Abstract
Parkinson's disease (PD), characterized by selective midbrain nigrostriatal dopaminergic degeneration, is consistently associated with moderate systemic mitochondrial dysfunction. Downstream degeneration of spinal cord has also been suggested in PD, although the mechanisms have not been much investigated. In the present study, two mitochondrial toxicants, 1-methyl-4-phenylpyridinium ion (MPP+) and rotenone were tested in ventral spinal cord (VSC 4.1) motoneuronal cells. Cell death was assessed by morphological and biochemical means to discern a lower apoptosis-inducing concentration and LC50, which were subsequently compared in further cytoprotection experiments. Mitochondrial toxicants dose-dependently induced increase in intracellular free Ca2+ level, which was conducive for increased expression and activities of Ca2+-activated neutral protease calpain and downstream caspase-3. Thus, mitochondrial damage triggered apoptotic mechanisms in spinal cord motoneurons. Inhibition of calpain by calpeptin significantly attenuated damaging effects of MPP+ and rotenone on motoneurons, especially at low apoptosis-inducing concentrations of toxicants and partly at their LC50, as demonstrated by absence of DNA ladder formation and decrease in TUNEL-positive cells. Cytoprotection by calpeptin was observed with marked decreases in Bax:Bcl-2 ratio and activities of calpain and caspase-3, which affirmed the role of mitochondrial dysfunction and involvement of intrinsic pathway in mediation of apoptosis. These findings strongly suggested that parkinsonian toxicants MPP+ and rotenone at low doses induced cascade of cell damaging effects in spinal cord motoneurons, thus, highlighting the possibility of induction of apoptotic mechanisms in these cells, when subjected to mitochondrial stress. Cytoprotection rendered by calpeptin further validated the involvement of calpain in apoptosis and suggested calpain inhibition as a potential neuroprotective strategy.
Keywords: calpain, caspase-3, calpeptin, parkinsonian toxicants, spinal motoneuronal cells
Introduction
Parkinson' disease (PD) is a movement disorder, characterized by disturbances in motor functions, which are predominantly ascribed to reduction in dopamine levels in midbrain substantia nigra, and consequent limitation of dopaminergic neurotransmission in striatum (Hornykiewicz, 2008). PD is associated with consistent systemic mitochondrial dysfunction (Schapira et al., 1990; Mizuno et al., 1998). Hence, mitochondrial toxicants, such as 1-methyl-4-phenylpyridinium ion (MPP+) and rotenone are used to generate experimental PD models in vivo and in vitro. As mitochondria play a decisive role in regulation of intracellular Ca2+ level, mitochondrial dysfunction causes aberrant Ca2+ homeostasis (Szabadkai and Duchen, 2008; Duchen and Szabadkai, 2010). Unregulated elevation of intracellular free Ca2+ may lead to a series of cell signaling cascades and generate environment that is highly conducive for up-regulation of cysteine proteases calpain and caspase-3 (Samantaray et al., 2008c). These proteases may degrade the cytoskeletal proteins and lead to cellular degradation and death (Ray and Banik, 2003; Tamada et al., 2005). These pathways were described in several neurodegenerative conditions including PD, where the apoptotic mechanisms were predominantly involved in demise of nigral dopaminergic neurons in midbrain, hence, were targeted for therapeutic intervention.
Recently, we reported that along with nigrostriatal degeneration, there was damage to spinal cord neurons in experimental parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone (Samantaray et al., 2007, 2008b). Nevertheless, different mechanisms of spinal cord degeneration were not explicitly investigated in experimental parkinsonism. The present study addressed the vulnerability of spinal cord motoneurons in culture upon exposure to parkinsonian toxicants and also examined mechanisms of such degeneration and protective strategies. We tested the effects of MPP+ and rotenone in ventral spinal cord motoneurons (VSC 4.1). Differentiation of VSC 4.1 cells induces specific enzymes, such as choline acetyltransferase (ChAT), neuron-specific enolase, immunoreactive 200 kDa neurofilament protein, and synaptophysin, which specify them as mammalian motoneurons (Smith et al., 1994).
Focus of the present study was to determine whether intrinsic mitochondrial pathway of apoptosis coupled to aberrant intracellular Ca2+-homeostatsis and activation of proteolytic enzymes calpain and caspase-3 was involved in the process of motoneuron damage and death, as previously found in brain and spinal cord of animals with experimental parkinsonism (Ray et al., 2000; Chera et al., 2002, 2004; Crocker et al., 2003; Samantaray et al., 2006, 2007, 2008c). Calpeptin, a cell-permeable peptide aldehyde inhibitor of calpain, was examined for its efficacy to render cytoprotection. Neuroprotective efficacy of calpeptin has previously been demonstrated in vitro and in vivo in experimental autoimmune encephalomyeitis, an experimental model of multiple sclerosis (Butler et al., 2009; Guyton et al., 2010), muscle cells exposed to inflammatory stress (Nozaki et al., 2010), motoneurons subjected to glutamate excitotoxicity (Das et al., 2005; Sribnick et al., 2009), retinal ganglion cells stressed with Ca2+ influx (Das et al., 2006). Protection rendered by calpeptin via inhibition of calpain activity suggested calpain involvement in MPP+ and rotenone mediated apoptosis of motoneurons and also corroborated calpain inhibition as a potential therapeutic target.
Experimental Procedures
Materials
The hybrid motoneuron-neuroblastoma cell line (VSC 4.1) was a gift from Dr. Stanley H. Appel (Houston, TX, USA). Cells were cultured in Dulbecco's Modified Earle's Medium (DMEM)/Ham's F12 50/50 Mix with L-glutamine and 15 mM HEPES supplemented with penicillin (100 IU/mL) and streptomycin (100 μg/mL) (Cellgro, Mediatech, Manassas, VA, USA). Complete medium contained 2% Sato's components and 2% of heat inactivated fetal bovine serum (FBS; HyClone, Logan, UT, USA). Cell differentiating agents (dibutyryl cAMP and aphidicolin) and neurotoxic compounds (MPP+ and rotenone) were obtained from Sigma-Aldrich (St. Louis, MO, USA); calpain inhibitor calpeptin was procured from EMD Biosciences (Gibbstown, NJ, USA). Toxicants were handled according to our institutional Health and Biosafety Committee.
The primary IgG antibodies: rabbit anti-caspase-3 polyclonal (1:250), mouse anti-Bax monoclonal (1:250), and mouse anti-Bcl-2 monoclonal (1:250) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-m-calpain polyclonal (1:500) that we used in the study was raised in our laboratory (Banik et al., 1983). Mouse anti-β-actin monoclonal (1:15,000) was from Sigma, and mouse monoclonal anti-α-spectrin (1:10,000) was from Biomol International (Plymouth Meeting, PA, USA). Peroxidase-conjugated goat anti-rabbit and anti-mouse secondary IgG antibodies (1:2000) were obtained from MP Biomedicals (Solon, OH, USA).
Cell culture, differentiation and treatments
VSC 4.1 cells were cultured in 75-cm2 flasks (Corning, NY, USA) pre-coated with 0.01% poly-L-ornithine (Sigma) in 0.6% boric acid solution (pH 8.4). Cells were grown in complete medium at 37°C in a humidified atmosphere of 95% air and 5% CO2, and were refreshed every alternative day. Sixty to 70% of confluence was attained in 3-4 days. Cells were redistributed at density of 106 cells in 75-cm2 flasks and differentiation was induced with dibutyryl cAMP (0.5 mM) over 5-7 days. Bystander killing was attained with aphidicolin (0.4 μg/mL) on every alternative day. By the 7th day VSC 4.1 cells were terminally differentiated into spinal motoneurons (further in the text mentioned as motoneurons or motoneuronal cells). Motoneuronal cells were then either exposed to toxicants, or pre-treated before exposure with calpeptin, a calpain inhibitor; all treatment procedures were conducted in low-serum medium (0.5% FBS). To analyze time- and dose-dependent effects, MPP+ and rotenone were added in concentration range of 10-500 μM and 10-500 nM, respectively. For certain experiments, cells were collected and disseminated in 6-well plates at density of 2.5 - 5 × 104 cells/well, in which treatments were performed. Calpeptin was tested for its cytoprotective efficacy at two doses, low (100 nM) and high (1 μM); the later was chosen for pre-treatment in subsequent studies.
Cell viability assay
3-(4, 5-Dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) was used to assess cell viability. The assay is based on the conversion of bright yellow MTT dye to dark blue formazan crystals by living cell mitochondria, which when dissolved in dimethylsulfoxide (DMSO; Sigma), has specific absorption maximum at 570 nm (Mosmann, 1983). Following toxicant exposure, medium was replaced with low serum medium containing MTT reagent (0.1 mg/mL) and incubated at 37°C for 1 h. Formazan crystals were precipitated by centrifugation at 1900 × g (Eppendorf Centrifuge 5804R, Germany), medium was aspirated, and crystals were dissolved in DMSO. Plates were read in Emax Precision Microplate reader at 570 nm with reference wavelength set at 630 nm using SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA). Optical density was compared setting the control at 100% (Sur et al., 2003).
