Abstract
Increasing resistance to quinolones, aminoglycosides, and/or cephamycins in extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae exacerbates the already limited antibiotic treatment options for infections due to these microbes. In this study, the presence of resistance determinants for these antimicrobial agents was examined by PCR among ESBL-producing Klebsiella pneumoniae (ESBL-KP) isolates that caused bacteremia. Pulsed-field gel electrophoresis was used to differentiate the clonal relationship among the isolates studied. Transferability and the location of the resistance genes were analyzed by conjugation experiments, followed by DNA-DNA hybridization. Among the 94 ESBL-KP isolates studied, 20 isolates of flomoxef-resistant ESBL-KP were identified. They all carried a DHA-1 gene and were genetically diverse. CTX-M genes were found in 18 of the isolates. Among these DHA-1/CTX-M-producing K. pneumoniae isolates, ISCR1 was detected in 13 (72%) isolates, qnr genes (1 qnrA and 17 qnrB genes) were detected in 18 (100%), aac(6′)-Ib-cr was detected in 11 (61%), and 16S rRNA methylase (all armA genes) was detected in 14 (78%). Four transconjugants were available for further analysis, and qnrB4, aac(6′)-Ib-cr, armA, and blaDHA-1 were all identified on these self-transferable blaCTX-M-carrying plasmids. The genetic environments of ISCR1 associated with armA, blaDHA-1, and qnrB4 genes in the four transconjugants were identical. Replicon-type analysis revealed a FIIA plasmid among the four self-transferable plasmids, although the other three were nontypeable. The cotransfer of multiple resistance genes with the ISCR1 element-carrying plasmids has a clinical impact and warrants close monitoring and further study.
INTRODUCTION
Klebsiella pneumoniae is an opportunistic pathogen associated with a wide spectrum of infections. Increasing resistance to multiple antimicrobial agents in the bacterium has compromised the effectiveness of treatment. Production of extended-spectrum cephalosporinases, either extended-spectrum β-lactamases (ESBLs) or plasmid-mediated AmpC β-lactamases (PMABLs), confers resistance to broad-spectrum penicillins and cephalosporins (13, 27, 29). While the coexistence of ESBL and PMABL genes in Enterobacteriaceae is common (42), multidrug resistance to other antimicrobial agents was less frequently studied among K. pneumoniae isolates that simultaneously produced both ESBLs and PMABLs.
Plasmid-mediated quinolone resistance (PMQR) determinants, such as qnr, aac(6′)-Ib-cr, and qepA genes, might cause low-level resistance or reduced susceptibility to quinolones because they could facilitate the selection of mutants with higher-level resistances (31, 40). Aminoglycoside resistance usually resulted from the presence of aminoglycoside-modifying enzymes (33). Recent reports highlighted the significance of emerging plasmid-mediated 16S rRNA methylase genes (armA and rmtB) in conferring high-level resistance to all aminoglycosides (3).
Integrons that contain resistance genes, such as aac(6′)-Ib-like genes in a class 1 integron cassette, could disseminate resistance characteristics (25). Interestingly, qnr is often present in complex class 1 integrons, located between common region 1 (CR1) that comprised the orf513 recombinase and a second copy of the 3′ conserved segment (3′-CS2), together with gene cassettes encoding resistance to other antimicrobial agents, such as aminoglycosides (31). Toleman et al. proposed that orf513, later termed insertion sequence common region 1 (ISCR1), may mobilize both a nearby sequence and a truncated 3′-CS from one integron to the 3′-CS of another integron using rolling circle (RC) transposition, thus facilitating the formation of complex class 1 integrons associated with ISCR1 (36). In addition, ISCR1 is associated with many antimicrobial resistance genes, including qnr (11) and other genes for aminoglycoside and β-lactam resistance (23, 36, 37). It was suggested that these antimicrobial resistance genes were added to the 3′-CS of the class 1 integron via comobilization with the nearby ISCR1 from other integrons, using RC transposition and homologous recombination (36).
Flomoxef, a cephamycin unique in having a difluoromethylthioacetamido group at position 7, has been reported to be as clinically effective as a carbapenem in treating flomoxef-susceptible ESBL-producing K. pneumoniae (ESBL-KP) bacteremia (20). In 2004, a sudden increase from less than 10% to 20% in bloodstream infections caused by flomoxef-resistant ESBL-KP isolates at our hospital was noted. These isolates also presented concomitant resistance to ciprofloxacin and amikacin (18). The presence of PMQR determinants, PMABL, and plasmid-mediated 16S rRNA methylase genes among these isolates and the associated genetic structures was further studied.
MATERIALS AND METHODS
Setting, bacterial isolates, and clinical features.
