Abstract
BACKGROUND AND PURPOSE
Cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) interact with transient receptor potential (TRP) channels and enzymes of the endocannabinoid system.
EXPERIMENTAL APPROACH
The effects of 11 pure cannabinoids and botanical extracts [botanical drug substance (BDS)] from Cannabis varieties selected to contain a more abundant cannabinoid, on TRPV1, TRPV2, TRPM8, TRPA1, human recombinant diacylglycerol lipase α (DAGLα), rat brain fatty acid amide hydrolase (FAAH), COS cell monoacylglycerol lipase (MAGL), human recombinant N-acylethanolamine acid amide hydrolase (NAAA) and anandamide cellular uptake (ACU) by RBL-2H3 cells, were studied using fluorescence-based calcium assays in transfected cells and radiolabelled substrate-based enzymatic assays. Cannabinol (CBN), cannabichromene (CBC), the acids (CBDA, CBGA, THCA) and propyl homologues (CBDV, CBGV, THCV) of CBD, cannabigerol (CBG) and THC, and tetrahydrocannabivarin acid (THCVA) were also tested.
KEY RESULTS
CBD, CBG, CBGV and THCV stimulated and desensitized human TRPV1. CBC, CBD and CBN were potent rat TRPA1 agonists and desensitizers, but THCV-BDS was the most potent compound at this target. CBG-BDS and THCV-BDS were the most potent rat TRPM8 antagonists. All non-acid cannabinoids, except CBC and CBN, potently activated and desensitized rat TRPV2. CBDV and all the acids inhibited DAGLα. Some BDS, but not the pure compounds, inhibited MAGL. CBD was the only compound to inhibit FAAH, whereas the BDS of CBC > CBG > CBGV inhibited NAAA. CBC = CBG > CBD inhibited ACU, as did the BDS of THCVA, CBGV, CBDA and THCA, but the latter extracts were more potent inhibitors.
CONCLUSIONS AND IMPLICATIONS
These results are relevant to the analgesic, anti-inflammatory and anti-cancer effects of cannabinoids and Cannabis extracts.
LINKED ARTICLES
This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: cannabinoids, cannabis, botanical extract, TRP channel, TRPV1, TRPA1, TRPM8, endocannabinoid, monoacylglycerol lipase, diacylglycerol lipase
Introduction
The plant Cannabis sativa L. has been used for millennia as a medicinal agent for pain relief, as well as for recreational purposes. It contains over 100 well-characterized compounds derived from a diterpene structure, known as ‘cannabinoids’ (ElSohly and Slade, 2005; Mehmedic et al., 2010). The large number of cannabinoids present in the plant – and the low naturally occurring levels of some of them – rendered it difficult to obtain sufficient quantities of such pure chemical entities in the past, thus explaining in part the slow progress of our understanding of the pharmacology of most of these compounds. Among them, (-)-trans-Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN) and Δ9-tetrahydrocannabivarin (THCV) are the best characterized (Mechoulam and Shvo, 1963; Gaoni and Mechoulam, 1964). THC largely accounts for the psychoactive properties of marijuana (Mechoulam, 2000), and most of its effects are attributed to the activation of the cannabinoid CB1 and CB2 receptors, two G-protein-coupled receptors (GPCR) widely expressed in mammalian tissues (Matsuda et al., 1990; Munro et al., 1993; Van Sickle et al., 2005; Gong et al., 2006; Pertwee, 2008). The identification of CB1 and CB2 led to the isolation of their endogenous agonists, N-arachidonoyl-ethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) (Devane et al., 1992; Mechoulam et al., 1995; Sugiura et al., 1995). Together with their receptors, these ‘endocannabinoids’ (EC) constitute the ‘endocannabinoid system’ (ECS), which also includes enzymes for EC biosynthesis and inactivation (Di Marzo et al., 2005), the most studied of which are N-acylphosphatidylethanolamine-specific phospholipase D and diacylglycerol lipase α (DAGLα), for AEA and 2-AG biosynthesis, respectively, and fatty acid amide hydrolase (FAAH) and monoacyl glycerol lipase (MAGL), for AEA and 2-AG hydrolysis (Di Marzo et al., 2005). Tissues produce several EC-like compounds with no direct activity at cannabinoid receptors, one of the most representative of which is N-palmitoylethanolamine (PEA), degraded by N-acylethanolamine acid amide hydrolase (NAAA) as well as FAAH (Ueda et al., 2010).
Pharmacological evidence suggests that additional targets for cannabinoids and EC exist. Among the proposed non-CB1/non-CB2 receptors for cannabinoids, transient receptor potential (TRP) channels and some orphan GPCR (GPR55, GPR119) were suggested to bind EC or EC-related endogenous compounds (for a recent review see De Petrocellis and Di Marzo, 2010). Furthermore, less specific targets – ranging from nuclear receptors to mitochondria and lipid rafts – were also proposed. The highly lipophilic nature of cannabinoids allows them to access intracellular sites of action, and non-CB1/TRPV1-receptor-mediated increases of [Ca2+]i by CBD have been observed in a variety of cell types (Ligresti et al., 2006), including hippocampal neurones (Drysdale et al., 2006). CBD actions on mitochondria and Ca2+ homeostasis provide a potential basis for CBD neuroprotective and anti-cancer properties (Massi et al., 2004; García-Arencibia et al., 2007; Iuvone et al., 2009; Ryan et al., 2009). However, CBD exhibits several other potentially beneficial properties, including strong anti-inflammatory actions (Costa et al., 2004; 2007; Pertwee, 2004; Carrier et al., 2006; Capasso et al., 2008; Pan et al., 2009). This compound has little or negligible affinity at CB1 and CB2 cannabinoid receptors (Pertwee, 2004), and might behave as a functional antagonist of CB receptor-mediated agonist effects in mouse brain and in membranes from cells transfected with human CB2 receptors (Thomas et al., 2007). Conversely, CBD potently increases the tissue levels of AEA by suppressing FAAH activity and/or by inhibiting the cellular uptake of AEA (Watanabe et al., 1998; Bisogno et al., 2001; Ligresti et al., 2006), whereas when combined with THC it is able to increase CB1 receptor expression (Hayakawa et al., 2008). TRP channels of the ankyrin type-1 (TRPA1, the ‘mustard oil’ receptor), vanilloid-type 1 (TRPV1, the ‘capsaicin receptor’) and 2 (TRPV2) are activated by CBD (Bisogno et al., 2001; De Petrocellis et al., 2008; Qin et al., 2008), whereas the TRP channel of melastatin type-8 (TRPM8, the ‘menthol receptor’) is antagonized by CBD (De Petrocellis et al., 2008). Importantly, the activation, and subsequent desensitization of TRPV1 and TRPV2, which are deeply involved in transducing inflammatory and chronic pain at the peripheral and spinal level (Levine and Alessandri-Haber, 2007), underlie some of the anti-hyperalgesic actions of CBD (Costa et al., 2004; 2007; Qin et al., 2008). Possibly as a result of these effects, or of its more recently reported direct or indirect stimulant actions on adenosine (Carrier et al., 2006) and 5-HT (5-HT1A) (Russo et al., 2005; Campos and Guimarães, 2008; Resstel et al., 2009; Magen et al., 2010; Yang et al., 2010) receptors, CBD potentiates some therapeutic effects of THC (Varvel et al., 2006; Marcu et al., 2010), while counteracting some of its undesirable effects (i.e. sedation, psychotropic effects, tachycardia, hyperphagia, etc.), thus allowing the use of higher and more efficacious co-administered doses of THC (Russo and Guy, 2006). These observations led to the clinical development of Sativex®, the first cannabinoid botanical drug product, based on an approximate 1:1 ratio of THC- and CBD-enriched Cannabis extracts, which was suggested to exhibit a higher therapeutic index than the corresponding pure cannabinoids (Russo and Guy, 2006).
Other cannabinoids, for example, CBN (a degradation product of THC) and cannabichromene (CBC), both demonstrate potent anti-inflammatory actions in the carrageenan paw oedema model of acute inflammation in rats (Sofia et al., 1974; Turner and Elsohly, 1981; Delong et al., 2010). As some strains of Cannabis contain a considerable amount of CBC, its effects on THC actions were investigated (Hatoum et al., 1981). Very recently it was shown that cannabigerol (CBG) activates α2-adrenoceptors and blocks G protein-coupled CB1 and 5-HT1A receptors (Cascio et al., 2010). Finally, THCV, the propyl homologue of THC, is a CB1 and CB2 receptor antagonist (Thomas et al., 2005) and a CB2 agonist at higher doses, which attenuate inflammation and hyperalgesia in mice (Bolognini et al., 2010). However, still very little is known of the mechanism of action of most of these and other ‘minor’ cannabinoids. Strains of cannabis (of defined genotype) expressing higher levels of these ‘minor’ cannabinoids as the principal cannabinoid have now been produced by GW Pharma Ltd.
In summary, non-psychotropic cannabinoids might contribute to several therapeutic benefits ascribed to Cannabis. On the other hand, the therapeutic use of THC is limited by its psychoactivity and potential for inducing dependence and tolerance, thus drawing further attention towards non-THC cannabinoids. The aim of the present study was to investigate the effects of 11 such compounds on TRPA1, TRPM8, TRPV1 and TRPV2 channels, as well as on DAGLα, FAAH, MAGL, NAAA and AEA cellular uptake (ACU). Apart from CBD, CBG, CBN and CBC, we also tested several cannabinoid propyl analogues and acids. For almost every cannabinoid tested, we also evaluated the activities of the corresponding cannabinoid extracts [‘botanical drug substance’ (BDS)]. The comparison between the effects observed with pure cannabinoids and the corresponding BDS might reveal the presence of potentially important synergistic effects that might be useful from a therapeutic point of view.
