Abstract
Serological studies of patients with pertussis and the identification of antigenic Bordetella pertussis proteins support the hypothesis that B. pertussis perceives an iron starvation cue and expresses multiple iron source utilization systems in its natural human host environment. Furthermore, previous studies using a murine respiratory tract infection model showed that several of these B. pertussis iron systems are required for colonization and persistence and are differentially expressed over the course of infection. The present study examined genome-wide changes in B. pertussis gene transcript abundance in response to iron starvation in vitro. In addition to known iron source utilization genes, we identified a previously uncharacterized iron-repressed cytoplasmic membrane transporter system, fbpABC, that is required for the utilization of multiple structurally distinct siderophores including alcaligin, enterobactin, ferrichrome, and desferrioxamine B. Expression of type III secretion system genes was also found to be upregulated during iron starvation in both B. pertussis strain Tohama I and Bordetella bronchiseptica strain RB50. In a survey of type III secretion system protein production by an assortment of B. pertussis laboratory-adapted and low-passage clinical isolate strains, iron limitation increased the production and secretion of the type III secretion system-specific translocation apparatus tip protein Bsp22 in all Bvg-proficient strains. These results indicate that iron starvation in the infected host is an important environmental cue influencing not only Bordetella iron transport gene expression but also the expression of other important virulence-associated genes.
INTRODUCTION
Iron regulatory mechanisms in the mammalian host serve to limit the amount of free iron available to pathogenic microbes. Host iron homeostasis is controlled by the peptide hormone hepcidin, the production of which is further upregulated during infection and inflammation (38, 41, 62). The action of hepcidin, coupled with the extracellular iron-binding proteins transferrin (Tf) and lactoferrin (Lf), results in an effective innate iron sequestration defense against microbes. Pathogens are able to scavenge iron in the host, and expression studies of a variety of host-pathogen systems have identified iron acquisition genes as a prevalent class of bacterial in vivo-expressed genes (51). Bacterial iron acquisition system genes are negatively regulated by Fur, or a functionally similar bacterial repressor, and are derepressed under conditions of iron starvation (11, 40). Microbial iron starvation is a key signal controlling the expression of other known virulence factors (49, 55). To obtain ferric iron, bacteria may produce siderophores and their cognate transporters or they may produce transporters allowing utilization of xenosiderophores (59). Some organisms have surface receptors allowing direct uptake of heme or the iron from transferrin and lactoferrin (72). In Gram-negative bacteria, TonB-dependent outer membrane receptors are required for transfer of iron chelates and heme to the periplasm, followed by transport to the cytoplasm by ATP-binding cassette (ABC)-type transporters. Inorganic iron in the periplasm is transported to the cytoplasm by membrane transporters such as the Sfu ABC system (6).
Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica are highly genetically related respiratory pathogens of mammals (52, 66). B. pertussis is a strictly human-adapted species and is the agent of whooping cough or pertussis. B. parapertussis causes respiratory infections in humans and sheep, and B. bronchiseptica causes respiratory diseases in a range of mammalian hosts, including rare cases in humans. These pathogenic bordetellae attach to the ciliated cells of the host respiratory epithelium by using multiple adhesins and produce a variety of virulence factors including multiple potent toxins, resulting in disease symptoms. Most of the known Bordetella virulence factor genes, including the type III secretion system (T3SS) genes, are regulated by a two-component phosphorelay system consisting of the BvgS transmembrane sensor kinase and the BvgA DNA-binding response regulator (8, 86, 91). Expression of the T3SS genes is coregulated by the BtrS alternative sigma factor, and btrS expression is BvgAS dependent (53, 93). T3SS-associated proteins are cytotoxic for a variety of eukaryotic cell types, cause release of hemoglobin from erythrocytes, and have diverse immunomodulatory effects on the host, thus contributing to virulence (1, 34, 46, 54, 92).
In order to acquire iron, B. pertussis, B. parapertussis, and B. bronchiseptica produce and utilize the siderophore alcaligin (22, 57, 63). Additionally, B. pertussis and B. bronchiseptica can use the xenosiderophores enterobactin (14), ferrichrome, and desferrioxamine B (13); B. bronchiseptica can also use aerobactin, ferrichrysin, ferricrocin, ferrirubin, protochelin, schizokinen, vicibactin, and pyoverdin (71). The three Bordetella species have from 14 to 19 predicted TonB-dependent receptor- mediated transport systems (5, 66), but only the alcaligin, enterobactin, and heme utilization systems have been characterized to date. Expression of each of these systems is repressed by Fur and is also activated under iron starvation conditions by mechanisms involving dedicated positive regulators that require the cognate iron source as the inducer (3, 16, 23, 87, 88).
Several observations indicate that Bordetella cells rely on their iron uptake systems for growth in vivo and that iron starvation is a key host environmental cue. Iron-starved B. pertussis was shown to adhere more effectively to A549 human alveolar epithelial cells compared with iron-replete bacteria (89). A B. pertussis tonB mutant was attenuated in a mouse model of infection (70), and a B. bronchiseptica alcaligin siderophore biosynthesis mutant showed decreased virulence in neonatal swine (73). Studies of a B. pertussis alcaligin receptor mutant showed that alcaligin utilization was critical for bacterial growth in mice throughout infection (20). Similar analyses using a B. pertussis heme receptor mutant demonstrated an in vivo growth defect in the late stages of infection, suggesting that persistence in the host depends on heme utilization (24). The BfeA enterobactin receptor was required for growth of B. pertussis in the early stages of mouse infection (21). Recent studies indicated that B. pertussis upregulates expression of the alcaligin, enterobactin, and heme utilization genes in vivo, confirming that the mammalian host is an iron-restricted environment and that the cognate iron sources are likely present in the host during infection (21). Expression patterns of these iron systems were correlated with the stage of infection during which the iron systems appear to have their greatest impact on Bordetella in vivo fitness. Serum antibodies from pertussis patients recognized numerous Bordetella iron-repressible cell envelope proteins including the receptors for alcaligin, enterobactin, and heme. Together, these results suggest that B. pertussis is iron starved in its natural human host environment and responds to the availability of diverse iron sources by expressing multiple iron utilization systems.
In the present study, we sought to understand better the responses of B. pertussis to the host iron-restricted environment and to identify other B. pertussis genes exhibiting increased expression under iron starvation conditions. In addition to identifying the genes responsible for alcaligin, enterobactin, and heme utilization, transcriptome analyses identified a previously uncharacterized cytoplasmic membrane transporter system that we then show is required by Bordetella cells for the utilization of multiple siderophores, including alcaligin and enterobactin. These studies also revealed repression of T3SS genes in B. pertussis and B. bronchiseptica by iron. Furthermore, we show that production of secreted T3SS proteins, including Bsp22, BteA, BopD, BopB, and BopN, is increased upon iron starvation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bordetella strains and plasmids used in this study are listed in Table 1. Bordetella strains were grown on Bordet-Gengou agar plates. Escherichia coli strains were grown using Luria-Bertani (LB) broth or LB agar. Stainer-Scholte (SS) medium (76) was used for broth cultures, and all B. pertussis SS cultures were supplemented with 0.5% Casamino Acids. Glassware was acid cleaned and rinsed in distilled deionized water prior to use. Iron-replete SS medium contained 36 μM ferrous sulfate. For iron-depleted SS cultures, SS medium lacking iron supplement was further deferrated using Chelex 100 resin (Bio-Rad Laboratories, Richmond, CA). The chrome azurol S assay (77) was used to monitor alcaligin production as reported previously (9). Final concentrations of antibiotics used for plasmid marker selection were as follows: tetracycline, 15 μg/ml; ampicillin, 100 μg/ml; gentamicin, 10 μg/ml; kanamycin, 50 μg/ml; and streptomycin, 90 μg/ml. Growth was monitored spectrophotometrically at a wavelength of 600 nm or by using a Klett colorimeter.
Table 1.
Bordetella strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Source |
|---|---|---|
| B. pertussis strains | ||
| Tohama I | Clinical isolate, ca. 1954 | C. Parker |
| UT25 | Clinical isolate, 1975 | C. Parker |
| UT25-90 | Degraded, avirulent phase IV derivative of UT25 | S. Armstrong |
| BP6068 | Clinical isolate, 1997 | J. Miller |
| 165 | Office of Biologics, Food and Drug Administration | C. Manclark via C. Parker |
| 114 | Office of Biologics, Food and Drug Administration | C. Manclark via C. Parker |
| 3779 | Eli Lilly & Co. vaccine strain | C. Parker |
| 10536 | Connaught Laboratories vaccine strain | T. Merkel |
| 188 | Clinical isolate | T. Merkel |
| 461 | Clinical isolate | T. Merkel |
| B. bronchiseptica strains | ||
| RB50 | bvg+ rabbit isolate, wild type | P. Cotter |
| RB53 | bvgS-C3, bvg(Con), Bvg+ phenotypic phase-locked mutant derivative of RB50 | P. Cotter |
| RB54 | ΔbvgAS, Bvg− phenotypic phase-locked mutant derivative of RB50 | P. Cotter |
| Plasmid vectors | ||
| pGEM3Z | 2.7-kb cloning vector, Ampr | Promega |
| pRK415 | 10.5-kb broad-host-range cloning vector, Tetr | N. Keen |
| pRK2013 | 48-kb helper plasmid for mobilization of non-self-transmissible plasmids, Kanr | D. R. Helinski |
| pEG7 | 8.0-kb allelic exchange vector derived from pSS1129, Ampr Gentr | P. Cotter |
| pSS4245 | 8.3-kb allelic exchange vector, I-SceI+, Kanr Strepr | S. Stibitz |
| Recombinant plasmids | ||
| pEG7/fbpABb | 0.8-kb PCR-generated DNA fragment internal to B. bronchiseptica RB50 fbpA gene cloned into pEG7, BamHI-EcoRI primer/adapters, Ampr Gentr | This study |
| pEG7/fbpABp | 0.8-kb PCR-generated DNA fragment internal to B. pertussis Tohama I fbpA gene cloned into pEG7, BamHI-EcoRI primer/adapters, Ampr Gentr | This study |
| pRK/fbpABp | 2.4-kb PCR-generated fbpA+ DNA fragment of B. pertussis Tohama I cloned into pRK415, BamHI-EcoRI primer/adapters, Tetr | This study |
| p3Z/fbpABp | 2.4-kb PCR-generated fbpA+ DNA fragment of B. pertussis Tohama I cloned into pGEM3Z, BamHI-EcoRI primer/adapters, Ampr | This study |
| p3Z/ΔfbpABp | p3Z/fbpABp with 0.7-kb in-frame deletion in fbpA generated by whole-plasmid inverse PCR mutagenesis | This study |
| pSS/ΔfbpABp | 1.7-kb BamHI-EcoRI ΔfbpABp insert fragment of p3Z/ΔfbpABp subcloned into pSS4245, I-SceI+, Kanr, Strepr | This study |
| pRK/fbpABCBp | 6.5-kb fpr fbpABC+ PCR-generated DNA fragment of B. pertussis Tohama I cloned into pRK415, HindIII primer/adapters, Tetr | This study |
Routine DNA procedures.
