Abstract
The relationship between hormones and the pathogenesis of prostate cancer (PCa) has been studied extensively. All the mainstay targets for hormonal PCa therapies are based on negating androgen action. Recent epidemiologic and experimental data have clearly pinpointed the key roles of estrogens in PCa development and progression. Racial and geographical differences, as well as age-associated changes, in estrogen synthesis and metabolism contribute significantly to the etiology by increasing the ratio of circulating estrogen to androgen, sex hormone binding globulin synthesis, and aromatase activity and reducing androgen glucuronidation and tissue bioactivation. Promotion of aberrant cell growth, evasion of apoptosis, increased oxidative stress and inflammation, and gains in adiposity and bioactivation to genotoxic carcinogens during adulthood are probable mechanisms of estrogen carcinogenicity, while “estrogen imprinting” via epigenetics in early-life also determines PCa risk. Although the effects of estrogens are known to be mediated by genomic actions of the two estrogen receptor (ER) subtypes (ERα and ERβ), other non-canonical mediators, including the different ERβ isoforms, membrane and mitochondrial ERs, and G protein-coupled receptor 30, may have major actions diverging from classical ER actions. These new discoveries have led to renewed interest among the public and the medicinal field in estrogens and antiestrogens as singular and adjuvant PCa treatment and prevention regimens. This review summarizes current knowledge on how different estrogens/antiestrogens/estrogen mimics contribute to prostate carcinogenesis, the roles of the different mediators of estrogen in the process, and the potentials of new estrogenic/antiestrogenic compounds as targeted therapies for prevention and treatment of PCa.
Keywords: Racial disparity, aging, sex steroid–binding globulin, estrogen receptor, ER spliced variants, GPR30, aromatase, antiestrogens, selective estrogen receptor modulator (SERM), nuclear receptor co-regulators, DNA methylation, developmental reprogramming, apigenin, phytoestrogen, apoptosis, oxidative stress, genomic damages, prostate cancer risk, hormonal therapy, epigenetics, genistein, resveratrol, raloxifene, toremifene, fulvestrant, estramustine, catecholestrogen
Androgens are traditionally recognized as the major hormone promoting normal and aberrant growth of the prostate. Recent literature, however, suggests that estrogen could also be an important mediator of these processes. Estrogen alone or in synergy with androgen is responsible for the pathogenesis of prostate cancer (PCa). More important, recent experimental data suggest that estrogen or its mimics could determine the risk of PCa development as early as the prenatal stage via a process known as “estrogen imprinting.” Here we a) review research findings that support a role for estrogens, estrogen mimics and estrogen metabolites in prostate carcinogenesis; b) discuss how different estrogen receptors (ER) mediate the action of estrogen in promoting the development and progression of PCa; and c) evaluate the potentials of estrogens, xenoestrogens, phytoestrogens, antiestrogens, and SERMs as therapeutics for prevention and treatment of PCa.
Epidemiologic and Animal-model Studies of the Relationship between Estrogens and Pathogenesis of PCa
Results from epidemiologic studies have suggested a role for estrogen in the pathogenesis of PCa. Racial/ethnic and geographical differences in the levels of estrogens provide a probable explanation for the disparity in the prevalence of PCa among various populations throughout the world.1-3 Apropos to this view is the finding that levels of circulating estrogens in African-American men, whose incidence of PCa is the highest in United States, are higher than those in Caucasian Americans throughout their adult life.4-8 In contrast, Japanese men, whose incidence of PCa is low, have lower circulating levels of estrogen than do Dutch-European men.9 A global study on 5,003 men aged 65 years or older showed that blacks (in the US and in West Africa) had higher estrogen levels than Caucasians or Asians (in the US and in their homelands), with levels of total and free estradiol-17β (E2) 10–16% higher and levels of estrone (E1) 27–39% higher than in the latter group.10 Moreover, the ratios of total E2 to total testosterone (T) and E1 to androstenedione were higher in blacks than in the other groups.10 A comprehensive analysis of the levels of androgens, estrogens, and their metabolites in circulation led to the conclusion that two fundamental metabolic processes, increased aromatase activity and reduced androgen glucuronidation, are major factors governing the ratio of estrogen to androgen in elderly men.10
Age is also a key risk factor for PCa.1 The prevalence of PCa increases dramatically as men age; this is paralleled by a significant increase in the ratio of circulating estrogen to androgen levels, which may increase by up to 40%.11-17 This age-related hormonal change, often referred to as “andropause,” is caused by several endocrine events, including a decline in testicular function, and increases in adiposity, extragonadal aromatization, and the production of sex hormone–binding globulin (SHBG) as men age.12;18-21 The level of 5α-dihydrotestosterone (DHT) was found to decrease whereas those of estrogen (both E2 and E1) in the epithelial cells, to increase in the aging prostate (Figure 1).22 Since estrogens can be synthesized de novo via aromatase activity in the prostate,23 tissue estrogen levels may be more important than circulating estrogen levels in promoting prostate carcinogenesis and progression. In this regard, recent studies utilizing laser capture microdissection samples demonstrated that stromal rather than epithelial aromatase activity may be important in upregulating the E2:T ratio in the tumor site via an alternative promoter activation mechanism during prostate carcinogenesis.24;25 Furthermore, aromatase-knockout mice, which cannot produce E2 locally in the prostate, have elevated levels of circulating T and DHT and, with age, are prone to the development of benign prostatic hyperplasia (BPH) but not PCa.26 Collectively, increased estrogenic influences on the prostate due to racial differences or andropause27 may elevate the risk of neoplastic transformation of the prostatic epithelium in men.3;28;29
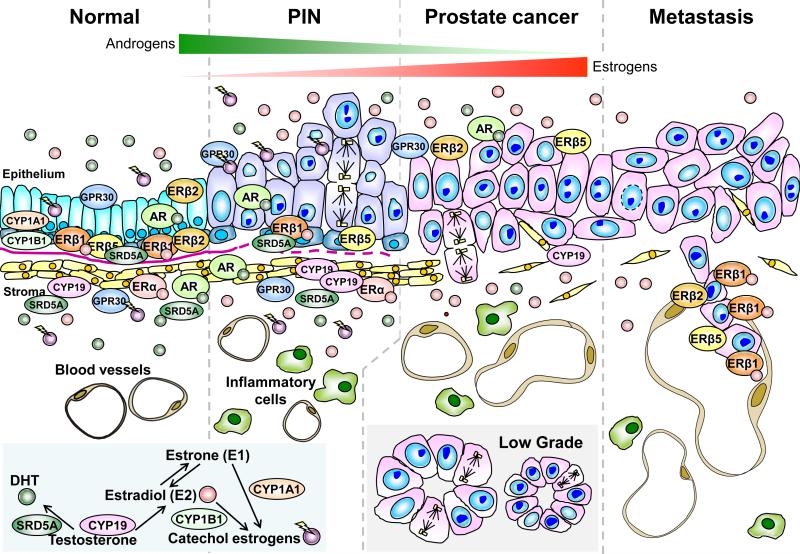
Figure. 1.
Expression of various hormone receptors and levels of hormones during the development and progression of prostate cancer (PCa). In the normal human prostate (far left panel), androgen receptor (AR), estrogen receptor alpha (ERα), different estrogen receptor beta (ERβ) isoforms, and G protein–coupled receptor 30 (GPR30) are expressed differentially in the stroma, and luminal and basal epithelium of the prostate. The epithelial cell compartment expresses only ERβ and AR, whereas the stromal compartment expresses both ERα and AR. GPR30 is expressed in both compartments. During tumor progression from high grade PIN (second to the far left panel) to prostate cancer (second to the far right panel), expression of AR and ERα remains unchanged, while expression of the antiproliferative ERβ1 is lost in the basal epithelial cells along with the disruption of the basement membrane (pink line). Two ERβ isoforms, ERβ2 and ERβ5, which have been shown to have pro-metastatic potential, are expressed in the normal epithelium, but their co-expression in PCa specimens is associated with a poor prognosis and shorter metastasis-free survival. In prostate metastases (far right panel), ERβ1 is re-expressed at high levels. Other non-canonical ERs such as GPR30 and ERRs are differentially expressed in normal and malignant prostate tissues but their roles in prostate carcinogenesis remains to be elucidated. Throughout the development and progression of PCa, tissue estrogen levels [estradiol-17β (E2) and estrone (E1)] increase with concomitant decreases in levels of 5α-dihydrotestosterone (DHT). These changes are due in part to an increase in CYP19 (aromatase) activity and a loss of SRD5A (5α-reductase) expression in the primary site (insert). SRD5A expression is completely lost in metastases. Additionally, an age-related reduction in testicular testosterone (T) synthesis, an increase in levels of sex hormone–binding globulin, and increases in adiposity and peripheral aromatase activity, collectively contribute to an increase in the estrogen to androgen ratio in circulation that further raise prostatic estrogen levels. In summary, a life-time over-exposure to estrogens and/or catecholestrogens could be an etiological factor in prostate carcinogenesis. The localizations of these enzymes as well as mediators of estrogen/androgen actions are indicated.
