Abstract
Genetic factors, externalizing personality traits such as impulsivity, and brain processing of salient stimuli all can affect individual risk for alcoholism. One of very few confirmed genetic association findings differentiating alcoholics from non-alcoholics is with variants in the inhibitory gamma-amino butyric acid α2 receptor subunit (GABRA2) gene. Here we report the association of two of these GABRA2 variants with measures of alcohol symptoms, impulsivity and with insula cortex activation during anticipation of reward or loss using functional magnetic resonance imaging (fMRI).
In a sample of 173 families (449 subjects), 129 of whom had at least one member diagnosed with alcohol dependence or abuse, carriers for the G allele in two SNPs and haplotypes were more likely to have alcohol dependence symptoms (rs279858 p = 0.01; rs279826 p = 0.05; haplotype p = 0.02) and higher NEO-PI-R Impulsiveness scores (rs279858 p = 0.016; rs279826 p = 0.012; haplotype p = 0.032) with a stronger effect in females (rs279858 p = 0.011; rs279826 p = 0.002; haplotype p = 0.006), all p values are corrected for family history and age. A subset of offspring from these families (n = 44, 20 females), genotyped for GABRA2, participated in an fMRI study using a monetary incentive delay task. Increased insula activation during reward (r2 = 0.4; p = 0.026) and loss (r2 = 0.38; p = 0.039) anticipation was correlated with NEO-PI-R Impulsiveness and further associated with the GG genotype for both SNPs (ps’ < 0.04). Our results suggest that GABRA2 genetic variation is associated with Impulsiveness through variation of insula activity responses, here evidenced during anticipatory responses.
Keywords: Impulsiveness, GABRA2, SNP, alcohol dependence, Insula, fMRI
Introduction
Gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter in the CNS, has a role in risk for developing alcohol use disorder (AUD) through the fast-acting receptor complex, GABAA 1, 2. Repeated alcohol exposure affects the GABA system 2 through binding sites at the GABA receptors reducing neural inhibitory action. Genetic variation in GABRA2, the gene encoding the GABAAα2 receptor subunit, has been reproducibly associated with both alcoholism 3–12 and an electroencephalography (EEG) measure, the β frequency band 6. Alcoholics 13 and their at risk offspring 14 have increased power in the β frequency band (13–28 Hz). Increased EEG-β activity is also a good predictor of relapse 15. In cortical networks, GABAergic transmission is critical for the maintenance of the excitation-inhibition homeostatic balance 16.
The insula cortex is an important area for integrating emotional and homeostatic information to and from limbic and cortical areas, and has been implicated in negative emotional states such as craving and anxiety. Evidence for a role of the insula in conscious urges to take drugs comes from studies involving damage to the insula in both human 17–19 and animals 20. Furthermore, insula activation has been associated with cue-induced drug craving 17, 21, 22, maintaining urge to the use of drugs 23 in addicts, and anticipation of aversive exposure in individuals with anxiety disorder 24. Studies in nonclinical samples have revealed an association between insula function and self-report measures of anxiety 25. These emotional stages of urge, anxiety, and anticipation of negative events may be related to Urgency, an impulsiveness trait involving rash actions under intense negative affect 26.
Impulsivity is a multidimensional behavioral construct which includes at least four different component traits: urgency, sensation seeking, lack of premeditation and lack of perseverance 26. Urgency (called Impulsiveness in the NEO-PI27) is related to the development of alcohol problems that are motivated by a coping strategy involving use of alcohol to deal with emotional distress, often with disregard for negative consequences 28.
Since variation in GABRA2 has been associated with childhood conduct disorder symptoms, EEG-β and alcoholism, we hypothesized that it may also influence behavioral traits such as impulsivity, an externalizing risk behavior for AUD. Here we report testing for a genetic association between two GABRA2 SNPs and the NEO-PI-R Impulsiveness facet. The test was carried out in a community-recruited sample of families which had at least one member with DSM-IV AUD diagnosis or were from an age and socioeconomically comparable group of control families 29. A subset of this sample comprising adolescent and young adult offspring underwent an fMRI study using a modification of the Monetary Incentive Delay Task 30. Given the role of the anterior insula cortex in the representation of interoceptive responses to emotionally salient and rewarding stimuli, the blood-oxygenation-level-dependent (BOLD) activation of this region was obtained during anticipation of a monetary reward or loss. We anticipated that genetic variation in GABRA2 would be associated with Impulsiveness, as well as with insula cortex activation, which in turn would be positively correlated with Impulsiveness.
