Abstract
Objective
Cardiovascular disease risk among persons with HIV is likely multifactorial, thus testing a variety of available noninvasive vascular ultrasound and other surrogate tests may yield differing results. To address this issue, we assessed multiple metabolic and clinical predictors of endothelial function and carotid intima–media thickness in HIV-infected subjects and compared results with HIV-negative controls.
Design
Prospective, cross-sectional study of 50 HIV-infected, healthy adults on stable highly active antiretroviral therapy matched to 50 HIV-negative controls by age, sex, race, and body mass index.
Methods
Flow-mediated vasodilation of the brachial artery, carotid intima–media thickness, dual energy X-ray absorptiometry (HIV-infected subjects), and fasting insulin, lipids, and oral glucose tolerance tests were performed. Results were compared between HIV-infected and control groups.
Results
Fifty percent of subjects were African–American with 34% women. Among HIV-infected, mean CD4 cell count was 547 cells/ µl; 90% had HIV RNA less than 50 copies/ml. There were no significant differences between HIV-infected and control subjects with regard to brachial artery flow-mediated vasodilation or carotid intima–media thickness. In multivariate analyses of the HIV cohort, independent predictors of endothelial dysfunction (lower flow-mediated vasodilation) were increasing insulin resistance, greater alcohol consumption, and higher baseline brachial artery diameter (P < 0.05); predictors of increased carotid intima–media thickness were hypertension, higher trunk/limb fat ratio, and insulin resistance (P < 0.05).
Conclusion
In this HIV cohort on modern highly active antiretroviral therapy with well controlled HIV, there were no significant differences with regard to preclinical markers of cardiovascular disease. Insulin resistance was a strong predictor of impaired brachial artery flow-mediated vasodilation and increased carotid intima–media thickness, and may be an important cardiovascular disease risk factor in the HIV population.
Keywords: endothelial dysfunction, HIV, insulin resistance
Introduction
The use of combination highly active antiretroviral therapy (HAART) has been associated with metabolic complications that may increase long-term risk of cardiovascular disease [1,2]. Some antiretroviral therapies have been associated with insulin resistance, increased visceral fat deposition, and increases in atherogenic dyslipidemia, all components of the metabolic syndrome [3–5]. Recent evidence indicates that cumulative exposure to protease inhibitors over time is an independent risk factor for myocardial infarction (MI) in a large, ongoing observational study [2]. Other data appear to implicate HIV infection itself rather than HAART as a potential contributor to elevated cardiovascular risk [6].
Surrogate markers of cardiovascular disease such as carotid intima–media thickness (CIMT) and brachial artery flow-mediated vasodilation (FMD) have been used in recent trials to assess incident risk. Increased CIMT is a marker of underlying atherosclerosis and correlates well with traditional risk factors; however, endothelial dysfunction as measured by brachial artery FMD may occur earlier in the development of atherosclerosis, independent of other cardiac risk factors [7–9]. Several small studies [10–13] have assessed endothelial dysfunction in HIV-infected persons with varying results, including association with metabolic abnormalities, protease inhibitor use, behavioral factors, or uncontrolled HIV infection. Another study [14] that used CIMT as a surrogate for cardiovascular disease risk in HIV-infected patients on HAART found no significant difference compared with a matched seronegative cohort. Given the likelihood that cardiovascular disease risk among persons with HIV is multifactorial, testing a variety of available noninvasive vascular ultrasound and other surrogate tests may yield differing results. To address this issue, brachial artery FMD, CIMT, body fat and lean mass, insulin resistance, and other metabolic and clinical parameters were obtained in otherwise healthy HIV-infected persons on stable HAART to determine the metabolic predictors of endothelial dysfunction and CIMT, examine the association between exposure to specific antiretrovirals and endothelial dysfunction, and compare results with an HIV-negative cohort matched by age, sex, race, and body mass index (BMI). We hypothesized that HIV-infected persons would have greater impairment in brachial artery FMD and increased CIMT compared with matched controls, and that endothelial dysfunction would be associated with evidence of insulin resistance.
Methods
Study design
The study population consisted of a prospective, cross-sectional cohort of randomly selected healthy HIV-infected adults on a stable HAART regimen for at least 6 months prior to the enrollment. HAART was defined as the use of at least two nucleosides with either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor (NNRTI). Patients were excluded if they had a current CD4 cell count of less than 100 cells/ µl, recent opportunistic infection, or use of chemotherapy or other immunomodulatory therapy within 60 days prior to enrollment, diagnosis of diabetes or known coronary artery disease, or pregnancy. Patients with other diagnoses that were felt to significantly affect endothelial function (i.e., uncontrolled hypertension, peripheral vascular disease) and patients actively using intravenous drugs, cocaine, or methamphetamines were also excluded. HIV-negative controls were healthy adults (nondiabetic, no signs/symptoms of vascular or renal disease, and low risk of HIV) randomly selected from subjects enrolled in clinical and observational studies at the Cardiovascular Imaging and Clinical Research Core Laboratory, Washington University School of Medicine. HIV-infected patients were matched to seronegative controls according to age, sex, race, and BMI. The study was reviewed and approved by the Washington University Human Research Protections Office.