Experimental models also support the suggestion that estrogens, alone or synergistically with androgens, are potent inducers of aberrant growth and neoplastic transformation in the prostate.1;2;30-33 Prenatal exposure to maternal estrogens or adult exposure to pharmacologic doses of estrogens induces a benign lesion termed squamous metaplasia, which is derived from the basal-cell proliferation of the prostates of various species, including humans.34 In a susceptible rat strain (Noble rats), chronic exposure to T plus E2 in adulthood promoted the evolution of a precancerous lesion similar to human prostatic intraepithelial neoplasia (PIN) and a high incidence of full-blown PCa31;35-37 via the alteration of levels of estrogen to androgen in a manner mimicking that in aging men.35-38 Paradoxically, dietary soy that is rich in phytoestrogens can mitigate the tumor-promoting effect of T plus E2 in this rat model.39 Moreover, treatment of the nude mice with T plus E2 could promote the progression of PCa and metastasis at distant organs in the tissue recombinants composed of mouse mensenchyme and a human prostatic epithelial cell line.40 Cellular and molecular changes implicated as the mechanisms leading to prostate carcinogenesis include a) a dramatic increase in the proliferation of epithelial cells;37 b) upregulation of growth factor–signaling pathways (e.g. TGFα/EGF receptor signaling;41 TGFβ signaling,42 IGF-1 and VEGF signaling,43 and ER signaling);44 c) prolactinemia or increased prolactin-receptor signaling;45 d) mitogen-activated protein kinase (MAPK) activation, possibly through Id-1;46;47 e) increased cell-survival potential through the overexpression of anti-apoptotic mediators (e.g., metallothionein48 and TRPM-2/clusterin);49;50 f) elevation in oxidative stress-induced DNA damage;51-53 g) changes in gene-expression profiles related to cell proliferation, DNA damage, activation of proto-oncogenes and transforming factors, IL-1B signaling, and TNF-α activation;54;55 and h) breakdown of epithelial basement membrane and stromal extracellular matrix due to the increase in gelatinolytic proteinase activity and altered expression of glycoconjugates in smooth muscles and their associated extracellular matrix.56-58 Another potential culprit associated with estrogen-induced/promoted PCa is hormone-induced chronic inflammation,38;59-61although this view is not uniformly supported by all studies.62
Influence of Estrogen Bioactivation and Detoxification on PCa Risk
The carcinogenic action of estrogen could be caused, in part, by metabolic activation of the natural estrogens E1 and E2 to genotoxic metabolites, such as 2- and 4-hydroxyl catechol estrogens, and their quinone/semiquinone intermediates that act as chemical carcinogens.63 The bioactivation process is mediated principally by two cytochrome P450 enzymes, CYP1A1 and CYP1B1, whereas the enzyme catechol-O-methyltransferase (COMT) is responsible for the inactivation and removal of these genotoxic estrogen-derived intermediates.63-66 Once formed, these genotoxic metabolites can cause genomic damages through processes such as the formation of DNA adducts.67 Supporting the suggestion that the genotoxicity of estrogen metabolites is involved in prostate carcinogenesis is the observation of increased DNA strand breakage52 and nuclear staining of 8-hydroxy-2′-deoxy-guanosine60 in the prostates of rats treated with T plus E2. Conversely, increased expression or activity of COMT was shown to protect against estrogen-induced cancer by mediating the conversion of catechol estrogens into methoxyestrogens that have potent apoptotic activity against rapidly growing PCa cells,68 prompting the testing of combinatory therapies involving methoxyestrogens and other standard therapies, such as hormone deprivation,69 docetaxel,70 and eugenol71 against PCa growth in model systems.
Furthermore, the aforementioned estrogen metabolites and their intermediates induce oxidative stress and likely promote the generation of high levels of reactive oxygen species (ROS), with damaging effects on proteins, lipids, and DNA in target tissues.29;66 Therefore, several isoforms of glutathione S-transferases (GSTs) have been reported to have protective effects against estrogen genotoxicity through the detoxification of ROS. Genetic polymorphisms that alter enzyme activity and epigenetics- or mutation-mediated silencing of the cognate genes are expected to affect the PCa risk by modulating the extent of lifelong exposure to genotoxic estrogen metabolites.29;72 Thus, polymorphisms of the CYP1A1m1 allele, the CYP1B1-Leu432Val, COMT at codon 62 and 158, and the GSTM1-null genotype have been shown to modify PCa risk in certain populations.73-80 In addition, a CYP1A1 variant (CYP1A1v) that resides preferentially in the nucleus and mitochondria was found to confer higher carcinogenic potential than its wild-type cytosolic counterpart.81 This finding is in agreement with the hypothesis that genotoxic estrogen metabolites produced in nuclei are potent tumor initiators.
Another metabolic pathway of relevance to estrogen carcinogenicity in the prostate is the in situ production of estrogen from androgen via aromatase encoded by CYP19.25 The enzyme and its activity have been demonstrated in the specimens of PCa and BPH.25;82 Clinical trials designed to test the efficacy of aromatase inhibitors in treating BPH83-85 and PCa86-89 have been reported. Polymorphisms in CYP19 including intron 4[TTTA]n repeat90 and C/T versus T/T genotype91 are associated with familial PCa. Continued investigation of the use of aromatase inhibitors as monotherapies or adjuvants for the treatment of PCa is warranted.26;92
Determination of PCa Risk during Early Life
The risk of developing PCa as an adult could be determined by early-life exposure to natural or environmental estrogens through a mechanism known as “estrogen imprinting.”93 Perinatal and neonatal exposure of rats94-97 or mice98-100 to estrogens or estrogen mimics induces inflammation, permanent changes in the levels of androgen and estrogen receptors, stromal hypertrophy, and elevated proliferative potentials in the prostatic epithelium of the adult aged gland.96;101-103 If the “estrogenized” adult glands are exposed to an elevated estrogen challenge during adult life (the “second hit”), full-blown PIN develops in the affected glands.104 An environmental estrogen, bisphenol A (BPA), is equally effective in sensitizing the adult prostate to increased estrogenic influence during adulthood with regard to the induction of PIN. An unbiased screening has identified permanent alterations in the methylation status of a CpG island in the promoter of phosphodiesterase 4D4 (Pde4e4), an enzyme responsible for regulating cellular cAMP.104 This finding suggests estrogen imprinting may involve epigenetic reprogramming of prostatic transcription programs in early life. Whether analogous phenomena exist in humans is unclear. However, circulating E2 levels have been found to be higher in pregnant African-American women than in pregnant Caucasian-American women.105;106 These data are consistent with the hypothesis that exposure to higher levels of E2 in utero may explain some of the differences in PCa risk among ethnic groups. Moreover, some indicators of high levels of estrogen during pregnancy, such as high birth weight and jaundice in the newborn, are associated with increased risk of PCa, whereas indicators of low estrogen levels, such as pre-eclampsia, are related to decreased risk.107;108 The male offspring of women who took diethylstilbestrol (DES) during pregnancy may have a higher risk of PCa.109 These data, taken together, indicate that PCa may be considered a fetus-based disease. In addition, the window of susceptibility for early-life reprogramming may extend to perinatal and peripubertal periods and exposures to environmental estrogens may have the same impact as natural estrogens. Of relevance to these hypotheses are studies reporting significant exposure of human fetuses to BPA, likely caused by maternal use of BPA-containing products.110-116 Perinatal exposure to BPA has also been documented.117-119 Paradoxically, concerns about developmental reprogramming of PCa risk by soy infant formulas containing high concentrations of phytoestrogens have seldom been raised.120-122 Yet, the results of numerous human studies examining the risks and benefits of adult consumption of soy and phytoestrogens for prevention of PCa have been inconclusive.123-127 In summary, if PCa risk can be reprogrammed in early life, cancer prevention strategies should be directed at the aforementioned early developmental stages.
