Abstract
NK cells lyse virus-infected cells by degranulation; however, alterations in NK cell degranulation in persistent viral infections have not been directly studied. Earlier reports have documented a decrease in NK activity in patients with frequently recurring herpes (FRH). We corroborate these findings by showing that the degranulation responses of blood NK cells from patients with FRH, both during relapse and during remission, are significantly lower than those in healthy donors. The impaired degranulation was probably not caused by defective target cell recognition, since it was observed upon stimulation both with K562 cells and with a receptor-independent stimulus (phorbol 12-myristate 13-acetate plus ionomycin). We also show that the intracellular expression of perforin and CD107a by NK cells from patients with FRH is not different from that in healthy donors, thus excluding that the low NK cell degranulation in FRH is caused by a smaller size of the lytic granule compartment. We confirm previous reports on lowered NK activity in FRH patients and show that NK activity is significantly impaired only during remission, but not relapse; the causes for the discrepancy between the low degranulation and “normal” NK cell activity during relapse are discussed. In all, these data point at the deficit of NK cell degranulation in FRH. Whether this is a predisposing factor or a consequence of herpes simplex virus infection requires further investigation.
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) and HSV-2, the causative agents of herpes labialis and genitalis, are examples of the most common human pathogens. After primary invasion through epithelial surfaces, HSVs establish a latent infection in trigeminal or sacral ganglia. Up to 90% of humans are lifelong HSV carriers (11). The latent infection can be reactivated by a number of factors, such as psychological stress, fever, UV irradiation, or immunosuppression (38). Upon reactivation, the virus is transported to the epithelia of the mouth cavity, lips, or genitals, more seldom to epithelia at other locations, where it replicates causing vesicular rash and ulceration (38); the virus may also be shed asymptomatically (37). Frequently recurring herpes (FRH), defined here as a disease with two or more symptomatic relapses a year, represents a significant socioeconomic problem; in the most severe cases of FRH, asymptomatic periods are virtually absent.
NK cells play an important role in the control of HSV infection (1, 19, 36). Mice lacking NK cells, as well as humans with primary or secondary immune deficiencies affecting NK cells, are very sensitive to HSV and develop severe forms of HSV infection (1, 6, 25, 28). At the same time, HSVs are able to counteract NK cell activation or evade from NK cell recognition (16, 31).
NK cells recognize virus-infected target cells by means of inhibitory and activating receptors (26, 27). Activation of an NK cell can ensue from stimulation of activating receptors that recognize viral proteins or stress-induced molecules on the surfaces of infected cells, from the insufficient stimulation of inhibitory receptors due to reduced major histocompatibility complex (MHC) class I expression, or from both (27). NK cells are also activated by antibodies coating virus-infected targets, which results in antibody-dependent cell-mediated cytotoxicity (14). A hallmark of NK cell activation is degranulation, that is, the release of lytic granule contents (perforin and granzymes) onto the surface of the target cell. The inner surface of the granules is coated with CD107a (lysosome-associated membrane protein 1), a highly glycosylated protein that constitutes ca. 50% of membrane proteins of the lysosomes and their derivatives, including lytic granules (20, 23, 29, 39). After degranulation, CD107a is exposed on the surface of the cytotoxic lymphocyte, where it might protect the outer membrane from perforin-mediated damage (20). Externalization of CD107a has been proven to be a marker of degranulation of NK cells (2, 7), CD8+ T cells (5), and CD4+ T cells (10). An NK cell degranulation assay, based on externalization of CD107a, enables the direct detection and enumeration of NK cells that respond to a particular stimulus by the release of cytotoxic proteins. The results of NK cell degranulation assays have been demonstrated to correlate with those of the standard cytotoxicity assay (2).
We took advantage of the degranulation assay to enumerate degranulating NK cells in patients with FRH during recurrence and remission, in comparison to healthy donors. In parallel, the intracellular expression of perforin and CD107a in NK cells was examined. As an integral parameter of NK cell function, we analyzed NK activity, i.e., the ability of mononuclear cells (MNCs) from patients and donors to kill K562 target cells in a standard cytotoxicity assay.
MATERIALS AND METHODS
Patients and donors.
A total of 34 patients with FRH (11 males and 23 females) were examined. Their median age was 32.5 years (range, 24 to 50 years). (The age here and below is expressed as the median [10th to 90th percentiles].) Included were patients with a history of FRH and no other active infectious or inflammatory diseases at the time of examination. Diagnosis of FRH was verified by an experienced immunodermatologist (I.N.Z.). Most patients (n=30) suffered from herpes genitalis, two had herpes labialis, and another two had a combined form of the disease. The majority of the patients (n=25) had two to six recurrences a year, while nine patients had more severe disease (more than six recurrences a year).