Laboratory and metabolic testing
After a 10-h overnight fast, HIV-infected subjects were examined at the Washington University General Clinical Research Center. A 75-g, 2-h oral glucose tolerance test (OGTT) was performed: venous blood samples were obtained at baseline and at 30-min intervals to obtain fasting and area-under-the-curve (AUC) measures for glucose and insulin levels. Samples were iced, centrifuged, and stored at −70°C until time of batched assay. Insulin testing was performed by double-antibody radioimmunoassay and C-peptide concentrations determined by radioimmunoassay (Diabetes Research and Training Center Radioimmunoassay Core Laboratory, Washington University School of Medicine) for all subjects. Insulin resistance was calculated using the homeostatic model assessment (HOMA-IR) [15] and the Belfiore OGTT index, which utilizes both insulin and glucose AUC [16]. Fasting lipid/lipoprotein levels were measured by enzymatic methods (Roche Diagnostics, Indianapolis, Indiana, USA). Highly sensitive C-reactive protein (hsCRP) was quantified by nephelometry (Dade-Behring BN-II System, Ramsey, Minnesota, USA) in HIV-infected subjects. hsCRP in control subjects was quantified by enzyme-linked immunosorbent assay (ELISA) (Life Diagnostics, Inc., West Chester, Pennsylvania, USA); the intraclass correlation coefficient between the two assays was 0.99. CD4 cell count and HIV RNA were performed by commercial assay (with lower limit of detection for HIV RNA 40 copies/ml). A Hologic Discovery W enhanced-array whole-body dual energy X-ray absorptiometry (DEXA) scanner and software (version 12.3:7; Hologic, Waltham, Massachusetts, USA) were used to measure whole body, trunk, and limb lean and fat tissue mass in HIV-infected subjects. Each scan was acquired and processed by a Hologic-certified radiology technologist. Vital signs, waist circumference, complete medication history, sociodemographics, and risk factors for cardiovascular disease were also obtained for all subjects. National Cholesterol Education Program criteria were used to identify the presence of the metabolic syndrome [17].
Endothelial function measurements
After a 10-h overnight fast, all subjects (HIV-infected subjects and seronegative controls) were examined at the Washington University Cardiovascular Imaging and Clinical Research Core Laboratory for assessment of endothelium-dependent brachial FMD. FMD values were presented as the percentage change in brachial artery diameter after reactive hyperemia, by vascular ultrasound according to a previously published protocol designed to control for as much variability as possible [18]. Prior to testing, all subjects were instructed to refrain from caffeine, alcohol, antioxidants, antacids, and nonsteroidal medications for 12 h before the FMD examination and to refrain from smoking on the morning of the study. The examination was conducted after a 15-min rest period in a quiet, temperature-controlled room with the subject in a supine position. A 14-MHz linear array transducer (Siemens Acuson Sequoia 256 Ultrasound System, Oceanside, California, USA) was used to visualize the brachial artery in the right arm1 cm above the antecubital fossa. The vessel diameter was determined in longitudinal views prior to placement of an occlusion cuff just proximal to the transducer location. The cuff was inflated to 200 mmHg for 5 min and then rapidly deflated. Images of the brachial artery diameter were acquired immediately, 1, 2, and 3 min following cuff deflation. For each time point, the diameter represents the mean of five equidistant measurements on the B-mode ultrasound image. Images of the brachial artery were stored on a magnetic optical disk for off-line analysis using Prosolv software (Problem Solving Concepts, Indianapolis, Indiana, USA). All measurements were performed by the same vascular sonographer. The intraobserver, intraclass correlation coefficient for FMD at our laboratory is greater than 0.98, which is similar to previously published studies [19].
Carotid intima–media thickness measurement
Carotid ultrasound was performed using the ultrasound system described above; B-mode images of both carotid arteries were acquired along the longitudinal axis. A 1-cm region of the common carotid artery just proximal to the bifurcation was identified and magnified for maximal resolution prior to export for off-line digital analysis with Prosolv software. CIMT measures were expressed as the average of four sites: the near-wall and far-wall intima–media thicknesses from both right and left common carotid arteries. Each site represented the average of three separate measurements, and all images were acquired by the same vascular sonographer. The intraobserver, intraclass correlation coefficient for CIMT at our laboratory is 0.89.