Material and Methods
Subjects and assessment
The population sample consisted of 449 subjects from the Michigan Longitudinal Study (MLS) who were genotyped for two GABRA2 SNPs and for whom NEO-PI-R data were available. This is an ongoing multi-wave, community recruited prospective study of families of men with a drunk-driving conviction and AUD diagnosis who were living with a 3–5 year old son/daughter and the biological mother at time of recruitment (Mean age 32; range 22–46 at baseline). The study began recruitment in 1985. In addition, control families without a history of substance abuse were recruited from the same or socioeconomically comparable neighborhoods. Families identified during the community canvass for controls who also had an AUD diagnosis were recruited as well 31. Both the parental (246) and the offspring (203) generation are being followed (see Table 1 for detailed demographic information). 323 subjects were from 129 families with risk for alcoholism and 126 from 44 control families. The great majority were of Caucasian origin, with only 1.8% (8) of other ethnicity. All subjects were extensively assessed at 3-year intervals with behavioral and alcohol measures appropriate for age. 130 (51 female; 14 adult offspring) subjects had a lifetime alcohol dependence/abuse diagnosis.
Table 1.
Summary of the sample demographics and traits by AUD family history and diagnosis
| No AUD family history (N = 126) | AUD Family history (N = 323) | DSM-IV Lifetime AUD diagnosis (N = 130) | |
|---|---|---|---|
| Adult age at time of last interview (mean, s.d.) | N = 67 48 ± 4.1 |
N = 179 47 ± 5.7 |
N = 116 47.3 (6.17) |
| Adult offspring age at time of last interview (mean, s.d.) | N = 59 20 ± 1.0 |
N = 144 20 ± 0.98 |
N = 14 16.9 ± 1.02 |
| Females | 47 | 131 | 51 |
| Males | 79 | 192 | 79 |
| % AD symptoms (mean, s.d.) | 0.05 ± 0.12 | 0.21 ± 0.26 | 0.38 ± 0.29 |
| Impulsiveness (mean, s.d.) | 15.66 ± 4.08 | 16.04 ± 3.71 | 16.17 ± 3.92 |
AUD, alcohol use disorder
AD, alcohol dependence
For the present study we used the maximum value (best estimate of maximum level of alcoholic symptomatology capable of being achieved by the individual) from all waves of the percentage of alcohol dependence symptoms (tolerance, withdrawal, craving) and problems (legal and social complications) reported from a 31-item checklist, the MLS Drinking and Drug History 32 which contains a list of all symptoms listed in DSM-IV and others from earlier diagnostic systems 33 (See Supplementary materials). It incorporates non-overlapping items from the 1978 NIDA Survey 34, from the American Drinking Practices Survey35 and from the V.A. Medical Center (University of California, San Diego) Research Questionnaire for Alcoholics36. They provide data on quantity, frequency and variability of alcohol consumption, frequency of drug use, and multiple questions on symptoms, consequences and troubles related to the use of these substances and have been extensively used in a variety of national surveys and clinical settings 37. The modal value is zero. Alcohol dependence and abuse lifetime diagnosis was made by a trained clinician based on DSM–IV criteria at each wave using three instruments: Diagnostic Interview Schedule (DIS-III), the Short Michigan Alcohol Screening Test (SMAST) and the Drinking and Drug History. On the basis of information collected by all three instruments, a lifetime diagnosis was made (Kappa = 0.81).
Personality traits were assessed using the NEO-PI-R questionnaire 27 at all waves for parents and starting at wave 6 (older than age 18) for offspring. Analyses compared the percentage of alcohol dependence symptoms with the personality trait Impulsiveness, a Neuroticism facet of the NEO-PI-R 27 and two GABRA2 SNPs.
In summary, our sample consists of 449 subjects with both genotype and Impulsiveness data. Impulsiveness and percentage of alcohol dependence symptoms was available for 415 subjects. Genotype data and percentage of alcohol dependence symptoms was available for 448 subjects.
Participants in the fMRI study
Twenty nine young adult children of alcoholics (COA) (17 males and 12 females; mean age: 20.1 ± 1.2; range: 18–22) and fifteen controls (7 males and 8 females; mean age 20.0 ± 1.3; range: 18–22) who were genotyped for the GABRA2 SNPs also participated in the fMRI study. All were Caucasian, right-handed, and were a sub-set of unrelated MLS offspring. All had passed the exclusionary screen criterion of no fetal alcohol effects when recruited as children. Control subjects also were unrelated and had no parental history of AUD.
Exclusion criteria for the present study were: any neurological, acute, uncorrected or chronic medical illness; any Axis I psychiatric or developmental disorders; any current or recent (within six months) treatment with centrally active medications; a history of psychosis or schizophrenia in first-degree relatives; and a positive urine drug screen on the day of the study. Three of the participants had a diagnosis of alcohol abuse; this was not exclusionary given our interest in predicting range of variation in risk for alcohol use disorder. Written informed consent, approved by the University of Michigan Medical School Institutional Review Board, was obtained prior to the study.