A subgroup consisting of 24 patients was examined prospectively: once during relapse (within 48 h after first symptoms) and once in remission (∼1 month after the first symptoms of relapse). The remaining 10 patients were studied once during relapse. During relapses, all patients received acyclovir in a dose of 400 mg three times a day for 10 days.
The control group consisted of 56 healthy subjects (21 males, 35 females). Their median age was 27 years (range, 23 to 35 years). All subjects had no known chronic infectious or inflammatory diseases and no acute infections for at least 1 month preceding the blood sampling.
The study was approved by the local Ethical Committee at the Institute of Immunology (Moscow, Russia). All subjects gave informed consent to the study. Samples (10 ml) of venous heparinized blood were obtained from all subjects.
NK cell degranulation assay.
Degranulation of NK cells following in vitro stimulation was assessed by a flow cytometry-based assay as described previously (2, 30), with minor modifications. MNCs were isolated from venous blood by using density gradient centrifugation, washed three times, and resuspended in complete culture medium (RPMI containing 2 mM l-glutamine and 10% fetal calf serum; all from PAA, Pasching, Austria). MNCs were plated in 96-well U-bottom plates at 5 × 105 cells/well in the presence of monensin (10 μM) and a fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (MAb) against CD107a (clone H4A3, at a 1/200 dilution; BD Pharmingen, San Diego, CA). The anti-CD107a MAb was present in the medium throughout the stimulation period, because CD107a that has been externalized by NK cells upon degranulation is rapidly reinternalized (7). Degranulation was induced by adding K562 target cells (5 × 105 per well, effector/target [E:T] ratio=1:1) or phorbol 12-myristate 13-acetate plus ionomycin (PMA+ION) to final concentrations of 100 ng/ml and 0.5 μg/ml, respectively (both from Sigma, St. Louis, MO). As a positive control of degranulation, we used PMA+ION, together with cytochalasin B (CB; final concentration, 5 μg/ml [Sigma]). A negative control well received complete culture medium instead of degranulation stimuli. One more control well (staining control) received neither stimuli nor the anti-CD107a MAb. After all of the ingredients were combined, the contents of each well were mixed once with a pipette, and the plates were then centrifuged at 200 × g for 1 min, followed by incubation for 4 h in a CO2 incubator (5% CO2, 37°C). The plates were then centrifuged again (450 × g, 1 min), the supernatants were discarded, and the cells were resuspended in phosphate-buffered saline (PBS) containing 0.02% sodium azide and 0.02% EDTA in order to dissociate cell-cell aggregates (5 min, room temperature). The cells were then washed in PBS with 0.5% bovine serum albumin and stained with a PC5-labeled MAb against CD3 and a phycoerythrin (PE)-labeled MAb against CD56 (both from Beckman Coulter, Miami, FL). After another wash, the cells were analyzed by a Cytomics FC500 flow cytometer equipped with CXP software (Beckman Coulter). NK cells were identified as CD3− CD56+ events with light scatter characteristics of lymphocytes. Three readout parameters were measured: (i) the percentage of CD107a+ NK cells among all NK cells, (ii) the percentage of CD107a+ NK cells among all MNCs, and (iii) the mean fluorescence intensity (MFI) of externalized CD107a on CD107a+ NK cells.
NK activity assay.
The NK activity of the MNCs was measured by using a flow cytometry-based assay. Briefly, K562 target cells were labeled with CFSE (carboxyfluorescein diacetate succinimidyl ester; Invitrogen, Paisley, United Kingdom) and plated in 96-well U-bottom plates at 8 × 103/well. Effector MNCs were added in graded numbers such that the E:T ratio ranged from 3.125:1 to 50:1. Each E:T ratio was tested in duplicate. To control for spontaneous target cell death, an additional two wells containing only target cells were prepared. The cells were incubated 4 h at 37°C in the presence of 5% CO2 and then resuspended and stained for 10 min with propidium iodide (PI) at 1 μg/ml. Duplicates were pooled, and the cells were analyzed by using a Cytomics FC500 flow cytometer. In each sample, the percentage of dead targets was determined as the percentage of PI+ events among CFSE+ events. Specific killing was calculated as follows: % specific killing=[(% experimental target death − % spontaneous target death)/(100 − the % spontaneous target death)] × 100.