The study was conducted at Kaohsiung Chang Gung Memorial Hospital, a 2,300-bed medical center providing both primary care and tertiary referral service in southern Taiwan, between March 2004 and November 2005. Flomoxef-resistant, ESBL-producing K. pneumoniae clinical isolates recovered from blood cultures were collected for further laboratory investigation. Review of medical records among the patients from whom the isolates were recovered was performed.
Susceptibility testing.
ESBL production was confirmed by the double-disk diffusion method recommended by the Clinical and Laboratory Standards Institute (CLSI) (7). MICs of amikacin, cefotaxime, cefotaxime-clavulanate, ciprofloxacin, ertapenem, and tigecycline were examined with Etest (AB Biodisk, Solna, Sweden). The results were interpreted according to the CLSI's recommendations (8), except that breakpoints recommended by the U.S. Food and Drug Administration were used for tigecycline susceptibility (susceptible, ≤2 μg/ml; resistant, ≥8 μg/ml) (22). MICs of flomoxef were determined by a standard broth microdilution method and interpreted according to a previous report (12). Quality control for the MIC determination was performed using reference strains Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853.
PFGE.
Genetic relationships of the flomoxef-resistant ESBL-KP isolates were characterized by pulsed-field gel electrophoresis (PFGE) as previously described (17). Genomic DNA of the bacteria precast in plugs was digested with XbaI and subjected to PFGE in a CHEF Mapper XA system (Bio-Rad Laboratories, Hercules, CA). An auto-algorithm mode was selected for running molecular masses ranging from 50 to 500 kb. Band patterns were analyzed using Fingerprinting II software, version 3.0 (Bio-Rad Laboratories), and interpreted according to previously defined criteria (35).
Analysis of the QRDRs among gyrase genes.
PCR amplification of the quinolone resistance-determining regions (QRDRs) among gyrA, gyrB, parC, and parE genes was performed on ciprofloxacin-resistant isolates as previously described (9). DNA sequencing was performed in both directions among the purified PCR products. The results were compared with the published sequence of K. pneumoniae (GenBank accession no. AP006725).
Analysis of OMP profiles and PCR sequencing of specific porin and β-lactamase genes.
Outer membrane protein (OMP) profiles of the isolates were examined with a method described previously (16). To examine the DNA sequences of the OmpK35 and OmpK36 genes, two pairs of previously described primers were used for PCR amplification and sequence analysis (17). For the PCR detection of specific ESBL genes (blaSHV, blaTEM, and blaCTX-M), four sets of previously reported primers were used (19). Multiplex PCR was performed to detect the presence of plasmid-mediated AmpC β-lactamase genes using the method described previously (28). DNA sequencing was performed as described above with the primers used for the respective PCR amplification.
Detection of genes for the class 1 integron, plasmid-mediated quinolone resistance, and 16S rRNA methylases.
PCR amplification was performed to detect the presence of the class 1 integron structure and genes qnrA, qnrB, qnrS, qepA, aac(6′)-Ib, armA, and rmtB, with primers listed in Table 1 (1, 21, 32, 34, 39–41). PCR-restriction fragment length polymorphism (PCR-RFLP) was used to examine the presence of qnrB variants, the wild-type allele aac(6′)-Ib, and the mutant allele aac(6′)-Ib-cr, as previously described (1, 34). RFLP analysis of the 496-bp qnrB PCR products was performed by using MvaI (Fermentas Inc., Hanover, MD), BglII (New England BioLabs, Beverly, MA), HindIII (New England BioLabs), and FokI (New England BioLabs). The 514-bp aac(6′)-Ib PCR products were digested by the NdeI and TaaI enzymes (Fermentas Inc.). All amplified products were sequenced using an automated sequencer (ABI Prism 3730 XL), and the results were compared with sequences published in the GenBank nucleotide database (www.ncbi.nlm.nih.gov/blast/).
Table 1.
