Abstract
Human papillomaviruses (HPVs) are the causative agents of several important genital and other mucosal cancers. The HPV16 E7 gene encodes a viral oncogene that is necessary for the continued growth of cancer cells, but its role in the normal, differentiation-dependent life cycle of the virus is not fully understood. The function of E7 in the viral life cycle was examined using a series of mutations of E7 created in the context of the complete HPV16 genome. The effect of these E7 mutations on key events of the viral life cycle, including immortalization, episomal maintenance, late promoter activation, and infectious virion synthesis, was examined. Our studies show that the pRb binding domain is indispensable for early viral activities, whereas the C-terminal zinc finger domain contributed primarily to very late events. Mutations of the casein kinase II phosphorylation site caused a complex phenotype involving both the function of E7 protein and a cis element necessary for the activation of the late promoter, identifying for the first time a promoter element important for late promoter function in the context of the viral genome. All mutant genomes tested showed reduced viral titers following growth in organotypic raft cultures. These studies clarify the role of E7 as a regulator of late events in the differentiation-dependent HPV life cycle.
INTRODUCTION
Human papillomaviruses (HPVs) are small, nonenveloped DNA viruses that are the causative agents of cervical cancer and also contribute to other anogenital and oral malignancies (51, 52). HPVs persistently infect stratified epithelia, and their life cycles are tightly linked to the differentiation program of the host keratinocytes (29). In HPV infections, a stable pool of infected cells is maintained in the basal epithelial layer, with low levels of viral genomes and gene expression. As infected daughter cells detach from the basement membrane and initiate squamous differentiation, viral proteins block normal cell cycle exit and activate the expression of host DNA replication enzymes to facilitate late events in the life cycle, such as viral genome amplification (29). However, the relationship between differentiation and the cell cycle in the context of the HPV life cycle is not well characterized.
The viral factor primarily responsible for maintaining replication competence in differentiating cells is E7 (37). The high-risk E7 proteins bind to pRb family members, resulting in the activation of E2F transcription factors and consequent rapid entry of the cell into S phase (38). E7 has a wide range of additional targets (37), but the extent to which these additional interactions contribute to the HPV life cycle is not fully understood. While many studies have used a variety of cell culture and animal model systems to characterize various functional domains in E7, little is known about the impact of E7 mutations in the context of the complete HPV genome during the productive life cycle. Previous studies have suggested that E7 is needed for episomal maintenance and immortalization of human foreskin keratinocytes (HFKs), and these activities depend on pRb binding and degradation domains (48). However, in HFKs that are already immortalized, the binding and degradation functions of E7 are not needed for episomal maintenance (8). In differentiating cells, E7 also is needed for the induction of viral genome amplification (13, 32), which may be due to the binding of pocket proteins, although the role of pRb degradation in this process is not fully understood (8). Mutants of E7 unable to bind pRb do not induce suprabasal DNA synthesis, but they still express L1 and E1̂E4, suggesting that amplification is not needed for L1 expression (8), and so the role of E7 in the activation of late gene expression remains unclear.
The activation of the viral late promoter is dependent on differentiation and drives the expression of the capsid proteins as well as regulatory factors, such as E1̂E4. The differentiation-dependent late promoter of HPV16, referred to as p670, is located in a region overlapping with the E7 open reading frame (ORF) (18, 43). Only a few studies have addressed the transcriptional control of HPV late promoters, and these have relied on transient reporter assays to suggest transcriptional repressors, such as CDP and YY1 (1, 2, 25), and transcriptional activators, such as hSkn-1a or C/EBPβ, as potential regulators (25, 26). Overall, relatively little is understood about which factors actually bind to the late promoter sequence in the viral genome or the particular cellular environment which leads to promoter activation during differentiation.
To understand how E7 functions in the differentiation-dependent phase of the virus life cycle, a series of mutations of E7 were created in the context of the complete HPV16 genome. The effect of these E7 mutations on key events of the viral life cycle, including immortalization, episomal maintenance, late promoter activation, and infectious virion synthesis, was examined. We found that the pRb binding domain is indispensable for early viral activities, whereas the C-terminal zinc finger domain contributed primarily to very late events. Mutations of the casein kinase II phosphorylation site caused a complex phenotype involving both the function of E7 protein and the activation of the late promoter in cis. This analysis identified the first promoter element that is necessary for late promoter function in the context of the viral genome. These studies identify important activities of E7 as a regulator of late events in the differentiation-dependent HPV life cycle.
MATERIALS AND METHODS
Cloning of mutant genomes and late promoter reporter.
pEGFP Ni HPV16 is a plasmid vector containing the W12 variant of HPV16 flanked by LoxP sites. Site-directed mutagenesis in the E7 open reading frame of pEGFP Ni HPV16 was performed using the QuikChange II site-directed mutagenesis kit (Agilent) using primers listed in Table S1 in the supplemental material and confirmed by sequencing. The late promoter reporter pGL2 16 Late was cloned by PCR using the primers 16 late pro 5′ and 16 late pro 3′ (see Table S1), digestion with KpnI and HindIII, and ligation into pGL2B.
Cell culture and creation of cell lines.
HFKs and HFK-derived cell lines were cultured in E medium with NIH 3T3 J2 fibroblast feeders as described previously (35). Cell lines containing HPV16 episomes were created by the cotransfection of pEGFP Ni HPV16 with an expression vector for Cre recombinase (a gift of Greg Smith, Northwestern University School of Medicine) and a neomycin resistance plasmid. Transfections were performed using polyethyleneimine (PEI; Polysciences). Cells were plated at a density of 2 to 3 million per 10-cm dish and transfected with plasmid DNA for 6 to 8 h at 37°C, followed by feeding with fresh medium and the addition of feeder cells. Starting the next day, cells were selected with G418 and expanded as described previously (49). Differentiation was induced by suspending cells in 1.5% methylcellulose (MC) for 24 h, followed by washes in phosphate-buffered saline (44).
Transient-transfection and luciferase assays.
Transient transfections of keratinocytes with reporter plasmids were performed using PEI as described above, except that transfection was performed overnight. The next day, cells were trypsinized and half plated in six-well plates and the other half suspended in 5 to 10 ml 1.5% MC. Following 24 h of incubation, lysates were prepared and luciferase activity was measured using the dual-luciferase reporter assay system (Promega) according to manufacturer's instructions, with Renilla luciferase as an internal control.
Western blotting.
Whole-cell extracts were prepared and subjected to SDS-PAGE and Western blotting as described previously (4, 40). Antibodies used were glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-47724; Santa Cruz), pRb (9309; Cell Signaling), cyclin A (C4710; Sigma), and E7 (28-0006; Invitrogen).
Southern analysis, Northern analysis, and quantitative real-time RT-PCR.