SNP genotyping and analysis
We selected two SNPs, rs279826 (intron 4) and rs279858 (exon 5, K132K) that capture the information of a long haplotype block of 109 Kb where previous associations have been reported with alcohol dependence and related traits. The two SNPs (rs279826 and rs279858) were genotyped by TaqMan using inventoried assays of primers and probes (Applied Biosystems, ABI, Foster City, CA). The PCR reactions were run in 5ul volume using ABI standard protocol 2.5ul of 2x Master Mix, 0.25ul of 20X primers and probes and 10–20 ng of genomic DNA. The cycling conditions for PCR consisted of 95°C for 10 min followed by 40 cycles of 92°C for 15 sec and 60°C for 1 min. The reactions and fluorescence scan were run on an ABI PRIZM 7900HT Sequence Analyzer. Allelic determination was done using SDS 2.1 (ABI). Probe sequences and allele information are publicly available online www.appliedbiosystems.com. We included twelve duplicates and no discrepancies were observed.
Linkage disequilibrium (LD) between markers was analyzed with Haploview 38. Both SNPs were in Hardy-Weinberg equilibrium. To test for an association between the SNPs and Impulsiveness we applied a regression test implemented in MERLIN-1.1-alpha program that takes family relationships into account in the association test 39. First, since the scores on the Impulsiveness facet from the Neuroticism domain did not differ significantly across assessment waves, we composed a new dependent variable for the Impulsiveness facet by averaging the individual’s scores across the different data waves. Second, we analyzed the correlation between the Impulsiveness scores and percentage of alcohol dependence symptoms using Spearman’s correlation since the alcohol variable was not normally distributed. To test the association of GABRA2 SNPs and percentage of alcohol dependence symptoms, a Zero-Inflated Poisson Regression model was applied to account for the excessive number of zeros (240/448) using SAS. The Zero-inflated Poisson test includes all subjects. The zero-inflated Poisson regression generates two separate models and then combines them. First, a model is generated for the “certain zero” cases. Then, a Poisson model is generated to predict the counts for those subjects who are not certain zeros. Finally, the two models are combined. This test does not consider family relationship.
Neuroimaging measures
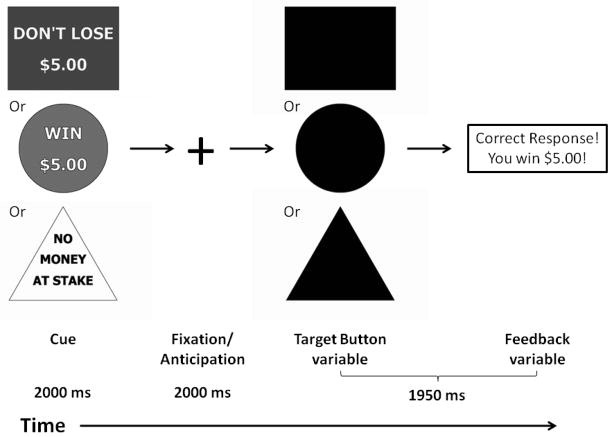
Brain response during anticipation of incentive stimuli was probed during fMRI using a modified version of the Monetary Incentive Delay task 30. Modifications were designed to eliminate the memory component from the original task, by replacing symbols for “reward” and “loss” trials with text stating trial type. A schematic of the modified paradigm is presented in Figure 1. Each 6 second trial consisted of four events. First, subjects were presented with an incentive cue (2000 ms) of five possible values (gain of $0.2, $5.0; loss of $0.2, $5.0; or no change $0). This was followed by a 2000 ms anticipation delay. Next, a target appeared for a variable length of time (200 to 300 ms) during which, subjects made a button press response in an attempt to gain or avoid losing said incentive. Subjects were instructed to respond to neutral targets despite the lack of incentive value. A feedback message then informed subjects of the trial outcome. The incentive trials were presented contiguously in a pseudorandom order. Subjects performed two runs of the task, each lasting 5 minutes. A total of twenty trials of each condition were recorded. The duration of the response target was calculated based on individual subject’s reaction time during a practice session prior to scanning. The allotted duration was calibrated such that the overall success rate was approximately 60%. Participants were paid a fixed rate to participate in the study and additionally received any money they won during the task. Reaction time and success rate for each incentive condition were calculated. Independent sample t-tests were used to assess possible performance differences between GABRA2 A-carriers and GG genotype groups for both SNPs and haplotypes.
Figure 1.
A schematic illustration of the monetary incentive delay task performed by subjects in the fMRI scanner.