Sequences of primers used for specific gene detection
| Target | Primer | Sequence (5′–3′) | Annealing temp (°C) | Position | GenBank accession no. | Reference |
|---|---|---|---|---|---|---|
| qnrA | qnrA-1A | TTCAGCAAGAGGATTTCTCA | 60 | 325–344 | AY070325 | 39 |
| qnrA-1B | GGCAGCACTATTACTCCCAA | 952–933 | ||||
| qnrB | qnrB-F | GATCGTGAAAGCCAGAAAGG | 53 | 175–194 | DQ351241 | 32 |
| qnrB-R | ACGATGCCTGGTAGTTGTCC | 624–643 | ||||
| qnrS | qnrS-1A | CAATCATACATATCGGCACC | 60 | 9748–9767 | AB187515 | 39 |
| qnrS-1B | TCAGGATAAACAACAATACCC | 10389–10369 | ||||
| qepA | qepA-F | GCAGGTCCAGCAGCGGGTAG | 60 | 4179–4198 | EF150886 | 40 |
| qepA-R | CTTCCTGCCCGAGTATCGTG | 4377–4396 | ||||
| aac(6′)-Ib | qac1 | GGCATCACTGCGTGTTCGCTCG | 65 | 1862–1841 | AF034958 | 1 |
| qac2 | GACTGAGCATGACCTTGCG | 1367–1349 | ||||
| armA | armA-F | CCGAAATGACAGTTCCTATC | 55 | 1931–1950 | AY220558 | 41 |
| armA-R | GAAAATGAGTGCCTTGGAGG | 2757–2775 | ||||
| rmtB | rmtB-F | ATGAACATCAACGATGCCCT | 50 | 1410–1429 | AB103506 | 41 |
| rmtB-R | CCTTCTGATTGGCTTATCCA | 2178–2159 | ||||
| Class 1 integron | 5′CS-F | GGCATCCAAGCAGCAAG | 55 | 1236–1252 | M73819 | 21 |
| 3′CS-R | AAGCAGACTTGACCTGA | 2813–2830 | ||||
| blaDHA-1 | DHA-MF | AACTTTCACAGGTGTGCTGGGT | 65 | 1244–1265 | Y16410 | 28 |
| DHA-MR | CCGTACGCATACTGGCTTTGC | 1648–1628 | ||||
| ISCR1 | orf513-F | CTTTTGCCCTAGCTGCGGT | 60 | 7329–7347 | DQ831141 | 21 |
| orf513-R | CTCACGCCCTGGCAAGGTTT | 7923–7904 | ||||
| ISCR1 and armA junction | orf513-F | CTTTTGCCCTAGCTGCGGT | 53 | 8953–8971 | FJ715937 | 21 |
| armA-R | GAAAATGAGTGCCTTGGAGG | 12391–12410 | 41 | |||
| 3′-CS2 | sul1-F1 | GTATTGCGCCGCTCTTAGAC | 55 | 7618–7637 | FJ715937 | This study |
| orf513-R1 | CGGTTAATGCCAGACGTTTT | 9682–9701 | ||||
| ampR-F | GATTACTGCCGGTGCTCAAT | 55 | 6164–6183 | FJ715937 | This study | |
| sul1-R1 | AGGGTTTCCGAGAAGGTGAT | 8196–8125 | ||||
| pap operon and ampC junction | pspD5′ | CCGGCAATCAGGCTAAATAA | 55 | 19982–20001 | AJ971344 | 38 |
| ampC3′ | TCCGGAAAAACAGGTGGCGA | 21161–21180 | ||||
| pap operon and qnrB junction | qnrB5′ | ACAGTGCGTAACGTTTGCTG | 50 | 67–86 | FJ715937 | This study |
| pspA3′ | ACGAACATCACGGGAAGAAC | 2255–2275 |
Conjugation.
Transferability of the antimicrobial resistance among the clinical isolates of K. pneumoniae was performed using an azide-resistant strain, E. coli J53Azr, as the recipient. Donors and the recipient were inoculated separately into LB broth and incubated at 37°C for 5 h. The bacterial cultures were then mixed together in a ratio of 1:3 (by volume) and were incubated overnight at 37°C. A portion (0.1 ml) of the overnight broth culture was spread onto an LB agar plate containing cefotaxime (2 μg/ml) and sodium azide (100 μg/ml). Transconjugants were then selected from the agar plate.
Plasmid analysis, replicon typing, and DNA-DNA hybridization.
Plasmids of the transconjugants were extracted by the method described by Kado and Liu (15), followed by electrophoresis on a 0.8% agarose gel (SeaKem LE agarose). A multiplex PCR replicon typing system was used to analyze the conjugative resistance plasmids (4). DNA-DNA hybridization was performed with a digoxigenin labeling and detection kit (Roche, Mannheim, Germany).
Analysis of the genetic environments of transferable resistant determinants.
The genetic context of the mobile element (ISCR1) associated with qnrB and armA was investigated by PCR mapping and subsequent sequencing. Plasmid DNA was extracted from the transconjugants by alkaline lysis (2) and amplified by PCR using the primers listed in Table 1 (21, 38, 41).
RESULTS
Bacterial strains and clinical features of the patients.