Methods
Compounds and extracts
CBC; CBD; CBG; CBN; cannabidiol acid (CBDA); cannabigerol acid (CBGA); cannabidivarin (CBDV); cannabigevarin (CBGV); cannabidivarin acid (CBDVA); Δ9-tetrahydrocannabinol (THC); Δ9-tetrahydrocannabinol acid (THCA); Δ9-tetrahydrocannabivarin (THCV); Δ9-tetrahydrocannabivarin acid (THCVA); and the corresponding BDS (extracts prepared from Cannabis sativa L. botanical raw material) were provided by GW Pharma Ltd (Salisbury, UK). The compounds were of at least 95% purity. The amount of each principal cannabinoid in the corresponding BDS varied between 40% and 70% (% w/w of extract) depending upon the BDS tested. The amount of each major cannabinoid in the BDS, expressed as a %, was used to calculate the amount of the BDS necessary to obtain the equimolar amount of the corresponding pure cannabinoid in the various experiments. Thus, for example, if the amount of a given cannabinoid in a given BDS was 70%, the amount of BDS to be administered to cells to yield a given final concentration of the cannabinoid was calculated from the molecular weight of the cannabinoid and the amount in milligrams of the BDS (as if the BDS only contained the given cannabinoid) divided by 0.7. The chemical profile of minor cannabinoids present in each BDS was unique to each BDS, as was that of non-cannabinoid components. Thus, each BDS has a unique chemical profile (‘chemical fingerprint’).
TRP calcium assays
HEK-293 cells stably over-expressing recombinant rat TRPA1 rat TRPM8, rat TRPV2 or human TRPV1 were selected by G-418 (Geneticin; 600 µg·mL−1), grown on 100 mm diameter Petri dishes as monolayers in minimum essential medium supplemented with non-essential amino acids, 10% fetal bovine serum and 2 mM glutamine, and maintained under 5% CO2 at 37°C. Stable expression of each channel was checked by quantitative-PCR. On the day of the experiment, the cells were loaded for 1 h at 25°C with the cytoplasmic calcium indicator Fluo-4AM (Invitrogen) 4 µM in dimethyl sulphoxide containing 0.02% Pluronic F-127 (Invitrogen, Carlsbad, CA, USA). After loading, cells were washed twice in Tyrode's buffer (145 mM NaCl, 2.5 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 10 mM d-glucose and 10 mM HEPES, pH 7.4), resuspended in the same buffer, and transferred to the quartz cuvette of the spectrofluorimeter (ex λ = 488 nm; em λ = 516 nm) (Perkin-Elmer LS50B equipped with PTP-1 Fluorescence Peltier System; Perkin-Elmer Life and Analytical Sciences, Waltham, MA, USA) under continuous stirring. Experiments were carried by measuring cell fluorescence before and after the addition of various concentrations of phytocannabinoids. The values of the effect on [Ca2+]i in wild-type (i.e. not transfected with any construct) HEK-293 cells were taken as baselines and subtracted from the values obtained from transfected cells. The potency of test compounds (EC50 values) were determined as the concentration of test substances required to produce half-maximal increases in [Ca2+]i. The efficacy of the agonists was determined by comparing their effect with the analogous effect observed with 4 µM ionomycin. The effects of the phytocannabinoids on TRPA1 are expressed as a percentage of the effect obtained with 100 µM allylisothiocyanate [mustard oil (MO)]. Antagonist/desensitizing behaviour was evaluated against capsaicin (0.1 µM) for TRPV1; icilin (0.25 µM) for TRPM8; MO (10 µM) for TRPA1; or lysophosphatidylcholine (LPC) (3 µM) for TRPV2 by adding the compounds in the quartz cuvette 5 min before stimulation of cells with agonists. Data are expressed as the concentration exerting a half-maximal inhibition of agonist effect (IC50). The effect on [Ca2+]i exerted by the agonist alone was taken as 100%. Some experiments were also carried out to antagonize the effects of CBDV and CBC on TRPA1-mediated elevation of [Ca2+]i with AP18 (Petrus et al., 2007) or HC030031 (McNamara et al., 2007), which were added to cells 5 min before the agonists. All determinations were at least performed in triplicate. Dose-response curves were fitted by a sigmoidal regression with variable slope. Curve fitting and parameter estimation were performed with GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA).
DAGL-α assay
Human recombinant DAGL-α was originally over-expressed with high efficiency in COS-7 cells and therefore these cells were used for this assay instead of HEK-293 cells, which, moreover, also express the native enzyme (Bisogno et al., 2003). COS-7 cells, over-expressing DAGL-α, were homogenized in Tris–HCl buffer, pH 7. The homogenates were centrifuged at 4°C at 800×g (5 min) and then at 10 000×g (25 min). The 10 000×g membrane fraction was incubated in Tris–HCl 50 mM at pH 7.0 at 37°C for 20 min, with synthetic 1-[14C]-oleoyl-2-arachidonoyl-glycerol (1.0 mCi·mmol−1, 25 µM) in the presence of vehicle or varying concentrations of test compounds. After the incubation, lipids were extracted with two volumes of CHCl3/MeOH 2/1 (by volume). The extracts were lyophilized under vacuum and then purified by TLC on silica on polypropylene plates using CHCl3/CH3OH/NH4OH (85/15/1 by volume) as the eluting system. Bands corresponding to [14C]-oleic acid were cut, and their radioactivity was counted with a β-counter.
MAGL assay
MAGL activity was measured using the cytosol derived from the 10 000×g fraction of homogenates from wild-type COS cells and synthetic 2 [3H]-arachidonoylglycerol; 100 µg of protein and 10 µM (10 000 cpm) [3H]-2-AG for each assay were incubated for 20 min a 37°C in the presence or absence of various concentrations of phytocannabinoids in Tris-HCl buffer, pH 7. [3H]-glycerol produced from the MAGL hydrolysis of [3H]-2-AG was measured by scintillation counting of the aqueous phase after extraction of the incubation mixture with two volumes of CHCl3/CH3OH (1:1 by volume) and used as a measure of enzyme activity.
FAAH assay
The effect of increasing concentrations of the compounds on the enzymatic hydrolysis of [14C]-AEA was studied using membranes prepared from rat brain. Membranes (70–100 µg) were incubated in the presence or absence of varying concentrations of the compounds and [14C]-AEA (10 000 cpm, 2.5 µM) in 50 mM Tris-HCl, pH 9, for 30 min at 37°C. [14C]-ethanolamine produced from [14C]-AEA hydrolysis was used to calculate FAAH activity and was measured by scintillation counting of the aqueous phase after extraction of the incubation mixture with two volumes of CHCl3/CH3OH (1:1 by volume). Data are expressed as the concentration exerting a half maximal inhibition (IC50).
NAAA assays
HEK-293 cells in which the cDNA encoding the human enzyme was stably over-expressed (HEK-NAAA cells), were used for a specific enzymatic assay of NAAA as described previously (Saturnino et al., 2010). Briefly, the 12 000 membrane fraction of homogenized cells was suspended in phosphate-buffered saline (pH 7.4) and subjected to two cycles of freezing and thawing and used in the assay. The enzyme preparation (50 µg per sample) was incubated at 37°C for 30 min with 100 µM [14C]-N-palmitoylethanolamine (10 000 cpm per sample) in a solution of citrate/sodium phosphate 50 mM (pH 5.2) and 0.1% Triton X-100, containing varying concentrations of the phytocannabinoids. The reaction was terminated by the addition of chloroform/methanol (1:1 by volume) and quantification of [14C]-ethanolamine was carried out by using a β-counter (TRI-carb 2100TR; Perkin-Elmer) to quantify the [14C]-ethanolamine formed by the enzymatic activity.
ACU assay
The effect of compounds on ACU was analysed on rat basophilic leukaemia cells by using 2.5 µM (10 000 cpm) [14C]-AEA. Cells were incubated with [14C]-AEA for 90 s at 37°C, in the presence or absence of varying concentrations of the compounds. Residual [14C]-AEA in the incubation medium after extraction with CHCl3/CH3OH 2:1 (by volume) was determined by scintillation counting of the lyophilized organic phase and was used as a measure of the AEA taken up by cells. Specific AEA uptake was determined by subtracting uptake at 4°C from uptake at 37°C. Data are expressed as the concentration exerting 50% inhibition of AEA uptake (IC50) calculated by GraphPad software (Graph-Pad Software Inc., San Diego, CA, USA).
