Abstract
The intracellular parasite Toxoplasma gondii develops inside a parasitophorous vacuole (PV) that derives from the host cell plasma membrane during invasion. Previous electron micrograph images have shown that the membrane of this vacuole undergoes an extraordinary remodeling with an extensive network of thin tubules and vesicles, the intravacuolar network (IVN), which fills the lumen of the PV. While dense granule proteins, secreted during and after invasion, are the main factors for the organization and tubulation of the network, little is known about the source of lipids used for this remodeling. By selectively labeling host cell or parasite membranes, we uncovered evidence that strongly supports the host cell as the primary, if not exclusive, source of lipids for parasite IVN remodeling. Fluorescence recovery after photobleaching (FRAP) microscopy experiments revealed that lipids are surprisingly dynamic within the parasitophorous vacuole and are continuously exchanged or replenished by the host cell. The results presented here suggest a new model for development of the parasitophorous vacuole whereby the host provides a continuous stream of lipids to support the growth and maturation of the PVM and IVN.
INTRODUCTION
Toxoplasma gondii is an apicomplexan parasite that infects almost any warm-blooded animal. This ubiquitous parasite is able to proliferate inside the host cell within a unique and dynamic parasitophorous vacuole (PV). This vacuole is established during invasion initially as an invagination of the host cell plasma membrane that surrounds the invading parasite and then pinches off to create a PV (18). During this process, parasite secretory organelles, called dense granules, discharge specific protein factors (GRAs) that remodel the vacuole into a sheltered niche for the parasite.
Toxoplasma's ability to acquire essential nutrients from its environment is fundamental to its intracellular lifestyle. It has been postulated that Toxoplasma gains access to various host metabolites (<1,300 Da) via diffusion through small pores in the PV membrane (PVM) (14). While no direct connection between the PV and the host vesicular transport system has been observed (13), Toxoplasma incorporates low-density lipoprotein (LDL)-derived cholesterol from host endolysosomes (4). Host mitochondria are also recruited efficiently to the cytosolic face of the PV soon after invasion, allowing Toxoplasma to scavenge host lipoic acid (5, 16).
In addition to nutrient acquisition from the host cytosol, Toxoplasma facilitates its intracellular lifestyle by actively remodeling the PV. The purpose of this remodeling and the origin of the lipid constituents it uses have not been established. Electron micrographs of the Toxoplasma PV show membrane-like material within the PV and close to the posterior end of the parasites at very early times during invasion (15). A complex of secreted GRA2/GRA4/GRA6 proteins interacts with this material and seems to be required for its reorganization and tubulation (9, 12). Later during infection, similar thin tubules fill the lumen of the PV to form an intravacuolar network (IVN) (10, 15). While electron micrographs suggested that the IVN might be primarily parasitic in origin (12, 15), no direct biochemical analysis has been reported, and its true origin has been a mystery. Tantalizingly, host endoplasmic reticulum (ER) proteins also appear to have access to the vacuolar space, and in particular, host glucose-6-phosphatase has been observed in the posterior membrane-like material seen in the nascent PV described above (7, 11). This suggests that there might be a flow of lipids between the host ER and the PVM but gives no hint as to the magnitude of that flow and the relative contributions that host and parasite lipids might each represent within the IVN.
In this study, by selectively labeling host cell or parasite membranes and using fluorescence confocal microscopy, we provide evidence that host lipids are the primary, if not exclusive, contributors to the IVN. Furthermore, results from fluorescence recovery after photobleaching (FRAP) experiments underscore the dynamic nature of the parasitophorous vacuole and suggest a continuous flow of lipids from the host cell into the PVM and IVN.
MATERIALS AND METHODS
Cells and parasites.
The SPCherry Toxoplasma strain was obtained by cloning the coding region of mCherry N-terminally fused to the signal peptide of GRA1 and C-terminally tagged with hemagglutinin (HA) into the pGRA-HA_HPT vector (3). The resulting plasmid was linearized, and 25 μg of DNA was transfected by electroporation into Toxoplasma strain RH Δhpt as previously described (17). Following selection in mycophenolic acid-xanthine medium (6), the resultant strain and the parental strain were maintained by serial passage in human foreskin fibroblast (HFF) cells grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (cDMEM).