In Situ Wright staining
Cells were differentiated and subsequently treated on sterile glass cover-slips inserted within 6-well plates. Plates were gently spun (150 × g, 10 min) to settle down less adherent apoptotic cells on cover slips. Post-treatment cells were fixed with 95% ethanol for 10 min and washed twice with phosphate-buffered saline (PBS). Cells were stained with 2 mL/well of each stain: Hema 3 Fixative, Hema 3 Solution 1 (eosin), and Hema 3 Solution 2 (hematoxylin), according to manufacturer's protocol. Next, cells were gently washed with water. The cells on cover slips were dried within the 6-well plates. The cover slips were flipped over onto a slide containing a drop of mount and viewed at 200 × magnification in Olympus BH-2 microscope (Olympus, Melville, NY, USA). Images were captured by a non-confocal camera and organized by Photoshop software (Adobe Systems, San Jose, CA, USA). The cells were deemed apoptotic based on whether or not they exhibited reduction in cell volume, shortening of neuritis, and cell membrane blebbing and/or chromatin condensation.
TUNEL assay
Post-treatment, DNA fragmentation and cell death was assessed by terminal deoxynucleotidyl transferase dUTP-mediated nicked end labeling (TUNEL). Cells were grown and treated as previously described. They were further fixed with 4% methanol-free formaldehyde in PBS for 15 min and washed twice with PBS. Cells were equilibrated and then incubated with fluorescein-conjugated 12-dUTP reagent (Apoptosis Detection System, Promega, Madison, WI, USA) at 37°C for 1 h. Labeling reaction was terminated by addition of NaCl/Na-citrate (SSC) (Promega). Cells were washed with PBS, and fragmentation of DNA was visualized by fluorescent microscope (Olympus BH2) at 200 × magnification. The green fluorescence correlated with DNA fragmentation. Experiments were done in triplicate; the percentage (%) of arbitrary fluorescence unit (% AFU) of TUNEL positive immunoreactivity (IR) was detected.
Intracellular free Ca2+ assay
The ratiometric dye Fura-2 was used to assess intracellular free Ca2+ in motoneurons exposed to gradation of toxicants. The original method (Grynkiewicz et al., 1985) was used with some modification (Butler et al., 2009). Post-exposure cells were suspended in fresh medium and washed in 2 mL of modified Locke's buffer (NaCl: 154 mM, KCl: 5.6 mM, NaHCO3: 3.4 mM, MgCl2: 1.2 mM, glucose: 5.6 mM, Hepes: 5 mM [pH 7.4], and CaCl2: 2.3 mM), originally introduced as a strong capacity buffer (Acker et al., 1987). Cell suspension in 1 mL of Locke's buffer plus 0.5% bovine serum albumin (Sigma) were counted on a hemocytometer. As cell death was higher in toxicant-exposed sets, cells were pooled to render equal number of cells in each set prior to fluoroprobe loading. At this step, 1 × 106 cells/mL were taken. Fura-2 AM (5 μM) from (Molecular Probes, Carlsbad, CA, USA), was loaded into the cells at 37°C for 30 min. Cells were spun and washed twice in ice-cold Locke's buffer. Concentration of [Ca2+]i was calculated using the equation [Ca2+]i = Kd(R −Rmin)/(Rmax−R). Spectrophotometric analysis of the fluorescence ratio (R) was done using SLM 8000 fluorometer at 340 nm and 380 nm wavelengths (Thermospectronic, USA). Maximal (Rmax) and minimal (Rmin) ratios were determined using 25 μM digitonin and 5 mM EGTA, respectively. The % increase in [Ca2+]i upon toxicant exposure compared to control was plotted.
Western blot analysis
The levels of expression of active proteases - calpain (inactive 80 kDa and active 76 kDa), caspase-3 (proenzyme 32 kDa, active 20 kDa and 12 kDa), pro-apoptotic Bax (24 kDa and 21 kDa) and anti-apoptotic Bcl-2 (26 kDa) were determined by Western blot analysis. Furthermore, proteolytic activities of calpain and caspase-3 were determined by appearance of calpain-specific spectrin breakdown product (SBDP, 145 kDa band) and caspase-3-specific SBDP (120 kDa band). Cells were cultured in 75-cm2 flasks, post-treatment harvested into 15 mL tubes, and centrifuged at 500 × g for 10 min. Cell pellets were re-suspended in 250 μL of ice-cold homogenizing buffer containing 50 mM Tris-HCl (pH 7.4) and 5 mM EGTA. Phenylmethylsulfonyl fluoride (1 mM) was freshly added to homogenizing buffer. Cells were homogenized by sonication with Kontes Micro Ultrasonic Cell Disrupter. Samples were kept on ice for 15 min to ensure total protein extraction. Protein concentration was estimated using Coomassie Plus Protein Assay Reagent (Pierce, Rockford, IL, USA) in spectrophotometer at 595 nm (Spectronic, Rochester, NY, USA). Samples were then diluted 1:1 in sample buffer [62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 5 mM β-mercaptoethanol, 10% glycerol] and boiled for 10 min. Samples were equilibrated to a final protein concentration of 1.5 mg/mL with 1:1 v/v mix of sample and homogenizing buffer, containing bromophenol blue dye (0.01%). Protein samples were resolved in 4-20% pre-cast sodium dodecyl sulfate–polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA, USA) at 100 V for 1 h, and transferred to the Immobilon™-P polyvinylidene fluoride micro-porous membranes (Millipore, Bedford, MA, USA) in a Genie transfer apparatus (Idea Scientific, Minneapolis, MN, USA). For resolution of SBDP bands, 5% pre-cast gels were used; electrophoresis was carried out at 100 V for 2 h. Membranes were blocked in 5% non-fat milk in Tris-buffer (20 mM Tris–HCl, pH 7.6, containing 0.1% Tween-20), incubated overnight at 4°C with appropriate primary IgG antibodies, followed by incubation with horseradish peroxidase–conjugated corresponding secondary IgG antibodies at room temperature. In between, membranes were washed 3 times for 5 min each in Tris-buffer. Membranes were shortly incubated with enhanced chemiluminescent reagents (ECL or ECL Plus, Amersham Biosciences) and imaged on Alpha-Innotech using FluorChem FC2 Imaging System (Cell Biosciences). All immunoblots except those for spectrin were re-probed with antibody against β-actin, which served as the corresponding protein loading controls.
DNA ladder assay
Genomic DNA was isolated using Wizard® Genomic DNA Purification Kit (Promega). Briefly, differentiated cells were exposed to MPP+ and rotenone and/or pre-treated with calpeptin. Cells were harvested, centrifuged and pellets were washed with PBS. Samples were incubated with nuclei lysis buffer (600 μL) and RNase (3 μL) at 37 °C for 30 min and then cooled at room temperature. Next, protein precipitation solution (200 μL) was added, and the mixture was chilled on ice for 15-30 min. Cell debris was sedimented and supernatant was transferred into fresh tubes containing 600 μL of isopropanol (Sigma). DNA was sedimented by centrifugation; pellets were washed with 75% ethanol (600 μL). All centrifugations were performed at 14,000 × g for 5 min in Thermo Scientific Sorvall Legend Micro 21 centrifuge (Germany). The final DNA pellets were rehydrated with rehydration solution (100 μL) at 65 °C for 1 h. DNA fragmentation was analyzed by resolving on 1% agarose gel, containing 1 mg/mL of ethidium bromide at 80 V for 1 h. DNA ladder formation was imaged using Alpha-Innotech UV transilluminator and FluorChem FC2 Imaging System (Cell Biosciences, Santa Clara, CA).
Statistical analysis
Each assay represents a minimum of 3 independent experiments performed in triplicate. Results were assessed in Stat View software (Abacus Concepts, Berkley, CA, USA) and compared by using one-way analysis of variance (ANOVA) with Fisher's protected least significant difference (PLSD) post-hoc test at 95% confidence interval. The difference between two treatments was considered significant at p ≤ 0.05.