During the study period, 546 patients were reported to have K. pneumoniae bacteremia. ESBL-producing K. pneumoniae isolates were recovered from the blood culture of 94 (17.2%) patients. Among them, 20 (22.9%) isolates were resistant to flomoxef and were included for further study. The median age of these patients was 42 years (range, 30 to 91 years), and 13 (65%) were male patients. No temporal or geographical relationship between the subjects was found. The majority of the patients were seriously ill with underlying diseases, had a prolonged hospital and/or intensive care unit stay, and received multiple antimicrobial agents before the development of the K. pneumoniae bacteremia. Lungs were the most common infection source of bacteremia (35%). β-Lactam antibiotics were the most common agent for empirical therapy. Three of the five patients treated with carbapenems as empirical therapy had favorable outcomes. The raw mortality rate was 70%.
Susceptibility testing, genotyping, β-lactamases, and OMP analysis.
The 20 flomoxef-resistant ESBL-KP strains were fully susceptible to tigecycline and showed high resistance to ciprofloxacin (18 isolates; 90%) and amikacin (16; 80%) and some resistance to ertapenem (6; 30%). PFGE revealed 20 genotypes, indicating the diversity of the isolates. Several ESBLs were identified: SHV-5 (n = 1), SHV-12 (n = 4), CTX-M-3 (n = 4), and CTX-M-14 (n = 14). DHA-1 was the only PMABL discovered among the 20 isolates. OmpK35 deficiency was found in six isolates that carried either blaCTX-M-14 alone (n = 5) or blaCTX-M-14 plus blaSHV-12 (n = 1); three of them were resistant to ertapenem at low MICs of 1 to 2 μg/ml (8). Another isolate that harbored both blaCTX-M-3 and blaSHV-5 genes was found to be deficient in both OmpK35 and OmpK36 and was resistant to ertapenem (MIC, 4 μg/ml) (8). In three of these isolates, a single nucleotide deletion at different positions, leading to a frameshift in the amino acid sequence, was found to be the major mechanism for the OmpK35 deficiency. For the remaining four isolates, no apparent mutational changes were found in either the promoter or the coding regions of the OmpK genes.
Detection of genes for the class 1 integron, plasmid-mediated quinolone resistance, and 16S rRNA methylase.
An aminoglycoside-modifying gene aac(6′)-Ib-containing class 1 integron was detected in 13 (65%) of the 20 isolates. The presence of qnrB4 was detected in 17 isolates, including one coexisting with a qnrA1 gene. In another single isolate, qnrB2 alone was detected. The aac(6′)-Ib-cr gene was present in 11 (55%) isolates that also harbored a qnr gene. No qepA was found. DHA-1-producing ESBL-KP isolates carrying plasmid-mediated quinolone resistance genes all harbored blaCTX-M genes, and three of them also possessed a blaSHV-12 gene. Among the 16 amikacin-resistant isolates, 2 carried the aac(6′)-Ib gene, and the other 14 carried the plasmid-mediated 16S rRNA methylase gene armA. Various combinations of blaCTX-M, blaDHA-1, qnr, aac(6′)-Ib-cr, and armA genes were found in 11 isolates.
Conjugation and plasmid restriction enzyme digestion profile.
Conjugation experiments were performed on all 20 isolates, and only four transconjugants were generated successfully (Table 2). The four donor strains expressed different PFGE patterns but all carried the blaCTX-M-14, blaDHA-1, qnrB4, aac(6′)-Ib-cr, and armA genes simultaneously. After conjugation, all these resistance genes were transferred together into the respective transconjugants, as evidenced by PCR amplification using specific primers. A blaSHV-12 gene was also carried by two of the donors, KP134 and KP148, but was not found in their transconjugants. Accordingly, the MICs of cefotaxime, cefotaxime-clavulanate, flomoxef, and amikacin all increased considerably compared to those of the recipient E. coli J53. Two donor strains, KP148 and KP243, were found to have 2 to 3 mutations in their QRDRs of the gyrA and parC genes, hence contributing to the high MICs of ciprofloxacin observed.
Table 2.