Whole-brain blood oxygen level-dependent (BOLD) functional images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI) using a T2*-weighted single-shot combined spiral in/out sequence 40 with the following imaging parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90; field-of-view (FOV) = 200 mm; matrix size = 64 × 64; in plane resolution = 3.12 × 3.12 mm; and slice thickness = 4 mm. A high resolution anatomical T1 scan was obtained for spatial normalization (three-dimensional spoiled gradient recalled echo [3-DSPGR], TR=25 msec, min TE, FOV=25 cm, 256×256 matrix, slice thickness=1.4 mm). Participant motion was minimized with the use of foam pads placed around the head along with a forehead strap. In addition, the importance of keeping as still as possible was emphasized during the Informed Consent process and at scanner entry.
Functional images were reconstructed using an iterative algorithm 41, which is more robust against image distortions caused by off-resonance effects than conventional methods. Subject head motion and slice-acquisition timing were corrected using the FSL 4.0 analysis tools library (Analysis Group, FMRIB, Oxford, UK) 42. Analysis of estimated motion parameters confirmed that overall head motion within each run did not exceed 2 mm translation or 2 degree rotation in any direction. All remaining image processing was completed using statistical parametric mapping SPM2 package (Wellcome Institute of Cognitive Neurology, London, UK). Functional images were spatially normalized to a standard stereotactic space as defined by the Montreal Neurological Institute (MNI). A 6 mm full width half maximum (FWHM) Gaussian spatial smoothing kernel was applied to improve signal to noise ratio and to account for individual differences in anatomy.
Statistical analysis
Individual analysis was completed using a general linear model (GLM) in SPM2. Five regressors of interest (anticipation of win $0.2, win $5.0, lose $0.2, lose $5.0 and neutral $0) were convolved with the canonical hemodynamic response function (HRF). Motion parameters were modeled as nuisance regressors to remove residual motion artifacts. Scanner drift and other low frequency noise were removed from the image time series using a 128 second high-pass filter. Two contrasts of interest, anticipation of reward ($0.2 and $5.0 combined) minus neutral incentive and anticipation of loss ($0.2 and $5.0 combined) minus neutral incentive, were calculated for each individual.
The present report focuses only on differences in the insula, due to a priori interest in this brain region. Volume of interest (VOI) analyses of BOLD signal data were conducted on bilateral insula. Left and right insula masks were generated based on the Anatomical Automatic Labeling (AAL) atlas using WFU PickAtlas software toolbox 43–45. Effect sizes for reward and loss anticipation activation in the VOIs were extracted from individual contrasts of interest using MarsBaR Region of interest toolbox 46. T-tests were conducted in SPSS to compare the insula effect sizes between A-carriers and GG GABRA2 genotypes and haplotypes. Pearson correlation was conducted in SPSS to test the relationship between Impulsiveness and insula effect sizes.
Results
GABRA2 variants, Impulsiveness and alcoholism
Personality and genotype data were available for 436 subjects (rs279826) and 431 subjects (rs279858) respectively (449 total). LD for both SNPs in our sample is r2 = 0.78, resulting in two major haplotypes, A-A and G-G, corresponding to 93% of the total haplotypes. We found both GABRA2 SNPs and the haplotype associated with the Impulsiveness facet of the Neuroticism domain in our overall sample (Table 2). These polymorphisms explain about 1.6% of the total variance of Impulsiveness. Overall, subjects with the GG genotype for both SNPs and haplotypes showed higher scores on the NEO-PI-R Impulsiveness facet (rs279858 F = 6.3; p = 0.02; rs279826 F = 6.9; p = 0.008; haplotype F = 5.2; p = 0.01). These results remain significant when controlled for family history and age (rs279858 p = 0.016; rs279826 p = 0.012; haplotype p = 0.032). Regressing out the effect of each SNP confirms that they are not independent. Genotype*sex interaction was observed as a secondary analysis for rs279826 (F = 2.9; p = 0.05) indicating that the genetic effect was observed in females (rs279858 p = 0.012; rs279826 p = 0.006; haplotype p = 0.009) and the association was stronger when controlled for family history and age (rs279858 p = 0.011; rs279826 p = 0.002; haplotype p = 0.006) (Table 2).
Table 2.
