Abstract
Desorption electrospray ionization-mass spectrometry (DESI-MS) has advantages for rapid sample analysis with little or no sample pretreatment, but performance for large biomolecules has not been demonstrated. In this study, liquid sample DESI, an extended version of DESI used for analysis of liquid sample, was shown to have capabilities for direct ionization of large noncovalent protein complexes (>45 kDa) and proteins (up to 150 kDa). Protein complex ions (e.g., superoxide dismutase, enolase, and hemoglobin) desorbed from solution by liquid sample DESI were measured intact, indicating the capability of DESI for preserving weak noncovalent interactions. Doping the DESI spray solvent with supercharging reagents resulted in protein complex ions having increased multiple charging without complex dissociation. Ion mobility measurements of model protein cytochrome c showed that the supercharging reagent favored the more compact conformation for the lower charged protein ions. Liquid sample DESI of hydrophobic peptide gramicidin D suggests that the ionization mechanism involves a droplet pick-up mixing process. Measurement of liquid samples significantly extends the mass range of DESI-MS, allowing the analysis of high-mass proteins such as 150 kDa immunoglobulin G (IgG), and thus represents the largest protein successfully ionized by DESI to date.
Keywords: desorption electrospray ionization, mass spectrometry, noncovalent protein complexes, ion mobility
INTRODUCTION
Protein complexes, such as those formed by noncovalent protein-protein and protein-ligand interactions, are the cornerstone of many biological processes and together they form various types of molecular machines that perform a vast array of biological functions. Because of their biological significance, the ability to structurally characterize protein complexes is of great importance. In this regard, mass spectrometry (MS) has demonstrated its utility for characterizing noncovalent protein complexes, particularly with the development of electrospray ionization (ESI).1 MS measurements of biomolecular assemblies provide information on binding partners, binding stoichiometries, and binding affinities.2-6 With the more recent development of tandem mass spectrometry (MS/MS) tools to interrogate intact protein-ligand complexes (i.e., top-down MS), the spatial or positional information on ligand binding sites can be assessed directly.7-9 The conceptual simplicity of this ESI-MS method, along with its speed, sensitivity, and low sample consumption makes MS an attractive tool compared to other spectroscopic techniques for monitoring binding and probing protein conformation.
A recent breakthrough in the MS field is the advent of ambient mass spectrometry techniques, such as desorption electrospray ionization (DESI)10 and direct analysis in real time (DART),11 which provide direct ionization of analytes with little or no sample preparation. It has been shown that DESI promotes the rapid analysis of a wide variety of analytes, ranging from pharmaceuticals to imaging small molecules directly from tissue.10, 12-15 However, previous investigations using traditional DESI have been limited to the examination of small proteins (≤ 27 kDa)14 and small protein-substrate complexes (≤ 15 kDa, e.g., lysozyme with its natural hexa-N-acetyl chitohexaose substrate),10 while large protein complexes have not been extensively explored by DESI.
Recently, in addition to being used regularly for solid sample analysis on surfaces, DESI has been extended to the direct analysis of liquid samples.16-20 In this “liquid sample DESI” technique, analyte desorption/ionization occurs via interactions of the liquid sample with charged microdroplets generated by a pneumatically-assisted DESI spray with subsequent desolvation of the resulting secondary microdroplets containing the sample analyte. It was found that liquid sample DESI can be used for the analysis of a wide range of compounds including amino acids, peptides, protein digests, and intact proteins directly from solution without extensive sample pretreatment. Several analytically useful features of liquid sample DESI have been uncovered, such as an increased tolerance to salt and other matrices that allows direct analysis of biological samples, e.g., raw urine. The direct sampling capability of liquid sample DESI allows electrolyzed samples from an electrochemical cell to be directly probed, enabling a convenient on-line coupling of MS with electrochemistry.17, 21 Liquid sample DESI is amenable for analysis of very small volume samples (e.g., nL size)20, 22 and continuous-flow samples eluting from a chromatographic column.23 The capability of integrating ionic reactions with the ionization event, i.e., reactive DESI,17, 20, 24-29 in which a chemical reagent is doped with the spray solvent to react with analytes, allows on-line post-column chromatographic derivatization for enhanced detection specificity.23
The present study focuses on the direct ionization of protein complexes and large proteins under ambient conditions by liquid sample DESI. The motivations for this study are multifold. Previously reported traditional DESI-MS studies of proteins and protein complexes dried on a surface demonstrated a molecular mass limit of less than 27 kDa.14 Interestingly, liquid sample DESI has been found to increase the mass range, as evidenced by the ionization of 66 kDa bovine serum albumin (BSA). Given this likelihood that DESI of proteins from solution is easier than from dried samples on a surface and the significance of mass spectrometric characterization of protein complexes, it is of interest to further explore the application of DESI to the analysis of larger proteins and protein complexes.
