Abstract
Differences in carbon assimilation pathways and reducing power requirements among organisms are likely to affect the role of the storage polymer poly-3-hydroxybutyrate (PHB). Previous researchers have demonstrated that PHB functions as a sole growth substrate in aerobic cultures enriched on acetate during periods of carbon deficiency, but it is uncertain how C1 metabolism affects the role of PHB. In the present study, the type II methanotroph Methylocystis parvus OBBP did not replicate using stored PHB in the absence of methane, even when all other nutrients were provided in excess. When PHB-rich cultures of M. parvus OBBP were deprived of carbon and nitrogen for 48 h, they did not utilize significant amounts of stored PHB, and neither cell concentrations nor concentrations of total suspended solids changed significantly. When methane and nitrogen both were present, PHB and methane were consumed simultaneously. Cells with PHB had significantly higher specific growth rates than cells lacking PHB. The addition of formate (a source of reducing power) to PHB-rich cells delayed PHB consumption, but the addition of glyoxylate (a source of C2 units) did not. This and results from other researchers suggest that methanotrophic PHB metabolism is linked to the supply of reducing power as opposed to the supply of C2 units for synthesis.
INTRODUCTION
Poly-3-hydroxybutyrate (PHB) is a biologically produced, biodegradable polyester with properties similar to those of polypropylene and mechanical properties that can be tailored for different applications by changing the copolymer content of the polymer (1, 5, 27). Many bacteria accumulate PHB as a carbon storage polymer under conditions of unbalanced growth (i.e., nutrient deficiency and/or carbon excess) (1, 27). Although considerable efforts are currently being devoted to commercializing microbial PHB production, commercialization thus far has been limited because of the relatively high production cost of PHB compared to that of traditional petrochemical-based plastics, such as polyethylene and polypropylene (28). A major fraction of the production cost (30%) (7) is due to feedstocks, which are typically sugars such as glucose and sucrose (27).
Methane is both an inexpensive feedstock for PHB production and a potent greenhouse gas; its use as a substrate for PHB production is therefore an effective means of carbon sequestration and an attractive alternative to sugar-based feedstocks. Methanotrophs have been shown to produce PHB (2, 18, 37, 41–44), and Wendlandt et al. (42) have reported PHB levels of up to 30 g liter−1 in a culture dominated by Methylocystis sp. GB 25 and maintained under nonaseptic conditions.
While PHB production is well characterized in a variety of bacteria, comparatively few studies have investigated why bacteria accumulate PHB or the process of PHB consumption (16, 20, 33, 36, 41). In nonmethanotrophs, PHB consumption is linked to both short-term replication and long-term survival under carbon starvation. Handrick et al. (16) concluded that Ralstonia eutropha replicated using stored PHB when no external carbon source was available but other nutrients needed for growth were present. When PHB was present without exogenous nitrogen or carbon, the concentration of cells did not increase. Activated sludge enrichments also replicated using stored PHB as a carbon source when no external carbon was present during cyclic pulse feeding of carbon (3, 4, 9, 12, 13, 31). In the absence of both carbon and nitrogen, PHB degraded more slowly than when nitrogen was present (36). It was hypothesized that these cells used PHB for cell maintenance in the absence of carbon and nitrogen (36). In cultures of Sinorhizobium meliloti, stored PHB enabled the replication of cells in the absence of exogenous carbon and aided in the long-term survival of cells (up to 160 days of starvation) (33).
Previous authors have proposed that PHB functions as a source of reducing power in methanotrophs during cometabolic oxidation of non-growth substrates, such as trichloroethylene (TCE), in the absence of methane (8, 14, 19, 20), and metabolic modeling suggests that PHB functions as a source of reducing power (38). It is not known, however, whether PHB can act as the sole growth substrate in methanotrophs. The growth of most methanotrophs requires reducing power and C2 units normally derived from the metabolism of C1 substrates (29). PHB metabolism could conceivably meet both of these needs and thus enable growth in the absence of methane.
Understanding the conditions under which PHB consumption occurs and consequently the advantages that PHB production confers may enable the selection of pure cultures and communities capable of high levels of PHB production. Through pulse feeding of acetate in a sequencing bioreactor, Johnson et al. (23) were able to confer a selective advantage upon bacteria capable of high levels of PHB production: carbon was stored as PHB when acetate was present, and cells reproduced using the stored PHB as a carbon source when acetate became limiting. A similar strategy could be adopted for other substrates, such as methane, but PHB may have a different role in methanotrophs given their greater demand for reducing power (e.g., NADH is required for the methane monooxygenase-mediated oxidation of methane [17, 29]). To assess this possibility, we investigated PHB production and consumption in Methylocystis parvus OBBP in the presence and absence of methane and nitrogen and explored the effects of formate (a supply of reducing power [20]) and glyoxylate (a supply of C2 compounds [26]). We find evidence that PHB acts as a source of reducing equivalents but does not serve as a sole growth substrate.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
M. parvus OBBP was obtained from M. Kalyuzhnaya (Lidstrom laboratory, University of Washington) and used for all experiments in this study. Sterile conditions were maintained throughout all experiments. All glassware was acid washed with 10% hydrochloric acid for at least an hour and triple rinsed in Milli-Q water to remove trace metals. M. parvus OBBP was grown in medium W1 (containing 0.8 mM MgSO4·7H2O, 10 mM NaNO3, 0.14 mM CaCl2, 1.2 mM NaHCO3, 2.35 mM KH2PO4, 3.4 mM K2HPO4, 20.7 μM Na2MoO4·2H2O, 1 μM CuSO4·5H2O, 10 μM Fe-EDTA, 1 ml trace metal solution [containing, per liter, 500 mg FeSO4·7H2O, 400 mg ZnSO4·7H2O, 20 mg MnCl2·7H2O, 50 mg CoCl2·6H2O, 10 mg NiCl2·6H2O, 15 mg H3BO3, 250 mg EDTA], and 10 ml vitamin solution [containing, per liter, 2.0 mg biotin, 2.0 mg folic acid, 5.0 mg thiamine·HCl, 5.0 mg calcium pantothenate, 0.1 mg vitamin B12, 5.0 mg riboflavin, and 5.0 mg nicotinamide]). Nitrogen-free W1 medium (which was identical to medium W1, except that it contained 0 mM NaNO3) was used to induce PHB production. Cultures were incubated under a 1:1 methane-oxygen atmosphere at 30°C on orbital shakers at 150 rpm.
Evaluation of cell growth in conjunction with PHB metabolism in the presence and absence of methane and nitrogen.