Statistical analysis
Data were analyzed using χ2 or Fisher’s exact tests for categorical variables. Continuous variables were compared using the Student’s t-test or Mann–Whitney U-test for normally and nonnormally distributed variables, respectively. Bivariate correlation testing was used to test the strength of association between FMD, CIMT, and other clinical variables. Significant variables were then entered stepwise into a linear regression model to determine the best multivariable model. Variables were assessed for collinearity and adjusted appropriately. HIV RNA levels, insulin resistance by HOMA-IR and Belfiore OGTT index, trunk/limb fat ratio, and triglycerides were log-transformed for analysis. Spearman correlation was performed between FMD, CIMT, and Framingham risk score. Correlation was also performed between HOMA-IR and Belfiore OGTT index. All P-values were two-tailed. Data were analyzed with use of the SPSS software package version 14.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Population characteristics
The study population included 50 HIV-infected subjects; 50% were African–American and 34% were women. The mean current CD4 cell count was 547 cells/ µl and 45 (90%) had HIV RNA less than 50 copies/ml. A group of 50 seronegative controls were matched by age, race, sex, and BMI to the 50 HIV-infected subjects. Additional characteristics of the HIV-infected and control subjects are shown in Tables 1 and 2.
Table 1.
Comparison between HIV-infected and control groups.
| Characteristic | HIV-infected group (N = 50) No. (%) |
HIV-uninfected group (N = 50) No. (%) |
P value |
|---|---|---|---|
| Age (mean ± SEM) | 42 ± 1.2 | 42 ± 1.3 | 0.98 |
| Sex | 1.00 | ||
| Men | 33 (66%) | 33 (66%) | |
| Women | 17 (34%) | 17 (34%) | |
| Race | 1.00 | ||
| White | 24 (48%) | 24 (50%) | |
| African–American | 25 (50%) | 25 (50%) | |
| Other | 1 (2%) | 1 (2%) | |
| Mean BMI (kg/m2 ± SEM) | 28.7 ± 0.9 | 30.2 ± 0.9 | 0.23 |
| Current smoker | 15 (30%) | 9 (18%) | 0.16 |
| Diagnosis of hypertension | 15 (30%) | 14 (28%) | 0.83 |
| Family history of cardiovascular disease | 16 (32%) | 11 (22%) | 0.23 |
| Use of lipid-lowering therapy | 12 (24%) | 3 (6%) | 0.03* |
| Diagnosis of metabolic syndrome | 15 (30%) | 9 (18%) | 0.24 |
| Mean fasting LDL (mg/dl ± SEM) | 98 ± 4 | 114 ± 4 | <0.01* |
| Mean fasting HDL (mg/dl ± SEM) | 47 ± 2 | 56 ± 2 | <0.01* |
| Mean fasting triglycerides (mg/dl ± SEM) | 141 ± 11 | 103 ± 7 | <0.01* |
| Mean fasting glucose (mg/dl ± SEM) | 89 ± 1 | 84 ± 2 | <0.01* |
| Mean fasting insulin (µU/ml) | 13.9 ± 1.9 | 13.0 ± 1.7 | 0.72 |
| Mean HOMA-IR | 3.1 ± 0.5 | 2.7 ± 0.3 | 0.50 |
| Mean hsCRP (mg/l) | 4.1 ± 0.6 | 6.0 ± 1.0 | 0.10 |
| Mean 10-year Framingham risk score | 0.3 ± 0.8 | −0.8 ± 0.8 | 0.38 |
| Resting brachial artery diameter (mm) | 3.9 ± 0.1 | 3.8 ± 0.1 | 0.56 |
| Mean brachial FMD (% ± SEM) | 12.0 ± 0.9 | 13.3 ± 1.0 | 0.30 |
| Mean CIMT (mm ± SEM) | 0.68 ± 0.02 | 0.68 ± 0.02 | 0.96 |
BMI, body mass index; CIMT, carotid intima–media thickness; FMD, flow-mediated vasodilation; HDL, high-density lipoprotein; HOMA-IR, homeostatic model of insulin resistance; hsCRP, highly-sensitive C-reactive protein; LDL, low-density lipoprotein.
P ≤ 0.05.
Table 2.
HIV cohort characteristics according to current HAART.
