Abstract
Several key developmental events occur in the first mitotic cell cycle of Xenopus; consequently this cycle has two gap phases and is ∼60–75 min in length. In contrast, embryonic cycles 2–12 consist only of S and M phases and are 30 min in length. Xe-Wee1 and Mos are translated and degraded in a developmentally regulated manner. Significantly, both proteins are present in the first cell cycle. We showed previously that the expression of nondegradable Mos, during early interphase, delays the onset of M phase in the early embryonic cell cycles. Here we report that Xe-Wee1 is required for the Mos-mediated M-phase delay. We find that Xe-Wee1 tyrosine autophosphorylation positively regulates Xe-Wee1 and is only detected in the first 30 min of the first cell cycle. The level and duration of Xe-Wee1 tyrosine phosphorylation is elevated significantly when the first cell cycle is elongated with nondegradable Mos. Importantly, we show that the tyrosine phosphorylation of Xe-Wee1 is required for the Mos-mediated M-phase delay. These findings indicate that Mos positively regulates Xe-Wee1 to generate the G2 phase in the first cell cycle and establish a direct link between the MAPK signal transduction pathway and Wee1 in vertebrates.
Keywords: Mos, Xe-Wee1, Xenopus, embryonic cell cycle
The oocytes, eggs, and embryos of Xenopus undergo an extraordinary series of cell-cycle transitions that are critical for the propagation and development of the organism (Gerhart 1980; Kirschner and Gerhart 1981; Kirschner et al. 1981). In oocytes, the meiotic cell cycle is initiated in response to progesterone and consists of two consecutive M phases without an intervening S phase. The resultant eggs are arrested at metaphase of meiosis II until fertilization, which releases the arrest and initiates the series of mitotic cycles. The first mitotic cell cycle following fertilization is ∼60–75 min long and has two gap phases. In contrast, mitotic cycles 2–12 are ∼25–30 minutes long and consist only of alternating S and M phases. The gap phases in the first cycle are necessary to accommodate the completion of meiosis II, the fusion of the sperm and egg pro-nuclei and the cortical rotation that determines the second axis of asymmetry (Gerhart 1980; Kirschner and Gerhart 1981; Kirschner et al. 1981). The early embryonic cell cycles proceed in the absence of growth and transcription, therefore the gap phases in the first cycle are unlike those found in somatic cell cycles (Gerhart 1980; Newport and Kirschner 1982). We have studied the mechanism that regulates the length of the first mitotic cell cycle and propose that the G2 phase in this cycle is generated by the developmentally regulated translation of Xe-Wee1 and degradation of Mos.
The cyclin B/cdc2 complex regulates the entry into M phase (for review, see Norbury and Nurse 1992; Morgan 1997). The Wee1 and Myt1 protein kinases inhibit entry into M phase by mediating the phosphorylation of cdc2 on two inhibitory sites, T14 and Y15. Wee1 is localized to the nucleus and phosphorylates only the Y15 residue, whereas Myt 1 is localized to the endoplasmic reticulum and phosphorylates both T14 and Y15 (Russell and Nurse 1987; Featherstone and Russell 1991; Igarashi et al. 1991; Parker et al. 1991, 1992; Parker and Piwnica-Worms 1992; Heald et al. 1993; McGowan and Russell 1993, 1995; Atherton-Fessler et al. 1994; Kornbluth et al. 1994; Mueller et al. 1995a, 1995b; Watanabe et al. 1995; Liu et al. 1997). These inhibitory phosphorylations are dominant and are removed by the Cdc25 phosphatase (for review, see Solomon 1993; Dunphy 1994; Morgan 1997). In contrast to Xe-Myt1, which appears to be present at all stages of early Xenopus development, the expression of Xe-Wee1 protein is developmentally regulated (Mueller et al. 1995b; Murakami and Vande Woude 1998). Xe-Wee1 is absent in stage VI oocytes, is translated at meiosis II and is present at relatively constant levels through gastrulation (Murakami and Vande Woude 1998). This pattern of expression is interesting given that the tyrosine phosphorylated form of cdc2 is observed during the interphase of first mitotic cell cycle but not during the meiotic ‘interphase’ or the interphases of cycles 2–12 (Ferrell et al. 1991). On the basis of these observations, we postulated that Xe-Wee1 was involved in regulating the length of the first mitotic cell cycle (Murakami and Vande Woude 1998). Although Xe-Wee1 was shown to regulate first cell cycle length, the ratio of Xe-wee1 to Cdc25, however, does not decline after the first cell cycle (Izumi et al. 1992; Hartley et al. 1996; Murakami and Vande Woude 1998). Therefore, the expression pattern of Xe-Wee1 does not fully explain the length of the first cycle (relative to cycles 2–12). This led us to propose that an additional factor was necessary to generate the gap phases and cdc2 tyrosine phosphorylation observed in the first cycle (Murakami and Vande Woude 1998).
In Xenopus oocytes, the Mos proto-oncogene is required for entry into meiosis I and meiosis II and is key component of cytostatic factor (CSF), the activity that maintains the metaphase arrest at meiosis II (Masui and Markert 1971; Sagata et al. 1988, 1989; O’Keefe et al. 1989; Daar et al. 1991; Kanki and Donoghue 1991; Yew et al. 1992). Mos phosphorylates and activates MAPK kinase (MEK), which in turn phosphorylates and activates MAPK (Nebreda and Hunt 1993; Posada et al. 1993; Shibuya and Ruderman 1993; Pham et al. 1995; Resing et al. 1995; Huang and Ferrell 1996; for review, see Kosako et al. 1994). We proposed that Mos could be involved in generating the gap phases in the first mitotic cell cycle (Murakami and Vande Woude 1998) because Mos protein is degraded 20–30 min after fertilization (Watanabe et al. 1991). In contrast, cyclin B/MPF (maturation promoting factor) is rapidly degraded within 5–10 minutes (Gerhart et al. 1984; Murray et al. 1989). Accordingly, the injection of a nondegradable form of Mos extended the length of the first cycle from 70 to 140 min. The delay occurred in the G2 phase of the cell cycle, concomitant with a dramatic increase in the level of cdc2 tyrosine phosphorylation. Moreover, a rapid 30-min embryonic cycle could be converted into a 60-min cell cycle by arresting the cycle with Mos and releasing the arrest with calcium ionophore (Murakami and Vande Woude 1998). Similar results were also observed in cell free extracts, indicating that the prolonged activation of Mos/MAPK [after maturation-promoting factor (MPF) inactivation] in the early portion of the first mitotic cell cycle extends the G2 phase of that cycle (Abrieu et al. 1997; Walter et al. 1997; Bitangcol et al. 1998). Interestingly, these results highlight the fact that the effects of Mos/MAPK vary dramatically as the oocyte, egg, and embryo progress through development. The activation of MAPK promotes entry into the first meiotic M phase, arrests the second meiotic M phase, and delays the first mitotic M phase (Kosako et al. 1994; Haccard et al. 1993, 1995; Minshull et al. 1994; Abrieu et al. 1996, 1997; Jones and Smythe 1996; Sagata 1996, 1997; Walter et al. 1997; Bitangcol et al. 1998; Murakami and Vande Woude 1998). In Xenopus, it appears that the developmentally regulated translation of specific substrates (e.g., Xe-Wee1) allows the same signal (MAPK) to produce different results at various stages of development.
In this study, we investigate the functional relationship between Xe-Wee1 and Mos. We show that Xe-Wee1 is necessary and sufficient for the Mos induced M-phase delay. Xe-Wee1 is regulated positively by tyrosine autophosphorylation and Xe-Wee1 tyrosine phosphorylation can up-regulated by Mos in the first mitotic cell cycle. Furthermore, we also show that the tyrosine phosphorylation of Xe-Wee1 is required for the Mos mediated M-phase delay. These finding indicate that Mos positively regulates Xe-Wee1 to generate the G2 phase in the first mitotic cell cycle.