Estrogen Receptors (ERα and ERβ) as Functionally Divergent Mediators of Estrogen Action in the Prostate
The actions of estrogens are now believed to be mediated primarily by two ER subtypes, ERα and ERβ and their variant forms.28;32;128;129 Both of the ERs have six common domains (A-F). They share a highly homologous DNA-binding domain (97% amino acid homology) but have very dissimilar N- and C-termini.130 In addition, their ligand-binding domains are 56% homologous. These structural differences are the basis of the reported significant functional differences between the two receptor subtypes. Furthermore, it has been reported that the binding of the two receptors to the same ligand can initiate recruitments of different co-regulators and trigger the utilization of different cis-regulatory elements, thus further increasing the functional diversity of these two receptors.130-135 Finally, the recent discovery of variant forms of ERα and ERβ (isoforms) caused by alternative splicing or mutations has added complexity to estrogen action because these variants clearly show distinct functional disparity and patterns of tissue-/cell type-specific distribution.129;132;136;137
Many reports have shown differential expression patterns of the two receptors in the epithelial and stromal compartments of the normal and malignant human prostate (Figure 1).33;137-144 In the normal human prostate, the wild-type ERβ (also known as ERβ1) is localized mainly in the basal epithelial compartment, where ERα is almost never found, while ERα, along with ERβ, is expressed in the stroma.141 Both ER subtypes are absent in the luminal epithelial compartment.141 A gradual loss of ERβ1 expression is apparent during the progression to high-grade PCa in the primary site 115;139;140;145;146 The progressive silencing of ERβ is accompanied by hypermethylation of a CpG island in the proximal promoter of the gene.147 Paradoxically, ERβ, but not ERα, is expressed at high levels in PCa metastasized to the bone and regional lymph nodes,141 with a concomitant loss of methylation in the CpG island of ERβ.147 Fine mapping of the CpG island revealed that this reversible silencing and reactivation of ERβ expression is attributable to cytosine methylation of an AP2 site within the CpG island of the ERβ promoter.148 Moreover, antiestrogen-based therapies involving DES or PC-SPES lowered the level of ERβ1 expression in bone metastases of the treated patients.149
It is now established that ERβ1 exerts an antiproliferative effect on the prostate epithelia131;133 and inhibits epithelial-mesenchymal transition.150 However, less is known about the biological roles of its spliced variants. We recently demonstrated that ERβ2 and ERβ5 promote metastasis and that their expression is associated with shorter metastasis-free survival in patients with PCa.137 In support of a divergent functional role of various ERβ isoforms, when a pan-ERβ antibody was used, the retention of ERβ expression in primary PCa was associated with increased mortality.139 The co-expression of ERβ with endothelial nitric oxide synthase (eNOS) and hypoxia-inducible factor 2α (HIF-2α) suggests that an estrogen-mediated NO-enriched environment may influence the aggressive phenotype of PCa significantly.151 Thus, ERβ appears to play differential roles in human prostate carcinogenesis through differential expression of the various spliced variants and possibly alternative promoter utilization.152 Differential binding of the receptor to different ligands and crosstalk with other transcriptional factor-signaling cascades may also introduce additional divergence in ERβ action.131;153;154
Studies in animal models have provided strong evidence of the antiproliferative role of ERβ in the prostate gland. ERβ knockout mice have been found to develop prostatic hyperplasia in old age, a phenomenon not seen in ERα knockout mice.155 Jiang et al.156 observed an age-dependent decline in ERβ expression in the canine prostate. Chang and Prins157 reported that neonatal exposure of rats to estrogen causes the downregulation of ERβ; the upregulation of ERα; and the development of hyperplastic, dysplastic, and neoplastic lesions in the adult ventral prostates. Consistent with the hypothesis of the antiproliferative role ERβ, Risbriger et al. demonstrated that the administration of ERβ agonists, but not of an ERα agonist, to aromatase knockout mice suppressed the prostate epithelial cell growth and promoted apoptosis158 and that the action of the receptor is mediated via TNF-α signaling.133
Apart from ERβ expression, ERα, which is expressed primarily in the stromal compartment of the prostate, may contribute to the pathogenesis of PCa. Higher levels of ERα were observed in the prostatic stroma of Hispanic and Asian men than in that of Caucasian and African-American men, who are at a higher risk for PCa.159 In a genotyping and allelic frequency analysis of six different polymorphic loci of ERα in a Japanese population, polymorphism in codon 10 was found to be associated with a higher PCa risk.160 These findings are in stark contrast to results from studies of genetic polymorphisms of ERβ161-164 that reported no strong association of various polymorphic loci with PCa risk, with the exception of one promoter single-nucleotide polymorphism (SNP).165;166
Finally, during fetal development of the human prostate, ERβ is the first to appear in the prostate (by the seventh week of gestation) and is the only ER subtype expressed in the epithelial and stromal cells during the early ductal morphogenesis.34;167 ERβ expression begins by week 15 of gestation and is strongly associated with the squamous metaplasia in the distal periurethral ducts and utricle.167 These findings suggested that ERβ, perhaps in concert with the AR, mediates the very early stage of fetal prostate development, followed by the action of ERα. Thus, selective ERβ-activating compounds may play an important role in prenatal estrogen imprinting of PCa risk.
Non-canonical Actions of Estrogen and a New Therapeutic Target (G Protein-Coupled Receptor 30)
Apart from acting as transcription factors, ERα and ERβ can trigger rapid non-genomic signal transduction at the cell membrane level through the activation of specific kinase activity or induction of a calcium influx.168-170 These membrane ER-mediated events can activate gene-transcription activities with or without synergy with the classical genomic actions of nuclear ERs.171 In this regard, membrane ERα has been shown to tether onto the epidermal growth factor receptor (EGFR) signaling pathway for the activation of the MAPK172 and phosphoinositide 3-kinase (PI3K) signaling.173 In addition, ERβ found localized in the mitochondria has been demonstrated to act as a mitochondrial transcription factor that regulates mitochondrial gene expression.174 ERβ has been reported to move from the mitochondria to the nucleus during neoplastic transformation.175 However, the functions of cell membrane or mitochondrial ERα and ERβ in the prostate and PCa remain unknown and need further elucidation.
Estrogen has recently been found to be able to exert its action via the G protein-coupled receptor 30 (GPR30).176 GPR30 has been localized in the cell membrane and endoplasmic recticulum of various estrogen-sensitive tissues and cell lines176-178 and to mediate the non-genomic action of E2 in breast, endometrial, and ovarian cancer cells through the activation of Erk1/2 and cAMP pathways.179;180 GPR30 is expressed in the immortalized normal prostate stroma cell line WPWY-1 but does not appear to mediate the growth response induced by high-dose E2.181 A recent study demonstrated positive staining of GPR30 in both plasma membrane and cytoplasm in the normal and cancerous prostate, and its predominant expression in the luminal epithelium.182 Activation of GPR30 by a non-estrogenic, synthetic, GPR30-specific ligand G-1 inhibited PCa cell growth in vitro by activating Erk1/2 and upregulating p21-mediated G2-M arrest and attenuated the growth of human PCa xenografts in nude mice.183 These new findings suggest that GPR30 is a novel estrogen mediator in the prostate and that a specific GPR30 ligand such as G-1 may have therapeutic potential for PCa.
Estrogen-related receptors (ERRs) are orphan nuclear receptors that are highly homologous to ERs, especially in the DNA- and ligand-binding domains.184 However, they do not bind natural estrogens and may have yet-to-be discovered endogenous ligands. There are at least three EERs, namely ERR-α, -β,185 and -γ.186;187 They regulate gene expression through transactivation via cis-elements such as the ERE, steroidogenic factor-1 response element (SFRE), and estrogen-related receptor response element (ERRE).188-192 Similar to ERs, different ERRs have distinct differential expression patterns in normal and cancerous prostate tissues/cells.193 Finally, although they do not bind natural estrogens, ERRβ and ERRγ do bind DES, tamoxifen, 4-hydroxytamoxifen, flavones, and isoflavone phytoestrogens194-196 and therefore should be considered in devising estrogen- or antiestrogen-based therapies for PCa.
Prevention of and Therapy for PCa with Selective ER Modulators (SERMs), Aromatase Inhibitors, Phytoestrogens, and Other Estrogen-/Antiestrogen-based Treatments
The first effective drug therapy for PCa was found about 70 years ago. DES, a xenoestrogen, was applied for the treatment of metastatic PCa197 but fell out of favor because of its cardiovascular toxicity and other serious adverse effects.33;198;199 Other ER-based treatments for PCa include antiestrogens, selective ER modulators (SERMs), and aromatase inhibitors. SERMs are estrogenic compounds that act as either ER agonists or antagonists according to the presence of different co-regulators in a cell-/tissue-specific manner. Consequently, the ERs can be either stimulated or inhibited in the cell/tissue.200 However, clinical trials have shown that different generations of SERMs, such as tamoxifen, toremifene, raloxifene, and fulvestrant, have limited efficacy as alternatives to DES.33;201-207 Their lack of efficacy could be due to their original design for targeting the transactivation of ERα on an ERE and/or blocking the traditional genomic action of estrogen. With our growing knowledge of the wide range of genomic and non-genomic actions of estrogen and the variable expression of ERβ and its isoforms in the prostate and PCa, as well as of the significance of the non-canonical ER, GPR30, the future design of estrogen/antiestrogen therapies and chemopreventive agents will have to take into account the complex nature of estrogen action in the development and progression of PCa.
In this regard, it is important to recognize that many specificities or off-target effects of estrogenic/antiestrogenic drugs are due to their broad spectrum of activities not restricted to their estrogenicity. Traditionally, estrogen-induced PCa regression is believed to be mediated by its action on the hypothalamic-pituitary axis, thereby inhibiting testosterone synthesis.208 However, it is now known that many estrogens/antiestrogens/phytoestrogens/SERMs including DES, 2-methoxy-E2, genistein, resveratrol, licochalcone, raloxifene, toremifene, fulvestrant, and estramustine, have antitumor effects independent of this pathway.209-225 The ability of these compounds to suppress PCa cell growth has been attributed to a broad range of actions, including direct cytotoxicity,223 interruption of cell-cycle progression,210;221;226 induction of apoptosis,211;221;224;225 depolymerization of microtubules,209 inhibition of DNA synthesis,213 inhibition of topoisomerase II,217 blockade of tyrosine kinase,217;218 disruption of apoptotic regulators,222 and activation of death-domain receptors.214;219 Some of these estrogenic/antiestrogenic compounds are also potent inhibitors of angiogenesis and metastasis, through their actions in upregulating expression of genes related to anti-angiogenesis or anti-metastasis,215 activating the cell adhesion–signaling molecule focal adhesion kinase,216 and reduction in metastatic spreading via the lymphatic system.220
As a recent development in the treatment of PCa, the application of estrogen patches has grown in popularity.227-229 The advantages include reduction in cardiovascular toxicity and the maintenance of adequate hormonal levels for a convenient time period.230 as well as the additional advantage of allevating hot flashes and improving bone density after endocrine treatment of PCa. 231;232 Estrogen patches have recently been tested in a phase II clinical trial as a first-line hormonal therapy in patients with locally advanced or metastatic PCa. Preliminary results produced the levels of testosterone and a PSA response similar to those of castration.228
Aside from treating PCa, the potential of SERMs for preventing PCa has turned out to be a promising proposition. The rationale stems from several lines of investigation. SERMS were shown to decrease testosterone levels by suppressing the hypothalamic-pituitary axis.233 Furthermore, age-related increases in PCa prevalence parallel increases in serum estrogen levels, and the incidence of PCa is low in countries with diets rich in phytoestrogens.234 A multicentered, double-blind randomized study involving 514 men with high-grade PIN and no cancer reported a significant reduction in PCa incidence in patients treated daily with 20 mg of toremifene for 6-12 months as compared with placebo.235 These promising results have prompted the initiation of a large phase III study to examine the potential for toremifene as a chemopreventive agent.