| Characteristic | Current PI use (N = 21) No. (%) |
Current NNRTI use (N = 29) No. (%) |
P value |
|---|---|---|---|
| Age (mean ± SEM) | 42 ± 1.2 | 43 ± 1.5 | 0.63 |
| Sex | |||
| Men | 15 (71%) | 18 (62%) | 0.56 |
| Women | 6 (29%) | 11 (38%) | |
| Race | |||
| White | 9 (43%) | 15 (52%) | 0.77 |
| African–American | 11 (52%) | 14 (48%) | |
| Time since HIV diagnosis (mean years ± SEM) | 10.3 ± 1.3 | 9.9 ± 1.0 | 0.82 |
| Current smoker | 5 (24%) | 10 (35%) | 0.55 |
| Mean CD4 cell count (cells/ µl ± SEM) | 477 ± 53 | 598 ± 42 | 0.78 |
| Mean nadir CD4 cell count | 213 ± 74 | 224 ± 36 | 0.89 |
| Number with HIV RNA < 50 copies/ml | 16 (74%) | 29 (100%) | 0.01* |
| Diagnosis of hypertension | 6 (29%) | 9 (31%) | 0.85 |
| Family history of cardiovascular disease | 6 (29%) | 10 (35%) | 0.60 |
| History of illicit drug use | 5 (24%) | 2 (7%) | 0.12 |
| Use of lipid-lowering therapy | 3 (14%) | 9 (31%) | 0.20 |
| Diagnosis of metabolic syndrome | 7 (33%) | 8 (28%) | 0.76 |
| Mean BMI (kg/m2 ± SEM) | 29.9 ± 1.6 | 27.8 ± 1.1 | 0.27 |
| Mean fasting LDL (mg/dl ± SEM) | 93 ± 6 | 101 ± 5 | 0.31 |
| Mean fasting HDL (mg/dl ± SEM) | 45 ± 2 | 49 ± 2 | 0.33 |
| Mean fasting triglycerides (mg/dl ± SEM) | 135 ± 14 | 145 ± 15 | 0.64 |
| Mean fasting glucose (mg/dl ± SEM) | 89 ± 2 | 89 ± 2 | 0.98 |
| Mean HOMA-IR | 3.3 ± 0.8 | 3.1 ± 0.5 | 0.88 |
| Mean hsCRP (mg/l) | 3.3 ± 0.7 | 4.7 ± 0.9 | 0.26 |
| Trunk/limb fat ratio | 1.7 ± 0.2 | 1.7 ± 0.2 | 0.82 |
| Time ever on HAART (months ± SEM) | |||
| Total time | 214.7 ± 29.2 | 204.6 ± 21.7 | 0.78 |
| NRTI | 155.5 ± 23.9 | 109.3 ± 15.7 | 0.62 |
| NNRTI | 13.54 ± 4.6 | 54.2 ± 5.4 | <0.01* |
| Ritonavir-boosted PI | 27.9 ± 5.1 | 1.4 ± 1.1 | <0.01* |
| Mean 10-year Framingham risk score | 0.3 ± 1.1 | 0.2 ± 1.2 | 0.98 |
| Mean FMD (% ± SEM) | 13.1 ± 1.4 | 11.2 ± 1.2 | 0.31 |
| Mean CIMT (mm ± SEM) | 0.69 ± 0.03 | 0.67 ± 0.02 | 0.69 |
BMI, body mass index; CIMT, carotid intima–media thickness; FMD, flow-mediated vasodilation; HDL, high-density lipoprotein; HOMA-IR, homeostatic model of insulin resistance; hsCRP, highly-sensitive C-reactive protein; LDL, low-density lipoprotein; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
P ≤ 0.05.
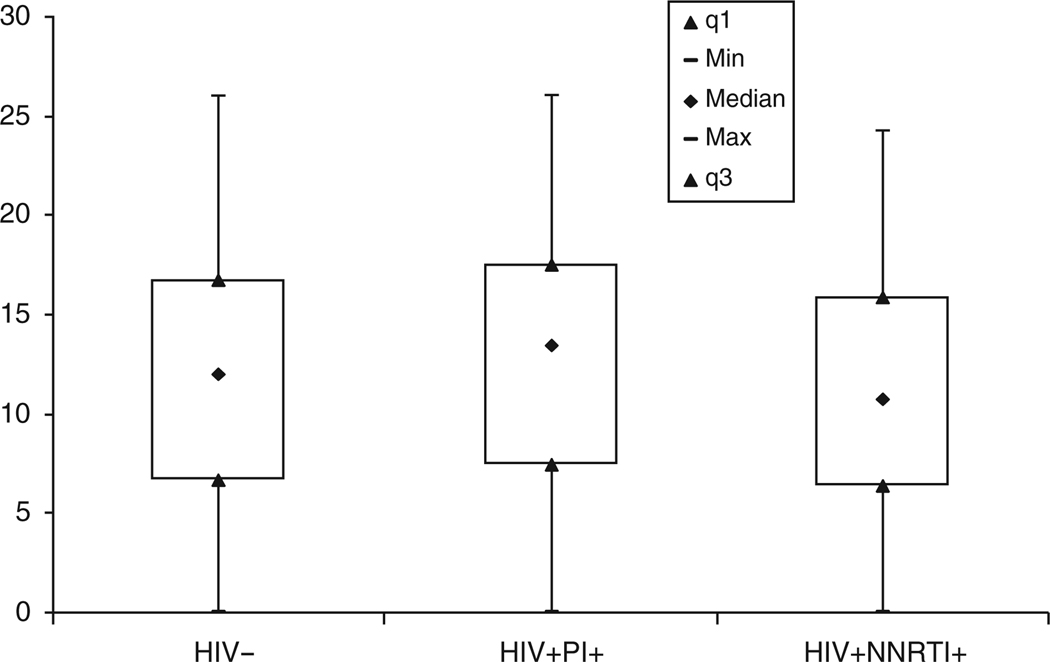
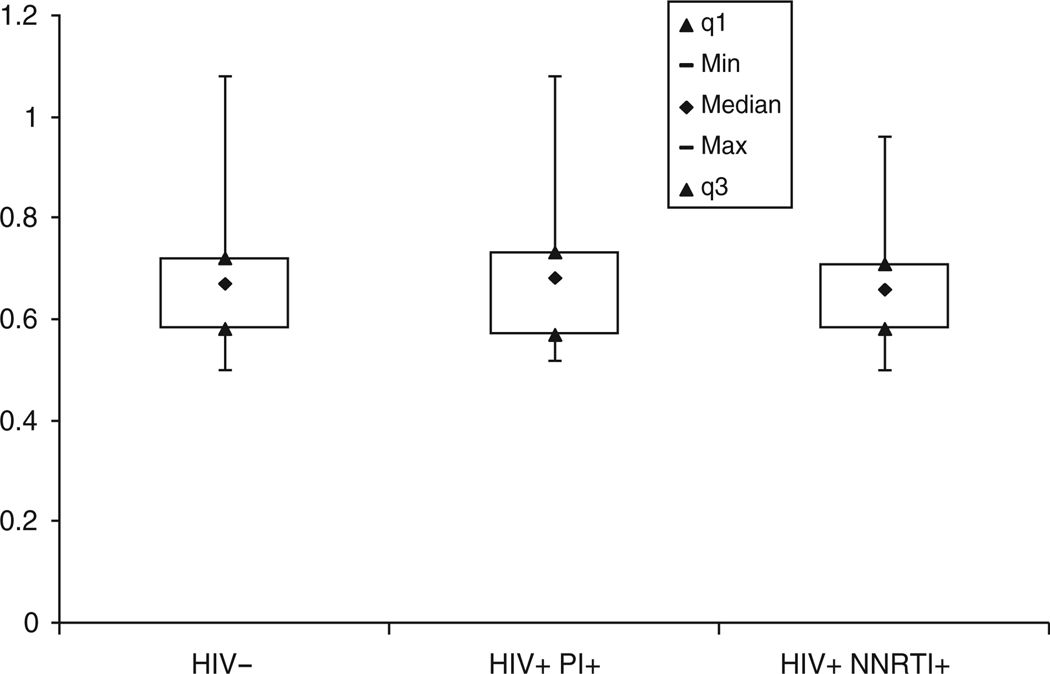