Results
Mos-mediated G2 arrest requires Xe-Wee1
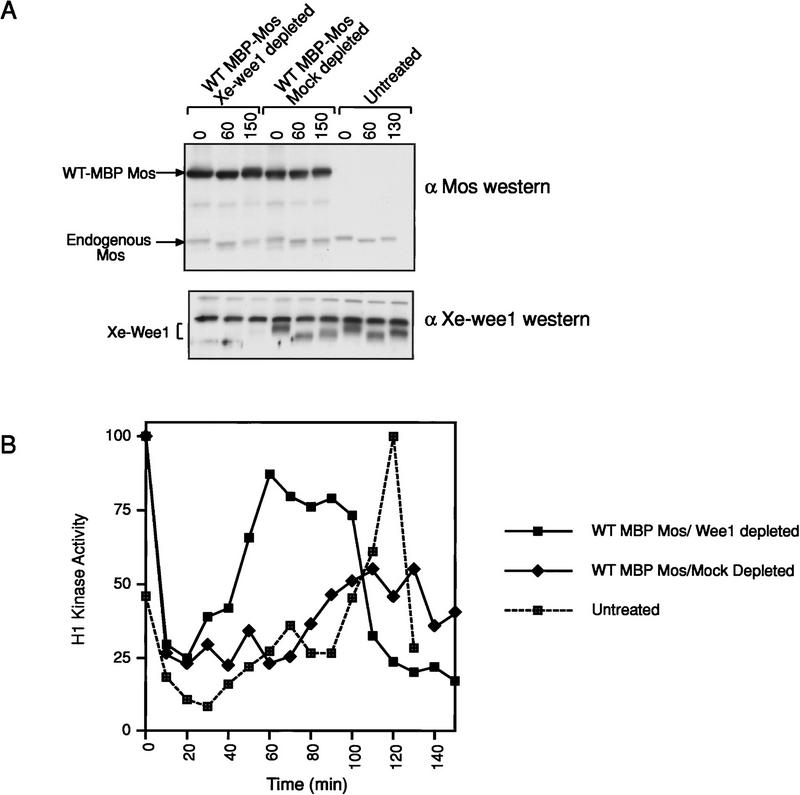
In intact eggs the injection of nondegradable Mos results in a protracted G2-phase or M-phase delay (Murakami and Vande Woude 1998). The addition of Mos to mitotic cell free extracts results in a G2 arrest (Abrieu et al. 1997; Walter et al. 1997; Bitangcol et al. 1998). To determine if the Mos-mediated G2 arrest was dependent on Xe-Wee1, we examined the effects of Mos after the removal of Xe-Wee1 by immunodepletion. Following the addition of nondegradable Mos to a CSF extract (Fig. 1A, top), the cell cycle was initiated by the addition of calcium. Extracts to which the kinase mutant (KM) version of nondegradable Mos had been added, cycled normally; whereas extracts that contained wild-type nondegradable Mos, did not cycle and no activation of MPF was observed (Abrieu et al. 1997; Walter et al. 1997; Bitangcol et al. 1998; data not shown). Using anti-Xe-Wee1 antisera, we depleted these CSF extracts of Xe-Wee1 (Fig. 1A, bottom). Control mock-depletions were performed with preimmune sera (Fig. 1A, bottom). Reduced MPF activation was observed after the addition of wild-type nondegradable Mos to mock-depleted extracts (Fig. 1B). In contrast, normal levels of MPF activation were observed after the addition of wild-type nondegradable Mos to Xe-Wee1 depleted extracts (Fig. 1B). MPF activation was faster in the Xe-Wee1 depleted extracts as compared to the untreated extracts (Fig. 1B). These results indicate that Xe-Wee1 regulates cell-cycle length and that the G2 arrest generated by Mos requires Xe-Wee1.
Figure 1.
G2 arrest mediated by Mos requires Xe-Wee1. (A) CSF extracts were prepared and supplemented with wild-type MBP-Mos (top). Xe-Wee1 was depleted from the CSF extract or the extract was mock depleted with preimmune sera (bottom). (B) The cell cycle was initiated by the addition of calcium, samples were taken at 10-min intervals and assayed for histone H1 kinase activity. Note that the endogenous Mos does not degrade efficiently in these extracts as compared to intact eggs (see Fig. 6B,F; Watanabe et al. 1991).
Autophosphorylation activity of Xe-Wee1 in the first mitotic cell cycle
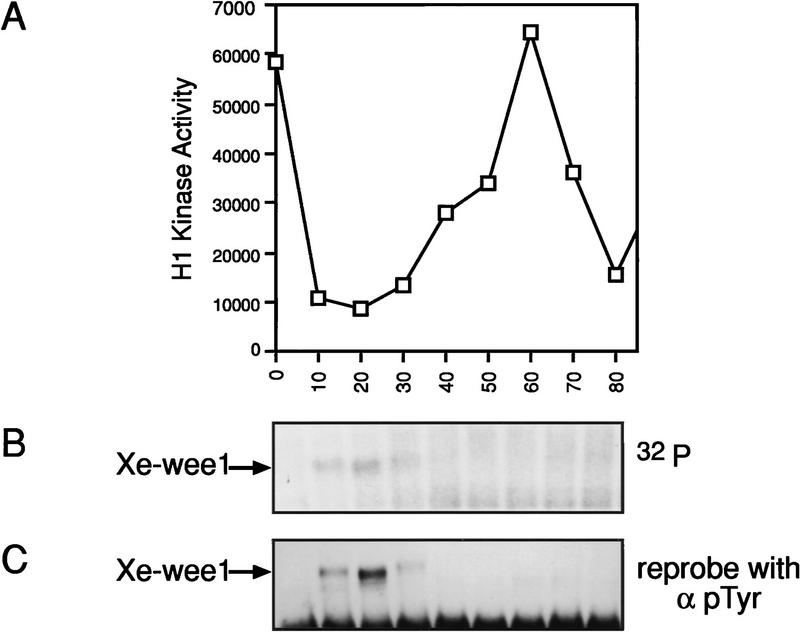
To determine if Xe-Wee1 activity could be modulated by Mos, we needed to characterize mechanisms of Xe-Wee1 regulation. Autophosphorylation has been shown to functionally regulate many protein kinases (Ullrich and Schlessinger 1990; Johnson et al. 1996) and Xe-Wee1 was observed to possess auto-kinase activity during interphase in cell-free extracts (Mueller et al. 1995a). We assessed Xe-Wee1 autokinase activity in the first mitotic cell cycle using lysates prepared from intact eggs. Unfertilized eggs were activated with calcium ionophore, lysates were prepared at 10-min intervals, and progression through the cell cycle was monitored by histone H1 kinase activity (Fig. 2A). Xe-Wee1 was isolated by immunoprecipitation and autophosphorylation reactions were performed on the immune complexes. Xe-Wee1 autophosphorylation activity was observed only in the first 30 min of the first cell cycle (Fig. 2B) even though the level of Xe-Wee1 protein is constant in this cycle (Murakami and Vande Woude 1998). The autophosphorylated Xe-Wee1 was recognized by an anti-phosphotyrosine antibody, indicating that some or all of the phosphorylations occurred on tyrosine residues (Fig. 2C).
Figure 2.
Xe-Wee1 has autophosphorylation activity in the first 30 min of the first mitotic cell cycle. The first mitotic cell cycle was initiated by treating unfertilized eggs with calcium ionophore (A23187). Ten eggs were collected at 10-min intervals and lysates were prepared in modified EB. Histone H1 kinase assays were used to monitor the progression through the cell cycle (A). Xe-Wee1 was immunoprecipitated (Ab 725:Ab 1532) from the remainder of each sample, and subjected to an immune complex autokinase assay with [γ-32P]ATP. The reactions were processed for SDS-PAGE, and the proteins were transferred to Immobilon membranes. After exposure to autoradiographic film (B), the filters were processed for immunoblotting with an anti-phosphotyrosine antibody (C).