Phytoestrogens are another important class of estrogen-based chemopreventive agents. The most common phytoestrogens, soy isoflavones such as genistein, equol, and daidzein, which are abundant in soy beans and its products, have been found to have estrogenic or antiestrogenic activity.236-239 They have been some of the most popular agents in studies of the potential of PCa chemoprevention and therapy because of the low incidence of PCa, along with the high levels of phytochemicals, in the diets of Asian populations.240 Although there is an abundance of in vitro and in vivo experimental data about the anti-tumor properties of phytoestrogens, the value of their protective or therapeutic effects in cohort studies still remains controversial.241-248 Nevertheless, a number of phase I clinical trials evaluating the use of phytoestrogen supplementation in patients with PCa have generally seen beneficial effects without any toxicity.212;249-252
Summary
Traditionally, androgens have been considered to be the major sex hormones regulating the normal and malignant growth of the prostate. However, recent epidemiologic findings and experimental data suggest that estrogens and their mimics can be responsible for the pathogenesis of PCa. The carcinogenicity of estrogens in the prostate during adulthood is believed to be mediated by the combined effects of the hormone-induced unscheduled cell proliferation and epigenetic silencing of anti-tumor genes, along with the bioactivation of estrogens to genotoxic carcinogens. Thus, individuals or ethnic groups with polymorphisms in genes encoding ERs and/or estrogen-metabolizing enzymes can modify the risk for PCa caused by altered responsiveness to the hormone and exposure to its carcinogenic metabolites over a lifetime. The age-dependent hormonal shift from androgen to estrogen could also be an important contributing factor to increased estrogen bioavailability. Although PCa has a long latency and starts to develop in men around middle age, recent data strongly suggest that PCa risk could be determined even as early as during pre- and perinatal life stages by a process known as the “estrogen imprinting.” Thus, primary PCa prevention should probably begin in early life. Among the various cellular mediators, ERβ appears to be a key determinant in the pathogenesis, progression, and metastasis of PCa. Therapeutic approaches targeting its activation/inactivation may have important ramifications in the prevention and treatment of PCa. Epigenetic mechanisms such as DNA methylation play important roles in regulating the expression of the two ER subtypes. A change in the methylation status of proximal promoters of these genes constitutes an “on/off” switch for reversible gene regulation. Moreover, the differential expression of different ER-spliced variants (isoforms) could explain some conflicting observations related to estrogen action in the initiation and progression of PCa. Apart from the canonical genomic action of ERα and ERβ, the therapeutic potential of ER and its variants that function in multiple non-genomic pathways, such as membrane ERα, mitochondrial ER, ERRs, and GPR30, may further contribute to the pathogenesis of PCa. Finally, various estrogenic/antiestrogenic/SERM-like compounds have demonstrable efficacies in causing PCa regression through the utilization of pathways independent of the hypothalamic-pituitary axis. As the treatment of advanced PCa with transdermal estrogen has gained in popularity, several SERMS, including toremifene, have shown promise as chemopreventive or therapeutic agents in clinical trials. Although data from clinical trials are not conclusive, phytoestrogen supplements, including dietary soy, continue to be used by patients as complementary alternative medicine for PCa. With a greater understanding of the molecular mechanism underlying estrogen carcinogenicity in the prostate, the applicability of estrogen/antiestrogen-based prevention and treatment therapies, as first-line or adjuvant therapies, will gain ground in the clinic. Thus, it is timely in devising a new generation of estrogenic/antiestrogenic therapies with higher specificity against PCa and fewer off-target effects.
Acknowledgements
We thank Nancy K. Voynow for her professional editorial assistance and Dr. Xiang Zhang for helpful critiques of the manuscript. Research supported in part by NIH grants CA112532; ES006096; ES015584; ES018758; and ES018789.
Funding support: NIH grants: DK061084, CA112532, CA015776, ES006096, and ES015584 to Shuk-mei Ho; Department of Defense Prostate Cancer Program grant: PC030595 to Shuk-mei Ho and Department of Defense Breast Cancer Program grant: BC094017 to Ming-tsung Lee
Footnotes
Declaration of interest The authors have nothing to disclose.
Contributor Information
Shuk-Mei Ho, Department of Environmental Health, Center for Environmental Genetics, and the Cancer Institute, College of Medicine, University of Cincinnati, Cincinnati, Ohio.
Ming-tsung Lee, Department of Environmental Health, College of Medicine, University of Cincinnati, Cincinnati, Ohio. Telephone 513-558-0595, Fax 513-558-0071, lee2mg@mail.uc.edu
Hung-Ming Lam, Department of Environmental Health, College of Medicine, University of Cincinnati, Cincinnati, Ohio. Telephone 513-558-0595, Fax 513-558-0071, lamhg@uc.edu
Yuet-Kin Leung, Department of Environmental Health, Center for Environmental Genetics, and The Cancer Institute, College of Medicine, University of Cincinnati, Cincinnati, Ohio. Telephone 513-558-5181, Fax 513-558-0071, ricky.leung@uc.edu
Reference List
- 1.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 2.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91(3):491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 3.Ho SM, Leung YK, Chung I. Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann N Y Acad Sci. 2006:1089177–193. doi: 10.1196/annals.1386.005. [DOI] [PubMed] [Google Scholar]
- 4.Abdelrahaman E, Raghavan S, Baker L, Weinrich M, Winters SJ. Racial difference in circulating sex hormone-binding globulin levels in prepubertal boys. Metabolism. 2005;54(1):91–96. doi: 10.1016/j.metabol.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Hui SL, DiMeglio LA, Longcope C, Peacock M, McClintock R, Perkins AJ, et al. Difference in bone mass between black and white American children: attributable to body build, sex hormone levels, or bone turnover? J Clin Endocrinol Metab. 2003;88(2):642–649. doi: 10.1210/jc.2002-020653. [DOI] [PubMed] [Google Scholar]
- 6.Richards RJ, Svec F, Bao W, Srinivasan SR, Berenson GS. Steroid hormones during puberty: racial (black-white) differences in androstenedione and estradiol--the Bogalusa Heart Study. J Clin Endocrinol Metab. 1992;75(2):624–631. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- 7.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92(7):2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986;76(1):45–48. [PubMed] [Google Scholar]
- 9.de Jong FH, Oishi K, Hayes RB, Bogdanowicz JF, Raatgever JW, van der Maas PJ, et al. Peripheral hormone levels in controls and patients with prostatic cancer or benign prostatic hyperplasia: results from the Dutch-Japanese case-control study. Cancer Res. 1991;51(13):3445–3450. [PubMed] [Google Scholar]
- 10.Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR, et al. Evidence for Geographical and Racial Variation in Serum Sex Steroid Levels in Older Men. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118(4-5):246–251. doi: 10.1016/j.jsbmb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 13.Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol. 1991;44(7):671–684. doi: 10.1016/0895-4356(91)90028-8. [DOI] [PubMed] [Google Scholar]
- 14.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73(5):1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths K. Estrogens and prostatic disease. International Prostate Health Council Study Group. Prostate. 2000;45(2):87–100. doi: 10.1002/1097-0045(20001001)45:2<87::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RO, Jacobson DJ, Rhodes T, Klee GG, Leiber MM, Jacobsen SJ. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate. 2004;61(2):124–131. doi: 10.1002/pros.20080. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab. 1972;34(4):730–735. doi: 10.1210/jcem-34-4-730. [DOI] [PubMed] [Google Scholar]
- 18.Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1041–1047. [PubMed] [Google Scholar]
- 19.Krieg JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 20.Moretti C, Frajese GV, Guccione L, Wannenes F, De Martino MU, Fabbri A, et al. Androgens and body composition in the aging male. J Endocrinol Invest. 2005;28(3 Suppl):56–64. [PubMed] [Google Scholar]
- 21.Starka L, Pospisilova H, Hill M. Free testosterone and free dihydrotestosterone throughout the life span of men. J Steroid Biochem Mol Biol. 2009;116(1-2):118–120. doi: 10.1016/j.jsbmb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993;77(2):375–381. doi: 10.1210/jcem.77.2.7688377. [DOI] [PubMed] [Google Scholar]
- 23.Farnsworth WE. Roles of estrogen and SHBG in prostate physiology. Prostate. 1996;28(1):17–23. doi: 10.1002/(SICI)1097-0045(199601)28:1<17::AID-PROS3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89(5):2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- 25.Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31(1):1–12. [PubMed] [Google Scholar]