DNA cloning procedures used standard methods (75). Conjugal transfer of plasmids to Bordetella strains, with E. coli DH5α as the plasmid donor strain and DH5α(pRK2013) as the source of mobilization functions, has been described previously (17). Bordetella transconjugants were selected on agar containing the appropriate antibiotics and colicin B (18). PCR was performed using standard procedures and Bordetella DNA templates in the presence of 5% dimethyl sulfoxide.
Nucleotide and protein sequence analysis.
Annotated Bordetella genome sequences (GenBank accession numbers: B. pertussis Tohama I, NC_002929; B. bronchiseptica RB50, NC_002927) were accessed at the GeneDB website (http://www.genedb.org/), developed and maintained by the Sanger Institute's Pathogen Sequencing Unit. Genome sequence features were visualized using the Artemis genome sequence viewer and annotation tool (74) (http://www.sanger.ac.uk/Software/Artemis/). Other DNA and protein analyses for sequence data management and PCR primer design relied on the Lasergene software package version 5.53 for Mac OS X (DNASTAR, Inc., Madison, WI).
Growth stimulation assays.
B. bronchiseptica utilization of iron sources was assessed using an agar bioassay method as described previously (15, 22), with iron-restricted LB agar plates containing the nonutilizable iron chelator EDDA {ethylenediaminedi-[(o-hydroxyphenyl)acetic acid]} at a final concentration of 100 μg/ml. B. pertussis LB agar bioassays used a modification of this method described previously (87). Test iron sources included the siderophores alcaligin and enterobactin purified as described previously (3, 22), the siderophores desferrioxamine B mesylate (Desferal) (Ciba Agrochemicals AG, Basel, Switzerland) and ferrichrome (Sigma-Aldrich, St. Louis, MO), ferrous sulfate (Sigma), and bovine hemin chloride (Sigma). Twofold serial dilutions of the iron sources were prepared in water, and five concentrations of each were tested in triplicate: FeSO4, 2.50, 1.25, 0.63, 0.31, and 0.16 mM; alcaligin, 619, 310, 155, 77, and 39 μM; enterobactin, 2.50, 1.25, 0.63, 0.31, and 0.16 μM; and bovine hemin chloride, desferrioxamine B mesylate, and ferrichrome, 100, 50, 25, 13, and 6 μM. Diameters of bacterial growth zones surrounding the sample wells were measured after 24 h (for B. bronchiseptica) or 48 h (for B. pertussis) of incubation at 37°C. Linear regression analysis confirmed a strong correlation between the log10 concentration of the iron source and the diameter of the growth zone it produced (correlation coefficients r ≥ 0.99). The ratio of the iron source concentrations necessary to produce equivalent growth diameters for the wild-type (WT) and mutant strains as determined by linear regression analysis was taken as a measure of their relative iron source utilization ability.
Utilization of lactoferrin (Lf) and transferrin (Tf) was assessed by measuring growth in liquid SS cultures with partially iron-saturated Tf or Lf as the sole iron source. Human Lf and Tf (Sigma) were loaded with ferric iron to 30% saturation as described by Agiato and Dyer (2). The solutions were adjusted to 2 mg of protein per ml in 50 mM Tris (pH 7.5), 150 mM sodium chloride, 20 mM sodium bicarbonate buffer. SS cultures were supplemented with 10 mM sodium bicarbonate and Tf or Lf at a 200-μg/ml protein concentration, yielding total iron concentrations estimated at 1.5 μM.
Hemolysis assays.
Hemolytic activity of B. bronchiseptica was measured essentially as described previously (48, 54, 64), using rabbit red blood cells (rRBC, HemoStat Laboratories). Briefly, washed B. bronchiseptica RB50 cell suspensions were prepared from 16-h iron-replete and iron-depleted SS cultures, mixed with washed rRBC suspensions at various bacterial cell/RBC ratios, and incubated at 37°C for 45 min. Controls included rRBC incubated without bacteria and rRBC lysed with water (100% lysis control).
Insertional mutagenesis of fbpA in B. pertussis and B. bronchiseptica.
DNA regions (800 bp) internal to the fbpA coding sequences of B. pertussis Tohama I (BP1605) and B. bronchiseptica RB50 (BB2946) were amplified by PCR using PCR primer/adapter pair 5′-ggccggatccGTCGACCAAGCGTTCCTCCCTGAT-3′ and 5′-ggccgaattcCGGCGCCGCTGATGTTGACG-3′ (underlining indicates restriction sites, and lowercase letters indicate adapter ends). Adapter ends were trimmed using BamHI and EcoRI, and the products were cloned into the mobilizable suicide plasmid pEG7. The resulting pEG7/fbpABp and pEG7/fbpABb plasmids were delivered to Tohama I or RB50 recipient strains by conjugation, and fbpA insertion mutants were selected on the basis of gentamicin and colicin B resistance.
Cloning the fbpA and fbpABC genes.
Chromosomal DNA regions (2.4 kb) including the 1-kb fbpA genes of B. pertussis Tohama I and B. bronchiseptica RB50 along with approximately 0.7 kb of flanking DNA sequences both upstream and downstream were PCR amplified using PCR primer/adapters 5′-ggccggatccGTGCCGGTGCCGAACAGATACAGG-3′ and 5′-ggccgaattc CGACGCGGCCGGCTCCCAGTG-3′, and the BamHI-EcoRI-digested PCR products were cloned into plasmid vectors pGEM3Z and pRK415 to yield fbpA+ plasmids p3Z/fbpABp, p3Z/fbpABb, pRK/fbpABp, and pRK/fbpABb. PCR primer/adapters 5′-ggccaagcttGCGCGGCCAAAGAAAAACC-3′ and 5′-ggccaagctt ACGGGGCCGGCAATGTCC-3′ were used to PCR amplify the 6.5- and 5.5-kb chromosomal DNA regions encompassing the fbpABC genes of B. pertussis Tohama I and B. bronchiseptica RB50, respectively, and the HindIII-digested products were cloned into plasmid vectors pGEM3Z and pRK415 to yield fbpABC+ plasmids p3Z/fbpABCBp, p3Z/fbpABCBb, pRK/fbpABCBp, and pRK/ fbpABCBb. The fbpABC DNA region cloned from Tohama I includes the IS481 insertion sequence located downstream of fbpC; this IS element is absent from the 5.5-kb RB50 DNA region.
Construction of a B. pertussis Δfbp mutant strain.
Plasmid p3Z/fbpABp was used as the DNA template for in vitro deletion mutagenesis by inverse PCR using 5′-phosphorylated DNA oligonucleotide primers 5′-GAAGGCGTCGAGCAGAGG-3′ and 5′-TACGCCCAGGCCAACTACGA-3′. The reaction products were circularized by ligation using T4 DNA ligase and then digested with DpnI before transformation of E. coli to yield plasmids carrying the ΔfbpABp allele with 729 bp of fbpA coding sequences deleted. Putative p3Z/ΔfbpABp plasmids were identified by restriction enzyme mapping, and the desired in-frame deletion mutation was confirmed by nucleotide sequencing. The ΔfbpABp allele was subcloned to allelic exchange plasmid vector pSS4245 (S. Stibitz, unpublished data) to create plasmid pSS/ΔfbpABp, and the ΔfbpA mutation was transferred to the chromosome of B. pertussis strains Tohama I and UT25 by using a method adapted by Stibitz from that of Posfai et al. (69) for use in B. pertussis. Briefly, pSS/ΔfbpABp was conjugally transferred to B. pertussis that had been modulated to Bvg− phenotypic phase by growth in the presence of 50 mM MgSO4. Primary selection for B. pertussis pSS/ΔfbpABp integrants used kanamycin, streptomycin, and colicin B under modulating (Bvg−) growth conditions to allow stable maintenance of the integrated plasmid. Integrants were pooled and restreaked on the same selective medium with MgSO4 and then subcultured to standard BG plates (Bvg+ growth conditions) to allow expression of the plasmid-encoded I-SceI restriction endonuclease, which is under the control of the Bvg-dependent ptx promoter. I-SceI production results in cleavage at the pSS4245 plasmid-encoded SceI site to produce a double-stranded break in the chromosome. Repair of this lesion involving recombination between the integrated plasmid and homologous chromosomal sequences results in allelic exchange at high frequency. Colonies were scored for loss of plasmid antibiotic resistance markers, and ΔfbpA mutants were confirmed by PCR mapping.
Electrophoretic analysis of Bordetella proteins.
SDS-PAGE analysis of proteins was performed using standard methods; proteins were visualized by staining with Coomassie blue dye. Extracts enriched in B. pertussis periplasmic proteins were produced by the osmotic shock method described by Shouldice and coworkers (79). Cell proteins for immunoblot analysis were prepared from B. pertussis strain Tohama I and B. bronchiseptica strains RB50 and RB54 cultured in iron-replete and iron-depleted SS media. Cells were suspended at an optical density at 600 nm (OD600) of 10 in 1 ml of SDS-PAGE sample buffer containing 2-mercaptoethanol and boiled for 5 min. Samples (40 μg of protein) were separated by SDS-PAGE on 7.5% polyacrylamide gels and transferred to nitrocellulose membranes. Mouse monoclonal antibody 8E7, which is specific for B. pertussis Vag8 (10, 35, 36), was used and reactivity detected using goat anti-mouse IgG conjugated to horseradish peroxidase (Cappel) with 4-chloronaphthol as the substrate.
LC-MS/MS analysis of secreted proteins.