Total DNAs were isolated and digested with XhoI (which does not cut the HPV16 genome) or BamHI (which cuts the HPV16 genome once) before being analyzed by Southern analysis as described previously (12). Total RNAs were harvested using RNA-STAT 60 (TelTest, Inc.) and subjected to Northern blotting using the linearized HPV16 genome as a probe as described previously (12). cDNAs were synthesized using the SuperScript first-round synthesis system (Invitrogen), and quantitative PCR (qPCR) was performed using LightCycler 480 SYBR green I master mix (Roche) on a LightCycler 480 instrument. Primers used to detect E1̂E4 spliced transcripts and cyclophilin A (as an internal reference) (47) are shown in Table S1 in the supplemental material.
EMSA.
For electrophoretic mobility shift assays (EMSA), nuclear extracts were prepared from HPV16-containing keratinocytes according to the method of Schreiber et al. (45). Oligonucleotide probes were annealed in Tris-EDTA (TE) buffer containing 50 mM NaCl, labeled with 32P using T4 polynucleotide kinase (New England Biological), and purified using Microspin G-25 columns (GE Healthcare). Twenty μg nuclear extract of was incubated for 10 min at 25°C with 1 μg poly(dI-dC):poly(dI:dC) with or without 50× excess unlabeled oligonucleotides in a buffer containing 20 mM HEPES, 100 mM KCl, 20% glycerol, 0.1 mM EDTA, 0.2 mM ZnSO4, 0.05% NP-40, and 1 μg/μl bovine serum albumin. Probes were added and incubated for 10 additional minutes at 25°C before the addition of loading dye and electrophoresis in a 6% polyacrylamide 0.25× Tris-borate-EDTA (TBE) gel. Following electrophoresis, gels were dried and subjected to autoradiography.
Organotypic cultures, virion preparation, and infectivity assays.
Organotypic raft cultures were made as previously described (34). Briefly, a dermal equivalent was prepared by mixing rat tail type 1 collagen (BD Biosciences) with NIH 3T3 fibroblasts, 10× Dulbecco's modified essential medium (DMEM), and equilibration buffer, and allowing the mixture to solidify. One million keratinocytes were seeded onto the surface of the collagen, and the next day the construct was lifted onto a stainless steel grid in a culture dish. Culture medium was added to the dish so that the construct was exposed to the medium from below and the air above, and medium was changed every other day. Rafts harvested for histology were fixed in 4% paraformaldehyde for 1 h and embedded in paraffin, sectioned, and stained by the Northwestern University Pathology Core Facility. Rafts harvested for viral preparation were treated at every feeding with 20 mM C8 (1,2 dioctanoyl-sn-glycerol; Cayman Chemical). The epithelial portion of the raft culture was removed from the dermal equivalent and quickly frozen in an ethanol–dry-ice bath.
Virus was isolated, titers were determined, and infectivity was measured as previously described (9), with some variations. For each preparation, a total of three rafts were Dounce homogenized in 500 μl phosphate buffer (0.06 M sodium phosphate, pH 8.0). Homogenizers were rinsed with 250 μl phosphate buffer. MgCl2 was added to 2 mM, and 375 U benzonase was added to 750 μl of viral cell lysate. Lysates were incubated at 37°C for 1 h to remove any unencapsidated viral genomes. NaCl (5 M) was added to a final concentration of 1 M NaCl, followed by centrifugation at 4°C for 10 min at 10,500 rpm. Viral supernatants were stored at −70°C.
To determine viral titers, 2 μl of proteinase K, 10 μl 10% SDS, 2 μl 140 ng/μl sonicated salmon-sperm carrier DNA, and Hirt buffer were added to 10 μl of a viral prep to a total of 200 μl. Samples were incubated with rotation at 37°C for 2 h. DNA was harvested by phenol-chloroform-isoamyl alcohol extraction followed by ethanol precipitation overnight at −20°C. The DNA pellet was washed with 70% ethanol and resuspended in 20 μl Tris-EDTA. A Qiagen Quantitect SYBR green PCR kit was used to detect viral genomes. One μl from each viral preparation was analyzed in triplicate in a total reaction volume of 25 μl. A portion of the HPV16 genome in E2 was amplified using the Titer 5′ and Titer 3′ primers as shown in Table S2 in the supplemental material. A standard curve was generated by amplifying serially diluted HPV16 genomes at 108, 107, 106, 105, and 104 copies. A Bio-Rad iQ5 multicolor real-time qPCR machine and software were utilized for PCR amplifications and data analysis. The PCR thermocycling profile was a 15-min hot start at 95°C followed by 40 cycles at 15 s at 94°C, 30 s at 52°C, and 30 s at 72°C.
For infectivity assays, HaCaT cells were grown in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 1 mM pyruvate, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and were seeded at 50,000 cells/well in 24-well plates 2 days prior to infections. Infections were performed at approximately 10 viral genomes per cell by diluting viral preparations in a total volume of 0.5 ml culture medium. Infections were allowed to continue for 48 h at 37°C. Total mRNA was harvested using the SurePrep TrueTotal RNA purification kit (Fisher Scientific). The Quantitect probe reverse transcription-PCR (RT-PCR) kit (Qiagen) was used for the RT-PCR and qPCRs. HPV16 E1̂E4 transcript was amplified using Inf E1̂E4 5′ and Inf E1̂E4 3′ (see Table S2 in the supplemental material) at a final concentration of 4 μM. A fluorogenic, HPV16 E1̂E4 probe (5′-6-carboxyfluorescein-CACCGGAAACCCCTGCCACACCACTAAG-black hole quencher [BHQ]-1-3′; nucleotides [nt] 3493 to 3520) was utilized at a final concentration of 0.2 μM to detect E1̂E4 cDNA. TATA-binding protein (TBP) was amplified using TBP 5′ and TBP 3′ (see Table S2) at a final concentration of 0.125 μM. TBP amplicons were detected by using a fluorogenic TaqMan probe 5′-5′-hexachlorofluorescein-TGTGCACAGGAGCCAAGAGTGAAGA-BHQ-1-3′ at 0.2 μM. Cycling conditions were 50°C for 30 min, 95°C for 15 min, 42 cycles of 94°C for 15 s, and 54.5°C for 1 min. The amplification efficiency of each primer set was 93% for E1̂E4 and 97% for TBP. REST software was used to analyze the data.
RESULTS
In this study, we sought to investigate the role of E7 in regulating differentiation-dependent events in the HPV16 life cycle. Many studies have used mutational analyses to examine the effects of HPV16 E7 on activities such as immortalization, Rb degradation, and effects on cell cycle regulators, but few studies have investigated the effects of this viral protein on differentiation-dependent events in the context of complete viral genomes. Figure 1 shows a schematic diagram of E7, including important functional domains as well as the mutations examined in this study, and a summary of previously reported effects of these mutations is shown in Table 1. For our analysis, we chose mutations in each of the characterized domains of E7 and in some cases made multiple mutations in the same domain. We also constructed an E7 knockout mutant genome consisting of three sequential stop codons at amino acids 9 to 11.
Fig. 1.