The lytic unit 20 (LU20) was defined as the amount of MNCs required to kill 20% of the target cells. To calculate the LU20, E:T ratios at which 20% targets would be killed were determined from killing curves and multiplied by 8 × 103 (the number of targets per well). For convenience, the data were expressed as the amount of LU20 per 105 MNCs. If a 20% killing was not reached even at the highest E:T ratio (50:1), then the amount of LU20 per 105 MNCs in the sample was assigned a value of “0”.
Intracellular staining for perforin and CD107a.
Freshly isolated MNCs were surface stained with a PC5-labeled MAb against CD3 and a PE-labeled MAb against CD56 (4°C, 20 min), washed in PBS, and fixed by 4% paraformaldehyde (Sigma) in PBS (15 min, 4°C). Fixed cells were permeabilized with 0.1% saponin in PBS containing 0.5% bovine serum albumin and stained with FITC-labeled MAbs against CD107a (1/50) or perforin (1/100, clone δG9; Abcam, Cambridge, United Kingdom) for 25 min at room temperature. Control samples were stained intracellularly with isotype-matched FITC-labeled mouse IgG (Caltag/Invitrogen). The cells were analyzed using a Cytomics FC500 flow cytometer and CXP software. Expression of intracellular molecules was presented as the MFI of NK cells in the FITC channel after staining with specific MAbs minus the MFI after staining with an isotype control.
Statistics.
Data were processed using Microsoft Excel 2003 (Microsoft) and GraphPad Instat 3 (GraphPad Software, La Jolla, CA). Independent groups were compared by using the Mann-Whitney U-test. Paired measurements were compared by using the Wilcoxon signed-rank test. Correlations were analyzed using Pearson criteria. All data here are presented as the median (10th to 90th percentiles).
RESULTS
NK cell degranulation in patients with FRH.
To induce NK cell degranulation, two kinds of stimuli were used: (i) standard NK-sensitive K562 target cells (receptor-dependent activation) and (ii) PMA+ION (receptor-independent activation). We observed that in the presence of CB, the PMA+ION combination triggers a very strong degranulation response of healthy donor NK cells (Fig. 1), which is in line with previously published data (3). Therefore, the combination of PMA+ION+CB was used as a positive control.
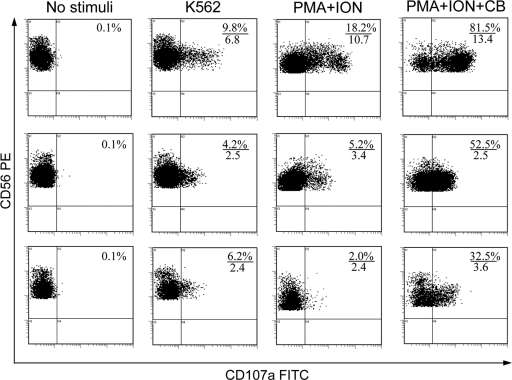
Fig. 1.
Typical pattern of the degranulation of NK cells from a healthy donor (upper row) and a patient with FRH in recurrence (middle row) and in remission (lower row) without stimulation and in response to K562 cells, phorbol 12-myristate 13-acetate plus ionomycin (PMA+ION), and phorbol 12-myristate 13-acetate plus ionomycin plus cytochalasin B (PMA+ION+CB). NK cell degranulation assay was performed as described in Materials and Methods, and the results were assessed by flow cytometry. Shown are the results for gated CD3− CD56+ NK cells. The numerators indicate percentages of NK cells that have externalized CD107a during the 4-h incubation period, and the denominators indicate the mean fluorescence intensity (MFI) of CD107a on CD107a+ NK cells (in arbitrary units).
Both during the recurrence of FRH and during remission, the percentages of NK cells degranulating in response to K562, PMA+ION, or PMA+ION+CB were significantly lower than in healthy donors (Table 1 and Fig. 1). Upon paired comparisons of responses obtained in the same patients during relapse and remission (n=24), no statistically significant differences were revealed.
Table 1.