Bordetella cells from SS cultures were pelleted by centrifugation, and the supernatant fluids were clarified by filtration using 0.2-μm pore size, low-protein-binding filters. Proteins were precipitated by addition of 100% trichloroacetic acid (wt/vol) to 15% final concentration, and incubation on ice for 4 h. Protein precipitates were recovered by centrifugation and solubilized by boiling for 5 min in SDS-PAGE sample buffer containing 2-mercaptoethanol. Protein bands were excised from Coomassie blue-stained SDS-PAGE gels and treated with trypsin. Trypsin treatment and reverse-phase liquid chromatography-tandem mass spectrometry (LC-MS/MS) were performed at the University of Minnesota Center for Mass Spectrometry and Proteomics facility. Peptide products were separated on a C18 Nanotrap column (Michrom BioResources, Auburn, CA) and subjected to tandem mass spectrometry using a ThermoFinnigan (ABI, Inc., Foster City, CA) LTQ ion trap mass spectrometer (MS).
Mass spectrometry data were analyzed using Sequest (ThermoFinnigan, version 27, rev. 13) and X! Tandem software (version 2006.04.01.2, The Global Proteome Machine Organization, http://www.thegpm.org). X! Tandem and Sequest were set up to search the NCBI Bordetella protein sequence database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=protein) assuming trypsin cleavage, and with a fragment ion mass tolerance of 1.00 Da and parent ion tolerance of 0.80 Da. The iodoacetamide derivative of cysteine was specified as a fixed modification, and oxidation of methionine as a variable modification. Scaffold (version Scaffold-01_06_19, Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 90.0% probability as specified by the Peptide Prophet algorithm (43). Protein identifications were accepted if they could be established at greater than 95.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (60). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
RNA isolation.
B. pertussis Tohama I or B. bronchiseptica RB50 cells were harvested from iron-replete SS cultures at early stationary growth phase, washed extensively with iron-free SS medium, and then subcultured to iron-replete or iron-depleted SS medium at an initial cell density corresponding to an OD600 of 0.1. Cultures were grown with rotary shaking at 300 rpm at 37°C. At various times ranging from 2 to 36 h, cultures were sampled and bacterial cells were collected by centrifugation. Supernatant fluids were discarded or collected for recovery of extracellular proteins, and cell pellets were rapidly frozen in liquid nitrogen and stored at −80°C for subsequent RNA isolation. Total RNA was isolated using the RNAqueous-4PCR kit (Ambion, Austin, TX) with an additional lysis step using lysozyme (0.4 mg/ml).
Microarray hybridization and analysis.
Bordetella RNA was reverse transcribed into Cy5-labeled cDNA and then cohybridized along with a Cy3-labeled mixture of B. pertussis, B. bronchiseptica, and B. parapertussis genomic DNA to a Bordetella microarray representing 97.4% of the B. pertussis Tohama I open reading frames (ORFs), 97.9% of the B. parapertussis strain 12822 ORFs, and 98.5% of the B. bronchiseptica RB50 ORFs, as previously described (25). The use of a common reference across different cDNA microarray experiments improves the reproducibility of hybridization signals and allows comparisons of gene expression levels determined in separate experiments (80). Arrays were scanned using a GenePix 4000B scanner and the GenePix Pro 5.0 software (Axon Instruments, Union City, CA). By using the GenePix Pro 6.0 software (Axon Instruments), spots were screened visually to exclude the low-quality spots, and background-subtracted Cy5/Cy3 intensity ratios were calculated. Data were filtered to include only spots containing more than 30 pixels and having a mean background-subtracted Cy3 signal greater than 150 U. Cy5/Cy3 ratios were averaged for replicate spots, log transformed, and normalized by calculating the median log ratio and subtracting the value obtained from each data point.
For the analysis of the iron starvation response in Tohama I and RB50, significance analysis of microarrays (SAM; version 1.0) (85) was performed for the subset of array elements whose data passed filtering criteria for at least 50% of the arrays in the experiment. For this analysis, data were assigned to two groups, iron replete and iron depleted, in order to identify transcripts with significant differences in abundance between the two nutritional growth conditions. Only time points subsequent to a detectable iron depletion response (16 h and later for RB50, 24 h and later for Tohama I) were used in the SAM analysis.
Hierarchical clustering of data was performed with Cluster (version 2.11.0.1) (32), using only those array elements whose data passed filtering criteria for at least 80% of the arrays. Transcript abundance was considered to vary significantly according to iron availability if there was a difference of at least 2 between the maximum and minimum log values across the data set (4-fold variation). Results were displayed using Treeview (version 1.60). Functional classifications of genes whose transcript abundances varied significantly in response to iron availability were as assigned by Parkhill and coworkers (66), and by the descriptive multifunctional classification of Serres and Riley (78).
Microarray data accession numbers.
Microarray data have been deposited in ArrayExpress under accession numbers E-MEXP-3263 (B. pertussis Tohama I) and E-MEXP-3076 (B. bronchiseptica RB50).
RESULTS
B. pertussis transcriptional response to iron restriction.
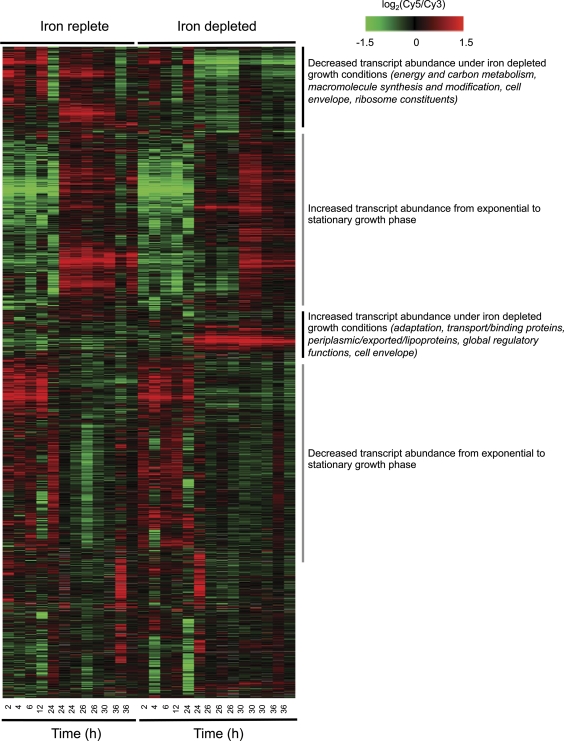
To analyze the B. pertussis transcriptional response to iron limitation, cDNA microarray expression analysis using Bordetella genomic DNA as the common reference was employed. Strain Tohama I was cultured in parallel in iron-replete and iron-depleted SS media and RNA was isolated at time points from 2 h to 36 h, during the different stages of growth (Fig. 1 and 2; also see Tables S1 and S2 in the supplemental material). This sampling scheme allowed growth phase-related changes in gene expression to be distinguished from those related to the iron starvation response. B. pertussis genes showing increased transcript abundance under iron-replete growth conditions (downregulated by iron limitation) included genes encoding 30S and 50S ribosomal proteins, ATP synthase subunits, and iron-containing proteins including Nuo subunits of the NADH-ubiquinone oxidoreductase, cytochromes, and catalase. Transcripts for capsular biosynthesis genes and two bacterioferritin genes, the products of which are predicted to play an important role in the storage of iron, were also increased in abundance in the presence of iron.
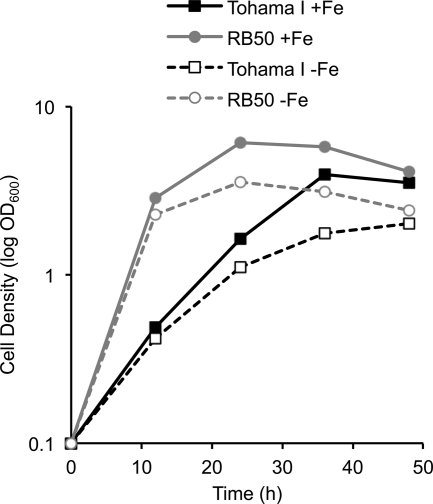
Fig. 1.
Growth of B. pertussis Tohama I and B. bronchiseptica RB50 in iron-replete (+Fe) and iron-depleted (−Fe) SS media. Typical growth curves (as monitored spectrophotometrically at 600 nm wavelength over a 48-h culture period) are shown.
Fig. 2.
Gene transcript abundance patterns in B. pertussis Tohama I. Gene transcript abundance patterns in B. pertussis Tohama I are shown for cultures grown under iron-replete and iron-depleted conditions. Rows correspond to array elements, and major functional categories for groups of genes and their predicted products are indicated on the right. Some genes are represented by multiple array elements. Gray indicates missing data. The elapsed time since the culture was started is indicated at the bottom.
As anticipated, iron-repressed genes (upregulated by iron limitation) included the alcaligin, enterobactin, and heme iron acquisition system genes, tonB and exbBD, and genes encoding predicted TonB-dependent receptors. The alcaligin, enterobactin, and heme utilization system genes are known to be iron repressible by Fur, in addition to being positively regulated (3, 16, 23, 87, 88). Of the 12 genes encoding uncharacterized TonB-dependent receptors, bfrB (BP2016) and bfrC (BP3663) were significantly upregulated upon iron starvation in vitro. Other genes predicted to be involved in iron transport, BP1136, BP1139, and BP1140, were also upregulated when iron was restricted. BP1136 encodes an extracytoplasmic function sigma factor; this gene is an ortholog of the B. bronchiseptica ecfI gene adjacent to and required for expression of bfrH (BB3658/BP1138), encoding a TonB-dependent receptor of unknown ligand specificity (27). Other upregulated genes adjacent to predicted TonB-dependent iron source receptor genes included BP1961 near bfrI (BP1962) and BP0454 near hemC (BP0456). BP1605, a gene encoding a predicted periplasmic ferric iron-binding component of an ABC superfamily transporter system, also showed increased expression during iron starvation. In this report, a detailed functional analysis of this gene is presented.