Results
Activity at TRPA1 channels
In Table 1, the efficacies and potencies of cannabinoids at eliciting a TRPA1-mediated increase in intracellular Ca2+ in HEK-293 cells over-expressing the channel are reported. HEK-293 cells were stably transfected with the plasmid pcDNA3 containing the rat recombinant TRPA1 cDNA, thus generating cells (denoted hereafter as ‘TRPA1-HEK-293’) that express high levels of TRPA1 transcript (De Petrocellis et al., 2008). The compounds tested induced TRPA1-mediated Ca2+ elevation with efficacy comparable with that of allylisothiocyanate, the most potent being CBC (EC50 = 90 nM) and CBD (EC50 = 110 nM), in good agreement with the EC50 values of 60 nM and 96 nM, respectively, previously published by our research group (De Petrocellis et al., 2008). The rank order of potency of the pure compounds was as follows: CBC > CBD > CBN > CBDV > CBG > THCV > CBGV > THCA > CBDA > CBGA > THCVA. Therefore, none of the pure compounds was more potent than CBC, CBD and CBN as TRPA1 agonists. These compounds as well as CBDV and CBG were more potent than allylisothiocyanate, whereas the acid cannabinoids were the least active in this test. Except for THCA and CBN, all the pure cannabinoids also exhibited higher efficacy than allylisothiocyanate. Interestingly, THCV-BDS was the most potent (EC50 = 70 nM), and other BDS (i.e. those of THCV, THCA, CBGV, CBDA, THCVA) were more potent (EC50 = 0.07–5.8 µM) than the corresponding pure compounds in this assay (Table 1). At high concentrations (>10 µM), some compounds also caused an effect on [Ca2+]i in cells that had not been transfected with any TRP construct (wild-type HEK-293 cells), and the values obtained from the latter cells were subtracted from those obtained in transfected cells. Importantly, all the compounds found here to activate TRPA1 also desensitized this channel to subsequent (5 min after addition of the cannabinoid) stimulation by MO allyisothiocyanates (Table 1). Finally, to provide conclusive evidence for the specificity of the effects, some compounds (i.e. CBDV and CBC) were also given to transfected cells that had been pre-incubated for 5 min with the TRPA1 antagonists AP18 (Petrus et al., 2007) or HC030031 (McNamara et al., 2007). The effect of CBDV (10 µM) was reduced by AP18 (50 µM) by 63.6 ± 7.1% and by HC030031 (50 µM) by 42.0 ± 3.4%. These antagonists reduced the effect of MO isothiocyanate (10 µM) by 51.0 ± 8.2% and 51.5 ± 5.2%,respectively. The effect of CBC (10 µM) was reduced by AP18 (50 µM) by 55.8 ± 6.0%. AP18 (10 µM) reduced the effects of CBC and MO isothiocyanate by 43.2 ± 3.8 and 48.6 ± 4.1% respectively (data are means ± SD of n = 3 determinations).
Table 1.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as functional agonists at TRPA1 channels, and the potency of pure cannabinoids as desensitizers of TRPA1 to the effect of allyl isothiocyanate
| Cannabinoid | Efficacy (% allyl isothiocyanate 100 µM) | Potency shown as EC50 (µM) | Desensitization (% allyl isothiocyanate 100 µM) IC50 (µM) |
|---|---|---|---|
| CBC | 119.4 ± 3.1 | 0.09 ± 0.01 | 0.37 ± 0.05 |
| CBC-BDS | 144.1 ± 6.0 | 0.25 ± 0.08 | NT |
| CBD | 115.9 ± 4.6 | 0.11 ± 0.05 | 0.16 ± 0.05 |
| CBD-BDS | 163.6 ± 11.9 | 0.38 ± 0.15 | NT |
| CBG | 99.9 ± 1.1 | 0.7 ± 0.03 | 13.0 ± 4.8 |
| CBG-BDS | No effect | ND | NT |
| CBN | 83.3 ± 4.0 | 0.18 ± 0.02 | 0.40 ± 0.04 |
| CBDA | 113.0 ± 11.0 | 5.3 ± 1.5 | 4.92 ± 0.09 |
| CBDA-BDS | 118.4 ± 11.9 | 1.0 ± 0.3 | NT |
| CBGA | 182.8 ± 0.2 | 8.4 ± 3.5 | 7.14 ± 0.17 |
| CBDV | 105.0 ± 0.7 | 0.42 ± 0.01 | 1.29 ± 0.38 |
| CBDV-BDS | 163.4 ± 28.3 | 0.5 ± 0.3 | NT |
| CBGV | 151.4 ± 0.9 | 1.6 ± 0.01 | 2.02 ± 0.25 |
| CBGV-BDS | 106.4 ± 4.0 | 0.8 ± 0.1 | NT |
| THC | 117 ± 12a | 0.23 ± 0.03a | NT |
| THCA | 41.6 ± 2.1 | 2.7 ± 0.9 | 95.25 ± 0.01 |
| THCA-BDS | 79.4 ± 2.0 | 0.22 ± 0.02 | NT |
| THCV | 243.0 ± 16.5 | 1.5 ± 0.6 | 3.07 ± 0.24 |
| THCV-BDS | 197.6 ± 7.9 | 0.07 ± 0.02 | NT |
| THCVA | 170.2 ± 15.9 | 16.4 ± 2.4 | 13.14 ± 0.85 |
| THCVA-BDS | 214.4 ± 20.6 | 5.8 ± 2.2 | NT |
| Allyl isothiocyanate | 100 | 1.4 ± 0.01 | NT |
The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods and was assessed in HEK-293 cells stably over-expressing the rat recombinant TRPA1 channel, and as a control, in wild-type HEK-293 cells. Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 100, 1000, 10 000 and 25 000 nM) of the pure compounds, or amounts of BDS estimated to contain equimolar concentrations of the major cannabinoid, were given to cells. Efficacy was calculated as % of the effect obtained with allyl isothiocyanate (100 µM), the effect of which was ∼30% of that of ionomycin (4 µM). In the antagonism-desensitization experiments, the same concentrations of the pure compounds were given to cells 5 min prior to allyl isothiocyanate (100 µM).
Data are from De Petrocellis et al. 2008.
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid; NT, not tested.
Activity at TRPM8 channels
All the TRPM8 experiments were carried out at 22°C. None of the compounds tested activated TRPM8-mediated Ca2+ elevation in HEK-293 cells stably transfected with cDNA encoding the rat TRPM8 (denoted hereafter as ‘TRPM8-HEK-293’), and overexpressing high levels of TRPM8 transcript (De Petrocellis et al., 2007). Instead, when the compounds were given to cells 5 min before the known TRPM8 agonist, icilin (0.25 µM), they all, with the exception of CBC, antagonized the Ca2+ elevation response. As shown previously, icilin dose dependently elevated intracellular Ca2+ in TRPM8-HEK-293 cells, but not in non-transfected cells, with an EC50 of 0.19 ± 0.03 µM (De Petrocellis et al., 2007) (Table 2). The rank order of potency of the pure compounds against icilin was: CBD > CBG > THCA > CBN > THCV > CBDV > CBGA > THCVA > CBGV > CBDA (IC50 = 0.06–4.8 µM), but also some BDS (THCV, CBG, THCA) were very potent (IC50 < 0.1 µM) at antagonizing TRPM8 (Table 2). A ‘CBG-free’ CBG-BDS was found to be inactive per se, but when added to pure CBG, the activity of CBG-BDS was restored and this compound was significantly more potent and efficacious at antagonizing TRPM8 than pure CBG (Figure 1), thus pointing to a synergistic effect between this cannabinoid and some of the components of its corresponding Cannabis extract.
Table 2.
Potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as functional antagonists at TRPM8 channels
| Cannabinoid | IC50µM (against icilin 0.25 µM) |
|---|---|
| CBC | 40.7 ± 0.6 |
| CBC-BDS | 13.0 ± 1.1 |
| CBD | 0.06 ± 0.01 |
| CBD-BDS | 1.29 ± 0.04 |
| CBG | 0.16 ± 0.02 |
| CBG-BDS | 0.034 ± 0.003 |
| CBN | 0.21 ± 0.05 |
| CBDA | 4.78 ± 0.01 |
| CBDA-BDS | 1.2 ± 0.1 |
| CBGA | 1.31 ± 0.09 |
| CBDV | 0.90 ± 0.01 |
| CBDV-BDS | 1.2 ± 0.05 |
| CBGV | 1.71 ± 0.04 |
| CBGV-BDS | 0.95 ± 0.03 |
| THC | 0.16 ± 0.01a |
| THCA | 0.15 ± 0.02 |
| THCA-BDS | 0.056 ± 0.0026 |
| THCV | 0.87 ± 0.006 |
| THCV-BDS | 0.02 ± 0.006 |
| THCVA | 1.33 ± 0.019 |
| THCVA-BDS | 0.45 ± 0.057 |
The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods and was assessed in HEK-293 cells stably over-expressing the rat recombinant TRPM8 channel, and as a control, in wild-type HEK-293 cells. Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 100, 1000, 10 000 and 25 000 nM) of the pure compounds, or amounts of BDS estimated to contain equimolar concentrations of the major cannabinoid, were given to cells 5 min prior to icilin (0.25 µM). None of the compounds exerted any significant TRPM8-mediated effect on intracellular calcium per se (not shown).
Data are from De Petrocellis et al., 2008.
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid.
Figure 1.