GABRA2 SNPs and Impulsiveness
| Impulsiveness | ||||||
|---|---|---|---|---|---|---|
| N | Overall Mean (s.e.m) |
N | Females Mean (s.e.m) |
N | Males Mean (s.e.m) |
|
| rs279858 | ||||||
| GG | 81 | 16.8 (0.41) | 35 | 17.3 (0.66) | 46 | 16.4 (0.53) |
| GA | 212 | 16.0 (0.26) | 84 | 16.6 (0.43) | 128 | 15.7 (0.32) |
| AA | 138 | 15.5 (0.32) | 55 | 15.3 (0.53) | 83 | 15.7 (0.39) |
| p value | 0.02 (*0.016) | 0.012 (*0.011) | 0.422 (*0.379) | |||
| rs279826 | ||||||
| GG | 99 | 16.6 (0.38) | 42 | 17.2 (0.60) | 57 | 16.1 (0.49) |
| GA | 214 | 15.9 (0.26) | 84 | 16.7 (0.42) | 130 | 15.5 (0.32) |
| AA | 123 | 15.2 (0.34) | 48 | 14.7 (0.56) | 75 | 16.1 (0.49) |
| p value | 0.008 (*0.012) | 0.006 (*0.002) | 0.557 (*0.588) | |||
| Haplotype | ||||||
| GG/GG | 83 | 16.7 (0.43) | 35 | 17.2 (0.69) | 48 | 16.3 (0.54) |
| AA/GG | 198 | 16.0 (0.28) | 76 | 16.6 (0.47) | 122 | 15.6 (0.34) |
| AA/AA | 124 | 15.2 (0.35) | 50 | 14.7 (0.57) | 74 | 15.6 (0.44) |
| p value | 0.01 (*0.032) | 0.009 (*0.006) | 0.490 (*0.531) | |||
p values adjusted for family history and age
Since GABRA2 variants were previously found to be associated with alcoholism, we also tested these Impulsiveness-associated SNPs for association with percentage of alcohol dependence symptom. In the presence of any alcoholic symptoms the GG genotype for both SNPs rs279858 (N = 443, β = −0.676; p = 0.02) and rs279826 (N = 448, β = −0.562; p = 0.05) and GG/GG haplotype (N = 408, β = −0.703; p = 0.02) were likely to have alcohol dependence symptoms compared to A carriers. In the case of the haplotype, this association became significant when gender was included as covariate where females with the GG/GG haplotype were likely to have a higher percentage of alcoholic symptoms compared to A carriers (β = 0.7653; p = 0.0053).
The initial stimulus for this study was the well established association of Impulsiveness with AUD 47. Percentage of alcohol symptoms (N = 415; r2 = 0.128; p = 0.009) was significantly positively correlated with Impulsiveness (Spearman’s rho correlation). No gender differences were observed for Impulsiveness.
Insula activity during anticipation of monetary reward and loss and GABRA2 variation
During reward anticipation, individuals with the GG genotype of either SNP and carriers of the G-G haplotype showed significantly higher activation in the left Insula compared to those with other genotypes/haplotypes (rs279858 p = 0.028; rs279826 p = 0.009; haplotype p = 0.020) and remained significant after corrected for family history and age (rs279858 p = 0.049; rs279826 p = 0.016; haplotype p = 0.028) (Table 3). In the right Insula, rs279826 G homozygotes showed higher activation (p = 0.05) during reward anticipation, whereas neither rs279858 nor the haplotype were associated (data not shown). During loss anticipation, rs279826 G homozygotes and carriers of the G-G haplotype were associated with higher left insula activation (rs279826 p = 0.024; haplotype p=0.05). We report no association for either SNP or the haplotype with right insula activation during loss anticipation. The association between rs279826 and right insula response to reward anticipation loses some significance when controlling for family history and age (p=.07), whereas the remainder of the findings remain significant (p<.05). However, children of alcoholics (COA) showed greater activation of right insula to reward (p=.022) and loss (p=.038). By regressing out each SNP we confirm that they are not independent. For both SNPs we observed that A homozygotes and heterozygotes have similar means and, because of the small sample size in the fMRI study, grouped them together and compared them to GG mean values.
Table 3.
Insula activation and GABRA2 genotypes
| Left insula | Mean effect size for reward anticipation | |||||
|---|---|---|---|---|---|---|
| N | Overall Mean (s.e.m) |
N | Females Mean (s.e.m) |
N | Males Mean (s.e.m) |
|
| rs279858 | ||||||
| GG | 10 | 0.73 (0.36) | 5 | 1.03 (0.35) | 5 | 0.44 (0.65) |
| AA/GA | 32 | −0.12 (0.18) | 15 | 0.02 (0.21) | 17 | −0.25 (0.28) |
