Abstract
BACKGROUND AND PURPOSE
The endogenous cannabinoid system participates in oligodendrocyte progenitor differentiation in vitro. To determine the effect of synthetic cannabinoids on oligodendrocyte differentiation, we exposed differentiating cultures of oligodendrocytes with cannabinoid CB1, CB2 and CB1/CB2 receptor agonists and antagonists. The response of the PI3K/Akt and the mammalian target of rapamycin (mTOR) signalling pathways were studied as effectors of cannabinoid activity.
EXPERIMENTAL APPROACH
Purified oligodendrocyte progenitor cells (OPC) obtained from primary mixed glial cell cultures were treated for 48 h with CB1, CB2 and CB1/CB2 receptor agonists (ACEA, JWH133 and HU210, respectively) in the presence or absence of the antagonists AM281 (CB1 receptor) and AM630 (CB2 receptor). Moreover, inhibitors of the phosphatidylinositol 3-kinase (PI3K)/Akt and mTOR pathways (LY294002 and rapamycin, respectively) were used to study the involvement of these pathways on cannabinoid-induced OPC maturation.
KEY RESULTS
ACEA, JWH133 and HU-210 enhanced OPC differentiation as assessed by the expression of stage specific antigens and myelin basic protein (MBP). Moreover, this effect was blocked by the CB receptor antagonists. ACEA, JWH133 and HU210 induced a time-dependent phosphorylation of Akt and mTOR, whereas the inhibitors of PI3K/Akt (LY294002) or of mTOR (rapamycin) reversed the effects of HU-210 on oligodendrocyte differentiation and kinase activation.
CONCLUSIONS AND IMPLICATIONS
Activation of cannabinoid CB1 or CB2 receptors with selective agonists accelerated oligodendrocyte differentiation through the mTOR and Akt signalling pathways.
LINKED ARTICLES
This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: oligodendrocyte progenitor, ACEA, JWH133, HU210, PI3K/Akt, mTOR, MBP
Introduction
The discovery of the psychoactive principle of Cannabis sativa L., Δ9-tetrahydrocannabinol (Δ9-THC), by Mechoulam over 46 years ago, marked the beginning of a new field of research into the pharmacological and physiological role of the cannabinoids (Gaoni and Mechoulam, 1964). Over the years, the importance of cannabinoid research has grown and developed, and it is currently considered by many to be one of the most exciting areas of neuropharmacology. Indeed, a specific endocannabinoid system has been shown to exist in the brain and the therapeutic potential of this system through its pharmacological manipulation has been explored (Devane et al., 1992; Mechoulam et al., 1995; Pertwee, 2005a; Fernandez-Ruiz et al., 2010a; Scotter et al., 2010). Accordingly, and in addition to the well-established behavioural effects of Δ9-THC, many other synthetic, plant-derived and endogenous cannabinoids exert profound effects on the immune system and the CNS (Cabral and Griffin-Thomas, 2008).
The therapeutic effects of cannabinoids in models of neurodegeneration have long been recognized (Grundy, 2002; Marsicano et al., 2003; Fernandez-Ruiz et al., 2010b), and it is believed that they may slow the neurodegeneration that ultimately leads to chronic disability in patients (Gowran et al., 2010). However, the role of cannabinoids in brain repair remains less clear, although several laboratories have found compelling evidence that cannabinoids may well play a significant role in both neuroregeneration and cell differentiation. Indeed, it was recently demonstrated that activation of the brain endocannabinoid system restored adult neurogenesis in the brain (see Molina-Holgado and Molina-Holgado, 2010), and that activation of the cannabinoid CB1 and CB2 receptors (nomenclature follows Alexander et al., 2009) up-regulates neurogenesis in vivo and in vitro (Aguado et al., 2006; Palazuelos et al., 2006; Molina-Holgado et al., 2007; Goncalves et al., 2008). Moreover, the neurogenic actions of the cannabinoids appear to influence the proliferation and differentiation of adult neural precursor cells in mice and rats, and in oligodendrocytes, cannabinoid receptors also affect progenitor survival and differentiation through phosphatidylinositol-3 kinase (PI3K)/Akt signalling (Arevalo-Martin et al., 2007; Molina-Holgado et al., 2002). Accordingly, endocannabinoids in the brain exert an important influence in neural development and brain repair.
2-Arachidonoyl glycerol (2-AG) is a ligand for the CB1 and CB2 receptors (Mechoulam et al., 1995; Sugiura et al., 1995; Sugiura and Waku, 2000), and two closely related diacylglycerol lipases (DAGLα and DAGLβ) that synthesize 2-AG have now been cloned (Bisogno et al., 2003). DAGL activity hydrolyses DAG into 2-AG, the most abundant endocannabinoid in the CNS (Sugiura and Waku, 2000). We recently showed endogenous 2-AG to be involved in the complex process of oligodendrocyte differentiation, also demonstrating that oligodendroglial cells express DAGLα, DAGLβ and monoacylglycerol lipase (MAGL), two enzymes responsible for the synthesis and degradation of 2-AG (Gomez et al., 2010). The inhibition of DAGL activity with specific pharmacological inhibitors, or disruption of 2-AG synthesis with specific siRNAs against DAG lipases impairs oligodendrocyte progenitor differentiation (Gomez et al., 2010), clearly demonstrating that 2-AG is essential for oligodendrocyte maturation.
Here, we confirm and expand on these previous studies demonstrating the relevance of basal cannabinoid activity on the differentiation of oligodendrocytes. Indeed, we now show that the activation of CB1 or CB2 receptors by selective exogenous agonists accelerates oligodendrocyte differentiation via the PI3K/Akt and mammalian target of rapamycin (mTOR) signalling pathways.