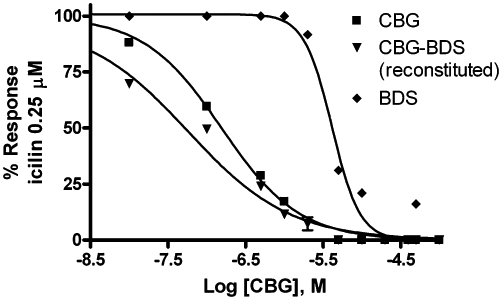
Inhibition of TRPM8-mediated elevation of intracellular calcium by cannabigerol (CBG), a CBG-botanical drug substance rendered free of CBG [denoted as botanical extract (BDS)] and the reconstituted CBG-BDS (denoted as CBG-BDS reconstituted). Experiments were carried out in HEK-293 cells stably over-expressing the rat recombinant TRPM8 channel and are means of at least n = 3 separate experiments. ‘CBG-BDS reconstituted’ was obtained by mixing the amount of CBG present in a given amount of CBG-BDS and the remaining amount of the ‘CBG-free’ BDS. The concentrations tested of ‘CBG-BDS reconstituted’ were calculated to contain equimolar concentrations of CBG as in pure CBG. The potency of ‘CBG-BDS reconstituted’ was slightly lower than that shown in Table 2 for CBG-BDS but still higher than that of CBG. SEM are not shown for the sake of clarity and were never higher than 10% of the means.
Activity at TRPV1 channels
Given the previously reported effects of some cannabinoids on TRPV1 (Bisogno et al., 2001; Ligresti et al., 2006), it was interesting to assess whether those compounds belonging to this class that had not been tested before on TRPV1 can interact with this target. Apart from the previously reported stimulating effects of CBD and CBG on TRPV1 (Bisogno et al., 2001; Ligresti et al., 2006), we found here that THCV and CBGV, but much less so their acid analogues, also stimulated human TRPV1 (EC50 = 1.0–2.0 µM), with the BDS being less efficacious than the corresponding cannabinoid, except for THCV-BDS (0.2 µM) (Table 3, Figure 2). Many compounds exhibited very weak efficacy at TRPV1 receptors, often hardly measurable. Therefore, the EC50 values calculated for these compounds may have little pharmacological meaning. Nevertheless, several compounds also desensitized TRPV1 to its subsequent (5 min after addition of the cannabinoid) stimulation by capsaicin (Table 3).
Table 3.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as functional agonists at TRPV1 channels, and the potency of pure cannabinoids as desensitizers of TRPV1 to the effect of capsaicin
| Cannabinoid | Efficacy (% ionomycin 4 µM) | Potency shown as EC50 (µM) | Desensitization (% capsaicin 0.1 µM) IC50 (µM) |
|---|---|---|---|
| CBC | <10 | 24.2 ± 3.1 | >50 |
| CBC-BDS | 29.1 ± 0.9 | 8.6 ± 1.2 | NT |
| CBD | 44.7 ± 0.02 | 1.0 ± 0.1 | 0.6 ± 0.05 |
| CBD-BDS | 59.9 ± 1.4 | 1.2 ± 0.4 | NT |
| CBG | 33.8 ± 2.3 | 1.3 ± 0.5 | 2.6 ± 0.2 |
| CBG-BDS | 23.0 ± 0.1 | 2.7 ± 0.1 | NT |
| CBN | <10 | 6.2 ± 3.7 | 81.7 ± 9.0 |
| CBDA | <10 | 19.7 ± 3.9 | 89.1 ± 0.3 |
| CBDA-BDS | 15.2 ± 0.01 | 10.3 ± 0.02 | NT |
| CBGA | 72.8 ± 2.0 | 21.0 ± 1.25 | 20.6 ± 0.5 |
| CBDV | 21.4 ± 0.6 | 3.6 ± 0.7 | 10.0 ± 0.5 |
| CBDV-BDS | 48.3 ± 1.1 | 4.5 ± 0.6 | NT |
| CBGV | 58.8 ± 0.9 | 2.0 ± 0.1 | 2.3 ± 0.3 |
| CBGV-BDS | 67.5 ± 1.8 | 3.9 ± 0.2 | NT |
| THC | <10 | ND | NT |
| THCA | <10 | ND | 19.2 ± 5.3 |
| THCA-BDS | <10 | 17.3 ± 0.1 | NT |
| THCV | 68.0 ± 1.6 | 1.5 ± 0.2 | 1.3 ± 0.1 |
| THCV-BDS | 48.9 ± 1.5 | 0.2 ± 0.04 | NT |
| THCVA | 20.0 ± 0.5 | 25.6 ± 1.1 | >50 |
| THCVA-BDS | 17.0 ± 1.4 | 29.0 ± 7.2 | NT |
The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods and was assessed in HEK-293 cells stably overexpressing the human recombinant TRPV1 channel, and as a control, in wild-type HEK-293 cells. Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 100, 1000, 10 000 and 25 000 nM) of the pure compounds, or amounts of BDS estimated to contain equimolar concentrations of the major cannabinoid, were given to cells. Efficacy was calculated as % of the effect obtained with ionomycin (4 µM). Capsaicin efficacy in this test is ∼75% of the effect of ionomycin. In the antagonism-desensitization experiments, the same concentrations of the pure compounds were given to cells 5 min prior to capsaicin (0.1 µM). CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid; ND, not determined; NT, not tested.
Figure 2.
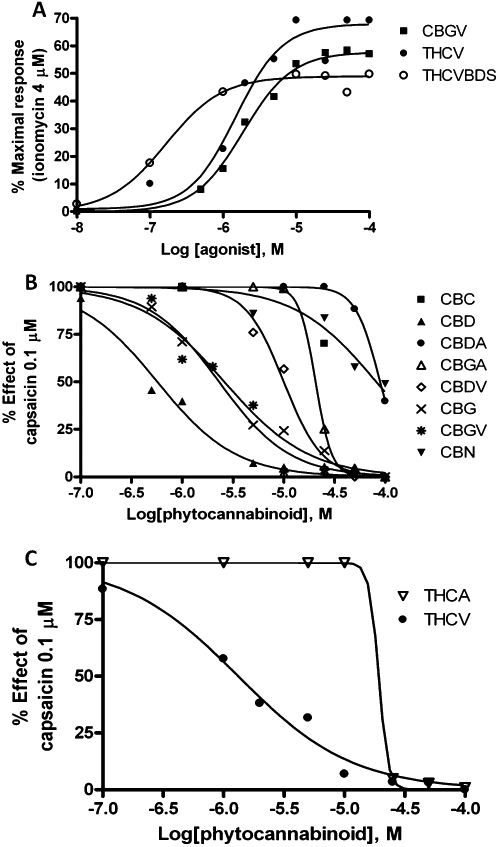
(A) Dose-dependent effects of cannabigivarin (CBGV), Δ9-tetrahydrocannabiverin (THCV) and THCV-botanical drug substance (THCV-BDS) on TRPV1-mediated elevation of intracellular calcium in HEK-293 cells stably over-expressing the human recombinant TRP channel of vanilloid type-1 (TRPV1) channel (HEK-293-TRPV1 cells). (B) Dose-dependent inhibitory effects of a 5 min pre-incubation of HEK-293-TRPV1 cells with several pure cannabinoids on the TRPV1-mediated elevation of intracellular calcium induced by capsaicin (0.1 µM). (C) Dose-dependent inhibitory effects of a 5 min pre-incubation of HEK-293-TRPV1 cells with THCV and THCA on the TRPV1-mediated elevation of intracellular calcium induced by capsaicin (0.1 µM). Data are means of at least n = 3 separate experiments. SEM are not shown for the sake of clarity and were never higher than 10% of the means. CBC, cannabichromene; CBD, cannabidiol; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; THCV, propyl analogue of THC or tetrahydrocannabivarin.
Activity at TRPV2 channels
The pure compounds available were also tested on TRPV2, based on the previously reported agonistic effect of CBD on this channel (Qin et al., 2008). We stably transfected HEK-293 cells with the plasmid pcDNA3 containing the rat recombinant TRPV2 cDNA, thus generating cells (denoted hereafter as ‘TRPV2-HEK-293’) that, as assessed by real-time PCR, express high levels of TRPV2 transcript (data not shown). This transcript was absent in HEK-293 cells that had not been transfected with the pcDNA3 plasmid and were treated with only Lipofectamine (data not shown). Using our fluorometric test, we showed that rat TRPV2-HEK293 cells exhibit a sharp increase in intracellular [Ca2+]i upon application of LPC or 2-aminoethoxydiphenyl borate, two well-established TRPV2 activators (Neeper et al., 2007; Monet et al., 2009). Using this test, we determined the concentration for half-maximal activation to be 3.4 ± 0.025 µM for LPC (Table 4, Figure 3). Analogous to the findings of Qin et al. (2008), THC and CBD, as well as several of the pure cannabinoids that were tested, increased [Ca2+]i in TRPV2-HEK-293 cells. The EC50 values are shown in Table 4, and the rank of potency was THC > CBD > CBGV > CBG > THCV > CBDV > CBN. Conversely, all the carboxylic acid cannabinoids and CBC were inactive or showed a very weak effect, as in the case of THCA, 18.4 ± 0.9 (3.0 ± 0.02) (Table 4). However, a 5 min preincubation of TRPV2-HEK-293 cells with CBGV, THC, CBG, CBD and THCV dose dependently abolished the elevation of [Ca2+]i induced by LPC 3 µM (Table 4). Importantly, the order of potency for desensitization did not merely reflect the rank order of potency for channel activation.
Table 4.
