Abstract
Constitutive activation of NF-κB signaling is a key event in virus- and non-virus-induced carcinogenesis. We have previously reported that cutaneous human papillomavirus type 38 (HPV38) displays transforming properties in in vitro and in vivo experimental models. However, the involvement of NF-κB signaling in HPV38-induced cell growth transformation remains to be determined. In this study, we showed that HPV38 E6 and E7 activate NF-κB and that inhibition of the pathway with the IκBα superrepressor sensitizes HPV38E6E7-immortalized human keratinocytes to tumor necrosis factor alpha (TNF-α)- and UVB radiation-mediated apoptosis. Accordingly, inhibition of NF-κB signaling resulted in the downregulation of NF-κB-regulated antiapoptotic genes, including cIAP1, cIAP2, and xIAP genes. These findings demonstrate a critical role of NF-κB activity in the survival of HPV38E6E7-immortalized human keratinocytes exposed to cytokine or UV radiation. Our data provide additional evidence for cooperation between beta HPV infection and UV irradiation in skin carcinogenesis.
INTRODUCTION
Human papillomaviruses (HPVs) are small double-stranded DNA viruses that infect epithelial cells of different anatomical regions (16). More than 100 HPV types have been isolated so far, and they can be classified as mucosal or cutaneous types according to their abilities to infect the mucosa of the genital and upper respiratory tracts or the skin, respectively. A subgroup of mucosal HPVs, referred to as high-risk (HR) HPV types, are the causal agents for the development of cervical cancer and other anogenital malignancies (16, 43). A vast number of studies on the HR HPV type 16 (HPV16) and others have clearly shown that the products of two early genes, E6 and E7, play a critical role in HPV-mediated carcinogenesis by altering the regulation of key cellular events, such as the cell cycle, DNA repair, apoptosis, and senescence (41). HPV16 E6 and E7 exert their transforming activities by targeting the products of tumor suppressor genes. E6 promotes the degradation of p53, affecting DNA repair and apoptosis, while E7 inactivates retinoblastoma protein (pRb) and its related proteins p107 and p130, leading to cell cycle deregulation (37, 42).
In addition to the mucosal HR HPV types, the cutaneous HPV types belonging to the genus beta of the HPV phylogenetic tree are also suspected to play a role in the development of human cancer (16). The beta HPV types were initially isolated from nonmelanoma skin cancers (NMSCs) of patients affected by a cancer-prone genetic disease called epidermodysplasia verruciformis (EV) (47). However, it is now clear that these HPV types can also be found in NMSCs of non-EV patients, in particular in immunosuppressed individuals (6). Similar to the HR mucosal HPV types, E6 and E7 from certain beta HPV types target p53- and pRb-regulated pathways and display transforming activities in in vitro and in vivo experimental models (1, 10, 17, 21, 38, 40, 48, 51). In particular, E6 and E7 from the beta HPV38 are able to immortalize the human keratinocytes that are the natural hosts of the virus (1, 10, 21).
NF-κΒ is composed of a homo- or heterodimer of proteins that belong to the NF-κΒ/REL family of transcription factors (27), and NF-κB can be activated in two distinct pathways, referred to as the canonical and noncanonical NF-κΒ pathways. In the canonical pathway, the prototypical NF-κΒ, with a p65 (RELA)/p50 heterodimer, is kept inactive in the cytoplasm of quiescent cells by its inhibitor, IκBα (27). Stimulation by proinflammatory cytokines or many other NF-κB-inducing agents leads to the activation of the IκB kinase (IKK) complex, consisting of kinase subunits IKKα and IKKβ and the regulatory subunit IKKγ, or NEMO, which then phosphorylates IκBα at two N-terminal serine residues to promote its polyubiquitination and degradation. Free NF-κB then accumulates in the nucleus to activate target genes that play an important role in cell survival, cell proliferation, and the immune system (27). The noncanonical pathway is triggered by specific ligands, such as CD40 ligand/lymphotoxin β or BAFF, and is mediated through NIK-induced activation of IKKα, phosphorylation and processing of p100 to p52, and nuclear translocation of the p52/RelB complex for gene activation (12, 13, 14). In certain cell types, activation of NF-κB by UV radiation can also occur via an IKK-independent pathway that involves casein kinase 2 (CK2)-mediated phosphorylation of IκBα at its C terminus, followed by its degradation to liberate NF-κB for nuclear entry and transcriptional gene activation (30).
Several oncogenic viruses, including Epstein-Barr virus (EBV), human herpesvirus 8 (HHV-8), and human T-cell leukemia virus 1 (HTLV-1), as well as HR mucosal HPV types, are able to activate NF-κB, which in turn promotes cellular proliferation, immortalization, and transformation via upregulation of NF-κB-responsive genes (5, 9, 20, 23, 25, 26, 31, 44).
In contrast to the mucosal HR HPV types, it is not known whether beta HPV types have the ability to target NF-κB pathways or whether this event plays a role in beta HPV-mediated cellular immortalization and transformation.
In the present study, we show that E6 and E7 of HPV38 are potent activators of NF-κB. Most importantly, this event appears to be important for protecting HPV38-immortalized keratinocytes against apoptosis induced by tumor necrosis factor alpha (TNF-α) and/or UV.
MATERIALS AND METHODS
Plasmid constructs.
The retroviral expression of pLXSN-HPV38E6E7, pLXSN-HPV38E6, and pLXSN-HPV38E7 has been described previously (1, 10). pLXSN-HPV10E6, pLXSN-HPV10E7, pLXSN-HPV20E6, and pLXSN-HPV20E7 were generated by molecular cloning. The expression plasmid pcDNA3-CYLD was obtained from G. Mosialos (School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece). pcDNA3-myc-p100 was obtained from S. Ley (NIMR, London, United Kingdom). p-EGFP-C1 was obtained from BD Clontech (Palo Alto, CA). pcDNA3-LMP1 and anti-LMP1 antibody were obtained from E. Kieff (Harvard Medical School, Boston, MA). The expression retrovirus vector containing the NF-κB superrepressor ΔN-IκBα, which lacks the sequence that codes for the first 36 N-terminal amino acids (pBabe-puro-ΔN-IκBα), was generated by introduction of the PCR-amplified DNA fragment from pcDNA3-Flag-IκBα (obtained from T. Gilmore, Boston University, Boston, MA, and E. Kieff, Harvard Medical School, Boston, MA) into BamHI and EcoRI sites of pBabe-puro. The construct was verified by sequencing. Bay-11 was purchased from Calbiochem, San Diego, CA.
Cell culture and transfection.
