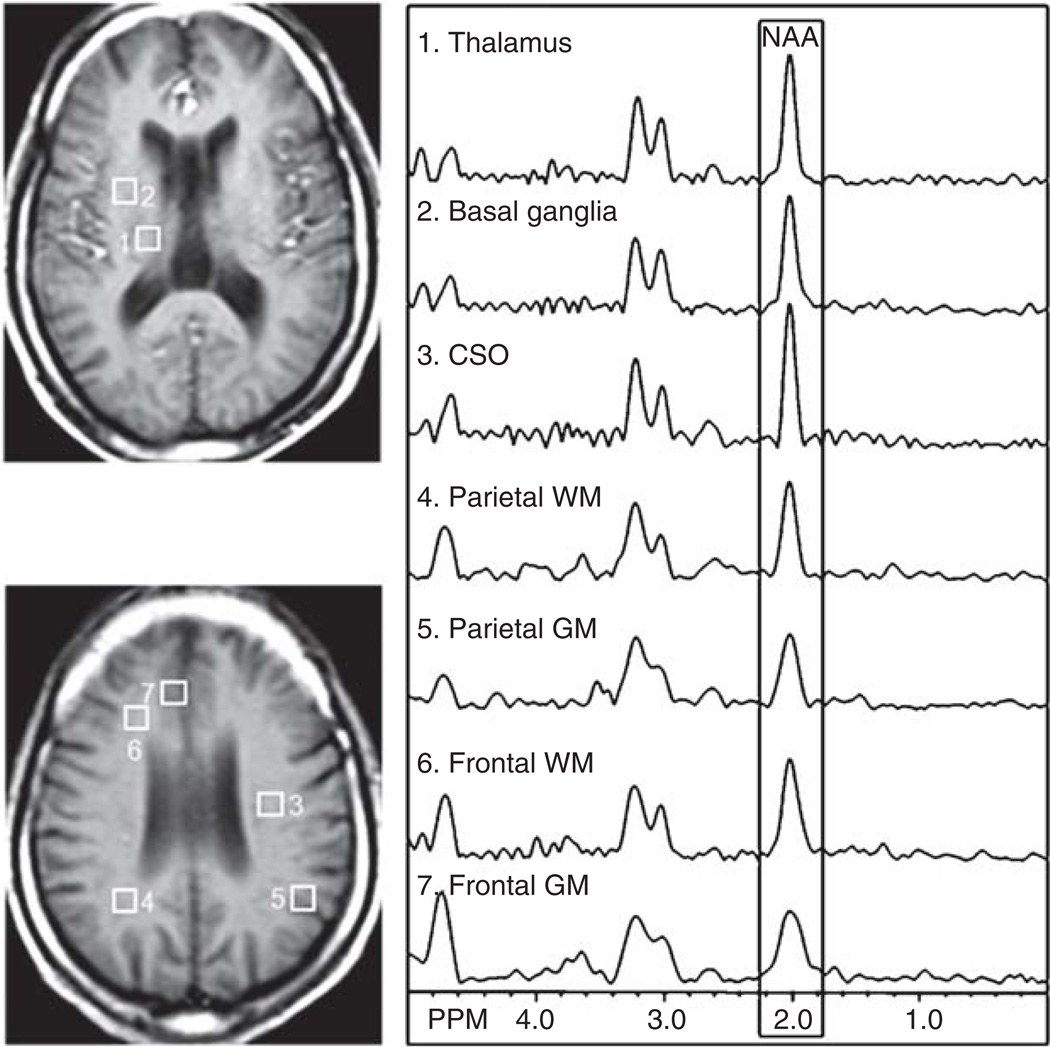
Figure 1.
T1-weighted images of a chronically HIV-infected male with dementia (MSK = 2) indicate voxel placement used in this study. NAA metabolite concentrations were evaluated within the following regions: basal ganglia (BG), thalamus (TH), frontal gray matter (FGM), frontal white matter (FWM), centrum semiovale (CSO), and both parietal gray (PGM) and white matter (PWM).
Figure 2.