Methods
Purification and culture of oligodendrocyte progenitor cells (OPCs)
All animal care and experimental procedures complied with current Spanish and European Union legislation (RD 1201/2005; 86/609/EEC). Primary mixed glial cultures were prepared as described previously (Molina-Holgado et al., 2002) and according to the modified technique of McCarthy and de Vellis (1980). Briefly, the forebrain of newborn Wistar rats (Harlan, Spain) was dissociated in 0.25% trypsin by trituration. The cell suspension was filtered through a 150 µm nylon mesh and the filtrate centrifuged at 190×g for 10 min. The cells were then resuspended in Dulbecco's modified Eagle medium (DMEM) containing 10% FCS and plated on poly-l-ornithine (15 µg·mL−1) coated 75 cm2 flasks (Nunc, Roskilde, Denmark). After 10 days in culture, the flasks were shaken at 225 rpm at 37°C for 2 h to remove the loosely adherent microglia, and the remaining OPCs present on the top of the confluent monolayer of astrocytes were dislodged by shaking overnight at 260 r.p.m. The cell suspension was filtered through a 30 µm nylon mesh and then pre-plated on bacterial grade Petri dishes for 2 h. The non-adherent OPCs that remained in suspension were recovered and further purified by immunopanning (Barres et al., 1992). Briefly, two 100 mm Petri dishes were incubated overnight at 4°C in 10 mL Tris (50 mM, pH 9.5) containing affinity-purified goat anti-mouse IgM (10 µg·mL−1). The next day, each dish was washed three times with PBS, and 10 mL of the primary A2B5 antibody was added (culture supernatants diluted 1:10 in PBS plus 0.2% BSA) for 1 h at room temperature. After a further three washes with PBS, 10 mL of DMEM plus 10% goat serum was added (20 min) to block non-specific binding to the dishes, and it was removed just before the addition of the cell suspension. Cells were added to the plates and after 1 h at room temperature, and the plates were rinsed repeatedly with Hank's balanced salt solution (HBSS; without calcium and magnesium). Finally, the adherent cells were released by incubating them in a 0.125% trypsin solution and then manually pipetting DMEM plus 10% FCS onto the surface of the dish. The purified cells were plated onto poly-d-lysine-coated glass coverslips (PDL; 5 µg·mL−1) at a density of 40 × 103 cells per cm2 in 6-well (9.6 cm2 per well) and 24-well (2 cm2 per well) tissue culture dishes, and they were cultured in serum-free defined medium (SFM) containing 5 ng·mL−1 platelet derived growth factor–AA (PDGF-AA) + 5 ng·mL−1 basic fibroblast growth factor (b-FGF) for 2 days to expand the number of OPCs and prevent their differentiation before use. The SFM used in oligodendroglial cultures was DMEM supplemented with 50 µg·mL−1 apo-transferrin, 20 nM hydrocortisone, 60 ng·mL−1 progesterone, 10 ng·mL−1d-biotin, 40 ng·mL−1 selenium, 10 µg·mL−1 insulin, 16 µg·mL−1 putrescine, 0.1% BSA, 50 U·mL−1 penicillin and 50 U·mL−1 streptomycin. The purity of the oligodendroglial cultures was assessed by examining cell morphology by phase-contrast microscopy and confirmed by immunostaining with cell-type-specific antibodies. More than 98% of the cells were positive for the A2B5 monoclonal antibody, a marker of OPCs, while less than 2% were GFAP-positive astrocytes or OX-42-positive microglia.
Incubation of OPCs with cannabinoids
To initiate differentiation of OPCs, cultures were switched to SFM lacking mitogenic growth factors but with 30 ng·mL−1 triiodothyronine (SFM + T3), in the presence or absence of experimental drugs for the times indicated (up to 48 h). HU210 and JWH133 were prepared in ethanol, whereas LY294002, rapamycin, ACEA, AM630 and AM281 were dissolved in DMSO and further diluted in SFM to the required concentrations. Control cultures received the vehicle alone (0.002%).
The concentrations of the cannabinoid agonists used in the present study were higher than would be expected based solely on their in vitro affinity constants. For example, ACEA has 1400-fold selectivity for CB1 over CB2 receptors (Ki for CB1, 1.4 nM), JWH133 has a 200-fold selectivity for CB2 over CB1 receptors (Ki for CB2, 3.4 nM and >10 µM for CB1) and HU210 displays high affinity for CB1 and CB2 receptors, as well as potent and relative intrinsic activity as a cannabinoid receptor agonist (Ki values are 0.061 and 0.52 nM at cloned human CB1 and CB2 receptors respectively). The Ki values of cannabinoid receptor ligands are calculated for the in vitro displacement of tritiated cannabinoid compounds from specific binding sites on rat, mouse or human CB1 and CB2 receptors, usually using membrane preparations (Pertwee et al., 2010). It should be noted that our experimental paradigm involves the incubation of live cells with CB receptor agonists for up to 48 h. This makes it necessary to increase the drug concentrations above those indicated by their in vitro pharmacological values in order to reveal specific effects and to avoid excessive loss of the compound by degradation in culture. Thus, the concentrations used in our study were selected on the basis of previous reports (see Pertwee, 2005b) and according to our dose–response experiments (see Figure 1).
Figure 1.
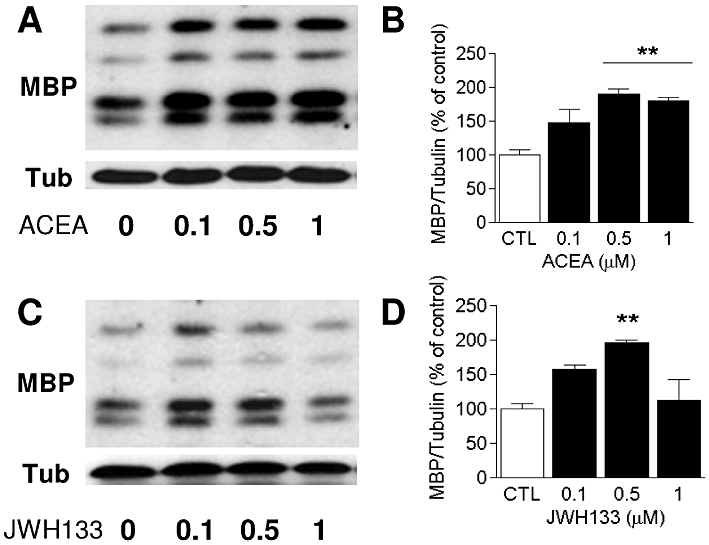
Treatment of differentiating OPC with selective CB receptor agonists stimulates MBP expression in a dose-dependent manner. Cultures were maintained for 48 h in the presence of ACEA and JWH133 (0, 0.5 and 1 µM) in SFM + T3. The levels of MBP after ACEA (A–B) or JWH133 (C–D) treatment relative to α-tubulin were assessed in Western blots by densitometry, and they are shown as the percentage of untreated controls. Values were obtained as the means ± SEM from three independent experiments (**P < 0.01, control vs. 0.5 or 1 µM ACEA or JWH133, one-way anova followed by Tukey's post hoc test).
Immunofluorescence in cultured cells
For immunostaining of oligodendrocytes, live cells plated onto PDL-coated coverslips were incubated for 15 min at room temperature with the mouse monoclonal antibodies A2B5 or O4 (culture supernatants diluted 1:10). After rinsing with PBS, cells were incubated for 15 min at room temperature with secondary Alexa-conjugated anti-mouse IgM. Subsequently, coverslips were washed with PBS, fixed with 4% paraformaldehyde and mounted on slides or processed for multiple labelling. For the latter, the coverslips were incubated overnight at 4°C with antibodies against α-tubulin (1:1000), CB1 (1:3000) or CB2 receptors (1:1000) in PBS containing 5% FCS and 0.1% Triton X-100. Subsequently, the coverslips were rinsed and incubated for 2 h at room temperature with Alexa-conjugated anti-mouse IgG. The nuclei were labelled with bis-benzimide (Hoechst 33258; 1 mg·mL−1 for 10 min at room temperature), and the coverslips were mounted on glass slides with fluorescent mounting medium. Non-specific interactions of secondary antibodies were verified by omitting primary antibodies.