Results
MPP+ and rotenone reduced cell viability
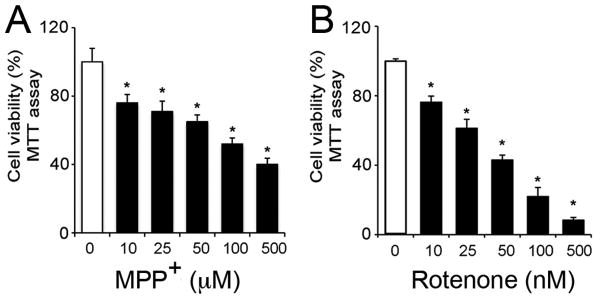
Neurotoxicity of MPP+ and rotenone was tested in motoneurons following 24 h exposure (Fig. 1). The effects were discernable as early as 6 h and were tested until 36 h. A 24 h time-point was chosen for subsequent experiments. Cell viability assay showed that motoneurons responded to these toxicants in a dose-dependent manner (Fig. 1). Micromolar concentrations of MPP+ were found to be effective (1-500 μM); 25 μM of MPP+ reduced cell viability by 25 %, whereas, LC50 was attained at concentrations ≥ 100 μM (p ≤ 0.05) compared to control (Fig. 1A). Rotenone was found to be toxic at nano-molar concentrations; 10 nM reduced cell viability by 24 %, whereas 50 nM of rotenone concentration was found to be LC50 (p ≤ 0.05) compared to control (Fig. 1B). Subsequently, apoptotic concentrations (25 μM and 10 nM), and LC50 (100 μM and 50 nM) for MPP+ and rotenone that induced ∼25 % and ∼50 % decrease in cell viability at 24 h, respectively, were compared in further experiments.
Fig. 1.
Effect of MPP+ (A) and rotenone (B) on motoneuronal cell viability. Cell viability was tested by MTT assay after 24 h exposure to toxicants. MPP+ (0 – 500 μM) and rotenone (0 – 500 nM) diminished the cell viability in a dose-dependent manner. Bar graphs represent data expressed as mean ± SEM of cell viability (%), obtained from 4 to 6 independent measures; *p ≤ 0.05, significantly different from viability of control cells.
MPP+ and rotenone induced apoptosis
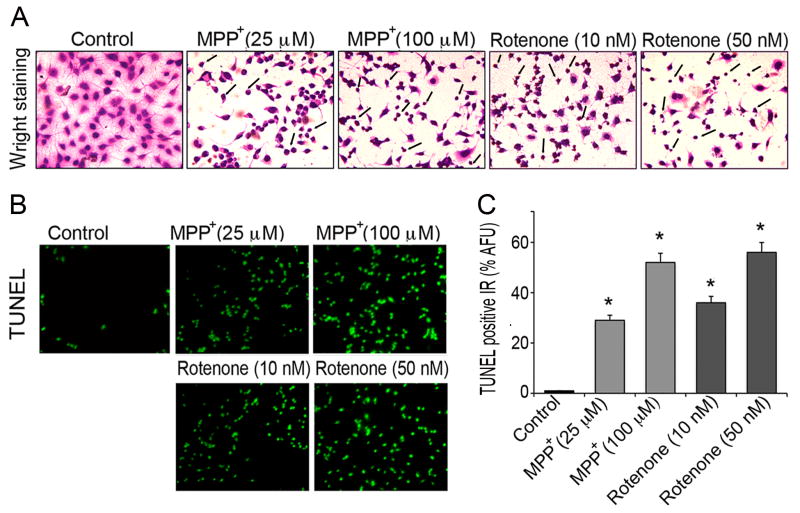
Morphological and biochemical indices of apoptosis in motoneurons were assessed with Wright staining and TUNEL assay (Fig. 2). In situ Wright staining and further examination by light microscope demonstrated deeply stained dead cells following toxicant exposure. As seen from representative microphotographs (Fig. 2A), both, low and high concentrations of MPP+ and rotenone substantially affected morphological features of motoneuronal cells compared with unexposed control cells. Both toxicants caused reduced cell size, shortened neurites, shrunken cell cytoplasm, and condensed nuclei. Loss of neurites was more pronounced in rotenone than MPP+. At lower concentrations of MPP+ (25 μM) and rotenone (10 nM) shrunken rounded cell bodies were found, which were deemed as apoptotic cells. Marked amplification in the number of dead cells (up to 50%) was found at higher concentrations of MPP+ (100 μM) and rotenone (50 nM), respectively. In the remaining attached cells, neurites were degenerated and shortened. The integrity of intact cell surfaces and membranes was found altered and disorganized.
Fig. 2.
Assessment of morphological and biochemical indices of apoptosis in motoneurons. In situ Wright staining and TUNEL assay were used to distinguish apoptotic cells following 24 h exposure to MPP+ (25 and 100 μM) and rotenone (10 and 50 nM). (A) Representative microphotographs of control and cells exposed to toxicants; images were taken by light microscope after in situ Wright staining at 200 × magnification. Arrows indicate damaged and apoptotic cells (B) Representative microphotographs of TUNEL positive motoneurons; images were viewed under fluorescent microscope and captured at 200 × magnification (green fluorescence). Only few TUNEL positive cells were found among control cells, whereas TUNEL positive IR prominently emerged in motoneurons after MPP+ and rotenone exposure, depicting motoneuronal cell death. (C) Bar graphs of % AFU of TUNEL positive IR compared to those of control cells. Data are expressed as mean ± SEM of % AFU, obtained from four different foci (n ≥ 4); *p ≤ 0.05, significantly different from control.
Further, fluorescein isothiocyanate-conjugated TUNEL assay was performed to quantify extent of cell death; cells were observed under fluorescent microscope at 200 × magnification (green fluorescence, Fig. 2B). Only a few TUNEL positive cells were found among control motoneuronal cells at 24 h. Increased TUNEL positive IR was detected in motoneurons exposed to toxicants. Estimated % AFU indicated that while low concentrations of MPP+ (25 μM) and rotenone (10 nM) increased TUNEL positive IR in motoneurons up to 3 to 4-fold (p ≤ 0.05), high doses of MPP+ (100 μM) and rotenone (50 nM) increased it by more than 5 to 6-fold (p ≤ 0.05) compared to control cells (Fig. 2C).
MPP+ and rotenone elevated intracellular free Ca2+
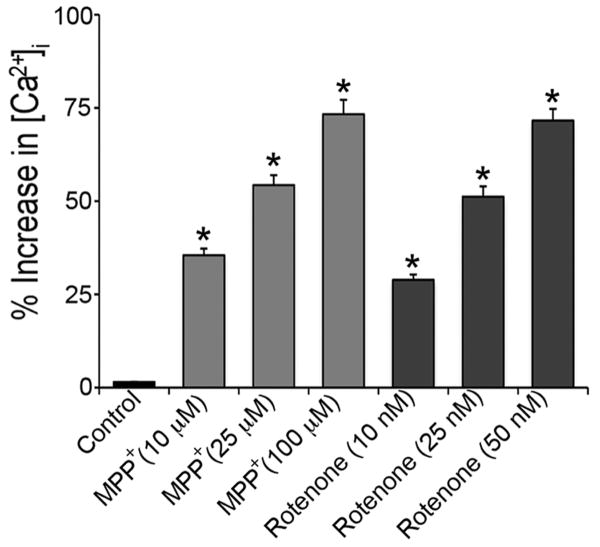
Fura-2 AM, a ratiometric dye, was used to evaluate the load of intracellular free Ca2+ within motoneurons after exposure to MPP+ or rotenone. Cells, pre-exposed to MPP+ (25, 50, and 100 μM) or rotenone (10, 25, and 50 nM) for 24 h, were loaded with Fura-2 AM, and the intracellular free Ca2+ levels were recorded. The data represented significant dose-dependent elevation of intracellular free Ca2+ level due to toxicants exposure (Fig. 3). Rise in concentration of MPP+ (25 to 100 μM) or rotenone (10 to 50 nM) evoked significant elevation of intracellular free Ca2+ levels by 2-2.5 folds (p ≤ 0.05) in exposed motoneurons compared to control cells.
Fig. 3.
Elevation of free [Ca2+]i levels in motoneuronal cells. After exposure to different concentrations of MPP+ (25, 50 and 100 μM) and rotenone (10, 25 and 50 nM) for 24 h, cells were incubated with ratiometric dye Fura-2AM. [Ca2+]i was measured by monitoring the emission at 510 nm with dual excitement at 340 nm and 380 nm. Bar graphs represent the % of [Ca2+]i increase compared to control cells; data are expressed as mean ± SEM of the % increase (n ≥ 3); *p ≤ 0.05, significantly different from control. Absolute values of [Ca2+]i in control cells were (80 ± 7.3) nM as observed in 3 independent measurements.