Identification of Xe-Wee1 autophosphorylation sites
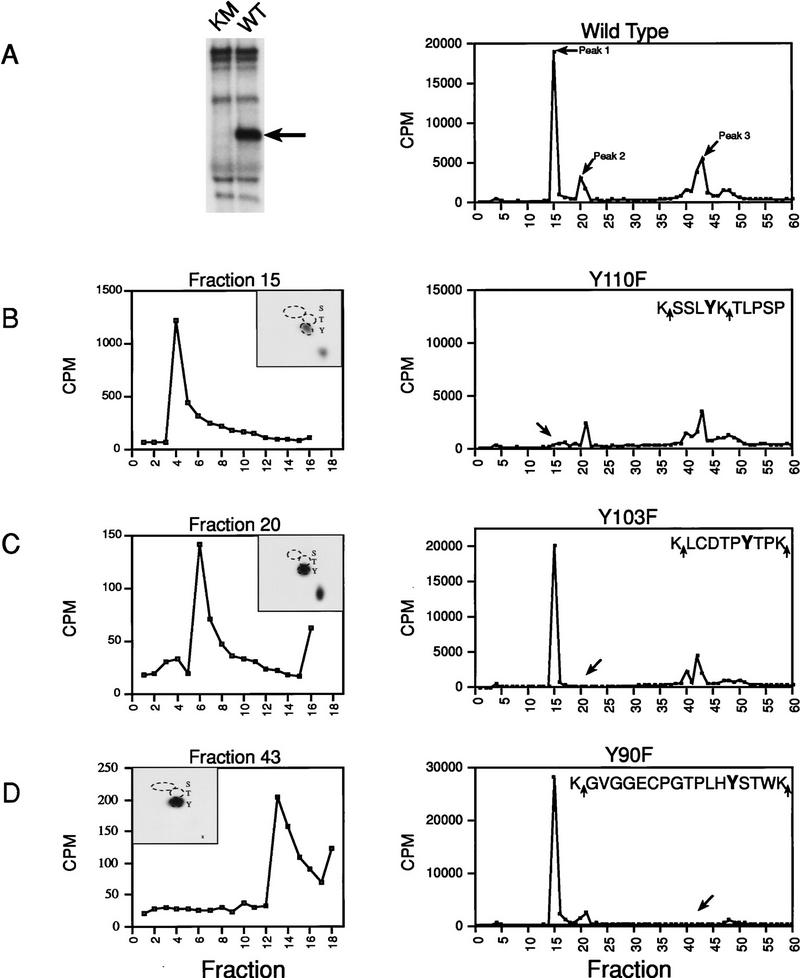
To identify the sites that were phosphorylated, wild-type or kinase-mutant Xe-Wee1 was immunoprecipitated from an in-vitro translation reaction and autophosphorylation reactions were performed in the presence of [γ-32P]ATP. Only wild-type Xe-Wee1 was phosphorylated (Fig. 3A, left panel) indicating that the phosphorylations were autophosphorylations rather than trans-phosphorylations by contaminating kinases. The phosphoproteins were isolated, subjected to proteolytic cleavage with trypsin or endopeptidase Lys-C, and the resultant phospho-peptides were subjected to reverse-phase HPLC analysis using a C-18 column. Quantification of the radioactivity released from the column revealed the presence of three major peaks (Fig. 3A, right). Aliquots from each of these three fractions were subjected to phospho-amino acid analysis, and the remainder of these fractions were subjected to Edman degradation. The phospho-amino acid analysis of the peptides contained in all three fractions demonstrated that autophosphorylation occurred exclusively on tyrosine residues (Fig. 3B–D, left panels, insets). Edman degradation revealed that the peptides eluting in fractions 15, 20, and 43, were phosphorylated on the fourth, sixth, and thirteenth residues, respectively (Fig. 3B–D, left panels). Based on this analysis, we determined that two of the autophosphorylation sites were the tyrosine residues at amino acid positions 110 and 90 (for fractions 15 and 43, respectively). For the peptide eluting in fraction 20, there were two candidate tyrosine residues; Y103 and Y236. Examination of the sequence surrounding the tyrosine residues at positions 110 and 90 indicated that each had a threonine residue at the +2 position (Fig. 3, B and D, right panels, sequence). The tyrosine residue of Cdc2 (Y15), phosphorylated by Wee1 (Gould and Nurse 1989), also has a nearby threonine residue (T14 at the −1 position); therefore, we predicted that the tyrosine residue at position 103 was the more likely candidate as it possessed threonine residues at the +1 and −2 positions. (Fig. 3C, right panel, sequence). We confirmed that these tyrosine residues (Y90, Y103, and Y110) were bona fide autophosphorylation sites, by substituting each tyrosine residue with a phenylalanine residue separately or in combination (Y90F; Y103F; Y110F; YYYFFF). The mutant proteins were then labeled with 32P in an autokinase assay and examined by HPLC analysis as described above. In each case, mutation of the tyrosine residue at the predicted position resulted in the absence of radioactivity in the corresponding fraction, thereby confirming each residue as a bona fide phosphorylation site (right panels in Fig. 3B, fraction 15, Y110F; 3C, fraction 20, Y103F; 3D, fraction 43, Y90F).
Figure 3.
Identification of Xe-Wee1 autophosphorylation sites. Wild type (WT) and kinase mutant (KM) Xe-Wee1 were translated in vitro and following immunoprecipitation, immune complex autokinase reactions were performed. After separation by SDS-PAGE (A, left), the wild-type 32P-labeled protein was eluted from the gel matrix, precipitated with TCA, and digested with either trypsin (which cleaves after Lys and Arg residues) or Lys-C (which cleaves after Lys residues). In the analysis of invitro-phosphorylated Xe-Wee1, both enzymes generated identical cleavage patterns, as the sites flanking the phosphopeptides were always lysine residues (B–D, right panels, sequences). The profile generated from the HPLC analysis of the wild-type Xe-Wee1 revealed three peaks of radioactivity (A, right). The peptides in fractions 15, 20, and 43, were subjected to phospho-amino acid analysis and two-dimensional thin-layer electrophoresis (B–D, left panels, insets), and Edman degradation (B–D, left panels). Each of the predicted tyrosine autophosphorylation sites was substituted with a phenylalanine residue, and the mutated versions of Xe-Wee1 were labeled with 32P in an autokinase assay and processed for HPLC analysis as described above; Y110F (B, right), Y103F (C, right), and Y90F (D, right). The amino acid sequence of the phosphopeptides is shown in the insets, arrows indicate LysC/trypsin cleavage sites.
Analysis of Xe-Wee1 phosphorylation in cell-free extracts
To show that the autophosphorylation sites identified in vitro are phosphorylated in vivo, we characterized the endogenous Xe-Wee1 protein that was labeled with [γ-32P]ATP in cell-free extracts. Cell-free extracts were used for this analysis because the 32P labeling of endogenous Xe-Wee1 in intact eggs was insufficient for extensive biochemical analysis (data not shown). Interphase extracts were prepared by adding calcium to CSF extracts (Lohka and Masui 1983; Murray 1991). Consistent with our observations in vivo (Fig. 2), immunoblotting with an anti-phosphotyrosine antibody showed that Xe-Wee1 was tyrosine-phosphorylated 30 min after the addition of calcium to a CSF extract (data not shown). Following the addition of 10 mCi of [γ-32P]ATP, endogenous Xe-Wee1 was collected by immunoprecipitation 30 min after the addition of calcium. The 32P-labeled protein was isolated, digested with trypsin, and the resultant phosphopeptides were subjected to reverse-phase HPLC analysis as described above. The HPLC profile generated from the Xe-Wee1 labeled in an interphase extract revealed ∼8 peaks of radioactivity (Fig. 4B). When compared with the HPLC profile generated from Xe-Wee1 labeled in an in vitro autokinase assay (Fig. 4A), the HPLC profile of the Xe-Wee1 labeled in the extracts contained a prominent novel peak of radioactivity in fractions 4–6 (Fig. 4B). The phospho-amino acid analysis of the peptides in fractions 4–6 revealed phosphorylation on serine residues in interphase and CSF extracts (M.S. Murakami, unpubl.); suggesting that the peptides in fractions 4–6 are constitutively phosphorylated on serine. Common peaks (fractions 15 and 19–20) of radioactivity were observed when the HPLC profile of the Xe-Wee1 labeled in an in vitro autokinase assay was compared to the profile generated from the Xe-Wee1 labeled in an interphase extract (Fig. 4A,B). A phospho-amino acid analysis of the peptide in the fifteenth fraction generated from the Xe-Wee1 labeled in an interphase extract revealed that this peptide was phosphorylated on tyrosine as well as serine (Fig. 4C, left). The phospho-amino acid analysis of the peptides in fraction 20 revealed phosphorylations on serine, threonine, and tyrosine residues (Fig. 4C, middle). A two-dimensional thin-layer electrophoresis/chromatography analysis of fraction 15 showed that the peptide generated from the Xe-Wee1 labeled in an interphase extract migrated exactly with the peptide generated from the Xe-Wee1 labeled in an in vitro kinase assay (Y110; Fig. 4D). A similar two-dimensional thin-layer electrophoresis and chromatography analysis was performed on the peptides that eluted in fractions 19 and 20. The migration pattern of the peptides that were generated from the Xe-Wee1 labeled in an interphase extract was similar to that of the peptides isolated from the Xe-Wee1 labeled in an in vitro autokinase assay (Y103; Fig. 4E). The analysis of the peptides in fractions 38–41 (Y90) was inconclusive (see legend to Fig. 4; data not shown). However, a phospho-amino acid analysis of the peptide(s) that eluted in fraction 41 demonstrated that the peptides in those fractions were phosphorylated on tyrosine, threonine, and serine residues (Fig. 4C, right panel). Taken together, these results strongly indicate that at least two of the tyrosine autophosphorylation sites that were mapped in vitro (Y110 and Y103) are also phosphorylated in cell-free extracts, and presumably in vivo.
Figure 4.