- 26.McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142(6):2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 27.Bain J. Testosterone and the aging male: to treat or not to treat? Maturitas. 2010;66(1):16–22. doi: 10.1016/j.maturitas.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73(3):233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh PB, Matanhelia SS, Martin FL. A potential paradox in prostate adenocarcinoma progression: oestrogen as the initiating driver. Eur J Cancer. 2008;44(7):928–936. doi: 10.1016/j.ejca.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 30.Bosland MC. Use of animal models in defining efficacy of chemoprevention agents against prostate cancer. Eur Urol. 1999;35(5-6):459–463. doi: 10.1159/000019879. [DOI] [PubMed] [Google Scholar]
- 31.Ho SM, Lane K, Lee K. Neoplastic transformation of the prostate. In: Naz Rajesh K., editor. Prostate: Basic and Clinical Aspects. CRC Press; New York: 1997. pp. 74–114. [Google Scholar]
- 32.Ricke WA, Wang Y, Cunha GR. Steroid hormones and carcinogenesis of the prostate: the role of estrogens. Differentiation. 2007;75(9):871–882. doi: 10.1111/j.1432-0436.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 33.Taplin ME, Ho SM. Clinical review 134: The endocrinology of prostate cancer. J Clin Endocrinol Metab. 2001;86(8):3467–3477. doi: 10.1210/jcem.86.8.7782. [DOI] [PubMed] [Google Scholar]
- 34.Adams JY, Leav I, Lau KM, Ho SM, Pflueger SM. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52(1):69–81. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- 35.Ho SM, Leav I, Merk FB, Yu M, Kwan PW, Ziar J. Induction of atypical hyperplasia, apoptosis, and type II estrogen-binding sites in the ventral prostates of Noble rats treated with testosterone and pharmacologic doses of estradiol-17 beta. Lab Invest. 1995;73(3):356–365. [PubMed] [Google Scholar]
- 36.Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst. 1988;80(13):1045–1053. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- 37.Leav I, Merk FB, Kwan PW, Ho SM. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate. 1989;15(1):23–40. doi: 10.1002/pros.2990150104. [DOI] [PubMed] [Google Scholar]
- 38.Yatkin E, Bernoulli J, Talvitie EM, Santti R. Inflammation and epithelial alterations in rat prostate: impact of the androgen to oestrogen ratio. Int J Androl. 2009;32(4):399–410. doi: 10.1111/j.1365-2605.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 39.Hsu A, Bruno RS, Lohr CV, Taylor AW, Dashwood RH, Bray TM, et al. Dietary soy and tea mitigate chronic inflammation and prostate cancer via NFkappaB pathway in the Noble rat model. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, et al. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118(9):2123–2131. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan PJ, Leav I, Greenwood J, Kwan PW, Ho SM. Involvement of transforming growth factor alpha (TGFalpha) and epidermal growth factor receptor (EGFR) in sex hormone-induced prostatic dysplasia and the growth of an androgen-independent transplantable carcinoma of the prostate. Carcinogenesis. 1996;17(12):2571–2579. doi: 10.1093/carcin/17.12.2571. [DOI] [PubMed] [Google Scholar]
- 42.Wong YC, Xie W, Tsao SW. Structural changes and alteration in expression of TGF-beta1 and its receptors in prostatic intraepithelial neoplasia (PIN) in the ventral prostate of noble rats. Prostate. 2000;45(4):289–298. doi: 10.1002/1097-0045(20001201)45:4<289::aid-pros2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-I (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35(3):165–177. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Lau KM, Leav I, Ho SM. Rat estrogen receptor-alpha and -beta, and progesterone receptor mRNA expression in various prostatic lobes and microdissected normal and dysplastic epithelial tissues of the Noble rats. Endocrinology. 1998;139(1):424–427. doi: 10.1210/endo.139.1.5809. [DOI] [PubMed] [Google Scholar]
- 45.Leav I, Merk FB, Lee KF, Loda M, Mandoki M, McNeal JE, et al. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am J Pathol. 1999;154(3):863–870. doi: 10.1016/S0002-9440(10)65333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leav I, Galluzzi CM, Ziar J, Stork PJ, Ho SM, Loda M. Mitogen-activated protein kinase and mitogen-activated kinase phosphatase-1 expression in the Noble rat model of sex hormone-induced prostatic dysplasia and carcinoma. Lab Invest. 1996;75(3):361–370. [PubMed] [Google Scholar]
- 47.Ling MT, Wang X, Ouyang XS, Lee TK, Fan TY, Xu K, et al. Activation of MAPK signaling pathway is essential for Id-1 induced serum independent prostate cancer cell growth. Oncogene. 2002;21(55):8498–8505. doi: 10.1038/sj.onc.1206007. [DOI] [PubMed] [Google Scholar]
- 48.Ghatak S, Oliveria P, Kaplan P, Ho SM. Expression and regulation of metallothionein mRNA levels in the prostates of noble rats: lack of expression in the ventral prostate and regulation by sex hormones in the dorsolateral prostate. Prostate. 1996;29(2):91–100. doi: 10.1002/(SICI)1097-0045(199608)29:2<91::AID-PROS4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 49.Ho SM, Leav I, Ghatak S, Merk F, Jagannathan VS, Mallery K. Lack of association between enhanced TRPM-2/clusterin expression and increased apoptotic activity in sex-hormone-induced prostatic dysplasia of the Noble rat. Am J Pathol. 1998;153(1):131–139. doi: 10.1016/S0002-9440(10)65553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouyang XS, Wang X, Lee DT, Tsao SW, Wong YC. Up-regulation of TRPM-2, MMP-7 and ID-1 during sex hormone-induced prostate carcinogenesis in the Noble rat. Carcinogenesis. 2001;22(6):965–973. doi: 10.1093/carcin/22.6.965. [DOI] [PubMed] [Google Scholar]
- 51.Ghatak S, Ho SM. Age-related changes in the activities of antioxidant enzymes and lipid peroxidation status in ventral and dorsolateral prostate lobes of noble rats. Biochem Biophys Res Commun. 1996;222(2):362–367. doi: 10.1006/bbrc.1996.0749. [DOI] [PubMed] [Google Scholar]
- 52.Ho SM, Roy D. Sex hormone-induced nuclear DNA damage and lipid peroxidation in the dorsolateral prostates of Noble rats. Cancer Lett. 1994;84(2):155–162. doi: 10.1016/0304-3835(94)90370-0. [DOI] [PubMed] [Google Scholar]
- 53.Tam NN, Ghatak S, Ho SM. Sex hormone-induced alterations in the activities of antioxidant enzymes and lipid peroxidation status in the prostate of Noble rats. Prostate. 2003;55(1):1–8. doi: 10.1002/pros.10169. [DOI] [PubMed] [Google Scholar]
- 54.Tam NN, Szeto CY, Sartor MA, Medvedovic M, Ho SM. Gene expression profiling identifies lobe-specific and common disruptions of multiple gene networks in testosterone-supported, 17beta-estradiol- or diethylstilbestrol-induced prostate dysplasia in Noble rats. Neoplasia. 2008;10(1):20–40. doi: 10.1593/neo.07889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM. Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology. 2002;143(6):2093–2105. doi: 10.1210/endo.143.6.8846. [DOI] [PubMed] [Google Scholar]
- 56.Chan FL, Ho SM. Comparative study of glycoconjugates of the rat prostatic lobes by lectin histochemistry. Prostate. 1999;38(1):1–16. doi: 10.1002/(sici)1097-0045(19990101)38:1<1::aid-pros1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Chan FL, Choi HL, Ho SM. Analysis of glycoconjugate patterns of normal and hormone-induced dysplastic Noble rat prostates, and an androgen-independent Noble rat prostate tumor, by lectin histochemistry and protein blotting. Prostate. 2001;46(1):21–32. doi: 10.1002/1097-0045(200101)46:1<21::aid-pros1004>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 58.Li SC, Chen GF, Chan PS, Choi HL, Ho SM, Chan FL. Altered expression of extracellular matrix and proteinases in Noble rat prostate gland after long-term treatment with sex steroids. Prostate. 2001;49(1):58–71. doi: 10.1002/pros.1118. [DOI] [PubMed] [Google Scholar]
- 59.Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate. 2008;68(12):1296–1306. doi: 10.1002/pros.20791. [DOI] [PubMed] [Google Scholar]
- 60.Tam NN, Leav I, Ho SM. Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am J Pathol. 2007;171(4):1334–1341. doi: 10.2353/ajpath.2007.070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yatkin E, Bernoulli J, Lammintausta R, Santti R. Fispemifene [Z-2-{2-[4-(4-chloro-1,2- diphenylbut-1-enyl)-phenoxy]ethoxy}-ethanol], a novel selective estrogen receptor modulator, attenuates glandular inflammation in an animal model of chronic nonbacterial prostatitis. J Pharmacol Exp Ther. 2008;327(1):58–67. doi: 10.1124/jpet.108.139501. [DOI] [PubMed] [Google Scholar]
- 62.Bernoulli J, Yatkin E, Laakso A, Anttinen M, Bosland M, Vega K, et al. Histopathological evidence for an association of inflammation with ductal pin-like lesions but not with ductal adenocarcinoma in the prostate of the noble rat. Prostate. 2008;68(7):728–739. doi: 10.1002/pros.20719. [DOI] [PubMed] [Google Scholar]