(A) Average NAA concentrations as measured by MRSI at study entry in subjects with HIV-associated dementia (N = 24) and those without (N = 30). Reported P values represent the results from nonparametric Wilcoxon rank sum test performed between the two cohorts for each region. (B) Average NAA concentrations in all seven brain regions of the 39 subjects who underwent repeat imaging at 3 and 10 months after initiating combination ART. RM ANOVA performed indicated that NAA levels recover with therapy, with significant increases seen in the parietal gray matter, frontal white matter, and parietal white matter (P = .004, P = .001, P = .0002, respectively). All error bars represent standard error of the mean.
Relationship between cognition and M-CSF
MSK scores, which were used initially to group dementia cohorts, did not change in this cohort over 10 months (Table 2). However, there was a trend for improved NPZ-8 scores after 10 months of therapy (RM ANOVA: P < .08), specifically between 3 and 10 months (t test: P = .03). Examining cognitive scores in the 39 subjects at study entry, those with lower M-CSF concentrations in the CSF were less likely to have cognitive impairment (NPZ-8: R = −.44, P = .005; MSK: P < .003, logistic regression). Subjects who had improved NPZ-8 scores after 10 months of combination ART use tended to exhibit larger decreases in CSF M-CSF concentrations (R = −.44, P = .08). This may help explain why baseline levels of M-CSF in the CSF had a tendency toward predicting cognitive improvement or outcome (MSK improvement: P = .04; NPZ-8 improvement: R = .31, P < .07; NPZ-8 outcome: R = −.31, P < .07). Concentrations of M-CSF in the plasma appeared to have no significant relationship to cognitive measures at any time point, or be able to predict cognitive outcome.
Relationship between neuronal metabolism and M-CSF
Spearman rank correlations were performed to examine the relationship between NAA and M-CSF concentrations in both the blood and the CSF (Table 3). At baseline, higher M-CSF concentrations were associated with lower NAA concentrations, the strongest of which were in the subcortical gray matter and white matter areas. Most interestingly, M-CSF measured in both the peripheral and CSF compartments had a similar relationship to NAA concentrations. After initiation of combination ART regimens, the correlation disappeared slowly over time in the CSF and weakened in the plasma (Table 3). Although it is suspected that antiretroviral therapies change the relationship between NAA and M-CSF over time, there is a significant cohort drop-out that may have contributed. Changes in M-CSF were not associated to changes in NAA between time points. Neither the initial concentrations nor the change in M-CSF concentration were able to predict improved neuroaxonal metabolism.
Table 3.
Correlations between M-CSF and in vivo NAA concentrations in various brain regions over 10 months of antiretroviral therapya
| M-CSF levels in blood | M-CSF levels in CSF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (N = 53) |
3 months (N = 39)b |
10 months (N = 34)b |
Baseline (N = 54) |
3 months (N = 36)b |
10 months (N = 30)b |
|||||||
| Brain regions | RS | P value | RS | P value | RS | P value | RS | P value | RS | P value | RS | P value |
| Basal ganglia | −.48 | <.0003 | −.52 | .0006 | −.31 | .08 | −.53 | <.00004 | −.51 | .001 | — | — |
| Thalamus | −.45 | <.0008 | −.39 | .01 | −.26 | .1 | −.52 | <.00006 | −.36 | .03 | — | — |
| Centrum semiovale | −.35 | .01 | — | — | −.37 | .03 | −.47 | .0003 | — | — | — | — |
| Parietal GM | −.29 | .03 | −.42 | <.008 | −.43 | .01 | −.38 | .005 | −.53 | <.001 | — | — |
| Parietal WM | — | — | — | — | — | — | −.34 | .01 | — | — | — | — |
| Frontal WM | — | — | — | — | — | — | −.32 | <.02 | −.25 | .1 | — | — |
| Frontal GM | −.41 | .002 | — | — | — | — | — | — | — | — | — | — |
Subjects imaged at study entry and 3 and 10 months after new therapy initiation. Only P values < .10 are shown to provide clarity.
N indicates number of subjects from the 39 who underwent repeat imaging who had M-CSF data available to perform correlations.