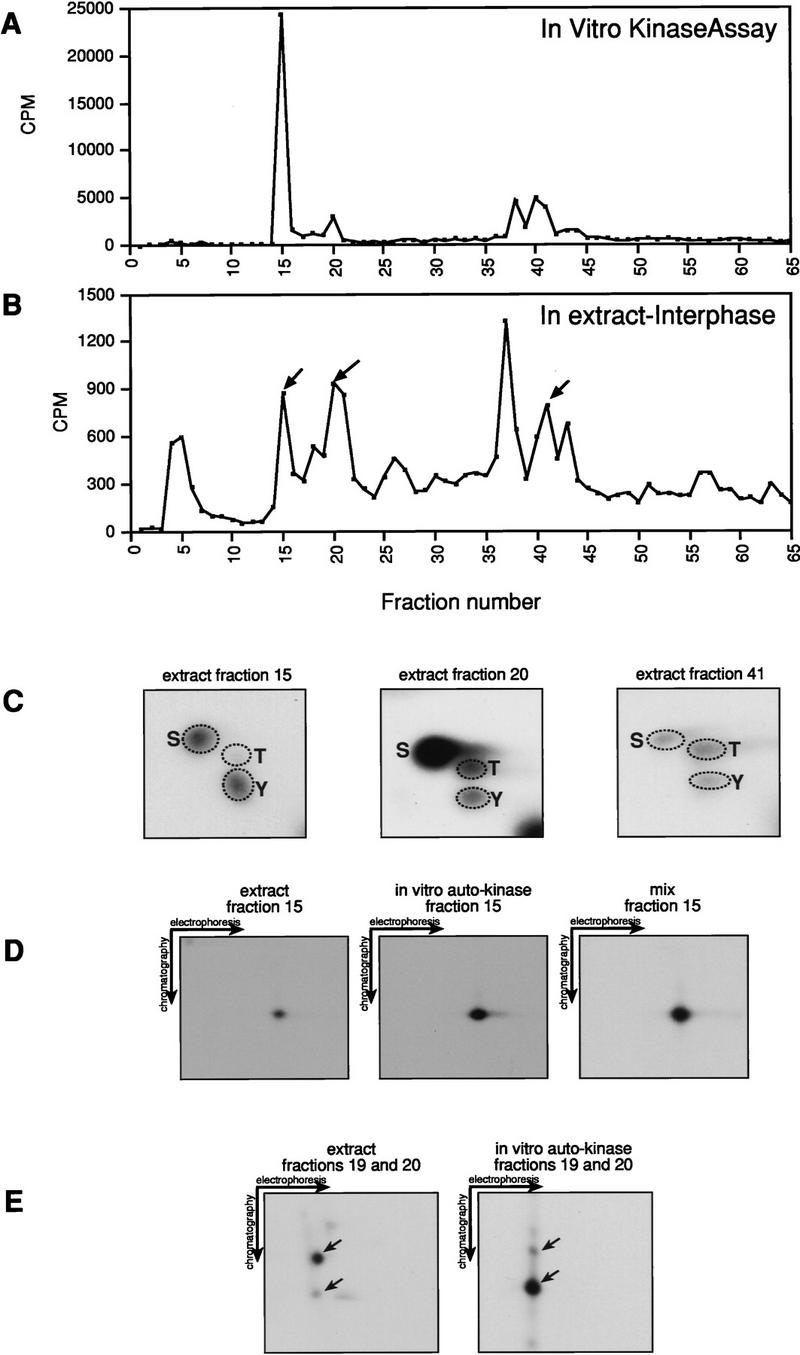
Analysis of Xe-Wee1 phosphorylation in cell-free extracts. Interphase extracts were prepared as described in the Materials and Methods. [γ-32P]ATP (10 mCi) was added to 150–200 μl of these extracts, and endogenous Xe-Wee1 was isolated by immunoprecipitation (antibodies 725 and 1532) 30 min after the addition of calcium. The labeled proteins were isolated, digested with trypsin, and subjected to reverse-phase HPLC analysis (B; interphase Xe-Wee1). The HPLC profile for the wild-type Xe-Wee1 labeled in an in vitro autokinase assay (A) was generated as described in Fig. 3. The peptides in fractions 15, 20, and 41 were subjected to phospho-amino acid analysis. Tyrosine (as well as serine and threonine) phosphorylation was detected (4C). The peptide in fraction 15 from the Xe-Wee1 protein labeled in the interphase extracts (D, left) or from the Xe-Wee1 labeled in an in vitro autokinase assay (D, middle) was subjected to two-dimensional thin-layer electrophoresis and chromatography separately or as a mixture (D, right). In addition, the peptides from fraction 19 and 20 were also subjected to a two-dimensional thin-layer electrophoresis/chromatography analysis (E, left: extract; E, right: in vitro kinase). We were unable to perform a conclusive two-dimensional TLC analysis on the Y90 peptide. In our analysis, we compared the peptides isolated from the endogenous Xe-Wee1 labeled in an extract with those generated from our recombinant Xe-Wee1 labeled in an in vitro autophosphorylation reaction. Two sequences of Xe-Wee1 have been reported (Mueller et al. 1995a; Murakami and Vande Woude 1998). We believe that endogenous Xe-Wee1, in any given extract, is a mixture of these two isoforms. Importantly, the Xe-Wee1 sequence reported by the Dunphy laboratory (Mueller et al. 1995a) lacks the tryptic cleavage site just upstream of Y90 (which is present in our clone). Therefore, the tryptic digestion of the radiolabeled endogenous ‘Dunphy’ Xe-Wee1 would generate a peptide with a different mobility in both the HPLC analysis and two-dimensional TLC analysis, precluding comigration with the peptide generated from our recombinant Xe-Wee1.
Functional analysis of Xe-Wee1 tyrosine autophosphorylation
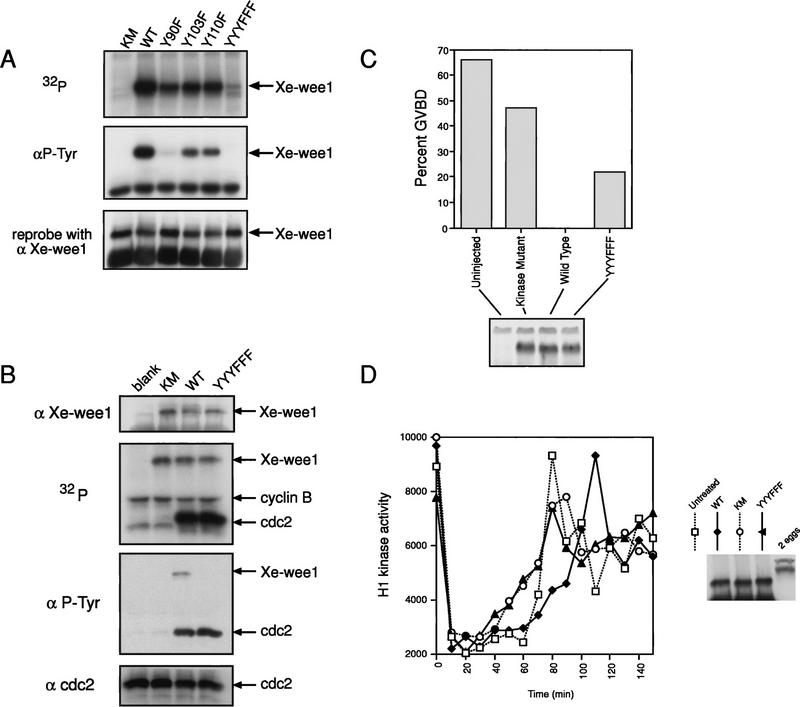
We examined the autokinase activity of the mutated Xe-Wee1 proteins using the in vitro-translated proteins in an autokinase assay. Each of the Xe-Wee1 proteins with single site substitutions (Y90F; Y103F and Y110F) showed reduced autokinase activity while the mutant in which all three sites had been substituted (YYYFFF) showed very little autokinase activity (Fig. 5A, top panel). When the autophosphorylated proteins were analyzed by Western analysis with anti-phosphotyrosine antibody, the Y103F and Y110F mutants showed reduced reactivity, whereas the Y90F and the YYYFFF mutants showed little or no reactivity with the antibody (Fig. 5A, middle panel).
Figure 5.
Analysis of Xe-Wee1 mutant proteins. Tyrosine residues at position 90 (Y90F), 103 (Y103F), and 110 (Y110F), were substituted with phenylalanine residues singly or in combination (YYYFFF). The proteins were translated in vitro and immune complex autokinase assays were performed. Following separation by SDS-PAGE, the proteins were transferred to Immobilon membrane and exposed to autoradiographic film (A, top). The membranes were then processed for immunoblotting with anti-phosphotyrosine antibody 4G10 (A, middle) after which the membranes were stripped and re-probed for Xe-Wee1 (A, lower; antibody 725). The various forms of Xe-Wee1 were translated and immunoprecipitated as described above (B, top). The immune complexes were supplemented with recombinant cyclin B/cdc2 and [γ-32P]ATP. The reactions were subjected to SDS-PAGE and then transferred to Immobilon membrane and exposed to autoradiographic film (B; second panel). The filters were processed for Western analysis with antiphosphotyrosine antibody (B, third panel), then stripped and reprobed with anti-Xe-Wee1 antibody (B, first panel) and cdc2 (B, fourth panel). RNAs encoding the mutated versions of Xe-Wee1 were transcribed and capped in vitro. Approximately 40 ng of each RNA was injected into ∼75 stage-VI oocytes that were then left at 16°C overnight. Western analysis showed that equivalent amounts of Xe-Wee1 protein were present in the oocytes that had been injected with RNA (C, bottom). Progesterone was added at 10 μg/ml, and GVBD was scored by the appearance of a white spot in the animal hemisphere (C, top). CSF extracts were supplemented with exogenous Xe-Wee1 (D). CSF extracts were prepared and supplemented with wild-type or mutant Xe-Wee1. The cell cycle was initiated by the addition of calcium, aliquots were removed at 10-min intervals, and H1 kinase activity was assessed. The Western blot represents 1/13 of the exogenous Xe-Wee1 added to 60 μl of extract (right).
We examined the ability of the mutant forms of Xe-Wee1 to phosphorylate cdc2. The various forms of Xe-Wee1 were translated in a reticulocyte lysate and immunoprecipitated as described above (Fig. 5B, top panel). Recombinant baculovirus kinase mutant cdc2/cyclin B complex was added to the Xe-Wee1 immune complex with [γ32-P]ATP and reaction buffer. The wild-type and the YYYFFF forms of Xe-Wee1 were equivalent in their capacity to phosphorylate cdc2, whereas the kinase mutant form of Xe-Wee1 showed virtually no ability to phosphorylate cdc2 (Fig. 5B, second panel). cdc2 was phosphorylated on tyrosine and equal amounts of cdc2 protein were present in all reactions (Fig. 5B, bottom two panels).