Some of the B. pertussis genes that were increased in expression under conditions of iron limitation were not anticipated to be regulated in such a fashion. For example, fhaB (BP1879), ptlC (BP3790), and ptlI (BP3792) were classified as iron repressed. In B. pertussis, fhaB is monocistronic (29, 50) and the pertussis toxin structural (ptx) and secretion (ptl) genes are cotranscribed from a promoter upstream of ptxA (12, 45). Analysis of the B. pertussis Tohama I derivative BP953 (81), carrying chromosomal fhaB-lacZ and ptx-phoA transcriptional fusions, revealed only modest elevation of fhaB and ptxA expression under iron-depleted growth conditions as compared with iron-replete conditions (46% and 14% increases in reporter activity after 48 h of culturing, respectively) (data not shown). Also among the B. pertussis genes showing elevated transcript abundance under iron starvation conditions were Bvg-dependent genes of the T3SS, which have been extensively analyzed in B. bronchiseptica (26, 37, 47, 48, 53, 54, 64, 65, 92, 93). We examined the influence of iron starvation on Bordetella T3SS gene expression and protein production in greater detail (see below).
BP1605 encodes a ferric iron binding protein.
Compared with expression levels during iron-replete growth conditions, the BP1605 gene of Tohama I showed a 4.8-fold increase in transcript abundance under conditions of iron limitation. BP1605 is predicted to encode a 37.4-kDa protein, referred to here as FbpA, that is similar to ferric iron-binding proteins including those involved in utilization of Lf- and Tf-bound iron by Neisseria gonorrhoeae and Neisseria meningitidis (FbpA, 28% identity) (30) and Haemophilus influenzae (HitA, 30% identity) (44), as well as the Serratia marcescens Sfu protein (28% identity) (6) involved in the uptake of inorganic ferric iron and FbpA of Campylobacter jejuni (35% identity) (see Fig. S1 in the supplemental material). The B. pertussis FbpA is a predicted periplasmic solute-binding component of an ABC superfamily transporter system. The crystal structures of the apo- and holo- forms of B. pertussis FbpA were determined previously by Tom-Yew and coworkers (83), and used to aid the refinement of the structure of the ferric iron-binding protein of C. jejuni (83), but no functional studies of B. pertussis FbpA have been reported. Adjacent to fbpA (Fig. 3), the downstream fbpB (BP1604) and fbpC (BP1603) genes encode putative inner membrane permease and ATPase components of the ABC transporter system, respectively. The fbpABC genes are predicted to be cotranscribed from a putative Fur-repressible promoter located upstream of fbpA. BP1603 is annotated as a pseudogene that is interrupted by an IS481 element in B. pertussis Tohama I; however, alignment of the deduced BP1603 product with the protein sequence of the B. bronchiseptica RB50 FbpC ortholog (locus tag BB2948) indicates that the IS element does not impinge on the coding sequence and the 265-amino-acid BP1603 product is intact but has 6 amino acids (FbpCBp amino acid residues 119 to 124, inclusive) that are absent in FbpCBb. BP1604 was absent from the array data set, so its expression patterns are unknown; however, BP1603 also showed an increase in transcript abundance during iron limitation, albeit less than 2-fold. Since fbpABC were iron repressed and encoded a predicted ABC-type transporter system, we hypothesized that these genes could potentially be involved in alcaligin or enterobactin utilization. No cytoplasmic membrane transporter functions are encoded within the alcaligin and enterobactin system gene clusters, and none had been identified until the present study.
Fig. 3.
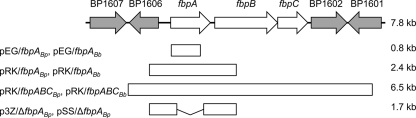
Genetic organization of the B. pertussis fbpA chromosomal region. The arrows in the uppermost diagram represent genetic limits and orientations of genes within the B. pertussis Tohama I DNA region that includes the fbpABC genes (white arrows): fbpA (BP1605), ferric iron-binding protein (periplasmic component of an ABC-type Fe3+ transport system); fbpB (BP1604), putative cytoplasmic membrane permease; fbpC (BP1603), probable ATP-binding component of ABC transporter. Flanking genes were as follows: BP1606, putative ferredoxin-NADP+ reductase; BP1607, probable LysR-family transcriptional regulator; BP1602, transposase; BP1601, conserved hypothetical protein. Cloned DNA fragments used for insertional mutagenesis (0.8 kb), complementation (2.4 kb and 6.5 kb), and construction of in-frame fbpA deletion mutations are shown. In B. bronchiseptica, the fragment analogous to the 6.5-kb region is only 5.5 kb in size because this region lacks the IS481 element.
fbpA mutants are defective in the utilization of multiple siderophores.
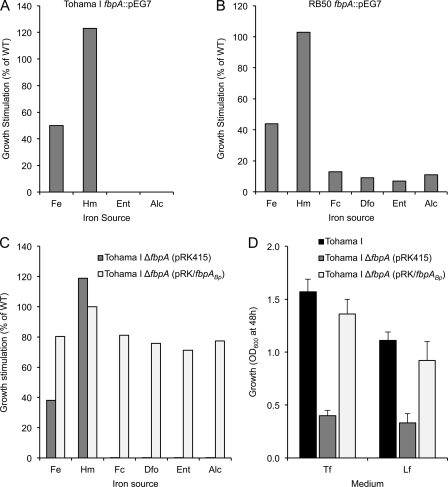
The fbpA gene of B. pertussis Tohama I was insertionally inactivated and the resulting mutant was tested for the ability to utilize a variety of known iron sources. Relative to the wild-type parent strain, the B. pertussis fbpA::pEG7 insertion mutant had severe defects in utilization of alcaligin and enterobactin and also showed reduced growth stimulation by iron (Fig. 4A). The mutant retained wild-type heme utilization levels, indicating that FbpA is not required for heme uptake; presumably that function is provided by the BhuT periplasmic heme binding protein encoded in the heme system gene cluster (87). fbpA::pEG7 insertion mutations were also constructed in B. pertussis strain UT25 and in B. bronchiseptica RB50. The B. pertussis UT25 (data not shown) and B. bronchiseptica RB50 (Fig. 4B) mutants had similar defective iron utilization phenotypes as did Tohama I fbpA::pEG7, indicating that these fbpABC-related functions are conserved in at least two B. pertussis strains and in these two Bordetella species. Other growth assays showed that, in addition to alcaligin and enterobactin, RB50 fbpA::pEG7 was also defective in the utilization of the siderophores ferrichrome and desferrioxamine B (Fig. 4B). Although fbpA disruption nullified growth stimulation by siderophores in B. pertussis, the B. bronchiseptica RB50 fbpA::pEG7 mutant retained limited siderophore utilization. The fbpA insertion mutants were complemented using plasmids carrying wild-type fbpABC genes but not by plasmids bearing fbpA alone (data not shown), suggesting that the fbpA::pEG7 insertion mutations exert polar effects on the downstream fbpB and fbpC genes.
Fig. 4.
Iron source utilization by WT and fbpA mutant strains of B. pertussis and B. bronchiseptica. (A) Growth stimulation of B. pertussis Tohama I fbpA::pEG7 mutant is shown relative to that of the WT parental strain. The mutant showed no measurable growth stimulation by enterobactin or alcaligin. (B) Growth stimulation of B. bronchiseptica RB50 fbpA::pEG7 mutant is shown relative to that of the WT parental strain. (C) Growth stimulation of B. pertussis Tohama I ΔfbpA(pRK415) (ΔfbpA) and the complemented strain Tohama I ΔfbpA(pRK/fbpABp) (ΔfbpA/fbpA+) compared to that of the WT parental strain. The ΔfbpA mutant showed no measurable growth stimulation by any siderophore tested. (D) Growth of B. pertussis in SS medium with Tf or Lf as the sole source of iron. Growth yield was measured as the optical density at 600 nm (mean ± SD, n = 3) after 48 h of culture. Iron sources: Fe, ferrous sulfate; Hm, hemin chloride; Fc, ferrichrome; Dfo, desferrioxamine B; Ent, enterobactin; Alc, alcaligin; Tf, transferrin; Lf, lactoferrin.
The requirement for FbpA in the TonB-dependent utilization of siderophores was not anticipated since no other bacterial ferric iron-binding protein with this particular function has been described previously. To further establish the role of FbpA in siderophore utilization by B. pertussis, nonpolar in-frame fbpA deletion mutations were constructed in strains Tohama I and UT25. Both B. pertussis ΔfbpA mutants had the same defective phenotype as did the B. pertussis fbpA::pEG7 insertion mutants; however, unlike the insertion mutants, the ΔfbpA mutants could be complemented in trans by the fbpA gene alone to greater than 70% of wild-type ability (Fig. 4C). These studies confirmed that fbpA is important for assimilation of iron delivered by multiple catecholate- and hydroxamate-type siderophores, and complementation results are consistent with predictions that fbpB and fbpC are operonic with fbpA.
FbpA is required for utilization of Tf and Lf as iron sources.
To retrieve the iron from Tf and Lf, Bordetella cells must use a siderophore such as alcaligin. Since fbpA is essential for the utilization of alcaligin by B. pertussis, it was hypothesized that fbpA mutants would display a growth defect in cultures having Lf or Tf as the sole iron source. Wild-type Tohama I was cultured in parallel with the ΔfbpA mutant, Tohama I ΔfbpA (pRK415 vector), and the complemented mutant, Tohama I ΔfbpA(pRK/fbpABp), in iron-depleted SS medium supplemented with partially iron-saturated human Tf or Lf. Under these iron-limited conditions, growth yields for the ΔfbpA mutant with Tf or Lf were significantly reduced compared with those of the wild-type parent (approximately 25% and 30% of wild-type growth levels, respectively) (Fig. 4D), despite production of alcaligin at normal levels by both wild-type and ΔfbpA strains; alcaligin concentrations consistently ranged from 11 to 16 μM in supernatant fluids of both wild-type and ΔfbpA strains after 48 h growth under iron-limited conditions. Growth with Tf or Lf was restored to the mutant to greater than 80% of the wild-type level by trans-complementation using fbpA. Since the ability to use a siderophore such as alcaligin to acquire iron from Lf and Tf is predicted to be important for host colonization, fbpABC may play a key role in this process.
FbpA is localized to the periplasm and its production is iron repressible.