For quantification, preparations were visualized by confocal microscopy (Leica TCS SP5, Leica Microsystems, Mannheim, Germany) with a 40× objective, and at least three independent cultures were examined for each experiment, five microscopic fields were counted per coverslip and two coverslips were examined from each culture. Cells were assigned (without knowledge of the treatments) to one of three categories of complexity (Figure 4P) according to Marin-Husstege et al. (2002): cells with simple morphology (A2B5+) and only a few short primary branches (Type A); O4+ cells with an intermediate morphology had abundant primary or secondary branches (type B); or O4+ cells with a complex morphology had profuse tertiary branches (type C).
Figure 4.

The selective CB1 and CB2 receptor agonists ACEA and JWH133 enhance oligodendrocyte maturation. (A–O) OPC cultures grown for 48 h in the presence of SFM plus PDGF-AA and b-FGF were switched to SFM without growth factors but supplemented with T3 (SFM + T3), and they were treated for 48 h with 0.5 µM ACEA or JWH133 with or without the CB receptor antagonists AM281 or AM630 (both at 1 µM). ACEA and JWH133 significantly increase the branching of cell processes as shown by O4 (green) and α-tubulin (red) staining (D–F and J–L), an effect that was reversed in the presence of AM281 (G–I) or AM630 (M–O). (P) After live staining with O4 (green), cells were fixed and processed for α-tubulin (red) labelling and then classified into the following categories according to Marin-Husstege et al. (2002): type A, cells with simple morphology and only a few short primary branches; type B, cells with an intermediate morphology (O4+) with abundant primary and secondary branches; type C, cells with a complex morphology (O4+) and profuse tertiary branches. (Q) The activation of cannabinoid receptors by ACEA or JWH133 is accompanied by an increase in cells classified as types B and C with a parallel decrease in the type A population, an effect that was reversed by the selective antagonists (*P < 0.05; **P < 0.01; ***P < 0.001 vs. control, one-way anova followed by Tukey's post hoc test).
Western blots
The cells were collected after treatment and lysed in Tris-buffered saline (TBS: pH 7.6) containing 10% glycerol, 1% Nonidet P-40, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 2 mM NaF, 5 mM dithiothreitol and a protease inhibitor cocktail (Molina-Holgado et al., 2002). The resulting cell extracts were mixed with 5× Laemmli sample buffer and boiled for 5 min, before equal amounts of protein (25 µg) were resolved on 10 or 12% SDS-polyacrylamide gels. After electroblotting the proteins to nitrocellulose at 4°C, the membranes were blocked for 1 h at room temperature in 5% (w/v) blotting grade non-fat dry milk in TBS plus 0.1% Tween-20. The membranes were then probed overnight with antibodies against: phospho-Akt (Ser473) (1:1000), phospho-mTOR (Ser2448) (1:1000), α-tubulin (1:100 000), myelin-associated glycoprotein (MAG; 1:500), 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase; 1:1000), myelin basic protein (MBP; 1:1000), CB1 (1:3000) or CB2 receptors (1:1000). Subsequently, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescence using SuperSignal West Pico Substrate Detection Kit. The blots were stripped in a 2% SDS and 0.7% β-mercaptoethanol solution in 62.5 mM Tris buffer (pH 6.8) and then reprobed. The optical density of protein labelling was quantified by densitometry using ImageJ software (NIH, Bethesda, MD).
Statistical analysis
Data are presented as the mean ± SEM of three to four independent experiments performed on separate cell preparations. All statistical analyses were performed and the graphs produced using GraphPad Prism software version 5.00 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Comparisons between experimental groups were made by anova followed by a post hoc Turkey's multiple comparison test.
Materials
The culture media and fetal calf serum (FCS) were obtained from Invitrogen (Barcelona, Spain), while human recombinant PDGF-AA and b-FGF came from PeproTech (London, UK). The anti-CB1 receptor antibody was from Frontier Science Ltd. (Ishikari Hokkaido, Japan), and anti-CB2 receptor antibody was from Cayman Chemical (Ann Arbor, MI). The anti-α-tubulin, anti-GFAP antibodies and the mTOR inhibitor, rapamycin, and the CB1 receptor agonist, ACEA, were from Sigma (Madrid, Spain). Anti-phospho-mTOR was from Cell signaling (Danvers, MA), and anti-MAG and anti-phospho-Akt antibodies were from Santa Cruz Biotechnology (Heidelberg, Germany). Anti-CNPase and anti-MBP antibodies were from Covance (Emeryville, CA), while the A2B5 mouse monoclonal antibody was from American Type Culture Collection (CRL 1520, LGC Standards, Barcelona, Spain). The blotting grade blocking agent, non-fat dry milk and the peroxidase-conjugated anti-mouse or anti-rabbit antibodies were from Bio-Rad Laboratories (Hercules, CA). The SuperSignal West Pico chemiluminescence Substrate Detection Kit was purchased from Thermo Scientific (Rockford, IL), and the secondary antibodies for immunofluorescence were from Molecular Probes (Eugene, OR). The CB receptor agonists HU-210 and JWH133, the CB receptor antagonists AM281 and AM630 and the selective inhibitor of PI3K, LY294002 were purchased from Tocris Bioscience (Bristol, UK). HU210 was kindly provided by Dr Javier Fernández-Ruiz (Universidad Complutense de Madrid, Spain).
Results
Exposure of OPCs in culture to selective cannabinoid receptor agonists increases myelin protein expression and their morphological complexity
To determine whether synthetic cannabinoid agonists accelerated OPC differentiation, we used the levels of MBP as an index of oligodendrocyte maturation (Cohen and Guarnieri, 1976), quantified from the Western blots. Cultures of differentiating OPC were treated for 48 h with different concentrations of the selective CB1 or CB2 receptor agonists, ACEA and JWH133 respectively (0.1, 0.5 and 1 µM). ACEA significantly increased MBP levels at 0.5 µM (P < 0.01 vs. control) and at 1 µM (Figure 1A and B). However, JWH133 only increased MBP levels significantly at 0.5 µM (P < 0.01 vs. control; Figure 1B and C). Thus, in subsequent experiments, these agonists were used at a concentration of 0.5 µM.
We next quantified the levels of the myelin proteins CNPase and MBP in Western blots, 24 or 48 h after exposure to the cannabinoid agonists. In control cultures, MBP was barely detected after 48 h of OPC differentiation, and it was not evident at all after 24 h (Figure 2A), whereas CNPase was found abundantly once OPC initiated differentiation. The incubation of cultures for 24 h with either ACEA or JWH133 had no effect on myelin protein expression (Figure 2A). However, when differentiating OPC were exposed for 48 h to ACEA or JWH133, we noticed a considerable increase in the levels of MBP (ACEA, P < 0.05 vs. control and JWH133, P < 0.001 vs. control; Figure 2B–E). These effects were specifically blocked by the selective CB1 or CB2 receptor antagonists AM281 and AM630 respectively (Figure 2B–E). No effect of AM630 was observed in cultures treated with ACEA, as seen with AM281 and JWH133 (data not shown).