Study design. (a) The early region of HPV16 showing the long control region (LCR), early and late promoters, and the open reading frames for E6, E7, and the 5′ end of E1. The position of the LoxP site at nucleotide 7465 is indicated. Also shown is the segment of the genome used in the late promoter reporter used in Fig. 4 and 5. (b) Regions of E7 and mutations examined in this study. The CR1, CR2, and C-terminal domains are shown, along with the CKII phosphorylation site. Mutated amino acids are listed with their positions within E7. (c) Protocol for deriving HPV16 genome-containing cell lines. The HPV16 genome flanked by LoxP sites was transfected into HFK cells along with a Neo resistance plasmid and an expression vector for Cre recombinase. The expression of Cre resulted in the excision and recircularization of the HPV16 genome.
Table 1.
E7 mutants in this study
| Mutant | pRb binding | pRb degradation | HFK immortalization | Comment(s) | Reference |
|---|---|---|---|---|---|
| H2P | + | − | − | Defective for p300, p600, cul2 binding | 21, 22 |
| LYCYE (Δ22-26) | − | −− | − | Defective for pRb binding and degradation | 28 |
| DLYC (Δ21-24) | − | − | − | Defective for pRb binding and degradation | 21, 39 |
| C24G | − | − | + | Competent for p107/p130 binding and degradation | 7, 23, 39, 48 |
| E26G | − | − | + | Competent for p107/p130 binding and degradation | 10, 23, 39 |
| SS31, 32RP, SS31, 32AA | + | + | + | No effect on episomal maintenance (HPV31) | 10, 19, 24, 48 |
| SS31, 32DD | + | + | + | Reduced S phase in differentiation | 17, 19 |
| CVQ(68-70)AAA | + | + | − | Defective for E2F6 binding and abrogating p21-mediated growth arrest | 20, 21, 33 |
| L67R | + | + | − | Defective for binding HDACs, Mi2β, pCAF; no episomal maintenance (HPV31) | 6, 30, 42 |
| C91S, C91G | + | + | − | Defective for E2F6, M2-PK, HDAC binding; prevents episomal maintenance (HPV31) | 31, 6, 28, 23, 33 |
To investigate the effects of E7 in the differentiation-dependent phase of the viral life cycle, we first generated keratinocyte cell lines that stably maintain HPV16 episomes by the transfection of cloned viral genomes. Previous methods to generate cell lines that stably maintained HPV episomes required the excision of genomes from plasmid sequences followed by religation. We investigated the use of a Cre-Lox recombination system that eliminated the need for these two steps. Lee et al. first described the use of a Cre/Lox system to generate infectious HPV16 in organotypic rafts (27). We used a variation of their system to create cell lines containing episomal HPV16 genomes with mutations in E7 (Fig. 1c). The plasmid pEGFP Ni HPV16, created by Lee et al. in the process of cloning the HPV16 adenovirus vector, contains HPV16 (W12 variant) flanked by LoxP sites. This plasmid was cotransfected into primary human foreskin keratinocytes along with an expression vector for Cre recombinase and a neomycin resistance plasmid. Following transfection, cells were selected with G418, and the resulting resistant colonies were pooled and expanded. Using this system, we observed a high rate of success in deriving cell lines stably maintaining HPV16 episomes. For the following studies, each E7 mutant was introduced into HFKs from four to five different donors. The resultant cell lines also were checked by PCR and sequencing and were found to maintain the expected E7 mutations.
Immortalization and growth.
We first investigated if there were differences between the E7 mutant genomes in their ability to form colonies following transfection and selection with G418 (Table 2). Whereas wild-type viral genomes generated numerous colonies readily following selection, the E7 stop mutant genomes and others containing mutations in the pocket protein binding CR2 domain did not, or did so at very low levels. Only a few colonies containing mutant genomes survived selection, and these senesced within the first or second passage after transfection. Of the mutations in the CR2 domain, none grew sufficiently to generate enough material to study, with the exception of a single colony of the C24G mutant that continued to expand for several passages. In addition, we observed that cells transfected with H2P mutant genomes generated cell lines at a lower efficiency than the wild type.
Table 2.
Phenotype summary
| Mutant | Class | No. surviving selection | No. surviving to passage 10 | No. harbored episomally | No. producing late transcripts |
|---|---|---|---|---|---|
| No DNA | 0/5 | 0/5 | |||
| WT | 5/5 | 5/5 | 4/5 | 5/5 | |
| Stop (aa 9-11) | Knockout | 1/5 | 0/5 | ||
| H2P | CR1-pRb degradation | 3/5 | 2/5 | 1/2 | 2/2 |
| LYCYE | CR2-pRb binding | 2/5 | 0/5 | ||
| DLYC | CR2-pRb binding | 2/5 | 0/5 | 0/1 | |
| C24G | CR2-pRb binding | 1/5 | 1/5 | 1/1 | 0/1 |
| E26G | CR2-pRb binding | 1/5 | 0/5 | ||
| SSRP | CKII | 3/4 | 3/3 | 3/3 | 0/3 |
| SSAA | CKII | 5/5 | 5/5 | 4/5 | 3/4 |
| SSDD | CKII | 5/5 | 4/4 | 3/4 | 1/4 |
| CVQ | C terminus | 5/5 | 4/4 | 4/4 | 2/4 |
| L67R | C terminus | 4/5 | 4/4 | 4/4 | 3/4 |
| C91G | C terminus | 4/5 | 2/4 | 2/2 | 1/2 |
| C91S | C terminus | 4/5 | 4/4 | 4/4 | 4/4 |
Pooled cell lines that survived selection were grown for 10 passages, or approximately 33 population doublings, at which point we considered surviving cell lines to be immortalized. We also continued to grow a subset of these cell lines up to passage 40, which was more than 6 months in culture, or 130 population doublings. All of the cell lines that survived to passage 10 continued to grow to passage 40, except for cells with C24G mutant genomes, which senesced at approximately passage 15. Cells with genomes containing the H2P mutation, which inactivates the CR1 domain and is reported to be necessary for pRb degradation, produced colonies at a lower efficiency than the wild type, but the cell lines that grew out were immortal, indicating that pRb degradation is not strictly required for immortalization in the context of the complete viral genome. The H2P, C24G, and C91 mutants were noticeably slower in growth rate than the wild type (see Fig. S1 in the supplemental material). In contrast, several SSDD lines grew noticeably faster. We conclude that in the context of the complete viral genome, the CR2 domain of E7 is necessary for the immortalization of HFKs, while the other domains we examined were not.
Episomal maintenance of E7 mutant genomes.
It was important to determine whether E7 mutant genomes were maintained as episomes, as previous studies indicated that this was necessary for the activation of late gene expression upon differentiation (15). For this analysis, total DNA was isolated from cell lines grown in monolayer culture and analyzed by Southern blotting to determine the state of the viral DNA. Representative Southern blots are shown in Fig. 2. In almost all cases, cells that grew out to form a cell line harbored the viral genome episomally (Table 2) with some modest variations in copy number, as we have observed in HPV31-containing cell lines (J. Bodily and L. Laimins, unpublished data). None of the mutant genomes consistently exhibited a lower copy number than the wild type. One exception was the C24G line, which contained the viral genome episomally but at reduced levels. In addition, the copy number of the C24G genome gradually diminished at later passages approaching senescence (not shown). Given that viral genomes were episomal until senescence, we believe that the C24G mutation does not intrinsically inhibit episomal replication and acts primarily to limit the ability to extend life span. One of the two lines containing the H2P mutation was episomal, and the other was integrated. These data indicate that pRb binding and degradation are not strictly necessary for replication as an episome beyond the requirement for immortalization. We did not identify any other regions of E7 that were necessary for episomal maintenance.