To assess the effects of the mutations on the biological activity of Xe-Wee1, we examined the ability of the mutant versions of Xe-Wee1 to inhibit progesterone induced oocyte maturation. Stage VI oocytes do not express Xe-Wee1, however, the premature expression of wild-type Xe-Wee1 in these oocytes prevents progesterone-induced oocyte maturation (Murakami and Vande Woude 1998). We injected RNAs encoding the various forms of Xe-Wee1 into stage VI oocytes. Following incubation at 16°C for 16 hr, all proteins were expressed at equivalent levels (Fig. 5C, bottom panel). Germinal vesicle breakdown (GVBD) was monitored following the addition of progesterone (Fig. 5C, top panel). The expression of wild-type Xe-Wee1 inhibited progesterone-mediated oocyte maturation, whereas expression of the kinase mutant did not. The inhibitory activity of the YYYFFF version of Xe-Wee1 was less than wild-type Xe-Wee1 but more than the kinase inactive Xe-Wee1 (Fig. 5C, top panel).
We also examined the effect of the mutations on the ability of Xe-Wee1 to delay M phase in a mitotic extract. As demonstrated previously (Mueller et al. 1995a), the addition of exogenous wild-type Xe-Wee1 delays the onset of M phase (Fig. 5D, ♦). Significantly, no delay was observed when the kinase mutant or the YYYFFF mutant Xe-Wee1 were added to the extracts (Fig. 5D, ○,▴). Taken together, these results indicate that tyrosine autophosphorylation regulates the activity of Xe-Wee1 in a positive manner as the mutation of these sites to nonphosphorylatable residues compromises the activity of the kinase.
Mos up-regulates Xe-Wee1 tyrosine phosphorylation
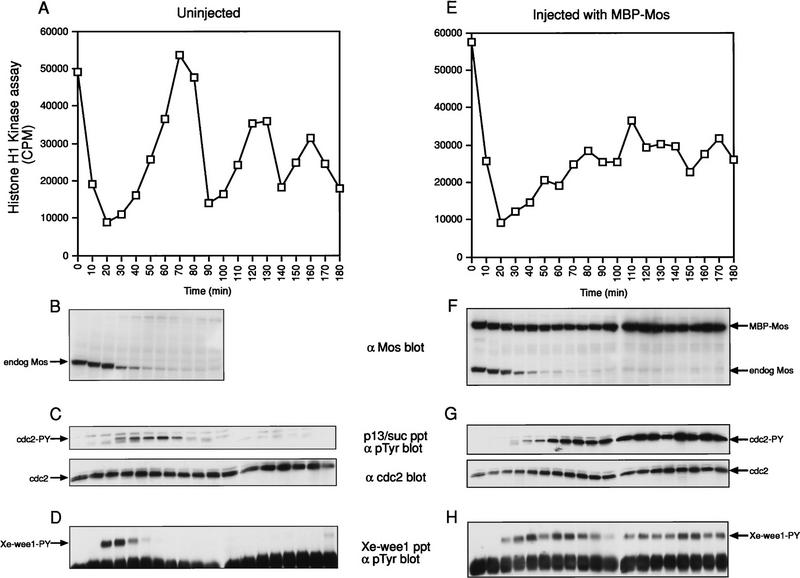
To determine the effect of Mos on the tyrosine phosphorylation state of Xe-Wee1, we examined Xe-Wee1 in the first three mitotic cell cycles and in a first cell cycle where we had injected nondegradable Mos (Murakami and Vande Woude 1998). Stage VI oocytes were injected with nondegradable Mos or left uninjected (Fig. 6B,F), and the oocytes were induced to mature in vitro with progesterone. After 16 hr, the mitotic cell cycles were initiated by treating meiosis II-arrested eggs with calcium ionophore, and lysates were prepared at 10-min intervals. We observed three peaks of MPF activity in the untreated eggs, indicating that they had progressed through three mitotic cell cycles (Fig. 6A). In contrast, the eggs that had been injected with nondegradable Mos failed to fully activate MPF activity (Fig. 6E). The M-phase delay was not attributable to slower cyclin translation or a delay in the onset of DNA replication (Murakami and Vande Woude 1998) but to higher levels of tyrosine-phosphorylated Cdc2 (Fig. 6, C vs. G). We isolated the Xe-Wee1 from these eggs and analyzed the state of Xe-Wee1 tyrosine phosphorylation with anti-phosphotyrosine antibody. In the case where the eggs were left untreated, we observed tyrosine phosphorylation of Xe-Wee1 only in the first 20–40 min of the first cell cycle (Fig. 6D), consistent with the results shown in Figure 2. Notably, we did not observe Xe-Wee1 tyrosine phosphorylation in cycles two or three. When the cell cycle was elongated with nondegradable Mos, we observed a dramatic and prolonged increase in Xe-Wee1 tyrosine phosphorylation (Fig. 6H). The levels of Xe-Wee1 protein were the same in all of the immunoprecipitates (data not shown). These results indicate that Mos positively regulates Xe-Wee1 in the first mitotic cell cycle.
Figure 6.
Analysis of Xe-Wee1 tyrosine phosphorylation in the first three mitotic cycles and in the first mitotic cycle with nondegradable Mos. Untreated eggs (A–D) and eggs that had been injected with wild-type nondegradable Mos (E–H) were activated with calcium ionophore and the progression through the cell cycle was monitored by histone H1 kinase activity (A,E). The levels of endogenous Mos and nondegradable Mos were detected by immunoblotting (B,F; one egg per lane). The level of tyrosine phosphorylated Cdc2 was determined following precipitation with p13/suc1 beads and immunoblotting with anti-phosphotyrosine antibody (C,G, top; 10 eggs per precipitate). The levels of Cdc2 in the p13 /suc1 precipitates are also shown (C,G, bottom). The levels of tyrosine-phosphorylated Xe-Wee1 were detected following immunoprecipitation with anti-Xe-Wee1 antibodies and immunoblotting with anti-phosphotyrosine antibody 4G10 (D,H; 10 eggs per precipitate).
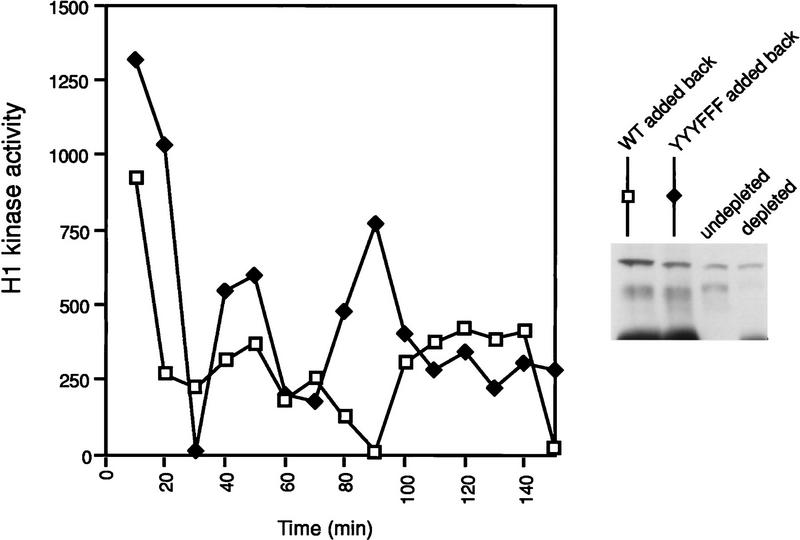
To show that Xe-Wee1 is sufficient for Mos-mediated G2 arrest, we added exogenous recombinant Xe-Wee1 back to a Xe-Wee1-depleted extract. Significantly, the addition of exogenous wild-type, but not YYYFFF Xe-Wee1, restored the ability of the depleted extracts to arrest in response to Mos. These results demonstrate that Xe-Wee1 is sufficient for the Mos-mediated M-phase delay and that the effects of Mos are mediated through the tyrosine phosphorylation of Xe-Wee1.
Discussion
The first mitotic cell cycle is ∼60–75 min in length and contains several critical developmental events: the completion of meiosis II; fusion of the sperm and egg pronuclei; and the rotation of the cortex that determines the second axis of asymmetry (Gerhart 1980; Kirschner et al. 1981; Kirschner and Gerhart 1981). These events are essential for the development of the organism and necessitate the introduction of gap phases, which are atypical in the sense that there are no transcriptional or growth requirements at this point in development. The developmental expression pattern of two key cell-cycle proteins, Xe-Wee1 and Mos, implicated these proteins as regulators of the first cell cycle. Xe-Wee1 is absent in stage VI oocytes, is synthesized at the onset of meiosis II, and persists until gastrulation. Xe-Wee1 does, in part, contribute to length of and cdc2 tyrosine phosphorylation observed in the first cycle (Murakami and Vande Woude 1998). The expression pattern of Xe-Wee1, however, does not fully explain the rapidity of cycles 2–12 as the ratio of Xe-Wee1 to Xe-Cdc25 does not change after the first cycle (Izumi et al. 1992; Hartley et al. 1996). We proposed that Mos might also regulate the length of this cycle (Murakami and Vande Woude 1998) because Mos protein is present in the first 30 min of this cycle. Injection of nondegradable Mos extended the length of the first mitotic cycle from 70 to 140 min. The longer cycle was not the consequence of slower cyclin translation or a delay in S phase, but higher levels of cdc2 tyrosine phosphorylation. Furthermore, a 30-min mitotic cell cycle could be converted to a 60-min cycle following an arrest with Mos and reactivation with calcium ionophore (Murakami and Vande Woude 1998). Although these results suggested that the effects of Mos were mediated through Xe-Wee1 and that Mos positively regulates Xe-Wee1 activity, this hypothesis had not been formally tested.