Discussion
The primary findings of this study indicate (1) M-CSF concentrations in the CSF have a relationship to cognitive impairment that is predictive of cognitive improvement (via MSK with trends for NPZ-8) in this small cohort; (2) at baseline, higher M-CSF levels were associated with decreased neuronal metabolism (lower NAA levels); a relationship that may be disrupted with the use of antiretroviral therapies; (3) neither baseline M-CSF levels nor the changes in M-CSF between time points were predictive of increases in NAA observed over the duration of the study; and (4) in nearly all subjects, increased NAA levels in many regions were observed within three months of initiating ART regimens.
Previous studies have observed the ability of M-CSF levels in the CSF to distinguish between dementia cohorts, as observed in this analysis (Gallo et al, 1990; McArthur et al, 2004). Moreover, this study indicated that M-CSF in the CSF demonstrated a direct relationship to composite neuropsychological scores (NPZ-8 at baseline), indicating those with higher levels had more cognitive impairment. Subjects who had improved NPZ-8 scores after 10 months of combination ART use tended to exhibit larger decreases in CSF M-CSF concentrations. Beyond the descriptive relationship, M-CSF concentrations in the CSF measured before therapy were capable of predicting improved MSK scores (with trends for NPZ-8) after 10 months of combination ART use. These results suggest CSF M-CSF levels (in the context of detectable viral levels) may be a useful predictor of active central nervous system CNS disease and the cognitive dysfunction caused by it. M-CSF levels may allow for a better assessment of those requiring ART medications with increased CNS penetration or specific efficacy against infected macrophages and microglia.
In contrast to the relationship found with cognition, baseline levels of M-CSF in both the CSF and blood were inversely correlated (using Spearman rank correlation) with NAA concentrations in many regions of the brain. It was interesting to discover a biologic marker associated with neuroaxonal metabolism in so many regions of the brain. MR studies to date have attempted to find such correlations with viral RNA, monocyte chemoattractant protein-1, CD4+ cell counts, and other molecules with varying degrees of success (Chang et al, 2002, 2003, 2004a). Notably, the strongest relationships at baseline and over time were observed within the subcortical regions within this study. It is possible that the stronger association in the basal ganglia and thalamus may be due to higher numbers of activated microglia in these areas (Kure et al, 1990). However, the spectra as shown in Figure 1 may also suggest that a lack of MR field homogeneity could have weakened the association in cortical regions, especially those near the air-tissue interfaces, such as the frontal cortex (Kreis, 2004). Regardless of this correlation observed at baseline, the relationship of CSF M-CSF to neuronal injury as measured by NAA was lost over time, indicating ART use may disrupt this association although subject drop-out may have contributed. Further analyses indicated that the changes in M-CSF and NAA between time points were not associated to one another, nor was M-CSF capable of predicting increases in NAA levels after 10 months of combination ART use.
Many MRS and MRSI studies have been performed on patients with HAD examining the neuronal integrity marker NAA, typically reported as a ratio to creatine levels in the same region rather than as an absolute concentration (Chang et al, 2004b; Sacktor et al, 2005). Speculation as to the changing nature of creatine levels in the brain due to HIV makes studies that can quantify absolute concentrations of NAA particularly relevant. Results in the present report indicate that NAA concentrations improved in PGM, PWM, and FWM regions of nearly all subjects and did so within the first 3 months of therapy, even though a cognitive response (NPZ-8) was not observed until months later. NAA may be a highly sensitive marker of neuronal dysfunction in the setting of unrestricted viral replication: one that indicates neuronal repair/recovery (e.g., reduced oxidative stress on mitochondria or remyelination in white matter regions) if therapy is initiated sufficiently early.
A limitation of this study includes a lack of history detailing drug abuse over the duration of the study, which could represent a confounding variable to these data and their interpretations. Also, the present study was not performed with respect to a specific antiretroviral therapy regimen and is representative of the many possible combinations of antiretroviral drugs currently used in treating HIV infection. The lack of consistency in regimens made further analysis or conclusions impossible due to the small sample sizes that had similar regimens. Future longitudinal studies should address the issue of inconsistent regimens since specific drugs such as zidovudine (AZT) may be more adept at reducing both the levels of M-CSF and viral production produced by macrophages (Kutza et al, 2000). The ability of AZT to control those events may provide some explanation as to the rapid decline in severe dementia observed in the mid to late 1990s. Interestingly, M-CSF antagonists have been proposed as a potential mutation-resistant therapeutic target that could prevent the maintenance and establishment of a macrophage reservoir (Bosch et al, 2006; Haine et al, 2006; Kutza et al, 2000).