Figure 2.
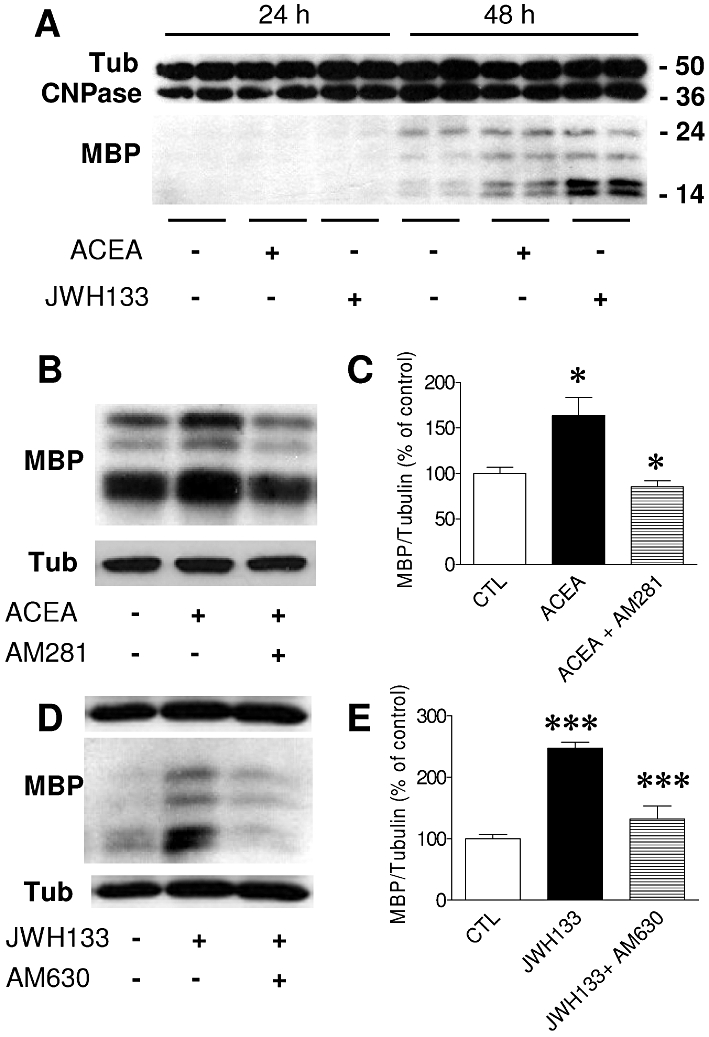
Treatment of differentiating OPC with the selective CB1 and CB2 receptor agonists, ACEA and JWH133, increases MBP levels. (A) Regulation of CNPase and MBP expression in cultures treated for 24 or 48 h in the presence of ACEA and JWH133 (0.5 µM) in SFM + T3. Molecular weights of marker proteins are indicated on the right. Western blots were quantified by densitometry, and the expression of MBP after ACEA (B–C) or JWH133 (D–E) treatment was calculated as the percentage of untreated controls relative to α-tubulin. Values were obtained as means ± SEM from three independent experiments (*P < 0.05, control vs. ACEA or ACEA vs. ACEA + AM281; ***P < 0.001, control vs. JWH133 or JWH133 vs. JWH133 + AM630, one-way anova followed by Tukey's post hoc test).
To test the effect of AM281 or AM630 alone on the differentiation of OPC, cultures were exposed to the antagonists for 48 h, and the accumulation of MAG was measured as an index of OPC differentiation (Gomez et al., 2010). In Western blots, there was no significant change in the levels of MAG after 48 h (Figure 3A), as confirmed by immunocytochemical staining with O4, a stage-specific antibody that recognizes sulphatide-positive pre-oligodendrocytes. After 48 h in the presence of AM281 or AM630, the proportion of O4+-cells remained unchanged, and the control values (40 ± 3% of total cells) were similar to those after treatment with AM281 (43 ± 6%), AM630 (39 ± 9%) or both antagonists together (43 ± 3%: Figure 3B).
Figure 3.
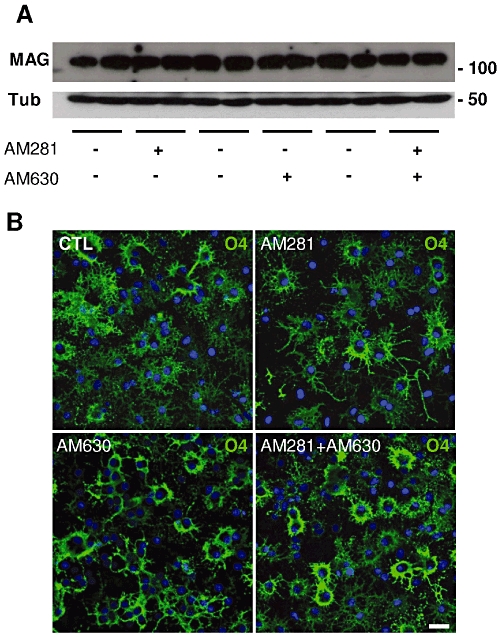
Effects of AM281 or AM630 on OPC differentiation. (A) In Western blots, there were no significant changes in MAG levels in cultures exposed to AM281 or AM630 (1 µM) in SFM + T3 for 48 h. Blots were quantified by densitometry, and the expression of MAG relative to α-tubulin was calculated as the percentage of the untreated controls. The values obtained (means ± SEM from three independent experiments run in duplicate) are shown in the corresponding text. Molecular weights of marker proteins are indicated on the right. (B) In accordance with the Western blots, immunocytochemical staining with O4 (green) did not reveal changes in the proportion of O4 + pre-oligodendrocytes or in the branching of cell processes after a 48 h exposure to AM281, AM630 or both antagonists. Scale bar: 25 µm.
In untreated cultures, OPC rapidly differentiate into oligodendrocytes in response to mitogen withdrawal, whereas in the presence of the selective CB1 or CB2 receptor agonists ACEA and JWH133 for 48 h (0.5 µM), the outgrowth of cellular processes was enhanced, and the cells presented a more mature phenotype (Figure 4A–O). These effects were quantified after immunocytochemical staining with the antibodies O4 and α-tubulin, which better defined the cell's morphology and the arborization of the processes. Thus, cells could be assigned to one of three categories of complexity: type A, cells with simple morphology and low branching; type B, cells with normal arborization; type C, cells with intense network of branched processes (Figure 4P). Both ACEA and JWH133 promoted the morphological differentiation of OPC as measured by an increase in the proportion of the mature cellular forms, types B and C, with a concomitant decrease in the type A cells. In control cultures, almost 80% of cells were scored as type A with a low complexity, whereas ACEA and JWH133 decreased the proportion of this type to 50% and 35% respectively (Figure 4Q). In contrast, the more mature type B cells doubled in number after activation of either receptor. Similarly, the more complex morphologies increased three- and fourfold after exposure to ACEA and JWH133 respectively (Figure 4Q).