Antimicrobial resistance genes and susceptibility profiles of four DHA-1/ESBL-producing K. pneumoniae isolates and their transconjugants in E. coli J53
| Bacterium | Resistance gene profile |
MIC (μg/ml)a |
Porin deficiencyb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bla | qnr | aac(6′)-Ib | 16S rRNA methylase | QRDRc |
CTX/CTX-CLA | FLO | CIP | AMK | TGC | ERT | |||
| gyrA | parC | ||||||||||||
| KP105 | blaCTX-M-14 | qnrB4 | aac(6′)-Ib-cr | armA | WT | >16/1 | 128 | 2 | >256 | 0.5 | 0.5 | None | |
| blaDHA-1 | |||||||||||||
| J53/pKP105 | blaCTX-M-14 | qnrB4 | aac(6′)-Ib-cr | armA | ND | >16/1 | 8 | 0.5 | >256 | 0.25 | 0.05 | ND | |
| blaDHA-1 | |||||||||||||
| KP134 | blaSHV-12 | qnrB4 | aac(6′)-Ib-cr | armA | WT | >16/1 | 128 | 2 | >256 | 0.5 | 0.5 | None | |
| blaCTX-M-14 | |||||||||||||
| blaDHA-1 | |||||||||||||
| J53/pKP134 | blaCTX-M-14 | qnrB4 | aac(6′)-Ib-cr | armA | ND | >16/1 | 64 | 0.25 | >256 | 0.25 | 0.5 | ND | |
| blaDHA-1 | |||||||||||||
| KP148 | blaSHV-12 | qnrB4 | aac(6′)-Ib-cr | armA | S83I | S80I | >16/1 | >128 | >32 | >256 | 0.5 | 2 | OmpK35 |
| blaCTX-M-14 | |||||||||||||
| blaDHA-1 | |||||||||||||
| J53/pKP148 | blaCTX-M-14 | qnrB4 | aac(6′)-Ib-cr | armA | ND | >16/1 | 8 | 1 | >256 | 0.25 | 0.012 | ND | |
| blaDHA-1 | |||||||||||||
| KP243 | blaCTX-M-14 | qnrB4 | aac(6′)-Ib-cr | armA | S83F | S80I | >16/1 | 64 | >32 | >256 | 1 | 1 | OmpK35 |
| blaDHA-1 | D87A | ||||||||||||
| J53/pKP243 | blaCTX-M-14 | qnrB4 | aac(6′)-Ib-cr | armA | ND | >16/1 | 32 | 0.5 | >256 | 0.5 | 1 | ND | |
| blaDHA-1 | |||||||||||||
| E. coli J53 | ND | 0.25/0.032 | 0.125 | 0.008 | 2 | 0.25 | 0.012 | ND | |||||
CTX, cefotaxime; CLA, clavulanic acid; FLO, flomoxef; CIP, ciprofloxacin; AMK, amikacin; TGC, tigecycline; ERT, ertapenem.
ND, not done.
QRDR, quinolone resistance-determining region; WT, wild type; ND, not done; S, serine; I, isoleucine; F, phenylalanine; D, aspartic acid; A, alanine.
Replicon typing of plasmid and DNA-DNA hybridization.
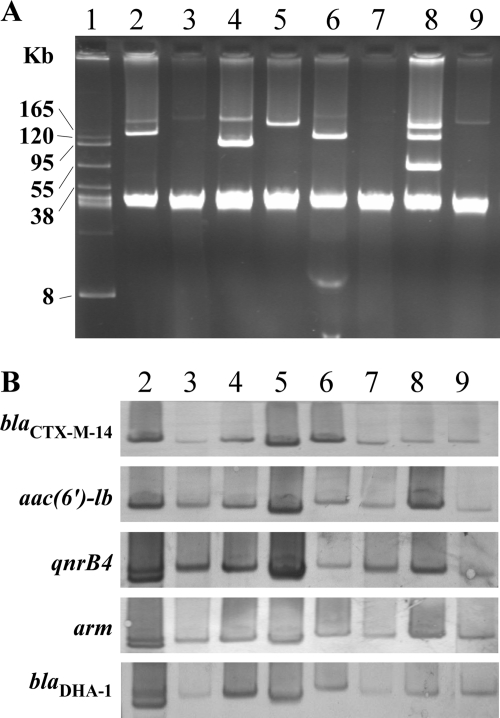
Figure 1 shows plasmid analysis and DNA-DNA hybridization for the four transconjugants and their donors. Although multiple plasmids were found among the donor isolates, a single plasmid that concomitantly harbored the resistance determinants, blaCTX-M-14, aac(6′)-Ib, qnrB, armA, and blaDHA-1, in the four transconjugants was found. In strain KP105, two bands were demonstrated by the DNA-DNA hybridization, using the amplicons of qnrB4, armA, and blaDHA-1 as the probes (Fig. 1B, lane 2). Enzyme digestion of the plasmid DNA by EcoRI, KpnI, and SalI (all obtained from New England BioLabs) was performed. The subsequent DNA-DNA hybridization using the same probes as mentioned above each produced only one single band with the use of each respective probe (data not shown), indicating that the two bands were very likely from the same plasmid. PCR-based replicon typing analysis indicated that the self-transferable plasmid from strain KP243 was a FIIA plasmid, while the remaining three transferable plasmids were nontypeable by the multiplex system.
Fig. 1.