MPP+ and rotenone induced activation of caspase-3 and calpain
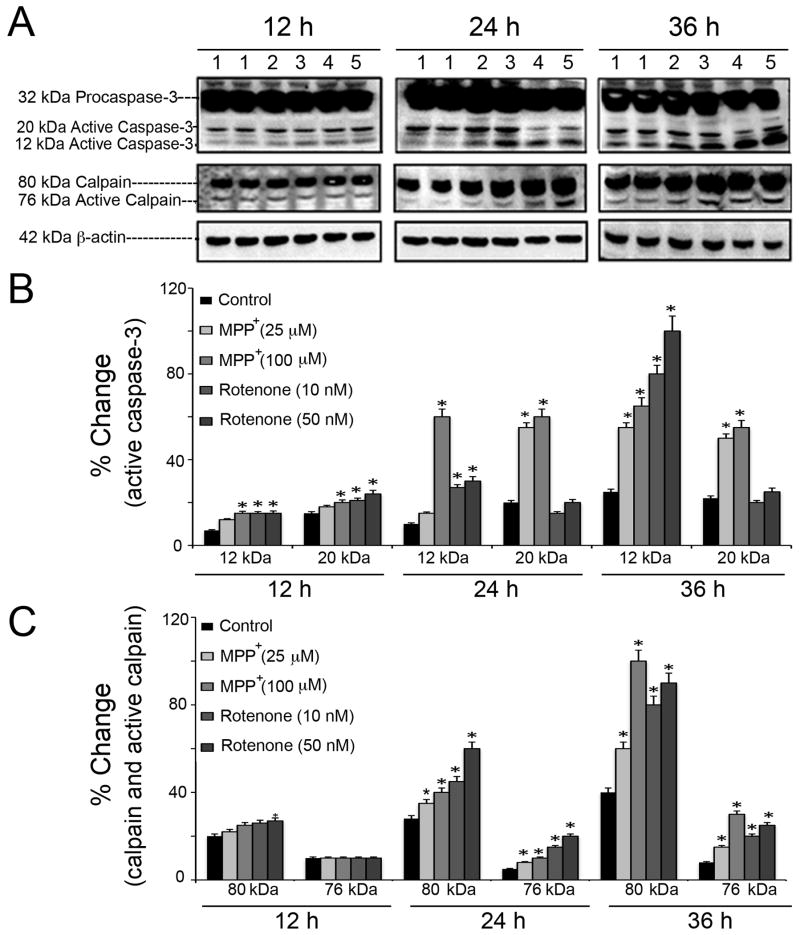
Involvement of proteolytic enzymes in neurotoxicity mediated by MPP+ and rotenone in motoneurons was evaluated detecting active fragments of caspase-3 and calpain (Fig. 4). Up-regulation of active caspase-3 (12 kDa and 20 kDa) and active calpain (76 kDa) immunoreactive bands were assessed via Western blotting (Fig. 4A). In order to fairly accommodate the multi-parametric comparisons (two distinct toxicants, their respective low and high doses, and three time-points), the overall data was grouped and represented as bar graphs under two heads – % changes of intensities for active caspase-3 fragments (Fig. 4B), and that for calpain and active peptide of calpain (Fig. 4C). Within each group the highest band intensities were set at 100% and all others were expressed relative to them; in caspase-3 group, the 12 kDa caspase-3 that was induced by rotenone (50 nM, at 36 h) was considered as 100% intensity; in calpain group, the 80 kDa calpain that was induced by MPP+ (100 μM, at 36h) was considered as 100% intensity. It was found that exposure of motoneurons to MPP+ (25 and 100 μM) and rotenone (10 and 50 nM) for 12, 24 and 36 h induced activation of these proteolytic enzymes in both time- and dose-dependent manners (p ≤ 0.05) compared with respective controls. While, MPP+ exposure caused gradual up-regulation of both 20 kDa and 12 kDa active caspase-3 fragments, rotenone induced elevation of 12 kDa fragment predominantly (Fig. 4B). Rotenone was more potent to induce 76 kDa active calpain at 24 h; both MPP+ and rotenone caused significant increase of active calpain bands at 36 h of exposure (Fig. 4C).
Fig. 4.
Time- and dose-dependent profiles of caspase-3 and calpain activation in motoneurons. (A) Representative immunoblots of 32 kDa pro-caspase-3, 20 kDa and 12 kDa active caspase-3, 80 kDa calpain and 76 kDa active calpain, after exposure of cells to MPP+ (25 and 100 μM) and rotenone (10 and 50 nM) for 12, 24 and 36 h. The listed lanes above the representative immunoblots correspond to: 1 = control, 2 = MPP+ (25 μM), 3 = MPP+ (100 μM), 4 = rotenone (10 nM), and 5 = rotenone (50 nM). Bar graphs designate the % change in intensities of immunoreactive bands for active caspase-3 (B), calpain and active calpain (C); data are expressed as mean ± SEM, obtained from three independent experiments (n ≥ 3); *p ≤ 0.05, significantly different from the respective controls. Data showed significant elevation in levels of active forms of proteases in motoneurons when exposed to toxicants as compared to control un-exposed cells; recorded changes increased proportionally with the doses of toxicants, and time of exposure. The monitoring of 42 kDa β-actin levels served as protein loading control.
Calpeptin protected motoneurons against MPP+ and rotenone
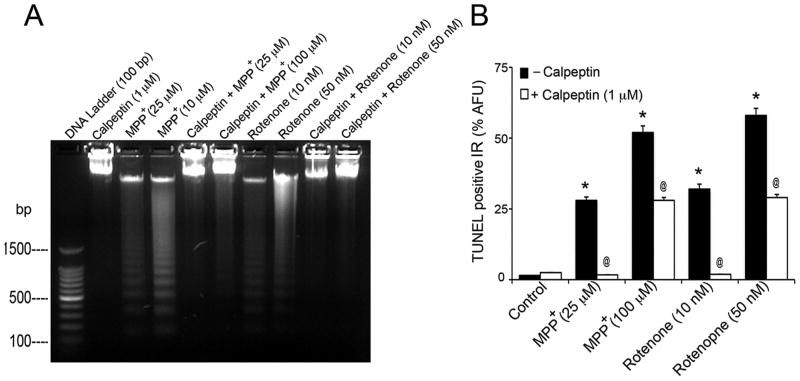
As MPP+ and rotenone induced activation of proteolytic enzymes calpain and caspase-3, we examined whether calpeptin, a synthetic inhibitor of calpain, is capable to block damaging effects of MPP+ and rotenone, and protect motoneurons. Calpeptin was initially tested for its cytoprotective efficacy at two doses, low (100 nM) and high (1 μM); in subsequent studies 1 μM dose was chosen for pre-treatment due to its higher efficacy. Earlier, we reported protective effects of calpeptin on cell viability, using quantitative MTT assay (Samantaray et al., 2008a). To further validate, motoneurons were pre-treated with 1 μM of calpeptin for 30 min and then exposed to MPP+ (25 and 100 μM) and rotenone (10 and 50 nM) for 24 h. The group of cells treated with 1 μM calpeptin alone served as calpeptin-treated control. DNA ladder formation and TUNEL IR were used to evaluate the cytoprotective efficacy of calpeptin (Fig. 5). Representative microphotograph in Fig. 5A illustrates dose-dependent DNA ladder formation in motoneuronal cells exposed to MPP+ and rotenone, respectively. Whereas pre-treatment with calpeptin preserved DNA, as no fragmentation was visualized in these cells.
Fig. 5.
Cytoprotective effects of calpeptin pre-treatment in motoneurons. (A) Representative microphotograph shows UV-transilluminated DNA ladder formation. While control samples did not indicate DNA ladder formation, preparations from cells exposed to MPP+ showed intense DNA laddering. Pre-treatment of cells with calpeptin preserved DNA, as no ladder formation was observed in these cases. (B) Bar graphs of % AFU of TUNEL positive IR, compared to those of control cells. Toxicant exposure increased TUNEL positive IR in motoneurons, compared to control (*p ≤ 0.05). Calpeptin protected cells and significantly reduced TUNEL IR compared to respective toxicants (@p ≤ 0.05). Data are expressed as mean ± SEM of % AFU, obtained from four different foci (n≥4).
Intense dose-dependent increase of % AFU of TUNEL positive IR was detected in motoneuronal cells after exposure to MPP+ and rotenone compared to control cells (p ≤ 0.05) (Fig 5B). Calpeptin significantly reduced TUNEL IR seen in motoneurons exposed to apoptotic and partly at LC50 doses of toxicants (p ≤ 0.05).