Cultures of M. parvus OBBP were incubated in the presence and absence of methane and nitrogen. An exponentially growing culture was diluted into fresh medium to give an optical density at 670 nm (OD670) of approximately 0.07 (total suspended solid [TSS] concentration of 0.03 mg ml−1). Fifty-milliliter aliquots of the dilute culture were transferred into 125-ml serum bottles (Wheaton Science Products, Millville, NJ), and the OD670 was measured. An uninoculated control contained 50 ml of sterile medium. Serum bottles were sealed with butyl rubber stoppers (Geo-Microbial Technologies, Inc.) and aluminum crimp seals, subjected to house vacuum, and supplemented with 55 ml each of filter-sterilized (0.2-μm-pore-size) methane and oxygen to restore atmospheric pressure. Cultures then were incubated at 30°C on an orbital shaker (New Brunswick Scientific, Edison, NJ) at 150 rpm. Duplicate serum bottles were sacrificed every 1.5 h for the first 13 h and every 4 to 6 h thereafter for a total of 35 h. The remaining bottles were periodically subjected to house vacuum and replenished with 55 ml each of methane and oxygen to maintain balanced growth.
To determine the effects of methane and nitrogen on PHB production and consumption, exponential-phase cultures of M. parvus OBBP were collected by centrifugation at 4,816 × g (4,700 rpm) for 8 min, washed once with nitrogen-free W1 medium, and resuspended in nitrogen-free W1 medium to induce PHB production. Using aseptic technique, 50-ml aliquots were transferred by pipette into previously autoclaved 125-ml serum bottles, and the optical density of each aliquot was measured. Serum bottles were sealed, and methane and oxygen were added as described above. Controls for time zero measurements were prepared containing 50 ml sterile medium. Serum bottles were then incubated at 30°C on orbital shakers at 150 rpm. Duplicate serum bottles were sacrificed every 1 to 2 h for the first 20 h.
At 20 h, the remaining bottles were divided into four groups: methane with nitrogen (M1N1), methane with no nitrogen (M1N0), nitrogen with no methane (M0N1), and no methane with no nitrogen (M0N0). The bottles were flushed with helium and subjected twice to a vacuum before the addition of gases as follows: 55 ml methane and 55 ml oxygen were added to M1N1 and M1N0, and 55 ml helium and 55 ml oxygen were added to M0N1 and M0N0. One milliliter of a 500 mM nitrate solution was injected into M1N1 and M0N1 incubations to give an initial concentration of 10 mM nitrate. All bottles were incubated at 30°C on an orbital shaker at 150 rpm and sacrificed in duplicate for a period of 45 h. Sterile controls were treated similarly and sacrificed at the end of the experiment.
To more thoroughly understand the behavior of M. parvus OBBP under M1N1 conditions, a portion of the experiment was repeated with several modifications. Exponentially growing cultures of M. parvus OBBP were transferred to nitrogen-free W1 medium and reincubated to induce PHB production. Cultures were incubated for approximately 22 h, and every 7 to 8 h the bottles were subjected to house vacuum and replenished with 55 ml each of filter-sterilized methane and oxygen. After 22 h, 40 ml of 500 mM nitrate solution was added to 2 liters of culture to give a final concentration of 10 mM, and 50-ml culture aliquots were transferred into serum bottles. Duplicates were sacrificed every 1.5 h for the first 7.5 h and every 3 to 6 h thereafter.
Five-milliliter gas samples were periodically withdrawn for gas-phase analysis using a gas-tight syringe (Restek, Bellefonte, PA). These samples were injected into 5-ml serum bottles previously subjected to a vacuum and flushed with helium four times and were analyzed for methane, oxygen, and carbon dioxide by gas chromatography. The liquid culture suspensions were immediately placed on ice, and subsamples (1 to 5 ml) were filtered for the determination of TSS. Approximately 5 ml of culture was removed and stored at 4°C for the analysis of cell concentration and fluorescence by flow cytometry. The remainder of the biomass was centrifuged, and the pellets were washed once with phosphate-buffered saline and then freeze-dried for the analysis of PHB and elemental composition (carbon, hydrogen, nitrogen, and sulfur [CHNS]). Freeze-dried cells were stored at −20°C in airtight containers. Supernatant was filtered through 0.2-μm filters and stored at 4°C for the analysis of nitrate and phosphate.
Effects of formate and glyoxylate on cell growth in the presence of PHB.
An exponentially growing culture of M. parvus OBBP was transferred to nitrogen-free W1 medium and reincubated for approximately 20 h to induce PHB production. Fifty-milliliter aliquots of the culture (OD670 of 0.88) were transferred to 125-ml serum bottles. Serum bottles were prepared in triplicate to test each of the following conditions: formate and methane (F1M1), no formate and methane (F0M1), formate and no methane (F1M0), and no formate and no methane (F0M0). Serum bottles were subjected to a vacuum and gases added as follows: F1M1 and F0M1, 55 ml methane and 55 ml oxygen, and F1M0 and F0M0, 55 ml helium and 55 ml oxygen. Sodium nitrate (Alfa Aesar, Ward Hill, MA) was added to a final concentration of 10 mM nitrate in all serum bottles, and sodium formate (Sigma-Aldrich) was added to a concentration of 2.5 mM formate in serum bottles under F1M1 and F1M0 conditions.
Serum bottles were incubated at 30°C on orbital shakers at 150 rpm. One-milliliter samples were taken from each bottle every 1.5 h for the first 4.5 h and every hour thereafter for 10 h. Fifty microliters of each sample was immediately diluted into 1.95 ml of sterile Milli-Q water and stored at 4°C for the subsequent analysis of cell concentration. The remaining sample was centrifuged. Pellets were stored at 4°C for subsequent fluorescence analysis by flow cytometry, and the supernatant was stored separately at −20°C for subsequent analysis of formate concentration.
To test the effects of formate at a higher concentration and to test the effects of glyoxylate, the experiment described above was repeated with several variations. Serum bottles were prepared in triplicate to test the following conditions: methane only; formate and methane; glyoxylate and methane; formate, glyoxylate, and methane; and formate only. Sodium formate, sodium glyoxylate (Santa Cruz Biotechnology, Santa Cruz, CA), and sodium nitrate all were added to an initial concentration of approximately 10 mM in serum bottles. All serum bottles were subjected to a vacuum and gases added as follows: all serum bottles containing methane received 55 ml methane and 55 ml oxygen, and all serum bottles without methane received 55 ml oxygen and 55 ml helium. Samples were processed as described above, and glyoxylate measurements were made for cultures containing gloxylate.
Analytical methods.