Compared to controls, HIV-infected subjects had significantly lower high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol and higher triglycerides and fasting glucose levels (P < 0.01 for all). On average, however, these values were within normal limits for both groups. There was no significant difference between groups with regard to other traditional cardiac risk factors; the mean Framingham 10-year risk scores were low (≤3%) in both groups. The brachial artery FMD and CIMT measurements were similar in HIV-infected subjects compared with control subjects (12 vs. 13% and 0.68 vs. 0.68 mm, respectively, P = NS; Figs 1 and 2).
Fig. 1. Percentage of brachial flow-mediated vasodilation (FMD) according to HIV status and current highly active antiretroviral therapy (HAART) regimen, P = NS between groups.
Data shown are 1st quartile (q1), minimum, median, maximum, and 3rd quartile (q3) values. NNRTI, nonnucleo-side reverse transcriptase inhibitor; PI, protease inhibitor.
Fig. 2. Carotid intima–media thickness (CIMT) according to HIV status and current highly active antiretroviral therapy (HAART) regimen, P = NS between groups.
Data shown are 1st quartile (q1), minimum, median, maximum, and 3rd quartile (q3) values. NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Predictors of flow-mediated vasodilation and carotid intima–media thickness
In the univariate analyses of HIV-infected subjects, a lower FMD was significantly associated with higher insulin resistance (by both HOMA-IR and Belfiore OGTT index), greater consumption of alcohol (≥1 drink/day), and higher baseline brachial artery diameter (P < 0.05 for all). In HIV seronegatives, higher insulin resistance, brachial artery diameter, and lower systolic blood pressure were predictive of lower FMD (P < 0.05 for all). Among HIV-infected and seronegative subjects, there was a significant correlation between CIMT and traditional cardiac risk factors including male sex, older age, history of hypertension, lower HDL cholesterol, higher glucose, higher insulin resistance, higher trunk/limb fat ratio (HIV-infected subjects with DEXA data), and the presence of an increasing number of metabolic syndrome criteria (P < 0.05 for all). In multivariate analyses (Table 3) among HIV-infected subjects, higher insulin resistance, greater consumption of alcohol, and higher resting brachial artery diameter remained significant independent predictors of FMD (adj. r2 = 0.40, P < 0.001 for model). History of hypertension, higher trunk/limb fat ratio, and insulin resistance remained significant predictors of CIMT (adj. r2 = 0.46, P < 0.001 for model). There was a strong correlation between the HOMA-IR and Belfiore OGTT indices (r = −0.82, P ≤ 0.001).
Table 3.