Efficacy and potency of pure cannabinoids as functional agonists at TRPV2 channels, and their potency as desensitizers of TRPV2 to the effect of lysophosphatidylcholine (LPC)
| Cannabinoid | Efficacy (% ionomycin 4 µM) | Potency shown as EC50 (µM) | Desensitization (% LPC 3 µM) IC50 (µM) |
|---|---|---|---|
| CBC | <10 | ND | 6.5 ± 1.6 |
| CBD | 40.5 ± 1.6 | 1.25 ± 0.23 | 4.5 ± 0.7 |
| CBG | 73.6 ± 1.2 | 1.72 ± 0.08 | 1.5 ± 0.2 |
| CBN | 39.9 ± 2.1 | 19.0 ± 3.7 | 15.7 ± 2.1 |
| CBDA | <10 | ND | 114.0 ± 18.0 |
| CBGA | <10 | ND | 87.3 ± 1.2 |
| CBDV | 49.9 ± 0.9 | 7.3 ± 0.4 | 31.1 ± 0.2 |
| CBGV | 75.4 ± 2.4 | 1.41 ± 0.36 | 0.7 ± 0.06 |
| THC | 53.0 ± 1.4 | 0.65 ± 0.05 | 0.8 ± 0.1 |
| THCA | 68.2 ± 1.0 | 18.4 ± 0.9 | 9.8 ± 2.6 |
| THCV | 73.8 ± 1.0 | 4.11 ± 0.11 | 0.8 ± 0.5 |
| THCVA | <10 | ND | 104.3 ± 9.9 |
| LPC | 91.71 ± 0.47 | 3.37 ± 0.025 | NT |
The effect of the compounds on the elevation of intracellular calcium was measured by fluorescence as described in Methods and was assessed in HEK-293 cells stably overexpressing the rat recombinant TRPV2 channel, and as a control, in wild-type HEK-293 cells. Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 100, 1000, 10 000 and 25 000 nM) of the pure compounds were given to cells. Efficacy was calculated as % of the effect obtained with ionomycin (4 µM). In the antagonism-desensitization experiments, the same concentrations of the pure compounds were given to cells 5 min prior to LPC (3 µM).
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid; ND, not determined; NT, not tested.
Figure 3.
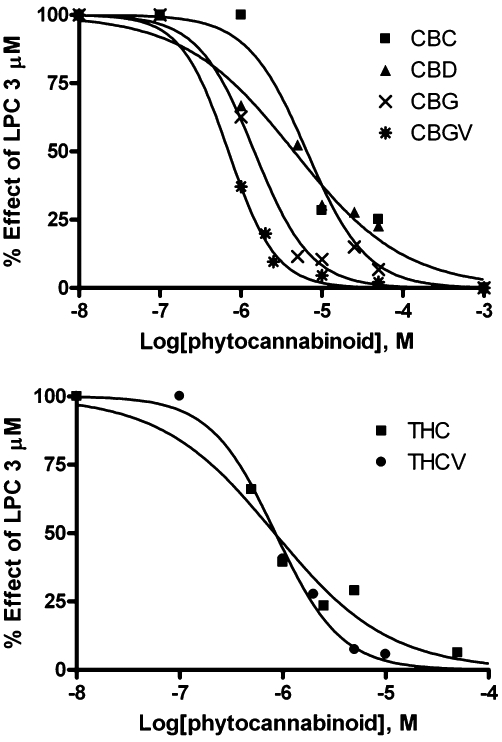
Dose-dependent effects of several pure cannabinoids on lysophosphatidylcholine (LPC, 3 µM)-induced, TRP channel of vanilloid type-2 (TRPV2)-mediated elevation of intracellular calcium in HEK-293 cells stably over-expressing the rat recombinant TRPV2 channel. Compounds were given to cells 5 min prior to LPC. Data are means of at least n = 3 separate experiments. SEM are not shown for the sake of clarity and were never higher than 10% of the means. CBC, cannabichromene; CBD, cannabidiol; CBG, cannabigerol; CBGV, cannabigivarin; THC, Δ9-tetrahydrocannabinol; THCV, propyl analogue of THC or tetrahydrocannabivarin.
Activity at 2-AG biosynthesizing and degrading enzymes
Two enzymes involved in the biosynthesis of 2-AG from diacylglycerol precursors were cloned and named sn-1-specific DAGLα and β. DAGLα was found in the adult brain to be mostly localized in post-synaptic neurones (Bisogno et al., 2003). An MAGL inactive on AEA has been suggested to act as a major 2-AG degrading enzyme (Dinh et al., 2002). Both DAGL and MAGL are members of the large lipase-3 family of Ser hydrolases. Several cannabinoids were screened here as possible DAGLα and MAGL inhibitors. The inhibitory effect of compounds on DAGLα was tested in COS cells over-expressing human DAGLα, the most abundant of the two DAGLs in the adult brain (Bisogno et al., 2003); wild-type (i.e. non-transfected) COS cells also exhibited DAGL activity but this was significantly lower that the activity of cells transfected with human DAGLα cDNA and was subtracted from the latter when calculating the IC50 values of the inhibitors (Bisogno et al., 2006). As reported in Table 5, CBDV (IC50 = 16.6 µM), and all the acids tested, exhibited inhibitory effects on DAGLα (IC50 = 19.4–65.7 µM). Of all the BDS tested, DAGLα inhibitory activity was found only in the CBDA-BDS and THCA-BDS. Because, of the pure compounds, only CBDV and all the acids inhibited DAGLα, we reasoned that the acid of CBDV might be even more potent. Therefore, we prepared a few milligrams of CBDVA and tested it only in this assay. However, this compound was not more potent than CBDV and CBDA (Table 5).
Table 5.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as inhibitors of human recombinant DAGLα
| Compounds | IC50 (µM) | Maximum concentration tested (% inhibition) |
|---|---|---|
| CBC | >100 µM | 100 µM |
| 39.5 ± 2.9 | ||
| CBC-BDS | >100 µM | 100 µM |
| 36.5 ± 7.1 | ||
| CBD | >100 µM | 100 µM |
| 0 | ||
| CBD-BDS | >50 µM | 50 µM |
| 8.5 ± 3.1 | ||
| CBG | >100 µM | 100 µM |
| 44.8 ± 4.1 | ||
| CBG-BDS | 65.7 ± 10.3 | 100 µM |
| 61.2 ± 4.9 | ||
| CBN | >50 µM | 50 µM |
| 26.9 ± 3.8 | ||
| CBDA | 19.4 ± 2.7 | 50 µM |
| 90.2 ± 2.6 | ||
| CBDA-BDS | 21.8 ± 1.3 | 50 µM |
| 87.4 ± 2.4 | ||
| CBGA | 30.5 ± 1.4 | 100 µM |
| 67.6 ± 0.3 | ||
| CBDV | 16.6 ± 4.1 | 100 µM |
| 83.3 ± 1.8 | ||
| CBDV-BDS | >50 µM | 50 µM |
| 16.2 ± 6.3 | ||
| CBGV | >50 µM | 50 µM |
| 18.9 ± 6.9 | ||
| CBGV-BDS | >50 µM | 50 µM |
| 47.1 ± 8.5 | ||
| THCA | 27.3 ± 1.6 | 50 µM |
| 86.2 ± 6.9 | ||
| THCA-BDS | 21.8 ± 2.8 | 50 µM |
| 97.6 ± 1.4 | ||
| THCV | >50 µM | 50 µM |
| 8.8 ± 0.7 | ||
| THCV-BDS | >50 µM | 50 µM |
| 0.5 ± 0.1 | ||
| THCVA | >50 µM | 100 µM |
| 71.6 ± 5.1 | ||
| THCVA-BDS | >50 µM | 100 µM |
| 52.1 ± 10.3 | ||
| CBDVA | 35.0 ± 5.6 | 50 µM |
| 61.0 ± 0.7 |
Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 25, 50 or 100 µM) of the pure compounds/BDS were incubated. Efficacy was calculated as % of DAGLα inhibition at the maximal concentration tested (which is shown in the first line of the cell).
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; DAGLα, diacylglycerol lipase α; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid.
The effect of the compounds on MAGL was studied in cytoplasmatic fractions of COS cells by using [3H]-2-AG as a substrate, as we had previously reported that the enzyme was abundantly expressed in this preparation (Bisogno et al., 2006). None of the pure compounds exhibited any significant inhibition up to 50 µM (Table 6), whereas some BDS exhibited significant MAGL inhibitory efficacy (IC50 = 6.2–29.6 µM), CBG-BDS and THCA-BDS being the most potent (Table 6). Indeed, a ‘CBG-free’ CBG-BDS was found to be weakly active per se, but, when added to pure CBG, the activity of CBG-BDS was restored and this reconstituted extract was significantly more potent and efficacious at inhibiting MAGL than pure CBG (Figure 4).