Plasmid profiles (A) and DNA-DNA hybridization (B) of four DHA-1/ESBL-producing K. pneumoniae isolates and their transconjugants. The probes used are as indicated. Lane 1, molecular mass marker, BAC-Tracker-supercoiled DNA ladder (Epicentre, Madison, WI); lanes 2 to 9, K. pneumoniae KP105, E. coli J53/pKP105, KP134, J53/pKP134, KP148, J53/pKP148, KP243, and J53/pKP243, respectively.
Prevalence of ISCR1 and the genetic environment flanking the ISCR1 element.
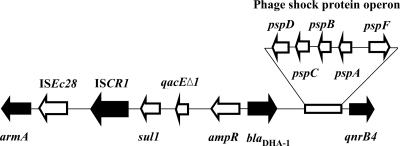
The presence of ISCR1 was confirmed by PCR amplification among the four self-transferable resistance plasmids. Further investigation indicated that the insertion of the ISCR1 truncated the su11-type integrons' incomplete 3′ conserved sequences (3′-CS). ISEc28 and the aminoglycoside resistance gene armA were found downstream of ISCR1, while the AmpC-type blaDHA-1 gene and its regulator, ampR, were located immediately after the incomplete 3′-CS. A phage shock protein operon and the qnrB4 gene were located downstream of the blaDHA-1 gene (Fig. 2). The genetic environments flanking the ISCR1 element in the four studied transconjugants were identical.
Fig. 2.
Genetic environment of ISCR1, associated with armA, blaDHA-1, and qnrB4 genes (black arrows).
ISCR1 was also detected in 9 of the remaining 16 clinical isolates tested. In 11 of the 13 ISCR1-containing plasmids, various combinations of blaCTX-M, blaDHA-1, qnr, aac(6′)-Ib-cr, and armA genes were also presented.
DISCUSSION
Currently, carbapenems are still regarded as the drug of choice for infections caused by ESBL-producing pathogens (27). However, the selective pressure from increasing use of carbapenems may lead to the development of carbapenem-resistant microbes or even the prevalence of other multidrug-resistant pathogens (6). An effective alternative to carbapenems, such as flomoxef, may relieve this selective pressure and offer an option to carbapenem-allergic patients, when necessary (20). Therefore, the increase of flomoxef resistance in ESBL-KP represents a clinical disturbance that requires further attention. Previously, we have found that patients with bacteremia caused by flomoxef-resistant ESBL-KP are more likely to receive inappropriate empirical therapy due to the multidrug resistance and are associated with a higher rate of mortality within 14 days (18). While no other clinical risk factors for the acquisition of flomoxef resistance among ESBL-KP isolates could be identified, laboratory investigation was conducted herein to reveal the related microbiological factors.
Most of the PMQR determinants or plasmid-mediated 16S rRNA methylase genes were shown to be associated with ESBL genes (31, 39, 43). To our knowledge, the present report is the first to show the simultaneous existence of blaDHA-1 with blaCTX-M, qnrB4, aac(6′)-Ib-cr, and armA genes carried by a single self-transferable plasmid. The diverse genotypes of the donor bacteria further indicated that the multidrug resistance among the organisms was associated with the resistance plasmids rather than the clonal expansion of a multiple resistant strain.
The armA gene is a part of the functional composite transposon Tn1548 in plasmid pIP1204 (10). Study of the flanking region of pIP1204 revealed that two insertion sequences, IS6100 and ISCR1, are located upstream of the armA gene (10). In the present study, ISEc28 and armA genes were found downstream of ISCR1 (Fig. 2), suggesting that the presence of ISCR1 may lead to the comobilization of the plasmid-mediated genes. Recently, researchers in China characterized the complete nucleotide sequence of K. pneumoniae plasmid pKP048 (GenBank accession no. FJ628167), which carries genes blaKPC-2, blaDHA-1, qnrB4, and armA (14). In the present study, ISCR1 was flanked by armA, blaDHA-1, and qnrB genes in the four self-transferable blaDHA-1-carrying plasmids (Fig. 2), similar to the 25-kbp region consisting of armA, ISCR1, blaDHA-1, and qnrB4 genes in the plasmid pKP048 detected in 2006 (14). Presumably, there is a relationship between ISCR1 and antimicrobial resistance genes. In our study, although self-transferable plasmids were successfully identified in four isolates, replicon typing revealed that the plasmids may not be derived from the same sources. On the other hand, 13 of the 20 isolates tested harbored ISCR1, and various combinations of blaCTX-M, blaDHA-1, qnr, aac(6′)-Ib-cr, and armA genes were found in 11 of these isolates. It appears that ISCR1 plays an important role in the transfer of blaDHA-1, together with an array of other resistance genes among our ESBL-KP isolates.