Also, DESI has the aforementioned capability to combine ionic reactions with its ionization process. This would allow one to increase the multiple charging of the resulting protein-complex ions by performing a variant form of reactive DESI17, 20, 24-26, 28-30 in which the DESI spray solvent is conveniently doped with “supercharging” reagents.31-37 Increasing multiple charging for MS analysis of large proteins could be of significant value to further structural analysis via top-down proteomic approaches using electron-based tandem mass spectrometry techniques, such as electron capture dissociation (ECD)7 and electron transfer dissociation (ETD).38
In the present study, several protein complexes and large proteins were examined using liquid sample DESI analysis and a quadrupole time-of-flight (QTOF) mass spectrometer with a broad m/z range, as well as an ion mobility function. In addition, by doping the DESI spray solvent or protein sample with supercharging reagents, the supercharging effect can be observed for protein-complex ions. This study demonstrates the potential of liquid sample DESI for protein-complex and large protein characterization.
EXPERIMENTAL SECTION
Materials
Cytochrome c (cytc, bovine), manganese superoxide dismutase (MnSOD from E. coli), enolase (from yeast), gramicidin D, antibody IgG1 kappa murine myeloma (IgG), m-nitrobenzyl alcohol (m-NBA), sulfolane, formic acid (FA), ammonium acetate (NH4OAc), and acetonitrile (ACN) were purchased from Sigma-Aldrich. Human hemoglobin (Hb) was provided by Professor Robert Clubb (UCLA Department of Chemistry and Biochemistry).
Apparatus
The liquid sample DESI source has been described previously,17, 21 in which a beam of charged microdroplets from the DESI spray probe is directed at the outlet of a sample introduction capillary for desorption and ionization. The sample introduction capillary outlet was positioned between the DESI spray probe and the mass spectrometer interface (Figure S-1, Supporting Information). A quadrupole-ion mobility-TOF instrument (Synapt HDMS, Waters Corporation, Milford, MA) was used for MS measurements. The standard nano-electrospray source was removed to accommodate the liquid sample DESI source. The DESI spray probe was supplied with a high voltage of +5 kV and high-pressure nebulizing nitrogen gas (120 psi) to generate the charged microdroplet beam for sample desorption and ionization. The DESI spray solvent was altered between ACN/H2O/FA acid (49.5:49.5:1 v/v/v) or 20mM NH4OAc, and was pumped at a flow rate of 10 μL/min through a gas-tight Hamilton syringe.
The Synapt HDMS parameters were: sampling cone voltage was kept at 80V, extraction cone voltage 5V, source temperature 80°C, trap collisional energy 4, and transfer collisional energy 6. Data were analyzed using MassLynx™ 4.1 and Driftscope v2.0 (Waters Corporation).
Sample Preparation
For protein-complex samples, the following conditions were implemented: MnSOD in 20 mM NH4OAc (or in 1% acetic acid for comparison); enolase in H2O; Hb in 20 mM NH4OAc, IgG in H2O/ACN/FA (90/10/0.1 v/v/v), and gramicidin D in methanol. The solutions were introduced into the DESI source through a deactivated fused silica capillary at a flow rate of 2 μL/min. Ion mobility measurements were exclusively conducted with cytochrome c, utilizing sample solvents of either H2O or 4% acetic acid both with and without the supercharging reagents. DESI spray solvents were kept at ACN/H2O/FA 49.5/49.5/1 (v/v/v), both with and without the supercharging reagents.