In conclusion, the relationship of M-CSF levels to NAA levels and cognitive measures is indirect. The reduction of viral antigen and RNA by ART results in the reduced activation/infection of monocytes that can traffic into the brain (Aquaro et al, 2006; Kaul et al, 2005). Decreasing the amount of virus brought into the brain results in reduced neurotoxicity caused by viral proteins. The M-CSF receptor is also found on microglial cells, which when activated creates another possible avenue for the production of neurotoxic viral proteins and cytokines (Fixe et al, 1998). Moreover, infected macrophages and microglia have increased cytokine production, such as tumor necrosis factor (TNF)-α. By reducing TNF-α production in the CNS, the extent of neuronal injury induced by radical oxygen species and glutamate excitatory pathways are greatly diminished. The argument that therapy disrupts the association between NAA and M-CSF by blocking those pathways is a valid interpretation, but it is possible that the loss of correlation is due to subject withdrawal from the CSF portion of the study. These results suggest that further study into the relationships of M-CSF to neuronal injury and cognitive impairment in larger cohorts of HIV+ individuals is warranted.
Methods
Subjects
Fifty-four HIV+ subjects were enrolled in this study, 34 of whom had AIDS (Center for Disease Control, Stage 3, CD4 T lymphocytes <200 cells/µl). Thirty-nine of those subjects returned for repeated evaluation after undergoing a documented therapy initiation/change due to failing health or complications including lack of adherence, intolerable side effects, or adverse reactions that prevented compliance with ART (Table 1). All subjects were placed on a combination ART regimen of three or more drugs, and nearly all (38/39) who participated in follow-up studies were on some form of highly active antiretroviral therapy (HAART). The present study was not performed with respect to a specific drug regimen and is more representative of the many combinations of ART drugs currently used in treating HIV infection. Subjects were excluded if they had evidence of any of the following: (1) past/current opportunistic central nervous system infection; (2) past/current severe or chronic neurologic disorder; (3) structural abnormality of the central nervous system; (4) current severe affective disorder believed to explain the subject’s cognitive status; or (5) history of head injury with loss of consciousness of greater than 1 hour. Subjects with a remote history of drug abuse were allowed into this study. However, if a subject arrived for testing and was acutely lethargic, the participant did not undergo testing and was rescheduled. The study was approved by the local Institutional Review Board and informed consent was received from all study participants. Subjects were recruited and studied at Johns Hopkins Medical Institutes, Baltimore, MD.
Neurologic and neuropsychological testing
All subjects underwent neurologic testing. The severity of dementia was determined by a neurologist and a neuropsychologist using a neuropsychological battery consisting of tests that covered executive function, verbal memory, informational processing speed, motor and psychomotor speed. Memorial Sloan-Kettering (MSK) scores and a composite neuropsychological z-score (NPZ-8) were determined for each subject. Those with MSK scores of 1 or 2 (N = 24 at study entry) were viewed as having dementia (HAD+). Neuropsychological impairment is defined as showing deficits (>1 standard deviation below the normal) in two or more cognitive domains. Of the 54 subjects enrolled at baseline, only 6 were not neuropsychologically impaired, and for those that underwent follow-up study, 5 of the 39 were neuropsychologically normal.
