Abstract
Complex defense signaling pathways, controlled by different hormones, are involved in the reaction of plants to a wide range of biotic and abiotic stress factors. We studied the ability of salicylic acid, jasmonate (JA), and ethylene (ET) to induce systemic defense in rice (Oryza sativa) against the root knot nematode Meloidogyne graminicola. Exogenous ET (ethephon) and JA (methyl jasmonate) supply on the shoots induced a strong systemic defense response in the roots, exemplified by a major up-regulation of pathogenesis-related genes OsPR1a and OsPR1b, while the salicylic acid analog BTH (benzo-1,2,3-thiadiazole-7-carbothioic acid S-methyl ester) was a less potent systemic defense inducer from shoot to root. Experiments with JA biosynthesis mutants and ET-insensitive transgenics showed that ET-induced defense requires an intact JA pathway, while JA-induced defense was still functional when ET signaling was impaired. Pharmacological inhibition of JA and ET biosynthesis confirmed that JA biosynthesis is needed for ET-induced systemic defense, and quantitative real-time reverse transcription-polymerase chain reaction data revealed that ET application onto the shoots strongly activates JA biosynthesis and signaling genes in the roots. All data provided in this study point to the JA pathway to play a pivotal role in rice defense against root knot nematodes. The expression of defense-related genes was monitored in root galls caused by M. graminicola. Different analyzed defense genes were attenuated in root galls caused by the nematode at early time points after infection. However, when the exogenous defense inducers ethephon and methyl jasmonate were supplied to the plant, the nematode was less effective in counteracting root defense pathways, hence making the plant more resistant to nematode infection.
The ultimate success or failure of any plant pathogen to invade and colonize its host is mainly dependent on the efficiency of the plant’s defense system. Plants generally respond to pathogen infection using several layers of constitutive and induced defenses (Adie et al., 2007; Robert-Seilaniantz et al., 2007; Schwessinger and Zipfel, 2008). Inducible plant defense mechanisms are mainly regulated by three types of signaling molecules or hormones: salicylate (SA), jasmonate (JA), and ethylene (ET; Loake and Grant, 2007; Balbi and Devoto, 2008; Grant and Jones, 2009; Pieterse et al., 2009). Their signaling pathways influence each other through a complex network of synergistic and antagonistic interactions (Koornneef and Pieterse, 2008), allowing the plant to efficiently tailor its defense reaction depending on the type of attacker encountered. In many cases, ET has been shown to act as an important modulator of plant responses to either SA or JA (Adie et al., 2007). In dicotyledons, mostly synergistic interactions between JA and ET signaling pathways have been reported, where both JA and ET are often required for the activation of defense-related genes, while SA and JA/ET defense pathways are mutually antagonistic (Thomma et al., 2001; Kunkel and Brooks, 2002; Rojo et al., 2003; Lorenzo and Solano, 2005; Beckers and Spoel, 2006; van Loon et al., 2006). The complex signaling network involved in the plant’s defense against bacterial, viral, and fungal pathogens has been extensively reviewed for dicotyledonous plants (Glazebrook, 2005; López et al., 2008; Bari and Jones, 2009), and although there are exceptions, it is generally accepted that SA-dependent defense is active against pathogens with a biotrophic lifestyle, whereas JA/ET-dependent defenses are active against necrotrophic pathogens (Glazebrook, 2005) and herbivorous insects (Kessler and Baldwin, 2002; Rojo et al., 2003; Howe and Jander, 2008).
Apart from reacting locally, plants can also mount a long-distance immune response termed systemic acquired resistance (SAR), in which naive tissues become more resistant to a broad spectrum of otherwise virulent pathogens. SAR can be induced both chemically and biologically and, at least in dicotyledonous plants, is characterized by local and systemic increases in endogenously synthesized SA and the concerted expression of a battery of defense-related genes, including those encoding pathogenesis-related (PR) proteins (Vlot et al., 2009). However, the roles of SA, JA, and ET, their interactions, and the overall mechanism of SAR in monocotyledonous plants have yet to be elucidated. Rice (Oryza sativa) is one of the most important crops worldwide and an attractive model plant for monocots with a tremendous amount of genomic and molecular information available, such as the Rice Genome Research Program (http//rgp.dna.affrc.go.jp; International Rice Genome Sequencing Project, 2005). The rice defense response has been studied for some major bacterial and fungal pathogens (Qiu et al., 2007; Yuan et al., 2007; De Vleesschauwer et al., 2008, 2010), but almost no research has been conducted on the molecular interaction between rice and nematodes, especially with regard to plant hormones. Studies involving plant-nematode interactions provide an opportunity to unravel plant defense signaling in root tissues, a research field that has received little attention so far.
Nematode problems on rice are likely to increase in the near future, mainly because of the increased use of so-called aerobic rice (Mew et al., 2004), which is being grown nowadays to increase water-use efficiency in rice production. In the tropics, aerobic rice can yield over 6 tons ha−1, but Peng et al. (2006) demonstrated a consistent yield decline in aerobic rice with continuous cropping, caused by so-called “soil sickness,” which is biologically linked to the buildup of nematode populations and other soil pathogens. The most damaging nematode attacking aerobic rice is the root knot nematode (RKN) Meloidogyne graminicola (Bridge et al., 2005). Although they cause important yield losses in rice-growing areas throughout the world (De Waele and Elsen, 2007), almost no fundamental research has been performed on these nematodes and their interaction with the rice plant. Infective second-stage juveniles of M. graminicola invade rice roots just behind the root tip and move toward the tip of the root, where they develop hook-like galls in which they complete their life cycle by laying eggs inside the root (Bridge et al., 2005). Plant parasitic nematodes puncture the cell wall with their stylet, inject secretions from their pharyngeal glands into the plant cell, and take up cytoplasm as their nutrition (Davis et al., 2000). RKN transform the punctured cells into so-called giant cells, which are kept alive as their food resource throughout their life cycle. During this infection process, nematodes induce changes in the plant’s gene expression patterns in both locally infected and systemic tissues (Gheysen and Mitchum, 2009). The role of plant defense pathways in nematode infection has only been investigated for dicotyledons, but even there merely fragmentary and often contradictory data are available. Some papers have shown the importance of SA in plant defense against RKN (Owen et al., 2002; Nandi et al., 2003; Branch et al., 2004) and cyst nematodes (Wubben et al., 2008), but Bhattarai et al. (2008) suggested that low levels of SA are already sufficient for tomato (Solanum lycopersicum) basal resistance to RKN. Concerning the JA/ET pathways, Lohar and Bird (2003) did not see changes in susceptibility in ET-resistant Lotus japonicus plants, but foliar JA application was shown to induce systemic defense against RKN in tomato (Cooper et al., 2005). Again contradicting this observation, Bhattarai et al. (2008) found that an intact JA signaling pathway is required for tomato susceptibility to RKN.
In this report, we present an in-depth characterization of the roles of SA, JA, and ET in mediating effective systemic rice defense to the root pathogen M. graminicola. We show that exogenous ET (ethephon) or JA (methyl jasmonate [MeJA]) application on the shoots is actively inducing systemic defense against RKN parasitism in rice roots. Studies with mutants and transgenics demonstrate that an intact JA biosynthesis pathway is a prerequisite for systemically induced resistance by both compounds, while ET signaling is not necessary for JA-induced defense. We also show that foliar ET treatment activates JA biosynthesis genes in roots. Our data favor a model in which the JA pathway is the key defense pathway involved in RKN interactions and is being efficiently activated and probably modulated by ET, while SA only plays a minor role in this. We also demonstrate that RKN infection elicits a local suppression of defense genes in the rice root to promote successful infection of the tissue.
