Abstract
While several distinct virulence factors of Helicobacter pylori have been shown to be associated with different clinical outcomes, there is still much to learn about the role of different bacterial factors in gastric carcinogenesis. This study looked at the distribution of the cagA, homA, and homB genes in strains isolated from patients suffering from gastroduodenal diseases in Iran and assessed if there was any association between disease state and the presence of the aforementioned virulence factors. Genomic DNA from 138 H. pylori strains was isolated and genotyped via PCR. Strains were obtained from dyspeptic patients (35 from gastritis patients, 62 from peptic ulcer patients, and 41 from gastric cancer patients) at the Teaching Touba Clinic and Imam Hospital of the Mazandaran University of Medical Sciences in Sari, Iran. The overall prevalence rates of cagA, homA, and homB were 58%, 54%, and 43%, respectively. Stratification of patients showed a significant difference in the prevalence of H. pylori virulence genes across the disease states. The frequency of homB was statistically significantly higher in gastric cancer patients (78%) than in patients suffering from peptic ulcers (20%) or gastritis (43%) (P < 0.0001). The presence of homB was also associated with the presence of cagA (r = 0.243). These data suggest that in this population the presence of homB may be a predictor of more virulent strains of H. pylori and influence the severity of disease manifestation.
INTRODUCTION
Helicobacter pylori is a Gram-negative, spiral-shaped, microaerophilic bacterium that infects more than 50% of the world's population (2, 30, 31). Colonization and long-term persistence of H. pylori can induce a complex immune response that can potentiate severe mucosal damage, including atrophy, intestinal metaplasia, and dysplasia, thereby making H. pylori the etiologic agent of acute and chronic gastritis, peptic ulcer disease (75% of gastric ulcers and 90% of duodenal ulcers), and two forms of gastric cancer (mucosa-associated lymphoid tissue lymphoma and gastric adenocarcinoma) (8, 15, 41, 42, 51). The association with the development of two forms of cancer led to the classification of H. pylori by the World Health Organization as the only bacterial class I carcinogen (24).
H. pylori's association with cancer, combined with the fact that gastric cancer is the second most common cause of cancer-associated death (33), has led to numerous studies designed to elucidate the bacterial factors responsible for progression to this disease. However, understanding why some individuals develop severe disease and others develop only mild disease has not been a straightforward task. This is due to the complex nature of interactions between the bacterium and the host. Important variables include variation in genetic content across bacterial strains and the role of host factors, such as host genetics, dietary intake, and other environmental factors in the disease process. Among the bacterial factors that have been implicated to impact disease development are a number of virulence factors that show heterogeneity across strains. Included among these are the toxins CagA and VacA, the IceA (for induced by contact with epithelium) protein, the proinflammatory outer membrane protein OipA, and the Helicobacter outer membrane proteins HomA and HomB (4, 11, 21, 37, 38, 43, 46, 53). The virulence factor that has emerged to be one of the major determinants of severe disease manifestations is cagA, and carriage of the gene is associated with peptic ulcers, atrophic gastritis, and adenocarcinoma (9, 17). In fact, H. pylori-infected gastric cancer patients are at least twice as likely to be infected with cagA-positive strains (9, 18). cagA is the last gene on the cag pathogenicity island (PAI), the majority of which encodes a type IV secretion apparatus that is used to directly inject CagA into host cells (10). Once injected, CagA is phosphorylated and forms a complex with SHP-2 (Src homology region 2-containing phosphatase 2 [22]), thereby altering multiple host signaling pathways (20–22, 32, 47, 52).
The Helicobacter outer membrane (Hom) adhesion molecules constitute a small paralogous family of proteins that contain alternating hydrophobic motifs and signal sequences in the C terminus, which are typical of other outer membrane proteins (1). homA and homB are the best-studied members of the Hom family, and H. pylori contains two possible genomic locations that can carry these two adhesion molecules (38). The homA and homB genes are 90% identical, and the variability between these genes is contained in the central domain (1). Adding to the complexity, six distinct different allelic variants within a 300-bp region within this central domain have been identified. Three of these variants are exclusively found in homB-positive strains, while only one of these variants is found exclusively in homA-positive strains (35, 36). There are also geographic differences in the sequences at both the 5′ and 3′ ends of the homB gene, and these differences can be divided among East Asian and Western strains (35). Strains can have a single homA or homB gene, in which the other locus remains empty; two copies of homA (homA and homA) or homB (homB and homB); a single copy of each gene (homA and homB); or neither of these genes (34). Studies have suggested that the number of homB genes affects the number of bacteria adhering to host cells and that the presence of homB is associated with secretion of the proinflammatory cytokine interleukin-8 (IL-8) (34). Studies to address whether the presence of homB is associated with increased disease severity have shown that the presence of homB is associated with peptic ulcer disease but not gastritis (34). More recently, the presence of homB has been suggested to be a discriminative factor between development of gastric cancer and duodenal ulcer (25).