Labeling of HFFs.
Unless otherwise stated, all culturing, labeling, and washes of live cells were performed at 37°C. HFFs growing on either chambered cover glass (for live-cell imaging) or 15-cm dishes (for lipid analysis) at 80 to 90% confluence were incubated for 1 h in a minimal volume of DMEM supplemented with 10% dialyzed fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (dDMEM). The fluorescent lipid probe 5-butyl-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-3-nonanoic acid (C4-BODIPY-C9; Molecular Probes) was then added to the medium at a final concentration of 20 μM. After 3 h of incubation in a humidified 37°C incubator with 5% CO2, the labeling medium was replaced and incubation was continued for another 3 h. Cells were washed 5 times with dDMEM, with incubation for 5 min between each wash. For live-cell imaging experiments, labeled HFFs were infected with unlabeled parasites for 18 h in dDMEM, and the medium was replaced by fresh dDMEM just before imaging. For lipid analysis, HFFs labeled as described above were washed and incubated in dDMEM without the fluorescent lipid probe for a further 18 h. Cells were washed twice with phosphate-buffered saline (PBS), scraped from the plate, and centrifuged at room temperature. The pellet was immediately flash-frozen and stored at −20°C until lipid extraction.
Labeling of intracellular parasites.
We slightly modified the protocol of Charron and Sibley (2) to obtain an adequate labeling of parasites that was compatible with the infection times used in our experiments. The protocol described above for HFFs was used to first saturate the lipid pools of HFFs with C4-BODIPY-C9, with the exception that no washes were performed after the second incubation in labeling medium. Instead, parasites were added to a very small volume of dDMEM and allowed to infect the cells for 18 h. The presence of the lipid probe had no apparent effect on parasite growth or morphology through the time course of the experiment. Infected cultures were washed 5 times with dDMEM, with incubation for 5 min between each wash. Cells were scraped, and parasites were released from their host cells by sequential passages through 25- and 27-gauge syringe needles at room temperature. Parasites were further purified from host debris by using a size-exclusion column (PD-10; General Electric). These labeled parasites were used either to infect fresh HFF monolayers for subsequent fluorescence microscopy or for lipid analysis. As a control for lipid analysis, HFFs were labeled and washed as mentioned above, followed by infection with unlabeled parasites. At 18 h postinfection, cultures were washed and parasites were released and purified from host cells as described before to determine the lipid uptake by the parasite under these conditions.
Lipid extraction and TLC analysis.
After labeling, frozen HFF or parasite samples were lyophilized followed by three sequential extractions with chloroform-methanol, at 2:1, 1:1, and 1:2. Organic phases were combined and dried under a nitrogen stream. Samples were resuspended in 20 μl and analyzed by one-dimensional thin-layer chromatography (TLC) on silica gel 60 plates, using chloroform-methanol-water-triethylamine (35:30:7:35) as the solvent. The following fluorescent standards were spotted and separated on the same plate: BODIPY-Cer, N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)sphingosine; BODIPY-FA, 5-butyl-4,4-difluoro-4-bora-3a,4a-diaza-s- indacene-3-nonanoic acid; BODIPY-PC, 2-(4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine; and BODIPY-SM, N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)sphingosyl phosphocholine (Molecular Probes). The fluorescent lipids were visualized with a Typhoon imager (GE Healthcare Life Sciences).
Confocal fluorescence microscopy and FRAP.