RESULTS AND DISCUSSION
Liquid sample DESI allows proteins in solution (e.g., water) to be directly desorbed and ionized with high sensitivity without addition of acids or organic solvents into sample solutions to generate native protein ions (e.g., cytochrome, ubiquitin, etc).39 Such a direct sampling feature could be advantageous for probing changes in protein conformation,40 and provides a complementary method for detecting noncovalent protein complexes to the well-known native ESI-MS.3, 41
The added flexibility of having different solvent systems tailored for the analyte and the DESI spray is a unique feature for liquid sample DESI. Analytes do not need to be soluble in the DESI solvent system. Gramicidin D is a mixture of linear, hydrophobic pentadecapeptides (gramicidin A, B, and C) with alternating D- and L-amino acids. The Val1-gramicidin A variety is the most abundant species (1881 Da). The peptides are not soluble in water, but can be dissolved in organic solvents (e.g., methanol). Gramicidin D (10 μM in methanol) was analyzed by liquid sample DESI using a spray solvent of 20 mM aqueous NH4OAc or a solvent spray comprised of 50/50 ACN/H2O, 0.1% FA. Both the singly and doubly charged ions were observed for both spray solvent systems (Figure S-2). This suggests that the methanolic droplets containing the analyte were picked up by the aqueous charged droplets from the DESI solvent spray probe, resulting in secondary fused microdroplets (as methanol and water are miscible) that subsequently enter the mass spectrometer for analysis.17 This droplet pick-up mechanism appears to be plausible for liquid sample DESI analyses, suggesting that ionization occurs after mixing of charged droplets generated from the liquid sample at the DESI source silica capillary.
Liquid Sample DESI of Noncovalent Protein Complexes
Protein complexes, some larger than 40 kDa, were tested using the quadrupole-ion mobility-TOF instrument equipped with a home-built liquid sample DESI source.
Superoxide Dismutase
Manganese superoxide dismutase (MnSOD from E. coli; monomer apo-protein, 22966 Da), the SOD isoform found in the mitochondrial matrix of eukaryotes as well as a variety of prokaryotes, is a metalloenzyme that plays an important role in immune defense by eliminating oxidative stress.42 In this study, 30 μM MnSOD in 20 mM NH4OAc (pH 6.8) in the sample capillary was desorbed/ionized by the DESI source using a 20 mM aqueous NH4OAc spray solvent. The 11+ to 14+ charged noncovalent 46 kDa dimer ions of MnSOD with two Mn ions bound per dimer (Mn2-MnSOD) were clearly detected (Figure 1A), confirming the soft ionization nature of liquid sample DESI. Interestingly, changing the DESI spray solvent to ACN/H2O/FA (49.5/49.5/1.0 v/v) also generated 11+ to 14+ charged MnSOD dimer ions (Figure 1B). This result is somewhat surprising, as the newly introduced spray contained a high fraction of organic solvent and acid that typically denature proteins and disrupt noncovalent interactions. It is most likely that only a limited interaction (i.e., partial mixing) or transient interaction (estimated to be less than 1 ms40) occurs between the DESI spray solvent and the analyte solution in liquid sample DESI analyses. This observation is also consistent with our previous observation of holo-myoglobin ions using denaturing DESI spray solvent conditions.40 An added advantage of including the organics and acid in the spray solvent is the gain in sensitivity when compared with those experiments conducted using ammonium acetate as the spray solvent. Using the ACN/H2O/FA DESI spray solvent (Figure 1B) increased the signal-to-noise ratio (S/N) of the MnSOD dimer ions by a factor of 2-3 relative to using NH4OAc (Figure 1A). This is also in-line with our previously reported data on cytochrome c40 that showed that increased sensitivity can be achieved with non-denaturing analyte solutions by using a denaturing solvent system (methanol/H2O/acetic acid) for the DESI spray solvent. By using both a denaturing solvent in the analyte solution (1% acetic acid) with the ACN/H2O/FA DESI spray solvent resulted in the observation of the denatured 22.9 kDa apo-SOD monomer (Figure 1C).
Figure 1.
Mass spectra showing liquid sample DESI analysis of 30 μM MnSOD. (A) MnSOD in 20 mM NH4OAc sampled by a DESI solvent composed of 20 mM NH4OAc aqueous solution. (B) MnSOD in 20 mM NH4OAc sampled by a DESI solvent of ACN/H2O/FA. (C) MnSOD (15 μM) in 1% HOAc sampled by a DESI solvent of 1:1 ACN/H2O with 1% FA.