Furthermore, OPC cultures incubated for 48 h with HU210 (0.5 µM), a high-affinity agonist of both CB1 and CB2 receptors, presented a more mature morphology (Figure 5A–F). There were more OPC with complex secondary and tertiary branching that were scored as types B and C (Figure 5P). Interestingly, blockade of either the CB1 or CB2 receptors abolished the effects of HU210, as occurred with both antagonists in combination (Figure 5G–P). In addition, HU210 increased the levels of MBP twofold when compared with the cells treated with the vehicle alone (Figure 5Q and R). Again, antagonism of the CB receptors overrode the effect of HU210 on MBP expression.
Figure 5.
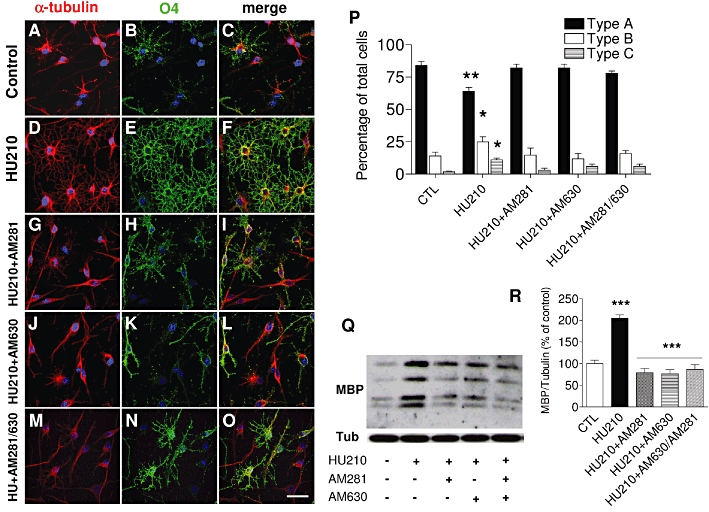
The CB1/CB2 receptor agonist HU210 augments oligodendrocyte maturation. (A–O) OPC cultures grown for 48 h in the presence of SFM plus PDGF-AA and b-FGF were switched to SFM + T3 and treated for 48 h HU210 (0.5 µM) with or without the CB receptor antagonists AM281, AM630 or both (1 µM). O4 (green) and α-tubulin (red) staining revealed a significant arborization of the cell processes after exposure to HU210 (D–F) with an increase in types B and C cells paralleled by a decrease in the type A population (*P < 0.05; **P < 0.01 vs. control, one-way anova followed by Tukey's post hoc test). These effects were abolished in the presence of AM281, AM630 or both (P). HU210 increased the expression of MBP (Q) in relation to the untreated control (R), as seen in Western blots quantified by densitometry and relative to α-tubulin. The values were obtained as the means ± SEM from three independent experiments (***P < 0.001, control vs. HU210; HU210 vs. HU210 + AM281; HU210 vs. HU210 + AM630 or HU210 vs. HU210 + AM281/AM630, one-way anova followed by Tukey's post hoc test). Scale bar: 25 µm.
Finally, we quantified the levels of CB1 and CB2 receptors in immunoblots of whole cell extracts following treatment with agonists and antagonists. A 48 h exposure to ACEA or JWH133 (0.1 and 0.5 µM), and to the antagonists AM281 and AM630 (1 µM), produced no significant differences in CB1 and CB2 receptors, suggesting that total receptor protein levels remained unchanged by these treatments (data not shown).
The cannabinoid agonists ACEA, JWH133 and HU210 activate PI3K/Akt and mTOR signalling
To investigate the involvement of the PI3K/Akt and mTOR cascades in agonist-induced signalling in oligodendrocyte progenitors, phosphorylation of these kinases was assessed by Western blotting with phospho-specific antibodies. Exposure of differentiating OPC cultures to HU210 (0.5 µM) caused the time-dependent phosphorylation of Ser473 in Akt (Figure 6A). HU210 increased Akt phosphorylation in as little as 5 min, reaching maximal levels after 10 min that were maintained for up to 1 h (Figure 6B). Similarly, Akt phosphorylation increased rapidly upon exposure to ACEA or JWH133 (0.5 µM), reaching maximal levels after 2 min but returning to control levels thereafter (Figure 6D–F). Exposing cultures to both ACEA and JWH133 (0.5 µM of each agonist) increased phospho-Akt levels by 182 ± 10% over the control values after 5 min, an effect not significantly different from that of either agonist alone (P > 0.05 vs. ACEA or JWH133; n = 3).
Figure 6.
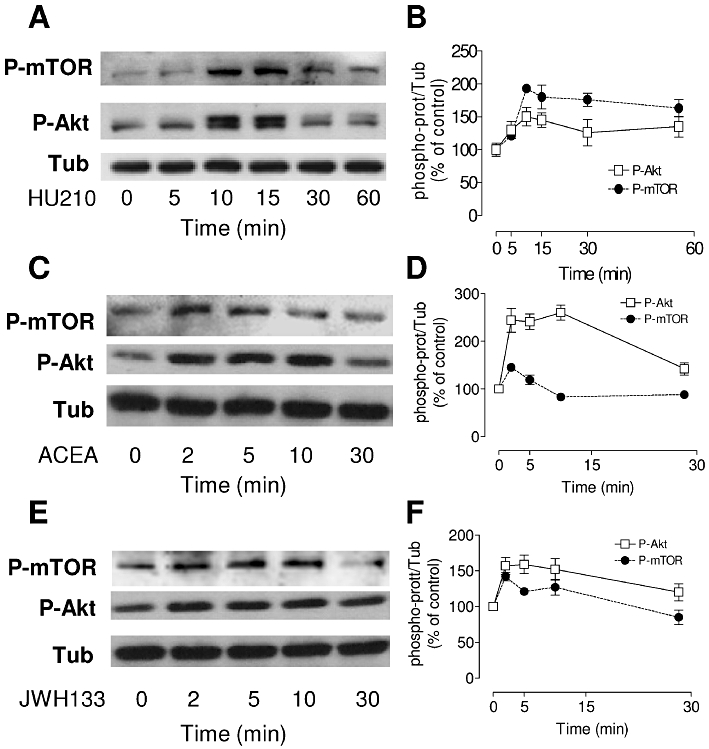
The CB receptor agonists ACEA, JWH133 and HU210 induce a time-dependent phosphorylation of Akt and mTOR. Cultures of OPC grown for 48 h in the presence of SFM plus PDGF-AA and b-FGF were switched to HBSS containing 1% FCS, and they were then stimulated with 0.5 µM of HU210, ACEA or JWH133 for different times. (A, C, E) Western blots probed with antibodies specific for phospho-Akt (Ser473) and phospho-mTOR (Ser2448). (B, D, F) Immunoblots were assessed by densitometry, and the values were expressed as the means ± SEM of three independent experiments.