- 63.Cavalieri EL, Rogan EG. A unified mechanism in the initiation of cancer. Ann N Y Acad Sci. 2002:959341–354. doi: 10.1111/j.1749-6632.2002.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 64.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;(27):75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 65.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996:36203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 66.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;(27):67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 67.Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr. 2000;(27):95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 68.Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23(2):165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 69.Sato F, Fukuhara H, Basilion JP. Effects of hormone deprivation and 2-methoxyestradiol combination therapy on hormone-dependent prostate cancer in vivo. Neoplasia. 2005;7(9):838–846. doi: 10.1593/neo.05145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reiner T, de Las PA, Gomez LA, Perez-Stable C. Low dose combinations of 2-methoxyestradiol and docetaxel block prostate cancer cells in mitosis and increase apoptosis. Cancer Lett. 2009;276(1):21–31. doi: 10.1016/j.canlet.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh R, Ganapathy M, Alworth WL, Chan DC, Kumar AP. Combination of 2-methoxyestradiol (2-ME2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2009;113(1-2):25–35. doi: 10.1016/j.jsbmb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Nock NL, Cicek MS, Li L, Liu X, Rybicki BA, Moreira A, et al. Polymorphisms in estrogen bioactivation, detoxification and oxidative DNA base excision repair genes and prostate cancer risk. Carcinogenesis. 2006;27(9):1842–1848. doi: 10.1093/carcin/bgl022. [DOI] [PubMed] [Google Scholar]
- 73.Acevedo C, Opazo JL, Huidobro C, Cabezas J, Iturrieta J, Quinones SL. Positive correlation between single or combined genotypes of CYP1A1 and GSTM1 in relation to prostate cancer in Chilean people. Prostate. 2003;57(2):111–117. doi: 10.1002/pros.10274. [DOI] [PubMed] [Google Scholar]
- 74.Caceres DD, Iturrieta J, Acevedo C, Huidobro C, Varela N, Quinones L. Relationship among metabolizing genes, smoking and alcohol used as modifier factors on prostate cancer risk: exploring some gene-gene and gene-environment interactions. Eur J Epidemiol. 2005;20(1):79–88. doi: 10.1007/s10654-004-1632-9. [DOI] [PubMed] [Google Scholar]
- 75.Quinones LA, Irarrazabal CE, Rojas CR, Orellana CE, Acevedo C, Huidobro C, et al. Joint effect among p53, CYP1A1, GSTM1 polymorphism combinations and smoking on prostate cancer risk: an exploratory genotype-environment interaction study. Asian J Androl. 2006;8(3):349–355. doi: 10.1111/j.1745-7262.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 76.Silig Y, Pinarbasi H, Gunes S, Ayan S, Bagci H, Cetinkaya O. Polymorphisms of CYP1A1, GSTM1, GSTT1, and prostate cancer risk in Turkish population. Cancer Invest. 2006;24(1):41–45. doi: 10.1080/07357900500449579. [DOI] [PubMed] [Google Scholar]
- 77.Sobti RC, Onsory K, Al-Badran AI, Kaur P, Watanabe M, Krishan A, et al. CYP17, SRD5A2, CYP1B1, and CYP2D6 gene polymorphisms with prostate cancer risk in North Indian population. DNA Cell Biol. 2006;25(5):287–294. doi: 10.1089/dna.2006.25.287. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki M, Mamun MR, Hara K, Ozeki T, Yamada Y, Kadowaki T, et al. The Val158Met polymorphism of the catechol-O-methyltransferase gene is associated with the PSA-progression-free survival in prostate cancer patients treated with estramustine phosphate. Eur Urol. 2005;48(5):752–759. doi: 10.1016/j.eururo.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka Y, Sasaki M, Shiina H, Tokizane T, Deguchi M, Hirata H, et al. Catechol-O-methyltransferase gene polymorphisms in benign prostatic hyperplasia and sporadic prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(2):238–244. doi: 10.1158/1055-9965.EPI-05-0550. [DOI] [PubMed] [Google Scholar]
- 80.Tang YM, Green BL, Chen GF, Thompson PA, Lang NP, Shinde A, et al. Human CYP1B1 Leu432Val gene polymorphism: ethnic distribution in African-Americans, Caucasians and Chinese; oestradiol hydroxylase activity; and distribution in prostate cancer cases and controls. Pharmacogenetics. 2000;10(9):761–766. doi: 10.1097/00008571-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Leung YK, Lau KM, Mobley J, Jiang Z, Ho SM. Overexpression of cytochrome P450 1A1 and its novel spliced variant in ovarian cancer cells: alternative subcellular enzyme compartmentation may contribute to carcinogenesis. Cancer Res. 2005;65(9):3726–3734. doi: 10.1158/0008-5472.CAN-04-3771. [DOI] [PubMed] [Google Scholar]
- 82.Hiramatsu M, Maehara I, Ozaki M, Harada N, Orikasa S, Sasano H. Aromatase in hyperplasia and carcinoma of the human prostate. Prostate. 1997;31(2):118–124. doi: 10.1002/(sici)1097-0045(19970501)31:2<118::aid-pros7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 83.Ito K, Fukabori Y, Shibata Y, Suzuki K, Mieda M, Gotanda K, et al. Effects of a new steroidal aromatase inhibitor, TZA-2237, and/or chlormadinone acetate on hormone-induced and spontaneous canine benign prostatic hyperplasia. Eur J Endocrinol. 2000;143(4):543–554. doi: 10.1530/eje.0.1430543. [DOI] [PubMed] [Google Scholar]
- 84.Radlmaier A, Eickenberg HU, Fletcher MS, Fourcade RO, Santos JM Reis, van Aubel OG, et al. Estrogen reduction by aromatase inhibition for benign prostatic hyperplasia: results of a double-blind, placebo-controlled, randomized clinical trial using two doses of the aromatase-inhibitor atamestane. Atamestane Study Group. Prostate. 1996;29(4):199–208. doi: 10.1002/(SICI)1097-0045(199610)29:4<199::AID-PROS1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki K, Okazaki H, Ono Y, Kurokawa K, Suzuki T, Onuma E, et al. Effect of dual inhibition of 5-alpha-reductase and aromatase on spontaneously developed canine prostatic hypertrophy. Prostate. 1998;37(2):70–76. doi: 10.1002/(sici)1097-0045(19981001)37:2<70::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 86.Brodie A, Njar V, Macedo LF, Vasaitis TS, Sabnis G. The Coffey Lecture: steroidogenic enzyme inhibitors and hormone dependent cancer. Urol Oncol. 2009;27(1):53–63. doi: 10.1016/j.urolonc.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Djurendic E, Daljev J, Sakac M, Canadi J, Santa SJ, Andric S, et al. Synthesis of some epoxy and/or N-oxy 17-picolyl and 17-picolinylidene-androst-5-ene derivatives and evaluation of their biological activity. Steroids. 2008;73(1):129–138. doi: 10.1016/j.steroids.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Kruit WH, Stoter G, Klijn JG. Effect of combination therapy with aminoglutethimide and hydrocortisone on prostate-specific antigen response in metastatic prostate cancer refractory to standard endocrine therapy. Anticancer Drugs. 2004;15(9):843–847. doi: 10.1097/00001813-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Sanderson T, Renaud M, Scholten D, Nijmeijer S, van den Berg M, Cowell S, et al. Effects of lactone derivatives on aromatase (CYP19) activity in H295R human adrenocortical and (anti)androgenicity in transfected LNCaP human prostate cancer cells. Eur J Pharmacol. 2008;593(1-3):92–98. doi: 10.1016/j.ejphar.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki K, Nakazato H, Matsui H, Koike H, Okugi H, Ohtake N, et al. Association of the genetic polymorphism of the CYP19 intron 4[TTTA]n repeat with familial prostate cancer risk in a Japanese population. Anticancer Res. 2003;23(6D):4941–4946. [PubMed] [Google Scholar]
- 91.Suzuki K, Nakazato H, Matsui H, Koike H, Okugi H, Kashiwagi B, et al. Genetic polymorphisms of estrogen receptor alpha, CYP19, catechol-O-methyltransferase are associated with familial prostate carcinoma risk in a Japanese population. Cancer. 2003;98(7):1411–1416. doi: 10.1002/cncr.11639. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, et al. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142(6):2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- 93.Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Long-term effects. Invest Urol. 1978;16(3):186–190. [PubMed] [Google Scholar]
- 94.Arai Y, Suzuki Y, Nishizuka Y. Hyperplastic and metaplastic lesions in the reproductive tract of male rats induced by neonatal treatment with diethylstilbestrol. Virchows Arch A Pathol Anat Histol. 1977;376(1):21–28. doi: 10.1007/BF00433082. [DOI] [PubMed] [Google Scholar]
- 95.Arai Y, Mori T, Suzuki Y, Bern HA. Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. Int Rev Cytol. 1983:84235–268. doi: 10.1016/s0074-7696(08)61019-0. [DOI] [PubMed] [Google Scholar]
- 96.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130(6):3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 97.Vorherr H, Messer RH, Vorherr UF, Jordan SW, Kornfeld M. Teratogenesis and carcinogenesis in rat offspring after transplacental and transmammary exposure to diethylstilbestrol. Biochem Pharmacol. 1979;28(12):1865–1877. doi: 10.1016/0006-2952(79)90638-5. [DOI] [PubMed] [Google Scholar]
- 98.McLachlan JA, Newbold RR, Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975;190(4218):991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- 99.McLachlan JA. Prenatal exposure to diethylstilbestrol in mice: toxicological studies. J Toxicol Environ Health. 1977;2(3):527–537. doi: 10.1080/15287397709529453. [DOI] [PubMed] [Google Scholar]
- 100.Pylkkanen L, Santti R, Newbold R, McLachlan JA. Regional differences in the prostate of the neonatally estrogenized mouse. Prostate. 1991;18(2):117–129. doi: 10.1002/pros.2990180204. [DOI] [PubMed] [Google Scholar]
- 101.Prins GS, Sklarew RJ, Pertschuk LP. Image analysis of androgen receptor immunostaining in prostate cancer accurately predicts response to hormonal therapy. J Urol. 1998;159(3):641–649. [PubMed] [Google Scholar]
- 102.Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61(16):6089–6097. [PubMed] [Google Scholar]
- 103.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997;94(5):2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ho SM, Tang WY, Belmonte de FJ, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 106.Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, et al. Pregnancy hormone concentrations across ethnic groups: implications for later cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1514–1520. doi: 10.1158/1055-9965.EPI-04-0869. [DOI] [PubMed] [Google Scholar]