RESULTS
Foliar Hormone Applications with Benzo-1,2,3-Thiadiazole-7-Carbothioic Acid S-Methyl Ester, MeJA, and Ethephon Activate Defense Pathways in Systemic Rice Roots
It has been well established that SA, JA, and ET play critical roles in plant defense signaling. To determine whether these hormones activate systemic defense against RKN parasitism in a compatible interaction with rice, we investigated the effects of foliar SA/JA/ET treatment on the activation of systemic rice defense pathways and subsequently on rice root susceptibility to the RKN M. graminicola. To verify the effectiveness of foliar hormone applications as an inducer of systemic defense pathways in the rice root system, the expression of four specific hormone-responsive genes and two general defense genes was investigated in the roots 24 h after foliar treatment using quantitative reverse transcription (qRT)-PCR.
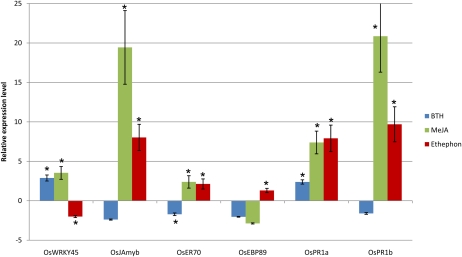
Figure 1 shows that foliar benzo-1,2,3-thiadiazole-7-carbothioic acid S-methyl ester (BTH) treatment did not induce a strong response of defense-related genes in the systemic root tissue. Only mRNA levels of pathogenesis-related gene OsPR1a and OsWRKY45, known to be a key regulator of the SA signaling pathway and involved in BTH-induced resistance to Magnaporthe grisea (Shimono et al., 2007), were higher in comparison with basal levels. OsPR1b, on the other hand, a gene that is commonly seen as SA inducible (Agrawal et al., 2000; Mitsuhara et al., 2008) and therefore generally accepted as a SAR marker gene, is not induced in systemic root tissue upon foliar application of BTH. In contrast, our data show that foliar supply of MeJA or ethephon results in a systemic up-regulation of OsPR1b, which is strongest upon MeJA treatment (factor = 20.9) and 9.7-fold upon ethephon application. Similarly, Figure 1 shows a systemic up-regulation of OsPR1a mRNA levels in the roots upon the three tested foliar hormone applications, with the highest induction observed upon ethephon treatment and the lowest upon BTH application.
Figure 1.
Effects of foliar hormone application of 500 μm ethephon, 100 μm MeJA, and 250 μm BTH on relative expression levels of six defense-related genes in systemic rice roots at 24 h after treatment. Fifteen-day-old Nipponbare rice shoots were sprayed until runoff with 500 μm ethephon, 100 μm MeJA, 250 μm BTH, or the corresponding control solution, and qRT-PCR was executed on RNA from root tissue 24 h after treatment. The relative expression levels of the selected genes in root tips of treated versus untreated plants are shown. Gene expression levels were normalized using two internal reference genes: OseIF4α and OsUBQ5. Bars represent means and se of two biological replicates, each containing a pool of eight plants. Asterisks indicate statistically significant differential expression in comparison with untreated plants. [See online article for color version of this figure.]
Twenty-four hours after foliar MeJA application, the roots of the treated plants showed a 19.4 times increase of mRNA levels of the JA-inducible rice myb transcription factor gene OsJAmyb (Lee et al., 2001), and also OsPR1a expression was strongly induced in the systemic root tissue (factor = 7.4). MeJA treatment had a minor effect on the systemic expression of OsWRKY45 and the ET-responsive factor OsERF70 (Cheong et al., 2003), while mRNA levels of OsEBP89 (Yang et al., 2002) were not significantly altered in comparison with basal expression levels.
Next to prevailing OsPR1a activation, the roots of ethephon-treated plants showed a strong up-regulation of OsJAmyb, a small but significant induction of the ET-responsive genes OsERF70 and OsEBP89, and a weak down-regulation of OsWRKY45 expression. OsERF70, also known as OsEREBP1, is an ET-induced ERF gene that activates PR defense genes (Cheong et al., 2003). Its expression was slightly increased in the systemic roots 24 h after MeJA and ethephon application (factor = 2.0 after both treatments). Although OsEBP89 has been reported to be more highly expressed upon continuous application of 100 μm 1-aminocyclopropane-1-carboxylic acid (Mao et al., 2006), our data show that this gene is only minorly up-regulated 24 h after foliar ethephon application. However, it cannot be ruled out that the expression of this gene may be more prominent at earlier time points and may have returned to near basal levels at 24 h after ethephon spraying.
Comparing all observations from the defense genes, we conclude that foliar application of MeJA or ethephon has a strong potentiating effect on systemic defense pathways in the roots, while foliar BTH treatment is less effective in activating the root defense system. Potential side effects from foliar hormone treatment on plant development were evaluated by monitoring the root and shoot growth of the plants 2 weeks after hormone spraying, but no observable phenotypic effects on plant growth and development were detected (data not shown).
Foliar ET/JA Application Triggers Systemic Resistance in Roots against the RKN M. graminicola
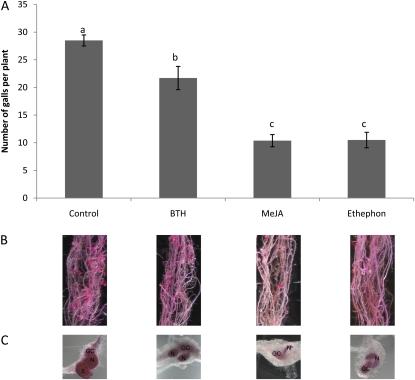
Given the observed up-regulation of systemic defense pathways in the rice roots upon foliar hormone application, we tested control and treated rice plants for their resistance against infection with RKN. Twenty-four hours after foliar hormone application, the RKN M. graminicola was inoculated on the rice roots and the number of galls per plant and per gram of root was evaluated 2 weeks after inoculation. Results are shown in Figure 2. Consistent with the observed systemic activation of defense pathways as revealed by qRT-PCR (Fig. 1), MeJA-supplied plants had significantly fewer (64% reduction) galls per plant and 55% fewer galls per gram of root than control plants. Significantly fewer galls per plant (63% reduction) and per gram of root (50%) were also observed for the ethephon-treated plants compared with control plants. Exogenous application of the SA analog BTH yielded a smaller reduction in the number of galls per plant (24%) and per gram of root (30%). Figure 2B shows a representative root system 2 weeks after infection with M. graminicola for the control plants and the three hormone applications. Roots were stained with acid fuchsin, which leads to intense pink staining of the galls. This figure illustrates the reduction of galls per root system upon foliar application with the different chemicals. Nematode development inside the galls as well as giant cells can be observed when the acid fuchsin-stained root system is destained for approximately 4 d in acid glycerol. Also in terms of nematode development inside the root galls, a delay was observed upon the different hormone treatments in comparison with nematode development in control plants (Fig. 2C). Two weeks after inoculation, the nematodes on control plants were producing egg masses, while nematodes infecting BTH-treated plants were still at the maturation phase and M. graminicola in MeJA- and ethephon-treated plants were only at the juvenile 3 or juvenile 4 phase (Fig. 2C). From these data, we can conclude that JA and ET are strong activators of systemically induced defense in rice roots against RKN, while BTH is less effective.