Multivariate analysis of HIV cohort.
| Predictor | Coefficient | 95% CI | P value |
|---|---|---|---|
| Significant predictors of percentage of brachial FMD | |||
| Baseline brachial diameter | −47.4 | −68.7, −26.2 | <0.01 |
| HOMA-IR | −6.4 | −10.4, −2.4 | <0.01 |
| ≥ 1 alcoholic drink/day | −3.3 | −6.4, −0.3 | 0.03 |
| Significant predictors of CIMT | |||
| Diagnosis of HTN | 0.11 | 0.05, 0.16 | <0.01 |
| Trunk/limb fat ratio | 0.27 | 0.14, 0.39 | <0.01 |
| HOMA-IR | 0.08 | 0.01, 0.16 | 0.05 |
CI, confidence interval; CIMT, carotid intima–media thickness; FMD, flow-mediated vasodilation; HOMA-IR, homeostatic model of insulin resistance; HTN, hypertension.
To further explore a potential confounding effect between ritonavir and insulin resistance, the relationship between current protease inhibitor use and brachial FMD or CIMT was also examined. All subjects on a protease-inhibitor-based regimen were on a ritonavir-boosted regimen (76% were on atazanavir/ritonavir). There was no significant association between either brachial FMD or CIMT and current use of a ritonavir-boosted protease inhibitor. Additionally, the cumulative exposure to HAART, as well as the cumulative exposure to individual drug classes (nucleosides, NNRTIs, protease inhibitors) revealed no significant association with either brachial FMD or CIMT. Studies have also found an association between thymidine analogue use and insulin resistance [20–22], but in the present study there was no significant correlation between cumulative exposure/use ever of zidovudine or stavudine and HOMA-IR/Belfiore index, brachial FMD, or CIMT. There have also been conflicting data with regard to effects of body fat changes (lipoatrophy) on FMD [13,23], but in our study we found no significant association between appendicular fat, trunk fat, or trunk/limb fat ratio and FMD. Finally, there was no significant correlation between HIV disease activity indicators (i.e., current/nadir CD4 cell count, HIV RNA, duration of HIV) and CIMT or brachial FMD. Overall, there were modest, significant correlations between FMD and CIMT (r = −0.40, P < 0.01) and between CIMT and 10-year Framingham score (r = 0.50, P < 0.01). There were no significant correlations between FMD and Framingham score, between FMD and hsCRP, and between CIMT and hsCRP. Traditional risk factors utilized in the Framingham risk score (age, smoking, HDL and total cholesterol, blood pressure) were more predictive of CIMT (r = 0.55, P = 0.01) than brachial FMD (r = 0.25, P = NS).
Discussion
The results of this study of HIV-infected subjects on modern HAART regimens and with well controlled viremia demonstrate no significant differences in either brachial FMD or CIMT compared with seronegative controls. Higher insulin resistance was a significant predictor of lower brachial FMD and higher CIMT.
A recent study examined endothelial function in HIV-infected subjects vs. controls, and found that HIV-infected subjects had significantly impaired brachial FMD, and that current intravenous drug use was significantly associated with FMD [10]. Although insulin resistance was not reported in that study, 63% of HIV-infected subjects had co-infection with hepatitis C, which has been associated with increased risk of insulin resistance in some [24,25] but not all studies [26]. Injection drug users were excluded from the present study.
Another prior study [12] in HIV-infected subjects suggested that protease inhibitor use is associated with endothelial dysfunction. Nevertheless, the predominant protease inhibitor used was indinavir, which has been associated with multiple metabolic complications including insulin resistance and lipodystrophy [27,28], and when given to HIV-seronegative controls, indinavir reduced endothelial function [29]. The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study reported that cumulative protease inhibitor exposure is an independent risk factor for MI [2], but the risk of specific protease inhibitors on MI incidence or surrogate measures of cardiovascular disease risk has not been well characterized. Although the HIV-infected subjects in the present study had considerable cumulative length of time on HAART, the brachial FMD was higher than in other cross-sectional studies of HIV-infected persons [10–13] and similar to that of normal controls; CIMT was also similar in both groups. These findings may be due, in part, to the long-term use of modern, metabolically ‘friendly’ antiretroviral regimens [30]. Furthermore, subjects in the current study had well controlled HIV, and the study protocol was designed to minimize confounders of endothelial function (as described in Methods).
Recent studies of HIV-infected, therapy-naive, and uninfected persons exposed to modern drugs such as lopinavir/ritonavir or efavirenz have shown improvements in endothelial function within a short period [13,31]. In one study, endothelial function was improved 6 months after HAART initiation, although still below normal values, and was independently predicted by the decline in HIV RNA [13]. Also, in-vitro studies of endothelial function have implicated HIV as potentially having direct and indirect effects on the endothelium, including direct infection and/or activation of endothelial cells by HIV, vascular injury as a result of chronic inflammation and immune activation, or dysregulation of the nitric oxide synthase system [32–35]. Both tat and gp120 proteins of the HIV virion may activate endothelial cells, resulting in increased expression of specific adhesion molecules (i.e., ICAM-1, E-selectin) that may contribute to endothelial dysfunction or a prothrombotic state [33–35]. Thus, it is possible that subjects in the current study had overall low cardiovascular risk due to good control of HIV viremia and adequate restoration of immunity. As only five subjects had detectable, low-level HIV viremia, we did not have statistical power to detect significant differences in CIMT/FMD due to differences in HIV viral load.