Fig. 2.
Southern analysis of cell lines containing HPV16 mutants. Total DNAs from cells grown in monolayer (Mo) or MC were cut with an enzyme that does not digest the HPV16 genome (U) or cuts it once (C). They then were subjected to Southern blotting and probed with a probe consisting of the full HPV16 genome. Standards corresponding to 100, 10, or 1 copy of the HPV16 genome per cell are included.
In contrast to studies with HPV31 and HPV18, which readily amplify genomes following suspension in methylcellulose (MC), we have been unable to observe the amplification of HPV16 genomes in any HPV16 cell lines following suspension in methylcellulose (Fig. 2) or grown in raft cultures (not shown). In addition to the floxed HPV16 genomes used in this study, we obtained the same results using different HPV16 genotypes and using genomes prepared using the excision-religation protocol before transfection (not shown). Despite this deficiency, the HPV16 lines were able to activate late transcripts upon differentiation in methylcellulose and produce infectious virus particles following growth in organotypic rafts (see below). The activation of the late promoter occurs upon differentiation, and some reports indicate that genome amplification enhances the levels of late gene expression (46). The use of the HPV16 genomes therefore allowed us to examine the specific effects of E7 on the regulation of late gene expression in the absence of complicating events due to amplification or the use of drugs to inhibit amplification.
Activation of late transcription.
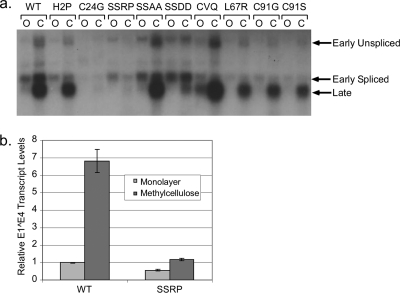
To investigate the effects of E7 on the activation of the HPV16 late viral promoter, total RNA was isolated from cells grown in monolayer or MC culture and examined by Northern blot analysis using a probe consisting of the complete viral genome (Fig. 3a). Cells containing wild-type viral genomes show a dramatic increase both in total transcript levels and in the levels of late spliced transcripts, as has been reported before (18). The C24G cell line, despite having episomal genomes, showed reduced total levels of viral transcripts and a reduced ability to increase the levels of these transcripts in MC culture. H2P, which inactivates the CR1 domain of E7 involved in pRb degradation, and mutations in the C terminus show the activation of viral late transcripts roughly equivalent to that of the wild type. Interestingly, the SSRP mutation in the casein kinase II (CKII) binding site was found to be defective for the activation of the late viral promoter (Fig. 3b). No E7 mutant genomes other than C24G and SSRP were consistently found to be defective for the activation of the late promoter upon differentiation.
Fig. 3.
Induction of late transcripts by mutant HPV genomes. (a) Total RNAs from cells grown in monolayer (O) or MC (C) were subjected to Northern blot analysis using a probe consisting of the full HPV16 genome. (b) Total RNAs from cells containing wild-type or SSRP mutant genomes were reverse transcribed and subjected to qPCR using primers that cover the E1̂E4 splice site. The data are the means from two separate HFK backgrounds and six PCRs. Bars represent ±1 standard error of the means.
SSRP mutation affects both E7 function and a cis element in the late promoter.
Genomes containing the SSRP mutation in the CKII phosphorylation site consistently exhibited a reduced ability to express high levels of late transcripts in response to differentiation (Fig. 3 and Table 2). We wished to understand the reason for this effect and considered two possibilities. One was that the E7 protein affects late transcription in trans and that the CKII phosphorylation site is important for that activity. Arguing against this possibility, cells containing genomes with the SSAA mutation, which also lacks the phosphoacceptor serines, were found not to be defective in the activation of late transcription. Moreover, the phosphomimetic SSDD mutant also was defective in activating late transcription. If the CKII site is important for the effects of the E7 protein on the late promoter, then the phosphomimetic and phosphorylation null mutants might be expected to have opposite effects, and certainly the SSAA and SSRP mutants should be similar in their behavior.
A second possibility was that by mutating the DNA sequence of the CKII phosphorylation site, we inadvertently altered a cis element in the differentiation-dependent HPV16 late promoter, which overlaps the E7 open reading frame. In generating the mutations in this study, we did not make any provision for avoiding promoter elements, primarily because little information is available on the identity of functional factor binding sites in this promoter. The analysis of the region containing the SSRP mutation did not identify any known transcription factor binding sites except for a previously noted CAATT motif located approximately 10 to 15 nucleotides upstream of the SSRP mutant sequence (Fig. 4a). To test whether the mutations we made in the CKII site affect late promoter activity in cis, a fragment of the HPV16 late promoter/E7 ORF, with or without the CKII mutations, was cloned into a luciferase reporter and transfected into cells harboring HPV16 episomes. These cells were chosen to ensure that wild-type E7 and all other viral factors that may be needed for late promoter activity would be present. Following transfection, the cells were divided and grown in monolayer culture or suspended in MC for 24 h. Luciferase activities then were measured, and the results of this analysis are shown in Fig. 4b. As expected, promoter activity was detected using the wild-type sequences, as shown by increased luciferase production relative to that of the empty vector. The activity of the wild-type reporter increased by approximately 2.5-fold in MC culture and is consistent with previous reports (5, 46). In contrast, each of the mutant reporters showed a defect in basal reporter activity as well as in the fold difference in activity between differentiated and undifferentiated conditions. The magnitude of the defects correlated with the reduced efficiency of late transcript induction in Northern blots (see Table 2).
Fig. 4.
cis element in the DNA sequence of the E7. (a) The DNA sequence and the changes made for each of the CKII mutations. The corresponding E7 amino acids are shown below the DNA sequence, including the LYCYE motif in the CRII domain. Also shown is a CCAAT sequence first identified in reference 26. (b) Inhibition of reporter activity by mutations in the CRII region. A luciferase reporter consisting of a portion of the HPV16 early region (Fig. 1) containing either the wild-type, mutant E7 sequence, or empty vector (V) was transfected overnight into cells containing HPV16 wild-type genome. The next day, cells were trypsinized and cultured in monolayer or suspended in MC. After 24 h, lysates were prepared and luciferase activities were measured. Each reading was normalized to the activity of Renilla luciferase and then to the activity of the wild-type reporter in monolayer. Each point represents the means from five experiments, and bars represent ±1 standard error of the means.
It was possible that the E7 open reading frame present in the luciferase reporter was expressed in transfected cells, and that the resultant wild-type or SSRP mutant E7 protein induced a trans effect on the reporter. We therefore created variants of the reporter containing stop codons early in the E7 open reading frame to preclude the cryptic production of E7 protein from the reporter construct. As shown in Fig. 5a, the same activity was seen with or without the E7 stop codons in both the wild-type and SSRP mutant reporters. Thus, the cryptic expression of E7 from the reporter does not influence reporter activity.