Enolase
Enolase is a metalloenzyme involved in the glycolytic pathway, catalyzing the dehydration of 2-phospho-D-glycerate. Enolase derived from yeast is a 93 kDa homodimeric complex (apo-monomer mass 46671 Da) that requires divalent metal ions, such as Mg2+, and first row divalent transition metals (e.g., Mn2+) for activity.5, 43 In this liquid sample DESI analysis, 10 μM enolase in H2O was analyzed with various DESI spray solvents. When using a DESI solvent of 20 mM aqueous NH4OAc, ions for the enolase 93 kDa noncovalent dimer were observed (93342 Da, theory; 93339 ± 6 Da, measured; 18+ to 22+ charge states), as well as protein monomer ions (Figure 2A). However, by using a potentially denaturing solvent composition (49.5/49.5/1.0 ACN/H2O/FA), the sensitivity was improved when compared to the 20mM aqueous NH4OAc solution (Figures 2 A,B). Moreover, using the ACN/H2O/FA spray solvent generated a noticeable increase in charge state for the dimer to 19+-26+. Although the acetonitrile/formic acid mixture did not cause complete denaturation to disrupt the noncovalent dimer, it may have cause partial unfolding of the enolase dimer exhibited by the higher degree of charging. In comparison to the MnSOD dimer, the enolase dimer appears to be more sensitive to unfolding in the presence of organic solvent and acid.
Figure 2.
Liquid sample DESI mass spectra of enolase (10 μM in water) with the DESI solvent composed of (A) 20 mM NH4OAc (aq), (B) 1:1 ACN/H2O with 1% FA, and (C) 1:1 ACN/H2O with 1% FA and 40 mM m-NBA supercharging reagent.
Currently, minimum protein concentrations of 1-10 μM have been analyzed using liquid sample DESI.17 For the protein enolase noncovalent dimer complex (Figure 2), a S/N ratio of ~9 was measured (for the 20+ dimer ion) from 4 pmol of protein injected. However, future experiments will explore the absolute sensitivity of the method for large protein analysis.
Hemoglobin
Human hemoglobin (Hb, 64.5 kDa) is a noncovalent tetrameric protein composed of two distinct polypeptide chains, two identical α subunits (141 amino acids, 15126 Da), and two identical β subunits (146 amino acids, 15867 Da), in addition to a heme molecule (protoporphyrin IX; 616 Da) noncovalently bound to each chain. The Hb tetramer associates from two αhβh heterodimers (where αh and βh represent the heme-bound α and β chains, respectively).44 Previous ESI-MS studies have shown that the intact Hb tetramer can be observed.44-48 The liquid DESI mass spectrum from 50 μM Hb in 20 mM NH4OAc and spraying a “native” solvent system (20 mM NH4OAc) enabled the observation of the intact Hb (αhβh)2 protein tetramer with charge states 14+ to 18+ (64451 Da, theory; 64449 ± 2 Da, measured) as well as the 32.2 kDa αhβh dimer (32226 Da, theory; 32225 ± 1 Da, measured; 11+ and 12+ charge states), with minor contributions from the αh and βh species (Figure 3). Changing the spray solvent system to one that contains acid (49.5/49.5/1 ACN/H2O/FA) and the Hb remaining in 20 mM NH4OAc resulted in the complete disruption of the noncovalent tetramer (data not shown). Thus, although the interaction between the spray solvent and the analyte is relatively short (likely less than 1 ms), the Hb tetramer appears to be the most sensitive to acid denaturation among the proteins studied.
Figure 3.
Liquid sample DESI mass spectra of human hemoglobin (50 μM) in 20 mM NH4OAc and a DESI spray solvent of (A) 20 mM NH4OAc and (B) 20 mM NH4OAc with 40 mM m-NBA. The “αhβh” notation refers to the heme-bound alpha and beta polypeptide chains. The free heme-bound alpha-chain is represented by the blue circle symbol ( ), and the red triangles (
), and the red triangles ( ) denote the heme-bound beta-chain.
) denote the heme-bound beta-chain.