Figure 2.
Effects of foliar application of plant hormones on systemic plant defense against M. graminicola infection. Shoots of 15-d-old rice plants were sprayed until runoff with 500 μm ethephon, 100 μm MeJA, 250 μm BTH, or the corresponding control solution. At 24 h after hormone treatment, plant roots were inoculated with 300 second-stage juveniles of M. graminicola. A, Number of galls per plant 2 weeks after inoculation. Bars represent means and se of eight plants. Different letters indicate statistically significant differences (Duncan’s multiple range test with α = 0.05). Data represent one of three independent experiments with similar results. B, Representative root system 2 weeks after infection with M. graminicola and with the corresponding foliar hormone application. C, Developing RKN inside root galls, visualized with acid fuchsin. Roots were boiled in acid fuchsin. This leads to intense pink staining of galls, giant cells (GC), nematodes (N), and egg mass (E).
JA Biosynthesis and ET Signaling Are Required for Systemically Induced Defense against M. graminicola
It is known that defense signaling pathways influence each other through a complex network of synergistic and antagonistic interactions (Koornneef and Pieterse, 2008). The interaction between the SA/JA/ET pathways in systemically induced plant defense of rice against RKN was evaluated by studying different rice mutants or transgenic plants that are impaired in one of these three pathways: the SA-deficient transgenic NahG plants (Yang et al., 2004), the JA biosynthesis mutant hebiba (Riemann et al., 2003), and a transgenic line with a silenced expression of OsEin2b, a central component in ET signaling (Bailey et al., 2009). Attraction and penetration of nematodes in the root system of these plants were tested, and no significant differences were observed (data not shown).
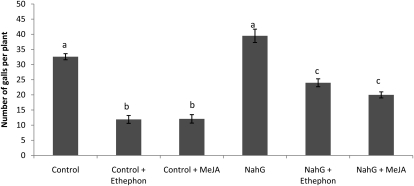
The NahG transgenics, expressing a bacterial salicylate hydroxylase that degrades SA to catechol (Gaffney et al., 1993) and therefore containing decreased SA levels, show similar but slightly higher levels of infection with RKN than the control plants (Fig. 3). However, when MeJA or ethephon is exogenously supplied to the NahG plants, a significantly lower susceptibility toward RKN than the control plants is observed (Fig. 3), although this reduction is lower than in the control plants. These data show that the JA/ET pathway has a strong positive role in systemically induced defense toward RKN, while the SA pathway is only minorly involved.
Figure 3.
Systemically induced defense against M. graminicola in SA-deficient transgenic NahG plants. Fifteen-day-old plants were sprayed until runoff with 500 μm ethephon, 100 μm MeJA, or the control solution. Plant roots were inoculated with 300 M. graminicola second-stage juveniles. Bars represent means and se of the number of galls at 2 weeks after inoculation recorded on eight plants. Different letters indicate statistically significant differences (Duncan’s multiple range test with α = 0.05). Data represent one of two independent experiments with similar results.
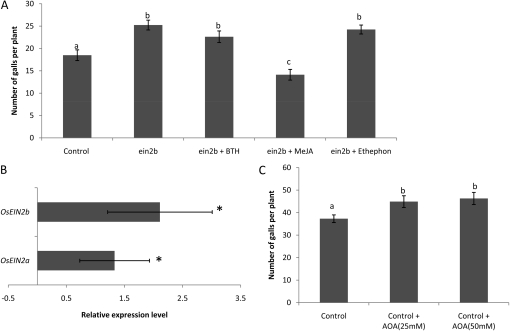
The OsEin2b RNA interference (RNAi) line, which has similar endogenous ET levels as wild-type plants, is significantly more susceptible to M. graminicola infection (Fig. 4A) than wild-type plants. Foliar treatment of the OsEin2b-silenced lines with BTH or ethephon does not protect these plants from M. graminicola infection, while MeJA is still able to induce systemic defense (Fig. 4A). From these data, we hypothesize that ET signaling, at least partly through OsEin2b, is responsible for ET-induced systemic defense of rice against RKN rather than de novo ET biosynthesis and corresponding endogenous ET levels. Using qRT-PCR, we confirmed that exogenous ET application significantly activates the systemic expression of OsEin2b and its paralog OsEin2a in rice roots 24 h after foliar treatment in wild-type Nipponbare plants (Fig. 4B). In planta inhibition of ET production through aminooxyacetic acid (AOA) application (Iwai et al., 2006) resulted in significantly higher susceptibility toward M. graminicola (Fig. 4C). This can be explained by the fact that blocking ET biosynthesis will automatically result in lower signal transduction in the ET pathway.
Figure 4.
Role of the ET pathway in systemically induced defense against RKN infection. A, Susceptibility for RKN after exogenous foliar hormone applications in the ET-insensitive line OsEin2b. B, qRT-PCR data showing the systemic up-regulation of ET signaling genes OsEin2a and OsEin2b in the root system at 24 h after foliar ethephon supply in wild-type Nipponbare plants. C, Number of galls per plant after treatment of wild-type plants with the ET biosynthesis inhibitor AOA (24 h before nematode inoculation). Different letters indicate statistically significant differences. Data represent one of two experiments with similar results.
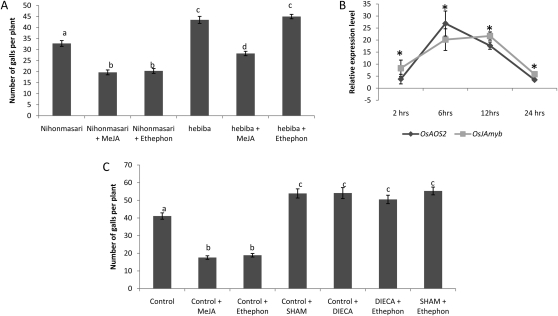
Figure 5A shows that the JA biosynthesis mutant hebiba (Riemann et al., 2003) is significantly more susceptible toward M. graminicola than its control, wild-type cv Nihonmasari. Exogenous ET or JA supplied onto Nihonmasari results in a similar systemic defense reaction as observed in previous experiments with wild-type cv Nipponbare. Exogenous MeJA application on hebiba makes the plants less susceptible for RKN, but this mutant does not respond to exogenous ET. Blocking JA biosynthesis in planta by three externally supplied lipoxygenase (LOX) inhibitors, 5,8,11,14-eicosatetraynoic acid (ETYA), salicylhydroxamic acid (SHAM), and diethyldithiocarbamic acid (DIECA), significantly increases the plants’ susceptibility toward RKN (Fig. 5C; data not shown for the ETYA treatment). Moreover exogenous ET failed to induce plant systemic defense against the nematodes when JA biosynthesis was impaired, either genetically (hebiba mutant) or chemically. These data indicate that ethephon-induced defense against M. graminicola requires de novo JA biosynthesis and demonstrate that JA plays a pivotal role in eliciting induced defense toward M. graminicola in rice roots. Our observations suggest that ET-induced defense works through activation of the JA pathway. In Figure 1, we have already demonstrated that exogenous ET strongly up-regulates systemic mRNA levels of the JA-responsive transcription factor OsJAmyb in the roots 24 h after treatment. To further confirm the hypothesis that ET would activate JA biosynthesis and to monitor the time course of this effect, mRNA levels of genes involved in JA biosynthesis (OsAOS2) and signaling (OsJAmyb) were monitored in systemic rice root tissue of wild-type Nipponbare plants at 2, 6, 12, and 24 h after foliar ET supply. The results (Fig. 5B) confirm a strong activation of both JA biosynthesis and JA signaling in systemic tissue already at 2 h after treatment and reaching its maximum after 6 h for JA biosynthesis and after 12 h for JA signaling.