Table 6.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as inhibitors of COS cell MAGL
| Compounds | IC50 (µM) | Maximum concentration tested (% inhibition) |
|---|---|---|
| CBC | 50.1 ± 12.1 | 100 µM |
| 64.5 ± 3.6 | ||
| CBC-BDS | 91.4 ± 13.8 | 100 µM |
| 58.5 ± 8.1 | ||
| CBD | >100 µM | 100 µM |
| 0 | ||
| CBD-BDS | >50 µM | 50 µM |
| 23.0 ± 3.8 | ||
| CBG | 95.7 ± 32.4 | 100 µM |
| 54.2 ± 3.8 | ||
| CBG-BDS | 24.6 ± 8.4 | 100 µM |
| 66.1 ± 3.4 | ||
| CBN | >50 µM | 50 µM |
| 31.5 ± 3.3 | ||
| CBDA | >50 µM | 50 µM |
| 18.5 ± 2.1 | ||
| CBDA-BDS | 29.6 ± 1.1 | 50 µM |
| 94.0 ± 10.5 | ||
| CBGA | >50 µM | 50 µM |
| 17.2 ± 1.8 | ||
| CBDV | >100 µM | 100 µM |
| 2.5 ± 0.1 | ||
| CBDV-BDS | >50 µM | 50 µM |
| 5.2 ± 0.6 | ||
| CBGV | >50 µM | 50 µM |
| 4.9 ± 1.9 | ||
| CBGV-BDS | >50 µM | 50 µM |
| 37.9 ± 5.7 | ||
| THCA | 46.0 ± 1.2 | 100 µM |
| 86.0 ± 4.1 | ||
| THCA-BDS | 6.2 ± 1.2 | 50 µM |
| 86.3 ± 13.1 | ||
| THCV | >50 µM | 50 µM |
| 18.2 ± 5.4 | ||
| THCV-BDS | >50 µM | 50 µM |
| 23.5 ± 5.2 | ||
| THCVA | >50 µM | 50 µM |
| 16.9 ± 9.6 | ||
| THCVA-BDS | >50 µM | 50 µM |
| 39.8 ± 5.7 |
Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 25, 50 or 100 µM) of the pure compounds/BDS were incubated. Efficacy was calculated as % of MAGL inhibition at the maximal concentration tested (which is shown in the first line of the cell).
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; MAGL, monoacylglycerol lipase; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid.
Figure 4.
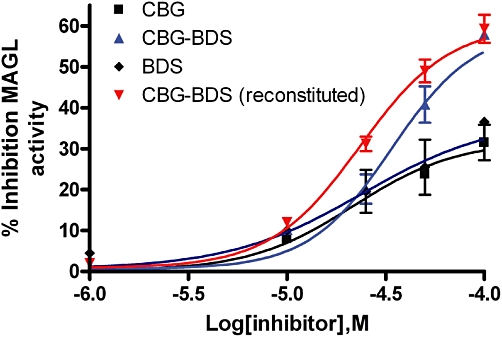
Inhibition of the effect of MAGL by cannabigerol (CBG), a CBG-botanical drug substance (CBG-BDS) as such or rendered free of CBG (denoted as BDS), and the reconstituted CBG-BDS (denoted as CBG-BDS reconstituted). Experiments were carried out in homogenates from COS cells and are means ± SEM of at least n = 3 separate experiments. ‘CBG-BDS reconstituted’ was obtained by mixing the amount of CBG present in a given amount of CBG-BDS and the remaining amount of the ‘CBG-free’ BDS. The concentrations tested of ‘CBG-BDS reconstituted’ were calculated to contain equimolar concentrations of CBG as in pure CBG. BDS, botanical drug substance; CBG, cannabigerol; MAGL, monoacylglycerol lipase.
Activity at AEA and PEA degrading enzymes
AEA and PEA are inactivated by enzymatic hydrolysis catalyzed by amidases, the best characterized of which is the FAAH (Cravatt et al., 1996). All compounds were therefore evaluated in assays of FAAH using [14C]-AEA hydrolysis by rat brain membranes (which express FAAH as the only AEA hydrolyzing enzyme). The effects are shown in Table 7. It is noteworthy that CBD was the only compound that inhibited FAAH, this effect having been already reported previously (Watanabe et al., 1998).
Table 7.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as inhibitors of rat brain FAAH
| Compounds | IC50 (µM) | Maximum concentration tested (% inhibition) |
|---|---|---|
| CBC | >100 µM | 100 µM |
| 46.5 ± 1.3 | ||
| CBC-BDS | 53.2 ± 11.3 | 100 µM |
| 68.6 ± 4.1 | ||
| CBD | 15.2 ± 3.2 | 50 µM |
| 87.2 ± 4.1 | ||
| CBD-BDS | 30.8 ± 6.2 | 100 µM |
| 89.4 ± 2.5 | ||
| CBG | >100 µM | 100 µM |
| 44.9 ± 1.4 | ||
| CBG-BDS | 62.9 ± 21.4 | 100 µM |
| 66.9 ± 5.0 | ||
| CBN | >50 µM | 50 µM |
| 29.1 ± 3.3 | ||
| CBDA | >50 µM | 50 µM |
| 42.2 ± 4.0 | ||
| CBDA-BDS | >50 µM | 50 µM |
| 43.9 ± 6.0 | ||
| CBGA | >50 µM | 50 µM |
| 46.2 ± 4.9 | ||
| CBDV | >100 µM | 100 µM |
| 36.2 ± 3.1 | ||
| CBDV-BDS | 53.4 ± 8.3 | 100 µM |
| 77.9 ± 1.1 | ||
| CBGV | >50 µM | 50 µM |
| 38.7 ± 3.8 | ||
| CBGV-BDS | >50 µM | 50 µM |
| 45.3 ± 6.5 | ||
| THCA | >50 µM | 50 µM |
| 38.8 ± 3.1 | ||
| THCA-BDS | >50 µM | 50 µM |
| 44.4 ± 4.0 | ||
| THCV | >100 µM | 100 µM |
| 26.8 ± 3.4 | ||
| THCV-BDS | >100 µM | 100 µM |
| 48.3 ± 3.4 | ||
| THCVA | >100 µM | 100 µM |
| 27.0 ± 3.6 | ||
| THCVA-BDS | >50 µM | 100 µM |
| 62.1 ± 1.4 |
Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 25, 50 or 100 µM) of the pure compounds/BDS were incubated. Efficacy was calculated as % of FAAH inhibition at the maximal concentration tested (which is shown in the first line of the cell).
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; FAAH, fatty acid amide hydrolase; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid.
PEA inactivation to palmitic acid and ethanolamine is catalyzed by FAAH and, more selectively, NAAA (Ueda et al., 2001). The screening of different cannabinoids allowed us to identify three BDS preparations that inhibited NAAA: CBC-BDS > CBG-BDS > CBGV-BDS (IC50 = 14.2–24.4 µM). The only pure compound with appreciable inhibitory activity was CBDA (IC50 = 23.0 µM) (Table 8). All these compounds were selective for NAAA, and were significantly much less active or inactive at FAAH, with IC50 values always higher than 50 µM (Table 7).
Table 8.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as inhibitors of human recombinant NAAA
| Compounds | IC50 (µM) | Max concentration tested (% inhibition) |
|---|---|---|
| CBC | >100 µM | 100 µM |
| 40.6 ± 2.1 | ||
| CBC-BDS | 14.2 ± 6.2 | 100 µM |
| 81.0 ± 5.1 | ||
| CBD | >100 µM | 100 µM |
| 47.2 ± 2.6 | ||
| CBD-BDS | >100 µM | 100 µM |
| 47.2 ± 3.4 | ||
| CBG | >100 µM | 100 µM |
| 19.4 ± 2.1 | ||
| CBG-BDS | 18.3 ± 9.4 | 100 µM |
| 68.5 ± 8.2 | ||
| CBN | >50 µM | 50 µM |
| 31.4 ± 2.9 | ||
| CBDA | 23.0 ± 1.3 | 50 µM |
| 62.5 ± 1.1 | ||
| CBDA-BDS | >50 µM | 50 µM |
| 43.8 ± 8.4 | ||
| CBGA | >50 µM | 50 µM |
| 33.2 ± 2.9 | ||
| CBDV | 72.3 ± 18.4 | 100 µM |
| 54.5 ± 6.1 | ||
| CBDV-BDS | >100 µM | 100 µM |
| 47.2 ± 8.2 | ||
| CBGV | >50 µM | 50 µM |
| 38.4 ± 4.0 | ||
| CBGV-BDS | 24.4 ± 2.1 | 100 µM |
| 75.0 ± 9.1 | ||
| THCA | >50 µM | 50 µM |
| 21.1 ± 1.8 | ||
| THCA-BDS | >50 µM | 50 µM |
| 25.5 ± 6.8 | ||
| THCV | >100 µM | 100 µM |
| 18.2 ± 9.1 | ||
| THCV-BDS | >100 µM | 100 µM |
| 21.4 ± 3.1 | ||
| THCVA | >100 µM | 100 µM |
| 20.5 ± 3.5 | ||
| THCVA-BDS | >100 µM | 100 µM |
| 44.7 ± 4.3 |
Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 10, 25, 50 or 100 µM) of the pure compounds/BDS were incubated. Efficacy was calculated as % of NAAA inhibition at the maximal concentration tested (which is shown in the first line of the cell).
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; NAAA, N-acylethanolamine acid amide hydrolase; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid.
The extent and duration of AEA actions are determined by its extracellular concentration, controlled in turn by the rate of AEA synthesis and degradation. As the hydrolytic enzymes are intracellularly located, an efficient transport system allowing for the rapid trafficking of AEA through these topographically separated sites was proposed. Although this putative ‘carrier’ has yet to be cloned, some indirect biochemical and pharmacological evidence suggests its existence (Di Marzo et al., 1994; Ligresti et al., 2004; 2010;). The 21 cannabinoid preparations were therefore evaluated for their activity on [14C]-AEA uptake by intact RBL-2H3 cells. Eleven were found to be active (Table 9), and interestingly none of the most potent preparations inhibited FAAH (Table 7). CBC = CBG > CBD inhibited AEA uptake most efficaciously and potently (IC50 = 11.3–25.3 µM), but some BDS (i.e. those of THCVA, CBGV, CBDA, THCA) were even more potent (IC50 = 5.8–12.5 µM) than the corresponding pure compounds in this assay (Table 9).
Table 9.