Supercharging of Protein Complexes by Reactive Liquid DESI
As the mass range of macromolecular mass spectrometry has increased, exemplified by use of ESI-TOF and ESI-quadrupole-TOF instruments, new methods to supplement the analysis of large molecules have been developed, e.g., ion mobility. Another such development is the incorporation of supercharging reagents for ESI-MS that effectively increase the overall charge on a protein, and thus lowers the observed mass-to-charge ratio to a value well within the detectable range of most analyzers.34 Recent experiments have identified a number of reagents that produce this supercharging phenomenon when applied in an ESI setting, such as m-nitrobenzyl alcohol (m-NBA) and tetramethylene sulfone (sulfolane).31, 32 These reagents have characteristics of low solution-phase basicity and non-volatility compared to water, as well as the ability to directly interact with proteins in solution (and possibly in the gas phase) to stabilize charging in the gas phase and to promote the supercharging phenomenon. These supercharging reagents are effective in both denaturing and native solution conditions.33-37 When paired with DESI,38 supercharging becomes a useful tool that drives the threshold of high molecular weight protein analysis towards ever-increasing limits. Recently, a novel method, continuous flow-extractive desorption electrospray ionization (CF-EDESI), has been used to control the protein charge state distributions using different additives, such as acetic acid and sulfolane, in the analysis of small proteins such as cytochrome c and lysozyme.38
Cytochrome c
To investigate the integration of supercharging with liquid sample DESI, bovine cytochrome c (cytc, 12231 Da) was selected as a sample specimen. The protein (50 μM) prepared in water with a spray solvent of 50/50 ACN/H2O and 0.1% FA (v/v) showed a native-type charge state distribution (7+ to 10+) that is consistent with previous work (Figure 4A).40 The supercharging reagent m-NBA (40 mM) was doped into the ACN/H2O/FA spray solvent, resulting in higher charging, ranging from 7+ to 17+ and centered at 12+ (Figure 4B). Using sulfolane (200 mM) in a similar fashion generated a cytc charge distribution with slightly higher charging than observed for m-NBA (Figure 4C). The introduction of supercharging agents and the resulting increase in charging for liquid DESI mimic the data observed for electrospray-assisted laser desorption/ionization (ELDI),49, 50 another ambient MS technique.
Figure 4.
Supercharging liquid sample DESI mass spectra of 50 μM cytochrome c in water with DESI spray solvents of (A) 1:1 ACN/H2O and 0.1% FA, (B) 1:1 ACN/H2O and 0.1% FA with 40 mM m-NBA and (C) 1:1 ACN/H2O and 0.1% FA with 200 mM sulfolane.
The effect of the addition of m-NBA to the liquid DESI spray on the structure of cytc was probed by ion mobility-mass spectrometry. Figure 5 shows the ion mobility data obtained for the 7+-9+ charge states of cytc. The protein dissolved in 4% acetic acid and coupled with a spray solvent of 50/50 ACN/H2O and 0.1% FA (v/v) served as a control to represent the denatured protein. The Driftscope ion mobility plots are shown in Figure 5 for the various cytc charge states generated from different solution and DESI spray solvent systems. A longer drift time is associated with a less compact, or more unfolded conformation. The ion mobility profiles for the 7+-9+ charge states are similar for cytc in either H2O or 50/50 ACN/H2O with 0.1% FA (v/v), with perhaps a slight increase in the more native or compact conformer present with cytc in H2O (represented by the purple trace and the small reduction of the extended conformer peak for the 8+ ion and the increase in the compact conformer for the 9+ ion). Adding 40 mM m-NBA to the DESI spray solvent (cytc in H2O) resulted in an increase in the more native, compact conformation of the protein (green trace in Figure 5). This trend was observed in triplicate measurements. Taken together, this trend suggests that, rather than causing unfolding and denaturation, the m-NBA supercharging reagent in the spray solvent aids in stabilization of the native conformation of a protein. However, additional experiments with many different proteins are needed to more firmly establish the effects of supercharging agents on protein conformation.
Figure 5.
Ion mobility drift time plots of the (A) 7+, (B) 8+, and (C) 9+ charged ions of cytochrome c (50 μM). The green ( ) and blue (
) and blue ( ) traces represent the ion mobility of cytc in 4% and 0% acetic acid (in water), respectively, and the DESI solvent of 1:1 ACN/H2O with 0.1% FA. The red line (
) traces represent the ion mobility of cytc in 4% and 0% acetic acid (in water), respectively, and the DESI solvent of 1:1 ACN/H2O with 0.1% FA. The red line ( ) represents the data from cytc in water and the DESI solvent of 1:1 ACN/H2O with 0.1% FA and 40 mM m-NBA.
) represents the data from cytc in water and the DESI solvent of 1:1 ACN/H2O with 0.1% FA and 40 mM m-NBA.