The mTOR pathway has recently been identified as a regulator of oligodendrocyte differentiation; however, the activation of mTOR by cannabinoid receptor agonists in oligodendrocytes has not yet been explored. We found that mTOR was phosphorylated on Ser2448 in a time-dependent manner after HU210 (0.5 µM) treatment. Maximal phosphorylation was observed after 10 min stimulation, and it was sustained for 60 min (Figure 6A and B). In contrast to Akt activation, incubation with ACEA or JWH133 provoked transient mTOR phosphorylation that peaked at 2 min, before falling below the basal level (Figure 6C–F).
The effects of HU210 on the differentiation of oligodendrocyte progenitor cells require PI3K/Akt and mTOR signalling
The results presented above indicated that HU210 activated the Akt and mTOR pathways. To explore the involvement of the PI3K/Akt and mTOR cascades in OPC differentiation, cultures were pretreated 30 min with LY294002 (2.5 µM), a reversible inhibitor of PI3K, and with rapamycin (1.5 nM), a macrolide immunosuppressant inhibitor of mTOR, before 10 min treatment with HU210 in the presence of these inhibitors, and the phosphorylation status of ERK, Akt and mTOR was examined in Western blots (Figure 7A). Both LY294002 and rapamycin abolished the phosphorylation of mTOR, Akt and ERK induced by HU210 (Figure 7A–D).
Figure 7.
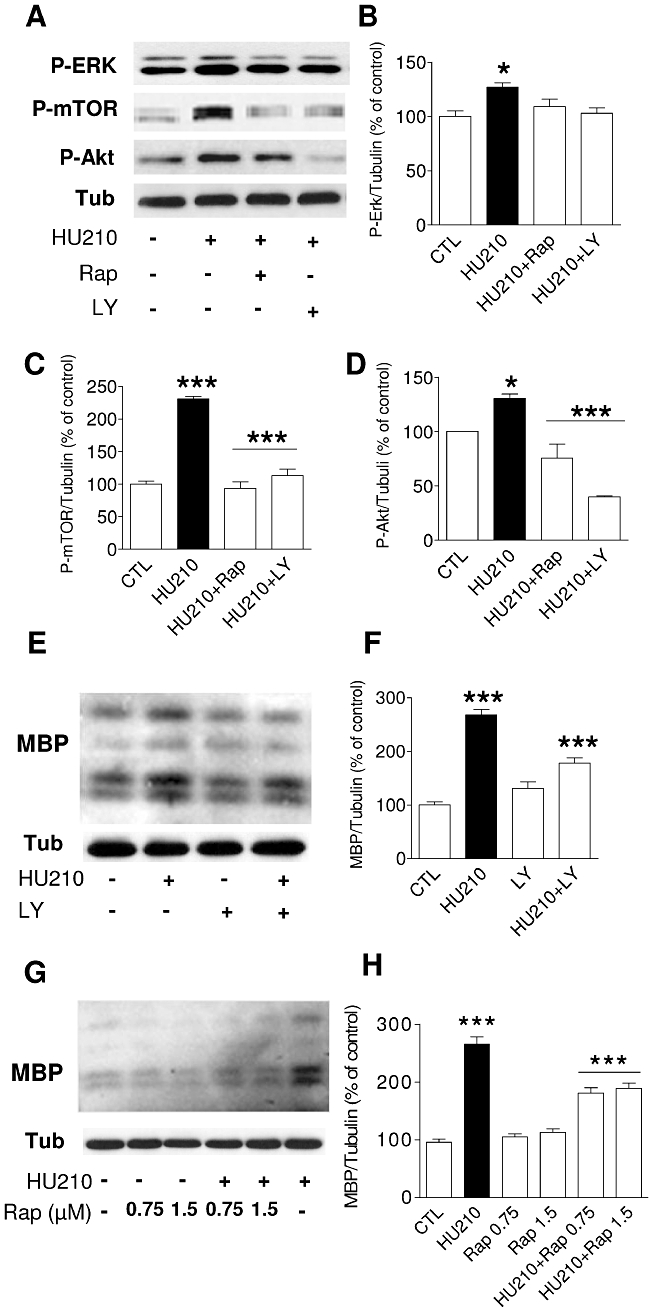
Inhibitors of PI3K and mTOR decrease the HU210-induced phosphorylation of Akt, mTOR and ERK, as well as MBP expression. (A) Cultures of OPC were treated for 30 min with either 0.75 nM rapamycin or 2.5 µM LY290042 and then stimulated for 10 min with HU210 (0.5 µM). (B–D) The densitometric data represent the mean ± SEM of three independent experiments (P-ERK: *P < 0.05 control vs. HU210; P-mTOR: ***P < 0.001, control vs. HU210; HU-210 vs. HU-210 + Rap or HU-210 + LY; P-Akt: *P < 0.05 control vs. HU210, ***P < 0.001 HU210 vs. HU-210 + Rap or HU-210 + LY, one-way anova followed by Tukey's post hoc test). (E–F) Western blot analysis of MBP expression 48 h after differentiation in the presence of HU210 showed a 2.5-fold increase in MBP levels that diminished significantly in the presence of LY294002 (2.5 µM: ***P < 0.001 control vs. HU210 or HU-210 vs. HU210 + LY294002, one-way anova followed by Tukey's post hoc test). After HU210 incubation, a partial reduction in the levels of MBP was observed with rapamycin (0.75 or 1.5 nM: ***P < 0.001 control vs. HU210 or HU210 vs. HU210 + rapamycin, one-way anova followed by Tukey's post hoc test, G–H).
To further characterize the signalling cascades through which the CB receptor agonist HU210 enhanced OPC differentiation, the cultures were exposed to the selective protein kinase inhibitors used before. First, to inhibit the actions of PI3K, OPC were treated for 48 h in differentiation media with 2.5 µM of LY294002 in the presence of HU210 (0.5 µM), which led to a 35% reduction in MBP levels (Figure 7E and F). To demonstrate a role for cannabinoid-induced mTOR phosphorylation in oligodendrocyte differentiation, we used rapamycin. Differentiating OPC were treated simultaneously with rapamycin (0.75 or 1.5 nM) and HU210 (0.5 µM), and in Western blots, a significant 30% reduction of HU210-stimulated MBP expression was observed (Figure 7G and H).
Similarly, immunocytochemical analyses revealed that after exposure to LY294002, the OPC exhibited a simple bipolar or multipolar morphology as when treated with HU210 (Figure 8A–F). Cells quantified as type A increased by 25%, while the more complex type B cells decreased by 40%, and the mature type C cells were nearly absent (Figure 8J). The results obtained following exposure to rapamycin indicated that O4+ cells displayed a more immature morphology than when treated with HU210 (Figure 8G–I), the proportion of type A cells increasing to 30% after rapamycin treatment.