- 107.Ekbom A, Hsieh CC, Lipworth L, Wolk A, Ponten J, Adami HO, et al. Perinatal characteristics in relation to incidence of and mortality from prostate cancer. BMJ. 1996;313(7053):337–341. doi: 10.1136/bmj.313.7053.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ekbom A, Wuu J, Adami HO, Lu CM, Lagiou P, Trichopoulos D, et al. Duration of gestation and prostate cancer risk in offspring. Cancer Epidemiol Biomarkers Prev. 2000;9(2):221–223. [PubMed] [Google Scholar]
- 109.Schrager S, Potter BE. Diethylstilbestrol exposure. Am Fam Physician. 2004;69(10):2395–2400. [PubMed] [Google Scholar]
- 110.Chapin RE, Adams J, Boekelheide K, Gray LE, Jr., Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 111.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 112.Kuroda N, Kinoshita Y, Sun Y, Wada M, Kishikawa N, Nakashima K, et al. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J Pharm Biomed Anal. 2003;30(6):1743–1749. doi: 10.1016/s0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 113.Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30(1):2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, et al. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab. 2003;88(3):1333–1340. doi: 10.1210/jc.2002-021015. [DOI] [PubMed] [Google Scholar]
- 116.Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, et al. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16(6):735–739. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 117.Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A. 2010;107(1):448–453. doi: 10.1073/pnas.0907697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prins GS, Tang WY, Belmonte J, Ho SM. Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: epigenetic mode of action is implicated. Fertil Steril. 2008;89(2 Suppl):e41. doi: 10.1016/j.fertnstert.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rubin BS, Soto AM. Bisphenol A: Perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304(1-2):55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Badger TM, Gilchrist JM, Pivik RT, Andres A, Shankar K, Chen JR, et al. The health implications of soy infant formula. Am J Clin Nutr. 2009;89(5):1668S–1672S. doi: 10.3945/ajcn.2009.26736U. [DOI] [PubMed] [Google Scholar]
- 121.Joeckel RJ, Phillips SK. Overview of infant and pediatric formulas. Nutr Clin Pract. 2009;24(3):356–362. doi: 10.1177/0884533609335309. [DOI] [PubMed] [Google Scholar]
- 122.Tuohy PG. Soy infant formula and phytoestrogens. J Paediatr Child Health. 2003;39(6):401–405. doi: 10.1046/j.1440-1754.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 123.Hamilton-Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer. 2008;60(1):7–13. doi: 10.1080/01635580701586770. [DOI] [PubMed] [Google Scholar]
- 124.Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, Muto S, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70(10):1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- 125.Kwan W, Duncan G, Van PC, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. 2010;62(2):198–207. doi: 10.1080/01635580903305318. [DOI] [PubMed] [Google Scholar]
- 126.Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8132 doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 128.Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol. 2007;39(3):183–188. doi: 10.1677/JME-07-0053. [DOI] [PubMed] [Google Scholar]
- 129.Warner M, Gustafsson JA. The role of estrogen receptor beta (ERbeta) in malignant diseases--a new potential target for antiproliferative drugs in prevention and treatment of cancer. Biochem Biophys Res Commun. 2010;396(1):63–66. doi: 10.1016/j.bbrc.2010.02.144. [DOI] [PubMed] [Google Scholar]
- 130.Katzenellenbogen BS, Sun J, Harrington WR, Kraichely DM, Ganessunker D, Katzenellenbogen JA. Structure-function relationships in estrogen receptors and the characterization of novel selective estrogen receptor modulators with unique pharmacological profiles. Ann N Y Acad Sci. 2001:9496–15. doi: 10.1111/j.1749-6632.2001.tb03998.x. [DOI] [PubMed] [Google Scholar]
- 131.Dondi D, Piccolella M, Biserni A, Della TS, Ramachandran B, Locatelli A, et al. Estrogen receptor beta and the progression of prostate cancer: role of 5alpha-androstane-3beta,17beta-diol. Endocr Relat Cancer. 2010;17(3):731–742. doi: 10.1677/ERC-10-0032. [DOI] [PubMed] [Google Scholar]
- 132.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006;103(35):13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, et al. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc Natl Acad Sci U S A. 2010;107(7):3123–3128. doi: 10.1073/pnas.0905524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res. 2001;7(12 Suppl):4338s–4342s. [PubMed] [Google Scholar]
- 135.Tremblay GB, Giguere V. Coregulators of estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12(1):1–22. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- 136.Barone I, Brusco L, Fuqua SA. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res. 2010;16(10):2702–2708. doi: 10.1158/1078-0432.CCR-09-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, et al. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010;17(3):675–689. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003;54(2):79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 139.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61(14):5331–5335. [PubMed] [Google Scholar]
- 140.Latil A, Bieche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, et al. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61(5):1919–1926. [PubMed] [Google Scholar]
- 141.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159(1):79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Royuela M, de Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Arenas MI, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol. 2001;168(3):447–454. doi: 10.1677/joe.0.1680447. [DOI] [PubMed] [Google Scholar]
- 143.Torlakovic E, Lilleby W, Torlakovic G, Fossa SD, Chibbar R. Prostate carcinoma expression of estrogen receptor-beta as detected by PPG5/10 antibody has positive association with primary Gleason grade and Gleason score. Hum Pathol. 2002;33(6):646–651. doi: 10.1053/hupa.2002.124033. [DOI] [PubMed] [Google Scholar]
- 144.Weihua Z, Warner M, Gustafsson JA. Estrogen receptor beta in the prostate. Mol Cell Endocrinol. 2002;193(1-2):1–5. doi: 10.1016/s0303-7207(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 145.Pasquali D, Staibano S, Prezioso D, Franco R, Esposito D, Notaro A, et al. Estrogen receptor beta expression in human prostate tissue. Mol Cell Endocrinol. 2001;178(1-2):47–50. doi: 10.1016/s0303-7207(01)00418-x. [DOI] [PubMed] [Google Scholar]
- 146.Pasquali D, Rossi V, Esposito D, Abbondanza C, Puca GA, Bellastella A, et al. Loss of estrogen receptor beta expression in malignant human prostate cells in primary cultures and in prostate cancer tissues. J Clin Endocrinol Metab. 2001;86(5):2051–2055. doi: 10.1210/jcem.86.5.7441. [DOI] [PubMed] [Google Scholar]
- 147.Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, et al. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164(6):2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26(52):7346–7354. doi: 10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- 149.Lai JS, Brown LG, True LD, Hawley SJ, Etzioni RB, Higano CS, et al. Metastases of prostate cancer express estrogen receptor-beta. Urology. 2004;64(4):814–820. doi: 10.1016/j.urology.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 150.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17(4):319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, et al. Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest. 2009;119(5):1093–1108. doi: 10.1172/JCI35079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leung YK, Lee MT, Wang J, Ho SM. Post-transcriptional regulation of estrogen receptor beta isoforms in prostate cancer. ENDO2010 92th Annual Meeting.2010. [Google Scholar]
- 153.Lee MT, Leung YK, Chung I, Ho SM. Regulation of ERbeta1 transcriptional activity by acetylation. ENDO 2010 92th Annual Meeting.2010. [Google Scholar]
- 154.Mak P, Leung YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia. 2006;8(11):896–904. doi: 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang J, Chang HL, Sugimoto Y, Lin YC. Effects of age on growth and ERbeta mRNA expression of canine prostatic cells. Anticancer Res. 2005;25(6B):4081–4090. [PubMed] [Google Scholar]
- 157.Chang WY, Prins GS. Estrogen receptor-beta: implications for the prostate gland. Prostate. 1999;40(2):115–124. doi: 10.1002/(sici)1097-0045(19990701)40:2<115::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 158.Risbridger GP, Bianco JJ, Ellem SJ, McPherson SJ. Oestrogens and prostate cancer. Endocr Relat Cancer. 2003;10(2):187–191. doi: 10.1677/erc.0.0100187. [DOI] [PubMed] [Google Scholar]
- 159.Haqq C, Li R, Khodabakhsh D, Frolov A, Ginzinger D, Thompson T, et al. Ethnic and racial differences in prostate stromal estrogen receptor alpha. Prostate. 2005;65(2):101–109. doi: 10.1002/pros.20272. [DOI] [PubMed] [Google Scholar]
- 160.Tanaka Y, Sasaki M, Kaneuchi M, Shiina H, Igawa M, Dahiya R. Polymorphisms of estrogen receptor alpha in prostate cancer. Mol Carcinog. 2003;37(4):202–208. doi: 10.1002/mc.10138. [DOI] [PubMed] [Google Scholar]
- 161.Chae YK, Huang HY, Strickland P, Hoffman SC, Helzlsouer K. Genetic polymorphisms of estrogen receptors alpha and beta and the risk of developing prostate cancer. PLoS One. 2009;4(8):e6523. doi: 10.1371/journal.pone.0006523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nicolaiew N, Cancel-Tassin G, Azzouzi AR, Grand BL, Mangin P, Cormier L, et al. Association between estrogen and androgen receptor genes and prostate cancer risk. Eur J Endocrinol. 2009;160(1):101–106. doi: 10.1530/EJE-08-0321. [DOI] [PubMed] [Google Scholar]