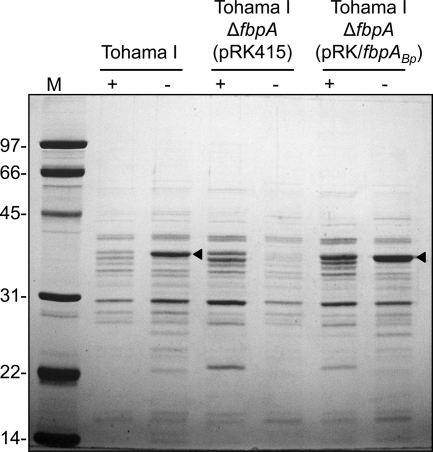
Osmotic shock fluids enriched in periplasmic components were prepared from B. pertussis cells and analyzed by SDS-PAGE. In support of the gene transcript abundance data, an iron-regulated periplasmic protein of ca. 37-kDa molecular mass (FbpA) was produced by B. pertussis Tohama I (Fig. 5). FbpA was not produced by Tohama I ΔfbpA(pRK415), and FbpA production was restored to the mutant by plasmid pRKfbpABp. Expression of fbpA from multicopy plasmid pRK/fbpABp resulted in modest overproduction of FbpA by iron-starved B. pertussis and in detectable production of FbpA by iron-replete cells. FbpA was the most abundant periplasmic protein in iron-starved B. pertussis cells, accounting for approximately 50% to 70% of the total protein released by osmotic shock.
Fig. 5.
Proteins released from B. pertussis cells by osmotic shock treatment. Shock fluids enriched in periplasmic proteins were prepared from cells harvested from iron-replete (+) and iron-depleted (−) SS cultures. A Coomassie blue-stained 12% polyacrylamide gel is shown. Sample loads were normalized to cell numbers based on optical density. Sizes of molecular mass (M) markers are shown in kDa. The arrowheads indicate the position of FbpA.
Effects of iron limitation on type III secretion system gene expression.
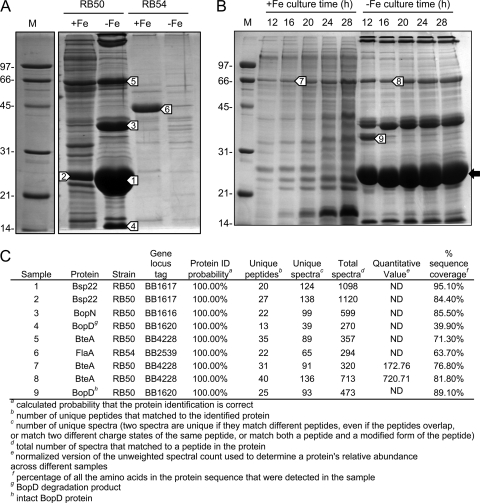
Gene expression patterns from microarray studies revealed that certain B. pertussis Tohama I T3SS bsc cluster genes, including bsp22, bopN, bopB, and bcrH2, as well as the T3SS effector gene bteA (also known as bopC), were significantly upregulated during iron limitation (1.8- to 2.7-fold increase in gene transcript abundance) (Fig. 2; also see Table S1 in the supplemental material). Since Tohama I had been reported to be deficient in T3SS protein production (34, 53), the potential iron regulation of T3SS gene expression and protein production was examined in B. bronchiseptica RB50, the strain in which the Bvg-dependent T3SS has been most extensively characterized. The effect of iron limitation on the protein secretion profiles of wild-type strain RB50 and its isogenic Bvg phenotypic phase-locked derivatives RB53 (Bvgc) and RB54 (Bvg−) (28) were analyzed by SDS-PAGE. Notably, iron limitation significantly altered the secretion profiles of RB50 (Fig. 6A) and RB53 (data not shown), resulting in the elevated production of prominent proteins of approximately 22-kDa and 39-kDa apparent molecular masses. In contrast, the secretion profiles of Bvg− strain RB54 were much less complex than those of Bvg+ strain RB50 and showed an overall reduction in total secreted protein levels under both iron-replete and iron-depleted conditions. However, a unique protein of an approximately 44-kDa apparent molecular mass appeared to be produced primarily by RB54 under iron-replete conditions.
Fig. 6.
Secretion profiles of B. bronchiseptica strain RB50 and isogenic Bvg− phenotypic phase-locked derivative RB54. Proteins were recovered from supernatant fluids of B. bronchiseptica strains cultured for 16 h in iron-replete (+Fe) or iron-depleted (−Fe) SS medium and separated by SDS-PAGE. Sizes of molecular mass markers (M) are given in kDa. (A) Secreted protein profiles of RB50 and RB54. Normalized sample loads represent proteins recovered from 1.2 ml of supernatant fluid (cell densities [OD600] of cultures: RB50 +Fe, 5.52; RB50 −Fe, 2.80; RB54 +Fe, 8.89; RB54 −Fe, 2.18). Bands (white arrows numbered 1 to 6) were excised from replicate gels for identification by LC-MS/MS. (B) Secreted proteins from RB50 cultures used as RNA sources in microarray analysis. The black arrow indicates the Bsp22 protein band in −Fe samples. Normalized sample loads represent proteins recovered from 1 ml of supernatant fluid. Bands (white arrows numbered 7 to 9) were excised from replicate gels for identification by LC-MS/MS. (C) Protein identities as determined by LC-MS/MS. Relative abundance of BteA (also known as BopC) in 16-h +Fe and −Fe sample bands was estimated by spectral counting (quantitative value). ND, not determined.
Based on published secretion profiles of strain RB50 (92, 93), it was hypothesized that the major ca. 22-kDa protein that was produced under both iron-replete and iron-depleted growth conditions was the T3SS tip complex structural protein, Bsp22. To confirm the identities of selected proteins secreted by B. bronchiseptica, protein bands were excised from one-dimensional SDS-PAGE gels (Fig. 6A) and analyzed (Fig. 6C). Reverse-phase liquid chromatography-tandem mass spectrometry (LC-MS/MS) confirmed the identity of the 22-kDa major extracellular protein as Bsp22 (22.1 kDa; BB1617) (bands 1 and 2). The second most abundant protein in iron-depleted culture supernatant fluids (band 3) was BopN (38.9 kDa; BB1616), the product of the bopN gene that is divergently transcribed from bsp22 in the T3SS bsc gene cluster. Bsp22 and BopN are typically the most abundant T3SS proteins secreted by B. bronchiseptica (48, 93). Analysis of density profile plots of stained SDS-PAGE gels determined that iron limitation resulted in an approximately 10-fold increase in Bsp22 protein levels in RB50 culture supernatant fluids when normalized to cell density (data not shown). Iron-regulated protein band 4 was identified as a degradation product of BopD (31.8 kDa intact; BB1620), as previously reported by Kuwae and coworkers (47). Band 5 was found to contain the T3SS effector protein BteA (BB4228) along with pertactin (both ca. 69 kDa in monomeric form). BteA is also thought to form large homomultimeric protein complexes or aggregates of greater than 200-kDa molecular mass (37, 47, 65). The extracellular protein (band 6) produced by the Δbvg mutant strain RB54 under iron-replete growth conditions was identified as flagellin (FlaA, 40.4 kDa; BB2539). FlaA levels in RB54 culture supernatant fluids were dramatically reduced under iron starvation growth conditions.
B. bronchiseptica shows elevated hemolytic activity during iron starvation.
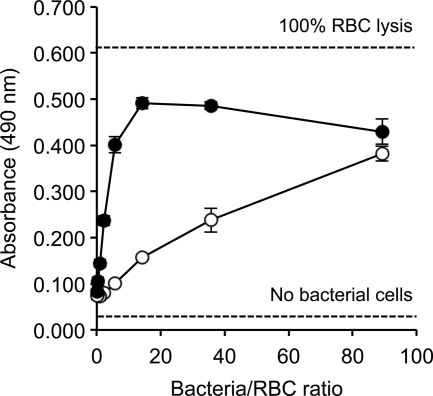
B. bronchiseptica Bsp22 is required for T3SS cytotoxicity and hemolytic activity that was previously shown by mutational analysis to be independent from that of adenylate cyclase (54). Medhekar and coworkers (54) showed that deletion of the cyaA adenylate cyclase toxin gene had no effect on hemolytic activity of B. bronchiseptica RB50 in this assay, whereas a cyaA bcsN double mutant, also lacking the T3SS ATPase activity, showed no hemolytic activity. Consistent with the elevated production of T3SS proteins during iron starvation, iron-starved RB50 cultures showed increased hemolytic activity against rabbit RBCs in vitro by the assay method of Medhekar and coworkers (54), compared with bacteria from iron-replete cultures (Fig. 7). The maximal hemolytic activity of iron-starved RB50 bacteria was 81% at a bacterial cell:RBC ratio of approximately 14:1, relative to that of water-lysed RBCs (100%, positive control). In contrast, the hemolytic activity of B. bronchiseptica cultured under iron-replete conditions was 26% at the same bacterial cell:RBC ratio.
Fig. 7.
Effect of iron starvation on T3SS-dependent hemolytic activity of B. bronchiseptica RB50. Dilutions of normalized bacterial suspensions prepared from iron-replete (open circles) and iron-depleted (filled circles) SS cultures were incubated with rabbit RBC, and liberated hemoglobin was measured by absorbance at a wavelength of 490 nm. Mean absorbance values (n = 3, ±1 SD) are shown. Dashed lines show control absorbance values corresponding to hemoglobin released in the absence of bacteria (no bacterial cells) and by hypotonic lysis of rabbit RBC using water (100% RBC lysis).
B. bronchiseptica iron limitation is associated with increased T3SS gene expression.
A limited analysis of iron-regulated T3SS gene expression patterns in B. bronchiseptica RB50 was performed using microarrays. Multiple RNA sample sets were generated from RB50 cultured in parallel in iron-replete and iron-depleted SS media at various times of the growth cycle (Fig. 1). As cells were collected for RNA isolation, secreted proteins were recovered from supernatant fluids and analyzed by SDS-PAGE (Fig. 6B). High-level production of Bsp22 by iron-starved RB50 was clearly evident by 8 h to 12 h of culturing (during early to mid-exponential growth phase) and thereafter up to 28 h (stationary growth phase), whereas Bsp22 production by iron-replete bacteria was comparatively low. In addition, a protein band (Fig. 6B, band 9) that had not been observed previously in this study was evident only in the 12-h sample (mid-exponential growth phase) from the iron-depleted culture. LC-MS/MS identified that protein as the intact BopD protein (31.8 kDa; BB1620) (Fig. 6C). The BopD protein complexes with BopB to form the pore in the host plasma membrane for translocation of T3SS effectors. Protein bands predicted to contain BteA were excised from the 16 h iron-replete and iron-depleted sample lanes (Fig. 6B, bands 7 and 8) and analyzed by LC-MS/MS. Estimates of BteA production levels based on spectral counting indicated that the specific level of BteA was more than 4-fold greater in the iron-depleted culture sample than in the iron-replete culture sample (Fig. 6C). Since protein gel loads had been normalized to culture volume and not culture cell density (OD600 at 16 h: iron replete, 3.94; iron depleted, 2.67), BteA production by iron-starved B. bronchiseptica on a per-cell basis is estimated to be more than 6-fold higher at 16 h than that by iron-replete cells.