An important finding is the association of insulin resistance with both endothelial dysfunction (i.e., lower FMD) and higher CIMT in HIV-infected subjects (Table 3). Although we did not find a significant association between current protease inhibitor or thymidine analogue use and insulin resistance, brachial FMD, or CIMT, the use of specific antiretrovirals has been strongly associated with insulin resistance in other studies [3,4,20–22,27,28]. The development of insulin resistance in some patients on HAART may have important long-term implications in determining cardiovascular disease risk. The traditional Framingham risk score calculator does not adequately account for insulin resistance (it only accounts for established diabetes) and in this study we did not find a significant correlation between brachial FMD and Framingham risk score. The Framingham score has not been validated in HIV and thus determination of insulin resistance or use of endothelial function testing could be a useful adjunct in assessing long-term cardiovascular disease risk in this population. Unfortunately larger, longitudinal cohort studies rarely incorporate the use of sensitive metabolic testing such as oral glucose tolerance testing or insulin profile. We found that a simple index of insulin resistance (HOMA-IR) was a stronger predictor of brachial FMD and CIMT than more indirect assessments, such as the diagnosis of metabolic syndrome. HOMA-IR was also strongly correlated with the sensitive Belfiore OGTT index, which requires repeated measures of insulin and glucose levels during a 2-h OGTT.
There were minor limitations. This study was cross-sectional and the number of subjects was low, but still higher compared with several other endothelial function studies in HIV [11–13,36]. In addition, several HIV-infected subjects were on statin therapy, which may have a positive effect on brachial FMD and CIMT. Nevertheless, two recent studies of statin therapy in HIV found no more than a 0.7–1.2% improvement in brachial artery FMD with statin therapy [37,38], and the inclusion of statin-treated, HIV-infected subjects in the present study was felt to best reflect current standard of care for persons with HIV [39]. When further controlling for use of lipid-lowering medication, there were still no significant differences in FMD or CIMT between HIV-infected and control groups.
In conclusion, in this HIV cohort on modern HAART and with well controlled HIV, we found no significant differences with regard to preclinical markers of cardiovascular disease risk such as endothelial function testing, CIMT, or Framingham risk score. Insulin resistance was a stronger predictor of brachial FMD and CIMT than lipid parameters, HAART regimen, indices of immune function, lifestyle factors, or body and limb adiposity. These findings suggest that insulin resistance is an important cardiovascular disease risk factor in the HIV-infected population, and that therapeutic strategies to improve insulin sensitivity may be warranted. Longitudinal studies of HIV-infected cohorts will be important to evaluate future changes in cardiovascular risk, especially as new HIV drug classes become available.
Acknowledgements
1 K23 AI065336-01 (K.E.M.), K12RR023249 (L.diF.), DK049393, DK059531, AT003083, DK056341, RR000036, RR000954, DK020579, AI025903.
We gratefully acknowledge the Barnes-Jewish Hospital Foundation for support to the Cardiovascular Imaging and Clinical Research Core Laboratory.
References
- 1.Holmberg SD, Tong TC, Ward DJ, Wood KC, Greenberg AE, Janssen RS HIV Outpatient Study (HOPS) investigators. Protease inhibitor drug use and adverse cardiovascular outcomes in ambulatory HIV-infected persons. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 2.The DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 3.Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 4.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 5.Yarasheski KE, Tebas P, Sigmund C, Dagogo-Jack S, Bohrer A, Turk J, et al. Insulin resistance in HIV-protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.Faulx MD, Wright AT, Hoit BD. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J. 2003;145:943–951. doi: 10.1016/S0002-8703(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 8.Kuvin JT, Karas RH. Clinical utility of endothelial function testing. Circulation. 2003;107:3243–3247. doi: 10.1161/01.CIR.0000075928.54461.33. [DOI] [PubMed] [Google Scholar]
- 9.Juonoala M, Viikari JSA, Laitinen T, Marniemi J, Helenius H, Rönnemaa T, et al. Interrelations between brachial endothelial function and carotid intima–media thickness in young adults. The Cardiovascular Risk in Young Finns Study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 10.Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven D, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Wijk JPH, de Koning EJP, Cabezas MC, Joven J, op’t Roodt J, Rebelink TJ, et al. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 12.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 13.Torriani FJ, Komarow L, Cotter BR, Murphy RL, Fichtenbaum CJ, Currier JS, et al. Control of HIV viral replication is associated with rapid improvement in endothelial function sustained over 24 weeks: A5152s, a substudy of A5152. Antivir Ther. 2007;12 Suppl 2:L15. [Abstract O-18] [Google Scholar]