The addition of Mos to cell-free extracts generates a G2 arrest (Abrieu et al. 1997; Walter et al. 1997; Bitangcol et al. 1998). By depleting these extracts of Xe-Wee1, we showed that the effects of Mos were mediated through Xe-Wee1 (Fig. 1). To understand the mechanism by which Mos regulates Xe-Wee1, we next analyzed possible mechanisms of Xe-Wee1 regulation. Autophosphorylation has been shown to regulate many protein kinases (Ullrich and Schlessinger 1990). We detected Xe-Wee1 autophosphorylation activity in the first 30 min of the first cell cycle (Fig. 2). We determined the sites of Xe-Wee1 autophosphorylation and showed that they were exclusively tyrosine residues (Fig. 3). This finding was in agreement with Parker and Piwnica-Worms (1992), but in conflict with other reports in which autophosphorylation was observed on tyrosine and serine residues (Featherstone and Russell 1991; Parker et al. 1991; Parker et al. 1992; McGowan and Russell 1993; Mueller et al. 1995a). These differences may be due to species specific variations or may be attributed to the stringency of the immune complex wash conditions in our experiments (0.75 m NaCl). All three autophosphorylation sites are in the amino terminus of the protein with a threonine residue at the +1 or +2 position. Although the Xe-Wee1 autophosphorylation sites are similar to the site that Wee1 recognizes on Cdc2 (T14, Y15; Gould and Nurse 1989) they are unusual in that they do not fit the consensus for other tyrosine kinase phosphorylation sites (Pearson and Kemp 1991). Kinases in which the enzymatic activity is activated by autophosphorylation often have an autophosphorylation site within the activation loop (Ullrich and Schlessinger 1990; Johnson et al. 1996). Consistent with the location of the autophosphorylation sites in the amino-terminal region, the mutation of the Xe-Wee1 tyrosine residues to phenylalanine did not alter the enzymatic activity of the kinase but did, however, compromise the biological activity of the protein (Fig. 5). We believe that our findings are consistent with a model in which the tyrosine phosphorylation of Xe-Wee1 serves to delay or retard the inactivation of Xe-Wee1 during the course of the cell cycle. We also note that Xe-Wee1 is phosphorylated on serine and threonine residues in interphase (Fig. 4C). At this point, we cannot conclusively rule out a regulatory role for these phosphorylations. In any case, all Wee1 homologs have tyrosine residues in the amino-terminal region and most Wee1 homologs have been reported to have autophosphorylation activity (Russell and Nurse 1987; Featherstone and Russell 1991; Igarashi et al. 1991; Parker et al. 1991, 1992; Parker and Piwnica-Worms 1992; Booher et al. 1993; McGowan and Russell 1993; Campbell et al. 1995; Mueller et al. 1995a; Watanabe et al. 1995). Furthermore, the regulatory role of the amino-terminal region appears to be conserved in the Wee1 family, several studies have shown that this region is important for negative regulation of the kinase (Tang et al. 1993; Aligue et al. 1997). Now it is clear that this region also has a positive regulatory function (Aligue et al. 1997; Boddy et al. 1998; and this work).
Significantly, we show that Xe-Wee1 tyrosine phosphorylation is tightly regulated during development. We find that Xe-Wee1 is normally tyrosine-phosphorylated in the first 30 min of the first cell cycle, the period when Mos/MAPK is active but MPF is not (Figs. 2 and 6). Although, we cannot exclude the possibility that other treatments that prolong interphase may also regulate Xe-Wee1 tyrosine phosphorylation, we show that the level and duration of Xe-Wee1 tyrosine phosphorylation increases dramatically in the presence of nondegradable Mos (Fig. 6) and that the effects of Mos are mediated through the tyrosine phosphorylation of Xe-Wee1 (Fig. 7). Taken together, our results suggest the protracted G2 phase induced by Mos is mediated through the positive regulation of Xe-Wee1. At this point we do not know if components of the Mos/MAPK pathway directly phosphorylate Xe-Wee1 to facilitate autophosphorylation. We note, however, that Xe-Wee1 autophosphorylation can be detected in the absence of Mos [following in vitro translation in reticulocyte lysates (Fig. 3A) and after translation in stage VI oocytes (M.S. Murakami, unpubl.)]. Further indication that the mechanism of Xe-Wee1 activation is likely to be more complicated is suggested by the fact that the Mos/MAPK pathway is fully active at meiosis II, yet we observe no tyrosine phosphorylation of Xe-Wee1 (Fig. 6D, lane 1). This observation also indicates that the negative regulation of Xe-Wee1 by M-phase kinases is dominant over the positive regulation mediated by Mos alone. Interestingly, our results show that Xe-Wee1 is tyrosine-dephosphorylated (Fig. 6D, 60 min), suggesting that a Xe-Wee1 tyrosine phosphatase exists; it is possible that the Mos/MAPK pathway down-regulates the activity of this tyrosine phosphatase.
Figure 7.
CSF extracts were first depleted of endogenous Xe-Wee1 as described in Fig. 1. Recombinant wild-type and YYYFFF Xe-Wee1 were added back to the depleted extracts (legend). The extracts were also supplemented with one-tenth volume of MBP–Mos. The cell cycle was initiated by the addition of calcium and progression through the cell cycle was monitored by analysis of histone H1 activity.
Studies aimed at understanding the mechanism of MPF activation have focused on the activation of positive regulators such as Cdc25 and the inactivation of negative regulators such as Wee1 (for review, see Norbury and Nurse 1992; Coleman and Dunphy 1994; King et al. 1994). Recent studies have revealed that these proteins are subject to additional levels of regulation that are critical for the DNA damage and replication checkpoint response (for reviews, see Enoch and Nurse 1991; Carr and Hoekstra 1995; Elledge 1996; Nurse 1997). The Chk1 kinase is activated in response to DNA damage and has been shown to phosphorylate the Cdc25 phosphatase (Walworth et al. 1993; Walworth and Bernards 1996; Furnari et al. 1997; Sanchez et al. 1997). This phosphorylation enhances the binding of 14-3-3 to Cdc25, and down-regulates Cdc25, thereby affecting a cell-cycle arrest (Peng et al. 1997; Kumagai et al. 1998). Both Chk1 kinase and Cds1 kinase (another kinase activated in response to DNA damage during S phase; Murakami and Okayama 1995; Lindsay et al. 1998) have been shown to phosphorylate Wee1 (O’Connell et al. 1997; Boddy et al. 1998). Genetic evidence indicates that these kinases may positively regulate Wee1 in response to replication defects or DNA damage during S phase (O’Connell et al. 1997; Boddy et al. 1998). G2/M regulatory components have also been shown to be downstream targets of MAPK following nutritional/environmental stress in yeast (Millar et al. 1995; Shiozaki and Russell 1995). Importantly, it has been shown that overexpression of the MAPK homolog MPK1 induces a G2/M arrest in a Wee1-dependent manner in Saccharomyces cerevisiae (Mizunuma et al. 1998). Furthermore, negative regulation of the cell cycle in response MAPK activation has been observed in mammalian systems. The expression of Mos in NIH3T3 cells was shown to produce a prolonged G2 phase (Okazaki et al. 1992) and the expression of Ras or Raf in primary cell lines has been shown to be growth inhibitory (for review, see Lloyd 1998). The early embryonic cycles of Xenopus are not subject to normal somatic checkpoint controls (Newport and Kirschner 1984). Here, it appears that these evolutionarily conserved signaling modules are used to generate developmentally important modifications of the cell cycle. Our findings show that Xe-Wee1 is required for the protracted G2 phase generated by Mos in the first cell cycle. We also find that Xe-Wee1 is positively regulated by tyrosine autophosphorylation that can be up-regulated by Mos. These findings underscore the remarkable conservation of cell cycle and signal transduction pathways as well as the versatility of the MAPK signaling cascade.
Materials and methods
Mutagenesis
The tyrosine residues at position 90, 103, and 110 were mutated to phenylalanine residues using the ‘Quick change’ site-directed mutagenesis kit (Stratagene, La Jolla, CA). Approximately 1kb of the region spanning the mutation was sequenced to verify the point mutation and to ensure that no additional mutations were introduced.