MRI and MRSI
All 54 HIV+ subjects enrolled were imaged at entry and 39 returned for follow-up imaging at both 3 and 10 months after change of therapy. All imaging experiments were performed on a 1.5-Tesla Philips ACS-NT Gyroscan MR scanner using a quadrature head coil. A routine brain MRI and multislice, proton two-dimensional (2D) MRSI at long echo time (TR/TE = 2000/280 ms) were performed. MRSI used a spin-echo (SE) sequence with 2D phase-encoding, CHESS pulse water suppression, and eight outer-volume saturation (OVS) pulses arranged in an octangular pattern for lipid suppression (Duyn et al, 1993). The full echo was sampled with 256 data points and a spectral width of 1000 Hz was used. Three 15-mm-thick slices (2.5-mm gap) were recorded parallel to the AC/PC line and a field of view of 24 cm with 28 × 28 circular phase-encoding steps was collected. The data acquisition time was 20 min and the nominal voxel size was approximately 1.1 cm3. Spectroscopic imaging data were reconstructed using software developed in-house (Soher et al, 1996). Multislice 2D MRSI data sets were processed via 3D Fourier transformation with cosine and Fermi filters in the spatial (phase-encoding) domains, and exponential line broadening of 3 Hz, zero-filling to 2048 data points, and a high-pass convolution filter to remove the residual water signal (50-Hz bandwidth) in the time domain. Quantification of MRSI data was performed using phantom replacement methodology, as described in detail previously (Soher et al, 1996), using a 20 mM solution of creatine for referencing. Signal intensities were corrected for relaxation time effects using relaxation time values from the literature (Kreis et al, 1993), and for different radiofrequency (RF) coil loading between the phantom and brain, based on the power required for a 90° pulse. As shown in Figure 1, NAA concentrations were calculated in the following regions of interest: thalamus, basal ganglia, frontal white matter, centrum semiovale, parietal white matter, frontal gray matter, and parietal gray matter.
Immunological assays
Blood and CSF samples were obtained at each imaging time point and processed in-house by the pathology laboratories at Johns Hopkins. All available blood samples underwent CD4 T-lymphocyte quantification by flow cytometry using the antibody combination of CD3, CD4, and CD45. Absolute T-cell counts were obtained using TruCount tubes (Becton Dickinson Biosciences, San Jose, CA), which contained a bead of known concentration. Samples were analyzed on a FACScan flow cytometer using Attractors software (Becton Dickinson Biosciences). HIV-1 RNA assays performed between 1999 and 2002 were done using the Roche Monitor Amplicor quantitative HIV-1 RNA assay version 1.0, using either the standard (detection limit <400 copies/ml) or ultrasensitive assay (detection limit <50 copies/µl). Blood and CSF samples underwent analysis for M-CSF quantification using Quantikine ELISA kits (R&D Systems, Minneapolis, MN).
Statistical analysis
All statistical analyses were performed using JMP (Version 8.0; SAS Institute, Cary, NC). Pearson chi-square tests were used to compare gender, race, and mode of infection distributions between the HAD− (N = 30) and HAD+ subject groups (N = 24). Nonparametric Wilcoxon rank sum tests were used to compare baseline viral RNA, CD4+ lymphocyte, M-CSF levels, and NAA concentrations between the dementia cohorts. The primary goal of this study was to explore the relationship of M-CSF on both NAA levels and cognitive measures and determine how these relationships change with the application of combination ART regimens. Initially, nonparametric Spearman rank correlations were used to compare M-CSF and NAA levels (N = 54) in the seven brain regions of interest. Bivariate linear regression was used to explore the relationship between NPZ-8 and M-CSF levels, and logistic regression for MSK and M-CSF. Repeated measures analysis of variance (RM ANOVA) was used to determine changes in NAA concentrations (N = 39), cognitive functioning, and immunologic markers across all three time points. If the time component of the RM ANOVA was found to be significant, matched pairs t tests were performed to identify the differences between all time points. A secondary objective was to determine if changes in M-CSF could predict cognitive outcome or changes in NAA levels. That was accomplished by performing univariate regressions between continuous predictive variables (baseline M-CSF levels, changes in M-CSF levels) and either continuous outcome variables (NPZ-8 score at 10 months or the change in NPZ-8 between study entry and 10 months; linear regression) or ordinal outcome variables (MSK score at 10 months; ordinal logistic regression). All analyses of viral loads were performed using the log transform of the values in order to normalize the distribution. All reported values within the tables are mean ± standard deviation (SD). Results were deemed significant if P < .05.