| p value | 0.028 (*0.027) | 0.027 (*0.018) | 0.279 (*0.318) | |||
| rs279826 | ||||||
| GG | 11 | 0.77 (0.32) | 6 | 1.05 (0.28) | 5 | 0.44 (0.65) |
| AA/GA | 33 | −0.19 (0.17) | 14 | −0.05 (0.21) | 19 | −0.29 (0.25) |
| p value | 0.009 (*0.016) | 0.008 (*0.006) | 0.229 (*0.226) | |||
| Haplotype | ||||||
| GG/GG | 10 | 0.73 (0.36) | 5 | 1.03 (0.35) | 5 | 0.44 (0.65) |
| AA/AA _AA/GG | 31 | −0.17 (0.18) | 13 | −0.03 (0.23) | 18 | −0.28 (0.27) |
| p value | 0.020 (*0.028) | 0.023 (*0.011) | 0.250 (*0.252) | |||
| Right insula | ||||||
| rs279826 | ||||||
| GG | 11 | 0.46 (0.37) | 6 | 0.77 (0.43) | 5 | 0.08 (0.65) |
| AA/GA | 33 | −0.29 (0.18) | 14 | −0.16 (0.22) | 19 | −0.38 (0.27) |
| p value | 0.05 (*0.07) | 0.046 (*0.043) | 0.462 (*0.490) | |||
| Mean effect size for loss anticipation
|
||||||
| Left insula | ||||||
| rs279826 | ||||||
| GG | 11 | 0.39 (0.36) | 6 | 0.57 (0.24) | 5 | 0.18 (0.78) |
| AA/GA | 33 | −0.35 (0.14) | 14 | −0.29 (0.14) | 19 | −0.40 (0.22) |
| p value | 0.024 (*0.026) | 0.004 (*0.004) | 0.335 (*0.337) | |||
| Haplotype | ||||||
| GG/GG | 10 | 0.30 (0.39) | 5 | 0.42 (0.23) | 5 | 0.17 (0.78) |
| AA/AA _AA/GG | 31 | −0.31 (0.12) | 13 | −0.36 (0.13) | 18 | −0.26 (0.19) |
| p value | 0.05 (*0.058) | 0.007 (*0.007) | 0.418 (*0.429) | |||
p values adjusted for family history and age
When stratifying by gender, the genetic difference in insula activation was apparent in females but not in males. Females with the GG genotype for both SNPs and haplotype showed higher left insula activation during reward anticipation compared to the A carriers (rs279858 p = 0.027; rs279826 p = 0.008; haplotype p = 0.023) and remain significant when corrected for family history and age (rs279858 p = 0.036; rs279826 p = 0.006; haplotype p = 0.011) while males did not show genetic differences in activation (all p’s > 0.23) (Table 3). Female rs279826 G homozygotes also showed greater right insula activation to reward anticipation (p = 0.046; corrected for family history and age p = 0.043). Also in females, greater left insula activation to loss anticipation was observed for rs279826 G homozygotes (corrected p = 0.004) and haplotype (corrected p= 0.007), whereas males showed no differences (all p’s>0.34). Given the association of these alleles with Impulsiveness, we examined whether Insula activation was also related to Impulsiveness. Indeed, Impulsiveness was correlated with left insula activation during reward anticipation (r2 = 0.399; p = 0.026) and loss anticipation (r2 = 0.361; p = 0.046). Impulsiveness and right insula activation approached significant correlation during reward anticipation (r2 = 0.338; p = 0.063) and was significantly correlated during loss anticipation (r2 = 0.373; p = 0.039). There were no significant differences between COA and controls or for gender for these correlations.
Discussion
This study was stimulated by three previously established findings: (a) an association of GABRA2 variants with alcoholism; (b) an association of impulsive behavior with alcoholism, and (c) an emerging role of the insula in conscious urges to take drugs and maintain the addictive behavior 18. We thus sought to determine whether alcoholism-associated GABRA2 variants were also associated with impulsive behaviors and with insula activation during reward and loss anticipation. In this study, the G allele and corresponding haplotype (GG/GG) for both SNPs previously described as the high-risk haplotype by four studies5, 6, 8, 9, are associated with higher probability to have alcoholic symptoms, higher scores of the NEO-PI-R Impulsiveness facet, and with greater insula activation during anticipation of reward and loss and this activation is correlated with NEO-PI-R Impulsiveness scores. The convergence of these findings suggests that variation in GABRA2 contributes to the risk of alcoholism through the influence on impulsive behaviors and supports previous studies that relate GABRA2 with impulsive-related traits such as conduct disorder 48 and this effect may take place at least in part in the insula.