Enolase and Hemoglobin
To further test the ability of liquid sample DESI to enable supercharging of larger proteins and protein complexes, 10 μM enolase in H2O was reacted with a spray solvent consisting of 40 mM m-NBA in 50/50 ACN/H2O and 0.1% FA. Figure 2C shows that the enolase dimer shift to higher charge, moving from 19+-26+ to the 23+-28+ range. (Note that the observed ions also have increased peak width, probably caused by the formation of adduct ions with m-NBA due to insufficient desolvation conditions used in the experiments). Similarly, by spraying 40 mM m-NBA in 20 mM NH4OAc, a charge increase was observed for both the Hb (αhβh)2 tetramer and αhβh dimer when compared to DESI lacking the supercharging reagent. Figure 3B shows a charge state distribution shift for the tetramer from a range of 15+-18+ to a range of 18+-21+; additionally, the dimer shifts from 11+-12+ to a higher range of 12+-16+. This further demonstrates that reactive DESI can be easily carried out using supercharging reagents to increase charging of proteins and protein complexes.
Immunoglobulin G (IgG) Measurement by Liquid Sample DESI
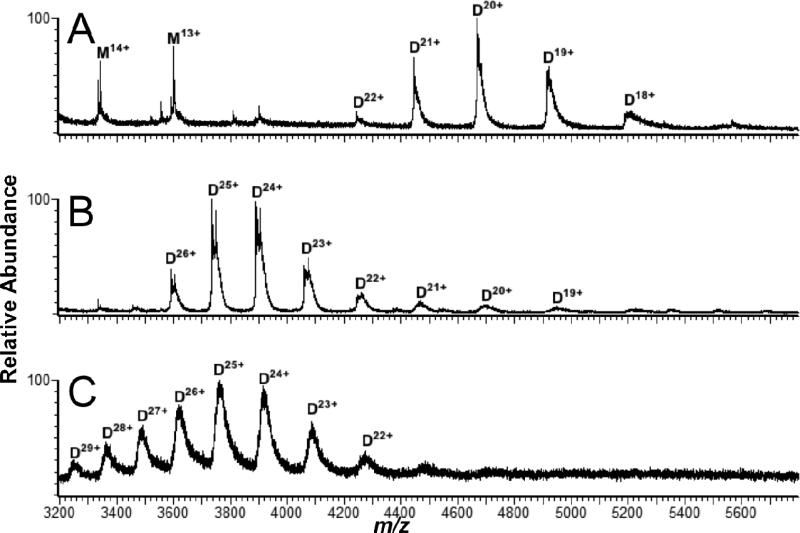
Traditional DESI of proteins desorbed from a dried state on a solid surface have molecular mass limits within the range of 27 kDa,14 whereas liquid sample DESI of proteins in a solution state, to date, have pushed this molecular mass limit to approximately 66 kDa (BSA).17 By coupling the liquid sample DESI apparatus to the higher mass range Q-TOF ion mobility mass spectrometer, this range for observable protein and protein-complex maximum molecular weights was extended to 150 kDa.
Immunoglobulin G (IgG) antibodies consist of 4 polypeptide chains: two identical heavy chains and two identical light chains all linked together by disulfide bonds that comprise the tetrameric quaternary structure. IgG (6 μM in 90/10 H2O/ACN, 0.1% FA) was analyzed using a spray solvent of 90/10 H2O/ACN, 0.1% FA, resulting in IgG ions ranging from 38+ to 55+ (Figure 6). Similar mass spectra were acquired with aqueous 20 mM NH4OAc as the spray solvent, albeit at slightly reduced signal-to-noise and charging (36+-51+) (data not shown).
Figure 6.
Liquid sample DESI mass spectrum of IgG (6 μM) in H2O/ACN/FA (90/10/0.1 by volume) with a DESI spray solvent composition the same as the analyte solvent.