Efficacy and potency of pure cannabinoids and of most of their corresponding ‘botanical drug substance’ (BDS) as inhibitors of anandamide cellular uptake by RBL-2H3 cells
| Compounds | IC50 (µM) | Max concentration tested (% inhibition) |
|---|---|---|
| CBC | 12.3 ± 2.7 | 25 µM |
| 83.2 ± 3.5 | ||
| CBC-BDS | 15.7 ± 4.2 | 25 µM |
| 83.6 ± 7.8 | ||
| CBD | 25.3 ± 1.8 | 25 µM |
| 49.9 ± 1.3 | ||
| CBD-BDS | 11.5 ± 3.2 | 25 µM |
| 76.8 ± 5.3 | ||
| CBG | 11.3 ± 4.2 | 25 µM |
| 85.4 ± 0.8 | ||
| CBG-BDS | 14.2 ± 2.5 | 25 µM |
| 89.3 ± 2.7 | ||
| CBN | ∼25 | 25 µM |
| 50.0 ± 31.8 | ||
| CBDA | >25 | 25 µM |
| 36.3 ± 3.3 | ||
| CBDA-BDS | 5.8 ± 1.3 | 50 µM |
| 70.3 ± 2.4 | ||
| CBGA | >25 | 25 µM |
| 43.6 ± 5.0 | ||
| CBDV | 21.3 ± 1.8 | 25 µM |
| 52.7 ± 3.0 | ||
| CBDV-BDS | >25 | 25 µM |
| 16.4 ± 3.2 | ||
| CBGV | >25 | 25 µM |
| 31.2 ± 4.0 | ||
| CBGV-BDS | 6.9 ± 1.5 | 25 µM |
| 78.7 ± 21.3 | ||
| THCA | >25 | 25 µM |
| 34.4 ± 2.8 | ||
| THCA-BDS | 9.7 ± 1.6 | 25 µM |
| 90.0 ± 4.6 | ||
| THCV | >25 | 25 µM |
| 46.9 ± 2.0 | ||
| THCV-BDS | >25 | 25 µM |
| 49.3 ± 2.2 | ||
| THCVA | >25 | 25 µM |
| 47.2 ± 2.1 | ||
| THCVA-BDS | 12.5 ± 5.2 | 25 µM |
| 71.0 ± 8.3 |
Data are means ± SEM of at least n = 3 separate experiments in which various concentrations (1, 5, 10, 25 µM) of the pure compounds/BDS were incubated. Efficacy was calculated as % of uptake inhibition at the maximal concentration tested (25 µM).
CBC, cannabichromene; CBD, cannabidiol; CBDA, cannabidiol acid; CBDV, propyl analogue of CBD or cannabidivarin; CBG, cannabigerol; CBGV, cannabigivarin; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; THCA, THC acid; THCV, propyl analogue of THC or tetrahydrocannabivarin; THCVA, tetrahydrocannabivarin acid.
Discussion
The results reported here can be summarized as follows:
Cannabinoids can activate TRPA1, TRPV1 and TRPV2, and functionally antagonize TRPM8 receptors in a manner dependent on their chemical structure – importantly, the structural prerequisites necessary to interact with the various TRPs seem to vary depending on the type of the channel, with CBC being the most potent at TRPA1 and the least potent at antagonizing TRPM8; CBN being relatively potent at TRPA1 and only weakly active at TRPV2; CBG and CBD the most potent at TRPM8 and TRPV1, and THC, THCV and CBD at TRPV2. With all TRP channels tested here, the cannabinoid acids were usually weakly active or inactive, whereas the propyl analogues were usually slightly less active than the corresponding pentyl cannabinoids. A notable exception to the former rule was the strong effect of THCA at TRPM8.
Several cannabinoids also interfered at mid-µM concentrations with enzymes catalyzing 2-AG biosynthesis via DAGLα, or with AEA inactivation via the putative membrane transporter or FAAH.
The effects of the BDS at TRP channels reflected in many cases those of the corresponding pure cannabinoids. However, in some cases, these extracts were endowed with pharmacological actions of their own at the investigated targets, as in the case of the inhibitory effects observed on MAGL and NAAA, or with a capacity to inhibit AEA uptake, antagonize TRPM8 or activate TRPA1 that was stronger than that of the corresponding pure cannabinoids.
Our current findings extend the concept that some TRP channels may serve as ionotropic cannabinoid receptors, which, in the context of primary afferent nerve fibres, may contribute to inflammatory hypersensitivity or vasodilatation. These results were not completely unexpected, because we had found previously that some cannabinoids activate TRPV1 (Ligresti et al., 2006) and TRPA1 (De Petrocellis et al., 2008) and antagonize TRPM8 (De Petrocellis et al., 2008), and others have reported that THC and CBN activate TRPA1 (Jordt et al., 2004), and that CBD activates TRPV2 (Qin et al., 2008). However, here we investigated for the first time the activity at these four targets of all pure plant cannabinoids, and of all Cannabis BDS, available to date, thus allowing an initial structure–activity relationship study of the effects of these natural compounds at TRP channels, and a direct comparison with extracts that can be easily obtained from Cannabis strains specifically bred to produce one principal cannabinoid over the others. A limitation of our study is that we did not investigate the effects of the compounds on native cells expressing the TRP channels, such as, for example, sensory neurones from dorsal root or trigeminal ganglia, which are likely to possess a level of cellular structure and subcellular organization with respect to TRP channel localization different from that of HEK-293 cells. Furthermore, we did not assess, for example by using patch clamp techniques, whether or not the effects of the compounds are exerted directly on the channels. However, it must be remembered that all effects at TRP channels reported so far for plant and synthetic cannabinoids were shown to occur also in dorsal root ganglia neurones (Jordt et al., 2004; De Petrocellis et al., 2008; Qin et al., 2008) and to be due to direct activation of the currents corresponding to these channels (Jordt et al., 2004; Akopian et al., 2008). Therefore, we have no reason to believe that the effects reported here are also not direct and do not occur in sensory neurones constitutively expressing the corresponding TRP channels. On the other hand, evidence has been obtained against cannabinoid interaction with TRP channels in cells with a level of expression of native TRP channels lower than in sensory neurones (Massi et al., 2004; Drysdale et al., 2006; Giudice et al., 2007).
We have also shown here that the stimulating effects of cannabinoids at TRPA1, TRPV1 and TRPV2 result in the desensitization of these channels. In fact, as only those compounds that activated TRPV1 or TRPV2 or TRPA1 per se also antagonized the action of capsaicin or LPC or allyl isothiocyanates, respectively, it is unlikely that these effects were due to antagonism, as in the case of TRPM8, and not to channel desensitization, although clearly the former possibility cannot be completely ruled out. However, the potency for desensitization for a given cannabinoid was not always proportional to its potency as an agonist per se. Indeed, it is now well established that the ability of TRP agonists to also desensitize these channels depends not only on their potency but also on their lipophilicity and facility to penetrate and cross the plasma membrane (Morita et al., 2006; Ursu et al., 2010). Desensitization might have important consequences for the potential use of cannabinoids as therapeutic agents in those disorders in which these four channels have been shown to be implicated and to play a permissive role, such as chronic and inflammatory pain and cancer. It was reported previously that CBD evokes a concentration-dependent release of CGRP from cultured rat DRG neurones, which depended on extracellular calcium, was blocked by ruthenium red (a non-selective inhibitor of TRP channels) and was due to TRPA1 and TRPV2 activation (Qin et al., 2008). Using selective knockdown of TRPV2 or TRPA1 mRNA levels, it was concluded that at least a significant component of the CBD-evoked CGRP release and antinociception was due to activation and desensitization of TRPV2 respectively (Qin et al., 2008). Importantly, synthetic cannabinoids that activate cannabinoid receptors were also shown to exert antinociceptive effects partly via TRPA1 desensitization (Akopian et al., 2008).
Also, the inhibitory effects of CBDV, CBDA, CBGA, THCA and CBDVA on DAGLα might have important therapeutic implications. The inhibition of DAGLα with a selective synthetic inhibitor was recently found to inhibit the intake of palatable food compared with normal chow in mice (Bisogno et al., 2009), and excessive 2-AG levels were associated with abdominal obesity and its metabolic consequences, such as dyslipidaemia and glucose intolerance (Di Marzo and Després, 2009). Therefore, if the above cannabinoids are also found to inhibit DAGLαin vivo and shown to lead to a reduction in the excessive 2-AG levels that accompany hyperphagia and abdominal obesity, one might propose the testing of, for example, CBDV, CBDA and CBDVA, in animal models of obesity and, eventually, in clinical trials in obese individuals.
As to the ability of THCA-BDS, CBDA-BDS and CBG-BDS to inhibit MAGL, this initially seemed to be due mostly to non-cannabinoid components of the extracts, as suggested by the fact that some plant natural products of terpenoid origin were recently found to potently inhibit this enzyme (King et al., 2009), and by the observation that pure THCA, CBDA and CBG were significantly less potent, when not inactive, at inhibiting MAGL. However, when we tested a ‘CBG-free’ CBG-BDS, we also found it to be less active than CBG-BDS, thus suggesting that both cannabinoid and non-cannabinoid-components of Cannabis extracts might cooperate at inhibiting the enzyme. These findings indicate that these extracts might be used to inhibit chronic and inflammatory pain, because URB602, a synthetic compound with lower potency at MAGL than THCA-BDS, CBDA-BDS and CBG-BDS, was previously reported to act as an efficacious anti-hyperalgesic and anti-inflammatory compound in animal models (Hohmann et al., 2005; Comelli et al., 2007; Guindon et al., 2007). A similar possibility might also exist for CBC-BDS, and CBGV-BDS as NAAA inhibitors, as synthetic compounds capable of inhibiting this enzyme, and subsequently of prolonging the lifespan of the anti-inflammatory endogenous endocannabinoid-like mediator, PEA, exert anti-inflammatory effects (Solorzano et al., 2009; Petrosino et al., 2010). In particular, CBG-BDS, due to its ability to inhibit both MAGL and NAAA, and antagonize TRPM8, should be tested in the future in animal models of chronic pain.