Fig. 5.
SSRP mutation of E7 does not act in trans on the late promoter. (a) Expression of E7 from the reporter is not a factor in reporter activity. Stop codons were engineered into either the SSRP or wild-type late promoter reporter at codons 9 to 11 of the E7 open reading frame. These plasmids were transfected overnight into HPV16 genome-containing cells, and cultures were assayed for luciferase activity as described for Fig. 4. Each point represents the means from seven experiments, and bars represent ±1 standard error of the means. (b) The expression of SSRP E7 does not affect reporter activity. The wild-type late promoter reporter was transfected into cells containing either wild-type HPV16 genomes (WT) or genomes containing the SSRP E7 mutation. Following transfection, luciferase activity was measured as described for panel a. Each point represents the means from 4 to 12 experiments, and bars represent ±1 standard error of the means. (c) The SSRP mutation affects binding of a protein complex to the DNA. Oligonucleotide probes consisting of the wild-type or SSRP sequence were allowed to bind to nuclear extracts from HPV16-containing cells in the presence or absence of a 50× excess of unlabeled wild-type or SSRP competitor oligonucleotide. Following electrophoresis and drying, the gel was subjected to autoradiography. NS, nonspecific complex; FP, free probe.
The experiments described above were performed in HPV-positive cells containing wild-type E7. We next investigated if the SSRP mutant E7 protein had a trans effect on late promoter activity by performing transient reporter assays in cells containing SSRP mutant genomes. We transfected cells that stably maintained the SSRP mutant genomes with the (wild-type) late promoter luciferase reporter and measured activity in monolayer and MC. As shown in Fig. 5b, the activity of the reporter in SSRP mutant cells was increased in MC at a level comparable to that of the wild type, indicating that the failure of SSRP mutant genomes to activate the late promoter was not due to an absence of a necessary trans factor in the SSRP mutant cell lines. These data rule out an effect of the SSRP mutation on the late promoter in trans and strongly suggest that the SSRP mutation affects a cis element. We next performed EMSA using nuclear extracts of differentiated HPV16-positive cells and detected a protein complex that formed on a probe with the wild-type sequence but was reduced on a mutant probe (Fig. 5c, open arrow). Wild-type competitor oligonucleotides could compete with the complex from the wild-type probe but the mutant competitor could not, whereas both wild-type and mutant competitor could compete with the complex from the mutant probe. This may be due either to the binding of two distinct complexes to the two probes or the same complex binding to both probes but with lower affinity to the mutant probe. This complex was less abundant in extracts from cells grown in MC (not shown). The identities of the proteins in this complex remain to be determined but are beyond the scope of this study.
Effects of E7 mutations on levels of cell cycle regulators following differentiation.
Another important activity of E7 in differentiating cells is the modulation of the expression of cell cycle regulators, such as pRb family members as well as cyclins and cyclin-dependent kinases. We next examined whether these activities were altered in cells containing mutant HPV16 genomes. We first examined the levels of E7 by Western analysis to determine if these changed upon differentiation. In our studies, a consistent reduction in E7 levels was observed upon differentiation in MC. In addition, the levels of some E7 mutants were reduced, which is consistent with the observations of others that these mutations reduce the stability of the E7 protein (31). We next examined the level of pRb family members in undifferentiated and differentiated cells to see how these were influenced by mutations in E7. In undifferentiated cells, we found that the levels of pRb were similar between HFKs and HPV16 wild-type-containing cells (Fig. 6). This lack of an effect of the viral genome on pRb levels was seen consistently and may reflect the low levels of E7 expressed from the episome compared to those of retroviral or other expression systems that have been used in other studies. Despite the lack of an effect of E7 on pRb in undifferentiated cells, pRb levels were observed to be significantly lower in HPV-containing cells than in HFKs following differentiation in MC. This is consistent with the idea that one important function of E7 is to prevent differentiation-dependent cell cycle withdrawal, and so E7's activities may be more apparent in differentiated conditions (13, 32). The C24G and H2P mutants of E7 were unable to mediate this differentiation-dependent reduction in pRb levels, which is consistent with the known defects of these mutants in mediating pRb degradation. The CKII phosphorylation mutants had an intermediate phenotype, with SSAA and SSRP degrading pRb in MC in approximately half of the experiments. SSDD and the C-terminal mutants showed reduced pRb levels in about two-thirds of the experiments. In the case of the C-terminal mutants, reduced pRb degradation efficiency may have been due to reduced E7 expression. E7 in the context of the complete genome had minimal effects on p107 and p130 in undifferentiated or differentiated cells (see Fig. S2 in the supplemental material).
Fig. 6.
Effect of E7 mutants on levels of pRb. Total lysates made from cells grown in monolayer (O) or MC (C) culture were subjected to SDS-PAGE and probed using the indicated antibodies.
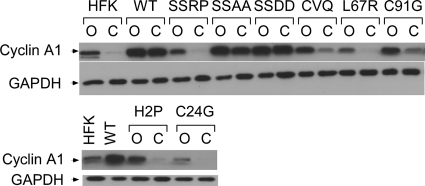
Progression through the cell cycle is regulated largely by the interaction of cyclins with cyclin-dependent kinases, so we next examined whether the expression of cyclins also was affected by the mutation of E7. As shown in Fig. 7, HPV-positive cells exhibited higher levels of cyclin A1 than HFKs in both undifferentiated and differentiated cells. This difference was more pronounced upon differentiation. Furthermore, the C-terminal mutations along with SSRP exhibited reduced levels of cyclin A1. Similar effects were seen with cyclins B1 and D1, but no differences were seen in the levels of cyclin E1 (see Fig. S3 in the supplemental material). The differences exhibited by the E7 mutant genomes in the protein levels of the various cyclins also was reflected in the levels of their transcripts, with the exception of cyclin D1, whose transcript levels did not differ significantly between the wild type and any of the mutants (not shown). Similar patterns of expression were seen for other cell cycle-related factors, such as PCNA, MCM7, and cdc25C (see Fig. S3). The levels of these cell cycle factors roughly correlated with the growth rate of the cells containing the mutant genomes. In the case of the C-terminal mutants, it is unclear whether lower levels of cyclins were due to the defective activity of the E7 mutant protein or to the lower levels of E7 seen in these lines. However, the reduction in the levels of these cell cycle factors did not seem to have a negative impact on the immortalization of the C-terminal mutant cells but may be related to the slower growth rate of these mutants.
Fig. 7.
Effect of E7 mutants on levels of cyclin A. Total lysates made from cells grown in monolayer (O) or MC (C) culture were subjected to SDS-PAGE and probed using the indicated antibodies.
SSRP is defective in inducing S phase factors.