Xe-Wee1 kinase assays
The various forms of Xe-Wee1 were translated in vitro (TNT, Promega) and incubated overnight at 4°C with antibodies generated against Xe-Wee1 (200 μl TNT reaction: 8 μl antibody 725 and 8 μl antibody 1532). The immune complexes were collected on protein A–Sepharose (Pharmacia) and washed twice with wash buffer (50 mm HEPES at pH 7.5, 5 mm EDTA, 5 mm EGTA) containing 0.75 m NaCl, twice with wash buffer containing 0.15 m NaCl and once with Xe-Wee1 kinase buffer (20 mm HEPES at pH 7.5, 150 mm NaCl, 5 mm MgCl2, 5 mm MnCl2, 1 mm DTT, 10% sucrose). For the in vitro autokinase assay, the Xe-Wee1 immune complexes were incubated for 20 min at room temperature in kinase buffer that had been supplemented with 40 μCi [γ-32P]ATP (6000 Ci/mmole, NEN). With lower salt (0.5 m NaCl) washes we detect serine phosphorylation of wild-type Xe-Wee1 and kinase mutant Xe-Wee1 (M.S. Murakami, unpubl.). The phosphorylated proteins were resolved by SDS-PAGE and visualized by autoradiography. The band corresponding to Xe-Wee1 was excised from the gel and the protein was eluted by incubation overnight at 37°C in 5 mm ammonium bicarbonate, 0.1% SDS, and 12.5 mmβ-mercaptoethanol. The protein was precipitated from the supernatant by incubation with 20% TCA and 16 μg/ml bovine serum albumin (BSA) (final concentrations) for 1 hr on ice and centrifugation for 15 min at 4°C. The pellet was washed once with acetone, dried, and treated with 10 μg of sequencing grade trypsin or endoproteinase Lys-C (Boehringer Mannheim) overnight at 37°C. The sample was then subjected to reverse phase HPLC analysis.
Exogenous substrate assay
The various forms of Xe-Wee1 were translated and subjected to immunoprecipitation as described above. The immune complexes were supplemented with Xe-Wee1 kinase buffer, 20 μCi [γ-32P]ATP, and 0.9 μg of cyclin B/cdc2 (gift from T. Stukenberg, Harvard Medical School, Boston, MA). The reactions were continued for 15 min at room temperature, stopped with sample buffer, and subjected to SDS-PAGE. Following electrophoretic transfer to Immobilon membrane, the filter was exposed to autoradigraphic film. The filter was then probed with an antiphosphotyrosine antibody, then stripped and reprobed with anti-Xe-Wee1 and anti-cdc2 antibodies.
Reverse-phase HPLC analysis
The analysis was performed essentially as described in Morrison (1993) and Cleghon (1996). Following digestion with trypsin or Lys-C, the sample was adjusted to pH 2.0 with 20% trifluoroacetic acid and then applied onto a Waters C18 column (3.9 × 300 mm). When the buffer salts began to elute, the column was developed with an increasing gradient of acetonitrile in 0.05% aqueous trifluoroacetic acid. The stepwise gradient at a flow rate of 1 ml/min was 0–30% CH3CN over 90 min, 30%–60% CH3CN for 5 min, and 60% CH3CN for 5 min. Fractions were collected at 1-min intervals and the radioactivity was quantified by Cherenkov counting in a scintillation counter.
Phosphoamino acid analysis
For the phosphoamino acid analysis, an aliquot of the HPLC fraction (at least 500 cpm) was dried under vacuum, subjected to acid hydrolysis with 5.7 n HCl for 1 hr at 110°C, and subjected to two-dimensional thin-layer electrophoresis as described by Boyle et al. (1991).
Edman degradation
Semiautomated amino-terminal sequence analysis was performed in a Beckman 890C spinning cup sequencer. Polybrene (2.5 mg, Aldrich) was applied to the spinning cup along with 120 nmoles of the dipeptide Tyr–Glu and subjected to four cycles of Edman degradation. Equine apomyoglobin (9 nmoles) along with the 32P-labeled peptide in CH3CN water was then added to the spinning cup, dried, and subjected to 20 cycles with no prewashes. Aliquots of the butyl chloride solution of the amino acid thiazoline derivatives in cycles 2 and 11 were removed to monitor the sequencing of the apomyoglobin. The remainder of each butyl chloride fraction was transferred to a glass scintillation vial, blown dry in a chemical hood with air, under a heat lamp, redissolved in 6 ml of PCS (Amersham) allowed to equilibrate in the dark for at least 2 hr. Radioactivity was quantified by counting in a scintillation counter for 20 min.
Two-dimensional thin-layer electrophoresis and chromatography
The fractions collected from the HPLC analysis were dried under vacuum then resuspended in pH 1.9 buffer (Boyle et al. 1991). The sample was spotted onto a TLC plate (EM4455), then subjected to thin layer electrophoresis in pH 1.9 buffer (25 min at 1000 V), followed by chromatography in N-butanol/pyridine/acetic acid/water (75:50:15:60). The plates were dried and the peptides were visualized by autoradiography.
Histone H1 kinase assays
Lysates were prepared from oocytes and eggs in modified EB (80 mm β-glycerophosphate, 20 mm HEPES at pH 7.5, 20 mm EGTA, 15 mm MgCl2, 1 mm sodium vanadate, 50 mm NaF, 20 mm sodium pyrophosphate, 2 mm DTT, 1 mm PMSF, 10 μg/ml aprotinin, 50 μg/ml leupeptin, 2 μg/ml pepstatin, 1 μm microcystin, 2.5 μm okadaic acid) at 10 μl/oocyte or egg. The lysates were clarified by centrifugation at 15,000 rpm for 15 min at 4°C. Five microliters of the resulting supernatant was added to 20 μl of H1 kinase reaction mix (final concentrations, 20 mm HEPES at pH 7.5, 5 mm EGTA, 10 mm MgCl2, 0.1 mm ATP, 5 μm PKA inhibitor peptide, 2 μg of histone H1) and 10 μCi [γ-32P]ATP. The reactions were incubated at room temperature for 15 min and resolved by SDS-PAGE. The bands corresponding to Histone H1 were excised and the incorporation was quantified by scintillation counting.
Oocytes, eggs, and extracts
Stage VI oocytes were isolated by manual dissection in 1× Modified Barth’s saline (Specialty Media, Lavallette, NJ) from unprimed females (Xenopus I, Ann Arbor MI). Maturation was induced with 10 μg/ml progesterone. GVBD was scored by the appearance of a white spot in the animal hemisphere and confirmed after fixation in 10% TCA. Injections into stage VI oocytes were done in Oocyte Culture Media (60% Lebovitz-15, 0.4 mm l-glutamine, 1% penicillin–streptomycin, 0.04% BSA). MBP–Mos fusion protein was prepared as described by Yew (1992) and injected to a final concentration of ∼9 ng/oocyte. RNAs were transcribed and capped in vitro using the mMessage machine kit (Ambion), resuspended in DEPC-treated H20, and injected at ∼40 ng/oocyte.
Unfertilized eggs were isolated either by in vitro maturation of stage VI oocytes, or by injecting female frogs with 800 units of Human Chorionic Gonadotropin 12–16 hr before to isolation. Eggs, which were laid, were dejellied in 2% cysteine/0.3× MMR. Before activation, the in vitro-matured oocytes or eggs were washed twice in 1× MMR, then activation was initiated by incubation in A23187 calcium ionophore for 5–10 min at room temperature (Boehringer Mannheim; 0.5 μg/ml in 1× MMR) after which time the eggs were washed three times in 0.1× MMR.
CSF extracts were prepared as described by Lohka and Masui (1983) and Murray (1991). Immunodepletions were done by incubating the CSF extracts with 0.5 volumes of protein A–Sepharose beads that were first coated with an equal volume of Xe-Wee1 antibody (1:1 Ab 725:Ab 1532). The extracts were supplemented with one-tenth volume (of extract) of MBP–Mos fusion protein (0.7 mg/ml). The release from the CSF arrest was initiated by the addition of 0.45 mm CaCl2. A 9- to 10-μl aliquot was removed at each time interval and diluted 10-fold with modified EB and frozen on dry ice. Two microliters of this was used in the histone H1 kinase assay. In some experiments, exogenous Xe-Wee1 was added to the extracts. In vitro-translated Xe-Wee1 was added back to the extract in the form of an immune complex.
32P-Labeling in the extracts was performed by adding 10 mCi of [γ-32P]ATP (which had been dried under vacuum, Dupont NEN) to 150–200 μl of extract. At the indicated time, the extract was ‘stopped’ by adding an equal volume of stop buffer (50 mm EDTA, 50 mm EGTA, 80 mm β-glycerol phosphate, 5 mm sodium vanadate, 50 mm NaF, 20 mm sodium pyrophosphate, 2 mm DTT, 20 mm HEPES at pH 7.5, 4 μm okadaic acid, 2 μm microcystin, and protease inhibitors). The diluted extracts were incubated with antibody for 3–4 hr at 4°C, and immune complexes were collected on protein A–Sepharose, washed, and subjected to SDS-PAGE. The 32P-labeled proteins were eluted from the gel, digested with trypsin and analyzed by reverse-phase HPLC analysis as described above.
Western blotting and immunoprecipitation
Lysates were prepared in EB and 1–1.5 oocyte or embryo equivalents were loaded per lane. Xe-Wee1 antibodies were characterized previously; antibodies 1532 and 725 were used at a dilution of 1:25 for immunoprecipitation and antibody 725 was used at a dilution of 1:1000 for immunoblotting. Mos (SC-86; used at 1:200 for immunoblotting) and cdc2 (SC-54; used at 1:200 for immunoblotting) antibodies were purchased from Santa Cruz Biotechnology. Immunoblotting was completed with an HRP-conjugated goat anti-rabbit secondary antibody (Boehringer Mannheim) and ECL (Amersham) detection.