Figure 8.
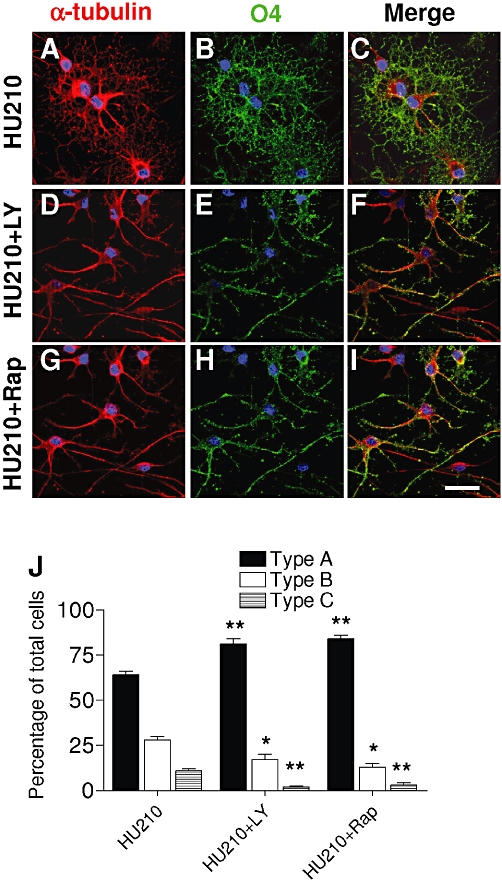
Inhibitors of PI3K and mTOR decrease HU210-mediated oligodendrocyte maturation. (A–I) OPC cultures grown for 48 h in the presence of SFM plus PDGF-AA and b-FGF were switched to SFM + T3 and treated for 48 h HU210 (0.5 µM) in the presence or absence of LY294002 (2.5 µM) or rapamycin (1.5 nM). O4 (green) and α-tubulin (red) staining revealed significant arborization of cell processes after HU210 (A–C) that was reversed after LY294002 or rapamycin (D–I). Inhibitors augmented the type A cells, while the mature types B and C populations diminished (**P < 0.01 vs. HU210; *P < 0.05 vs. HU210, one-way anova followed by Tukey's post hoc test) (J).
Discussion
The data presented here demonstrated that activation of CB1 or CB2 receptors with selective exogenous agonists accelerated oligodendrocyte differentiation. By pharmacologically activating CB receptors (CB1, CB2 or both CB1 and CB2 receptors) with specific synthetic CB receptor agonists (ACEA, JWH133 or HU210), we markedly accelerated oligodendrocyte progenitor differentiation in our in vitro system. In addition, we provide evidence that such an effect was exerted through a mechanism dependent on the activation of the PI3K/Akt and mTOR signalling pathways.
In the early nineties, classical autoradiographic studies demonstrated that CB receptors were expressed in several regions of the white matter in the CNS (Herkenham et al., 1991). Although oligodendrocytes are one potential cell type that might express CB receptors, the precise identification and the role of these receptors in these cells remained unexplored. The atypical distribution of CB receptors reported in the fetal brain (Romero et al., 1997) was confirmed by the observation of CB receptor binding, mRNA expression and activation of signal transduction mechanisms in non-neuronal cells of the white matter (Berrendero et al., 1998). However, compelling evidence that functional CB receptors are expressed in purified oligodendrocyte cultures, in the postnatal and adult corpus callosum, and in the spinal cord white matter, was later presented (Molina-Holgado et al., 2002; Arevalo-Martin et al., 2007; Garcia-Ovejero et al., 2009). The results presented herein further confirm the presence of CB receptors in oligodendrocytes, and they indicate that synthetic CB1, CB2 and mixed CB1/CB2 receptor agonists (ACEA, JWH133 and HU210, respectively) exert a strong effect on OPC, increasing MBP levels as a marker of oligodendrocyte maturity as soon as 48 h after the differentiation process starts, as well as increasing the proportion of differentiating oligodendrocyte morphologies. These effects were receptor specific since pharmacological blockade of either receptor with AM281 or AM630 abolished the action of ACEA, JWH133 and HU210. Thus, a primary function of CB receptors in oligodendroglial cells seems to be to control oligodendrocyte development. In support of this statement, previous studies indicate that the brain of postnatal rats exposed to the non-selective CB1/CB2 receptor agonist WIN 55,212-2 for 15 days augmented MBP expression in the subcortical white matter, an effect that was overridden with CB1 or CB2 receptor antagonists (Arevalo-Martin et al., 2007). These results demonstrate the specific functional association of brain endocannabinoids and oligodendrocyte development in a pathway regulated by CB receptors.
The CB receptors are the most abundant G protein-coupled receptors in the brain (see Scotter et al., 2010). However, despite recent advances in understanding the actions of endocannabinoids on CNS development (Fernández-Ruiz et al., 2000), the signal transduction pathways regulated by CB receptors in oligodendrocytes are poorly characterized. Cellular responses triggered by CB receptor activation include activation of the mitogen-activated protein kinase (ERK/MAPK), the Src family of non-receptor tyrosine kinases and the PI3K/Akt signalling pathways (Bouaboula et al., 1995; Gómez del Pulgar et al., 2000; Galve-Roperh et al., 2002). Previous studies from our laboratory suggest a role for ERK/MAPK signalling in the actions of endogenous 2-AG-induced OPC maturation (Gomez et al., 2010), as well as the involvement of PI3K/Akt signalling in OPC survival after the withdrawal of trophic support (Molina-Holgado et al., 2002). The present data extend these studies, indicating for the first time that the effects of synthetic CB receptor agonists in oligodendrocyte differentiation are mediated by the PI3K/Akt and mTOR signalling.
The initial observation that transgenic mice with constitutively active Akt in the oligodendrocyte lineage (Plp-Akt-DD) begin myelinating earlier and produce more myelin suggested that this serine/threonine kinase could be one of the signals regulating myelination (Flores et al., 2008). Interestingly, the only substrate that showed changes in phosphorylation in Plp-Akt-DD mice was mTOR. This kinase acts as a master switch in cell signalling, integrating inputs from multiple upstream stimuli to regulate cell growth (Wullschleger et al., 2006). Two different mTOR protein complexes exist, termed mTOR complexes 1 and 2 (mTORC1 and mTORC2), and both are related to the PI3K/Akt pathway. The mTORC2 phosphorylates and fully activates Akt, whereas the PI3K/Akt pathway is among the agents that triggers mTORC1 activation. It was recently revealed that activation of mTOR is essential for the generation of GalC+ immature oligodendrocyte in vitro (Tyler et al., 2009), consistent with mTOR acting as a primary target of Akt signalling in Plp-Akt-DD mice (Narayanan et al., 2009). However, the extrinsic signals that activate mTOR in differentiating OPC are currently unknown. As our study shows that CB receptors increase OPC maturation through the Akt and mTOR pathways, the endocannabinoids may be the extracellular signals that activate Akt and mTOR during oligodendrocyte differentiation.