As expected from the extensive literature, we observed that subjects with higher Impulsiveness scores also had higher percentage of alcohol dependence symptoms, replicating the established evidence of the relationship between Impulsiveness and alcoholism 28, 47, 49. We also replicate the association of two GABRA2 SNPs and the haplotype with the presence of alcoholic symptoms. A large number of subjects in this study do not have any alcoholic symptom resulting in an excessive number of zeroes (240/448) for which a Zero-inflated Poisson test was applied. Subjects with the GG allele for both SNPs and haplotype are more likely to have any alcoholic symptom compared to A carriers. Several studies have reported the association of GABRA2 variants and alcohol-related traits but the direction of the association is not consistent. Two major haplotypes have been identified for this high-LD region. The haplotype containing the G allele for both SNPs analyzed in this study, has been associated with alcohol dependence in four studies 5, 6, 8, 9 and with the complementary haplotype (the A allele from both SNPs) in three studies 3, 11, 12. The studies from Edenberg 6 and Agrawal 3 used the same data set from the Collaborative Study on the Genetics of Alcoholism (COGA) where Agrawal reported the A allele for both SNPs rs279858 and rs279826 associated with comorbid illicit drug dependence, while Edenberg later clarified that the G-allele-containing haplotype was associated with alcohol dependence 50. When the SNP rs279858 was compared to the efficacy of three psychosocial treatments, different drinking outcomes among treatments were observed for subjects with the A allele, while no difference in outcome among treatments was observed for G allele carriers 4. The authors suggested that an elevated anxiety level driven by the presence of the G allele may compromise the outcome as a result of enhanced stress and impulsivity. Individuals with the G allele may need to drink more alcohol to achieve the desired effects compared to AA subjects who experienced a higher subjective response to alcohol 4. One11 of the three studies that reported the A allele for the two SNPs in this study associated with alcohol-related measures, found the haplotype containing the A allele for both SNPs associated with alcoholism compared to controls but when the SNPs were analyzed independently, no association was observed. Our results may contribute to a better understanding of GABRA2 variation on Impulsiveness measures. The G allele of both SNPs and corresponding G-G haplotype was associated with a specific kind of Impulsiveness that assesses rash action in response to distress - with rashness indicating impulsive action along with later regret.
Furthermore, we established an association between the NEO-PI-R Impulsiveness facet and activation of the insula cortex during anticipation of reward and loss in a subset of samples. GABRA2 SNPs were associated with both the Impulsiveness facet in the large sample and with the insula cortex activation during reward and loss anticipation in the subset of samples. Greater insula activation during anticipation of reward and loss was observed in individuals with the G allele for both SNPs and this activation correlated with Impulsiveness scores. One SNP in particular, the rs279826 G homozygotes, was associated with left and right insula activation during reward anticipation and with the left insula during loss anticipation. This suggests that the effect of genetic variation in GABRA2 on rash actions under distress may occur via modulation of insula activation. The insula translates interoceptive signals into conscious feelings, which can lead to subjective pleasure and cue-induced urges, anxiety, and biased decision-making in the face of uncertain risk and reward 18. The present findings add to the weight of evidence for the role of the insula on emotional stages of urge to take drugs 18, and altered related anticipatory processing in anxious individuals 24. These traits are related to Impulsiveness which in turn is positively correlated with insula activation in our sample.
Interestingly, the genetic effect of GABRA2 on percentage of alcohol dependence symptom, Impulsiveness and insula activation was observed in females and not in males. No difference for this genetic effect was observed between subjects with higher risk for AUD compared to low risk subjects. Enoch et al, has reported association of anxiety disorders (lifetime DSM-III-R anxiety disorders) in an haplotype containing the rs279858 G allele in women in a case-control sample of Plain Indians alcoholics 7. This sex difference may be explained by the effect of neurosteroids, such as progesterone, on the GABA system modulating an array of behaviors including anxiety 51. Prolonged exposure to progesterone increased anxiety-like behavior in female rats 52. In addition, several cross-sectional, longitudinal and ecological momentary assessment studies have found that the association between distress and alcohol consumption was greater in women than in men 53–55. This may suggest that GABRA2 genetic variation may differentially influence the negative affect component in impulsive behavior in women; this in turn is an additional risk factor for problem alcohol consumption. Our results suggest that the alcohol dependence GABRA2 G-G haplotype captures the genetic susceptibility for Impulsiveness driven by anxiety in females.
We propose that the GABRA2 G-G haplotype influences the homeostatic imbalance toward excitation, as evidenced by the electrophysiological anomalies associated with this haplotype 6. This imbalance may intensify the activation of the interoceptive representations within the Insula (higher activation for GG during cue-induced urges), thus contributing to impulsive behaviors 48, 56, 57 related to distress. However, the applicability of these results across the entire sample must be treated with caution given that differences have been observed in the role of GABRA2 in alcoholism risk across development. Specifically, a strong association with alcohol dependence may not emerge until the mid-20’s, whereas association with conduct disorder symptoms is present at earlier ages 48. We interpret the present results to indicate an influence of GABRA2 on the underlying neural system that influences both early risk factors as well as later alcohol dependence. However, prospective, longitudinal studies will be required to support this. Larger samples along with replication of our findings are necessary to elucidate the specific mechanism by which GABRA2 exerts its effect on Impulsiveness-related traits and its role in alcohol addiction.
Supplementary Material
Acknowledgments
We acknowledge all the participants in the Michigan Longitudinal Study for their commitment to the study over the years. We thank Dr. Jennie Jester from the MLS study and Dr. Laura Klem from the Center for Statistical Consultation and Research (CSCAR) at the University of Michigan for their excellent statistical support.