Figure 5.
A, Effects of JA/ET-induced systemic defense against M. graminicola in the JA biosynthesis mutant hebiba and the corresponding wild-type Nihonmasari. B, qRT-PCR data showing the relative expression levels of JA biosynthesis (OsAOS2) and signaling (OsJAmyb) genes in the roots at different time points after systemic exogenous ET supply in wild-type plants. C, JA/ET-induced systemic defense after application of JA biosynthesis-inhibitors SHAM and DIECA. Fifteen-day-old plants were sprayed until runoff with 500 μm ethephon, 100 μm MeJA, or the control solution and with or without SHAM (200 μm) and DIECA (100 μm). Plant roots were inoculated with 300 M. graminicola juveniles. Bars represent means and se of the number of galls at 2 weeks after inoculation recorded on eight plants. For infection experiments, different letters indicate statistically significant differences (Duncan’s multiple range test with α = 0.05). For qRT-PCR data, asterisks indicate significant differential expression in comparison with untreated control tissue. Data represent one of two independent experiments with similar results.
M. graminicola Represses Defense Pathways in the Galls to Promote Plant Susceptibility
In order to obtain a more detailed understanding of the role of SA/JA/ET-mediated defense signaling during RKN infection on rice, the expression of six genes involved in different plant defense pathways was evaluated in root galls at 1, 2, and 3 d after inoculation (DAI). The expression of these genes was also monitored when systemic defense was induced by spraying the shoots with MeJA and ethephon 24 h prior to inoculation.
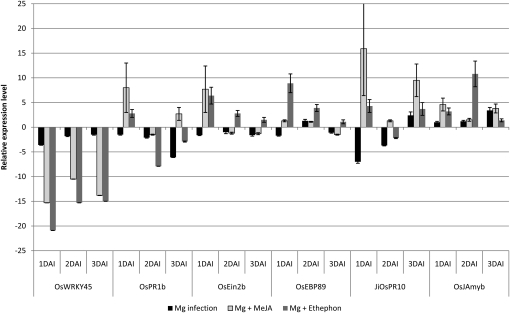
Relative expression levels of the defense genes in root galls caused by M. graminicola, compared with corresponding mock-inoculated tissue, are shown in black in Figure 6. Early upon infection (1 DAI), the mRNA levels of all genes, except for OsJAmyb, are significantly attenuated in the gall tissue. At 1 DAI, the strongest down-regulation is observed for the JA-responsive PR gene JiOsPR10 (Jwa et al., 2001), with a 7.3-fold reduction. At 2 DAI, the expression of OsWRKY45, OsPR1b, OsEin2b, and JiOsPR10 is still lower in RKN-infected gall tissue than in control root tips, although down-regulation is generally less strong than at 1 DAI. At 3 DAI, JiOsPR10 is slightly up-regulated (2.4-fold), while the expression level of the five other genes has almost returned to basal expression levels. These data demonstrate that RKN are either directly or indirectly suppressing defense pathways in the rice root tip at very early stages after penetration.
Figure 6.
qRT-PCR data on some selected defense-related genes in RKN galls on rice, and the effects of JA/ET-induced systemic defense on these genes. Bars represent mean expression levels and se from two biological and three technical replicates each containing a pool of eight plants. Data are shown as relative expression levels of infected and treated/untreated tissue in comparison with the control tissue (root tips of uninfected and untreated plants). Gene expression levels were normalized using two internal reference genes, OseIF4α and OsUBQ5.
When the systemic defense-inducing compounds MeJA and ethephon were administered onto the shoots 1 d prior to root infection with RKN (white and gray bars, respectively, in Fig. 6), all genes except OsWRKY45 were significantly up-regulated at 1 DAI in comparison with roots from untreated and noninfected plants. At 2 and 3 DAI, the trend of their expression is still generally higher than in nonsprayed infected plants (black bars), but mRNA levels are returning to control values. Although strongly down-regulated in the JA/ET-treated plants, mRNA levels of OsWRKY45 in the root galls were elevated after exogenous BTH application (data not shown), just as in noninfected tissue (Fig. 1). This is consistent with the fact that this gene is only known to be involved in BTH-induced resistance (Shimono et al., 2007). The up-regulation of all other tested genes confirms the defense-inducing capabilities of MeJA and ethephon in systemic root tissue, as already established in noninfected tissue (Fig. 1). When these compounds are administered to the systemic shoot tissue, the nematode encounters difficulties suppressing the fortified plant defense pathways, leading to a slower RKN development, reduced gall formation, and hence lower susceptibility to nematode infection.
Directly comparing the results from Figures 1 and 6 is complex, as we have worked on different tissues and are comparing infected with noninfected plants (whole roots versus galls). For example, there is a strong down-regulation of OsWRKY45 expression in galls upon MeJA or ethephon treatment (Fig. 6), while this gene was up-regulated in whole noninfected roots upon foliar MeJA treatment and down-regulated upon ethephon application (Fig. 1). It is hard to explain these differences, as we cannot distinguish whether these observations are due to nematode effects, systemic hormone signaling effects, or related to tissue-dependent differential expression of these genes.
DISCUSSION
Plant defense mechanisms are very complex and composed of multiple layers, which causes them to be effective against diverse types of pathogens (Spoel et al., 2007). SA, JA, and ET are known to play key roles for signaling networks involved in local and systemic plant defense responses (Robert-Seilaniantz et al., 2007; Bari and Jones, 2009). In dicotyledons, SA is generally linked with defense against biotrophic pathogens that feed and reproduce on living host cells, while the JA/ET pathways are involved in defense against necrotrophic pathogens that kill the host cells during infection (Pieterse et al., 2009). Broadly speaking, two systemically induced defense systems are recognized: (1) induced systemic resistance, induced by nonpathogenic rhizobacteria and involving JA/ET pathways but not SA; and (2) SAR, induced systemically after inoculation with necrotizing pathogens, hypersensitive response, pathogen-associated molecular patterns, or application of some chemicals (SA analogs or agonists), mediated by SA signaling and involving the activation of PR proteins (Vlot et al., 2009). The research described here aims to provide a characterization of the role of SA/JA/ET-mediated systemic defense signaling in rice and its importance in rice root defense against the RKN M. graminicola. Sedentary endoparasitic nematodes like the RKN (Meloidogyne spp.) are obligate biotrophs that induce the formation of complex feeding sites within the roots of their plant host (Williamson and Gleason, 2003).