An association between cannabinoid signalling and the mTOR pathway has been shown to modulate long term memory in the hippocampus (Puighermanal et al., 2009). Moreover, insulin-like growth factor 1 (IGF-1) stimulated protein synthesis and differentiation in oligodendrocyte progenitors require the PI3K/mTOR/Akt and MEK/ERK pathways (Bibollet-Bahena and Almazan, 2009). Therefore, our study confirmed that CB receptor stimulation influenced Akt phosphorylation and phosphorylation of mTOR in OPC cultures. Furthermore, in our in vitro system, we demonstrated that rapamycin and LY294002, the inhibitors of mTOR and PI3K, respectively, strongly inhibited the cannabinoid receptor-mediated increase in MBP levels and the appearance of mature oligodendrocyte phenotypes. In addition, both inhibitors abolished the phosphorylation of Akt and mTOR induced by HU210, in agreement with the inhibitory effect of rapamycin on mTOR and Akt in OPC (Tyler et al., 2009). Moreover, rapamycin treatment significantly reduces the effect of IGF-1 on Akt phosphorylation, suggesting that this drug can impair Akt activity by inhibiting mTOR in OPC cultures (Bibollet-Bahena and Almazan, 2009). We have now demonstrated that rapamycin inhibited the effect of HU210 on this kinase. Finally, mTOR is also phosphorylated via PI3k/AKT signalling (Navéet al., 1999), and LY294002 inhibited HU210-induced phosphorylation of mTOR. These observations illustrate the complex crosstalk between PI3K/Akt and mTOR during the process of cannabinoid-induced oligodendrocyte differentiation.
Together, the data presented here suggest that an up-regulation in endocannabinoid tone might be responsible for oligodendrocyte differentiation and provide proof-of-concept that CB receptors and 2-AG/DAGL act as potential therapeutic targets to counteract the loss of oligodendroglial cells. Therefore, acute activation of the local endocannabinoid system would have a profound positive effect on oligodendrocyte fate and subsequently, on brain repair. As a result, we propose that the brain endocannabinoid system might well modulate the progression of demyelinating diseases such as multiple sclerosis.
Acknowledgments
This research was funded by grants from the Gobierno de Castilla-La Mancha (Fundación para Investigación Sanitaria en Castilla-La Mancha; FISCAM, PI-2007/19) to EM-H and from Fundación Mutua-Madrileña to OG. AS-R is supported by FISCAM (MOV-2008_JI/7). MQU Le is supported by Instituto de Salud Carlos III (Ministerio de Ciencia e Innovación of Spain, Exp. N° CA09/00609). We are grateful to Drs JA Rodríguez-Alfaro, J Mazario and A Fernandez (Servicio de Microscopia, Hospital Nacional de Paraplejicos, Toledo) for their excellent technical assistance. We would also like to thank Dr Mark Sefton (BiomedRed) for critical reading of the manuscript and editorial assistance.
Glossary
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonoyl glycerol
- ACEA
N-(2-chloroethyl)-5Z, 8Z, 11Z, 14Z-eicosatetraenamide
- AM281
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- AM630
6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone
- bFGF
basic fibroblast growth factor
- CBs
cannabinoids
- DAGLα
diacylglycerol lipase α
- DAGLβ
diacylglycerol lipase β
- HU210
(6aR)-trans-3-(1,1-dimethylpeptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-ibenzo[b,d]pyran-9-methanol
- JWH133
(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethy-6H-dibenzo[b,d]pyran
- LY294002
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride
- MAGL
monoacylglycerol lipase
- mTOR
mammalian target of rapamycin
- OL
oligodendrocyte
- OPC
oligodendrocyte progenitor cells
- PDGF-AA
platelet-derived growth factor-AA
- PI3K
phosphatidylinositol 3-kinase
Conflict of interest
The authors state that they have no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–8 as PowerPoint slide.
References
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Martin A, Garcia-Ovejero D, Rubio-Araiz A, Gomez O, Molina-Holgado F, Molina-Holgado E. Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur J Neurosci. 2007;26:1548–1559. doi: 10.1111/j.1460-9568.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, et al. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-I-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signalling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Cannabinoids as therapeutic agents for ablating neuroinflammatory disease. Endocr Metab Immune Disord Drug Targets. 2008;8:159–172. doi: 10.2174/187153008785700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SR, Guarnieri M. Immunochemical measurement of myelin basic protein in developing rat brain: an index of myelin synthesis. Dev Biol. 1976;49:294–299. doi: 10.1016/0012-1606(76)90276-1. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Berrendero F, Hernández ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets. 2010a;14:387–404. doi: 10.1517/14728221003709792. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Hernandez M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010b;16:72–91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, et al. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I, Rueda D, Gómez del Pulgar T, Velasco G, Guzmán M. Mechanism of extracellular signal-regulated kinase activation by the CB (1) cannabinoid receptor. Mol Pharmacol. 2002;62:1385–1392. doi: 10.1124/mol.62.6.1385. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure elucidation and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;1646–47:11–20. [Google Scholar]
- Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, et al. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol Dis. 2009;33:57–71. doi: 10.1016/j.nbd.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Gómez del Pulgar T, Velasco G, Guzmán M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez O, Arevalo-Martin A, Garcia-Ovejero D, Ortega-Gutierrez S, Cisneros JA, Almazan G, et al. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia. 2010;58:1913–1927. doi: 10.1002/glia.21061. [DOI] [PubMed] [Google Scholar]
- Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00195.x. (in press, doi: 10.1111/j.1755-5949.2010.00195.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy RI. The therapeutic potential of the cannabinoids in neuroprotection. Expert Opin Investig Drugs. 2002;11:1365–1374. doi: 10.1517/13543784.11.10.1365. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Molina-Holgado F. Mending the broken brain: neuroimmune interactions in neurogenesis. J Neurochem. 2010;114:1277–1290. doi: 10.1111/j.1471-4159.2010.06849.x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Molina-Holgado F, Almazan G, Borrell J, et al. Expression of cannabinoid CB1 receptors in oligodendroglial cells: activation of PI-3KAkt signalling pathway promotes cell survival. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Rubio-Araiz A, Garcia-Ovejero D, Williams RJ, Moore JD, Arevalo-Martin A, et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2007;25:629–634. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin, mTOR, pathway to regulate central nervous system myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005a;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005b;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Romero J, García-Palomero E, Berrendero F, García-Gil L, Hernández ML, Ramos JA, et al. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Scotter EL, Abood ME, Glass M. The endocannabinoid system as a target for the treatment of neurodegenerative disease. Br J Pharmacol. 2010;160:480–498. doi: 10.1111/j.1476-5381.2010.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Waku K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids. 2000;108:89–106. doi: 10.1016/s0009-3084(00)00189-4. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29:6367–6378. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