B. bronchiseptica RB50 microarray expression analysis showed increased transcript abundance of T3SS genes similar to results obtained for B. pertussis (see Fig. S2 in the supplemental material). Expression of T3SS genes was strongly upregulated during iron starvation, with almost every gene in the T3SS cluster showing a large increase in transcript abundance over iron-replete levels. These findings are consistent with the proteomics-based evidence for elevated production of T3SS proteins by B. bronchiseptica under iron starvation conditions, and confirm that iron regulation of B. bronchiseptica T3SS gene expression occurs at the transcriptional level. Furthermore, the T3SS effector protein gene bteA (47, 65) and the vag8 gene BB1864, which encodes a T3SS accessory factor (53), were found to be coordinately upregulated with the RB50 T3SS locus. To corroborate the vag8 transcription results, Vag8 protein levels in both B. bronchiseptica and B. pertussis were examined by immunoblot analysis using Vag8-specific mouse monoclonal antibody 8E7 (see Fig. S3 in the supplemental material). Although Vag8 was detectable in the B. pertussis Tohama I samples, there was no significant effect of iron starvation on Vag8 production; similar results were obtained using B. pertussis strain UT25 (data not shown). In contrast, Vag8 production by B. bronchiseptica RB50 was estimated to be at least 3-fold higher in iron-starved cells than in cells from iron-replete cultures. As predicted, Vag8 protein production by the Bvg− strain RB54 was not detected in cells regardless of iron status.
The mechanistic basis of the association between iron starvation stress and upregulation of T3SS gene expression in B. pertussis and B. bronchiseptica is unknown. A bioinformatics-based approach was used to identify potential Fur repressor binding sites within the T3SS bsc and btr gene clusters that might account for the apparent iron regulation of T3SS genes, but no predicted strong Fur binding sites could be identified in the bsp22-bopN intergenic region or upstream of the bcr and btr loci. Four potential weak overlapping Fur binding sites (57.9 to 62.5% similarity with the E. coli consensus Fur binding site, 5′-GATAATGATAATCATTATC-3′) were identified in the DNA region located 20 to 61 nucleotides (nt) upstream of btrS (BP2234/BB1638), which encodes an extracytoplasmic function sigma factor required for transcription of the bsc T3SS genes (5′-AAACCGGCAGTCAGTATCTTGGTGACGTGAATTATCACC-3′). This btrS upstream DNA region (identical in B. pertussis and B. bronchiseptica) was tested for Fur binding activity using a functional Fur repressor titration assay (82), but it had no significant Fur binding activity in this assay (data not shown).
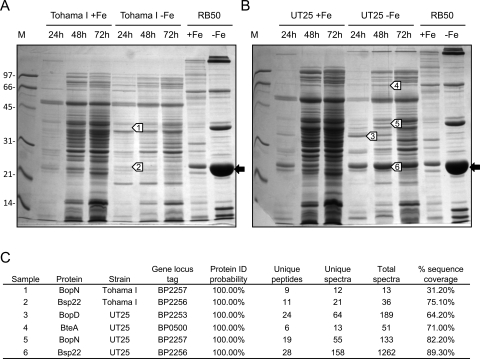
B. pertussis clinical and laboratory-adapted strains show increased production of T3SS proteins during iron limitation.
It was reported that B. pertussis strain Tohama I does not produce detectable Bsp22 or other T3SS proteins (33, 53). In a later study, Bsp22 was identified in low-passage clinical B. pertussis isolates but not the laboratory-adapted Tohama I strain (34). Importantly, none of the studies to date has examined the production of B. pertussis T3SS proteins during iron starvation. Since the current microarray study found that T3SS gene transcription was upregulated during iron starvation in both B. pertussis Tohama I and B. bronchiseptica RB50, it was hypothesized that secreted T3SS proteins might be detected in iron-starved Tohama I cultures. In initial studies, neither B. pertussis Tohama I nor the low-passage strain UT25 produced T3SS proteins by 16 to 18 h of growth in iron-replete or iron-depleted SS medium (data not shown). However, subsequent cultures showed that by 24 h B. pertussis UT25 produced multiple iron-regulated proteins with electrophoretic mobilities similar to those of the RB50 T3SS proteins. Tohama I also appeared to produce at least two putative T3SS proteins during iron starvation, including the major protein Bsp22, albeit at levels much lower than those of UT25 or RB50 (Fig. 8A and B). Putative B. pertussis T3SS proteins (Fig. 8A and B, bands 1 to 6) were excised from gels and analyzed by LC-MS/MS. UT25 protein bands were confirmed to be Bsp22, BopN, BopD, and BteA (Fig. 8C). Tohama I protein bands were identified as the major T3SS proteins Bsp22 and BopN. Despite low-level production of Bsp22 and BopN by Tohama I, protein identities could be assigned with the highest degree of statistical confidence (Fig. 8C). For all B. pertussis proteins that were identified by LC-MS/MS, spectra included ion fragments unique to B. pertussis T3SS proteins compared with their B. bronchiseptica orthologs (data not shown).
Fig. 8.
Secretion profiles of B. pertussis strains Tohama I (A) and UT25 (B) cultured in iron-replete (+Fe) or iron-depleted (−Fe) SS medium. Supernatant fluids were collected at various culture times and the secreted proteins were recovered as described in Materials and Methods. Coomassie blue-stained 12% polyacrylamide gels are shown, with sizes of molecular mass (M) markers given in kDa. Sample loads were normalized to culture cell density. B. bronchiseptica RB50 secreted proteins are shown for comparison. The prominent bands (black arrows) in the RB50 −Fe lanes are Bsp22. Bands (white arrows numbered 1 to 6) were excised from replicate gels and subjected to LC-MS/MS analysis. (C) Protein identities as determined by LC-MS/MS.
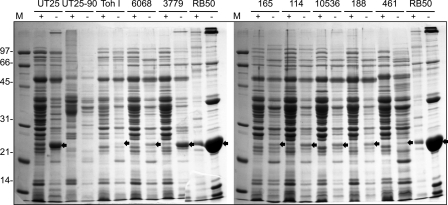
Since the low-passage isolate UT25 produced T3SS proteins at higher levels than the relatively high-passage Tohama I strain, a limited survey of various B. pertussis strains (Table 1) was performed to assess whether high-level T3SS protein production was a trait common only to low-passage isolates and whether T3SS protein production was increased by iron starvation in other B. pertussis strains. Strains were cultured in iron-replete and iron-depleted SS media for 46 h; all strains grew to similar levels and produced high levels of alcaligin in iron-depleted SS medium (data not shown). Using Bsp22 as a hallmark of T3SS production, all B. pertussis strains exam- ined except for the avirulent, laboratory-adapted derivative UT25-90 produced T3SS proteins, albeit at an apparent level lower than that of B. bronchiseptica RB50 (Fig. 9). There was no strict correlation between T3SS protein production level and the extent of laboratory passage. Of the strains examined, strains 3779 (high passage) and UT25 (low passage) appeared to produce the highest levels of Bsp22, whereas Tohama I produced the lowest level. All B. pertussis strains that produced Bsp22 appeared to produce elevated levels of it under iron-limited growth conditions.
Fig. 9.
Secretion profiles of various B. pertussis strains. Low-passage and high-passage strains of B. pertussis (Table 1) were cultured for 46 h in iron-replete (+) or iron-depleted (−) SS medium. Secreted proteins were recovered as described in Materials and Methods and analyzed using SDS-PAGE. Molecular mass (M) markers are shown in kDa. The black arrows indicate the positions of Bsp22. B. bronchiseptica RB50 secreted proteins are shown for comparison. Sample loads were normalized to culture cell density and represent proteins recovered from a volume of supernatant fluid originating from 1.0 OD600 of bacterial cells.
DISCUSSION
The natural host environment of B. pertussis is an exquisitely iron-depleted one in which homeostatic mechanisms severely limit the amount of extracellular iron available to microbes (38, 41, 62). Expression of the Bordetella alcaligin, enterobactin, and heme receptor genes in infected mice and production of those receptors in human hosts indicate a natural state of iron starvation stress in vivo (21). In the current study, B. pertussis genes encoding the anticipated alcaligin biosynthesis and heme uptake functions, the BfeR regulator of the bfeA enterobactin receptor gene, TonB system genes, and genes encoding TonB-dependent receptors of unknown specificity showed increased transcript abundance under iron starvation conditions.
In previous Bordetella studies, increased transcript abundance for alcABC and hurI was associated with glutamate starvation (58). Nakamura and colleagues (58) also detected increased transcript abundance for other iron-related genes (including bfrB, bfrC, bfrH, bhuR, hurIR, and alcABDE) upon transition from logarithmic to stationary growth phase, when the bacteria begin to respond to nutrient scarcity. Results from the current study using specific iron-depleted growth conditions indicate that B. pertussis responds to iron starvation stress in a manner distinct from that of cells transitioning to stationary growth. Production of the Bordetella TonB-dependent receptor BfrD is both Bvg activated and iron repressed (7, 67). Others have reported genes encoding several TonB-dependent receptors of unknown ligand specificity as variably Bvg activated or Bvg repressed (29, 61). The significance of the differential expression of those genes in Bvg-related array studies is uncertain, since the cultures were not specifically starved for iron to allow Fur derepression. Absolute transcript abundance would likely be minimal under standard, iron-replete growth conditions regardless of Bvg phenotypic phase, and only relative expression levels were determined in those studies.