Residents in the northern regions of Iran are at high risk for development of gastric cancer. Specifically, gastric cancer clusters exist within the Mazandaran region (29). Given the complexity of H. pylori virulence factors, the rather poorly understood distribution of these factors in Iran, and the high incidence of gastric cancer in this region, the prevalence of the virulence genes cagA, homA, and homB in patients with gastroduodenal disorders within a population at risk for gastric cancer from the northern section of Iran was assessed (29). In this population, we found that while cagA presence had no significant effect on disease type, there was a strong association between the presence of homB and the progression to gastric cancer. Additionally, there was an association between the presence of the homB and cagA genes. These data suggest that within this population, homB may serve as a better predictor of severe disease development than cagA.
MATERIALS AND METHODS
Study participants.
Biopsy samples were obtained from patients undergoing endoscopic procedures for dyspepsia at the Teaching Touba Clinic and Imam Hospital of Mazandaran University of Medical Sciences, Sari, Iran. Exclusion criteria for the study included previous gastric surgery; H. pylori eradication treatment; and previous use of antibiotics, nonsteroid anti-inflammatory drugs, proton pump inhibitors, or H2-receptor blockers in the previous 30 days. Additionally, patients who had received antisecretory drugs, bismuth salts, or sucralfate within the 2 weeks prior to the endoscopy procedure were excluded. Three biopsy specimens were obtained from the gastric antrum of each patient and were used for pathological examination, urease test (H. pylori Quick Test; Biohit Diagnostics Helsinki, Finland), and microbial culture, respectively. Patients were included in the study upon a positive urease test, and written informed consent was obtained from all participants. The study protocol was approved by the Medical Research Ethics Committee of Mazandaran University of Medical Sciences. Diagnosis of gastric disorders was based on clinical presentation, endoscopic findings, and histopathologic confirmation (14, 26). A total of 138 H. pylori-positive patients were enrolled in the study.
Isolation of H. pylori isolates.
Biopsy specimens were placed in sterile thioglycolate broth (Merck, Germany) and transferred to the microbiology laboratory for further processing. The specimens were dissected and then plated on Columbia agar (Mast, United Kingdom) plates containing 7% fetal calf serum (Gibco), 10% defibrinated sheep blood (Jihad Daneshgahi, Iran), and H. pylori-selective antibiotic tablets (Mast, United Kingdom). These plates were incubated under microaerobic conditions at 37°C for a maximum of 7 days. H. pylori colonies were confirmed via morphology, Gram stain, and positive oxidase, catalase, and urease activity tests, as well as by successful amplification of the housekeeping gene glmM (13). PCR amplification of the glmM gene for the detection of H. pylori isolates was performed with the glmM forward and reverse primers described by Lu et al. (28).
homA, homB, and cagA genotyping.
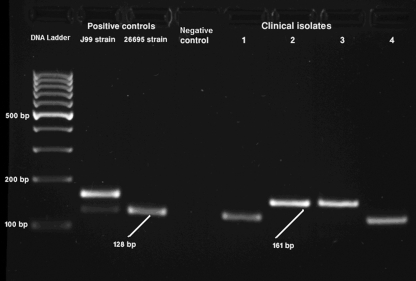
Genomic DNA was extracted from a single colony per patient using an AccuPrep genomic DNA extraction kit (Bioneer, South Korea) according to the manufacturer's directions. All primers used in this study are listed in Table 1. The presence of cagA was indicated by a 298-bp amplicon when the gene was amplified with primers D008 and R008 using the conditions described previously (19) (Fig. 1). Identification of the presence of the homA and/or homB gene was accomplished through a single PCR using the F1-jhp0870/jhp0649 and R1-jhp0870/jhp0649 primers, which yielded a 128-bp product denoting the presence of the homA gene and a 161-bp product denoting the presence of the homB gene using conditions described previously (38) (Fig. 1). These primers amplify an internal region of both genes that lies approximately 505 to 530 bp from the start of the genes. This region contains variations between the homA gene and the homB gene, but the conserved nature of the primers allows amplification of all homA and homB variants currently identified. Reference strain 26695 was used as a positive control for homA, and strain J99 was used as positive control for homA and homB (38).
Table 1.
Primer sequences
| Primer name | Sequence | Gene(s) amplified | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| glmM forward | 5′-AAGCTTTTAGGGGTGTTAGGGGTTT-3′ | glmM | 294 | 28 |
| glmM reverse | 5′-AAGCTTACTTTCTAACACTAACGC-3′ | |||
| F1-jph0870/jhp0649 | 5′-AGAGGGTFTTTFAAACGCTCAATA-3′ | homA | 128 | 38 |
| R1-jhp0870/jhp0649 | 5′-GGTGAATTCTTCTGCGGTTTG-3′ | homB | 161 | |
| D008 | 5′-ATAATCGTAAATTAGACAACTTGAGCGA-3′ | cagA | 298 | 19 |
| R008 | 5′-TTAGAATAATCAACAAACATCACGCCAT-3′ |
Fig. 1.