Human embryonic kidney 293 (HEK293) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (45). Primary human foreskin keratinocytes (HFKs) or human foreskin keratinocytes immortalized by HPV38 E6 and E7 oncogenes (HFK 38 E6E7) were cultured as previously described (1, 10). Cell transfection was performed using the Fugene transfection kit (Roche, Indianapolis, IN) according to the manufacturer's instructions. In all cases, total DNA levels were normalized with empty vector. Small interfering RNA (siRNA) for p65 (siGenome Smart pool M-003533-02-0005), human RELA (NM_021975; Dharmacon, Lafayette, CO), or scrambled RNA used as a control (Ambion, Austin, TX) was transfected using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Retroviral infection.
The generation of high-titer retroviral stocks (>5.0 × 106 IU/ml) by transfection of Phoenix cells, the infection of human keratinocytes, and the establishment of immortalized human keratinocytes were performed as previously described (1).
Cell proliferation assay.
To assess cell proliferation, HFK 38 E6E7 and HFK 38 E6E7-ΔNIκBα cells were seeded at a density of 2 × 103/cm2 in 6-cm dishes. After 24 h, the cells were incubated with or without TNF-α (10 ng/ml) in combination or not with Bay11-7082 (1 μM). The media were then changed every 48 h, and the cells were counted at regular intervals as indicated.
Luciferase reporter assay.
Cells in six-well plates were transfected with NF-κB-dependent firefly luciferase reporter construct (500 ng) together with a Renilla luciferase construct (50 ng). Forty-eight hours posttransfection, the cells were incubated with or without TNF-α (10 ng/ml) for 6 h. Whole-cell extracts were prepared, and the firefly, as well as the Renilla, luciferase activities were measured according to the protocol for the dual-luciferase system (Promega, Madison, WI).
TNF-α stimulation, UV light irradiation, and FACS analysis.
Cells were incubated with TNF-α (10 ng/ml), and the stimulation was terminated by replacing the medium with cold phosphate-buffered saline (PBS). UV irradiation was performed with UV-Bio-Spectra (Vilber Lourmat, Marne-La-Vallée, France). For irradiation, the medium was removed and replaced with PBS. The cells were irradiated with 80 mJ/cm2 of UV radiation at 312 nm. After irradiation, fresh medium was added to the cells. To assess survival by fluorescence-activated cell sorting (FACS), cells were seeded in 6-cm dishes. After 24 h, they were irradiated with or without UV (312 nm; 80 mJ/cm2) or not irradiated and incubated with or without TNF-α (10 ng/ml). Cells were then collected, washed twice with PBS, and fixed with 70% ethanol on ice for 30 min. Approximately 106 cells were washed with PBS and resuspended in 500 μl of PBS containing RNase A (0.1 mg/ml) and propidium iodide (5 μg/ml). After incubation for 30 min on ice, the percentage of apoptotic cells was analyzed with a Becton Dickinson FACSort to determine the percentage of sub-G0 cell population using the Cell Quest analysis software package.
Cell fractionation.
Cytoplasmic and nuclear extracts were prepared using the Panomics nuclear extraction kit (Panomics, Inc., Fremont, CA) according to the manufacturer's instructions. Proteins in different cellular fractions were analyzed by immunoblotting.
Antibodies, immunoblotting, and immunofluorescence.
Immunoblotting was performed as previously described (45). Briefly, cells were lysed in cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 3% glycerol, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM sodium fluoride, 1 mM sodium orthovanadate) for 30 min on ice. Insoluble material was removed by centrifugation at 14,000 × g for 15 min. Whole-cell extracts were analyzed by immunoblotting. The antibodies used were as follows: p65, phospho-p65, phospho-IκBα, p100, p52, PARP (Cell Signaling), β-tubulin, anti-Flag (Sigma), and actin (MP Biomedicals, Inc.). TNF-α was obtained from R&D Systems (Minneapolis, MN). For immunofluorescence, HFK 38 E6E7 and HFK 38 E6E7-ΔNIκBα cells were seeded on sterilized coverslips in 12-well plates at 1 × 105 cells per well. After exposure to TNF-α for the indicated periods, the cells were fixed with 4% paraformaldehyde for 10 min. The fixed cells were permeabilized with 0.1% Triton X-100 for 5 min and then blocked with 5% nonfat milk in 0.05% Tris-buffered saline–Tween at room temperature for 1 h, followed by incubation with the indicated primary antibodies and fluorescein isothiocyanate/tetramethyl rhodamine isothiocyanate-conjugated secondary antibodies (Interchim) as described previously. The cellular localization of p65 was visualized using a fluorescence microscope, and random fields were photographed.
Reverse transcription (RT)-PCR and quantitative RT-PCR (qRT-PCR) analyses.
Total RNA was extracted from cells with TRIzol reagent (Invitrogen), followed by DNase I treatment to prevent cellular DNA contamination in the PCR. cDNA was synthesized with the First Strand cDNA synthesis kit (MBI Fermentas, Heidelberg, Germany) with a random hexamer primer and Moloney murine leukemia virus reverse transcriptase. The primers used for detection of HPV38 E6 and E7, xIAP, cIAP1, cIAP2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have been described previously (10, 24). Real-time PCR was performed using the MesaGreen qPCR MasterMix Plus for SYBR Assay (Eurogentec, San Diego, CA). The following primers were used: xIAP, Fw (5′-TGGGGTTCAGTTTCAAGGAC-3′) and Rev (5′-CGCCTTAGCTGCTCTTCAGT-3′); cIAP1, Fw (5′-CTGGGAACCAAAGGATGATG-3′) and Rev (5′-ATTCGAGCTGCATGTGTCTG-3′); cIAP2, Fw (5′-CCAAGTGGTTTCCAAGGTGT-3′) and Rev (5′-TGGGCTGTCTGATGTGGATA-3′); HPV38 E6, Fw (5′-TCTGGACTCAAGAGGATTTTG-3′) and Rev (5′-CACTTTAAACAATACTGACACC-3′); HPV38 E7, Fw (5′-CAAGCTACTCTTCGTGATATAGTTC-3′) and Rev (5′-CAGGTGGGACACAGAAGCCTTAC-3′); and GAPDH, Fw (5′-GCCAAAAGGGTCATCATC-3′) and Rev (5′-TGCCAGTGAGCTTCCCGTTC-3′). GAPDH was used for normalization. Relative expression was calculated using the comparative threshold cycle (CT) method (2−ΔΔCT) (34).
EMSA.