Genes with elevated transcript abundance during iron-replete, versus iron-restricted, growth included those involved in energy production, ribosome structure, iron storage, and oxidative stress. Iron response array studies using bacteria such as Campylobacter jejuni (42), Burkholderia (84) and Neisseria (31, 39) spp., Helicobacter pylori (56), and Pasteurella multocida (68) have shown similar expression patterns, with genes such as those encoding ferritin, NADH dehydrogenase, cytochromes c, ribosomal proteins, and oxidative stress functions identified as having increased expression under iron-replete, versus depleted, growth conditions. Typically, many of these genes encode proteins with iron or heme cofactors; thus, their reduced expression during iron starvation may in part represent an iron conservation strategy.
The present study is, to our knowledge, the first global analysis of Bordetella gene transcription in response to iron starvation. A 2007 analysis of B. pertussis proteins produced during iron-replete versus iron-depleted conditions (90) identified some of the same iron-repressed proteins specified by genes found in our analysis: the BhuS heme utilization protein, the BfrB TonB-dependent receptor protein, AlcC alcaligin biosynthesis enzyme, FumC fumarate hydratase, and the BP1605 product that we describe herein as FbpA. Those investigators noted similarity of the BP1605 protein to FbpA family members and of the predicted products of BP1604 and BP1603 to the permease and ATPase components of an ABC transporter, respectively.
The Bordetella outer membrane alcaligin and enterobactin receptor genes are located within their cognate system gene clusters (3, 4, 14, 19). However, there are no genes in either cluster that are predicted to encode a cytoplasmic membrane transport apparatus, and none could be reliably predicted from the genome sequences. The present study identified fbpA, encoding a member of the family of periplasmic ferric iron-binding proteins. Bordetella FbpA is required for optimal utilization of inorganic iron. In addition, B. pertussis and B. bronchiseptica fbpA mutants were unable to utilize not only the alcaligin siderophore but also the xenosiderophores enterobactin, ferrichrome, and desferrioxamine B. Therefore, FbpA, and likely FbpB and FbpC, appear to constitute a binding protein-dependent ABC transporter for the uptake of iron. Since the use of these structurally diverse siderophores requires the Fbp system, it is likely that the iron is removed from the siderophores after transit to the periplasm. The Bordetella Fbp system may represent a critical nodal point in iron assimilation in these pathogens, providing an activity essential for iron delivery via siderophores including alcaligin and enterobactin, which are known to be important for multiplication in vivo (20, 21).
The B. pertussis T3SS genes specifying BcrH2, BopB, Bsp22, and BopN were also upregulated upon iron starvation. Comparative analyses showed that B. bronchiseptica T3SS proteins were produced and secreted at increased levels under iron starvation growth conditions; Bsp22 and BopN in particular were secreted in remarkable abundance. B. bronchiseptica gene expression profiling also indicated increased transcription of a number of T3SS-related genes. Included in this group were vag8, a Bvg-activated gene encoding an outer membrane protein implicated in secretion of type III substrates, and the T3SS effector gene bteA. Examination of secreted proteins from iron-replete versus iron-starved B. pertussis Tohama I confirmed the microarray results, revealing increased production of Bsp22 and BopN. In B. pertussis, vag8 was not iron regulated, nor were Vag8 production levels influenced by the iron status; however, in B. bronchiseptica, Vag8 production was enhanced during growth under iron-limited conditions.
A survey of B. pertussis strains revealed that they all produced and secreted the T3SS protein Bsp22 and perhaps others, and this production was enhanced by iron limitation. Some strains, including Tohama I, secreted modest levels of T3SS proteins and others secreted significantly higher levels; none of the B. pertussis strains produced the high Bsp22 levels observed in B. bronchiseptica RB50. It is possible that T3SS expression in B. pertussis is a strain- or lineage-variable trait, as has been proposed for B. bronchiseptica (26). No obvious correlation was observed between highly passaged laboratory-adapted B. pertussis isolates versus those that have been minimally passaged.
Another unusual observation was that significant levels of the FlaA flagellin subunit were found in the spent culture supernatant of the B. bronchiseptica RB54 ΔbvgS mutant grown under iron-replete, but not iron-depleted, conditions. Neither the flaA gene of B. pertussis nor that of B. bronchiseptica RB50 was transcriptionally upregulated specifically under iron-replete growth conditions. The RB54 FlaA result may represent an artifact of growth under high-iron conditions that are not encountered in vivo or be related to the Bvg− status of the mutant that leads to constitutive production of flagellin, the excess of which is found in the culture supernatant. The potential contribution of post-transcriptional regulation has not been examined.
Iron repression of the Bordetella T3SS has not been described previously, and the biological significance and mechanisms of iron regulation of the system are unknown; however, iron starvation does not appear to be associated with increased expression of all Bvg-dependent virulence genes. Bordetella type III proteins are required for a hemolytic activity that is distinct from that caused by the adenylate cyclase toxin (48, 54, 64), and the present study showed that hemolytic activity was increased in iron-starved Bordetella cells. It is possible that the T3SS plays a role in iron acquisition by making intracellular heme or iron available, necessitating iron-mediated control of the system. Alternatively, iron starvation may be an important host environmental cue affecting expression of the Bordetella T3SS. These observations illuminate a potentially important intersection of the Bvg virulence regulon and the iron starvation response. This response allows Bordetella cells to perceive a primary nutritional stress signal that not only triggers mechanisms to obtain essential iron but also informs the organism that it is in a host, whereupon appropriate bacterial virulence factors may be deployed.
Supplementary Material
ACKNOWLEDGMENTS
Support for this study was provided by Public Health Service grants AI057188 and AI54970 (to D.A.R.) and AI31088 (to S.K.A.) from the National Institute of Allergy and Infectious Diseases.
We thank Scott Stibitz and Noel Keen for plasmids and Peggy Cotter, Tod Merkel, Jeff F. Miller, and Charlotte Parker for Bordetella strains. We also thank Bruce Witthuhn for help with analysis of mass spectrometry data.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Abe A., Nagamatsu K., Watanabe M. 2008. The Bordetella type III secretion system: its application to vaccine development. Microbiol. Immunol. 52:128–133 [DOI] [PubMed] [Google Scholar]
- 2. Agiato L. A., Dyer D. W. 1992. Siderophore production and membrane alterations by Bordetella pertussis in response to iron starvation. Infect. Immun. 60:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson M. T., Armstrong S. K. 2004. The BfeR regulator mediates enterobactin-inducible expression of Bordetella enterobactin utilization genes. J. Bacteriol. 186:7302–7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson M. T., Armstrong S. K. 2006. The Bordetella bfe system: growth and transcriptional response to siderophores, catechols, and neuroendocrine catecholamines. J. Bacteriol. 188:5731–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson M. T., Armstrong S. K. 2008. Norepinephrine mediates acquisition of transferrin-iron in Bordetella bronchiseptica. J. Bacteriol. 190:3940–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angerer A., Gaisser G., Braun V. 1990. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J. Bacteriol. 172:572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antoine R., et al. 2000. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol. 182:5902–5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arico B., et al. 1989. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc. Natl. Acad. Sci. U. S. A. 86:6671–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong S. K., Clements M. O. 1993. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J. Bacteriol. 175:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armstrong S. K., Parker C. D. 1986. Heat-modifiable envelope proteins of Bordetella pertussis. Infect. Immun. 54:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagg A., Neilands J. B. 1987. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471–5477 [DOI] [PubMed] [Google Scholar]
- 12. Baker S. M., Masi A., Liu D.-F., Novitsky B. K., Deich R. A. 1995. Pertussis toxin export genes are regulated by the ptx promoter and may be required for efficient translation of ptx mRNA in Bordetella pertussis. Infect. Immun. 63:3920–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beall B., Hoenes T. 1997. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology 143:135–145 [DOI] [PubMed] [Google Scholar]
- 14. Beall B., Sanden G. N. 1995. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology 141:3193–3205 [DOI] [PubMed] [Google Scholar]
- 15. Beaumont F. C., Kang H. Y., Brickman T. J., Armstrong S. K. 1998. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport in Bordetella pertussis and Bordetella bronchiseptica. J. Bacteriol. 180:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brickman T. J., Armstrong S. K. 1995. Bordetella pertussis fur gene restores iron repressibility of siderophore and protein expression to deregulated Bordetella bronchiseptica mutants. J. Bacteriol. 177:268–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brickman T. J., Armstrong S. K. 1996. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J. Bacteriol. 178:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brickman T. J., Armstrong S. K. 1996. Colicins B and Ia as novel counterselective agents in interspecies conjugal DNA transfers from colicin-sensitive Escherichia coli donors to other gram-negative recipient species. Gene 178:39–42 [DOI] [PubMed] [Google Scholar]
- 19. Brickman T. J., Armstrong S. K. 1999. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J. Bacteriol. 181:5958–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brickman T. J., Armstrong S. K. 2007. Impact of alcaligin siderophore utilization on in vivo growth of Bordetella pertussis. Infect. Immun. 75:5305–5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brickman T. J., Hanawa T., Anderson M. T., Suhadolc R. J., Armstrong S. K. 2008. Differential expression of Bordetella pertussis iron transport system genes during infection. Mol. Microbiol. 70:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brickman T. J., Hansel J. G., Miller M. J., Armstrong S. K. 1996. Purification, spectroscopic analysis and biological activity of the macrocyclic dihydroxamate siderophore alcaligin produced by Bordetella pertussis and Bordetella bronchiseptica. Biometals 9:191–203 [DOI] [PubMed] [Google Scholar]