To analyze the concentrations of methane, oxygen, and carbon dioxide, 1 ml of gas from each sample bottle was injected onto a GOW-MAC gas chromatograph equipped with an Altech CTR 1 column and a thermal conductivity detector. The following method parameters were used: injector, 120°C; column, 45°C; detector, 120°C; and current, 150 mV. Peak areas of methane, oxygen, and carbon dioxide were compared to standards and quantified using the software ChromPerfect (Justice Laboratory Software, Denville, NJ). Concentrations of dissolved oxygen and dissolved methane were calculated using Henry's constant at 30°C and assuming equilibrium between the liquid and gas phases.
To analyze TSS, between 1 and 10 ml (the exact amount varied based on optical density) of culture from each serum bottle was filtered through a prewashed and preweighed 0.2-μm Pall membrane filter (Pall, Port Washington, NY). Filters were dried in an oven at approximately 80°C for ≥12 h, cooled to room temperature in a desiccator, and then weighed on a PerkinElmer AD6 Autobalance.
Concentrations of nitrate and phosphate were measured on a Dionex Ion chromatograph equipped with a GP50 gradient pump, CD25 conductivity detector, AS40 automated sampler, and AS11HC column. Samples were diluted for analysis by adding 0.5 ml of each sample to 5 ml of Milli-Q water and were compared against standards of NaNO3 and K2HPO4. Formate and glyoxylate concentrations were measured on the same instrument using an AA6 column. Samples were diluted in Milli-Q water when necessary and compared to standards of sodium formate and sodium glyoxylate.
To measure the concentration of cells, 50 μl of each sample was diluted to a total volume of 2 ml in Milli-Q water. Dilutions were analyzed at a constant flow rate for 30 s on a BD LSR II flow cytometer. Cell counts were calibrated to solutions containing 6 μm beads at known concentrations (Invitrogen).
Maximum specific growth rates and doubling times were calculated from the slope of a least-squares regression of the natural logarithm of cell counts versus time.
PHB concentration.
PHB concentration was measured directly via gas chromatography using a modified version of the protocol described by Braunegg et al. (6). For each sample, 5 to 10 mg of freeze-dried biomass was weighed out on an analytical balance, transferred to a 12-ml glass vial, and sealed with a polytetrafluoroethylene (PTFE)-lined plastic cap (Wheaton Science Products; Millville, NJ). Two milliliters of methanol acidified with sulfuric acid (3%, vol/vol) and containing 1.0 g liter−1 benzoic acid and 2 ml of chloroform were added to each vial. The vials were shaken gently and then heated at 100°C for 3.5 h. Once the vials had cooled to room temperature, 1 ml deionized water was added to each. The vials were subjected to vortex mixing for 30 s and allowed to stand until phase separation was complete. The organic phase was analyzed using an Agilent 6890N gas chromatograph equipped with an HP-5 column [containing (5% phenyl)-methylpolysiloxane; Agilent Technologies] and a flame ionization detector (FID). dl-β-Hydroxybutyric acid sodium salt (Sigma) was used as a standard. Unless otherwise specified, this gas-chromatographic measurement was used to make all reported measurements of PHB content.
Nile red fluorescence was tested as a more rapid assay for PHB. Five hundred microliters of each sample was centrifuged, and the resulting pellet was resuspended in 1.0 ml of a 40:60 ethanol-deionized water mixture. Ethanol was allowed to permeate the cells for 15 min, and samples were again pelleted and resuspended in 900 μl deionized water mixed with 100 μl Nile red solution (250 μg ml−1 Nile red suspended in dimethyl sulfoxide). Samples were incubated for 45 min, diluted 1:20 in deionized water, and then analyzed on a BD LSR II flow cytometer (BD Biosciences) equipped with a 532-nm laser. Fluorescence values indicating fluorescence per cell were captured using a phycoerythrin (PE) filter set, and the average values for each sample are compared to the percent PHB in cells (mg PHB·mg TSS−1).
CHNS analyses were performed on select samples. Approximately 1 to 2 mg of freeze-dried biomass was weighed out on an analytical balance. Samples were analyzed on a PerkinElmer series 2400 II CHNS/O analyzer operating in CHNS mode. The extended combustion time was set to 5 s. Cysteine powder was used as a standard.
Statistical analyses.
Spearman's rank correlation test (39) was used to determine whether trends were significant over time. Wilcoxon signed-rank tests were used to compare the PHB contents of cultures incubated with and without formate (39). The comparison-of-slopes method was used to test differences in growth rates and yields (32). Statistical analyses were carried out using PASW statistics release 18.0.0 (SPSS Inc., Chicago, Illinois). In summaries of statistical analyses, ρ represents Spearman's correlation coefficient, n represents the number of points used, and P represents the significance. Analyses are considered significant when P ≤ 0.05.
RESULTS
Proxy measurements for PHB content.
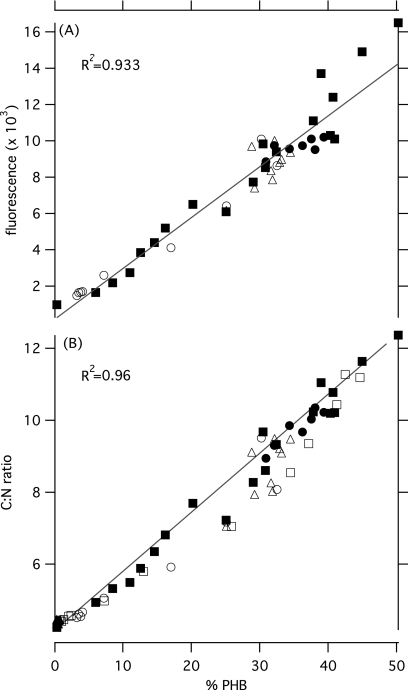
The average fluorescence of individual cells was compared to the percent PHB measured in the TSS of each sample (mg PHB mg TSS−1) as shown in Fig. 1A. There is a strong linear correlation between the values (R2 = 0.93), demonstrating a direct relationship between fluorescence and PHB content while cells are consuming and producing PHB.
Fig. 1.
Percent PHB in cells (100 · mg PHB·mg TSS−1) is plotted versus the fluorescence of cells after staining with Nile red (A) and the C/N weight ratio in the TSS (B). Lines represent best-fit linear regressions for each series. Each point represents the average of replicate measurements taken at each time point. The following conditions are depicted: methane and nitrogen excess with no initial PHB (balanced growth, ▴), methane excess and nitrogen absence (▪), methane and nitrogen absence in cells with stored PHB (●), methane absence and excess nitrogen in cells with stored PHB (▵), and methane and nitrogen excess in cells with stored PHB in the first (○) and second (□) experiments performed, respectively.
A plot of percent PHB in cells versus the C/N mass ratio is shown in Fig. 1B. There is a strong linear correlation (R2 = 0.96) between these values, indicating a direct relationship between the C/N ratio in cells and PHB content while cells are consuming and producing PHB.