Genotyping of homA and homB in H. pylori isolates. The image is from a representative gel electrophoresis of PCR amplification products of homA (128 bp) and homB (161 bp) genes from clinical isolates and J99 and 26695 as positive-control strains for both homA and homB and only homA, respectively. The negative-control lane represents a PCR performed with no template DNA.
Data analysis.
Isolates were assessed for the presence of the cagA, homA, and homB genes, according to disease state, age, and gender. Comparisons of continuous and discontinuous variables were performed with the Student t test, analysis of variance, the Fisher exact test, or the chi-square test. Logistic regression analysis was used for multivariate analysis, and log linear modeling was used to assess any higher-order associations, fitting a saturated model using categorical variables representing cagA, homA, homB, disease state, gender, and age. Higher-order associations were identified using a backward-selection algorithm with statistical significance assessed at the 5% level. Data were analyzed using SPSS (version 16) software (SPSS Inc., Chicago, IL).
RESULTS
Sample acquisition and virulence gene genotyping.
A complete list of isolates and epidemiological data (age and gender), disease state, and virulence factor status is available in Table S1 in the supplemental material. H. pylori was successfully cultured from 138 patients suffering from gastroduodenal disorders from northern Iran. The presence of H. pylori was verified via morphology, Gram stain, and positive oxidase, catalase, and urease activity tests and further confirmed by successful amplification of the housekeeping gene glmM. The mean age of the overall population was 42.9 ± 13.5 years. The population was fairly evenly distributed across gender, with 63 (45.7%) being female and 75 (54.3%) being male. Moreover, the H. pylori-positive samples were distributed across the different disease states: 35 (25.4%) from gastritis patients, 62 (44.9%) from peptic ulcer disease patients, and 41 (29.7%) from gastric cancer patients. The complete breakdown of the virulence gene status and epidemiological factors across the different disease states is shown in Table 2. There were no significant differences in mean age (P = 0.151) or gender (P = 0.500) across the disease spectrum.
Table 2.
Breakdown of epidemiological and virulence factors of a north Iranian population
| Characteristic | No. of patients with the following disease statea: |
|||
|---|---|---|---|---|
| Total | Gastritis | Peptic ulcers | Gastric cancer | |
| Overall population | ||||
| Total | 138 | 35 | 62 | 41 |
| Female | 63 | 15 | 26 | 22 |
| Male | 75 | 20 | 36 | 19 |
| cagA status | ||||
| Positive | 80 | 18 | 34 | 28 |
| Female | 39 | 7 | 16 | 16 |
| Male | 41 | 11 | 18 | 12 |
| Negative | 58 | 17 | 28 | 13 |
| Female | 24 | 8 | 10 | 6 |
| Male | 34 | 9 | 18 | 9 |
| homA status | ||||
| Positive | 75 | 20 | 49 | 6 |
| Female | 35 | 8 | 22 | 5 |
| Male | 40 | 12 | 27 | 1 |
| Negative | 63 | 15 | 13 | 35 |
| Female | 28 | 7 | 4 | 17 |
| Male | 35 | 8 | 9 | 18 |
| homB status | ||||
| Positive | 60 | 15 | 13 | 32 |
| Female | 26 | 7 | 4 | 15 |
| Male | 34 | 8 | 9 | 17 |
| Negative | 78 | 20 | 49 | 9 |
| Female | 37 | 8 | 22 | 7 |
| Male | 41 | 12 | 27 | 2 |
The mean ages ± 1 standard deviation are 42.9 ± 13.5, 43.6 ± 14.7, 44.7 ± 12.9, and 39.5 ± 13.2 years for all patients and patients with gastritis, peptic ulcers, and gastric cancer, respectively.
Virulence factors. (i) Cytotoxin-associated gene A, cagA.
The presence of cagA has been linked to the development of more severe disease states (9, 27, 40) and therefore is an important virulence factor to assess within this population. A majority of the strains (80 [58%]) were found to be cagA positive, while 58 (42%) were cagA negative. Of the 80 cagA-positive strains, 39 (48.75%) were from female patients and 41 (51.25%) were from male patients. Eighteen isolates were from gastritis patients, 34 were from peptic ulcer disease patients, and 28 were from patients presenting with gastric cancer. Of the 58 cagA-negative strains, 24 (41.4%) were from female patients and 34 (58.6%) were from male patients. Seventeen of these isolates were from gastritis patients, 28 isolates were from peptic ulcer disease patients, and 13 isolates were from gastric cancer patients (Table 2).