Live microscopy was performed in phenol-free dDMEM in a 4-well chambered cover glass (LabTek). The temperature was kept at 37°C during all experiments by using a stage heater (Carl Zeiss). Optical slices (0.5 μm) were collected with a laser scanning microscope (LSM 510; Carl Zeiss) by using an argon laser for BODIPY and a 543-nm laser for mCherry. All images were captured using a Plan Apo 63× oil immersion objective, and LSM 510 software was used for image acquisition. Image processing and data analysis were done with ImageJ, and when required, results were plotted using GraphPad Prism. For photobleaching, a region of interest (ROI) corresponding to the full vacuole was selected. Bleaching of the ROI was performed with 5 iterations of the 488-nm argon laser at 100% power. After photobleaching, images were taken at 10-s intervals for the first minute, followed by capture every minute using the argon laser at 2% power unless otherwise indicated. Three different regions were considered: the full vacuole (ROI), a region outside cells for fluorescence background correction (BG), and the total field of view (total) for bleaching correction during monitoring. Microsoft Excel was used to normalize the relative fluorescence intensity (I) for each experiment by using the following equation: %I = {[(ROIt − BGt) × (total0 − BG0)] × 100}/[(totalt − BGt) × (ROI0 − BG0)], where t indicates the time point examined and 0 represents the intensity before bleaching. The means ± standard deviations for 12 different experiments were plotted.
RESULTS AND DISCUSSION
Host cells are major lipid contributors to the intravacuolar network.
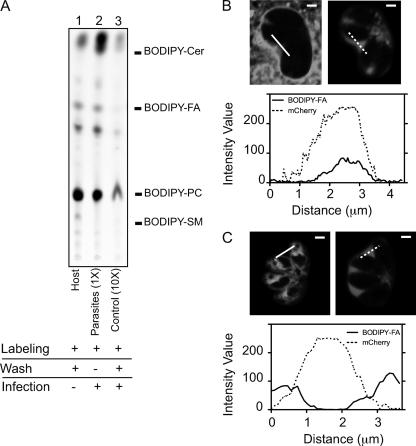
To assess the contribution of host lipids to the IVN, we prelabeled host membranes, allowed infection with unlabeled parasites, and then analyzed the cultures by fluorescence confocal microscopy. For the label, we used a fluorescent C4-BODIPY-C9-labeled fatty acid that, due to the lack of ionic charge of the BODIPY fluorophore, resembles endogenous fatty acids and has a reduced spontaneous transfer between membranes (1). We also confirmed previous reports (8) that the labeled fatty acid is converted mainly into phosphatidylcholine (PC) (Fig. 1 A, lane 1), one of the major lipid components of host endomembranes, including those of the ER and mitochondria (19). As a marker for the space within the PV, we used parasites that express mCherry N-terminally fused to the GRA1 signal peptide and expressed from the GRA1 promoter. In this strain, mCherry localizes to dense granules and is secreted into the PV, occupying the space between the thin tubules of the IVN. The results showed that under these conditions, the lipid probe is visualized readily not only at the PVM but also within the vacuolar space, as indicated by colocalization with the vacuolar mCherry (Fig. 1B). The extent of this colocalization was evident when we plotted the intensity of fluorescence across the vacuolar space, as the profiles corresponding to the fluorescence of the lipid probe and mCherry show coincident peaks.
Fig. 1.
Selective prelabeling of host or parasite membranes indicates that the host is the major lipid contributor to IVN development. (A) HFFs grown in 15-cm dishes were incubated with C4-BODIPY-C9-labeled fatty acid alone (lane 1; host), prelabeled with C4-BODIPY-C9-labeled fatty acid and infected with Toxoplasma for 18 h in the presence of the fluorescent probe [lane 2; parasites (1×)], or prelabeled with C4-BODIPY-C9-labeled fatty acid, washed, and infected with Toxoplasma for 18 h in medium without fluorescent probe [lane 3; control (10×)]. Lipids were extracted from host cells (lane 1) or purified parasites (lanes 2 and 3) and separated on silica plates by TLC using chloroform-methanol-water-triethylamine (35:30:7:35) as the solvent. For the control (10×), 10-fold more sample was spotted on the silica plate than for the parasite (1×). Fluorescence standards used were as follows: BODIPY-Cer, BODIPY-ceramide; BODIPY-FA, BODIPY fatty acid; BODIPY-PC, BODIPY-phosphatidylcholine; and BODIPY-SM, BODIPY-sphingomyelin. (B) HFFs grown on chambered cover glass were prelabeled with C4-BODIPY-C9-labeled fatty acid, washed, and infected with Toxoplasma secreting mCherry for 18 h. Optical sections (0.5 μm) of infected live cells were acquired by confocal microscopy. One section is shown. The left image represents the BODIPY label, and the right image shows secreted mCherry as a marker for the PV space. Intensity profiles are shown along the solid line for the BODIPY label and along the dashed line for mCherry, from left to right. Bars, 2 μm. (C) Same as panel B, except that parasites prelabeled with C4-BODIPY-C9-labeled fatty acid were used to infect unlabeled host cells.