Cell growth and PHB metabolism in the presence and absence of methane and nitrogen.
Under conditions of balanced growth, M. parvus OBBP maintained a PHB content of ≤1% (mg PHB mg TSS−1). The doubling time of the cells was 6.45 h, the yield of TSS was 0.45 mg TSS mg CH4−1, and methane and oxygen were consumed in a molar ratio of 1:1.5.
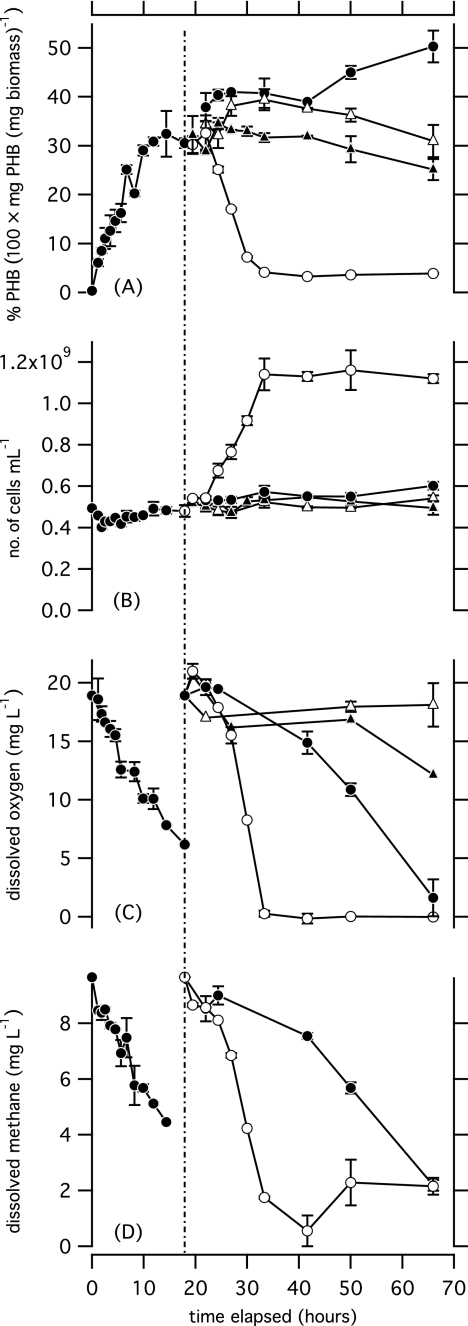
Figure 2A illustrates PHB content over time when cultures of M. parvus OBBP grown under balanced-growth conditions were incubated without nitrogen to induce PHB production and subsequently incubated with and without methane and with and without nitrogen. Table 1 provides details of PHB production and consumption, yield on methane, and dissolved oxygen levels for conditions of PHB production and consumption. When cultures were incubated with methane and without nitrogen (M1N0), PHB content increased. It is likely that, after a total of 66 h of M1N0 conditions, low dissolved oxygen levels limited further PHB production.
Fig. 2.
(A) Percent PHB, (B) concentration of cells, (C) dissolved oxygen concentration, and (D) dissolved methane concentration are plotted over time for cultures of M. parvus OBBP that were incubated initially with methane and no nitrogen and transferred at 18 h (dotted line) to conditions with and without methane and with and without nitrogen. Conditions of methane and no nitrogen (●), methane and nitrogen (○), no methane and nitrogen (▴), and no methane and no nitrogen (▵) are shown. Error bars represent the range of values for replicate samples. The absence of error bars on points in C and D indicates that only a single measurement was available.
Table 1.
Stoichiometry of PHB production under unbalanced growth conditions (nitrogen limitation) and subsequent PHB consumptiona
| PHB production and utilization | Time (h) | Yield of PHB (g g methane−1) | Methane/oxygen consumption ratio | Initial PHB concn (mg ml−1) | Final PHB concn (mg ml−1) | Initial % PHB | Final % PHB | Initial nitrate concn (mg N ml−1) | Final nitrate concn (mg N ml−1) |
|---|---|---|---|---|---|---|---|---|---|
| Production | |||||||||
| Methane, no nitrogen (M1N0) | 0–18 | 0.34 | 1:1.5 | 0 | 0.14 ± 0.01 | 0.4 | 30.5 ± 1.0 | 0 | 0 |
| Methane, no nitrogen (M1N0) | 18–66 | 0.24 | 1:1.4 | 0.14 ± 0.01 | 0.28 ± 0.03 | 30.5 ± 1.0 | 50.3 ± 3.3 | 0 | 0 |
| Utilization | |||||||||
| No methane, no nitrogen (M0N0) | 18–66 | NA | NA | 0.14 ± 0.01 | 0.12 ± 0.02 | 30.5 ± 1.0 | 30.9 ± 3.3 | 0 | 0 |
| No methane, nitrogen (M0N1) | 18–66 | NA | NA | 0.14 ± 0.01 | 0.14 ± 0.05 | 30.5 ± 1.0 | 25.1 ± 2.2 | 0.21 ± 0.002 | 0.19 |
| Methane, nitrogen (M1N1) | 18–66 | NA | 1:1.4 | 0.14 ± 0.01 | 0.02 ± 0.003 | 30.5 ± 1.0 | 3.9 ± 0.1 | 0.21 ± 0.002 | 0.11 ± 0.003 |
Errors represent the ranges of duplicate measurements. NA, not applicable.
When PHB-rich cultures were incubated without methane (M0N1 and M0N0), the PHB content showed little change. When both methane and nitrogen were absent (M0N0), no measurable changes occurred in the system. There was no significant trend in TSS, cell concentration, PHB content, percent PHB, or oxygen consumed. When nitrogen was added to PHB-rich cultures and methane was not present (M0N1), PHB content decreased slightly, but cell concentration, TSS concentration, and nitrate concentration did not show a significant trend over time. However, the concentration of nitrogen-associated TSS increased (ρ = 0.56, n = 20, P = 0.02), and the percent nitrogen in both the TSS (ρ = 0.7, n = 18, P = 0.001) and the non-PHB TSS (ρ = 0.695, n = 18, P = 0.001) increased. Cells were not replicating, but uptake of nitrogen occurred as PHB and oxygen were consumed.