Financial Sponsor: This work was supported in part by grants R37 AA07065 to RAZ, R01 DA02726 (RAZ, MMH, JKZ), R03 AA01957601 to SV, K01 DA020088 to MMH.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63(9):957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- 2.Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139(1–2):2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, et al. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36(5):640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 4.Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, et al. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcohol Clin Exp Res. 2007;31(11):1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129(1):104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 6.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141(6):599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16(1):9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- 9.Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, et al. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29(4):493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- 10.Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, et al. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30(6):1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- 11.Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42(3):184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Lind PA, Macgregor S, Agrawal A, Montgomery GW, Heath AC, Martin NG, et al. The role of GABRA2 in alcohol dependence, smoking, and illicit drug use in an Australian population sample. Alcohol Clin Exp Res. 2008;32(10):1721–1731. doi: 10.1111/j.1530-0277.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52(8):831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- 14.Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, et al. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51(3):239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25(3):332–340. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- 16.Le Roux N, Amar M, Moreau A, Baux G, Fossier P. Impaired GABAergic transmission disrupts normal homeostatic plasticity in rat cortical networks. Eur J Neurosci. 2008;27(12):3244–3256. doi: 10.1111/j.1460-9568.2008.06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 18.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60(2):207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 20.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318(5850):655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 21.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 22.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 23.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60(4):402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 26.Whiteside Stephen P, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- 27.Costa PTJ, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- 28.Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav. 2004;29(7):1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description and implications for a differentiated social policy. RoutledgeFalmer; New York: 2000. [Google Scholar]
- 30.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 31.Zucker RA, Ellis DA, Bingham CR, Fitzgerald HE, Sanford KP. Other evidence for at least two alcoholisms, II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology. 1996;8:831–848. [Google Scholar]
- 32.Zucker RA, Fitzgerald HE, Noll RB. Drinking and drug history. Michigan State University; East Lansing, Michigan: 1990. [Google Scholar]
- 33.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26(1):57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 34.Johnston LD, Bachman JG, O’Malley PM. Drugs and the class of 1978: Behaviors, attitudes, and recent national trends (DPEW Publication No. ADM 79–877) National Institute on Drug Abuse DoR, U.S. Department of Health, Education, and Welfare; Washington DC: 1979. [Google Scholar]
- 35.Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes (Monograph No. 6) New Brunswick, NJ: Publications Division, Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- 36.Schuckit MA. Research questionnaire. San Diego, CA: Alcoholism Treatment Program; V.A. Medical Center, University of California; San Diego: 1978. [Google Scholar]
- 37.Room R. Measuring drinking patterns: the experience of the last half century. J Subst Abuse. 2000;12(1–2):23–31. doi: 10.1016/s0899-3289(00)00038-9. [DOI] [PubMed] [Google Scholar]
- 38.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 39.Chen WM, Abecasis GR. Family-based association tests for genomewide association scans. Am J Hum Genet. 2007;81(5):913–926. doi: 10.1086/521580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 41.Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans Med Imaging. 2003;22(2):178–188. doi: 10.1109/tmi.2002.808360. [DOI] [PubMed] [Google Scholar]
- 42.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 43.Maldjian J, Laurienti P, Burdette J. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 44.Maldjian J, Laurienti P, Kraft R, Burdette J. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 46.Region of interest analysis using an SPM toolbox. Proceedings of the 8th International Conference on Functional Mapping of the Human Brain; June 2–6 2002; Sendai, Japan. [Google Scholar]
- 47.Magid V, Maclean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addict Behav. 2007;32(10):2046–2061. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 49.Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, et al. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32(9):1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- 50.Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11(3–4):386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 51.Engel SR, Grant KA. Neurosteroids and behavior. Int Rev Neurobiol. 2001;46:321–348. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- 52.Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305(2):541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- 53.Hartka E, Johnstone B, Leino EV, Motoyoshi M, Temple MT, Fillmore KM. A meta-analysis of depressive symptomatology and alcohol consumption over time. Br J Addict. 1991;86(10):1283–1298. doi: 10.1111/j.1360-0443.1991.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 54.Muraven M, Collins RL, Morsheimer ET, Shiffman S, Paty JA. One too many: predicting future alcohol consumption following heavy drinking. Exp Clin Psychopharmacol. 2005;13(2):127–136. doi: 10.1037/1064-1297.13.2.127. [DOI] [PubMed] [Google Scholar]
- 55.Poulin C, Hand D, Boudreau B, Santor D. Gender differences in the association between substance use and elevated depressive symptoms in a general adolescent population. Addiction. 2005;100(4):525–535. doi: 10.1111/j.1360-0443.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 56.Dick DM, Agrawal A, Schuckit MA, Bierut L, Hinrichs A, Fox L, et al. Marital status, alcohol dependence, and GABRA2: evidence for gene-environment correlation and interaction. J Stud Alcohol. 2006;67(2):185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- 57.Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, et al. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Arch Gen Psychiatry. 2009;66(6):649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.