Percentages of NK cells degranulating in response to the given stimuli, in relation to all NK cells
| Group | n | Median % NK cells degranulating (10th to 90th percentiles)a |
|||
|---|---|---|---|---|---|
| No stimuli | K562 | PMA+ION | PMA+ION+CB | ||
| Healthy donors | 40 | 0.4 (0.1–0.8) | 11.2 (5.1–21.8) | 11.8 (5.2–23.9) | 66.8 (32.5–88.5) |
| FRH, recurrence | 33 | 0.5 (0.1–1.2) | 7.7 (3.0–15.7)* | 4.3 (1.3–12.5)*** | 49.4 (16.9–68.0)** |
| FRH, remission | 24 | 0.3 (0.1–0.7) | 8.2 (4.8–14.5)* | 5.5 (1.7–11.0)*** | 39.1 (25.4–68.6)** |
*, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to the response to the same stimulus in healthy donors [Mann-Whitney U-test]).
Besides the percentages of degranulating NK cells, we also measured the MFI of externalized CD107a on CD107a+ NK cells after treatment with the same stimuli. Since the anti-CD107a MAb is present in the medium since the beginning of stimulation, the MFI of externalized CD107a can be interpreted as a measure of the mean number of granules discharged by individual degranulating NK cell during the stimulation period. The MFI of externalized CD107a in patients with FRH was significantly lower than in healthy donors, which was most obvious upon stimulation with PMA+ION+CB (Table 2 and Fig. 1). Thus, FRH is characterized both by lower numbers of NK cells capable of degranulation, as judged by percentages of CD107a+ cells, and by lower numbers of granules discharged by individual NK cells, as judged by the MFI of externalized CD107a. Upon prospective comparisons, the MFI of externalized CD107a in remission was not different from that of CD107a in relapse, irrespective of the stimulus used.
Table 2.
Mean fluorescence intensity of externalized CD107a on degranulating NK cells
| Group | n | Median MFI (10th to 90th percentiles)a |
||
|---|---|---|---|---|
| K562 | PMA+ION | PMA+ION+CB | ||
| Healthy donors | 40 | 3.6 (2.2–8.0) | 3.4 (1.7–10.4) | 8.4 (3.3–18.5) |
| FRH, recurrence | 33 | 2.7 (1.6–4.5)*** | 3.0 (1.8–4.8) | 3.6 (2.4–5.8)*** |
| FRH, remission | 24 | 2.6 (1.7–4.6)*** | 2.8 (1.8–3.9) | 2.9 (2.3–5.1)*** |
The mean fluorescence intensity (MFI) of externalized CD107a on degranulating NK cells is expressed in arbitrary units. **, P < 0.01; ***, P < 0.001 (compared to the response to the same stimulus in healthy donors [Mann-Whitney U-test]).
Intracellular expression of CD107a and perforin by NK cells from patients with FRH.
One cause of the impaired externalization of CD107a by NK cells in FRH could be the low intracellular content of CD107a. We therefore stained ex vivo NK cells for intracellular CD107a, but we found no significant differences between healthy donors and patients with FRH (Table 3). Furthermore, the intracellular levels of CD107a in ex vivo NK cells did not correlate with subsequent degranulation responses, measured either as percentages of degranulating NK cells or the MFI of externalized CD107a (data not shown).
Table 3.
Intracellular expression of CD107a and perforin
| Group | Median MFI (10th to 90th percentiles)a |
|
|---|---|---|
| CD107a | Perforin | |
| Healthy donors | 11.1 (6.6–14.1) | 38.7 (24.7–52.4) |
| FRH, recurrence | 9.1 (5.6–17.3) | 29.1 (19.3–51.2) |
| FRH, remission | 10.7 (5.0–17.6) | 32.8 (19.7–59.4) |
The intracellular expression of CD107a and perforin is indicated as the MFI in arbitrary units.
Since CD107a can be found in subcellular compartments other than lytic granules, its intracellular levels may not adequately reflect the sizes of the granule compartments in NK cells. Therefore, we stained NK cells for perforin, which is a more specific marker of the lytic granules (MAb δG9 was used for staining). Again, the intracellular levels of perforin were similar in NK cells from healthy donors and patients (Table 3). These data argue that reduced degranulation of NK cells in FRH, as measured by CD107a externalization, is not caused by a smaller size of the lytic granule compartment and/or by lower CD107a expression.
NK activity of MNCs in patients with FRH.