Calpeptin decreased Bax:Bcl-2 ratio following toxicant exposure
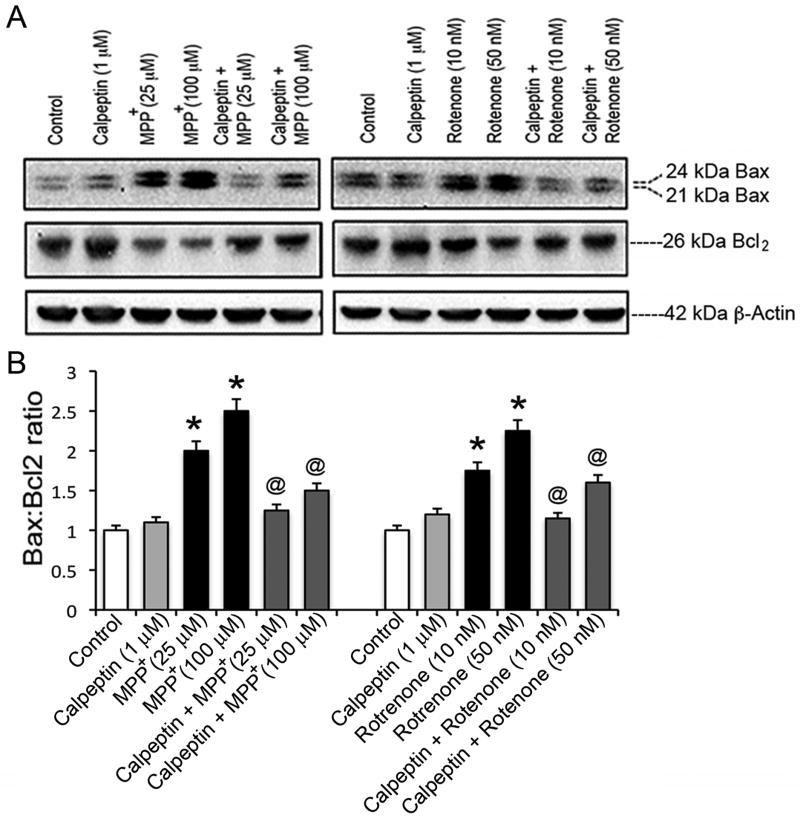
Levels of pro-apoptotic Bax and anti-apototic Bcl-2 were further tested to assess calpeptin efficacy (Fig. 6). Motoneurons were pre-treated with 1 μM calpeptin and then exposed to toxicants, as described above. Representative microphotographs of Western blots showed dose-dependent increase in Baxα (24 kDa) and Baxβ (21 kDa) immunoreactive bands in response to MPP+ or rotenone exposure compared to control cells (Fig. 6A). While detected levels of Bcl-2 protein in exposed cells were much lower than in controls, calpeptin promoted elevation of Bcl-2 and decreased Bax levels. Marked increase in Bax:Bcl-2 ratio (p ≤ 0.05) in response to apoptotic and partly LC50 doses of toxicants (Fig. 6B) was significantly restored by calpeptin (p ≤ 0.05).
Fig. 6.
Effects of calpeptin pre-treatment on expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins. Cells were exposed with two concentrations of toxicants for 24 h with or without calpeptin (1 μM) pre-treatment. (A) Representative immunoblots indicated increase in expression of 21 and 24 kDa Bax after exposure to MPP+ (left panel) or rotenone (right panel); effects of calpeptin pre-treatment are shown by increased 26 kDa Bcl-2. (B) Bar graphs represent Bax:Bcl-2 ratio, determined according to intensity of immunoreactive bands shown in panel (A). Data were expressed as mean ± SEM of IR intensity, obtained from three independent assays (n≥3); *p ≤ 0.05, significantly different from control; @p ≤ 0.05, significantly different from toxicants. Level of 42 kDa β-actin served as protein loading control.
Calpeptin inhibited formation of SBDPs
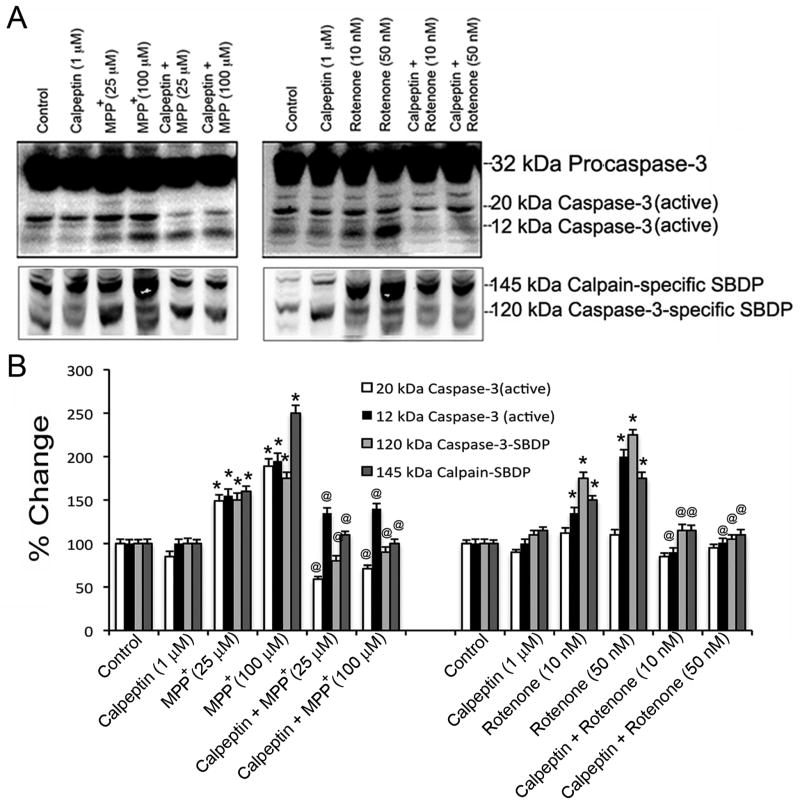
As calpeptin inhibits the proteolytic enzymatic activity, the formation of calpain-specific 145 kDa SBDP and caspase-3-specific 120 kDa SBDP were monitored to confirm cytoprotective efficacy of calpeptin (Fig. 7). Pre-treatment with 1 μM calpeptin was followed by exposure to MPP+ (25 and 100 μM) and rotenone (10 and 50 nM) for 24 h as previously described. Immunoreactive bands of pro-caspase-3, active caspase-3 fragments, and SBDPs were monitored by Western blot analysis. It was also recorded that calpeptin alone did not affect immunoreactive profiles of calpain, capase-3, and SBDPs.
Fig. 7.
Effects of calpeptin pre-treatment on activation of caspase-3 and calpain. Cells were pre-treated with calpeptin (1 μM) and exposed to toxicants for 24 h. The activation of proteolytic enzymes and protective effects of calpeptin were evaluated by appearance of calpain-specific and caspase-3-specific SBDPs. (A) Representative immunoblots showed expression of 32 kDa pro-caspase-3, 20 kDa and 12 kDa active caspase-3, after exposure of cells to MPP+ and rotenone (upper panel). Calpeptin pre-treatment caused decrease in immunoreactive bands for 20 and 12 kDa active caspase-3. Representative immunoblots of 145 kDa calpain-specific SBDP and 120 kDa caspase-3-specific SBDP indicated appearance of these proteins after exposure to MPP+ or rotenone, and decreased immunoreactive bands with calpeptin pre-treatment (lower panel). (B) Bar graphs designate the % change in intensities of immunoreactive bands; data expressed as mean ± SEM, obtained from three independent experiments (n ≥ 3); *p < 0.05, significantly different from respective controls; @p ≤ 0.05, significantly different from toxicants.
Significant activation of pro-caspase-3 in response to toxicant exposure, substantiated by appearance of 12 and 20 kDa fragments of active caspase-3, was accompanied by intense formation of 145 kDa calpain-specific SBDP and 120 kDa caspase-3-specific SBDP compared to control cells (Fig. 7A and B). Calpeptin reduced active caspase-3 levels compared to controls (p ≤ 0.05), and significantly reduced formation of SBDPs (p ≤ 0.05) as indicated in representative microphotographs and by corresponding quantification of changes (Fig. 7A and 7B). These results further indicate the crucial role of calpain and caspase-3 activation in MPP+- and rotenone-mediated apoptosis in motoneurons, wherein calpeptin rendered significant protection.