The data presented in this report show that exogenous ethephon and MeJA are potent inducers of systemic root defense against RKN attack in rice, while foliar application with the SA analog BTH only results in a minor induction of systemic defense pathways. Although the production of SA is generally linked to SAR in other plants (van Loon et al., 2006), SAR action in rice has not been completely elucidated, but SA probably plays a slightly different role in this monocotyledonous plant. It has been suggested that local endogenous SA protects rice from oxidative damage caused by aging as well as by biotic and abiotic stress (Yang et al., 2004), and BTH application has been shown to induce defense against many fungal and bacterial pathogens (Rohilla et al., 2002; Shimono et al., 2007; De Vleesschauwer et al., 2008). On the other hand, in contrast to dicotyledonous plants, the monocot rice accumulates very high levels of SA in young leaves and shoots (7–30 μg g−1 fresh weight, in comparison with only 20–200 ng g−1 fresh weight in tobacco [Nicotiana tabacum] and Arabidopsis [Arabidopsis thaliana]), which do not seem to increase after inoculation with Pseudomonas syringae, M. grisea, or Rhizoctonia solani, but low SA levels in roots and suspension cells (Silverman et al., 1995; Chen et al., 1997). This high endogenous SA level in the shoots probably explains why, under our experimental conditions, BTH application on the rice shoot induces only a weak systemic defense response in the roots and hence only has a minor effect on RKN susceptibility. We speculate that foliar BTH application does not create a strong enough SA signal to be efficiently transported to the root system; hence, our treatment cannot trigger a strong systemic defense reaction there.
In Arabidopsis and tobacco plants expressing the Pseudomonas putida NahG gene and thus containing a lower endogenous level of SA, SA was clearly demonstrated to play a central role in local and systemic resistance against pathogen infection (Gaffney et al., 1993; Delaney et al., 1994). Rice NahG plants, on the other hand, are only slightly more susceptible to RKN infection, again pointing to a positive but minor role for SA in rice defense against RKN. The fact that JA and ET can still induce defense against RKN in the NahG plants, although slightly less strong than in wild-type plants, signifies that the JA/ET-induced systemic defense only needs minor levels of SA in rice roots to be functional against RKN. However, since data on salicylate hydroxylase expression and corresponding SA levels in the root tissue of rice NahG plants are not available, and bearing in mind that SA levels in rice roots are generally already low (70 ng g−1 fresh weight; Chen et al., 1997), these observations should be interpreted with caution.
In rice, it has been suggested that JA would be an important mediator of SAR, rather than SA (Tamogami et al., 1997; Schweizer et al., 1998; Lee et al., 2004). Our data show that both MeJA and ethephon are effective elicitors of systemically induced defense mechanisms active against RKN in rice roots. Exogenous JA and ET supply on the rice shoots resulted in a strong induced defense in the systemic tissue, exemplified by the strong up-regulation of PR genes OsPR1a and OsPR1b, which are generally seen as SAR marker genes. This observation is in line with their local responsiveness to many endogenous and exogenous signals (Jwa et al., 2006; Mitsuhara et al., 2008), but their systemic up-regulation in rice roots upon foliar JA/ET supply has never been demonstrated before. Previously, it was reported that OsPR1b is locally up-regulated by JA but down-regulated by the ET precursor 1-aminocyclopropane-1-carboxylic acid (Agrawal et al., 2000; Mei et al., 2006; Mitsuhara et al., 2008), while we demonstrate an up-regulation of this gene in systemic tissues upon ethephon treatment.
Previous experiments on dicotyledonous plants showed that MeJA application on roots of oat (Avena sativa) and spinach (Spinacia oleracea) and shoots of tomato enhanced resistance to parasitic nematodes, possibly by elevating the level of compounds that are toxic to nematodes, like phytoectosteroids, flavonoids, and proteinase inhibitors (Soriano et al., 2004a, 2004b; Cooper et al., 2005). Despite the fact that Bhattarai et al. (2008) reported that JA signaling, but not endogenous JA levels, promotes susceptibility to RKN in tomato, our experiments with the JA biosynthesis mutant hebiba and chemical JA biosynthesis inhibitors reveal that JA rather plays a positive role in rice resistance against RKN.
ET biosynthesis inhibition as well as impaired ET signaling (OsEin2b RNAi line) result in a higher susceptibility of the rice plant toward RKN. Considering the fact that endogenous ET levels are not significantly altered in Ein2b RNAi plants (Bailey et al., 2009), we deduce that ET signaling, rather than ET biosynthesis, is responsible for ET-mediated systemic defense against M. graminicola. Since MeJA spraying still induces defense against RKN in ET-insensitive lines while exogenous ET fails to trigger defense against the nematodes when JA biosynthesis is impaired, our results favor a model in which ET-induced systemic defense against M. graminicola works through activation of the JA defense pathway in the systemic root tissue. Indeed, qRT-PCR data confirmed that exogenous ET on the shoots strongly activates the JA biosynthesis and signaling pathway in the root already from 2 h after treatment on. Previously, LOX enzymes, responsible for the biosynthesis of JA precursors, have been demonstrated to be highly induced in incompatible tomato-Meloidogyne javanica and soybean (Glycine max)-Heterodera glycines interactions (Bhattarai et al., 2008; Klink et al., 2009), and a ZmLOX3 knockout mutant line is more susceptible to RKN (Gao et al., 2008). These observations demonstrate that JA is a key signal involved in plant defense against RKN, and possibly other sedentary nematodes, and our data reveal that the JA pathway in roots is being efficiently induced and probably modulated by systemic ET in rice.
The importance of the JA/ET pathway in defense against RKN is a remarkable conclusion in light of the fact that sedentary nematodes are obligate biotrophs, while these pathways are generally considered to be mainly involved in plant defense against necrotrophic pathogens and herbivorous insects (Kessler and Baldwin, 2002; Rojo et al., 2003; Glazebrook, 2005; Howe and Jander, 2008). It has to be noted that other than the nematode papers described above, data on the roles of plant hormones in defense against root pathogens are limited (Gutjahr and Paszkowski, 2009). The role of JA is certainly not clear in this respect, as it seems to have no effect on root parasitic plants (Kusumoto et al., 2007), while for symbiotic interactions with arbuscular mycorrhizal fungi and rhizobacteria, results vary depending on the concentrations used, the stage of plant development, and the stage of the infection process (Gutjahr and Paszkowski, 2009).
In order to antagonize the host plant immune response, many pathogens try to manipulate the signaling network between these plant defense pathways for their own benefit (Pieterse et al., 2009; De Vleesschauwer et al., 2010). In this report, we demonstrate that defense signaling is suppressed in root galls caused by M. graminicola very early after infection of the rice root. Transcriptome studies already suggested that JA signaling and some genes involved in JA biosynthesis are suppressed during fully established compatible cyst nematode infections in soybean (Ithal et al., 2007a, 2007b). Data from RKN giant cells in Arabidopsis (Jammes et al., 2005; Barcala et al., 2010) revealed that many defense-related genes, previously shown to be induced in other plant-pathogen interactions, like PR proteins, coding enzymes of the phenylpropanoids, salicylate signaling, cytokinin signaling, genes involved in lignin biosynthesis, simple phenols, and shikimate biosynthetic pathways, follow an overall down-regulation in galls and in giant cells. In line with our experimental data, ET biosynthesis and signaling were generally down-regulated in giant cells on Arabidopsis (Barcala et al., 2010). Strikingly, ET has been suggested to be critical for syncytia formation during cyst nematode infections on Arabidopsis (Goverse et al., 2000; Wubben et al., 2001). However, for RKN, Lohar and Bird (2003) found no changes in susceptibility in ET-resistant Lotus japonicus, and the fact that ET was not detected in galls at 1 to 2 d post infection (Glazer et al., 1983) in combination with the transcriptional patterns observed in this study and by Barcala et al. (2010) argue against a crucial role for ET-activated pathways during early giant cell differentiation; rather, they suggest that the RKN is actively suppressing the ET pathway and other defense signaling pathways in the root. The contrasting roles of ET in RKN and cyst nematode infection might be explained by their different modes of action, as RKN migrate in a stealthy way between the root cells whereas cyst nematodes move through them, causing extensive necrosis.