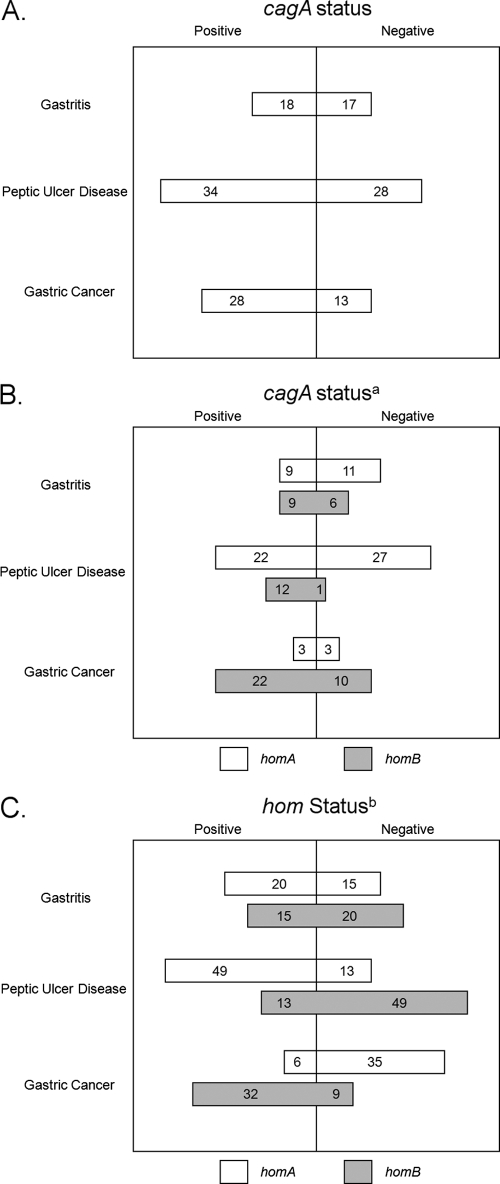
The presence or absence of the cagA gene was not statistically significantly linked to gender (P = 0.4888). Even though the cagA-positive strains were more prevalent in patients suffering from gastric cancer (68.3%; Table 2 and Fig. 2A), cagA status did not have a statistically significant effect on disease state in this population (P = 0.2654). In fact, cagA status did not statistically impact any breakdown of disease states: cancer versus gastritis and peptic ulcer disease (P = 0.3291), cancer versus peptic ulcer disease (P = 0.2185), cancer versus gastritis (P = 0.1618), peptic ulcer disease versus gastritis and gastric cancer (P = 0.6033), peptic ulcer disease versus gastritis (P = 0.8331), or gastritis versus gastric cancer and peptic ulcer disease (P = 0.4293) (Table 3). However, the presence of the cagA gene was significantly linked to the distribution of both homA (P = 0.0017) and homB (P = 0.0054) (Fig. 2B).
Fig. 2.
Schematic depiction of the distribution of the different virulence factors stratified by disease state within the Iranian population of this study. (A) The distribution of the cagA gene stratified by disease state is depicted. (B) The distribution of cagA, homA, and homB stratified by disease state is depicted. Only homA- or homB-positive strains are depicted among the cagA-positive or -negative isolates. a, positive correlation between the presence of the cagA gene and the homB gene (r = 0.243) and an inverse correlation between the presence of the cagA gene and the homA gene (r = −0.279). (C) The distribution of the different hom genes stratified by disease state is depicted. b, a significant association exists between the distribution of the homA gene and disease state (P < 0.0001) and the distribution of the homB gene and disease state (P < 0.0001), and the progression to gastric cancer is influenced by the presence of the homB gene.
Table 3.
Distribution of virulence factors across the different disease states
| Gene and groups compared | P valuea |
|---|---|
| Distribution of cagA | |
| Gastric cancer vs peptic ulcer disease and gastritis | 0.3291 |
| Gastric cancer vs peptic ulcer disease | 0.2185 |
| Gastric cancer vs gastritis | 0.1618 |
| Peptic ulcer disease vs gastric cancer and gastritis | 0.6033 |
| Peptic ulcer disease vs gastritis | 0.8331 |
| Gastritis vs gastric cancer and peptic ulcer disease | 0.4293 |
| Distribution of homA | |
| Gastric cancer vs peptic ulcer disease and gastritis | <0.0001 |
| Gastric cancer vs peptic ulcer disease | <0.0001 |
| Gastric cancer vs gastritis | 0.0001 |
| Peptic ulcer disease vs gastric cancer and gastritis | <0.0001 |
| Peptic ulcer disease vs gastritis | 0.0348 |
| Gastritis vs gastric cancer and peptic ulcer disease | 0.8445 |
| Distribution of homB | |
| Gastric cancer vs peptic ulcer disease and gastritis | <0.0001 |
| Gastric cancer vs peptic ulcer disease | <0.0001 |
| Gastric cancer vs gastritis | 0.0022 |
| Peptic ulcer disease vs gastric cancer and gastritis | <0.0001 |
| Peptic ulcer disease vs gastritis | 0.0348 |
| Gastritis vs gastric cancer and peptic ulcer disease | 1 |
Shading indicates P values that are statistically significant, as defined in the Materials and Methods.