Cells were collected and washed twice with PBS, and nuclear extracts were prepared as described previously (55). Briefly, cell pellets were resuspended in cold buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg/ml aprotinin, and 5% glycerol) and incubated on ice for 30 min with occasional mixing. Triton X-100 was then added to a final concentration of 0.2%, and the cell lysates were centrifuged at 5,000 rpm for 5 min to obtain nuclear pellets. The supernatants were collected as cytoplasmic fractions. The nuclear pellets were washed with buffer A, resuspended in buffer B (20 mM HEPES, pH 7.9, 450 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 1 μg/ml aprotinin, and 25% glycerol), and incubated on ice for 30 min with occasional mixing to extract nuclear proteins. Nuclear lysates were centrifuged at 14,000 rpm for 10 min, and the supernatants were collected as nuclear fractions. Protein was quantified using the Bradford assay. An electrophoretic mobility shift assay (EMSA) was performed using the EMSA Gel Shift kit (Panomics, Inc., Fremont, CA) according to the manufacturer's instructions. Briefly, 5 μg of total nuclear proteins was incubated at room temperature with biotin-labeled NF-κB binding oligonucleotide, poly(dI-dC), and binding buffer. Complexes were resolved on a 5% nondenaturing acrylamide gel, followed by blotting on a nylon membrane and visualization by using avidin-labeled horseradish peroxidase (HRP) conjugate.
Colony formation assay.
HFK 38 E6E7 and HFK 38 E6E7-ΔNIκBα cells were seeded at a density of 1 × 103/6-cm dish. After 24 h, the cells were incubated with or without TNF-α (10 ng/ml). The medium was changed every 48 h, and the cells were grown for 8 to 10 days. After this period, the cells were washed with PBS, and colonies were fixed and stained on the plates with crystal violet in 20% methanol. The number of colonies was quantified in each plate by direct counting.
RESULTS
HPV38 E6 and E7 activate NF-κB signaling.
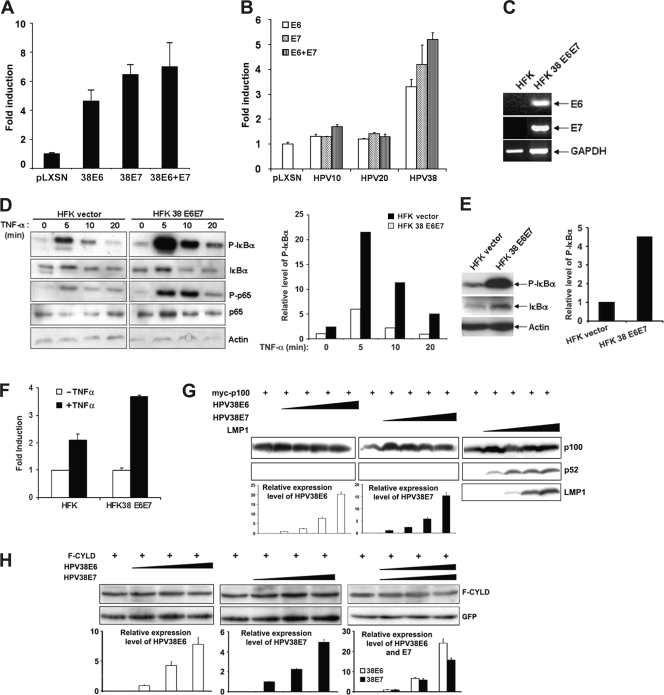
To determine whether HPV38 activates NF-κB, HEK293 cells were transfected with HPV38 E6 or E7 expression constructs, together with a luciferase reporter with four upstream NF-κB sites (49). As shown in Fig. 1A, both viral proteins, alone or in combination, induced κB site reporter gene activity by about 5- and 7-fold, respectively, compared to the empty-vector control. To evaluate whether cutaneous HPVs, such as HPV10 and HPV20, which are not able to immortalize keratinocytes (10) are impaired in NF-κB activation, HEK293 cells were transfected with HPV10 and HPV20 E6 and E7 expression plasmids, together with an NF-κB–luciferase reporter. As shown in Fig. 1B and in agreement with previous observations, induction of NF-κB was detected with HPV38 E6 and E7, but not with HPV10 and HPV20 oncoproteins. Thus, the capacity for HPV38 E6 and E7 to immortalize human primary keratinocytes correlates with their ability to activate NF-κB.
Fig. 1.
HPV38 E6 and E7 activate NF-κB and sensitize HFKs to TNF-α-mediated NF-κB activation. (A) HEK293 cells were transiently transfected with an NF-κB-dependent firefly luciferase reporter construct (500 ng), together with a Renilla luciferase construct (50 ng), with or without the different expression vectors for HPV38 E6 and HPV38 E7 oncogenes. The cells were harvested 48 h posttransfection, whole-cell extracts were prepared, and the firefly and Renilla luciferase activities were measured. The fold induction of NF-κB activity was determined. The experiment was performed at least three times independently in duplicate. The error bars indicate standard deviations. (B) HEK293 cells were transiently transfected with an NF-κB-dependent luciferase reporter construct, together with a Renilla luciferase construct with or without the different pLXSN expression vectors for HPV10 E6, HPV10 E7, HPV20 E6, HPV20 E7, HPV38 E6, and HPV38 E7 oncogenes. The Renilla luciferase activity was measured, and the fold induction of NF-κB activity was determined. The data are representative of at least three independent experiments performed in duplicate. (C) Total RNA was isolated from HFKs transduced with the empty vector pBabe (HFK) and immortalized HFKs expressing HPV38E6E7 (HFK 38 E6E7) using TRIzol reagent, and cDNA was prepared by RT-PCR. The cDNA obtained was used as a template for determination of the expression of HPV38 E6 and E7 genes with GAPDH expression as a control. (D) HFKs and HFKs immortalized by HPV38E6E7 were incubated with or without TNF-α (10 ng/ml) for the indicated periods, and whole-cell extracts were analyzed by immunoblotting with actin as a loading control. Quantification of the level of phosphorylated IκBα (P-IκBα) relative to time zero is presented (right). (E) HFKs containing empty vector and immortalized HFK 38 E6E7 established from keratinocytes from a different donor than in panel D were analyzed by immunoblotting for the expression of P-IκBα, with actin as a loading control. On the right is shown the quantification of the relative levels of P-IκBα. (F) HFKs and HPV38E6E7 HFKs (HFK38 E6E7) were transiently transfected with a NF-κB-dependent firefly luciferase reporter construct, and the luciferase activity and NF-κB fold induction were determined as in panel A. The experiment was performed at least three times independently in duplicate. (G) HEK293 cells were transiently transfected with a constant amount of myc-p100 expression plasmid with increased concentrations of expression vectors for HPV38 E6, HPV38 E7, or EBV LMP1. The expression of p100, p52, and LMP1 was monitored by immunoblotting. The expression levels of HPV38 E6 and HPV38 E7 were determined by qRT-PCR (bottom). (H) HEK293 cells were transiently transfected with a constant amount of F-CYLD expression plasmid with increased concentrations of expression vectors for HPV38 E6, HPV38 E7, or both together. F-CYLD protein was revealed by immunoblotting using anti-Flag antibodies. The expression levels of HPV38 E6 and HPV38 E7 were determined by qRT-PCR as in panel F (bottom). Green fluorescent protein (GFP) was used as an internal control for transfection and loading.