Discussion
PD is a movement disorder, characterized by disturbances in motor functions, which are predominantly ascribed to reduction in dopamine levels in midbrain substantia nigra and consequent limitation of dopaminergic neurotransmission in striatum. Nevertheless, several parkinsonian symptoms such as gait failure, somatic pain, orthostatic hypotension, and autonomic disturbances are attributed to disorganization and dysfunction of spinal systems (Dickson et al., 2009; Lim et al., 2009). Hence, spinal cord may also be involved in progression of parkinsonian disability. Neuromorphological alterations were reported in the spinal cord by Lewy body-mapping in elderly subjects with incidental Lewy body disease often regarded as symptomatic PD (Bloch et al., 2006; Klos et al., 2006), and in the spinal cord of human PD patients (Wakabayashi and Takahashi, 1997; Braak et al., 2007). Major findings of these studies indicated correlation between spinal cord and autonomic dysfunction. Furthermore, it was suggested that spinal cord might be one of the earliest and consistently affected sites in PD. The issue of spinal cord degeneration and dysfunction in PD, which has been demonstrated in animal models (Martin et al., 2006), currently receives much attention. Recently, our laboratory demonstrated degeneration of spinal cord neurons in two distinct models of experimental parkinsonism induced by parkinsonian toxicants MPTP and rotenone in C57BL/6N mice and Lewis rats, respectively (Ray et al., 2000; Chera et al., 2002, 2004; Samantaray et al., 2006, 2007, 2008a, 2008b). As molecular mechanisms of degeneration of dopaminergic neurons in PD have been extensively explored in vitro using MPP+ (the active metabolite of MPTP) and rotenone (an environmental toxin), these toxicants were employed to test the vulnerability of spinal motoneurons as cell culture model of parkinsonism. Both toxicants are known to affect mitochondrial function (Heikkila et al., 1985). Once within the cells, toxicants accumulate in mitochondria through inner membrane electrical gradient (Singer et al., 1988) and bind at the junction of NADH dehydrogenase and coenzyme Q, inhibiting NADH:quinone reductase (Complex I) activity, thus, blocking the mitochondrial respiration (Krueger et al., 1990; Kovacic et al., 1991). Subsequently, these toxicants instigate inhibition of oxidative phosphorylation, ATP depletion, loss of mitochondrial membrane potential, accumulation of intracellular free Ca2+, and release of mitochondrial cytochrome c. These events, associated with idiopathic parkinsonism, are well-documented in the midbrain nigrostriatal degeneration using experimental models of PD (Crocker et al., 2003; Samantaray et al., 2008c; Banerjee et al., 2009). Spinal cord is connected with upper movement control center in the brain by ascending and descending pathways, therefore spinal motoneurons, situated in ventral horn, play significant role in controlling muscle function. Any detrimental changes to this highly organized system might affect or disturb the transmission of motor impulses to muscles, ultimately affecting movement functions. The present study addressed the vulnerability of spinal motoneurons in culture upon exposure to two parkinsonian toxicants MPP+ and rotenone, and examined protective strategies. It affirmed apoptotic mechanisms of cell death in spinal motoneuronal cells, when exposed to low apoptotic and LC50 doses of toxicants. Inhibition of mitochondrial complex I by MPP+ and rotenone followed by mitochondrial dysfunction induced cascade of signaling pathways that provoked increase in intracellular free Ca2+ concentration. This led to up-regulation of expression and activity of calpain and caspase-3 proteases in dose- and time-dependent manners. Involvement of intrinsic mitochondrial pathway of apoptosis was inferred by elevation of Bax:Bcl2 ratio in motoneurons following toxicant exposure. Calpeptin rendered significant cytoprotection, which further confirmed the involvement of calpain in MPP+- and rotenone-mediated apoptosis in spinal motoneurons. This, in turn, corroborates calpain inhibition as a promising therapeutic target in the complex disorder PD, where downstream spinal cord damage may also be involved.
The hybrid cell line VSC 4.1 has been successfully used as an in vitro model to study mechanisms of degeneration of spinal motoneurons relevant to motoneuron diseases such as amyotropic lateral sclerosis (ALS) (Alexianu et al., 1994b; Smith et al., 1994). Several studies have emphasized the benefits of differentiating these hybrid cells into motor phenotype prior to examining the role of different factors that may be associated with motoneuron damage, such as effect of ALS patient IgG on Ca2+ currents (Mosier et al., 1995), rise in intracellular free Ca2+ concentration (Appel et al., 1995; Colom et al., 1997), role of Ca2+ binding proteins causing damage to motoneurons in ALS (Alexianu et al., 1994a, 1998; Ho et al., 1996). This cell line has also been used to study cell death mechanisms involving glutamate toxicity (Das et al., 2005; Sribnick et al., 2009), inflammatory stress (Smith et al., 2009; Das et al., 2011), and oxidative stress (Das et al., 2010). Importantly, the current study showed vulnerability of differentiated VSC 4.1 cells within apoptotic concentrations of toxicants, whereas undifferentiated cells were resistant at low doses and vulnerable at much higher concentrations of the same toxicants.
Aberrant Ca2+ homeostasis, one of the factors involved in pathophysiology of PD has been extensively investigated in midbrain nigrostriatal degeneration (Samantaray et al., 2008c); Ca2+-dependent cell death mechanism has been previously demonstrated in VSC 4.1 motoneuronal cells (Smith et al., 1994). The present study confirms elevation of intracellular free Ca2+ induced by parkinsonian toxicants, suggesting common degenerative pathways in spinal cord motoneurons and brain dopaminergic cells as putative parkinsonian mechanisms.
Although, the neurotoxic concentrations for MPP+ and rotenone varied on a log-scale (as μM and nM concentrations used, respectively), both toxicants induced identical apoptotic signaling pathway in motoneurons. The difference in concentration may be due to facilitated access of the lipophilic rotenone coupled to the fact that rotenone binds to the mitochondrial complex I with higher affinity, than MPP+ (Krueger et al., 1990). While entry of MPP+ in dopaminergic cells occurs via dopamine transporters, such transporters are not expressed in the cholinergic spinal motoneuronal cells. Hence, entry of MPP+ in motoneurons is most likely through alternate pathway such as cationic amino acid transporters as reported in cerebellar granule cells (Gonzalez-Polo et al., 2001). The effective doses of MPP+ found in the current study further support such an assumption. Both, MPP+ and rotenone inhibit mitochondrial complex I activity, consequently inducing intrinsic or direct pathway of apoptosis, where mitochondria play key role in cell death mechanism. This cell death pathway is initiated by the Bcl-2 family of mitochondrial membrane proteins, through increase of pro-apoptotic Bax and Bak, and decrease of anti-apoptotic Bcl-2 and Bcl-xL levels (Nicotra and Parvez, 2002). Imbalances between the two differentially acting membrane proteins are known to bring about chain of cell degenerative reactions. These proteins located between inner and outer mitochondrial membranes, undergo conformational changes to form specific channels, through which cytochrome c, apoptosis-inducing factor, and flavoprotein are released into the cytosol (Desagher et al., 1999). We found that exposure of motoneurons to MPP+ or rotenone increased Bax:Bcl-2 ratio, which strongly supported our hypothesis that parkinsonian toxicants could induce death in motoneurons through intrinsic mitochondrial pathway, particularly affecting levels of Bax and Bcl-2 proteins.
The primary mode of cell death induced by MPP+ has been variously reported based on the toxicant concentrations used, duration of exposure, and most important, the cell type. Relatively low doses of MPP+ (1-150 μM) were capable of inducing damage to cells in culture, including rat cerebellar granule neurons (Du et al., 1997; Gonzalez-Polo et al., 2001; Harbison et al., 2011), MN9D (Choi et al., 1999; Chu et al., 2005; Luo et al., 2009), and rat ventral mesencephalic neurons (Dodel et al., 1998; Hartmann et al., 2000; Han et al., 2003, Jourdi et al., 2009), while almost 1000-fold higher doses of MPP+ (mM range) were found deleterious for SH-SY5Y, PC12, N-27, and NT2 cells (Anantharam et al., 2007; Domingues et al., 2008; Wales et al., 2008; Cheng et al., 2009; Kim et al., 2009). These findings suggested diverse intracellular signaling pathways in MPP+-neurotoxicity. While earlier studies aimed at unraveling the mechanisms of midbrain nigrostriatal dopaminergic degeneration, here, the approach was to address impact of mitochondrial stress on spinal motoneurons, which might be helpful in addressing the degeneration outside brain in PD. In spinal motoneurons both calpain and caspase-3 were activated by low doses of MPP+, and calpain inhibition by calpeptin could significantly block neurotoxic effects at apoptotic and partly at LC50.
Motoneurons have been previously studied in vitro for effects of mitochondrial toxicant rotenone. Rotenone had no effect on ATP synthesis at concentrations less than 3 nM on primary spinal cord motor neuron cultures received from transgenic SOD1-G93A ALS mice. However, at 10 nM dose ATP production was reduced to 60%. Interestingly, neuroprotection was conferred by D-β-3 hydroxybutyrate against chronic rotenone-mediated mitochondrial toxicity (Complex I), recovering ATP production up to 90%, but not against malonate (Complex II) (Zhao et al., 2006). Rat spinal cord cultures exposed to rotenone for 24 h showed relatively selective motor neuron death at 20–30 nM; but rotenone caused neuronal death in both motor and non-motoneurons at higher concentrations (Kanki et al., 2004). Our current report on the apoptotic mechanisms at the lower rotenone dose of 10 nM infers a possibility of selective motoneuronal damage involving the cysteine proteases calpain and caspase-3. Thus, this study showed vulnerability of spinal motoneurons to mitochondrial toxins, which are accepted parameters of all parkinsonian toxins.
Calpain and caspases-3 were shown to play essential role in apoptosis through intrinsic pathway in different cell types (Donepudi and Grutter, 2002; Movsesyan et al., 2004). Caspase-3 has been characterized as an effector in the decision process. However, whether calpain or caspase-3 plays the primary role in cell death pathways remains debatable. Apoptosis in ciliary ganglion neurons requires the action of both calpain and caspase families (Villa et al., 1998). Caspase-3 either can be repressed (McGinnis et al., 1999) or directly activated (Blomgren et al., 2001) by calpains, probably depending on timing of calpain activation itself (Neumar et al., 2003). However, calpain has been suggested to be upstream of caspase-3 activity in cell death mechanism (Ray and Banik, 2003). This is further supported by the fact that calpain is required for caspase-3 activation (Blomgren et al., 2001). In motoneurons, calpain and caspase-3 were diversely up-regulated with MPP+ and rotenone, depending on toxicant dosage and time of exposure. Formation of 20 kDa and 12 kDa active caspase-3 fragments with both MPP+ and rotenone started early at 12 h of exposure and reached maximum at 36 h. This dynamics well correlated with formation of 76 kDa active calpain at 24 and 36 h. The 12 kDa, but not 20 kDa caspase-3 was gradually generated in response to both toxicants. In contrast, the 20 kDa caspase-3 was induced only with MPP+ at later time points, but not with rotenone. Calpain activation occurred similarly in response to both toxicants. These observations suggested cross-communication between active caspase-3 and up-regulated calpain during the course of toxicant exposure. Overall, MPP+ and rotenone demonstrated potent activation of calpain- and caspase-dependent pathways of cell degradation and death. Moreover; calpain could modulate the downstream caspase-3 indicating that calpain worked upstream of caspase-3, as previously proposed (Ray and Banik, 2003).