(ii) Helicobacter outer membrane family members homA and homB.
In a population where the presence of cagA is not a good indicator of progression to more severe disease manifestations, other bacterial factors must play a more important role in disease progression. Recently, the presence of different outer membrane proteins has been linked to more severe disease manifestations (25, 34, 38). In fact, the presence of the Helicobacter outer membrane protein B (homB) has been linked to progression to gastric cancer (25). In order to assess if this correlation existed within this population, the presence of either homA or homB was assessed. The presence of homA was identified by the presence of a 128-bp amplicon, whereas the presence of homB was indicated by the presence of a 161-bp amplicon, as previously described (38) (Fig. 1). Either homA or homB was present in 135 (97.8%) of all strains; 3 gastric cancer strains were negative for both the homA and homB genes. However, all three homA- and homB-negative strains carried cagA. Seventy five isolates (54.3%) were homA positive and 60 isolates (43.5%) were positive for homB. Of note, with the exception of the three homA- and homB-negative strains, strains carried just one or the other hom gene, and no strains carried both homA and homB.
(a) homA status.
Of the 75 homA-positive strains, 35 (46.7%) were from female patients and 40 (53.3%) were from male patients. Twenty isolates were from gastritis patients, 49 isolates were from peptic ulcer disease patients, and 6 were from patients diagnosed with gastric cancer. Of the 63 homA-negative strains, 28 (44.4%) were isolated from females and 35 (55.6%) were isolated from males. Fifteen isolates were from gastritis patients, 13 isolates were from peptic ulcer disease patients, and 35 were from gastric cancer patients (Table 2 and Fig. 2C).
The presence or absence of the homA gene was not statistically significantly linked to gender (P = 0.8643). However, the distribution of homA had a statistically significant effect on disease state (P < 0.0001; Fig. 2): cancer versus gastritis and peptic ulcer disease (P < 0.0001), cancer versus peptic ulcer disease (P < 0.0001), cancer versus gastritis (P = 0.0001), peptic ulcer disease versus gastritis and gastric cancer (P < 0.0001), and peptic ulcer disease versus gastritis (P = 0.0348). However, the presence of homA did not statistically significantly impact gastritis versus peptic ulcer disease and gastric cancer (P = 0.8445). The presence of the homA gene was significantly linked to the distribution of both cagA (P = 0.0017) and homB (P < 0.0001). The association with homB is due to the fact that, with the exception of the three homA-negative and homB-negative strains, strains that carry homA do not carry homB and vice versa.
(b) homB status.
Of the 60 homB-positive strains, 26 (43.3%) were from female patients and 34 (56.7%) were from male patients. Fifteen isolates were from gastritis patients, 13 isolates were from peptic ulcer disease patients, and 32 were from gastric cancer patients. Of the 78 homB-negative strains, 37 (47.4%) were from female patients and 41 (52.6%) were from male patients. Twenty isolates were from gastritis patients, 49 isolates were from peptic ulcer disease patients, and 9 were from patients diagnosed with gastric cancer (Table 2).
The presence or absence of the homB gene was not statistically significantly linked to gender (P = 0.7306). However, the status of homB had a statistically significant effect on disease state (P < 0.0001). In fact the presence or absence of homB status statistically significantly impacted most of the disease states (Fig. 2C and Table 3): cancer versus gastritis and peptic ulcer disease (P < 0.0001), cancer versus peptic ulcer disease (P < 0.0001), cancer versus gastritis (P = 0.0022), peptic ulcer disease versus gastritis and gastric cancer (P < 0.0001), and peptic ulcer disease versus gastritis (P = 0.0348). However, the presence of homB did not statistically significantly impact gastritis versus peptic ulcer disease and gastric cancer (P = 1.0000). Indeed, the majority of isolates from gastric cancer patients (78%) carried homB. The presence of the homB gene was significantly linked to the distribution of both cagA (P = 0.0054) and homA (P < 0.0001). Again, the association with homA is due to the fact that, with the exception of the three homA- and homB-negative strains, strains that carry homA do not carry homB and vice versa.
cagA, homA, and homB genotypes.