CONCLUSIONS
As demonstrated in this series of liquid sample DESI studies, noncovalent interactions among protein complexes can be detected. Sensitivity for measuring protein complexes is improved slightly by using a denaturing spray solvent (i.e., containing organic acids). In addition, the inclusion of supercharging reagents in the DESI spray solvent produced the desired effect by increasing multiple charging of proteins and protein complexes without disrupting their structure and conformation, further expanding the capabilities of liquid sample DESI. Proteins as large as 150 kDa IgG and with low micromolar concentrations can be successfully ionized by DESI. Additional refinement to the DESI system in the future should further improve the sensitivity for large molecule analysis. Because of its flexibility and its compatibility with an unlimited range of spray solvent and sample solvents as well as its useful conjunction with supercharging compounds, liquid sample DESI is emerging as a useful tool for mass spectrometric monitoring of protein conformational changes in solution over real-time for proteins, both small and large.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (CHE-0911160 to HC), the Ruth L. Kirschstein National Research Service Award (GM007185, UCLA Cellular and Molecular Biology Training Grant, for CNF), and the National Institutes of Health (R01 RR20004 to JAL).
REFERENCES
- 1.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 2.Barrera NP, Bartolo ND, Booth PJ, Robinson CV. Science. 2008;321:243–246. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- 3.Heck AJR, van den Heuvel RHH. Mass Spectrom. Rev. 2004;23:368–389. doi: 10.1002/mas.10081. [DOI] [PubMed] [Google Scholar]
- 4.Loo JA. Mass Spectrom. Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Loo JA. Int. J. Mass Spectrom. 2001;204:113–123. [Google Scholar]
- 6.van Duijn E. J. Am. Soc. Mass Spectrom. 2010;21:971–978. doi: 10.1016/j.jasms.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Xie Y, Zhang J, Yin S, Loo JA. J. Am. Chem. Soc. 2006;128:14432–14433. doi: 10.1021/ja063197p. [DOI] [PubMed] [Google Scholar]
- 8.Yin S, Loo JA. J. Am Soc. Mass Spectrom. 2010;21:899–907. doi: 10.1016/j.jasms.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Yin S, Loo JA. Int. J. Mass Spectrom. 2011;300:118–122. doi: 10.1016/j.ijms.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 11.Cody RB, Laramee JA, Durst HD. Anal. Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 12.Denes J, Katona M, Hosszu A, Czuczy N, Takats Z. Anal. Chem. 2009;81:1669–1675. doi: 10.1021/ac8024812. [DOI] [PubMed] [Google Scholar]
- 13.Kauppila T, Wiseman JM, Ketola RA, Kotiaho T, Cooks RG, Kostiainen R. Rapid Commun. Mass Spectrom. 2006;20:387–392. doi: 10.1002/rcm.2304. [DOI] [PubMed] [Google Scholar]
- 14.Shin YS, Drolet B, Mayer R, Dolence K, Basile F. Anal. Chem. 2007;79:3514–3518. doi: 10.1021/ac062451t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotte-Rodriguez I, Chen H, Cooks RG. Chem. Commun. 2006:953–955. doi: 10.1039/b515122h. [DOI] [PubMed] [Google Scholar]
- 16.Miao Z, Chen H. Proceedings of the 56th ASMS Conference on Mass Spectrometry and Allied Topics; Denver, CO. 2008. [Google Scholar]
- 17.Miao Z, Chen H. J. Am. Soc. Mass Spectrom. 2009;20:10–19. doi: 10.1016/j.jasms.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Zhao M, Lin Z, Zhang S, Yang C, Zhang X. Anal. Chem. 2008;80:6131–6136. doi: 10.1021/ac800803x. [DOI] [PubMed] [Google Scholar]
- 19.Chipuk JE, Brodbelt JS. J. Am. Soc. Mass Spectrom. 2008;19:1612–1620. doi: 10.1016/j.jasms.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Chen H. Int. J. Mass Spectrom. 2010;289:98–107. [Google Scholar]
- 21.Li J, Dewald HD, Chen H. Anal. Chem. 2009;81:9716–9722. doi: 10.1021/ac901975j. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Miao Z, Yuan Z, Harrington PB, Colla J, Chen H. Int. J. Mass Spectrom. 2011;301:102–108. [Google Scholar]