To exclude the possibility that the lipid in these experiments was derived from parasites that took up the label, metabolized it into a higher-order lipid, and then exported this lipid into the PV, we purified parasites from the cultures shown in Fig. 1B and analyzed the extent of lipid incorporation. Consistent with the fact that the parasites show no visible label in the images (Fig. 1B) and were not detectably labeled upon imaging of isolated parasites (data not shown), there was only a marginal lipid label present in a 10-fold excess of parasite material (Fig. 1A, lane 3). Hence, the labeled lipid material in the PV space seen in Fig. 1B appears to derive largely, if not exclusively, from the host cell.
Parasites provide little, if any, lipid to the intravacuolar network.
A major contribution of host cell lipid does not preclude a similar contribution from parasites. To assess this possibility, intracellular parasites were labeled, lysed by use of a syringe, and purified through a size-exclusion column. Figure 1A, lane 2, shows efficient incorporation of the label into parasites, as previously reported (2). Surprisingly, given previous suppositions based on electron micrographs (15), when these parasites were used to infect unlabeled HFF monolayers, there was no significant fluorescence in the area corresponding to the IVN, as indicated by the lack of colocalization with secreted mCherry (Fig. 1C). Hence, under the experimental conditions used here, it is the host cell and not the parasite that appears to contribute the vast majority of lipid within the developing IVN.
Continuous flow of lipids from host cell membranes to the Toxoplasma intravacuolar network.
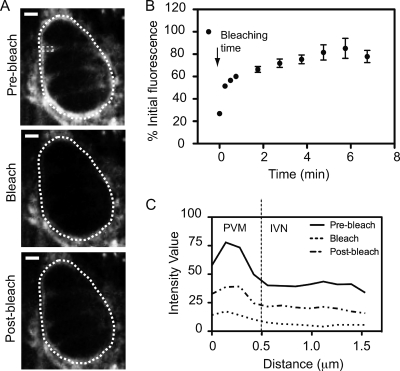
The finding that the host cell is the major source of lipids within the IVN indicates an important interaction in which the host provides the membrane and the parasite, perhaps through the activity of dense granule proteins, shapes the membrane into an intricate tubular network. To determine if there is a continuous supply of lipids from host membranes to the parasite IVN, host cells were prelabeled and then infected with unlabeled parasites. FRAP microscopy was used to assess the recovery of BODIPY fluorescence at the Toxoplasma PV and IVN after bleaching of the full vacuole. Figure 2 A shows images taken before bleaching (prebleach), immediately after bleaching (bleach), and at a subsequent time point during recovery (postbleach) for a given PV. The results (Fig. 2A and B) show a marked recovery of the fluorescence within the entire vacuole. Since the PV is decorated with host ER and mitochondria that were also labeled with this fluorescent probe, it is possible that the main contribution to the PVM fluorescence recovery was from host organelles in close apposition to the vacuole that may also have been bleached during the experiment. To determine whether there is a continuous incorporation of host lipids into the IVN, the fluorescence intensity values corresponding to a rectangular area across the PVM and IVN were plotted. Consistent with the image shown in Fig. 2A, Fig. 2C shows that fluorescence recovery after photobleaching also occurred within the space between the parasites, far from the PVM and PV perimeter. Since the IVN is the only known membranous material within the PV space, this labeling presumably represents the tubules of the IVN network.