As discussed above, cells containing SSRP mutant genomes were less efficient in inducing cyclins and S phase factors. Our previous analyses identified a cis element in the late promoter that also was affected by this mutation, and it thus was possible that some product of the late promoter, perhaps E1̂E4 or E5, is important for supporting high levels of cyclin A rather than the altered activity of E7 protein. To eliminate any possible effects due to the expression of E1̂E4 or E5, we created retroviral vectors expressing wild-type or mutant E7 in the absence of other HPV gene products. Following the infection of HFKs and selection, the levels of cyclin A were determined using Western blot analysis. We found that levels of cyclin A were reduced in cells expressing SSRP, SSAA, and SSDD mutant E7s (Fig. 8). Similar reductions were seen in levels of PCNA and MCM7 (not shown). Importantly, levels of wild-type and mutant E7s themselves were comparable as measured by Western blotting (Fig. 8). Given that there were no other HPV products expressed in these cells, this result indicates that the mutation of the CKII region creates a defect in the E7 protein that prevents high-level expression of S phase factors.
Fig. 8.
CKII mutants affect the ability of E7 protein to induce cyclin A. HFKs were infected with retroviral vectors encoding wild-type or CKII mutant E7s. Following selection, cells were expanded. Total lysates made from cells grown in monolayer (O) or MC (C) culture were subjected to SDS-PAGE and probed using the indicated antibodies.
Growth and production of infectious virions in raft culture.
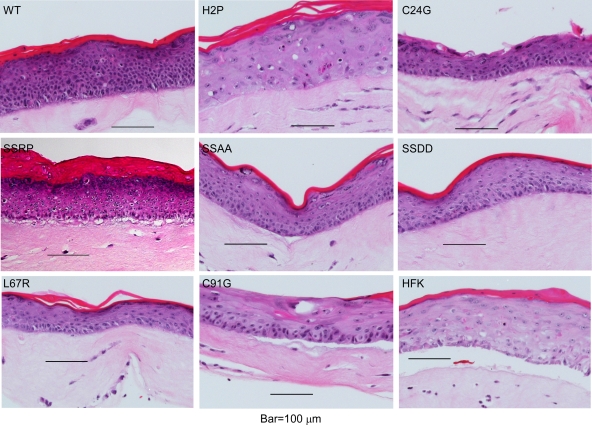
We next examined the growth of cell lines containing wild-type and mutant HPV16 genomes in raft cultures. As expected, cells containing wild-type HPV16 showed a very proliferative growth pattern, with nuclei retained into the differentiated layers (Fig. 9). Some variation in raft morphology was seen between the mutant cell lines, but the most consistent finding was that the growth of C91G/C91S was disrupted, with a reduced number of cells and less cornification than that of the wild type. Notably, the growth and stratification of these cells was less than that of HFKs, suggesting that the absence of these E7 functions causes a detrimental effect on differentiating cells.
Fig. 9.
Growth of wild-type and mutant cell lines in organotypic cultures. Organotypic cultures of each cell line were prepared and allowed to grow for 10 days. Tissues were harvested, fixed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Bars represent 100 μm.
To determine whether mutations in E7 would affect the production of infectious virus particles, we grew raft cultures for either 10 or 20 days and generated putative viral particle preparations. We analyzed these preparations in two ways. First, total levels of viral genome-containing particles (titers) were quantified by digesting unencapsidated DNA with benzonase and then determining the levels of encapsidated genomes using qPCR. This gives a measure of the efficiency of the mutant rafts in producing particles. The levels of particles isolated from SSRP mutant rafts were lower than that for the wild type (Fig. 10a), as would be expected with reduced late promoter activity and consequently less expression of L1 and L2. Several other mutants, including C24G, H2P, and L67R, did not yield genome-containing particles in appreciable amounts, whereas others, such as SSAA, SSDD, and CVQ, yielded particles at reduced levels compared to those of the wild type (see Fig. S4 in the supplemental material). To determine whether the particles produced by mutant cell lines were capable of initiating infection (infectivity), preparations containing equal numbers of viral genome equivalents were used to infect HaCaT cells for 48 h. Levels of E1̂E4 transcripts then were measured using quantitative RT-PCR (qRT-PCR) as a marker for the initiation of viral transcription. Although SSRP rafts produced virus particles at a reduced level, these particles were just as infectious as the wild type on a per-particle basis (Fig. 10b), which is consistent with the idea that the SSRP mutant affects events late in the life cycle but not the early events measured in the infectivity assay. SSAA, SSDD, and CVQ particles also were infectious at a level comparable to that of the wild type (see Fig. S4 in the supplemental material). Insufficient amounts of particles were produced from C24G, H2P, and L67R cell lines to reliably demonstrate infectivity. Although the titer of particles produced by the C91S cell line was low, these particles nonetheless were infectious (see Fig. S4).
Fig. 10.
Titer and infectivity of SSRP virus particles. Raft cultures of each cell line were grown for 10 or 20 days in the presence of the PKC activator C8. Tissues were harvested and putative viral stocks were generated. (a) The quantity of viral genome-containing particles (VGE) in each stock was measured by qPCR and normalized to the wild type. Only the values for 10-day rafts are shown. Values for two separate SSRP cell lines are shown. (b) Equal numbers of viral genome-containing particles were added to HaCaT cells. Following incubation for 48 h, total RNAs were isolated and analyzed for the expression of E1̂E4 transcripts using qRT-PCR. Values were normalized to TBP as an internal control and then to the value of wild-type virus isolated from 10-day rafts.
DISCUSSION
Our studies demonstrate that HPV 16 E7 protein has important activities in the viral life cycle in both differentiated and undifferentiated cells. In undifferentiated cells, we found that the mutation of the pRb binding site abrogated immortalization, indirectly affecting the ability to maintain episomes, which is consistent with previous reports (48). We were surprised to observe, however, that mutations in the histone deacetylase (HDAC) binding C terminus of HPV16 E7 (L67R, C91S, and C91G) were not defective in episomal maintenance or immortalization. This is in contrast to a previous report from our laboratory using HPV31, in which the L67R and C91G mutations were found to interfere with both episomal maintenance and immortalization (28). We do not understand the basis of this difference between the virus types. We speculate that HDAC binding by E7 serves a more important purpose in the HPV31 life cycle than in that of HPV16. It also is possible that the difference is due not to a property of E7 but to differences in E6 between the two virus types. The strong HPV16 E6 oncogene may be able to compensate for mutations in E7 by providing some immortalizing functions that HPV31 E6 cannot. We have previously observed that HPV16 E6 induces the efficient degradation of p53, while this is not seen with HPV31 E6 (unpublished data). These findings illustrate the importance of examining the functions of viral proteins in the context of the complete genome in which all of the viral factors are present, and that there are significant differences between high-risk types.