Next, we assessed the effect of HPV38 E6 and E7 on activation of NF-κB in human keratinocytes, which are the natural hosts of the virus. Primary HFKs were infected with empty retrovirus (pLXSN) or immortalized by transduction with a recombinant HPV38E6E7 retrovirus. RT-PCR analysis demonstrated the presence of mRNAs for both viral genes (Fig. 1C). A relative increase of phosphorylated IκBα was also detected in HPV38E6E7-immortalized cells in comparison to primary cells transduced with an empty vector (Fig. 1D, with quantification on the right). This difference was more evident in HPV38E6E7 HFKs following treatment with TNF-α than in the control cells (Fig. 1D). To corroborate the initial data, the experiments were repeated with an additional cell line of HPV38E6E7 HFKs that was obtained using primary keratinocytes from another donor (Fig. 1E, with quantification on the right). In these immortalized keratinocytes, the constitutive levels of phosphorylated IκBα were even higher than in the previously analyzed HPV38E6E7 HFKs (Fig. 1D and E). The effect of the oncoproteins on NF-κB was further corroborated in an NF-κB promoter reporter assay, which showed that an increase of NF-κB activation occurred upon TNF-α treatment (Fig. 1F). The HPV38 E6- and E7-mediated activation of NF-κB signaling was also confirmed in a third HPV38E6E7 HFK line that was obtained using primary cells from an additional donor (data not shown).
Since the phosphorylation and degradation of IκBα depend on the canonical pathway of NF-κB, we asked whether the noncanonical pathway can also be activated by HPV38 E6 and E7. To this end, we examined the effects of HPV38 E6 and E7 on the processing of p100/NF-κB2 to p52, a hallmark of noncanonical NF-κB activation (13, 14). Ectopic expression of EBV LMP1, a known inducer of both canonical and noncanonical NF-κB (36), increased the processing of p100/NF-κB2 to p52 (Fig. 1G). However, neither HPV38 E6 nor HPV38 E7 induced a significantly increased level of p52 (Fig. 1G). Thus, HPV38 E6 and E7 mainly induce the canonical pathway of NF-κB activation.
To gain further insight into the mechanisms by which HPV38 E6 and E7 activate NF-κB, we examined whether, like HPV16 E6 (3), HPV38 E6 and/or E7 can induce the degradation of the tumor suppressor CYLD, a deubiquitinating enzyme that inhibits NF-κB activation (50). We found that neither HPV38 E6 nor E7 downregulates the levels of CYLD (Fig. 1H).
Taken together, these results show that HPV38 oncoproteins E6 and E7 activate the canonical pathway of NF-κB and cooperate with TNF-α in NF-κB activation through phosphorylation of IκBα and p65.
Inhibition of NF-κB signaling affects cellular proliferation and cell survival of HPV38E6E7 keratinocytes upon TNF-α treatment.
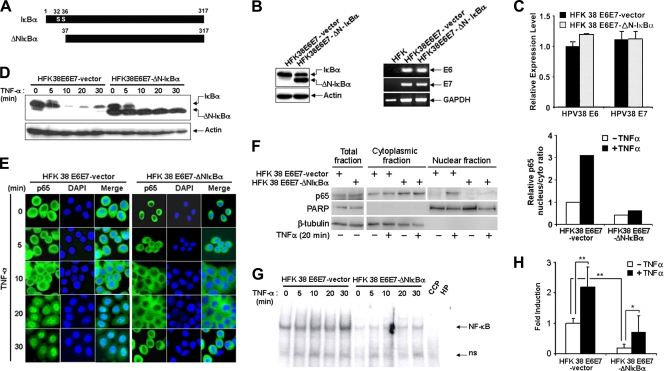
Due to the importance of NF-κB activity in cell survival, proliferation, and transformation, we next determined the effects of inhibition of NF-κB signaling on these events in HPV38E6E7 HFKs. To inhibit the NF-κB pathways, we used a deletion mutant of IκBα (ΔN-IκBα) that lacks the first N-terminal 36 amino acids (Fig. 2A) (7, 8). ΔN-IκBα cannot be phosphorylated and degraded and thus blocks the activation of NF-κB by retaining p65 in the cytoplasm (7). HPV38E6E7 HFKs were transduced with the empty retrovirus pBabe as a control or with the retrovirus expressing the superrepressor ΔN-IκBα. As shown in Fig. 2B (left), ΔN-IκBα was efficiently expressed in transduced cells, whereas no ΔN-IκBα protein band was detected in the vector control-transduced cells. ΔN-IκBα expression did not significantly alter the mRNA levels of HPV38 E6 and E7 (Fig. 2B, right, and C).
Fig. 2.
Nuclear translocation and activity of NF-κB (p65) induced by TNF-α is abrogated in HFK 38 E6E7 expressing the IκBα superrepressor ΔNIκBα. (A) Schematic representation of the generation of ΔN-IκBα by PCR. (B) Whole-cell extracts were prepared from HFK 38 E6E7-containing vector and HFK 38 E6E7 expressing ΔN-IκBα (HFK38E6E7-ΔNIκBα) and analyzed by immunoblotting for IκBα with actin as a loading control (left). Total RNA was isolated from HFKs, HFK 38 E6E7, and HFK 38 E6E7-ΔNIκBα using TRIzol reagent, and cDNA was prepared by RT-PCR. The cDNA obtained was used as a template for determination of the expression of HPV38 E6 and E7 genes with GAPDH expression as a control (right). (C) The expression levels of HPV38 E6 and E7 in HFK 38 E6E7-vector and in HFK 38 E6E7-ΔNIκBα were determined by qRT-PCR. The error bars indicate standard deviations. (D) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were incubated with or without TNF-α (10 ng/ml) for the indicated periods, and whole-cell extracts were analyzed by immunoblotting for IκBα with actin as a loading control. (E) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were incubated with or without TNF-α (10 ng/ml) for the indicated periods and stained for NF-κB (p65) and nucleus (DAPI [4′,6-diamidino-2-phenylindole]). (F) HFK 38 E6E7-vector and HFK 38 E6E7-ΔNIκBα were treated or not with TNF-α (10 ng/ml) for 20 min. Cellular cytoplasmic and nuclear fractions, as well as total lysate, were then prepared and analyzed by immunoblotting. β-Tubulin and PARP were used as controls for the cellular fractionation (left). Quantification of the relative nuclear p65 fraction is also shown (right). (G) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were incubated with TNF-α (10 ng/ml) for the indicated periods, and nuclear proteins were extracted and incubated with labeled or unlabeled (CCP) NF-κB-binding oligonucleotides. The DNA-protein complexes were separated on a polyacrylamide gel, followed by blotting and detection with an EMSA Gel shift kit (Panominc Inc.). (H) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were transiently transfected with an NF-κB-dependent firefly luciferase reporter construct, together with a Renilla luciferase construct. Forty-eight hours posttransfection, the cells were incubated with or without TNF-α (10 ng/ml) for 6 h. The firefly and Renilla luciferase activities and the NF-κB fold induction were then determined. The experiments were performed at least three times independently in duplicate. ** and *, P < 0.01 and P < 0.05, respectively.