- 14.Currier JS, Kendall MA, Henry WK, Alton-Smith B, Torriani FJ, Tebas P, et al. Progression of carotid-artery media thickening in HIV-infected and uninfected adults. AIDS. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 15.Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Belfiore F, Iannello S, Volpicelli G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Mol Genetics Metab. 1998;63:134–141. doi: 10.1006/mgme.1997.2658. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant CL. Definition of metabolic syndrome. Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 18.Corretti MC, Plotnick GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. Am J Physiol. 1995;286:H1397–H1408. doi: 10.1152/ajpheart.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, et al. Noninvasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. BMJ. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–1147. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 21.Fleischman A, Johnsen S, Systrom DM, Mirko H, Farrar T, Frontera WR, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–E1673. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Vonderen MGA, Blümer RME, Hassink E, Sutinen J, Ackermans MT, van Agtmael MA, et al. and the MEDICLAS study group. Persistent reduction in peripheral glucose disposal starting prior to fat distribution changes, only during first line ART with AZT/3TC/LPV/r but not NVP/LPV/r, suggests contribution of AZT/3TC to insulin resistance by a body composition-independent mechanism. Program and Abstracts of the 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. 2007. Jul, [Abstract TUPEB077] [Google Scholar]
- 23.Dubé MP, Shen C, Waltz JS, Greenwald ML, Mather K, Gupta SK. Relationship of body composition, antiretroviral use, and HIV disease factors to endothelial dysfunction in HIV-infected subjects. Antivir Ther. 2007;12 Suppl 2:L15–L16. doi: 10.1089/aid.2010.0007. [Abstract O-19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duong M, Petit JM, Piroth L, Grappin M, Buisson M, Chavanet P, et al. Association between insulin resistance and hepatitis C virus chronic infection in HIV-hepatitis C virus-coinfected patients undergoing antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:245–250. doi: 10.1097/00126334-200107010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Hadigan C, Chung R, Murray G, Purkis D, Grinspoon S. Hepatitis B and C co-infection and alanine aminotransferase are associated with increased insulin resistance and diabetes in patients with fat redistribution. Antivir Ther. 2002;7:L24. [Google Scholar]
- 26.Ledergerber B, Hansjakob F, Rickenbach M, Lehmann R, Elzi L, Hirschel B, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45:111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 27.Noor MA, Lo JC, Mulligan K, Schwarz JM, Halvorsen RA, Schambelan M, et al. Metabolic effects of indinavir in healthy HIV seronegative men. AIDS. 2001;15:11–18. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–875. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 29.Shankar SS, Dubé MP, Gorski JC, Klaunig JE, Steinberg HO. Indinavir impairs endothelial function in healthy HIV-negative men. Am Heart J. 2005;150:933.e1–933.e7. doi: 10.1016/j.ahj.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Hammer SM, Saag MS, Schecter M, Montaner JSG, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection. 2006 recommendations of the International AIDS Society-USA Panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 31.Grubb JR, Dejam A, Voell J, Blackwelder WC, Sklar PA, Kovacs JA, et al. Lopinavir–ritonavir: effects on endothelial cell function in healthy subjects. J Infect Dis. 2006;193:1516–1519. doi: 10.1086/503807. [DOI] [PubMed] [Google Scholar]
- 32.Chai H, Yang H, Ya S, Li M, Lin PH, Lumsden AB, et al. Effects of 5 HIV protease inhibitors on vasomotor function and super-oxide anion production in porcine coronary arteries. J Acquir Immune Defic Syndr. 2005;40:12–19. doi: 10.1097/01.qai.0000172368.05327.7b. [DOI] [PubMed] [Google Scholar]
- 33.Chi D, Henry J, Kelly J, Thorpe R, Smith JK, Krishnaswamy G. The effects of HIV infection on endothelial function. Endothelium. 2000;7:223–242. doi: 10.3109/10623320009072210. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 35.Paladugu R, Fu W, Conklin BS, Lin PH, Lumsden AB, Yao Q, et al. HIV tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38:549–555. doi: 10.1016/s0741-5214(03)00770-5. [DOI] [PubMed] [Google Scholar]
- 36.Nolan D, Watts GF, Herrmann SE, French MA, John M, Mallal S. Endothelial function in HIV-infected patients receiving protease inhibitor therapy: does immune competence affect cardiovascular risk? Q J Med. 2003;96:825–832. doi: 10.1093/qjmed/hcg145. [DOI] [PubMed] [Google Scholar]
- 37.Hürlimann D, Chenevard R, Ruschitzka F, Flepp M, Enseleit F, Béchir M, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor containing antiretroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92:110–112. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein JH, Merwood MA, Bellehumeur JL, Aeschlimann SE, Korcarz CE, Underbakke GL, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147:713. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Dubé MP, Stein J, Aberg J, Fichtenbaum CJ, Gerber JG, Tashima KT, et al. Guidelines for the evaluation and management of dyslipidemia in HIV-infected adults receiving antiretroviral therapy: recommendations of the HIV Medicine Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]