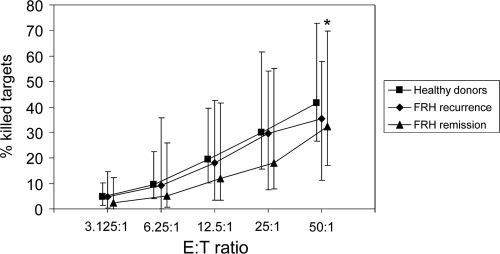
The NK activity during the remission of FRH tended to be lower than in healthy donors (Fig. 2); however, a statistically significant difference between the two groups was observed only at E:T ratio of 50:1. At this ratio, 41.5% (range, 26.4 to 72.7%) of the K562 cells were killed by MNCs from healthy donors, whereas only 32.4% (range, 16.9 to 69.7%) of the targets were killed by MNCs from FRH patients in remission (P < 0.05). Upon recurrence, however, 35.3% (range, 11.1 to 58.0%) of the targets were killed at an E:T ratio of 50:1 (Fig. 2), which was not significantly different from the donor group.
Fig. 2.
NK activity in healthy donors (n=51) and patients with FRH upon recurrence (n=28) and remission (n=23). MNCs from patients and donors were incubated for 4 h with K562 target cells at the indicated effector/target (E:T) ratios, and the percentages of killed K565 cells were determined as described in Materials and Methods. Squares, diamonds, and triangles denote medians of respective groups; bars represent the 10th and 90th percentiles. *, P < 0.05 (comparison between healthy donors and FRH patients in remission at this E:T ratio).
The amount of LU20 per 105 MNCs followed the same trends. MNCs from healthy donors contained 0.93 LU20 (range, 0.36 to 2.6 LU20) per 105 MNCs, which was not different from values in FRH patients during recurrence (0.77 LU20 [range, 0 to 2.73 LU20]) but was significantly higher than in FRH patients during remission (0.41 LU20 [range, 0 to 2.46 LU20]; P=0.03).
Percentages of NK cells among MNCs in patients with FRH.
The discrepancy between low NK cell degranulation and “normal” NK activity, which was observed during recurrence of FRH, could result from elevated percentages of NK cells among MNCs at this stage, which could compensate for deficient degranulation. However, percentages of CD3− CD56+ NK cells among MNCs in FRH patients did not differ from those in healthy donors, being 7.2% (range, 2.8 to 17.0%) during recurrence and 6.7% (range, 2.5 to 14.8%) in remission compared to 8.9% (range, 3.8 to 18.7%) in the donors. Furthermore, when percentages of degranulating NK cells were calculated in relation to all MNCs rather than to all NK cells (as in Table 1), they were still significantly lower in recurrence compared to healthy donors. For example, upon stimulation with K562, degranulating NK cells constituted 0.9% (range, 0.3 to 2.6%) of MNCs in healthy donors and 0.5% (range, 0.1 to 1.3%) of MNCs in the recurrence of FRH (P < 0.01) and 0.5% (range, 0.2 to 1.4%) in remission (P < 0.05). Thus, the lack of difference in NK activity between the donors and the FRH patients upon recurrence cannot be explained by a compensatory increase in percentages of the NK cells among MNCs during recurrence.
Correlation of NK activity with percentages and degranulation of NK cells.
The most direct measure of NK cell function, among those used here, is NK activity, i.e., the ability of freshly isolated MNCs to kill MHC-I− K562 cells. A simple model can be proposed, wherein the NK activity of MNCs depends on three main factors: (i) the percentages of NK cells among MNCs, (i) the ability of NK cells to degranulate, and (iii) the expression of cytotoxic molecules such as perforin by NK cells. We therefore analyzed whether NK activity correlates with any of the three parameters in healthy donors and patients with FRH. Patients in recurrence or remission were analyzed separately. As a measure of NK activity, we used target cell killing at E:T ratio of 50:1.
As expected, in all three groups there was a moderate positive correlation between the NK activity of MNCs and the percentages of NK cells among MNCs (Table 4). In addition, the NK activity correlated with the percentages of NK cells degranulating in response to K562 cells. These latter correlations were stronger when the percentages of degranulating NK cells taken into analysis were calculated in relation to all of the MNCs rather than to all the NK cells (Table 4), which is reasonable, since NK activity was tested using unfractionated MNCs and not isolated NK cells. In healthy donors and FRH patients in remission, correlations between NK activity and the frequencies of degranulating NK cells (in relation to all MNCs) were strong (r > 0.7), whereas only a moderate correlation was observed upon recurrence of FRH (r=0.53, Table 4). In all groups, the NK activity of the MNCs did not correlate with the MFI of the surface CD107a on degranulating NK cells (Table 4) or with the intracellular content of CD107a or perforin in NK cells (data not shown). Similar correlation coefficients were obtained when the amounts of LU20 per 105 MNCs were used as a measure of NK activity (data not shown).