Next, we wanted to examine the association between cagA and either of the hom genes. Through statistical analysis, a significant inverse correlation between the presence of the cagA and homA genes was identified (r = −0.279, P = 0.001). This means that homA positivity was more frequently found in strains lacking the cagA gene (Table 3). Moreover, a statistically significant positive correlation was observed between cagA positivity and homB status (r = 0.243, P = 0.004). This positive correlation indicates that there is an association between being positive for homB and being positive for cagA. Indeed, the majority of homB-positive strains were cagA positive (71.7%), whereas among homB-negative strains, the cagA status was evenly distributed: 47.4% were cagA positive, and 52.6% were cagA negative.
Since both the homA and homB genes showed a direct association with disease and cagA, we wanted to know if there was any association between homA, cagA, and disease state or homB, cagA, and disease state. However, neither of these associations reached statistical significance (P = 0.178 and P = 0.073, respectively). Next we wanted to know if either homA or homB was a predictor of gastric cancer independent of cagA. Logistic regression analysis revealed that the homB-positive genotype was a predictor of gastric cancer independent of cagA (odds ratio = 8.453, P < 0.001). Unfortunately, due to the almost inverse relationship of homA and homB status, we were unable to assess the association between these two genes.
DISCUSSION
Gastric cancer is the fourth most common malignancy and is responsible for over 700,000 deaths per year (16), and infection with H. pylori has been attributed to over 63% of all cases of gastric cancer worldwide (39). Additionally, H. pylori is responsible for 75% of all gastric ulcers and 90% of all duodenal ulcers (8), making H. pylori a medically important bacterium. Given only this evidence, eradication of this gastric intruder seems to be the most logical course of action. However, recent epidemiological studies have identified protective correlates between H. pylori colonization and different diseases, specifically, active tuberculosis, which is a worldwide health concern (44); asthma (45), which is on the rise in developed countries; and esophageal cancer, which is also on the rise (7). Thus, scientists are attempting to determine what makes some strains more virulent than other strains. If bacterial factors that are responsible for increased virulence are identified, then perhaps highly virulent strains can be eradicated without losing the protective effects generated through colonization with less virulent strains.
The bacterial factor that has emerged to be the major bacterial determinant for progression to more severe disease is cagA (9, 27, 40). In fact, cagA-positive H. pylori strains have been associated with the severe mucosal inflammation that underlies peptic ulcer, atrophic gastritis, and gastric carcinoma (9, 27). Even though the rate of gastric cancer is high in Iran, the prevalence of infection with cagA-positive H. pylori strains varies from 68% (48) to 76% (23). In our study, only 58% of isolates were cagA positive, and the distribution of the cagA gene was not statistically significantly linked to disease. While they are surprising, our results are congruent with those of another study that showed that cagA presence was not significantly associated with peptic ulcer disease in Iran (23). Indeed, Iran seems to represent a gastric cancer enigma in terms of H. pylori virulence factors; several studies have shown that the presence of cagA does not influence progression to peptic ulcers or gastric cancer (6, 12, 23, 29, 49, 50). However, we do note that though not significant in our population, the majority (68.3%) of isolates from gastric cancer patients did carry the cagA gene and the three gastric cancer isolates that were homA and homB negative also carried the cagA gene. These findings suggest that in this population, cagA alone is not an adequate predictor of disease and suggest that other virulence genes are important. Indeed, this finding presents the opportunity to assess the impact of other virulence factors on severe disease progression.
Outer membrane proteins are very important during infection and can influence the levels of bacterial colonization. Additionally, an increased number of H. pylori cells adherent to the gastric epithelium could induce greater changes in host cell signaling pathways. H. pylori carries two paralogous outer membrane proteins, HomA and HomB, which have recently been suggested to be important determinants of disease severity (25). While the homA and homB genes are 90% identical (1), the differences between homA and homB translate to different impacts on disease manifestations; homB presence is associated with gastric cancer (25).
In this population, the presence of homB was associated with progression to gastric cancer; homB was present in 78% of all isolates from gastric cancer patients (Table 2). In fact, logistic regression revealed that homB was a predictor of gastric cancer independent of cagA. However, we did note a positive correlation between the presence of cagA and homB (Table 2). This suggests that cagA and homB may interact in some fashion. Of note, homB has been shown to be prevalent in the majority of East Asian strains, while the presence of homA within these strains is rare (37): East Asian strains typically carry the most virulent form of CagA (5, 21). Despite these correlations, a three-way association between homB, cagA, and disease state was not identified within this population. If homB was influencing only adherence of the bacteria to the gastric epithelium, we would expect that gastric cancer development would still be linked to cagA, that there would be a three-way interaction, or that homB is not an independent contributor to the progression to gastric cancer. However, data from this population suggest that the presence of homB does more than just influence bacterial adherence. Interestingly, it has been demonstrated that the presence of homB increases secretion of the proinflammatory cytokine IL-8 (34), and increased inflammation is indicative of more severe disease presentations, such as gastric ulcers and gastric cancer (3). Thus, perhaps HomB promotes more severe inflammation, which in turn affects gastric cancer progression. Since the identification of allelic variants within the central domain of homB is recent (35), the impact of these alleles within both Western and East Asian populations still needs to be elucidated. For instance, it would be interesting to determine if different homB variants/mutants with point mutations are more prevalent among gastric cancer strains than noncancer strains. If this was the case, then isogenic strains that vary only in the homB variant carried could be created and then progression to gastric cancer could be monitored in an animal model. Clearly, the exact mechanistic role of the homB gene product in gastric pathology, particularly corpus inflammation, mucosal atrophy, and metaplasia, still needs to be defined.