Exposure of cells to TNF-α did not affect the phosphorylation and protein levels of ΔN-IκBα, in contrast to the endogenous wild-type IκBα (Fig. 2D). Consistent with the data presented in Fig. 1A on IκBα phosphorylation, partial localization of p65 in the nucleus was observed in unstimulated parental control HPV38E6E7-immortalized keratinocytes (Fig. 2E). This p65 nuclear accumulation gradually increased upon TNF-α treatment (Fig. 2E, left). In contrast, p65 was retained in the cytoplasm in HPV38E6E7 keratinocytes expressing ΔN-IκBα following treatment with TNF-α (Fig. 2E, right). Similar results were obtained by cellular fractionation followed by p65 immunoblotting (Fig. 2F). To more directly determine the effect of expression of ΔN-IκBα in keratinocytes on nuclear NF-κB activity, electromobility shift and luciferase reporter assays were performed. As shown in Fig. 2G, TNF-α-induced nuclear κB site binding activity was greatly decreased in ΔN-IκBα HPV38E6E7 HFKs compared to control cells. Consistent with these results, ΔN-ΙκΒα significantly inhibited NF-κB reporter gene activity in HPV38E6E7 keratinocytes (Fig. 2H).
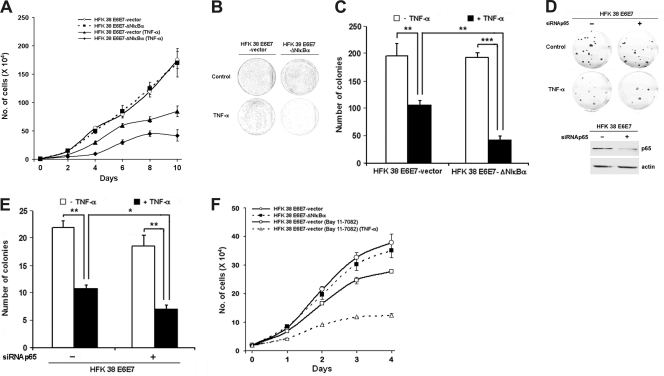
We next examined whether inhibition of NF-κB activity by ΔN-ΙκΒα affects the proliferation of HPV38E6E7 HFKs. Under normal growth conditions, ΔN-ΙκΒα expression did not significantly affect the proliferation rate of HPV38E6E7 HFKs in comparison to the control cells (Fig. 3A). Exposure to TNF-α resulted in significant inhibition of proliferation of both control cells and cells expressing the ΙκΒα superrepressor. However, the proliferation of HPV38E6E7-immortalized keratinocytes with impaired NF-κB was substantially more inhibited than that of the control cells (Fig. 3A). Additional experiments showed that cells expressing the IκBα superrepressor formed 4 to 10 times fewer colonies than the parental HPV38E6E7-immortalized keratinocytes following exposure to TNF-α (Fig. 3B and C). Next, we determined whether inhibition of the NF-κB complex by an alternative method could result in the same phenomenon. Indeed, p65 gene silencing by siRNAp65 in HPV38E6E7-immortalized keratinocytes led to a significant decrease in colony formation upon TNF-α exposure (Fig. 3D and E). To further corroborate these data, cells were exposed to the chemical NF-κB signaling pathway inhibitor Bay-11, which blocks the activation of IKK complex. Bay-11 significantly reduced the proliferation of HPV38E6E7-immortalized-keratinocytes in comparison to the control untreated cells (Fig. 3F) as a result of NF-κB inhibition. As observed for ΔN-ΙκΒα, the inhibition of proliferation is enhanced in Bay-11-treated cells exposed to TNF-α (Fig. 3F). The inhibition of proliferation observed in cells treated with Bay-11 in the absence of TNF-α contrasts with what was observed in cells where NF-κB was compromised with ΔN-ΙκΒα (Fig. 1A). This difference may be due to the key role of IKKβ in promoting accumulation of the p53 antagonist ΔNp73α, a function that is independent of NF-κB activation (2). However, the fact that Bay-11 treatment strongly enhanced the inhibition of cellular proliferation induced by TNF-α fully confirmed our initial data obtained in ΔN-ΙκΒα-expressing cells. Thus, HPV38 E6 and E7 attenuate the TNF-α-induced inhibition of cellular proliferation via activation of NF-κB signaling.
Fig. 3.
NF-κB contributes to the proliferation and survival of HFK 38 E6E7. (A) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were exposed or not exposed to TNF-α (10 ng/ml), and the cells were counted at regular intervals as indicated. The experiments were performed at least three times independently in duplicate. The error bars indicate standard deviations. (B) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were exposed or not to TNF-α as indicated, followed by a colony formation assay. (C) Quantification of the colony formation assay presented in panel B. ** and ***, P < 0.01 and P < 0.001, respectively. (D) HFK 38 E6E7 transfected with siRNA control or siRNA for p65 was exposed or not to TNF-α, followed by a colony formation assay (top). The expression level of p65 is shown below. (E) Quantification of the colony formation assay in panel D. *, P < 0.05. (F) Cells were treated with the chemical inhibitor of NF-κB, Bay-11, with or without TNF-α and processed as in panel A.
HPV38 E6 and E7 inhibit TNF-α-induced apoptosis by increasing the expression of NF-κB-regulated survival factor genes.