Table 4.
Pearson correlation coefficients between parameter 1 and parameter 2 in healthy donors and patients with FRH
| Parameter 1 | Parameter 2 | Pearson correlation coefficient (r)a |
||
|---|---|---|---|---|
| Healthy donors | FRH (recurrence) | FRH (remission) | ||
| % NK cells among MNCs | NK activity | 0.64*** | 0.59** | 0.63** |
| % NK cells degranulating in response to K562, in relation to all NK cells | NK activity | 0.42* | 0.42* | 0.7*** |
| % NK cells degranulating in response to K562, in relation to all MNCs | NK activity | 0.72*** | 0.53** | 0.79*** |
| MFI externalized CD107a on NK cells degranulating in response to K562 | NK activity | 0.03 | -0.06 | 0.02 |
Asterisks indicate the P values for the correlation coefficients as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Functional parameters of NK cells do not correlate with severity of FRH.
The subgroups with moderate disease (two to six relapses/year) and severe disease (more than six relapses/year) did not differ from each other with regard to any parameter studied, including the NK activity, the degranulation of NK cells in response to different stimuli, and the intracellular content of CD107a or perforin (data not shown). In the two separate subgroups, as in the entire FRH group, none of the studied parameters depended on the stage of the disease (recurrence or remission).
DISCUSSION
NK cells can lyse HSV-infected targets in vitro and are important in the control of HSV infection in vivo (13, 19, 36). The contribution of NK cell dysfunction to the pathogenesis of FRH has long been debated (12, 24, 33, 40). To our knowledge, the present study is the first to directly examine degranulation of NK cells in FRH. We found that FRH is associated with deficient NK cell degranulation responses both to classical NK cell targets (K562 cells) and to PMA+ION (Table 1). Since PMA and ION act downstream of the surface receptors, this finding suggests that the impaired degranulation in FRH was not caused by defective target cell recognition. This conclusion is supported by a recent study that reported no differences in NK cell receptor expression in FRH patients and healthy controls (9).
We found that NK activity in the FRH patients was significantly lower than in the donors only during remission, which is reminiscent of earlier findings (12, 24). The low NK activity, most likely caused by deficient NK cell degranulation, could hinder the elimination of HSV-infected cells and facilitate a recurrence. Upon recurrence, however, while NK cell degranulation responses remained low, the NK activity was not significantly different from that in healthy donors. Furthermore, the correlation between NK activity of MNCs and the percentages of degranulating NK cells among MNCs, which was strong in healthy donors and in patients with remission of FRH, was somewhat weaker upon recurrence of FRH (Table 4). One possible explanation for these findings is that NK cells, after being primed in vivo in response to the active HSV infection, use degranulation-independent mechanisms of killing, which may involve FasL, TRAIL, or arachidonic acid metabolites (15, 32, 34). Second, primed NK cells may produce gamma interferon (IFN-γ) (18), which in turn may sensitize K562 cells to perforin-dependent killing (4). We assume that NK cells may utilize same mechanisms to combat HSV-infected cells in vivo. Third, another MNC subpopulation might develop NK-like activity during recurrence. One candidate could be the CD3+ CD56+ “natural T” cells (17), which do degranulate (albeit very modestly) upon coculture with K562; however, the degranulation responses of these cells were somewhat lower in FRH patients than in the healthy donors and were not different in recurrence compared to remission (our unpublished observations).
It is currently unclear whether the low NK cell degranulation responses in patients with FRH are a consequence of the ongoing HSV infection or a primary event predisposing individuals to FRH. Reports on the ability of the HSVs to inhibit NK cell functions are scarce. An early study showed that NK cells can be disarmed (i.e., loose their cytotoxicity) upon cell-cell contact with HSV-infected targets (16), but the mechanisms of this effect remain unclear (41). A more recent report indicated that ICP27, an immediate-early gene product of HSV-1, makes HSV-infected cells secrete a type I IFN antagonist (22), which may potentially block the effects of type I IFNs on NK cells, resulting in lower degranulation. Type I IFN signaling is essential for NK cell responses to HSV infection (21). However, since the parameters of NK cell degranulation in FRH did not depend on the disease stage (Tables 1 and 2) or severity, it is questionable that the low NK cell degranulation in FRH is a direct consequence of the active HSV infection.