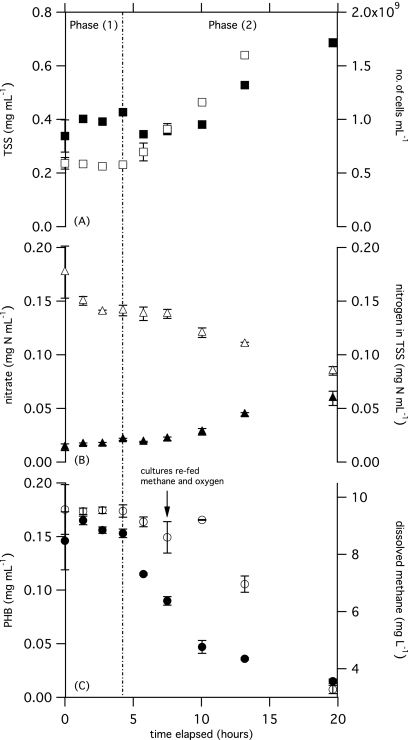
PHB content dropped off sharply when both methane and nitrogen were present (M1N1), and the concentration of cells increased exponentially (Fig. 2B) until the level of dissolved oxygen dropped to near 0 (Fig. 2C). It may be hypothesized that the depletion of oxygen limited cell growth after PHB reserves had been exhausted. In a second experiment, the headspace of serum bottles was replenished with methane and oxygen (at 7.5, 23.5, and 31 h), samples were taken more frequently to obtain a larger data set, and replicate TSS measurements were performed for each duplicate. PHB content dropped from 10.5% ± 1% to 4.1% ± 0.2% (0.14 ± 0.01 to 0.03 ± 0.005 mg ml−1) during 15.4 h in the first experiment (Fig. 3) and from 44.7% ± 1.1% to 2.4% ± 0.3% (0.15 to 0.02 ± 0.002 mg ml−1) during 19.6 h in the second experiment (see Fig. S1 in the supplemental material).
Fig. 3.
First two phases of metabolism in PHB-rich cells of M. parvus OBBP exposed to methane and nitrogen are depicted. Comparisons are drawn between TSS (▪) and concentration of cells (□) (A), concentration of dissolved nitrate as N (▵) and N-TSS (▴) (B), and PHB content (●) and dissolved methane concentration (○) (C). All error bars represent the range of values for replicate samples. No data are available for the concentration of cells at 19.6 h due to sample-processing error.
Phases of growth and PHB utilization.
Three phases of growth and PHB utilization were observed: phase 1 (0 to 4.3 h), nitrogen accumulation; phase 2 (4.3 to 19.6 h), cell reproduction and concurrent PHB and CH4 depletion; and phase 3 (19.6 to 35.5 h), continued cell reproduction with CH4 consumption after PHB consumption. Phase 1 ended when the cells began to replicate, and phase 2 ended when the cells had depleted nearly all of their PHB reserves (≤2% PHB remaining) and were utilizing primarily methane for growth. During phase 3, cells continued to replicate and consume methane.
Throughout phase 1, neither the concentration of M. parvus OBBP nor the PHB content of the cells exhibited significant positive or negative trends, nor was a significant amount of CH4 consumed. However, the percent PHB did decrease significantly (ρ = −0.88, n = 8, P = 0.004), and the PHB content as measured by fluorescence also decreased significantly (ρ = −0.83, n = 8, P = 0.01). Although there was no significant trend in the concentration of nitrate, the nitrate concentration appeared to decrease from 0.18 ± 0.02 to 0.14 ± 0.005 mg N ml−1, the percent nitrogen in the TSS increased from 4.2% ± 0.2% to 5.0% ± 0.2% (ρ = 0.77, n = 8, P = 0.03), and nitrogen assimilation measured as mg N ml−1 increased significantly from 0.014 ± 0.003 to 0.02 ± 0.001 (ρ = 0.88, n = 8, p = 0.004). TSS increased from 0.34 ± 0.06 to 0.43 ± 0.005 mg ml−1 (ρ = 0.75, n = 8, P = 0.05). These data indicate that the cells assimilated nitrogen in quantities sufficient to account for the increase in TSS as nitrogen and consumed stored PHB, but they did not consume exogenous CH4.
During the second phase, methane and PHB were used simultaneously as cosubstrates. The cell count increased, and PHB content decreased rapidly over a 15-h period, from 37.2% ± 1.5% to 2.4% ± 0.3% of the total biomass, or 0.16 ± 0.01 to 0.017 ± 0.002 mg ml−1. A total of 1.8 ± 0.09 mmol methane and 2.6 ± 0.13 mmol oxygen were consumed. The percent nitrogen in the TSS increased from 5.0% ± 0.2% to 8.7% ± 0.6%, and the percent nitrogen in the non-PHB TSS increased from 7.9% ± 0.5% to 8.9% ± 0.6%. The average doubling time of cells in phase 2, 6.08 h, was not statistically different from the average doubling time of cells without PHB under balanced growth conditions (6.45 h; n = 15, P = 0.16). However, the doubling time during the early part of this phase, from 4.3 to 7.5 h, was 4.94 h, which is significantly shorter than the doubling time observed under growth conditions without initial PHB reserves (n = 13, P = 0.02). This suggests that the doubling time of cells was more rapid in the earlier part of phase 2, when cells had ≥25% PHB. A yield of 0.71 mg non-PHB biomass (mg CH4)−1 was observed during phase 2, but this was not statistically different from the yield observed without PHB reserves, at 0.56 mg non-PHB biomass (mg CH4)−1 (n = 10, P = 0.4). A time of 5.7 h was chosen as the initial point for calculating changes in non-PHB TSS, because non-PHB TSS was lowest at this point.
During phase 3, the PHB content of the cells decreased to ≤1%, or 0.009 ± 0.001 mg ml−1. At 19.6 and 31 h, the amount of oxygen in the headspace of serum bottles decreased to ≤0.2 mmol. When the gas phase was replenished with methane and oxygen, an immediate increase in cell concentration and TSS occurred at both time points. When the experiment ended at 35.5 h, a total of 4.9 ± 0.1 mmol methane had been consumed, and the final TSS concentration was 1.2 ± 0.04 mg ml−1. A residual of 0.02 ± 2E−5 mg ml−1 of nitrate-N remained.
Effects of formate on cell growth in the presence of PHB.
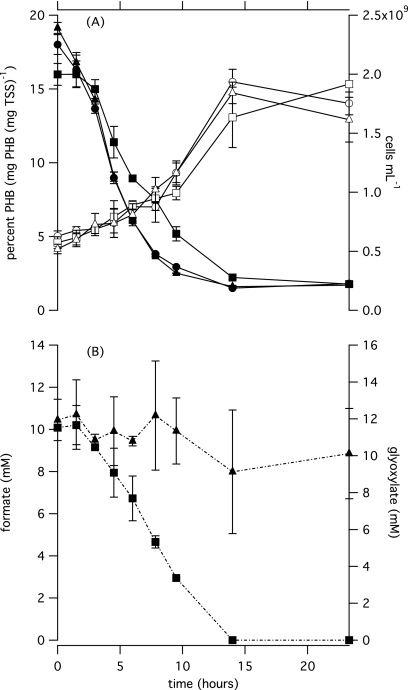
Figure 4 illustrates the effects of 10 mM formate on cultures of M. parvus OBBP with PHB and methane. Data from the initial experiment where 2.5 mM formate was provided show similar trends and are included in Fig. S2 in the supplemental material. Gas chromatography was used to measure the initial percentage of PHB (17.4% ± 5.4%), and all other PHB values were converted from fluorescence values to percent PHB based on the initial value and a direct linear relationship.