Our studies relied on the generation of keratinocyte cell lines that stably maintained HPV16 episomes, and for this we used a Cre-Lox system for the transfection of cloned genomes. The incorporation of this change eliminated the need for two steps in the original protocol that required excision from plasmid sequences and religation. The number of G418 colonies that arose following the transfection of Cre-Lox genomes was comparable (within 2-fold) to the number of colonies generated using the previous protocol. Interestingly, using both transfection protocols, we observed that although HPV16 genomes were readily established as episomes and activated high levels of late transcripts upon differentiation, they failed to amplify. This contrasts with similar studies with HPV31, HPV16, or HPV18 genomes, where both amplification and late gene induction were readily seen. Failure to amplify was not specific to methylcellulose-induced differentiation, as similar effects were seen in the Southern analysis of DNA from organotypic raft cultures. We tested different variant genotypes of HPV16 and found the same results for each. The amplification of HPV16 genomes is seen in biopsy samples as well as with cell lines containing HPV16 episomes (11, 13, 14, 16), although the number of cells supporting amplification in HPV16-containing lesions is few (36, 41). Despite the lack of significant amplification by Southern blotting, our transfected cell lines generated infectious virus particles (see below). This was an unexpected result that suggests that the abundance of viral genome copies is not necessarily limiting for virion production, which is consistent with previous observations in HPV31 that virion production can occur at high levels without concomitant viral genome amplification (3) and that the induction of suprabasal DNA synthesis by HPV16 E7 is not necessary for E1̂E4 or L1 protein expression (8). Furthermore, the lack of amplification with HPV16-positive cell lines allowed us to examine the role of E7 in activating late gene expression without the potentially complicating effects of increases in genome copy number.
The SSRP mutation of E7 is responsible for both a cis defect in the late promoter and a defect in the ability of the E7 protein to induce the expression of S phase factors in trans. The existence of cis activities in the E7 reading frame also was suggested by studies by McLaughlin-Drubin et al. in which mutations in the HPV18 E7 ORF could not be fully complemented in trans by retrovirus vectors expressing E7 (32). The investigation of the mechanisms and cellular factors that regulate the activation of late viral gene expression has been limited, and no sequences in the context of complete genomes have been identified to be necessary for this activity. Our study identified the first such factor binding site, corresponding to sequences around the CKII phosphorylation motif in the E7 ORF. The nucleotide sequence of this region is well conserved between HPV types 16, 31, and 11 (see Fig. S5 in the supplemental material). Computer analysis of this site did not identify any known transcription factor-binding motifs. A CAATT motif is found 10 to 20 nucleotides upstream of this region, but whether this site is functionally important or is affected by the SSRP mutation is unknown. Preliminary gel shift experiments using antibodies to C/EBP failed to show that this factor is part of the complex bound to the CKII motif (not shown). The major start site for the late promoter (around nucleotide 670) is very close to the mutated region, so perhaps the binding of the core transcription machinery is affected. Further analysis will be required to identify the binding factors, but this is beyond the scope of this study.
We investigated the ability of E7 to alter the expression and/or levels of important cell cycle regulators. While E7 has been shown to induce the rapid degradation of pRb in overexpression assays, we found significant effects of E7 on pRb levels only in differentiating cells. Since the levels of E7 also decreased upon differentiation, more complex mechanisms must be involved in regulating pRb levels than simply the recruitment of degradation factors. We found minimal effects of E7 on p130 and p107 levels, in contrast to previous reports that suggested a specific effect on p130 degradation (50). This difference might be due to the viral types studied or the different methods used. Our studies showed that E7 proteins containing mutations in the CKII site in the context of the complete genome were defective for the enhanced expression of cyclin A and D1 in differentiating cells (SSRP < SSAA and SSDD). Since these mutations had no trans effects on the induction of the late viral promoter, the ability to support late promoter activity and the ability to induce cyclin expression are not necessarily linked.
The ultimate test of the viral mutants is the ability to generate virions. Interestingly, we observed that cell lines with episomal HPV16 were able to synthesize virions despite the lack of significant differentiation-dependent genome amplification as measured by Southern blot analysis. Although raft cultures can be variable, as is typical for this technology, some conclusions can be drawn about the role of E7 in virus production. All of our mutant genomes were reduced to some degree in their ability to generate encapsidated virus particles. This suggests that different domains of E7 play distinct roles in creating a cellular environment suitable for late viral activities in differentiating keratinocytes. However, despite fewer virus particles, all mutants whose particle yields were high enough to assay were shown to be infectious. This is consistent with the idea that E7 acts primarily in later stages of the life cycle while having less of a role in the initial establishment of infection, which is the parameter measured by the infectivity assay. The mutation of the zinc finger domain of E7 clearly altered the growth of cells in raft culture in addition to inhibiting the production of virus particles. Whether the failure of these mutants to produce infectious particles was due to some activity of E7 necessary for virion assembly or to the general disruption of cell growth and differentiation is not clear. Interestingly, the SSAA mutant cell lines were able to produce reasonably high yields of infectious virus particles compared to the wild type, indicating that phosphorylation by CKII is not necessary for infectious HPV16 virion production under our conditions. It is possible that phosphorylation at this site is important in modulating the response to some stress signal present in vivo, such as hypoxia or immune responses, that are not modeled in our system.
In conclusion, we have found that different domains of HPV16 E7 contribute to the viral life cycle at various stages. The pocket protein-binding and degradation ability of E7 was needed early in the life cycle for efficient immortalization and maintenance of the episome. The C-terminal zinc finger domain was not necessary for early viral events but was needed late in the life cycle for normal tissue growth and production of virions. Although the CKII phosphorylation of E7 was not needed at any stage of the life cycle, we found a cis element in this area of the E7 coding region that was necessary for late promoter activity, which is the first such element identified in the context of the complete viral genome. Finally, several mutants affected the yield of virus particles produced, but the infectivity of these particles generally was unaffected. These results shed light on the complex and overlapping activities of the E7 region in the differentiation-dependent life cycle of HPV16.
Supplementary Material
ACKNOWLEDGMENTS
We thank Greg Smith (Northwestern University School of Medicine) for the pBS Cre plasmid and John Lee (Sanford School of Medicine Sioux Falls, SD) for the pEGFP Ni HPV16 plasmid. Sequencing services were performed at the Northwestern University Genomics Core Facility, and the Northwestern University Pathology Core Facility performed tissue processing and staining.