The use of several cannabinoids as analgesics is also suggested by their inhibitory effects on endocannabinoid inactivation mediated by either FAAH (which under certain conditions can also recognize 2-AG as a substrate) or AEA cellular reuptake (which occurs through an as yet uncharacterized mechanism that also recognizes 2-AG). Interestingly, several BDS and compounds were shown here to inhibit ACU even in the absence of any effect on FAAH, thus arguing in favour of the possibility that the re-uptake of endocannabinoids does not occur uniquely via FAAH-driven diffusion across the plasma membrane (Ligresti et al., 2004).
In conclusion, our data support the current concept (Izzo et al., 2009) that not only cannabinoids different from THC (and CBD), but also cannabis extracts enriched in such compounds, might be used in the future as potential therapeutic agents, especially if the procedures for extraction of plant materials and the selection of the appropriate strains of Cannabis are standardized. Our findings also provide evidence that these natural products can interact with proteins of the ECS as well as with TRPV1, TRPV2, TRPA1 and TRPM8 channels. The present results also reinforce the suggestion that the latter channels, along with their role as sensors of thermic, inflammatory, mechanical and chemical stimuli, should be regarded as ionotropic cannabinoid receptors (Akopian et al., 2009; De Petrocellis and Di Marzo, 2010). Finally, independently of their possible therapeutic implications, our present data suggest the use of some cannabinoids as pharmacological tools, especially for the study of those proteins, such as TRPV2 and TRPA1, for which very few selective agonists or antagonists have been reported. Future studies are needed to characterize those components of the various Cannabis extracts used here that inhibit the inactivation of EC and EC-like compounds, and the nature of their interactions with the effects of pure cannabinoids.
Acknowledgments
This study was partly funded by GW Pharma, UK.
Glossary
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 5-HT
5-hydroxy-tryptamine
- ACU
anandamide cellular uptake
- AEA
anandamide
- BDS
botanical drug substance
- CB1
cannabinoid receptor type-1
- CB2
cannabinoid receptor type-2
- CBC
cannabichromene
- CBD
cannabidiol
- CBDA
cannabidiol acid
- CBDV
propyl analogue of CBD or cannabidivarin
- CBDVA
acid of the propyl analogue of CBD
- CBG
cannabigerol
- CBGA
cannabigerol acid
- CBGV
propyl analogue of CBG or cannabigivarin
- CBN
cannabinol
- DAGLα
diacylglycerol lipase α
- EC
endocannabinoid
- FAAH
fatty acid amide hydrolase
- MAGL
monoacylglycerol lipase
- NAAA
N-acylethanolamine acid amide hydrolase
- THC
Δ9-tetrahydrocannabinol
- THCA
THC acid
- THCV
propyl analogue of THC or tetrahydrocannabivarin
- THCVA
tetrahydrocannabivarin acid
- TRP
transient receptor potential
- TRPA1
TRP channel of ankyrin type 1
- TRPM8
TRP channel of melastatin type-8
- TRPV1
TRP channel of vanilloid type-1
- TRPV2
TRP channel of vanilloid type-2
Conflict of interests
VD is the recipient of a research grant from GW Pharma, Ltd. CGS is an employee of GW Pharma, Ltd.
Supporting Information
Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2009;30:79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signalling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, et al. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta. 2006;1761:205–212. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Burston JJ, Rai R, Allarà M, Saha B, Mahadevan A, et al. Synthesis and pharmacological activity of a potent inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol. ChemMedChem. 2009;4:946–950. doi: 10.1002/cmdc.200800442. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol. 2010;160:677–687. doi: 10.1111/j.1476-5381.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154:1001–1008. doi: 10.1038/bjp.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci USA. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159:129–141. doi: 10.1111/j.1476-5381.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br J Pharmacol. 2007;152:787–794. doi: 10.1038/sj.bjp.0707425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, et al. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:294–299. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Starowicz K, Schiano-Moriello A, Vivese M, Orlando P, Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res. 2007;313:1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- Delong GT, Wolf CE, Poklis A, Lichtman AH. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010;112:126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Després JP. CB1 antagonists for obesity – what lessons have we learned from rimonabant? Nat Rev Endocrinol. 2009;5:633–638. doi: 10.1038/nrendo.2009.197. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. In: Pertwee RG, editor. Cannabinoids. Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. pp. 147–185. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AJ, Ryan D, Pertwee RG, Platt B. Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology. 2006;50:621–631. doi: 10.1016/j.neuropharm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, Fernández-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–170. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Giudice ED, Rinaldi L, Passarotto M, Facchinetti F, D'Arrigo A, Guiotto A, et al. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J Leukoc Biol. 2007;81:1512–1522. doi: 10.1189/jlb.1206738. [DOI] [PubMed] [Google Scholar]
- Gong J-P, Onaivi ES, Ishiguro H, Liu Q-R, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum NS, Davis WM, Elsohly MA, Turner CE. Cannabichromene and Δ9-tetrahydrocannabinol: interactions relative to lethality, hypothermia and hexobarbital hypnosis. Gen Pharmacol. 1981;12:357–362. doi: 10.1016/0306-3623(81)90090-2. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, et al. Cannabidiol potentiates pharmacological effects of Δ9-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- King AR, Dotsey EY, Lodola A, Jung KM, Ghomian A, Qiu Y, et al. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem Biol. 2009;16:1045–1052. doi: 10.1016/j.chembiol.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, et al. Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem J. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, Schiano-Moriello A, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- Ligresti A, De Petrocellis L, Hernán Pérez de la Ossa D, Aberturas R, Cristino L, Schiano-Moriello A, et al. Exploiting nanotechnologies and TRPV1 channels to investigate the putative anandamide membrane transporter. PloS One. 2010;5:e10239. doi: 10.1371/journal.pone.0010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol. 2010;159:950–957. doi: 10.1111/j.1476-5381.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, et al. Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308:838–845. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Looking back at Cannabis research. Curr Pharm Des. 2000;6:1313–1322. doi: 10.2174/1381612003399509. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shvo Y. Hashish – I: the structure of cannabidiol. Tetrahedron. 1963;19:2073–2078. doi: 10.1016/0040-4020(63)85022-x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Δ(9) -THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Monet M, Gkika D, Lehen'kyi V, Pourtier A, Vanden Abeele F, Bidaux G, et al. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Morita A, Iwasaki Y, Kobata K, Iida T, Higashi T, Oda K, et al. Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat TRPV1. Life Sci. 2006;79:2303–2310. doi: 10.1016/j.lfs.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu SM. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–714. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology and therapeutic potential of cannabidiol. In: Di Marzo V, editor. Cannabinoids. London: Kluwer Academic/Plenum Publishers; 2004. pp. 32–83. [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Iuvone T, Di Marzo V. N-palmitoyl-ethanolamine: biochemistry and new therapeutic opportunities. Biochimie. 2010;92:724–727. doi: 10.1016/j.biochi.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29:2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saturnino C, Petrosino S, Ligresti A, Palladino C, De Martino G, Bisogno T, et al. Synthesis and biological evaluation of new potential inhibitors of N-acylethanolamine hydrolyzing acid amidase. Bioorg Med Chem Lett. 2010;20:1210–1213. doi: 10.1016/j.bmcl.2009.11.134. [DOI] [PubMed] [Google Scholar]
- Sofia RD, Nalepa SD, Vassar HB, Knobloch LC. Comparative anti-phlogistic activity of Δ9-tetrahydrocannabinol, hydrocortisone and aspirin in various rat paw edema models. Life Sci. 1974;15:251–260. doi: 10.1016/0024-3205(74)90214-8. [DOI] [PubMed] [Google Scholar]
- Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Natl Acad Sci USA. 2009;106:20966–20971. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Comm. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Elsohly MA. Biological activity of cannabichromene, its homologs and isomers. J Clin Pharmacol. 1981;21:283S–291S. doi: 10.1002/j.1552-4604.1981.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamanaka K, Yamamoto S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem. 2001;276:35552–355527. doi: 10.1074/jbc.M106261200. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA) Prog Lipid Res. 2010;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Ursu D, Knopp K, Beattie RE, Liu B, Sher E. Pungency of TRPV1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur J Pharmacol. 2010;641:114–122. doi: 10.1016/j.ejphar.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, et al. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology. 2006;186:226–234. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ogi H, Nakamura S, Kayano Y, Matsunaga T, Yoshimura H, et al. Distribution and characterization of anandamide amidohydrolase in mouse brain and liver. Life Sci. 1998;62:1223–1229. doi: 10.1016/s0024-3205(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Yang KH, Galadari S, Isaev D, Petroianu G, Shippenberg TS, Oz M. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J Pharmacol Exp Ther. 2010;333:547–554. doi: 10.1124/jpet.109.162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