We next sought to determine whether the inhibition of NF-κB signaling in HPV38E6E7 HFKs influences the apoptosis induced by TNF-α and/or UV irradiation. The latter treatment was included in our experiments because it is known to be a strong inducer of TNF-α production and a key risk factor in skin carcinogenesis.
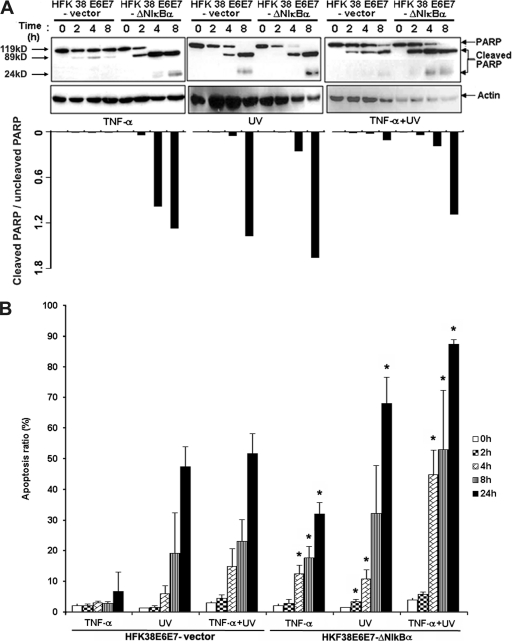
To evaluate the apoptotic response induced by TNF-α or UV irradiation in HPV38E6E7 HFKs, we first analyzed the cleavage of the caspase substrate PARP, which is an event closely associated with apoptosis. As observed previously in a different study on HPV16 (4), TNF-α treatment of HPV38E6E7 HFKs induced a low level of apoptosis with cleavage of a small proportion of PARP, while this event was strongly enhanced in cells expressing ΔN-IκBα (Fig. 4A, left). Similarly, PARP cleavage induced by UV irradiation alone or in combination with TNF-α was more evident in ΔN-IκBα HPV38E6E7 than in control cells (Fig. 4A, center and left). Quantification of the data clearly shows that more cleaved PARP was observed in cells with compromised NF-κB signaling than in control cells (Fig. 4A, bottom). In agreement with the PARP cleavage data, FACS analysis showed that upon TNF-α or UV exposure, the percentage of apoptotic cells with a sub-G0 content was higher in HPV38E6E7 HFKs with inhibited NF-κB signaling (Fig. 4B).
Fig. 4.
NF-κB inhibition induces apoptosis in immortalized HFK 38 E6E7. (A) (Top) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were incubated with TNF-α (10 ng/ml) or irradiated with UVB (312 nm; 80 mJ/cm2) or both for the indicated periods, and the whole-cell extracts were analyzed by immunoblotting for PARP with actin as a loading control. (Bottom) Quantification of relative cleaved PARP product obtained as shown above. (B) HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were incubated with TNF-α (10 ng/ml) or irradiated with UVB (312 nm; 80 mJ/cm2) for the indicated periods. Subsequently, the cells were fixed and stained with propidium iodide, and the percentage of sub-G0 cell population was detected by FACS. The experiments were performed at least three times independently in duplicate. An asterisk indicates that a statistical difference exists between HFK 38 E6E7-ΔN-IκBα and HFK 38 E6E7-vector at that time point with a P value of <0.05. The error bars indicate standard deviations.
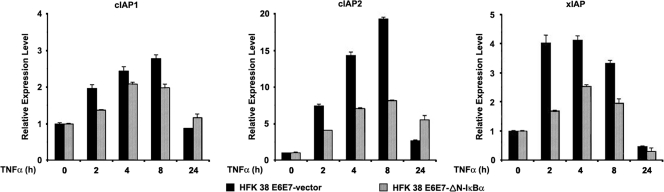
NF-κB activity protects cells against apoptosis by inducing the expression of a variety of cell survival genes (29, 54). Therefore, we sought to determine whether the increased apoptosis observed upon TNF-α or UV exposure is due to altered expression of three survival factors, cIAP, cIAP2, and xIAP, which are encoded by NF-κB-regulated genes (22). While TNF-α exposure, as expected, did increase the level of mRNA expression of cIAP1, cIAP2, and xIAP in the control cells, a substantial reduction of the expression of cIAP2 and xIAP was observed in ΔN-ΙκΒα HPV38E6E7 HFKs and to a lesser extent for cIAP1 (Fig. 5). Interestingly, inhibition of the expression of HPV38 E6 and E7 by using siRNA resulted in apoptosis (Accardi et al., unpublished), likely a result of inhibition of the expression of survival factors.
Fig. 5.
NF-κB-regulated anti-apoptotic genes are important for the survival of HFK 38 E6E7. HFK 38 E6E7 and HFK 38 E6E7-ΔN-IκBα were incubated with TNF-α (10 ng/ml) for the indicated periods, and the total RNA extracted was analyzed by qRT-PCR for expression of cIAP1, cIAP2, and xIAP. The data are representative of three independent experiments with a P value of <0.05. The error bars indicate standard deviations.
Altogether, these results show that HPV38-induced NF-κB signaling activation is important for immortalization and that this event plays a key role in favoring the survival of keratinocytes upon TNF-α or UV exposure via expression of NF-κB-regulated survival factor genes.
DISCUSSION
We have previously shown that E6 and E7 from beta cutaneous HPV38 displayed transforming activities in in vitro and in vivo experimental models (1, 17). Here, we report that HPV38 E6 and E7, similar to HPV16 E6 (28) and to the oncoprotein LMP1 of EBV (8, 49), are able to induce activation of NF-κB in human keratinocytes. Both HPV38 E6 and E7 can independently activate NF-κB (Fig. 1A). However, no significant increase in NF-κB activation was observed when the two oncoproteins were expressed together (Fig. 1A), probably as a result of a saturation effect. In addition, NF-κB activation upon TNF-α exposure was strongly increased in HPV38E6E7-immortalized keratinocytes in comparison to primary cells (Fig. 1D). Most importantly, we show that inhibition of NF-κB signaling by different means sensitizes HPV38E6E7-immortalized keratinocytes to TNF-α- and UV-induced apoptosis. This event is associated with downregulation of the expression of survival factors regulated by NF-κB. Interestingly, E6 and E7 from cutaneous HPVs that cannot promote keratinocyte immortalization are impaired in inducing NF-κB activation (Fig. 1B), indicating a link between NF-κB activation and E6- and E7-mediated cell immortalization.