- 23.Zhang Y, Yuan Z, Dewald HD, Chen H. Chem. Commun. 2011;47:4171–4173. doi: 10.1039/c0cc05736c. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Talaty N, Takats Z, Cooks RG. Anal. Chem. 2005;77:6915–6927. doi: 10.1021/ac050989d. [DOI] [PubMed] [Google Scholar]
- 25.Cotte RI, Chen H, Cooks RG. Chem. Commun. 2006:953–955. doi: 10.1039/b515122h. [DOI] [PubMed] [Google Scholar]
- 26.Cotte-Rodriguez I, Hernandez-Soto H, Chen H, Cooks RG. Anal. Chem. 2008;80:1512–1519. doi: 10.1021/ac7020085. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Cotte-Rodriguez I, Cooks RG. Chem. Commun. 2006:597–599. doi: 10.1039/b516448f. [DOI] [PubMed] [Google Scholar]
- 28.Nyadong L, Green MD, De Jesus VR, Newton PN, Fernandez FM. Anal. Chem. 2007;79:2150–2157. doi: 10.1021/ac062205h. [DOI] [PubMed] [Google Scholar]
- 29.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Anal. Chem. 2007;79:8327–8332. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Venter A, Cooks RG. Chem. Commun. 2006:2042–2044. doi: 10.1039/b602614a. [DOI] [PubMed] [Google Scholar]
- 31.Lomeli SH, Yin S, Ogorzalek Loo RR, Loo JA. J. Am. Soc. Mass Spectrom. 2009;20:593–596. doi: 10.1016/j.jasms.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomeli SH, Peng IX, Yin S, Ogorzalek-Loo RR, Loo JA. J. Am Soc. Mass Spectrom. 2010;21:127–131. doi: 10.1016/j.jasms.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iavarone AT, Jurchen JC, Williams ER. J. Am. Soc. Mass Spectrom. 2000;11:976–985. doi: 10.1016/S1044-0305(00)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iavarone AT, Jurchen JC, Williams ER. Anal. Chem. 2001;73:1455–1460. doi: 10.1021/ac001251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iavarone AT, Williams ER. Int. J. Mass Spectrom. 2002;219:63–72. [Google Scholar]
- 36.Iavarone AT, Williams ER. J. Am. Chem. Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iavarone AT, Williams ER. Anal. Chem. 2003;75:4525–4533. doi: 10.1021/ac034144i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang SH, Wijeratne AB, Li L, Edwards BL, Schug KA. Anal. Chem. 2011;83:643–647. doi: 10.1021/ac102327f. [DOI] [PubMed] [Google Scholar]
- 39.Miao Z, Chen H, Liu P, Liu Y. Anal. Chem. 2011;83:3994–3997. doi: 10.1021/ac200842e. [DOI] [PubMed] [Google Scholar]
- 40.Miao Z, Wu S, Chen H. J. Am. Soc. Mass Spectrom. 2010;21:1730–1736. doi: 10.1016/j.jasms.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaddis CS, Lomeli SH, Yin S, Berhane B, Apostol MI, Kickhoefer VA, Rome LH, Loo JA. J. Am Soc. Mass Spectrom. 2007;18:1206–1216. doi: 10.1016/j.jasms.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiujano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. J. Biol. Chem. 2001;276:11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- 43.Zhang E, Brewer JM, Minor W, Carreira LA, Lebioda L. Biochemistry. 1997;36:12526–12534. doi: 10.1021/bi9712450. [DOI] [PubMed] [Google Scholar]
- 44.Light–Wahl KJ, Schwartz BL, Smith RD. J. Am. Chem. Soc. 1994;116:5271–5278. [Google Scholar]
- 45.Apostol I. Anal. Biochem. 1999;272:8–18. doi: 10.1006/abio.1999.4140. [DOI] [PubMed] [Google Scholar]
- 46.Ofori-Acquah SF, Green BN, Davies SC, Nicolaides KH, Sarjeant GR, Layton DM. Anal. Biochem. 2001;298:76–82. doi: 10.1006/abio.2001.5358. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt A, Karas M. J. Am. Soc. Mass Spectrom. 2001;12:1092–1098. doi: 10.1016/S1044-0305(01)00294-X. [DOI] [PubMed] [Google Scholar]
- 48.Simmons DA, Wilson DJ, Lajoie GA, Doherty-Kirby A, Konermann L. Biochemistry. 2004;43:14792, 14801. doi: 10.1021/bi048501a. [DOI] [PubMed] [Google Scholar]
- 49.Peng IX, Ogorzalek Loo RR, Shiea J, Loo JA. Anal. Chem. 2008;80:6995–7003. doi: 10.1021/ac800870c. [DOI] [PubMed] [Google Scholar]
- 50.Peng IX, Ogorzalek Loo RR, Margalith E, Little MW, Loo JA. Analyst. 2010;135:767–772. doi: 10.1039/b923303b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.