For the analysis of cdc2 tyrosine phosphorylation, lysates from 10–15 oocytes or embryos were incubated with 30–50 μl p13/suc1 beads (Oncogene Science) overnight at 4°C. The beads were washed three times in wash buffer and processed for SDS-PAGE. The proteins were transferred to Immobilon membranes that were then incubated with the anti-phosphotyrosine antibody 4G10 (1:3000, generously provided by D. Morrison, ABL Basic Research Program, NCI-FCRDC). The 4G10 antibody was also used to analyze the tyrosine phosphorylation state of Xe-Wee1. Western analysis was completed with an HRP-conjugated goat anti-mouse secondary antibody and visualized following ECL detection.
Acknowledgments
We thank D. Morrison for advice, critical reading, and 4G10 antibody and T. Stukenberg for recombinant cyclin B/cdc2 and helpful discussions. We also thank A. Cline for manuscript preparation. This research was sponsored in part by the National Cancer Institute, Department of Health and Human Services, under contract with ABL.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL woude@ncifcrf.gov; FAX (301) 846-5038.
References
- Abrieu A, Lorca T, Labbe J-C, Morin N, Keyse S, Doree M. MAP kinase does not inactivate, but rather prevents the cyclin degradation pathway from being turned on in Xenopus egg extracts. J Cell Sci. 1996;109:239–246. doi: 10.1242/jcs.109.1.239. [DOI] [PubMed] [Google Scholar]
- Abrieu A, Fisher D, Simon M-N, Doree M, Picard A. MAPK inactivation is required for the G2 to M-phase transition of the first mitotic cell cycle. EMBO J. 1997;16:6407–6413. doi: 10.1093/emboj/16.21.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligue R, Wu L, Russell P. Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J Biol Chem. 1997;272:13320–13325. doi: 10.1074/jbc.272.20.13320. [DOI] [PubMed] [Google Scholar]
- Atherton-Fessler S, Liu F, Gabrielli B, Lee M S, Peng C-Y, Piwnica-Worms H. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994;5:989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitangcol JC, Chau AS-S, Stadnick E, Lohka MJ, Dicken B, Shibuya EK. Activation of the p42 mitogen-activated protein kinase pathway inhibits cdc2 activation and entry into M-phase in cycling Xenopus egg extracts. Mol Biol Cell. 1998;9:451–467. doi: 10.1091/mbc.9.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Booher RN, Deshaies RJ, Kirschner MW. Properties of Saccharomyces cerevisiae Wee1 and its differential regulation of p34cdc28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Campbell SD, Sprenger F, Edgar BA, O’Farrell PH. Drosophila wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila cdc2 in vitro. Mol Biol Cell. 1995;6:1333–1347. doi: 10.1091/mbc.6.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AM, Hoekstra MF. The cellular responses to DNA damage. Trends Cell Biol. 1995;5:32–40. doi: 10.1016/s0962-8924(00)88934-5. [DOI] [PubMed] [Google Scholar]
- Cleghon V, Gayko U, Copeland TD, Perkins LA, Perrimon N, Morrison DK. Drosophila terminal structure development is regulated by the compensatory activities of positive and negative phosphotyrosine signaling sites on the Torso RTK. Genes & Dev. 1996;10:566–577. doi: 10.1101/gad.10.5.566. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Daar I, Paules RS, Vande Woude GF. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991;114:329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG. The decision to enter mitosis. Trends Cell Biol. 1994;4:202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: Preventing an identity crisis. Science. 1996;274:1664–1671. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Enoch T, Nurse P. Coupling M phase and S phase: Controls maintaining the dependence of mitosis on chromosome replication. Cell. 1991;65:921–923. doi: 10.1016/0092-8674(91)90542-7. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Gerhart JG. Mechanisms regulating pattern formation in the amphibian egg and early embryo. In: Goldberger RF, editor. Biological regulation and development. New York, NY: Plenum Publishing; 1980. pp. 133–316. [Google Scholar]
- Gerhart J, Wu M, Kirschner M. Cell cycle dynamics of an M phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Rempel RE, Maller JL. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Heald R, McLoughlin M, McKeon F. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell. 1993;74:463–474. doi: 10.1016/0092-8674(93)80048-j. [DOI] [PubMed] [Google Scholar]
- Huang C-YF, Ferrell J. Dependence of Mos-induced Cdc2 activation on MAP kinase function in a cell-free system. EMBO J. 1996;15:2169–2173. [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1+-like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Noble MEM, Owen DJ. Active and inactive protein kinases: Structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Jones C, Smythe C. Activation of the Xenopus cyclin degradation machinery by full-length cyclin A. J Cell Sci. 1996;109:1071–1079. doi: 10.1242/jcs.109.5.1071. [DOI] [PubMed] [Google Scholar]
- Kanki JP, Donoghue DJ. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc Natl Acad Sci. 1991;88:5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kirschner MW, Gerhart JC. Spatial and temporal changes in the amphibian egg. BioScience. 1981;31:381–388. [Google Scholar]
- Kirschner MW, Butner KA, Newport JW, Black SD, Scharf SR, Gerhart JC. Spatial and temporal changes in early amphibian development. Netherlands J Zool. 1981;31:50–77. [Google Scholar]
- Kornbluth S, Sebastian B, Hunter T, Newport J. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol Biol Cell. 1994;5:273–282. doi: 10.1091/mbc.5.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Regulation and function of the MAP kinase cascade in Xenopus oocytes. J Cell Sci (Suppl.) 1994;18:115–119. doi: 10.1242/jcs.1994.supplement_18.17. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay HD, Griffiths DJF, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes & Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The Human Myt1 Kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AC. Ras versus cyclin-dependent kinase inhibitors. Cur Opin Genet Dev. 1998;8:43–48. doi: 10.1016/s0959-437x(98)80060-9. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–146. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JB, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes & Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Mizunuma M, Hirata D, Miyahara K, Tsuchiya E, Miyakawa T. Role of calcineurin and Mpk1 in regulating the onset of mitosis in budding yeast. Nature. 1998;392:303–306. doi: 10.1038/32695. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Heidecker G, Rapp UR, Copeland TD. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268:17309–17316. [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus wee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- Murakami MS, Vande Woude GF. Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-Wee1 and Mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- Murray AW. Methods of cell biology. San Diego, CA: Academic Press; 1991. Cell cycle extracts; pp. 581–605. [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Hunt T. The c-Mos protooncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- ————— Regulation of the cell cycle during early Xenopus development. Cell. 1984;37:731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Nishizawa M, Furuno N, Yasuda H, Sagata N. Differential occurrence of CSF-like activity and transforming activity of Mos during the cell cycle in fibroblasts. EMBO J. 1992;11:2447–2456. doi: 10.1002/j.1460-2075.1992.tb05309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe SJ, Wolfes H, Kiessling AA, Cooper GM. Microinjection of antisense c-mos oligonucleotides prevents meiosis II in the maturing mouse egg. Proc Natl Acad Sci. 1989;86:7038–7042. doi: 10.1073/pnas.86.18.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human Wee1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Parker LL, Atherton-Fessler S, Lee MS, Ogg S, Falk JL, Swenson KI, Piwnica-Worms H. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 1991;10:1255–1263. doi: 10.1002/j.1460-2075.1991.tb08067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Atherton-Fessler S, Piwnica-Worms H. p107Wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Peng C-Y, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25c on Serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Pham CD, Arlinghaus RB, Zheng C-F, Guan K-L, Singh B. Characterization of MEK1 phosphorylation by the v-Mos protein. Oncogene. 1995;10:1683–1688. [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resing KA, Mansour SJ, Hermann AS, Johnson RS, Candia JM, Fukasawa K, Vande Woude GF, Ahn NG. Determination of v-mos-catalyzed phosphorylation sites and autophosphorylation sites on MAP kinase kinase by ESI/MS. Biochemistry. 1995;34:2610–2620. doi: 10.1021/bi00008a027. [DOI] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sagata N. Meiotic metaphase arrest in animal oocytes: Its mechanisms and biological significance. Trends Cell Biol. 1996;6:22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- ————— What does Mos do in oocytes and somatic cells? BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: Linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Solomon MJ. Activation of the various cyclin/cdc2 protein kinases. Curr Opin Cell Biol. 1993;5:180–186. doi: 10.1016/0955-0674(93)90100-5. [DOI] [PubMed] [Google Scholar]
- Tang Z, Coleman TR, Dunphy WG. Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J. 1993;12:3427–3436. doi: 10.1002/j.1460-2075.1993.tb06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Walter SA, Guadagno TM, Ferrell JE., Jr Induction of a G2-phase arrest in Xenopus egg extracts by activation of p42 mitogen-activated protein kinase. Mol Biol Cell. 1997;8:2157–2169. doi: 10.1091/mbc.8.11.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Hunt T, Ikawa Y, Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991;352:247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human Wee1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N, Mellini ML, Vande Woude GF. Meiotic initiation by the mos protein in Xenopus. Nature. 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]