It was previously shown that HPV16E6E7 keratinocytes are protected from apoptosis through NF-κB activation (11, 28, 32, 44). Interestingly, cIAP-2, the main survival factor of human keratinocytes that is induced by HPV16 E6 through NF-κB activation (28, 56), is also significantly upregulated in HPV38E6E7-immortalized keratinocytes in an NF-κB-dependent manner. Thus, the two HPV types show some similarity in their molecular mechanisms. However, the ability to activate NF-κB appeared not to be shared with E6 and E7 from cutaneous HPV types that display weak in vitro transforming activities (Fig. 1B), suggesting that this function may be linked to cellular transformation.
The mechanisms by which HPV38 E6 and E7 activate NF-κB remain unclear. However, it is likely that E6 and E7 from HPV38 act at different levels of the NF-κB signaling pathway. It has been demonstrated that the PDZ binding motif present in HPV16 E6 is important for HPV16 E6-induced NF-κB activation (28). Activation of NF-κB by HPV16 E6 is also associated with the degradation of the deubiquitinating enzyme CYLD, an inhibitor of NF-κB signaling (3). The absence of a PDZ binding motif in HPV38 E6 and in the E6s of other cutaneous HPVs and the inability of HPV38 E6 and E7 to induce CYLD degradation (Fig. 1H) suggest that HPV38 E6 and E7 activate NF-κB by mechanisms different from those of HPV16 E6. Our data also reveal that, in contrast to another viral oncoprotein, LMP1 (36), the canonical pathway appears to be the major pathway used by HPV38 E6 and E7 to activate NF-κB.
NF-κB has been found to be an important modulator of the expression of various genes contributing to the malignant phenotype in human squamous cell carcinoma (SCC) (35). In addition, NF-κB is constitutively active in murine and human SCC (18, 19, 53), and inhibition of NF-κB in these cancer cells abrogates cell survival and tumor growth (19, 39). However, several studies have reported that transgenic mice expressing a nondegradable form of IκBα show increased susceptibility to developing squamous cell carcinoma following increased apoptosis (52). Inactivation of IKKα increases skin tumor formation (15, 46, 57). In addition, decreased expression of IKKα was also observed in human skin and lung SCC (33, 46, 53). This discrepancy can result in differences in the cell system environment and the nature of targeted cells (54). In virus-induced carcinogenesis, NF-κB might be required at the earliest stage of carcinogenesis of skin keratinocytes. However, inhibiting NF-κB in keratinocytes immortalized by HPV38E6E7 did not significantly affect the growth of cells in comparison to control cells (Fig. 3). It is possible that in this particular context, the lack of a requirement for NF-κB for cell growth may result from additional cellular events, such as activation of hTERT and chromosome rearrangements (21). Alternatively, HPV38E6E7 may activate a parallel cellular signaling pathway, such as AP-1, that has a partial protective effect, as demonstrated for HPV16E6E7 (32). This situation contrasts with the case, for example, of immortalization of primary B cells by EBV, which requires NF-κB activation. Here, blocking NF-κB spontaneously induced apoptosis in the EBV-immortalized lymphoblastoid cell lines (9).
In conclusion, our data show that NF-κB activation in HPV38 E6 and E7 keratinocytes strongly alleviates the antiproliferative response of UV and/or the proinflammatory cytokine TNF-α and that a correlation exits between NF-κB activation and the ability of cutaneous HPV oncoproteins to promote keratinocyte immortalization. Since UV irradiation is considered a key risk factor in skin carcinogenesis, our findings provide evidence for cooperation between UV and beta HPV infection in promoting skin cancer.
ACKNOWLEDGMENTS
We thank T. Gilmore for his critical reading of the manuscript and Annick Rivoire and John Daniel for editing. We thank T. Gilmore, E. Kieff, G. Mosialos, and S. Ley for reagents. We are grateful to all the members of our laboratory for their comments and cooperation.
The study was partially supported by grants from the Association pour la Recherche Contre le Cancer et La Ligue Contre le Cancer du Comité du Rhône and Comité de la Drôme (to B.S.S. and M.T.), from the Institut National du Cancer (INCa) (to B.S.S.), by the IARC fellowships program (to I.H.), and by a fellowship from La Cooperation Française of the French Government (to I.F.).
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Accardi R., et al. 2006. Skin human papillomavirus type 38 alters p53 functions by accumulation of deltaNp73. EMBO Rep. 7:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Accardi R., et al. 2011. I{kappa}B kinase {beta} promotes cell survival by antagonizing p53 functions through {Delta}Np73{alpha} phosphorylation and stabilization. Mol. Cell, Biol. 31:2210–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. An J., et al. 2008. Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-kappaB activation. Cancer Cell 14:394–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basile J. R., Zacny V., Munger K. 2001. The cytokines tumor necrosis factor-alpha (TNF-alpha) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J. Biol. Chem. 276:22522–22528 [DOI] [PubMed] [Google Scholar]
- 5. Boccardo E., Noya F., Broker T. R., Chow L. T., Villa L. L. 2004. HPV-18 confers resistance to TNF-alpha in organotypic cultures of human keratinocytes. Virology 328:233–243 [DOI] [PubMed] [Google Scholar]
- 6. Bouvard V., Gabet A.-S., Accardi R., Sylla B. S., Tommasino M. 2006. The cutaneous human papillomavirus types and non-melanoma skin cancer, p. 369–377 In Campo S. (ed.), Papillomavirus research: from natural history to vaccines and beyond. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 7. Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485–1488 [DOI] [PubMed] [Google Scholar]
- 8. Cahir-McFarland E. D., et al. 2004. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 78:4108–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahir-McFarland E. D., Davidson D. M., Schauer S. L., Duong J., Kieff E. 2000. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. U. S. A. 97:6055–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caldeira S., et al. 2003. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J. Virol. 77:2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaturvedi V., et al. 1999. Apoptosis in proliferating, senescent, and immortalized keratinocytes. J. Biol. Chem. 274:23358–23367 [DOI] [PubMed] [Google Scholar]
- 12. Claudio E., Brown K., Park S., Wang H., Siebenlist U. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 3:958–965 [DOI] [PubMed] [Google Scholar]
- 13. Coope H. J., et al. 2002. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 21:5375–5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dejardin E., et al. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17:525–535 [DOI] [PubMed] [Google Scholar]
- 15. Descargues P., Sil A. K., Karin M. 2008. IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer. EMBO J. 27:2639–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Villiers E. M., Fauquet C., Broker T. R., Bernard H. U., zur Hausen H. 2004. Classification of papillomaviruses. Virology 324:17–27 [DOI] [PubMed] [Google Scholar]