Acknowledgments
This work was funded by NIH grants R01 MH61438 (to M.G.P.) and K25 NS051129 (to M.R.L.).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aquaro S, Svicher V, Schols D, Pollicita M, Antinori A, Balzarini J, Perno CF. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80:1103–1110. doi: 10.1189/jlb.0606376. [DOI] [PubMed] [Google Scholar]
- Barker PB. N-acetyl aspartate—a neuronal marker? Ann Neurol. 2001;49:423–424. [PubMed] [Google Scholar]
- Bosch B, Clotet-Codina I, Blanco J, Pauls E, Coma G, Cedeno S, Mitjans F, Llano A, Bofill M, Clotet B, Piulats J, Este JA. Inhibition of human immunodeficiency virus type 1 infection in macrophages by an alpha-v integrin blocking antibody. Antiviral Res. 2006;69:173–180. doi: 10.1016/j.antiviral.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol. 2006;1:160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canque B, Rosenqwajg M, Gey A, Tartour E, Fridman WH, Gluckman JC. Macrophage inflammatory protein-1alpha is induced by human immunodeficiency virus infection of monocyte-derived macrophages. Blood. 1996;87:2011–2019. [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52:100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004a;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck O, Miller EN. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004b;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CT. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993;188:277–282. doi: 10.1148/radiology.188.1.8511313. [DOI] [PubMed] [Google Scholar]
- Fixe P, Praloran V. M-CSF: haematopoietic growth factor or inflammatory cytokine? Cytokine. 1998;10:32–37. doi: 10.1006/cyto.1997.0249. [DOI] [PubMed] [Google Scholar]
- Gallo P, Pagni S, Giometto B, Piccinno MG, Bozza F, Argentiero V, Tavolato B. Macrophage-colony stimulating factor (M-CSF) in the cerebrospinal fluid. J Neuroimmunol. 1990;29:105–112. doi: 10.1016/0165-5728(90)90152-d. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Haine V, Fischer-Smith T, Rappaport J. Macrophage colony-stimulating factor in the pathogenesis of HIV infection: potential target for therapeutic intervention. J Neuroimmune Pharmacol. 2006;1:32–40. doi: 10.1007/s11481-005-9003-1. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 Suppl 1:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kreis R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 2004;17:361–381. doi: 10.1002/nbm.891. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J Magn Reson. 1993;B102:9–19. [Google Scholar]
- Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80:393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- Kutza J, Crim L, Feldman S, Hayes MP, Gruber M, Beeler J, Clouse KA. Macrophage colony-stimulating factor antagonists inhibit replication of HIV-1 in human macrophages. J Immunol. 2000;164:4955–4960. doi: 10.4049/jimmunol.164.9.4955. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, Schifitto G, Selnes OA, Sacktor N, Stern Y, Albert SM, Kieburtz K, deMarcaida JA, Cohen B, Epstein LG. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MA, Lentz MR, Lee V, Halpern EF, Sacktor N, Selnes O, Barker PB, Pomper MG. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology. 2010;254:577–586. doi: 10.1148/radiol.09081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Ernst T, Mao X, Selnes O, Pomper MG, Chang L, Zhong K, Shungu DC, Marder K, Shibata D, Schifitto G, Bobo L, Barker PB. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. J Magn Reson Imaging. 2005;21:325–333. doi: 10.1002/jmri.20272. [DOI] [PubMed] [Google Scholar]
- Salvan AM, Vion-Dury J, Confort-Gouny S, Nicoli F, Lamoureux S, Cozzone PJ. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses. 1997;13:1055–1066. doi: 10.1089/aid.1997.13.1055. [DOI] [PubMed] [Google Scholar]
- Si Q, Cosenza M, Zhao ML, Goldstein H, Lee SC. GM-CSF and M-CSF modulate beta-chemokine and HIV-1 expression in microglia. Glia. 2002;39:174–183. doi: 10.1002/glia.10095. [DOI] [PubMed] [Google Scholar]
- Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magn Reson Med. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart JL, Payan C, Coutellier A, Herson S, Baril L, Bricaire F, Calvez V, Cabanis EA, Lacomblez L, Lubetzki C. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–115. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274:168–170. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–935. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]