Compared to the standard NK activity assay, which is based on the quantitation of killed targets, the degranulation assay offers valuable additional information about NK cells, namely, it reveals their functional heterogeneity. A consistent finding in all studies on the subject is that only a minor fraction of ex vivo NK cells degranulate upon coincubation with K562 or other target cells (2, 7, 8, 35); the same was observed in the present study (Fig. 1 and Table 1). Even the PMA+ION+CB combination, which is an exceptionally strong stimulus that triggers the degranulation of >90% of neutrophils (our unpublished observations), induces degranulation in only 66.8% (range, 32.5 to 88.5%) of the NK cells from healthy donors and in even fewer NK cells from FRH patients (Table 1). It could be hypothesized that only NK cells that can degranulate in response to PMA+ION+CB are “cytotoxically competent,” whereas FRH is accompanied by shrinkage of this “competent” NK cell subpopulation in peripheral blood (Table 1). This, in turn, may be caused by redistribution of the “competent” NK cells to the sites of active infection or by dilution of the circulating NK cell pool by cells that have been disarmed upon contact with HSV-infected targets (16). However, again, the lack of association between NK cell degranulation and stage or severity of FRH does not support such a scenario.
Given all of the above, it appears more likely that the moderate deficiency of NK cell degranulation, observed here, is a certain primary defect that might contribute to susceptibility to FRH. Since degranulation responses in the FRH group vary over a wide range and partly overlap with responses in healthy donors (Tables 1 and 2, Fig. 2), it is obvious that the impaired NK cell degranulation must combine with other factors to produce a clinical disease. Similarly, particular clinical characteristics of the disease, including its severity, are probably determined by factors other than the deficient degranulation of NK cells.
In conclusion, FRH is associated with a moderate deficiency in NK cell degranulation responses compared to healthy donors. The causes of this deficiency, as well as its role in the pathogenesis of FRH, require further in-depth investigation.
Footnotes
Published ahead of print on 6 July 2011.
REFERENCES
- 1. Adler H., et al. 1999. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J. Neuroimmunol. 93:208–213 [DOI] [PubMed] [Google Scholar]
- 2. Alter G., Malenfant J. M., Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15–22 [DOI] [PubMed] [Google Scholar]
- 3. Atkinson E. A., Gerrard J. M., Hildes G. E., Greenberg A. H. 1990. Studies of the mechanism of natural killer (NK) degranulation and cytotoxicity. J. Leukoc. Biol. 47:39–48 [DOI] [PubMed] [Google Scholar]
- 4. Berthou C., et al. 2000. Interferon-gamma-induced membrane PAF-receptor expression confers tumor cell susceptibility to NK perforin-dependent lysis. Blood 95:2329–2336 [PubMed] [Google Scholar]
- 5. Betts M. R., et al. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65–78 [DOI] [PubMed] [Google Scholar]
- 6. Biron C. A., Byron K. S., Sullivan J. L. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731–1735 [DOI] [PubMed] [Google Scholar]
- 7. Bryceson Y. T., March M. E., Barber D. F., Ljunggren H. G., Long E. O. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 202:1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bryceson Y. T., et al. 2007. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood 110:1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter C., Savic S., Cole J., Wood P. 2007. Natural killer cell receptor expression in patients with severe and recurrent herpes simplex virus-1 (HSV-1) infections. Cell. Immunol. 246:65–74 [DOI] [PubMed] [Google Scholar]
- 10. Casazza J. P., et al. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chayavichitsilp P., Buckwalter J. V., Krakowski A. C., Friedlander S. F. 2009. Herpes simplex. Pediatr. Rev. 30:119–130 [DOI] [PubMed] [Google Scholar]
- 12. Cheknev S. B., et al. 1994. The natural killer activity and indices of the interferon status of patients with recurrent genital herpes being treated with ridostin. Vopr. Virusol. 39:125–128 [PubMed] [Google Scholar]
- 13. Chisholm S. E., Howard K., Gomez M. V., Reyburn H. T. 2007. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J. Infect. Dis. 195:1160–1168 [DOI] [PubMed] [Google Scholar]
- 14. Chung A. W., Rollman E., Center R. J., Kent S. J., Stratov I. 2009. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J. Immunol. 182:1202–1210 [DOI] [PubMed] [Google Scholar]