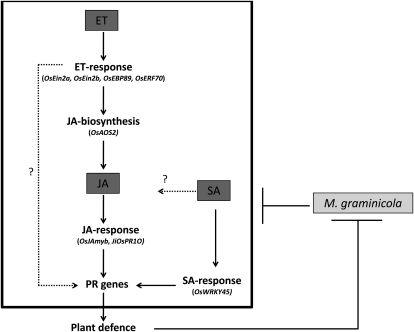
When considered together, our results support a model (Fig. 7) whereby exogenous ET and JA protect rice from M. graminicola attack at least in part by antagonizing pathogen-induced defense gene repression. Manipulating plant hormone signaling represents an extremely powerful virulence strategy, considering the global impact of hormone homeostasis on multiple cellular responses (de Torres-Zabala et al., 2007; Spoel and Dong, 2008).
Figure 7.
Model illustrating the interplay between SA, JA, and ET in systemically induced defense from shoot to root in rice. Genes shown to be involved in this mechanism are indicated in parentheses. Lines ending with arrows show activation. The line ending with a perpendicular short line indicates suppression. Dashed lines indicate possible effects, for which this paper does not provide evidence.
CONCLUSION
In summary, we have shown that JA or ET application onto the shoots induces a systemic defense pathway involving the up-regulation of PR genes and protecting the rice root from infection by the RKN M. graminicola. Our data demonstrate that ET-induced systemic defense involves ET signaling and a strong activation of JA biosynthesis and signaling genes, indicating that the JA pathway is a key defense pathway involved in RKN resistance of the rice root system. The SA pathway, on the other hand, has a minor positive effect on root defense against RKN, but we speculate that the high endogenous SA levels in rice shoots preclude a systemic SA signal from being efficiently transported to the root tissue.
Early upon infection, the RKN is manipulating defense pathways in the root galls to promote infection of the tissue. The experiments from this study suggest that exogenous ET or JA supplied to the rice shoot counteracts this down-regulation by inducing systemic JA/ET pathways in the root tissue, hence protecting the plant from infection with the root pathogen M. graminicola.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rice (Oryza sativa ‘Nipponbare’, japonica type; provided by the U.S. Department of Agriculture; GSOR-100) was used in all experiments as the wild-type plant (control), except for the hebiba experiments. Transgenic OsEin2b RNAi and NahG lines were kindly provided by Yinong Yang (Pennsylvania State University); the hebiba mutant and its corresponding wild-type cv Nihonmasari were kindly provided by P. Nick (Karlsruhe University). Seeds were germinated on wet filter paper for 5 d at 30°C, transferred to Synthetic Absorbent Polymer substrate (Reversat et al., 1999), and further grown at 26°C under a 12-h/12-h light regime (150 μmol m−2 s−1) and 70% to 75% relative humidity. Plants were watered with distilled water twice per week and fertilized once per week with 10 mL of Hoagland solution.
Infection Experiments
The Meloidogyne graminicola culture was provided by Prof. Dirk De Waele (Catholic University Leuven) and was originally isolated in the Philippines. A pure nematode culture was maintained in vivo on wild-type Nipponbare plants and grasses (Echinocloa crus-galli). Nematodes were extracted from 3-month-old infected plants using the modified Baermann method (Luc et al., 2005). The nematode suspension was collected after 48 h. Sixteen-day-old rice plants were inoculated with 300 second-stage juveniles of M. graminicola per plant or mock inoculated with water. The infection level of the plants was evaluated at 15 d post inoculation using acid fuchsin staining. We evaluated nematode susceptibility of the plants by counting the number of galls per plant and calculating the number of galls g−1 fresh root weight. To visualize the galls, roots were boiled for 3 min in 0.8% acetic acid and 0.013% acid fuchsin. They were washed with running tap water and then destained in acid glycerol.
Chemical Treatments
Ethephon, 2-chloroethyl phosphonic acid, and MeJA were purchased from Sigma, and BTH was a kind gift from Syngenta Crop Protection. For the pharmacological experiments, AOA, a potent inhibitor of ET biosynthesis, the LOX inhibitor ETYA, and two other JA biosynthesis inhibitors, SHAM and DIECA, were also purchased from Sigma. All hormone and inhibitor solutions were prepared in water containing 0.02% (v/v) Tween 20, with the exception of ETYA, which was dissolved in a few drops of ethanol prior to diluting in water. The chemicals and the concentrations used are as follows: MeJA (100 μm), BTH (250 μm), ethephon (500 μm), SHAM (200 μm), DIECA (100 μm), AOA (25–50 mm). For chemical treatment of plants, intact 15-d-old seedlings were sprayed with vaporizers until run off with a fine mist of either compound at the indicated concentrations. Distilled water containing 0.02% (v/v) Tween 20 was used as a control treatment. All experiments were repeated with similar results. In infection experiments, the chemicals were sprayed 24 h before nematode inoculation.
Data Collection and Statistical Analyses
All statistical analyses were performed in SPSS. Normality of the data was checked by applying the Kolmogorov-Smirnov test of composite normality (α = 0.05) and visualized by a box plot. Homoscedasticity of the data was checked by applying the Levene test (α = 0.05). The assumptions of normality and homoscedasticity of the data were checked and found to be fulfilled. Collected data were then analyzed using ANOVA. The means of the control and treated groups were compared by Duncan’s multiple mean comparison test.
RNA Extraction, cDNA Synthesis, and qRT-PCR
Root RNA was extracted using TRIzol (Invitrogen) following the manufacturer’s instructions. The RNA concentration and purity were measured using the NanoVue spectrophotometer (GE Healthcare). To remove all contaminating DNA, the extract was treated with DNaseI. One microgram of RNA was treated with 1 μL of DNaseI (1 unit μL−1; Fermentas), 1 μL of RiboLock RNase Inhibitor (40 units μL−1; Fermentas), and 1.8 μL of DNaseI buffer (10×; Fermentas) in a total volume of 18 μL. The mixture was incubated at 37°C for 30 min, after which 2 μL of 25 mm EDTA was added and incubated for 10 min at 65°C to stop the reaction.
First-strand cDNA synthesis was done in three steps: (1) addition of 1 μL of oligo(dT) (700 ng μL−1), 2 μL of 10 mm deoxyribonucleotide triphosphates, and 4 μL of RNase-free water to the DNase-treated RNA and incubation for 5 min at 65°C (to remove secondary structures); (2) addition of 8 μL of 5× first-strand buffer (Invitrogen) and 4 μL of 0.1 m dithiothreitol and incubation for 2 min at 42°C; and (3) addition of 1 μL of SuperScript II Reverse Transcriptase (200 units μL−1; Invitrogen) and incubation for 2 h at 42°C. The solution was diluted by adding 60 μL of water, and the quality of the cDNA was tested by performing a standard RT-PCR with some reference genes and checking the products on a 1.5% agarose gel.