Furthermore, cleavage of cytoskeletal protein alpha-spectrin and formation of SBDPs by calpain and caspase-3 additionally demonstrates the involvement of these proteases in degradation of motoneurons. Cleavage of cytoskeletal structural proteins destroys cellular functioning and survival, disabling cellular repair, inactivating inhibitors of DNA fragmentation.
Calpeptin, the calpain inhibitor, was capable of attenuating destructive effects of both these proteases in motoneurons induced by MPP+ and rotenone. Activated apoptotic events were silenced by calpetin; decreased calpain and caspase-3 expression, reduced Bax:Bcl-2 ratio, SBDP formation, TUNEL positive cells and DNA fragmentation, along with morphological observations distinctly indicated neuroprotective efficacy of calpeptin in motoneurons. Thus, the damaging effects of MPP+ and rotenone in spinal motoneurons, as shown in the present study, are in line with studies in mesencephalic dopaminergic neurons, tyrosine hydroxylase-immunopositive cells in substantia nigra and striatum, and MN9D dopaminergic cell line, correlating with PD related questions. Motoneurons exposed to MPP+ and rotenone committed apopotic cell death. Although, DNA fragmentation might also occur randomly in necrotic cells, morphological examination and DNA laddering in our study suggested apoptotic death of motoneurons. Also, the current study separately addressed the LC50 and necrosis in motoneurons by appropriately dosing MPP+ and rotenone.
In summary, parkinsonian toxicants induced apoptotic death in motoneurons, and increased proteolytic activities of calpain and caspase-3 were implicated as possible mechanisms involved in neurons outside brain. Calpeptin, the calpain inhibitor, was found to be neuroprotective and hence, could be a promising intervening agent against neurodegeneration in parkinsonism.
Highlights.
Mitochondrial toxin-induced damage was investigated in VSC 4.1 spinal motoneurons.
MPP+ and rotenone induced apoptosis in spinal motoneurons.
Apoptosis occurred via free Ca2+ elevation, calpain and caspase-3 up-regulation.
Calpeptin, a calpain inhibitor, protected motoneurons against MPP+ and rotenone.
Calpain inhibition is suggested as potentially neuroprotective.
Acknowledgments
This work was supported in part by the R01 grants (NS-57811, NS-62327, and NS-65456) from the NINDS-NIH (Bethesda, MD, USA).
Abbreviations
- ALS
amyotropic lateral sclerosis
- AFU
arbitrary fluorescence unit
- IR
immunoreactivity
- MPP+
1-methyl-4-phenylpyridinium ion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MTT
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PD
Parkison's disease
- PBS
phosphate-buffered saline
- %
percentage
- SBDP
spectrin breakdown product
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- VSC 4.1
ventral spinal cord cells clone 4.1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker H, Carlsson J, Holtermann G, Nederman T, Nylen T. Influence of glucose and buffer capacity in the culture medium on growth and pH in spheroids of human thyroid carcinoma and human glioma origin. Cancer Res. 1987;47:3504–3508. [PubMed] [Google Scholar]
- Alexianu ME, Ho BK, Mohamed AH, La Bella V, Smith RG, Appel SH. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994a;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- Alexianu ME, Mohamed AH, Smith RG, Colom LV, Appel SH. Apoptotic cell death of a hybrid motoneuron cell line induced by immunoglobulins from patients with amyotrophic lateral sclerosis. J Neurochem. 1994b;63:2365–2368. doi: 10.1046/j.1471-4159.1994.63062365.x. [DOI] [PubMed] [Google Scholar]
- Alexianu ME, Robbins E, Carswell S, Appel SH. 1Alpha, 25 dihydroxyvitamin D3-dependent up-regulation of calcium-binding proteins in motoneuron cells. J Neurosci Res. 1998;51:58–66. doi: 10.1002/(SICI)1097-4547(19980101)51:1<58::AID-JNR6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–997. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SH, Smith RG, Alexianu M, Siklos L, Engelhardt J, Colom LV, Stefani E. Increased intracellular calcium triggered by immune mechanisms in amyotrophic lateral sclerosis. Clin Neurosci. 1995;3:368–374. [PubMed] [Google Scholar]
- Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson's disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik NL, Hogan EL, Jenkins MG, McDonald JK, McAlhaney WW, Sostek MB. Purification of a calcium-activated neutral proteinase from bovine brain. Neurochem Res. 1983;8:1389–1405. doi: 10.1007/BF00964996. [DOI] [PubMed] [Google Scholar]
- Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- Butler JT, Samantaray S, Beeson CC, Ray SK, Banik NL. Involvement of calpain in the process of Jurkat T cell chemotaxis. J Neurosci Res. 2009;87:626–635. doi: 10.1002/jnr.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YF, Zhu GQ, Wang M, Cheng H, Zhou A, Wang N, Fang N, Wang XC, Xiao XQ, Chen ZW, Li QL. Involvement of ubiquitin proteasome system in protective mechanisms of Puerarin to MPP(+)-elicited apoptosis. Neurosci Res. 2009;63:52–58. doi: 10.1016/j.neures.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Chera B, Schaecher KE, Rocchini A, Imam SZ, Ray SK, Ali SF, Banik NL. Calpain upregulation and neuron death in spinal cord of MPTP-induced parkinsonism in mice. Ann N Y Acad Sci. 2002;965:274–280. doi: 10.1111/j.1749-6632.2002.tb04169.x. [DOI] [PubMed] [Google Scholar]
- Chera B, Schaecher KE, Rocchini A, Imam SZ, Sribnick EA, Ray SK, Ali SF, Banik NL. Immunofluorescent labeling of increased calpain expression and neuronal death in the spinal cord of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. Brain Res. 2004;1006:150–156. doi: 10.1016/j.brainres.2004.01.065. [DOI] [PubMed] [Google Scholar]
- Choi WS, Canzoniero LM, Sensi SL, O'Malley KL, Gwag BJ, Sohn S, Kim JE, Oh TH, Lee EB, Oh YJ. Characterization of MPP(+)-induced cell death in a dopaminergic neuronal cell line: role of macromolecule synthesis, cytosolic calcium, caspase, and Bcl-2-related proteins. Exp Neurol. 1999;159:274–282. doi: 10.1006/exnr.1999.7133. [DOI] [PubMed] [Google Scholar]
- Chu CT, Zhu JH, Cao G, Signore A, Wang S, Chen J. Apoptosis inducing factor mediates caspase-independent 1-methyl-4-phenylpyridinium toxicity in dopaminergic cells. J Neurochem. 2005;94:1685–1695. doi: 10.1111/j.1471-4159.2005.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom LV, Alexianu ME, Mosier DR, Smith RG, Appel SH. Amyotrophic lateral sclerosis immunoglobulins increase intracellular calcium in a motoneuron cell line. Exp Neurol. 1997;146:354–360. doi: 10.1006/exnr.1997.6541. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Garner DP, Del Re AM, Woodward JJ, Kumar DM, Agarwal N, Banik NL, Ray SK. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006;1084:146–157. doi: 10.1016/j.brainres.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Das A, McDowell M, Pava MJ, Smith JA, Reiter RJ, Woodward JJ, Varma AK, Ray SK, Banik NL. The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-alpha toxicity involves membrane melatonin receptors. J Pineal Res. 2010;48:157–169. doi: 10.1111/j.1600-079X.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Smith JA, Gibson C, Varma AK, Ray SK, Banik NL. Estrogen receptor agonists and estrogen attenuate TNF-alpha-induced apoptosis in VSC4.1 motoneurons. J Endocrinol. 2011;208:171–182. doi: 10.1677/JOE-10-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Dodel RC, Du Y, Bales KR, Ling ZD, Carvey PM, Paul SM. Peptide inhibitors of caspase-3-like proteases attenuate 1-methyl-4-phenylpyridinum-induced toxicity of cultured fetal rat mesencephalic dopamine neurons. Neuroscience. 1998;86:701–707. doi: 10.1016/s0306-4522(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Domingues AF, Esteves AR, Swerdlow RH, Oliveira CR, Cardoso SM. Calpain-mediated MPP+ toxicity in mitochondrial DNA depleted cells. Neurotox Res. 2008;13:31–38. doi: 10.1007/BF03033365. [DOI] [PubMed] [Google Scholar]
- Donepudi M, Grutter MG. Structure and zymogen activation of caspases. Biophys Chem. 2002;101-102:145–153. doi: 10.1016/s0301-4622(02)00151-5. [DOI] [PubMed] [Google Scholar]