- 17. Dong W., et al. 2005. Skin hyperproliferation and susceptibility to chemical carcinogenesis in transgenic mice expressing E6 and E7 of human papillomavirus type 38. J. Virol. 79:14899–14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong Z., et al. 1999. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-beta. Cancer Res. 59:872–879 [PubMed] [Google Scholar]
- 19. Duffey D. C., et al. 1999. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 59:3468–3474 [PubMed] [Google Scholar]
- 20. Filippova M., Brown-Bryan T. A., Casiano C. A., Duerksen-Hughes P. J. 2005. The human papillomavirus 16 E6 protein can either protect or further sensitize cells to TNF: effect of dose. Cell Death Differ. 12:1622–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabet A. S., et al. 2008. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J. 22:622–632 [DOI] [PubMed] [Google Scholar]
- 22. Gaur U., Aggarwal B. B. 2003. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem. Pharmacol. 66:1403–1408 [DOI] [PubMed] [Google Scholar]
- 23. Guasparri I., Keller S. A., Cesarman E. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Hasegawa T., et al. 2003. Expression of the inhibitor of apoptosis (IAP) family members in human neutrophils: up-regulation of cIAP2 by granulocyte colony-stimulating factor and overexpression of cIAP2 in chronic neutrophilic leukemia. Blood 101:1164–1171 [DOI] [PubMed] [Google Scholar]
- 25. Havard L., Delvenne P., Frare P., Boniver J., Giannini S. L. 2002. Differential production of cytokines and activation of NF-kappaB in HPV-transformed keratinocytes. Virology 298:271–285 [DOI] [PubMed] [Google Scholar]
- 26. Havard L., Rahmouni S., Boniver J., Delvenne P. 2005. High levels of p105 (NFKB1) and p100 (NFKB2) proteins in HPV16-transformed keratinocytes: role of E6 and E7 oncoproteins. Virology 331:357–366 [DOI] [PubMed] [Google Scholar]
- 27. Hayden M. S., Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell 132:344–362 [DOI] [PubMed] [Google Scholar]
- 28. James M. A., Lee J. H., Klingelhutz A. J. 2006. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 80:5301–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karin M. 2006. Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436 [DOI] [PubMed] [Google Scholar]
- 30. Kato T., Jr., Delhase M., Hoffmann A., Karin M. 2003. CK2 Is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol. Cell 12:829–839 [DOI] [PubMed] [Google Scholar]
- 31. Kfoury Y., Nasr R., Hermine O., de The H., Bazarbachi A. 2005. Proapoptotic regimes for HTLV-I-transformed cells: targeting Tax and the NF-kappaB pathway. Cell Death Differ. 12(Suppl. 1):871–877 [DOI] [PubMed] [Google Scholar]
- 32. Li J. J., Rhim J. S., Schlegel R., Vousden K. H., Colburn N. H. 1998. Expression of dominant negative Jun inhibits elevated AP-1 and NF-kappaB transactivation and suppresses anchorage independent growth of HPV immortalized human keratinocytes. Oncogene 16:2711–2721 [DOI] [PubMed] [Google Scholar]
- 33. Liu B., et al. 2006. A critical role for I kappaB kinase alpha in the development of human and mouse squamous cell carcinomas. Proc. Natl. Acad. Sci. U. S. A. 103:17202–17207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 35. Loercher A., et al. 2004. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 64:6511–6523 [DOI] [PubMed] [Google Scholar]
- 36. Luftig M., et al. 2004. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc. Natl. Acad. Sci. U. S. A. 101:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mantovani F., Banks L. 2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874–7887 [DOI] [PubMed] [Google Scholar]
- 38. Massimi P., et al. 2008. Comparative transforming potential of different human papillomaviruses associated with non-melanoma skin cancer. Virology 371:374–379 [DOI] [PubMed] [Google Scholar]
- 39. Mayo M. W., et al. 1997. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278:1812–1815 [DOI] [PubMed] [Google Scholar]
- 40. Michel A., Kopp-Schneider A., Zentgraf H., Gruber A. D., de Villiers E. M. 2006. E6/E7 expression of human papillomavirus type 20 (HPV-20) and HPV-27 influences proliferation and differentiation of the skin in UV-irradiated SKH-hr1 transgenic mice. J. Virol. 80:11153–11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Münger K., et al. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Münger K., et al. 2001. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888–7898 [DOI] [PubMed] [Google Scholar]
- 43. Muñoz N., et al. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518–527 [DOI] [PubMed] [Google Scholar]
- 44. Nees M., et al. 2001. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J. Virol. 75:4283–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niu Y., et al. 2006. A nuclear export signal and phosphorylation regulate Dok1 subcellular localization and functions. Mol. Cell. Biol. 26:4288–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park E., et al. 2007. Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res. 67:9158–9168 [DOI] [PubMed] [Google Scholar]
- 47. Pfister H. 2003. Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 31:5. [DOI] [PubMed] [Google Scholar]
- 48. Schaper I. D., et al. 2005. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 65:1394–1400 [DOI] [PubMed] [Google Scholar]
- 49. Sylla B. S., et al. 1998. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-kappaB through a pathway that includes the NF-kappaB-inducing kinase and the IkappaB kinases IKKalpha and IKKbeta. Proc. Natl. Acad. Sci. U. S. A. 95:10106–10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trompouki E., et al. 2003. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424:793–796 [DOI] [PubMed] [Google Scholar]
- 51. Underbrink M. P., Howie H. L., Bedard K. M., Koop J. I., Galloway D. A. 2008. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J. Virol. 82:10408–10417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Hogerlinden M., Rozell B. L., Ahrlund-Richter L., Toftgard R. 1999. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 59:3299–3303 [PubMed] [Google Scholar]
- 53. Van Waes C., Yu M., Nottingham L., Karin M. 2007. Inhibitor-kappaB kinase in tumor promotion and suppression during progression of squamous cell carcinoma. Clin. Cancer Res. 13:4956–4959 [DOI] [PubMed] [Google Scholar]
- 54. Wang C. Y., Mayo M. W., Korneluk R. G., Goeddel D. V., Baldwin A. S., Jr 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683 [DOI] [PubMed] [Google Scholar]
- 55. Wu Z. H., Shi Y., Tibbetts R. S., Miyamoto S. 2006. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311:1141–1146 [DOI] [PubMed] [Google Scholar]
- 56. Yuan H., et al. 2005. Human papillomavirus type 16 E6 and E7 oncoproteins upregulate c-IAP2 gene expression and confer resistance to apoptosis. Oncogene 24:5069–5078 [DOI] [PubMed] [Google Scholar]
- 57. Zhu F., et al. 2009. Critical role of IkappaB kinase alpha in embryonic skin development and skin carcinogenesis. Histol. Histopathol. 24:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]