The transconjugants showed only low-level quinolone resistance, suggesting that high-level quinolone resistance in the original isolates may result from the coordinating action of several resistance mechanisms, such as QRDR point mutations (Table 2). The qnr genes alone may not confer resistance to quinolone (24), but they provide the first step toward further in vivo selection of high-level resistance against quinolones (30). Park et al. also observed the presence of qnrB in isolates with a wide range of quinolone MIC values, including full susceptibility (26). The wide range of MICs observed among qnr-positive bacteria suggests that the phenotypic detection of qnr genes in clinical isolates might be difficult. The presence of aac(6′)-Ib-cr also does not always relate to high MICs of quinolones. Nine of the eleven isolates that possessed both qnr and aac(6′)-Ib-cr genes demonstrated high quinolone MICs of over 32 μg/ml. The phenomenon suggests that these two genetic factors may be synergistic in increasing quinolone resistance.
Our study revealed the coexistence of resistance genes encoding DHA-1, SHV, and CTX-M enzymes in K. pneumoniae and that the blaDHA-1 gene was associated with aac(6′)-Ib-cr, armA, and qnrB4 genes. The PCR-based replicon typing system provides a novel method to describe the dissemination and to follow the evolution of plasmid-mediated resistance (5). Through this method, one of the self-transferable plasmids identified in the present study was determined to be a rare FIIA type, while the remaining three remain to be defined. Because literature reports on plasmid replicon typings are still rare, further research is required to determine the influence of acquired resistance on plasmid trafficking. Horizontal gene transfer, especially that associated with ISCR1, appears to play an important role in the dissemination of the multidrug-resistant K. pneumoniae studied herein. Further investigations of the ISCR1 elements that may have contributed to the rapid dissemination of antibiotic resistance genes are warranted.
ACKNOWLEDGMENTS
We are grateful to Leung Kei Siu, National Health Research Institutes, for kindly providing Escherichia coli strain J53Azr.
This work was supported by grant CMRPG360863 from the Chang Gung Memorial Hospital and grant NSC 97-2314-B-182A-028 from the National Science Council, Executive Yuan, Taipei, Taiwan.
Footnotes
Published ahead of print on 11 July 2011.
REFERENCES
- 1. Ambrozic Avgustin J. A., Keber R., Zerjavic K., Orazem T., Grabnar M. 2007. Emergence of the quinolone resistance-mediating gene aac(6′)-Ib-cr in extended-spectrum beta-lactamase-producing Klebsiella isolates collected in Slovenia between 2000 and 2005. Antimicrob. Agents Chemother. 51:4171–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birnboim H. C., Doly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bogaerts P., et al. 2007. Emergence of ArmA and RmtB aminoglycoside resistance 16S rRNA methylases in Belgium. J. Antimicrob. Chemother. 59:459–464 [DOI] [PubMed] [Google Scholar]
- 4. Carattoli A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 5. Carattoli A., et al. 2006. Replicon typing of plasmids encoding resistance to newer β-lactams. Emerg. Infect. Dis. 12:1145–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chia J. H., et al. 2010. Development of high-level carbapenem resistance in Klebsiella pneumoniae among patients with prolonged hospitalization and carbapenem exposure. Microb. Drug Resist. 16:317–325 [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100-S19 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing, 20th informational supplement. M100-S20-U Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Eaves D. J., et al. 2004. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 48:4012–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galimand M., Courvalin P., Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garnier F., Raked N., Gassama A., Denis F., Ploy M. C. 2006. Genetic environment of quinolone resistance gene qnrB2 in a complex su11-type integron in the newly described Salmonella enterica serovar Keurmassar. Antimicrob. Agents Chemother. 50:3200–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grimm H. 1991. Interpretive criteria of antimicrobial disk susceptibility tests with flomoxef. Infection 19(Suppl. 5):258–263 [DOI] [PubMed] [Google Scholar]
- 13. Jacoby G. A. 2009. AmpC β-lactamase. Clin. Microbiol. Rev. 22:161–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Y., et al. 2010. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54:3967–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kado C. I., Liu S. T. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee C. H., et al. 2006. In vivo selection of OmpK35-deficient mutant after cefuroxime therapy for primary liver abscess caused by Klebsiella pneumoniae. J. Antimicrob. Chemother. 58:857–860 [DOI] [PubMed] [Google Scholar]