- Du Y, Dodel RC, Bales KR, Jemmerson R, Hamilton-Byrd E, Paul SM. Involvement of a caspase-3-like cysteine protease in 1-methyl-4-phenylpyridinium-mediated apoptosis of cultured cerebellar granule neurons. J Neurochem. 1997;69:1382–1388. doi: 10.1046/j.1471-4159.1997.69041382.x. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Szabadkai G. Roles of mitochondria in human disease. Essays Biochem. 2010;47:115–137. doi: 10.1042/bse0470115. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Mora A, Clemente N, Sabio G, Centeno F, Soler G, Fuentes JM. Mechanisms of MPP(+) incorporation into cerebellar granule cells. Brain Res Bull. 2001;56:119–123. doi: 10.1016/s0361-9230(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guyton MK, Das A, Samantaray S, Wallace GCt, Butler JT, Ray SK, Banik NL. Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. Neurosci Res. 2010;88:2398–2408. doi: 10.1002/jnr.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, Oh YJ. Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci. 2003;23:5069–5078. doi: 10.1523/JNEUROSCI.23-12-05069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison RA, Ryan KR, Wilkins HM, Schroeder EK, Loucks FA, Bouchard RJ, Linseman DA. Calpain Plays a Central Role in 1-Methyl-4-phenylpyridinium (MPP(+))-Induced Neurotoxicity in Cerebellar Granule Neurons. Neurotox Res. 2011 doi: 10.1007/s12640-010-9172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Nicklas WJ, Vyas I, Duvoisin RC. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci Lett. 1985;62:389–394. doi: 10.1016/0304-3940(85)90580-4. [DOI] [PubMed] [Google Scholar]
- Ho BK, Alexianu ME, Colom LV, Mohamed AH, Serrano F, Appel SH. Expression of calbindin-D28K in motoneuron hybrid cells after retroviral infection with calbindin-D28K cDNA prevents amyotrophic lateral sclerosis IgG-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1996;93:6796–6801. doi: 10.1073/pnas.93.13.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Basic research on dopamine in Parkinson's disease and the discovery of the nigrostriatal dopamine pathway: the view of an eyewitness. Neurodegener Dis. 2008;5:114–117. doi: 10.1159/000113678. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Hamo L, Oka T, Seegan A, Baudry M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology. 2009;56:876–885. doi: 10.1016/j.neuropharm.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki R, Nakamizo T, Yamashita H, Kihara T, Sawada H, Uemura K, Kawamata J, Shibasaki H, Akaike A, Shimohama S. Effects of mitochondrial dysfunction on glutamate receptor-mediated neurotoxicity in cultured rat spinal motor neurons. Brain Res. 2004;1015:73–81. doi: 10.1016/j.brainres.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Kim NK, Choi BH, Huang X, Snyder BJ, Bukhari S, Kong TH, Park H, Park HC, Park SR, Ha Y. Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced murine Parkinson's disease model. Eur J Neurosci. 2009;29:891–900. doi: 10.1111/j.1460-9568.2009.06653.x. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- Kovacic P, Edwards WD, Ming G. Theoretical studies on mechanism of MPTP action: ET interference by MPP+ (1-methyl-4-phenylpyridinium) with mitochondrial respiration vs. oxidative stress. Free Radic Res Commun. 1991;14:25–32. doi: 10.3109/10715769109088938. [DOI] [PubMed] [Google Scholar]
- Krueger MJ, Singer TP, Casida JE, Ramsay RR. Evidence that the blockade of mitochondrial respiration by the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) involves binding at the same site as the respiratory inhibitor, rotenone. Biochem Biophys Res Commun. 1990;169:123–128. doi: 10.1016/0006-291x(90)91442-u. [DOI] [PubMed] [Google Scholar]
- Lim SY, Fox SH, Lang AE. Overview of the extranigral aspects of Parkinson disease. Arch Neurol. 2009;66:167–172. doi: 10.1001/archneurol.2008.561. [DOI] [PubMed] [Google Scholar]
- Luo D, Zhang Q, Wang H, Cui Y, Sun Z, Yang J, Zheng Y, Jia J, Yu F, Wang X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur J Pharmacol. 2009;617:33–40. doi: 10.1016/j.ejphar.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Yoshino H, Ikebe S, Hattori N, Kobayashi T, Shimoda-Matsubayashi S, Matsumine H, Kondo T. Mitochondrial dysfunction in Parkinson's disease. Ann Neurol. 1998;44:S99–109. doi: 10.1002/ana.410440715. [DOI] [PubMed] [Google Scholar]
- Mosier DR, Baldelli P, Delbono O, Smith RG, Alexianu ME, Appel SH, Stefani E. Amyotrophic lateral sclerosis immunoglobulins increase Ca2+ currents in a motoneuron cell line. Ann Neurol. 1995;37:102–109. doi: 10.1002/ana.410370119. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Movsesyan VA, Stoica BA, Yakovlev AG, Knoblach SM, Lea PMt, Cernak I, Vink R, Faden AI. Anandamide-induced cell death in primary neuronal cultures: role of calpain and caspase pathways. Cell Death Differ. 2004;11:1121–1132. doi: 10.1038/sj.cdd.4401442. [DOI] [PubMed] [Google Scholar]
- Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem. 2003;278:14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]
- Nicotra A, Parvez S. Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol. 2002;24:599–605. doi: 10.1016/s0892-0362(02)00213-1. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Das A, Ray SK, Banik NL. Calpain inhibition attenuates intracellular changes in muscle cells in response to extracellular inflammatory stimulation. Exp Neurol. 2010;225:430–435. doi: 10.1016/j.expneurol.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Ray SK, Wilford GG, Ali SF, Banik NL. Calpain upregulation in spinal cords of mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson's disease. Ann N Y Acad Sci. 2000;914:275–283. doi: 10.1111/j.1749-6632.2000.tb05202.x. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Butler JT, Ray SK, Banik NL. Extranigral neurodegeneration in Parkinson's disease. Ann N Y Acad Sci. 2008a;1139:331–336. doi: 10.1196/annals.1432.002. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Knaryan VH, Butler JT, Ray SK, Banik NL. Spinal cord degeneration in C57BL/6N mice following induction of experimental parkinsonism with MPTP. J Neurochem. 2008b;104:1309–1320. doi: 10.1111/j.1471-4159.2007.05091.x. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Knaryan VH, Guyton MK, Matzelle DD, Ray SK, Banik NL. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats. Neuroscience. 2007;146:741–755. doi: 10.1016/j.neuroscience.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Ray SK, Ali SF, Banik NL. Calpain activation in apoptosis of motoneurons in cell culture models of experimental parkinsonism. Ann N Y Acad Sci. 2006;1074:349–356. doi: 10.1196/annals.1369.034. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Ray SK, Banik NL. Calpain as a potential therapeutic target in Parkinson's disease. CNS Neurol Disord Drug Targets. 2008c;7:305–312. doi: 10.2174/187152708784936680. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Singer TP, Ramsay RR, McKeown K, Trevor A, Castagnoli NE., Jr Mechanism of the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), the toxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicology. 1988;49:17–23. doi: 10.1016/0300-483x(88)90169-2. [DOI] [PubMed] [Google Scholar]
- Smith JA, Zhang R, Varma AK, Das A, Ray SK, Banik NL. Estrogen partially down-regulates PTEN to prevent apoptosis in VSC4.1 motoneurons following exposure to IFN-gamma. Brain Res. 2009;1301:163–170. doi: 10.1016/j.brainres.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci U S A. 1994;91:3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur P, Sribnick EA, Wingrave JM, Nowak MW, Ray SK, Banik NL. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003;971:178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- Tamada Y, Nakajima E, Nakajima T, Shearer TR, Azuma M. Proteolysis of neuronal cytoskeletal proteins by calpain contributes to rat retinal cell death induced by hypoxia. Brain Res. 2005;1050:148–155. doi: 10.1016/j.brainres.2005.05.048. [DOI] [PubMed] [Google Scholar]
- Villa PG, Henzel WJ, Sensenbrenner M, Henderson CE, Pettmann B. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998;111(Pt 6):713–722. doi: 10.1242/jcs.111.6.713. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. The intermediolateral nucleus and Clarke's column in Parkinson's disease. Acta Neuropathol. 1997;94:287–289. doi: 10.1007/s004010050705. [DOI] [PubMed] [Google Scholar]
- Wales SQ, Laing JM, Chen L, Aurelian L. ICP10PK inhibits calpain-dependent release of apoptosis-inducing factor and programmed cell death in response to the toxin MPP+ Gene Ther. 2008;15:1397–1409. doi: 10.1038/gt.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]