Fig. 4.
Depletion of PHB and formate and the increase in concentration of cells are depicted for cultures of M. parvus OBBP exposed to methane and nitrogen with and without the addition of formate. The concentration of PHB is shown for cultures with methane only (●), methane and formate (▪), and methane and glyoxylate (▴) in the upper panel. Cell concentration is shown for cultures with methane only (○), methane and formate (□), and methane and glyoxylate (▵) in the upper panel. The concentration of formate in cultures with methane and formate (▪) and the concentration of glyoxylate in cultures with methane and glyoxylate (▴) are shown in the lower panel. Error bars represent the standard deviations from triplicate cultures.
From 1.5 to 14 h, the utilization of PHB in cultures with formate was delayed compared to that of cultures without formate (P = 0.003 and P = 0.04 for 2.5 and 10 mM formate, respectively). Thereafter, formate was consumed concurrently with PHB and was completely consumed after 14 h. The delay in PHB consumption suggests that formate and PHB mediate a common function as electron donors, but simultaneous use thereafter suggests a rerouting of electron flow from PHB in the presence of formate, enabling the concomitant use of both substrates.
In the presence of nitrogen and the absence of methane, there was no significant increase in cell concentration due to the presence of formate. When 2.5 mM formate was added, there was a slight, significant positive trend in cell concentration with (ρ = 0.81, n = 12, P = 0.001) and without (ρ = 0.79, n = 12, P = 0.002) added formate, although no significant difference was observed between the two (P = 0.8). In cultures amended with 2.5 mM formate, the cell concentration increased by an average of 7% at the end of 22 h. In cultures amended with 10 mM formate, there was no significant increase in cell concentration (ρ = 0.7, n = 5, P = 0.2). This indicates that formate addition had little effect on cell growth, enabling only limited cell replication in the absence of methane.
In cultures provided with methane and nitrogen, there was no significant difference in PHB consumption between cultures with and without glyoxylate (P = 0.4) (Fig. 4). There was no significant glyoxylate consumption during 23 h.
DISCUSSION
PHB quantification.
This study included the use of two rapid, solvent-free techniques for the estimation of PHB content and their calibration against conventional measurements made by gas chromatography. Nile red staining combined with flow cytometry enabled rapid assay of PHB content (10, 15, 25, 40). The data indicate an excellent correlation between fluorescence and PHB content (Fig. 1A). A second technique comparing the relationship between carbon/nitrogen ratios and PHB content also was evaluated. Since PHB contains no nitrogen, the C/N ratio was expected to increase with increasing PHB content. This result was demonstrated by a high, direct correlation between C/N ratio and PHB content in cells that are accumulating and/or consuming PHB (Fig. 1B).
Physiological role of PHB.
Understanding the role of PHB in methanotrophs may enable the design of a stressed environment where high levels of PHB confer a competitive advantage. Repeated stresses may in turn enable the long-term maintenance of a nonsterile, methane-utilizing culture that can produce increasingly higher levels of PHB for commercial purposes.
Methanotrophs utilize PHB slowly under starvation conditions (22), and PHB consumption is known to aid in the long-term survival of other bacteria (33). In activated sludge enrichments, for example, the concentration of PHB decreased from 85 to ≤10% in just 40 h when there was no carbon (i.e., electron donor) or nitrogen source (36). In contrast, the utilization of PHB in M. parvus OBBP is relatively slow under similar starvation conditions. The concentration of PHB in starving cells decreased from 31 to 25% after 48 h, but this trend was not significant. This suggests that if PHB aids in the survival of M. parvus OBBP, it is over a relatively long timescale. Methanotrophs are known to survive for up to 10 weeks without methane (34), but their survival has not yet been linked to the presence of PHB. Further work should evaluate the relationship between PHB and long-term survival in methanotrophs.
In many bacteria, PHB is used as both a growth substrate and a source of reducing power under conditions of carbon starvation (3, 4, 9, 11, 13, 31, 33), enabling PHB-rich cells to replicate in the absence of an exogenous carbon source. In M. parvus OBBP, however, stored PHB did not enable replication in the absence of exogenous carbon when all other nutrients were present. In the absence of methane, the cell concentrations did not increase significantly in PHB-rich cultures fed nitrogen and formate compared to cultures incubated without formate. This indicates that growth on PHB is not prevented by a deficiency in reducing power. Others have proposed that the methylotrophic metabolism of PHB enables the regeneration of glyoxylate (26). In theory, PHB could itself be assimilated as a growth substrate if formaldehyde or a precursor were present. However, the addition of glyoxylate to PHB-rich cultures of M. parvus OBBP in the presence of methane did not affect PHB consumption, suggesting that PHB and glyoxylate do not contribute to the same metabolic pathway in M. parvus OBBP.
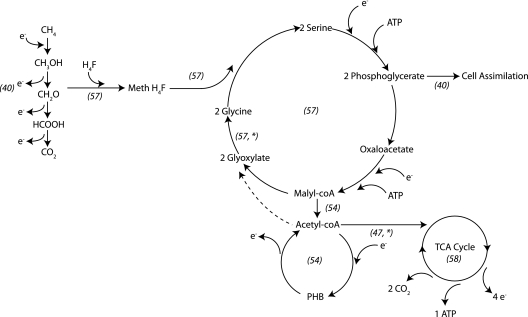
Type II methanotrophs possess a complete tricarboxylic acid (TCA) cycle, which functions as a source of reducing equivalents (21, 30). PHB is a sink for these equivalents (Fig. 5). In PHB-rich cells of M. parvus OBBP fed nitrogen but incubated without methane, the percentage of PHB decreased with an accompanying increase in nitrogen-associated TSS and in the percent nitrogen in the non-PHB TSS. Evidently, PHB is used under these conditions as a source of reducing power to assimilate nitrogen. When PHB-rich cells of M. parvus OBBP initially were exposed to both methane and nitrogen, a 4-h lag ensued before cell replication began. During this period, cells accumulated nitrogen but did not replicate or consume significant amounts of methane or PHB. A likely explanation is the induction and synthesis of proteins needed for replication. Cells under M1N1 and M0N1 conditions likewise accumulated nitrogen in the first 4 h following nitrate addition, suggesting that reducing power from PHB can be used for N assimilation in the presence or absence of methane. Johnson et al. (24) reported a similar role for PHB in acetate-fed communities subjected to repeated nitrogen limitation and pulses of acetate: PHB and acetate degraded concurrently when nitrogen was present.