- 17. Lee C. H., et al. 2007. Collateral damage of flomoxef therapy: in vivo development of porin deficiency and acquisition of blaDHA-1 leading to ertapenem resistance in a clinical isolate of Klebsiella pneumoniae producing CTX-M-3 and SHV-5 β-lactamases. J. Antimicrob. Chemother. 60:410–413 [DOI] [PubMed] [Google Scholar]
- 18. Lee C. H., et al. 2010. Bacteremia due to extended-spectrum β-lactamase-producing Klebsiella pneumoniae with or without plasmid-mediated AmpC β-lactamase (DHA-1): microbiologic and clinical implications. Antimicrob. Agents Chemother. 54:5395–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee C. H., et al. 2004. Recurrent Klebsiella pneumoniae mycotic aneurysm in a diabetic patient and emergence of an extended-spectrum beta-lactamase (CTX-M-24)-containing Klebsiella pneumoniae strain after prolonged treatment with first-generation cephalosporins for mycotic aneurysm. Microb. Drug Resist. 10:359–363 [DOI] [PubMed] [Google Scholar]
- 20. Lee C. H., Su L. H., Tang Y. F., Liu J. W. 2006. Treatment of ESBL-producing Klebsiella pneumoniae bacteremia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J. Antimicrob. Chemother. 58:1074–1077 [DOI] [PubMed] [Google Scholar]
- 21. Levesque C., Piche L., Larose C., Roy P. H. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J. W., et al. 2008. In vitro activity of tigecycline against clinical isolates of Acinetobacter baumannii in Taiwan. Int. J. Antimicrob. Agents 32(Suppl.):S188–S191 [DOI] [PubMed] [Google Scholar]
- 23. Mammeri H., Van De Loo M., Poirel L., Martinez-Martinez L., Nordmann P. 2005. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob. Agents Chemother. 49:71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martínez-Martínez L., Pascual A., Jacoby G. A. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797–799 [DOI] [PubMed] [Google Scholar]
- 25. Nemec A., Dolzani L., Brisse S., van den Broek P., Dijkshoorn L. 2004. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J. Med. Microbiol. 53:1233–1240 [DOI] [PubMed] [Google Scholar]
- 26. Park Y., Yu J. K., Lee S., Oh E. J., Woo G. J. 2007. Prevalence and diversity of qnr alleles in AmpC-producing Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii and Serratia marcescens: a multicentre study from Korea. J. Antimicrob. Chemother. 60:868–871 [DOI] [PubMed] [Google Scholar]
- 27. Paterson D. L., Bonomo R. A. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pérez-Pérez F. J., Hanson N. D. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiple PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Philippon A., Arlet G., Jacoby G. A. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poirel L., et al. 2006. In vivo selection of fluoroquinolone-resistant Escherichia coli isolates expressing plasmid-mediated quinolone resistance and expanded-spectrum β-lactamase Antimicrob. Agents Chemother. 50:1525–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robicsek A., Jacoby G. A., Hooper D. C. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629–640 [DOI] [PubMed] [Google Scholar]
- 32. Robicsek A., Strahilevitz J., Sahm D. F., Jacoby G. A., Hooper D. C. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw K. J., Rather P. N., Hare R. S., Miller G. H. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamang M. D., et al. 2008. Plasmid-mediated quinolone resistance determinants qnrA, qnrB, and qnrS among clinical isolates of Enterobacteriaceae in a Korean hospital. Antimicrob. Agents Chemother. 52:4159–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toleman M. A., Bennett P. M., Walsh T. R. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tran J. H., Jacoby G. A. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc. Natl. Acad. Sci. U. S. A. 99:5638–5642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verdet C., et al. 2006. Emergence of DHA-1-producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating from Morganella morgannii. Antimicrob. Agents Chemother. 50:607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu J. J., Ko W. C., Tsai S. H., Yan J. J. 2007. Prevalence of plasmid-mediated quinolone resistance determinants qnrA, qnrB, and qnrS among clinical isolates of Enterobacter cloacae in a Taiwanese hospital. Antimicrob. Agents Chemother. 51:1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamane K., Wachino J., Suzuki S., Arakawa Y. 2008. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 52:1564–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan J. J., et al. 2004. Plasmid-mediated 16S rRNA methylases conferring high-level aminoglycoside resistance in Escherichia coli and Klebsiella pneumoniae isolates from two Taiwanese hospitals. J. Antimicrob. Chemother. 54:1007–1012 [DOI] [PubMed] [Google Scholar]
- 42. Yan J. J., et al. 2004. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a teaching hospital in Taiwan. J. Clin. Microbiol. 42:5337–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu F., et al. 2009. Prevalence of 16S rRNA methylase genes in Klebsiella pneumoniae isolates from a Chinese teaching hospital: coexistence of rmtB and armA genes in the same isolate. Diagn. Microbiol. Infect. Dis. 64:57–63 [DOI] [PubMed] [Google Scholar]