- 23. Brickman T. J., Kang H. Y., Armstrong S. K. 2001. Transcriptional activation of Bordetella alcaligin siderophore genes requires the AlcR regulator with alcaligin as inducer. J. Bacteriol. 183:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brickman T. J., Vanderpool C. K., Armstrong S. K. 2006. Heme transport contributes to in vivo fitness of Bordetella pertussis during primary infection in mice. Infect. Immun. 74:1741–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brinig M. M., et al. 2006. Significant gene order and expression differences in Bordetella pertussis despite limited gene content variation. J. Bacteriol. 188:2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buboltz A. M., Nicholson T. L., Weyrich L. S., Harvill E. T. 2009. Role of the type III secretion system in a hypervirulent lineage of Bordetella bronchiseptica. Infect. Immun. 77:3969–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burgos J. M., King-Lyons N. D., Connell T. D. 2010. Expression of BfrH, a putative siderophore receptor of Bordetella bronchiseptica, is regulated by iron, Fur1, and the extracellular function sigma factor EcfI. Infect. Immun. 78:1147–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cotter P. A., Miller J. F. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cummings C. A., Bootsma H. J., Relman D. A., Miller J. F. 2006. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J. Bacteriol. 188:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhungana S., Anderson D. S., Mietzner T. A., Crumbliss A. L. 2005. Kinetics of iron release from ferric binding protein (FbpA): mechanistic implications in bacterial periplasm-to-cytosol Fe3+ transport. Biochemistry 44:9606–9618 [DOI] [PubMed] [Google Scholar]
- 31. Ducey T. F., Carson M. B., Orvis J., Stintzi A. P., Dyer D. W. 2005. Identification of the iron-responsive genes of Neisseria gonorrhoeae by microarray analysis in defined medium. J. Bacteriol. 187:4865–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eisen M. B., Spellman P. T., Brown P. O., Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fauconnier A., et al. 2001. Characterization of the type III secretion locus of Bordetella pertussis. Int. J. Med. Microbiol. 290:693–705 [DOI] [PubMed] [Google Scholar]
- 34. Fennelly N. K., et al. 2008. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect. Immun. 76:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finn T. M., Amsbaugh D. F. 1998. Vag8, a Bordetella pertussis bvg-regulated protein. Infect. Immun. 66:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frank D. W., Parker C. D. 1984. Isolation and characterization of monoclonal antibodies to Bordetella pertussis. J. Biol. Stand. 12:353–365 [DOI] [PubMed] [Google Scholar]
- 37. French C. T., et al. 2009. The Bordetella type III secretion system effector BteA contains a conserved N-terminal motif that guides bacterial virulence factors to lipid rafts. Cell. Microbiol. 11:1735–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ganz T. 2009. Iron in innate immunity: starve the invaders. Curr. Opin. Immunol. 21:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grifantini R., et al. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. U. S. A. 100:9542–9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hantke K. 1981. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 182:288–292 [DOI] [PubMed] [Google Scholar]
- 41. Hentze M. W., Muckenthaler M. U., Andrews N. C. 2004. Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297 [DOI] [PubMed] [Google Scholar]
- 42. Holmes K., et al. 2005. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology 151:243–257 [DOI] [PubMed] [Google Scholar]
- 43. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. 2002. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74:5383–5392 [DOI] [PubMed] [Google Scholar]
- 44. Khan A. G., et al. 2007. High-affinity binding by the periplasmic iron-binding protein from Haemophilus influenzae is required for acquiring iron from transferrin. Biochem. J. 404:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kotob S. I., Hausman S. Z., Burns D. L. 1995. Localization of the promoter for the ptl genes of Bordetella pertussis, which encode proteins essential for secretion of pertussis toxin. Infect. Immun. 63:3227–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kozak N. A., Panina E. M., Miller J. F. 2007. Type III secretion in Bordetella subspecies, p. 119–139 In Locht C. (ed.), Bordetella molecular microbiology. Horizon Bioscience, Norfolk, United Kingdom [Google Scholar]
- 47. Kuwae A., et al. 2006. BopC is a novel type III effector secreted by Bordetella bronchiseptica and has a critical role in type III-dependent necrotic cell death. J. Biol. Chem. 281:6589–6600 [DOI] [PubMed] [Google Scholar]
- 48. Kuwae A., Ohishi M., Watanabe M., Nagai M., Abe A. 2003. BopB is a type III secreted protein in Bordetella bronchiseptica and is required for cytotoxicity against cultured mammalian cells. Cell. Microbiol. 5:973–983 [DOI] [PubMed] [Google Scholar]
- 49. Litwin C. M., Calderwood S. B. 1993. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 6:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Locht C., Geoffroy M. C., Renauld G. 1992. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 11:3175–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mahan M. J., Heithoff D. M., Sinsheimer R. L., Low D. A. 2000. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu. Rev. Genet. 34:139–164 [DOI] [PubMed] [Google Scholar]
- 52. Mattoo S., Cherry J. D. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mattoo S., Yuk M. H., Huang L. L., Miller J. F. 2004. Regulation of type III secretion in Bordetella. Mol. Microbiol. 52:1201–1214 [DOI] [PubMed] [Google Scholar]
- 54. Medhekar B., Shrivastava R., Mattoo S., Gingery M., Miller J. F. 2009. Bordetella Bsp22 forms a filamentous type III secretion system tip complex and is immunoprotective in vitro and in vivo. Mol. Microbiol. 71:492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mekalanos J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Merrell D. S., et al. 2003. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect. Immun. 71:6510–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moore C. H., Foster L. A., Gerbig D. G., Jr., Dyer D. W., Gibson B. W. 1995. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J. Bacteriol. 177:1116–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura M. M., et al. 2006. Growth phase- and nutrient limitation-associated transcript abundance regulation in Bordetella pertussis. Infect. Immun. 74:5537–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Neilands J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723–26726 [DOI] [PubMed] [Google Scholar]
- 60. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. 2003. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75:4646–4658 [DOI] [PubMed] [Google Scholar]
- 61. Nicholson T. L. 2007. Construction and validation of a first-generation Bordetella bronchiseptica long-oligonucleotide microarray by transcriptional profiling the Bvg regulon. BMC Genomics 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nicolas G., et al. 2001. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc. Natl. Acad. Sci. U. S. A. 98:8780–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nishio T., et al. 1988. Isolation and structure of the novel dihydroxamate siderophore alcaligin. J. Am. Chem. Soc. 110:8733–8734 [Google Scholar]
- 64. Nogawa H., Kuwae A., Matsuzawa T., Abe A. 2004. The type III secreted protein BopD in Bordetella bronchiseptica is complexed with BopB for pore formation on the host plasma membrane. J. Bacteriol. 186:3806–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Panina E., et al. 2005. A genome-wide screen identifies a Bordetella type III secretion effector and candidate effectors in other species. Mol. Microbiol. 58:267–279 [DOI] [PubMed] [Google Scholar]
- 66. Parkhill J., et al. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32–40 [DOI] [PubMed] [Google Scholar]
- 67. Passerini de Rossi B. N., et al. 2003. Vir90, a virulence-activated gene coding for a Bordetella pertussis iron-regulated outer membrane protein. Res. Microbiol. 154:443–450 [DOI] [PubMed] [Google Scholar]
- 68. Paustian M. L., May B. J., Kapur V. 2001. Pasteurella multocida gene expression in response to iron limitation. Infect. Immun. 69:4109–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Posfai G., Kolisnychenko V., Bereckzki Z., Blattner F. R. 1999. Markerless gene replacement in Escherichia coli stimulated by a double-strand break in the chromosome. Nucleic Acids Res. 27:4409–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pradel E., Guiso N., Menozzi F. D., Locht C. 2000. Bordetella pertussis TonB, a Bvg-independent virulence determinant. Infect. Immun. 68:1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pradel E., Locht C. 2001. Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J. Bacteriol. 183:2910–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ratledge C., Dover L. G. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881–941 [DOI] [PubMed] [Google Scholar]
- 73. Register K. B., Ducey T. F., Brockmeier S. L., Dyer D. W. 2001. Reduced virulence of a Bordetella bronchiseptica siderophore mutant in neonatal swine. Infect. Immun. 69:2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rutherford K., et al. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945 [DOI] [PubMed] [Google Scholar]
- 75. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 76. Schneider D. R., Parker C. D. 1982. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect. Immun. 38:548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schwyn B., Neilands J. B. 1987. Universal chemical-assay for the detection and determination of siderophores. Anal. Biochem. 160:47–56 [DOI] [PubMed] [Google Scholar]
- 78. Serres M. H., Riley M. 2000. MultiFun, a multifunctional classification scheme for Escherichia coli K-12 gene products. Microb. Comp. Genom. 5:205–222 [DOI] [PubMed] [Google Scholar]
- 79. Shouldice S. R., et al. 2003. High resolution structure of an alternate form of the ferric ion binding protein from Haemophilus influenzae. J. Biol. Chem. 278:11513–11519 [DOI] [PubMed] [Google Scholar]
- 80. Sterrenburg E., Turk R., Boer J. M., van Ommen G. B., den Dunnen J. T. 2002. A common reference for cDNA microarray hybridizations. Nucleic Acids Res. 30:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stibitz S. 1994. Mutations in the bvgA gene of Bordetella pertussis that differentially affect regulation of virulence determinants. J. Bacteriol. 176:5615–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stojiljkovic I., Baumler A. J., Hantke K. 1994. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J. Mol. Biol. 236:531–545 [DOI] [PubMed] [Google Scholar]
- 83. Tom-Yew S. A., Cui D. T., Bekker E. G., Murphy M. E. 2005. Anion-independent iron coordination by the Campylobacter jejuni ferric binding protein. J. Biol. Chem. 280:9283–9290 [DOI] [PubMed] [Google Scholar]
- 84. Tuanyok A., et al. 2005. Genome-wide expression analysis of iron regulation in Burkholderia pseudomallei and Burkholderia mallei using DNA microarrays. FEMS Microbiol. Lett. 252:327–335 [DOI] [PubMed] [Google Scholar]
- 85. Tusher V. G., Tibshirani R., Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Uhl M. A., Miller J. F. 1994. Autophosphorylation and phosphotransfer in the Bordetella pertussis BvgAS signal transduction cascade. Proc. Natl. Acad. Sci. U. S. A. 91:1163–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vanderpool C. K., Armstrong S. K. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 183:4278–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vanderpool C. K., Armstrong S. K. 2003. Heme-responsive transcriptional activation of Bordetella bhu genes. J. Bacteriol. 185:909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vidakovics M. L., et al. 2007. Iron stress increases Bordetella pertussis mucin-binding capacity and attachment to respiratory epithelial cells. FEMS Immunol. Med. Microbiol. 51:414–421 [DOI] [PubMed] [Google Scholar]
- 90. Vidakovics M. L., et al. 2007. Profiling the Bordetella pertussis proteome during iron starvation. J. Proteome Res. 6:2518–2528 [DOI] [PubMed] [Google Scholar]
- 91. Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yuk M. H., Harvill E. T., Cotter P. A., Miller J. F. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991–1004 [DOI] [PubMed] [Google Scholar]
- 93. Yuk M. H., Harvill E. T., Miller J. F. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945–959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.