Fig. 5.
Pathway for production and consumption of PHB in M. parvus OBBP. The pathways are based primarily on the work of Lidstrom (29) and Hanson and Hanson (17), and all references are included in italics on the figure. An asterisk indicates that the results of this work support the proposed step in the pathway. The conversion of acetyl coenzyme A (acetyl-coA) to glyoxylate is shown as a dotted line because it was not observed under conditions of the present study. Note that PHB is used as a source of reducing power upon entering the TCA cycle.
Previous studies have shown that PHB provides reducing power for the cometabolic oxidation of non-growth substrates in the absence of methane (8, 14, 19, 20). Methanotrophic cells with higher levels of PHB have higher transformation capacities for trichloroethylene (TCE) than cells with lower levels of PHB, and the effects of PHB mimicked those of formate in M. parvus OBBP. In the present study, the presence of formate delayed the consumption of PHB when methane and nitrogen were also present, suggesting that PHB and formate mediate the same role in the metabolism and that formate is consumed as an additional source of reducing power. Metabolic models assuming that PHB is used as a source of reducing equivalents in Methylosinus trichosporium OB3b have shown good agreement with experimental data (38), suggesting that PHB function is conserved among type II methanotrophs.
The pattern of concurrent PHB and methane utilization in M. parvus OBBP is unlike that of other bacteria. Typically, when an exogenous carbon source is supplied in excess, most PHB-producing bacteria produce PHB rather than utilize it (1). The degradation of PHB takes place only after the exhaustion of exogenous carbon supplies (35) and under unbalanced growth conditions (38). However, in M. parvus OBBP, PHB was consumed when methane was present in excess, and the consumptions of PHB and methane were concurrent. Others (38) have hypothesized that PHB serves as a sink of reducing equivalents and is consumed only when NADH is limiting. NADH would logically be growth limiting following a period of methane absence, but in this work, PHB was not consumed at appreciable rates under such conditions.
The coutilization of PHB and methane suggests that PHB utilization confers a physiological advantage under growth conditions, likely in the form of readily available reducing power for methane monooxygenase activity, carbon dioxide assimilation, and nitrogen assimilation. This work establishes that PHB increases the specific growth rate of exponentially growing cultures of M. parvus OBBP that have ≥20% PHB (P = 0.02), providing a significant short-term competitive advantage over cells that lack PHB.
For microorganisms that use PHB as a growth substrate, the absence of exogenous carbon stimulates the consumption of PHB (16). The repeated pulsing of exogenous carbon, therefore, confers a selective advantage on those cells that have high levels of stored PHB. The sustained pulsing of acetate into an acetate-utilizing community increased the rate and ultimate capacity of PHB production (3, 4, 9, 12, 13, 31). However, the results of the present study suggest the need for a different selection strategy in the case of methanotrophs. A plausible strategy is the pulsing of nitrogen into a sequencing batch reactor with a sustained supply of methane. This would alternately stimulate the production and utilization of PHB, and those cells able to store high quantities of PHB presumably would gain a competitive advantage under balanced growth conditions. Further study is needed to examine the effects of repeated nitrogen limitation on methanotrophic cultures and the potential of such strategies to enhance PHB production.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Kalhuznaya for providing M. parvus OBBP and Katherine Rostkowski, Andrew Pfluger, Joseph Burg, and Andrew Baltay for their help in the laboratory. We also thank several anonymous reviewers whose comments assisted in improving the manuscript.
This work was supported by a Graduate Research Fellowship from the National Science Foundation to A.J.P. and by the California Environmental Protection Agency, Department of Toxic Substances Control, under contract 07T3451.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Anderson A. J., Dawes E. A. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asenjo J. A., Suk J. 1986. Microbial conversion of methane into poly-beta hydroxybutrate (PHB)-growth and intracellular product accumulation in a type-II methanotroph. J. Ferment. Technol. 64:271–278 [Google Scholar]
- 3. Beccari M., Majone M., Massanisso P., Ramadori R. 1998. A bulking sludge with high storage response selected under intermittent feeding. Water Res. 32:3403–3413 [Google Scholar]
- 4. Beun J. J., Dircks K., Van Loosdrecht M. C., Heijnen J. J. 2002. Poly-beta-hydroxybutyrate metabolism in dynamically fed mixed microbial cultures. Water Res. 36:1167–1180 [DOI] [PubMed] [Google Scholar]
- 5. Braunegg G., Lefebvre G., Genser K. F. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127–161 [DOI] [PubMed] [Google Scholar]
- 6. Braunegg G., Sonnleitner B., Lafferty R. 1978. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur. J. Appl. Microbiol. Biotechnol. 6:29–37 [Google Scholar]
- 7. Choi M. H., Yoon S. C., Lenz R. W. 1999. Production of poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid) and poly(4-hydroxybutyric acid) without subsequent degradation by Hydrogenophaga pseudoflava. Appl. Environ. Microbiol. 65:1570–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu K., Alvarez-Cohen L. 1998. Effect of nitrogen source on growth and trichloroethylene degradation by methane-oxidizing bacteria. Appl. Environ. Microbiol. 64:3451–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciðgin A. S., Karahan O., Orhon D. 2007. Effect of feeding pattern on biochemical storage by activated sludge under anoxic conditions. Water Res. 41:924–934 [DOI] [PubMed] [Google Scholar]
- 10. Degelau A., Scheper T., Bailey J., Guske C. 1995. Fluorometric measurement of poly-beta hydroxybutyrate in Alcaligenes eutrophus by flow-cytometry and spectrofluorometry. Appl. Microbiol. Biotechnol. 42:653–657 [Google Scholar]
- 11. Dionisi D., et al. 2005. Storage of biodegradable polymers by an enriched microbial community in a sequencing batch reactor operated at high organic load rate. J. Chem. Technol. Biotechnol. 80:1306–1318 [Google Scholar]
- 12. Dionisi D., Majone M., Papa V., Beccari M. 2004. Biodegradable polymers from organic acids by using activated sludge enriched by aerobic periodic feeding. Biotechnol. Bioeng. 85:569–579 [DOI] [PubMed] [Google Scholar]