While it is clear that H. pylori-induced disease pathology is influenced by multiple host, dietary, environmental, and bacterial factors, it is clear from this study that the presence of homB correlates with severe disease progression. We have shown that there is a statistically significant association between development of gastric cancer and the presence of homB. Currently, the reason for this correlation is unclear, and further studies are required to elucidate the molecular role that homB plays in cancer development. This study also demonstrates the importance of H. pylori virulence factor polymorphism in disease progression, since there is a functional discrepancy between two highly similar genes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jeannette M. Whitmire for critical reading of the manuscript and Cara H. Olsen for assistance with the statistical analysis. We thank the reviewers for their helpful comments on our manuscript.
This work was supported by a grant of the Molecular and Cell Biology Research Center (MCBRC), Mazandaran University of Medical Sciences (grant 88-45).
The contents of the manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or the DOD. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 6 July 2011.
REFERENCES
- 1. Alm R. A., et al. 2000. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect. Immun. 68: 4155–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous. 1993. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. The EUROGAST Study Group Gut 34: 1672–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atherton J. C. 2006. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 1: 63–96 [DOI] [PubMed] [Google Scholar]
- 4. Atherton J. C., et al. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270: 17771–17777 [DOI] [PubMed] [Google Scholar]
- 5. Azuma T., et al. 2004. Association between diversity in the Src homology 2 domain-containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J. Infect. Dis. 189: 820–827 [DOI] [PubMed] [Google Scholar]
- 6. Baghaei K., et al. 2009. Determination of Helicobacter pylori virulence by analysis of the cag pathogenicity island isolated from Iranian patients. Dig. Liver Dis. 41: 634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blaser M. J. 2008. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev. Res. (Phila.) 1: 308–311 [DOI] [PubMed] [Google Scholar]
- 8. Blaser M. J. 1998. Helicobacter pylori and gastric diseases. BMJ 316: 1507–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaser M. J., et al. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55: 2111–2115 [PubMed] [Google Scholar]
- 10. Censini S., et al. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. U. S. A. 93: 14648–14653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Covacci A., et al. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. U. S. A. 90: 5791–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dabiri H., et al. 2010. Analysis of Helicobacter pylori genotypes in Afghani and Iranian isolates. Pol. J. Microbiol. 59: 61–66 [PMC free article] [PubMed] [Google Scholar]
- 13. De Reuse H., Labigne A., Mengin-Lecreulx D. 1997. The Helicobacter pylori ureC gene codes for a phosphoglucosamine mutase. J. Bacteriol. 179: 3488–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dixon M. F., Genta R. M., Yardley J. H., Correa P. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20: 1161–1181 [DOI] [PubMed] [Google Scholar]
- 15. Ernst P. B., Gold B. D. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54: 615–640 [DOI] [PubMed] [Google Scholar]
- 16. Ferlay J., et al. 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127: 2893–2917 [DOI] [PubMed] [Google Scholar]
- 17. Figura N., et al. 1989. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J. Clin. Microbiol. 27: 225–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gwack J., et al. 2006. CagA-producing Helicobacter pylori and increased risk of gastric cancer: a nested case-control study in Korea. Br. J. Cancer 95: 639–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamlet A., Thoreson A. C., Nilsson O., Svennerholm A. M., Olbe L. 1999. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology 116: 259–268 [DOI] [PubMed] [Google Scholar]
- 20. Higashi H., et al. 2004. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J. Biol. Chem. 279: 17205–17216 [DOI] [PubMed] [Google Scholar]
- 21. Higashi H., et al. 2002. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. U. S. A. 99: 14428–14433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higashi H., et al. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295: 683–686 [DOI] [PubMed] [Google Scholar]
- 23. Hussein N. R., et al. 2008. Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H. pylori-associated disease. J. Clin. Microbiol. 46: 1774–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. International Agency for Research on Cancer 1994. Infection with Helicobacter pylori, p. 177–240In Monographs on the evaluation of carcinogenic risks to humans, vol. 61 International Agency for Research on Cancer, Lyon, France: [PMC free article] [PubMed] [Google Scholar]