The qPCR Core Kit for SYBR Green I no ROX (Eurogentec) was used in all qRT-PCR analyses. The reaction mixture contained the following ingredients: 1 μL of first-strand cDNA, 2 μL of reaction buffer (10×), 1.4 μL of MgCl2 (50 mm), 0.8 μL of deoxyribonucleotide triphosphates (5 mm), 0.6 μL of SYBR Green, 0.1 μL of HotGoldStar DNA polymerase (5 units μL−1), and 600 nm of each primer in a total volume of 20 μL (Table I). All PCRs were performed in three technical replicates. Two independent biological replicates, each containing a pool of eight plants, were analyzed. The reactions were performed in the Rotor-Gene 3000 (Corbett Life Science) using Rotor Discs (Qiagen), and results were generated by the Rotor-Gene 6 software. PCR was performed under the following conditions: 10 min at 95°C, and 45 cycles of 25 s at 95°C, 60 s at 58°C, and 20 s at 72°C. After the PCR, a melting curve was generated by gradually increasing the temperature to 95°C to test the amplicon specificity. Data were analyzed using the REST 384 software (Corbett Research; Pfaffl et al., 2002). This software uses a permutation analysis to compare the relative expression between a sample and a control group and to determine the statistical significance of the results.
Table I.
Overview of the target genes used in this study, showing their GenBank accession numbers and the primer pair used for qRT-PCR
| Target Gene | GenBank Accession No. | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
| OsUBQ5 | AK061988 | ACCACTTCGACCGCCACTACT | ACGCCTAAGCCTGCTGGTT |
| OseIF4α | AK073620 | TTGTGCTGGATGAAGCTGATG | GGAAGGAGCTGGAAGATATCATAGA |
| OsERF70 | AF193803.1 | ACCTTGGGGGTAGCATATCG | AGGGAACAGGTCCAATCACC |
| OsEBP89 | AJ304840 | TGACGATCTTGCTGAACTGAA | CAATCCCACAAACTTTACACA |
| OsEin2a | Os07g06130 | TAGGGGGACTTTGACCATTG | TGGAAGGGACCAGAAGTGTT |
| OsEin2b | Os07g06190 | GCGCATGTTGTAGAAGACGA | CAGGCAGCTTCGAATCAAGT |
| OsPR1a | Os07g0418500 | TCGTATGCTATGCTACGTGTTT | CACTAAGCAAATACGGCTGACA |
| OsPR1b | Os01g28450 | GGCAACTTCGTCGGACAGA | CCGTGGACCTGTTTACATTTT |
| JiOsPR10 | AF395880 | CGGACGCTTACAACTAAATCG | AAACAAAACCATTCTCCGACAG |
| OsJAmyb | AY026332 | GAGGACCAGAGTGCAAAAGC | CATGGCATCCTTGAACCTCT |
| OsAOS2 | NM_001055971.1 | TGCGCGACCGCCTCGATTTC | GGCCAGGCGGGACGTTGATG |
| OsWRKY45 | Os05g0323900 | AATTCGGTGGTCGTCAAGAA | AAGTAGGCCTTTGGGTGCTT |
References
- Adie B, Chico JM, Rubio-Somoza I, Solano R. (2007) Modulation of plant defenses by ethylene. J Plant Growth Regul 26: 160–177 [Google Scholar]
- Agrawal GK, Rakwal R, Jwa NS. (2000) Rice (Oryza sativa L.) OsPR1b gene is phytohormonally regulated in close interaction with light signals. Biochem Biophys Res Commun 278: 290–298 [DOI] [PubMed] [Google Scholar]
- Bailey TA, Zhou XJ, Chen JP, Yang Y. (2009) Role of ethylene, abscisic acid and MAP kinase pathways in rice blast resistance. Wang G, Valent B, , Advances in Genetics, Genomics and Control of Rice Blast Disease. Springer, Dordrecht, The Netherlands, pp 185–190 [Google Scholar]
- Balbi V, Devoto A. (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177: 301–318 [DOI] [PubMed] [Google Scholar]
- Barcala M, García A, Cabrera J, Casson S, Lindsey K, Favery B, García-Casado G, Solano R, Fenoll C, Escobar C. (2010) Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J 61: 698–712 [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JD. (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Beckers GJM, Spoel SH. (2006) Fine-tuning plant defense signalling: salicylate versus jasmonate. Plant Biol (Stuttg) 8: 1–10 [DOI] [PubMed] [Google Scholar]
- Bhattarai KK, Xie QG, Mantelin S, Bishnoi U, Girke T, Navarre DA, Kaloshian I. (2008) Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol Plant Microbe Interact 21: 1205–1214 [DOI] [PubMed] [Google Scholar]
- Branch C, Hwang CF, Navarre DA, Williamson VM. (2004) Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol Plant Microbe Interact 17: 351–356 [DOI] [PubMed] [Google Scholar]
- Bridge J, Plowright RA, Peng D. (2005) Nematode parasites of rice. Luc M, Sikora RA, Bridge J, , Plant-Parasitic Nematodes in Subtropical and Tropical Agriculture. CAB International, Wallingford, UK, pp 87–130 [Google Scholar]
- Chen ZX, Iyer S, Caplan A, Klessig DF, Fan BF. (1997) Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol 114: 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, Park CY, Kim JC, Park BO, Koo SC, et al. (2003) BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol 132: 1961–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WR, Jia L, Goggin L. (2005) Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. J Chem Ecol 31: 1953–1967 [DOI] [PubMed] [Google Scholar]
- Davis EL, Hussey RS, Baum TJ, Bakker J, Schots A, Rosso MN, Abad P. (2000) Nematode parasitism genes. Annu Rev Phytopathol 38: 365–396 [DOI] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al. (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bögre L, Grant M. (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 26: 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleesschauwer D, Djavaheri M, Bakker PA, Höfte M. (2008) Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol 148: 1996–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleesschauwer D, Yang YN, Cruz CV, Höfte M. (2010) Abscisic acid-induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase-mediated repression of ethylene signaling. Plant Physiol 152: 2036–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele D, Elsen A. (2007) Challenges in tropical plant nematology. Annu Rev Phytopathol 45: 457–485 [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756 [DOI] [PubMed] [Google Scholar]
- Gao XQ, Starr J, Göbel C, Engelberth J, Feussner I, Tumlinson J, Kolomiets M. (2008) Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol Plant Microbe Interact 21: 98–109 [DOI] [PubMed] [Google Scholar]
- Gheysen G, Mitchum MG. (2009) Molecular insights in the susceptible plant response to nematode infection. Plant Cell Monogram 15: 45–81 [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Glazer I, Orion D, Apelbaum A. (1983) Interrelationships between ethylene production, gall formation, and root-knot nematode development in tomato plants infected with Meloidogyne javanica. J Nematol 15: 539–544 [PMC free article] [PubMed] [Google Scholar]
- Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J. (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant Microbe Interact 13: 1121–1129 [DOI] [PubMed] [Google Scholar]
- Grant MR, Jones JDG. (2009) Hormone (dis)harmony moulds plant health and disease. Science 324: 750–752 [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Paszkowski U. (2009) Weights in the balance: jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol Plant Microbe Interact 22: 763–772 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436: 793–800 [DOI] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Hearne L, Maier T, Baum TJ, Mitchum MG. (2007a) Parallel genome-wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Mol Plant Microbe Interact 20: 293–305 [DOI] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG. (2007b) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact 20: 510–525 [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y. (2006) Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol 142: 1202–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette ML, Renou JP, Abad P, Favery B. (2005) Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J 44: 447–458 [DOI] [PubMed] [Google Scholar]