- 13. Dircks K., Henze M., van Loosdrecht M. C., Mosbaek H., Aspegren H. 2001. Storage and degradation of poly-beta-hydroxybutyrate in activated sludge under aerobic conditions. Water Res. 35:2277–2285 [DOI] [PubMed] [Google Scholar]
- 14. Fitch M. W., Speitel G. E., Georgiou G. 1996. Degradation of trichloroethylene by methanol-grown cultures of Methylosinus trichosporium OB3b PP358. Appl. Environ. Microbiol. 62:1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorenflo V., Steinbuchel A., Marose S., Rieseberg M., Scheper T. 1999. Quantification of bacterial polyhydroxyalkanoic acids by Nile red staining. Appl. Microbiol. Biotechnol. 51:765–772 [DOI] [PubMed] [Google Scholar]
- 16. Handrick R., Reinhardt S., Jendrossek D. 2000. Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J. Bacteriol. 182:5916–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanson R. S., Hanson T. E. 1996. Methanotrophic bacteria. Microbiol. Rev. 60:439–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helm J., Wendlandt K. D., Jechorek M., Stottmeister U. 2008. Potassium deficiency results in accumulation of ultra-high molecular weight poly-beta-hydroxybutyrate in a methane-utilizing mixed culture. J. Appl. Microbiol. 105:1054–1061 [DOI] [PubMed] [Google Scholar]
- 19. Henry S. M., Grbic-Galic D. 1991. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer. Appl. Environ. Microbiol. 57:236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henrysson T., McCarty P. L. 1993. Influence of the endogenous storage lipid poly-beta-hydroxybutyrate on the reducing power availability during cometabolism of trichloroethylene and naphthalene by resting methanotrophic mixed cultures. Appl. Environ. Microbiol. 59:1602–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins I. J., Best D. J., Hammond R. C., Scott D. 1981. Methane-oxidizing microorganisms. Microbiol. Rev. 45:556–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James B. W., Mauchline W. S., Dennis P. J., Keevil C. W., Wait R. 1999. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl. Environ. Microbiol. 65:822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson K., Jiang Y., Kleerebezem R., Muyzer G., van Loosdrecht M. C. M. 2009. Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity. Biomacromolecules 10:670–676 [DOI] [PubMed] [Google Scholar]
- 24. Johnson K., Kleerebezem R., van Loosdrecht M. C. 2010. Influence of the C/N ratio on the performance of polyhydroxybutyrate (PHB) producing sequencing batch reactors at short SRTs. Water Res. 44:2141–2152 [DOI] [PubMed] [Google Scholar]
- 25. Kacmar J., Carlson R., Balogh S., Srienc F. 2006. Staining and quantification of poly-3-hydroxybutyrate in Saccharomyces cerevisiae and Cupriavidus necator cell populations using automated flow cytometry. Cytometry A 69A:27–35 [DOI] [PubMed] [Google Scholar]
- 26. Korotkova N., Lidstrom M. E. 2001. Connection between poly-beta-hydroxybutyrate biosynthesis and growth on C(1) and C(2) compounds in the methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 183:1038–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee S. 1996. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49:1–14 [DOI] [PubMed] [Google Scholar]
- 28. Lee S. Y., Park S. J., Park J. P., Lee Y., Lee S. H. 2005. Economic aspects of biopolymer production, vol. 2 Wiley-VCH, Weinheim, Germany [Google Scholar]
- 29. Lidstrom M. E. 2006. Aerobic methylotrophic prokaryotes, p. 618–634 In Falkow S., et al. (ed.), The prokaryotes. Springer, New York, NY [Google Scholar]
- 30. Madigan M. T., Martinko J. M., Parker J. 2003. Brock biology of microorganisms, 10th ed. Pearson Education, Inc., Upper Saddle River, NJ [Google Scholar]
- 31. Majone M., Massanisso P., Ramadori R. 1998. Comparison of carbon storage under aerobic and anoxic conditions. Water Sci. Technol. 38:77–84 [Google Scholar]
- 32. Neter J., Waserman W., Kutner M. 1990. Qualitative independent variables, 3rd ed. Richard D. Irwin, Inc., Chicago, IL [Google Scholar]
- 33. Ratcliff W. C., Kadam S. V., Denison R. F. 2008. Poly-3 hydroxybutyrate (PHB) supports survival and reproduction in starving rhizobia. FEMS Microbiol. Ecol. 65:391–399 [DOI] [PubMed] [Google Scholar]
- 34. Roslev P., King G. M. 1994. Survival and recovery of methanotrophic bacteria starved under oxic and anoxic conditions. Appl. Environ. Microbiol. 60:2602–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Senior P. J., Dawes E. A. 1973. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 134:225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Serafim L. S., Lemos P. C., Oliveira R., Reis M. A. 2004. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 87:145–160 [DOI] [PubMed] [Google Scholar]
- 37. Shah N. N., Hanna M. L., Jackson K. J., Taylor R. T. 1995. Batch cultivation of Methylosinus trichosporium OB3B: IV. Production of hydrogen-driven soluble or particulate methane monooxygenase activity. Biotechnol. Bioeng. 45:229–238 [DOI] [PubMed] [Google Scholar]
- 38. Sipkema E. M., de Koning W., Ganzeveld K. J., Janssen D. B., Beenackers A. A. 2000. NADH-regulated metabolic model for growth of Methylosinus trichosporium OB3b: model presentation, parameter estimation, and model validation. Biotechnol. Prog. 16:176–188 [DOI] [PubMed] [Google Scholar]
- 39. Snedecor G., Cochran W. G. 1989. Statistical methods, 8th ed. Iowa State University Press, Ames, IA [Google Scholar]
- 40. Tyo K. E., Zhou H., Stephanopoulos G. N. 2006. High-throughput screen for poly-3-hydroxybutyrate in Escherichia coli and Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 72:3412–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vecherskaya M., Dijkema C., Stams A. J. 2001. Intracellular PHB conversion in a type II methanotroph studied by 13C NMR. J. Ind. Microbiol. Biotechnol. 26:15–21 [PubMed] [Google Scholar]
- 42. Wendlandt K. D., Jechorek M., Helm J., Stottmeister U. 2001. Producing poly-3-hydroxybutyrate with a high molecular mass from methane. J. Biotechnol. 86:127–133 [DOI] [PubMed] [Google Scholar]
- 43. Xin J. Y., Zhang Y. X., Zhang S., Xia C. G., Li S. B. 2007. Methanol production from CO2 by resting cells of the methanotrophic bacterium Methylosinus trichosporium IMV 3011. J. Basic Microbiol. 47:426–435 [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y., Xin J., Chen L., Song H., Xia C. 2008. Biosynthesis of poly-3-hydroxybutyrate with a high molecular weight by methanotroph from methane and methanol. J. Natural Gas Chem. 17:103–109 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.