- 15. Cifone M. G., et al. 1993. Involvement of phospholipase A2 activation and arachidonic acid metabolism in the cytotoxic functions of rat NK cells. Cell. Immunol. 148:247–258 [DOI] [PubMed] [Google Scholar]
- 16. Confer D. L., et al. 1990. Herpes simplex virus-infected cells disarm killer lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 87:3609–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doherty D. G., et al. 1999. The human liver contains multiple populations of NK cells, T cells, and CD3+ CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J. Immunol. 163:2314–2321 [PubMed] [Google Scholar]
- 18. Fehniger T. A., et al. 2003. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101:3052–3057 [DOI] [PubMed] [Google Scholar]
- 19. Fitzgerald P. A., Mendelsohn M., Lopez C. 1985. Human natural killer cells limit replication of herpes simplex virus type 1 in vitro. J. Immunol. 134:2666–2672 [PubMed] [Google Scholar]
- 20. Fukuda M. 1991. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 266:21327–21330 [PubMed] [Google Scholar]
- 21. Gill N., Chenoweth M. J., Verdu E. F., Ashkar A. A. 2011. NK cells require type I IFN receptor for antiviral responses during genital HSV-2 infection. Cell. Immunol. 269:29–37 [DOI] [PubMed] [Google Scholar]
- 22. Johnson K. E., Knipe D. M. 2010. Herpes simplex virus-1 infection causes the secretion of a type I interferon-antagonizing protein and inhibits signaling at or before Jak-1 activation. Virology 396:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kannan K., et al. 1996. Lysosome-associated membrane proteins h-LAMP1 (CD107a) and h-LAMP2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell. Immunol. 171:10–19 [DOI] [PubMed] [Google Scholar]
- 24. Leszczyszyn-Pynka M. 1995. Natural cytotoxicity of peripheral blood mononuclear cells in herpes simplex and varicella-zoster virus infections. Acta Haematol. Pol. 26:393–402 [PubMed] [Google Scholar]
- 25. Lopez C., et al. 1983. Correlation between low natural killing of fibroblasts infected with herpes simplex virus type 1 and susceptibility to herpesvirus infections. J. Infect. Dis. 147:1030–1035 [DOI] [PubMed] [Google Scholar]
- 26. Moretta L., et al. 2003. Surface receptors and functional interactions of human natural killer cells: from bench to the clinic. Cell. Mol. Life Sci. 60:2139–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moretta L., Moretta A. 2004. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23:255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orange J. S. 2002. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 4:1545–1558 [DOI] [PubMed] [Google Scholar]
- 29. Peters P. J., et al. 1991. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 173:1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubio V., et al. 2003. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 9:1377–1382 [DOI] [PubMed] [Google Scholar]
- 31. Schepis D., et al. 2009. Herpes simplex virus infection downmodulates NKG2D ligand expression. Scand. J. Immunol. 69:429–436 [DOI] [PubMed] [Google Scholar]
- 32. Screpanti V., Wallin R. P., Grandien A., Ljunggren H. G. 2005. Impact of FASL-induced apoptosis in the elimination of tumor cells by NK cells. Mol. Immunol. 42:495–499 [DOI] [PubMed] [Google Scholar]
- 33. Sirianni M. C., et al. 1986. Immunological responses of patients with recurrent herpes genitalis. Diagn. Immunol. 4:294–298 [PubMed] [Google Scholar]
- 34. Smyth M. J., et al. 2005. Activation of NK cell cytotoxicity. Mol. Immunol. 42:501–510 [DOI] [PubMed] [Google Scholar]
- 35. Tomescu C., Chehimi J., Maino V. C., Montaner L. J. 2009. Retention of viability, cytotoxicity, and response to IL-2, IL-15, or IFN-α by human NK cells after CD107a degranulation. J. Leukoc. Biol. 85:871–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vollstedt S., et al. 2004. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J. Virol. 78:3846–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wald A., et al. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844–850 [DOI] [PubMed] [Google Scholar]
- 38. Whitley R. J., Roizman B. 2001. Herpes simplex virus infections. Lancet 357:1513–1518 [DOI] [PubMed] [Google Scholar]
- 39. Winchester B. G. 2001. Lysosomal membrane proteins. Eur. J. Paediatr. Neurol. 5(Suppl. A):11–19 [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto T., Osaki T., Yoneda K., Ueta E. 1993. Immunological investigation of adult patients with primary herpes simplex virus-1 infection. J. Oral Pathol. Med. 22:263–267 [DOI] [PubMed] [Google Scholar]
- 41. Zahariadis G., et al. 2008. Cell-type-specific tyrosine phosphorylation of the herpes simplex virus tegument protein VP11/12 encoded by gene UL46. J. Virol. 82:6098–6108 [DOI] [PMC free article] [PubMed] [Google Scholar]