- Jwa NS, Agrawal GK, Rakwal R, Park CH, Prasad Agrawal V. (2001) Molecular cloning and characterization of a novel jasmonate inducible pathogenesis-related class 10 protein gene, JIOsPR10, from rice (Oryza sativa L.) seedling leaves. Biochem Biophys Res Commun 286: 973–983 [DOI] [PubMed] [Google Scholar]
- Jwa NS, Agrawal GK, Tamogami S, Yonekura M, Han O, Iwahashi H, Rakwal R. (2006) Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol Biochem 44: 261–273 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Klink VP, Hosseini P, Matsye P, Alkharouf NW, Matthews BF. (2009) A gene expression analysis of syncytia laser microdissected from the roots of the Glycine max (soybean) genotype PI 548402 (Peking) undergoing a resistant reaction after infection by Heterodera glycines (soybean cyst nematode). Plant Mol Biol 71: 525–567 [DOI] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ. (2008) Cross talk in defense signaling. Plant Physiol 146: 839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Kusumoto D, Goldwasser Y, Xie X, Yoneyama K, Takeuchi Y. (2007) Resistance of red clover (Trifolium pratense) to the root parasitic plant Orobanche minor is activated by salicylate but not by jasmonate. Ann Bot (Lond) 100: 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Cho K, Jang S, Rakwal R, Iwahashi H, Agrawal GK, Shim J, Han O. (2004) Inverse correlation between jasmonic acid and salicylic acid during early wound response in rice. Biochem Biophys Res Commun 318: 734–738 [DOI] [PubMed] [Google Scholar]
- Lee MW, Qi M, Yang YO. (2001) A novel jasmonic acid-inducible rice myb gene associates with fungal infection and host cell death. Mol Plant Microbe Interact 14: 527–535 [DOI] [PubMed] [Google Scholar]
- Loake G, Grant M. (2007) Salicylic acid in plant defence: the players and protagonists. Curr Opin Plant Biol 10: 466–472 [DOI] [PubMed] [Google Scholar]
- Lohar DP, Bird DM. (2003) Lotus japonicus: a new model to study root-parasitic nematodes. Plant Cell Physiol 44: 1176–1184 [DOI] [PubMed] [Google Scholar]
- López MA, Bannenberg G, Castresana C. (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11: 420–427 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Solano R. (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8: 532–540 [DOI] [PubMed] [Google Scholar]
- Luc M, Sikora RA, Bridge J. (2005) Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, Ed 2. CAB International, Wallingford, UK [Google Scholar]
- Mao CZ, Wang SM, Jia QJ, Wu P. (2006) OsEIL1, a rice homolog of the Arabidopsis EIN3 regulates the ethylene response as a positive component. Plant Mol Biol 61: 141–152 [DOI] [PubMed] [Google Scholar]
- Mei CS, Qi M, Sheng GY, Yang YN. (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19: 1127–1137 [DOI] [PubMed] [Google Scholar]
- Mew TW, Leung H, Savary S, Cruz CMV, Leach JE. (2004) Looking ahead in rice disease research and management. Crit Rev Plant Sci 23: 103–127 [Google Scholar]
- Mitsuhara I, Iwai T, Seo S, Yanagawa Y, Kawahigasi H, Hirose S, Ohkawa Y, Ohashi Y. (2008) Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol Genet Genomics 279: 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi B, Kundu K, Banerjee N, Babu SPS. (2003) Salicylic acid-induced suppression of Meloidogyne incognita infestation of okra and cowpea. Nematology 5: 747–752 [Google Scholar]
- Owen KJ, Green CD, Deverall BJ. (2002) A benzothiadiazole applied to foliage reduces development and egg deposition by Meloidogyne spp. in glasshouse-grown grapevine roots. Australas Plant Pathol 31: 47–53 [Google Scholar]
- Peng SB, Bouman B, Visperas RA, Castaneda A, Nie LX, Park HK. (2006) Comparison between aerobic and flooded rice in the tropics: agronomic performance in an eight-season experiment. Field Crops Res 96: 252–259 [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. (2002) Relative Expression Software Tool (REST(c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Qiu DY, Xiao J, Ding XH, Xiong M, Cai M, Cao Y, Li XH, Xu CG, Wang SP. (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20: 492–499 [DOI] [PubMed] [Google Scholar]
- Reversat G, Boyer J, Sannier C, Pando-Bahuon A. (1999) Use of a mixture of sand and water-absorbent synthetic polymer as substrate for the xenic culturing of plant-parasitic nematodes in the laboratory. Nematol 1: 209–212 [Google Scholar]
- Riemann M, Muller A, Korte A, Furuya M, Weiler EW, Nick P. (2003) Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol 133: 1820–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JD. (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10: 372–379 [DOI] [PubMed] [Google Scholar]
- Rohilla R, Singh US, Singh RL. (2002) Mode of action of acibenzolar-S-methyl against sheath blight of rice, caused by Rhizoctonia solani Kühn. Pest Manag Sci 58: 63–69 [DOI] [PubMed] [Google Scholar]
- Rojo E, Solano R, Sanchez-Serrano JJ. (2003) Interactions between signaling compounds involved in plant defense. J Plant Growth Regul 22: 82–98 [Google Scholar]
- Schweizer P, Buchala A, Dudler R, Metraux JP. (1998) Induced systemic resistance in wounded rice plants. Plant J 14: 475–481 [Google Scholar]
- Schwessinger B, Zipfel C. (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11: 389–395 [DOI] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, Takatsuji H. (2007) Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 19: 2064–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P, Seskar M, Kanter D, Schweizer P, Metraux JP, Raskin I. (1995) Salicylic acid in rice: biosynthesis, conjugation, and possible role. Plant Physiol 108: 633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano IR, Asenstorfer RE, Schmidt O, Riley IT. (2004a) Inducible flavone in oats (Avena sativa) is a novel defense against plant-parasitic nematodes. Phytopathology 94: 1207–1214 [DOI] [PubMed] [Google Scholar]
- Soriano IR, Riley IT, Potter MJ, Bowers WS. (2004b) Phytoecdysteroids: a novel defense against plant-parasitic nematodes. J Chem Ecol 30: 1885–1899 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Dong XN. (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3: 348–351 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X. (2007) Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA 104: 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamogami S, Rakwal R, Kodama O. (1997) Phytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid. FEBS Lett 412: 61–64 [DOI] [PubMed] [Google Scholar]
- Thomma BP, Penninckx IA, Broekaert WF, Cammue BPA. (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ. (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44: 135–162 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Williamson VM, Gleason CA. (2003) Plant-nematode interactions. Curr Opin Plant Biol 6: 327–333 [DOI] [PubMed] [Google Scholar]
- Wubben MJE, Jin J, Baum TJ. (2008) Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Mol Plant Microbe Interact 21: 424–432 [DOI] [PubMed] [Google Scholar]
- Wubben MJE, II, Su H, Rodermel SR, Baum TJ. (2001) Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Mol Plant Microbe Interact 14: 1206–1212 [DOI] [PubMed] [Google Scholar]
- Yang HJ, Shen H, Chen L, Xing YY, Wang ZY, Zhang JL, Hong MM. (2002) The OsEBP-89 gene of rice encodes a putative EREBP transcription factor and is temporally expressed in developing endosperm and intercalary meristem. Plant Mol Biol 50: 379–391 [DOI] [PubMed] [Google Scholar]
- Yang YN, Qi M, Mei CS. (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40: 909–919 [DOI] [PubMed] [Google Scholar]
- Yuan YX, Zhong SH, Li Q, Zhu ZR, Lou YG, Wang LY, Wang JJ, Wang MY, Li QL, Yang DL, et al. (2007) Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J 5: 313–324 [DOI] [PubMed] [Google Scholar]