Fig. 2.
Continuous flow of host lipids to Toxoplasma PVM and IVN. (A) Typical FRAP experiment with prelabeled HFFs infected with unlabeled parasites. A 1-μm optical slice was taken before bleaching (prebleach), right after the photobleach (bleach), and 2 min into recovery (postbleach), using an argon laser at 5% power. The white dotted line shows the perimeter of the area that was photobleached; the small dotted rectangle shows the portion of the IVN that was also analyzed in panel C. (B) Same as panel A, except that the argon laser was used at 2% power and the fluorescence intensity of the PV was normalized to prebleach values for prelabeled host cells infected with unlabeled parasites for 18 h and plotted over time. Error bars indicate standard deviations (n = 12 vacuoles). (C) The recovery of fluorescence within the PV and IVN after photobleaching of the full vacuole is shown by plotting of the intensity profile corresponding to the rectangular area indicated in panel A before, during, and 2 min after photobleaching. The dotted line at 0.5 μm represents the approximate boundary between the PVM at the periphery of the PV and the IVN occupying the space between the parasites.
Different mechanisms might be responsible for the diffusion or transport of host lipids to the PVM and IVN. We envision a model in which the parasite drives the host cell to consider the PV, which is initially derived from the host cell plasma membrane, a host organelle. Thus, Toxoplasma may somehow coopt host vesicular and/or nonvesicular mechanisms for lipid transport between intracellular membranes to supply the required lipids for PVM growth and IVN remodeling. One clue to the mechanism involved comes from the observation that host ER proteins can be detected within the Toxoplasma vacuolar space (7, 11), suggesting a potential fusion or continuity between the lumens and, perhaps, membranes of the host ER and the PV. This continuity provides an attractive model for the continuous supply of host lipids to at least the PVM seen here.
How these lipids might move from the PVM to the IVN is not clear. The detailed structure of the IVN is not yet known, but rare tubules have been observed in apparent contiguity with the PVM (15). Hence, a lipid that flows to the PVM might be able to diffuse laterally into at least some portion of the IVN. While there was almost full recovery of IVN fluorescence by FRAP, we cannot rule out the existence of a small immobile fraction, which would indicate that not all of the network is connected to the PVM, i.e., some of it may exist as tubovesicular islands. It has also been postulated that the IVN might be connected with the parasite plasma membrane (10). Our data argue against this possibility, as we saw little, if any, labeling of parasites grown in prelabeled host cells, despite very strong labeling of the IVN. However, parasite lipids were efficiently labeled when the parasites were grown in host cells in the presence of excess labeled fatty acid. Together, these results suggest that the parasites scavenge host fatty acid rather than phosphatidylcholine to feed the growth of their own plasma and internal membranes.
The tubular architecture of the IVN has been shown to be at least partially dependent on GRA2 and GRA6 (12), in the absence of which the tubular organization of the IVN is reduced and instead appears more vesicular. Nevertheless, when host cells prelabeled with C4-BODIPY-C9 were infected with unlabeled wild-type or Δgra2 parasites, the resulting parasitophorous vacuoles were indistinguishable with regard to the intensity of fluorescence at the intravacuolar network (data not shown). This result suggests that deletion of GRA2 does not significantly abrogate the flow of lipids from the host to the parasite vacuolar network. Another dense granule protein (GRA7) has been implicated in the invaginations of the PVM that confine host endolysosomes. These structures, named host organelle-sequestering tubulo-structures (HOST structures), have been suggested to play a role in parasite uptake of host cholesterol (3). Whether these HOST structures are the same as the tubules of the network has not yet been established. To assess a potential role of GRA7 in the recruitment of host fatty acids and/or phospholipids to the parasite IVN, we prelabeled host cells with C4-BODIPY-C9, followed by infection with unlabeled wild-type or Δgra7 parasites. We saw no significant differences in the intensity of fluorescence within the vacuolar network between wild-type and Δgra7 vacuoles (data not shown). This result indicates that deletion of GRA7 does not interrupt the flow of host BODIPY-labeled fatty acids and/or BODIPY-phosphatidylcholine to the parasite IVN. Regardless of the particular proteins involved, our results show that Toxoplasma's IVN is derived largely, if not exclusively, from lipids obtained from the host cell. How the host accommodates and responds to this substantial draw on its resources remains to be determined.