- 25. Jung S. W., Sugimoto M., Graham D. Y., Yamaoka Y. 2009. homB status of Helicobacter pylori as a novel marker to distinguish gastric cancer from duodenal ulcer. J. Clin. Microbiol. 47: 3241–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kajitani T. 1981. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn. J. Surg. 11: 127–139 [DOI] [PubMed] [Google Scholar]
- 27. Kuipers E. J., Perez-Perez G. I., Meuwissen S. G., Blaser M. J. 1995. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J. Natl. Cancer Inst. 87: 1777–1780 [DOI] [PubMed] [Google Scholar]
- 28. Lu J. J., et al. 1999. Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastric tissues. J. Clin. Microbiol. 37: 772–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malekzadeh R., Derakhshan M. H., Malekzadeh Z. 2009. Gastric cancer in Iran: epidemiology and risk factors. Arch. Iran Med. 12: 576–583 [PubMed] [Google Scholar]
- 30. Marshall B. J., Warren J. R. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i: 1311–1315 [DOI] [PubMed] [Google Scholar]
- 31. Matysiak-Budnik T., Megraud F. 1997. Epidemiology of Helicobacter pylori infection with special reference to professional risk. J. Physiol. Pharmacol. 48(Suppl. 4): 3–17 [PubMed] [Google Scholar]
- 32. Neel B. G., Gu H., Pao L. 2003. The ′Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28: 284–293 [DOI] [PubMed] [Google Scholar]
- 33. Neugut A. I., Hayek M., Howe G. 1996. Epidemiology of gastric cancer. Semin. Oncol. 23: 281–291 [PubMed] [Google Scholar]
- 34. Oleastro M., et al. 2008. Evaluation of the clinical significance of homB, a novel candidate marker of Helicobacter pylori strains associated with peptic ulcer disease. J. Infect. Dis. 198: 1379–1387 [DOI] [PubMed] [Google Scholar]
- 35. Oleastro M., Cordeiro R., Menard A., Gomes J. P. 2010. Allelic diversity among Helicobacter pylori outer membrane protein genes homB and homA generated by recombination. J. Bacteriol. 192: 3961–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oleastro M., et al. 2009. Allelic diversity and phylogeny of homB, a novel co-virulence marker of Helicobacter pylori. BMC Microbiol. 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oleastro M., et al. 2009. Disease association with two Helicobacter pylori duplicate outer membrane protein genes, homB and homA. Gut Pathog. 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oleastro M., Monteiro L., Lehours P., Megraud F., Menard A. 2006. Identification of markers for Helicobacter pylori strains isolated from children with peptic ulcer disease by suppressive subtractive hybridization. Infect. Immun. 74: 4064–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parkin D. M. 2006. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118: 3030–3044 [DOI] [PubMed] [Google Scholar]
- 40. Parsonnet J., Friedman G. D., Orentreich N., Vogelman H. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parsonnet J., et al. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325: 1127–1131 [DOI] [PubMed] [Google Scholar]
- 42. Parsonnet J., et al. 1994. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330: 1267–1271 [DOI] [PubMed] [Google Scholar]
- 43. Peek R. M., Jr., et al. 1998. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc. Assoc. Am. Physicians 110: 531–544 [PubMed] [Google Scholar]
- 44. Perry S., et al. 2010. Infection with Helicobacter pylori is associated with protection against tuberculosis. PLoS One 5: e8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reibman J., et al. 2008. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One 3: e4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rhead J. L., et al. 2007. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133: 926–936 [DOI] [PubMed] [Google Scholar]
- 47. Roovers K., Assoian R. K. 2000. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22: 818–826 [DOI] [PubMed] [Google Scholar]
- 48. Salehi Z., et al. 2009. Helicobacter pylori cagA status and peptic ulcer disease in Iran. Dig. Dis. Sci. 54: 608–613 [DOI] [PubMed] [Google Scholar]
- 49. Salehi Z., Mollasalehi H., Jelodar M. H., Kazemi M., Zahmatkesh R. 2010. The relationship between Helicobacter pylori infection and gastric adenocarcinoma in northern Iran. Oncol. Res. 18: 323–328 [DOI] [PubMed] [Google Scholar]
- 50. Shokrzadeh L., et al. 2010. Analysis of 3′-end variable region of the cagA gene in Helicobacter pylori isolated from Iranian population. J. Gastroenterol. Hepatol. 25: 172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Talley N. J., et al. 1991. Gastric adenocarcinoma and Helicobacter pylori infection. J. Natl. Cancer Inst. 83: 1734–1739 [DOI] [PubMed] [Google Scholar]
- 52. Tsutsumi R., Takahashi A., Azuma T., Higashi H., Hatakeyama M. 2006. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 26: 261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamaoka Y., Kwon D. H., Graham D. Y. 2000. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 97: 7533–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.