This work was supported by grants from the National Cancer Institute to L.A.L.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Ai W., Narahari J., Roman A. 2000. Yin yang 1 negatively regulates the differentiation-specific E1 promoter of human papillomavirus type 6. J. Virol. 74:5198–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ai W., Toussaint E., Roman A. 1999. CCAAT displacement protein binds to and negatively regulates human papillomavirus type 6 E6, E7, and E1 promoters. J. Virol. 73:4220–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alam S., Conway M. J., Chen H. S., Meyers C. 2008. The cigarette smoke carcinogen benzo[a]pyrene enhances human papillomavirus synthesis. J. Virol. 82:1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bodily J. M., Mehta K. P., Laimins L. A. 2011. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Res. 71:1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodily J. M., Meyers C. 2005. Genetic analysis of the human papillomavirus type 31 differentiation-dependent late promoter. J. Virol. 79:3309–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brehm A., et al. 1999. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 18:2449–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chien W. M., Parker J. N., Schmidt-Grimminger D. C., Broker T. R., Chow L. T. 2000. Casein kinase II phosphorylation of the human papillomavirus-18 E7 protein is critical for promoting S-phase entry. Cell Growth Differ. 11:425–435 [PubMed] [Google Scholar]
- 8. Collins A. S., Nakahara T., Do A., Lambert P. F. 2005. Interactions with pocket proteins contribute to the role of human papillomavirus type 16 E7 in the papillomavirus life cycle. J. Virol. 79:14769–14780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conway M. J., et al. 2009. Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions. J. Virol. 83:10515–10526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demers G. W., Espling E., Harry J. B., Etscheid B. G., Galloway D. A. 1996. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 70:6862–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doorbar J., et al. 1997. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology 238:40–52 [DOI] [PubMed] [Google Scholar]
- 12. Fehrmann F., Klumpp D. J., Laimins L. A. 2003. Human papillomavirus type 31 E5 protein supports cell cycle progression and activates late viral functions upon epithelial differentiation. J. Virol. 77:2819–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flores E. R., Allen-Hoffmann B. L., Lee D., Lambert P. F. 2000. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J. Virol. 74:6622–6631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flores E. R., Allen-Hoffmann B. L., Lee D., Sattler C. A., Lambert P. F. 1999. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 262:344–354 [DOI] [PubMed] [Google Scholar]
- 15. Frattini M. G., Lim H. B., Laimins L. A. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. U. S. A. 93:3062–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garner-Hamrick P. A., Fisher C. 2002. HPV episomal copy number closely correlates with cell size in keratinocyte monolayer cultures. Virology 301:334–341 [DOI] [PubMed] [Google Scholar]
- 17. Genovese N. J., Banerjee N. S., Broker T. R., Chow L. T. 2008. Casein kinase II motif-dependent phosphorylation of human papillomavirus E7 protein promotes p130 degradation and S-phase induction in differentiated human keratinocytes. J. Virol. 82:4862–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grassmann K., Rapp B., Maschek H., Petry K. U., Iftner T. 1996. Identification of a differentiation-inducible promoter in the E7 open reading frame of human papillomavirus type 16 (HPV-16) in raft cultures of a new cell line containing high copy numbers of episomal HPV-16 DNA. J. Virol. 70:2339–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heck D. V., Yee C. L., Howley P. M., Munger K. 1992. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. U. S. A. 89:4442–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helt A. M., Funk J. O., Galloway D. A. 2002. Inactivation of both the retinoblastoma tumor suppressor and p21 by the human papillomavirus type 16 E7 oncoprotein is necessary to inhibit cell cycle arrest in human epithelial cells. J. Virol. 76:10559–10568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helt A. M., Galloway D. A. 2001. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J. Virol. 75:6737–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huh K. W., et al. 2005. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc. Natl. Acad. Sci. U. S. A. 102:11492–11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jewers R. J., Hildebrandt P., Ludlow J. W., Kell B., McCance D. J. 1992. Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J. Virol. 66:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones D. L., Thompson D. A., Munger K. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97–107 [DOI] [PubMed] [Google Scholar]
- 25. Kukimoto I., Kanda T. 2001. Displacement of YY1 by differentiation-specific transcription factor hSkn-1a activates the P(670) promoter of human papillomavirus type 16. J. Virol. 75:9302–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kukimoto I., Takeuchi T., Kanda T. 2006. CCAAT/enhancer binding protein beta binds to and activates the P670 promoter of human papillomavirus type 16. Virology 346:98–107 [DOI] [PubMed] [Google Scholar]
- 27. Lee J. H., et al. 2004. Propagation of infectious human papillomavirus type 16 by using an adenovirus and Cre/LoxP mechanism. Proc. Natl. Acad. Sci. U. S. A. 101:2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Longworth M. S., Laimins L. A. 2004. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J. Virol. 78:3533–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longworth M. S., Laimins L. A. 2004. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 68:362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Longworth M. S., Wilson R., Laimins L. A. 2005. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 24:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McIntyre M. C., Frattini M. G., Grossman S. R., Laimins L. A. 1993. Human papillomavirus type 18 E7 protein requires intact Cys-X-X-Cys motifs for zinc binding, dimerization, and transformation but not for Rb binding. J. Virol. 67:3142–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLaughlin-Drubin M. E., Bromberg-White J. L., Meyers C. 2005. The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology 338:61–68 [DOI] [PubMed] [Google Scholar]
- 33. McLaughlin-Drubin M. E., Huh K. W., Munger K. 2008. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. J. Virol. 82:8695–8705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyers C. 1996. Organotypic (raft) epithelial tissue culture system for the differentiation-dependent replication of papillomavirus. Methods Cell Sci. 18:201–210 [Google Scholar]
- 35. Meyers C., Laimins L. A. 1994. In vitro systems for the study and propagation of human papillomaviruses. Curr. Top. Microbiol. Immunol. 186:199–215 [DOI] [PubMed] [Google Scholar]
- 36. Middleton K., et al. 2003. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J. Virol. 77:10186–10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Münger K., et al. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Münger K., et al. 2001. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888–7898 [DOI] [PubMed] [Google Scholar]
- 39. Münger K., et al. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakamura M., et al. 2009. Hypoxia-specific stabilization of HIF-1alpha by human papillomaviruses. Virology 387:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peh W. L., et al. 2002. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 76:10401–10416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phelps W. C., Munger K., Yee C. L., Barnes J. A., Howley P. M. 1992. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J. Virol. 66:2418–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rohlfs M., Winkenbach S., Meyer S., Rupp T., Durst M. 1991. Viral transcription in human keratinocyte cell lines immortalized by human papillomavirus type-16. Virology 183:331–342 [DOI] [PubMed] [Google Scholar]
- 44. Ruesch M., Stubenrauch F., Laimins L. 1998. Activation of papillomavirus late gene transcription and genome amplification upon differentiation in semisolid medium is coincident with expression of involucrin and transglutaminase but not keratin 10. J. Virol. 72:5016–5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schreiber E., Matthias P., Muller M. M., Schaffner W. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spink K. M., Laimins L. A. 2005. Induction of the human papillomavirus type 31 late promoter requires differentiation but not DNA amplification. J. Virol. 79:4918–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steele B. K., Meyers C., Ozbun M. A. 2002. Variable expression of some “housekeeping” genes during human keratinocyte differentiation. Anal. Biochem. 307:341–347 [DOI] [PubMed] [Google Scholar]
- 48. Thomas J. T., Hubert W. G., Ruesch M. N., Laimins L. A. 1999. Human papillomavirus type 31 oncoproteins E6 and E7 are required for the maintenance of episomes during the viral life cycle in normal human keratinocytes. Proc. Natl. Acad. Sci. U. S. A. 96:8449–8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson R., Laimins L. A. 2005. Differentiation of HPV-containing cells using organotypic “raft” culture or methylcellulose. Methods Mol. Med. 119:157–169 [DOI] [PubMed] [Google Scholar]
- 50. Zhang B., Chen W., Roman A. 2006. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc. Natl. Acad. Sci. U. S. A. 103:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. zur Hausen H. 1996. Papillomavirus infections–a major cause of human cancers. Biochim. Biophys. Acta 1288:F55–F78 [DOI] [PubMed] [Google Scholar]
- 52. zur Hausen H. 1999. Papillomaviruses in human cancers. Proc. Assoc. Am. Physicians 111:581–587 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.