ACKNOWLEDGMENTS
We are grateful to Michael Reese and other members of the Boothroyd lab for reagents and helpful discussions. We also acknowledge Jon Mulholland in the Cell Science Imaging Facility at Stanford University for technical assistance.
This work was supported by the National Institutes of Health (grant AI21423), and Carolina Caffaro was supported in part by a fellowship from the American Heart Association.
Footnotes
Published ahead of print on 17 June 2011.
REFERENCES
- 1. Bai J., Pagano R. E. 1997. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry 36:8840–8848 [DOI] [PubMed] [Google Scholar]
- 2. Charron A. J., Sibley L. D. 2002. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115:3049–3059 [DOI] [PubMed] [Google Scholar]
- 3. Coppens I., et al. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125:261–274 [DOI] [PubMed] [Google Scholar]
- 4. Coppens I., Sinai A. P., Joiner K. A. 2000. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149:167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crawford M. J., et al. 2006. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 25:3214–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donald R. G. K., Carter D., Ullman B., Roos D. S. 1996. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J. Biol. Chem. 271:14010–14019 [DOI] [PubMed] [Google Scholar]
- 7. Goldszmid R. S., et al. 2009. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J. Exp. Med. 206:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasurinen J. 1992. A novel fluorescent fatty acid, 5-methyl-BDY-3- dodecanoic acid, is a potential probe in lipid transport studies by incorporating selectively to lipid classes of BHK cells. Biochem. Biophys. Res. Commun. 187:1594–1601 [DOI] [PubMed] [Google Scholar]
- 9. Labruyere E., Lingnau M., Mercier C., Sibley L. D. 1999. Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii. Mol. Biochem. Parasitol. 102:311–324 [DOI] [PubMed] [Google Scholar]
- 10. Magno R. C., Lemgruber L., Vommaro R. C., De Souza W., Attias M. 2005. Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc. Res. Tech. 67:45–52 [DOI] [PubMed] [Google Scholar]
- 11. Melo E. J., de Souza W. 1997. Relationship between the host cell endoplasmic reticulum and the parasitophorous vacuole containing Toxoplasma gondii. Cell Struct. Funct. 22:317–323 [DOI] [PubMed] [Google Scholar]
- 12. Mercier C., et al. 2002. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol. Biol. Cell 13:2397–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mordue D. G., Hakansson S., Niesman I., Sibley L. D. 1999. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 92:87–99 [DOI] [PubMed] [Google Scholar]
- 14. Schwab J. C., Beckers C. J. M., Joiner K. A. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U. S. A. 91:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sibley L., Niesman I., Parmley S., Cesbron-Delauw M. F. 1995. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J. Cell Sci. 108:1669–1677 [DOI] [PubMed] [Google Scholar]
- 16. Sinai A. P., Webster P., Joiner K. A. 1997. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 110:2117–2128 [DOI] [PubMed] [Google Scholar]
- 17. Soldati D., Boothroyd J. C. 1993. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science 260:349–352 [DOI] [PubMed] [Google Scholar]
- 18. Suss-Toby E., Zimmerberg J., Ward G. E. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. U. S. A. 93:8413–8418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thumser A. E., Storch J. 2007. Characterization of a BODIPY-labeled fluorescent fatty acid analogue. Binding to fatty acid-binding proteins, intracellular localization, and metabolism. Mol. Cell. Biochem. 299:67–73 [DOI] [PubMed] [Google Scholar]