- 163.McIntyre MH, Kantoff PW, Stampfer MJ, Mucci LA, Parslow D, Li H, et al. Prostate cancer risk and ESR1 TA, ESR2 CA repeat polymorphisms. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2233–2236. doi: 10.1158/1055-9965.EPI-07-0481. [DOI] [PubMed] [Google Scholar]
- 164.Chen YC, Kraft P, Bretsky P, Ketkar S, Hunter DJ, Albanes D, et al. Sequence variants of estrogen receptor beta and risk of prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2007;16(10):1973–1981. doi: 10.1158/1055-9965.EPI-07-0431. [DOI] [PubMed] [Google Scholar]
- 165.Hedelin M, Balter KA, Chang ET, Bellocco R, Klint A, Johansson JE, et al. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate. 2006;66(14):1512–1520. doi: 10.1002/pros.20487. [DOI] [PubMed] [Google Scholar]
- 166.Thellenberg-Karlsson C, Lindstrom S, Malmer B, Wiklund F, Augustsson-Balter K, Adami HO, et al. Estrogen receptor beta polymorphism is associated with prostate cancer risk. Clin Cancer Res. 2006;12(6):1936–1941. doi: 10.1158/1078-0432.CCR-05-0269. [DOI] [PubMed] [Google Scholar]
- 167.Shapiro E, Huang H, Masch RJ, McFadden DE, Wilson EL, Wu XR. Immunolocalization of estrogen receptor alpha and beta in human fetal prostate. J Urol. 2005;174(5):2051–2053. doi: 10.1097/01.ju.0000176472.90432.5b. [DOI] [PubMed] [Google Scholar]
- 168.Kampa M, Pelekanou V, Castanas E. Membrane-initiated steroid action in breast and prostate cancer. Steroids. 2008;73(9-10):953–960. doi: 10.1016/j.steroids.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 169.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 170.Zhalilo LI, Novominskaia IM. Tissue respiratory activity with a varying potassium ion content in the incubation medium. Fiziol Zh. 1976;22(3):410–412. [PubMed] [Google Scholar]
- 171.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 172.Zhang Z, Maier B, Santen RJ, Song RX. Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activation. Biochem Biophys Res Commun. 2002;294(5):926–933. doi: 10.1016/S0006-291X(02)00348-0. [DOI] [PubMed] [Google Scholar]
- 173.Song RX, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERalpha and Src. Trends Endocrinol Metab. 2005;16(8):347–353. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 174.O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21(6):1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 175.Chen JQ, Russo PA, Cooke C, Russo IH, Russo J. ERbeta shifts from mitochondria to nucleus during estrogen-induced neoplastic transformation of human breast epithelial cells and is involved in estrogen-induced synthesis of mitochondrial respiratory chain proteins. Biochim Biophys Acta. 2007;1773(12):1732–1746. doi: 10.1016/j.bbamcr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 176.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 177.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, et al. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75(8-9):603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 178.Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, et al. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71(4):310–316. doi: 10.1016/j.steroids.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 179.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol. 2010;204(2):105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 180.Park II, Zhang Q, Liu V, Kozlowski JM, Zhang J, Lee C. 17Beta-estradiol at low concentrations acts through distinct pathways in normal versus benign prostatic hyperplasia-derived prostate stromal cells. Endocrinology. 2009;150(10):4594–4605. doi: 10.1210/en.2008-1591. [DOI] [PubMed] [Google Scholar]
- 181.Zhang Z, Duan L, Du X, Ma H, Park I, Lee C, et al. The proliferative effect of estradiol on human prostate stromal cells is mediated through activation of ERK. Prostate. 2008;68(5):508–516. doi: 10.1002/pros.20722. [DOI] [PubMed] [Google Scholar]
- 182.Lam HM, Ouyang B, Ho SM. Activation of the G-coupled Protein Receptor 30 (GPR30) by the non-estrogenic ligand G-1 inhibits prostate cancer cell growth and regulation of its expression via an epigenetic mechanism. ENDO 2009 91st Annual Meeting.2010. [Google Scholar]
- 183.Chan QK, Lam HM, Ng CF, Lee AY, Chan ES, Ng HK, et al. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death Differ. 2010;17(9):1511–1523. doi: 10.1038/cdd.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, et al. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab. 2005;90(3):1830–1844. doi: 10.1210/jc.2004-1421. [DOI] [PubMed] [Google Scholar]
- 185.Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 186.Chen F, Zhang Q, McDonald T, Davidoff MJ, Bailey W, Bai C, et al. Identification of two hERR2-related novel nuclear receptors utilizing bioinformatics and inverse PCR. Gene. 1999;228(1-2):101–109. doi: 10.1016/s0378-1119(98)00619-2. [DOI] [PubMed] [Google Scholar]
- 187.Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14(3):382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- 188.Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14(3):382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- 189.Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274(32):22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- 190.Vanacker JM, Pettersson K, Gustafsson JA, Laudet V. Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. EMBO J. 1999;18(15):4270–4279. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, et al. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol. 1999;13(12):2151–2162. doi: 10.1210/mend.13.12.0381. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Z, Teng CT. Estrogen receptor-related receptor alpha 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem. 2000;275(27):20837–20846. doi: 10.1074/jbc.M001880200. [DOI] [PubMed] [Google Scholar]
- 193.Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, et al. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab. 2005;90(3):1830–1844. doi: 10.1210/jc.2004-1421. [DOI] [PubMed] [Google Scholar]
- 194.Suetsugi M, Su L, Karlsberg K, Yuan YC, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1(13):981–991. [PubMed] [Google Scholar]
- 195.Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci U S A. 2001;98(15):8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tremblay GB, Bergeron D, Giguere V. 4-Hydroxytamoxifen is an isoform-specific inhibitor of orphan estrogen-receptor-related (ERR) nuclear receptors beta and gamma. Endocrinology. 2001;142(10):4572–4575. doi: 10.1210/endo.142.10.8528. [DOI] [PubMed] [Google Scholar]
- 197.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 198.Cox RL, Crawford ED. Estrogens in the treatment of prostate cancer. J Urol. 1995;154(6):1991–1998. [PubMed] [Google Scholar]
- 199.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. 2000;18(1):52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 200.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 201.Bergan RC, Reed E, Myers CE, Headlee D, Brawley O, Cho HK, et al. A Phase II study of high-dose tamoxifen in patients with hormone-refractory prostate cancer. Clin Cancer Res. 1999;5(9):2366–2373. [PubMed] [Google Scholar]
- 202.Chadha MK, Ashraf U, Lawrence D, Tian L, Levine E, Silliman C, et al. Phase II study of fulvestrant (Faslodex) in castration resistant prostate cancer. Prostate. 2008;68(13):1461–1466. doi: 10.1002/pros.20813. [DOI] [PubMed] [Google Scholar]
- 203.Hamilton M, Dahut W, Brawley O, Davis P, Wells-Jones T, Kohler D, et al. A phase I/II study of high-dose tamoxifen in combination with vinblastine in patients with androgen-independent prostate cancer. Acta Oncol. 2003;42(3):195–201. doi: 10.1080/02841860310010718. [DOI] [PubMed] [Google Scholar]
- 204.Lissoni P, Vigano P, Vaghi M, Frontini L, Giuberti C, Manganini V, et al. A phase II study of tamoxifen in hormone-resistant metastatic prostate cancer: possible relation with prolactin secretion. Anticancer Res. 2005;25(5):3597–3599. [PubMed] [Google Scholar]
- 205.Shazer RL, Jain A, Galkin AV, Cinman N, Nguyen KN, Natale RB, et al. Raloxifene, an oestrogen-receptor-beta-targeted therapy, inhibits androgen-independent prostate cancer growth: results from preclinical studies and a pilot phase II clinical trial. BJU Int. 2006;97(4):691–697. doi: 10.1111/j.1464-410X.2006.05974.x. [DOI] [PubMed] [Google Scholar]
- 206.Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184(4):1316–1321. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stein S, Zoltick B, Peacock T, Holroyde C, Haller D, Armstead B, et al. Phase II trial of toremifene in androgen-independent prostate cancer: a Penn cancer clinical trials group trial. Am J Clin Oncol. 2001;24(3):283–285. doi: 10.1097/00000421-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 208.el-Rayes BF, Hussain MH. Hormonal therapy for prostate cancer: past, present and future. Expert Rev Anticancer Ther. 2002;2(1):37–47. doi: 10.1586/14737140.2.1.37. [DOI] [PubMed] [Google Scholar]
- 209.Dahllof B, Billstrom A, Cabral F, Hartley-Asp B. Estramustine depolymerizes microtubules by binding to tubulin. Cancer Res. 1993;53(19):4573–4581. [PubMed] [Google Scholar]
- 210.Fu Y, Hsieh TC, Guo J, Kunicki J, Lee MY, Darzynkiewicz Z, et al. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2004;322(1):263–270. doi: 10.1016/j.bbrc.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 211.Kim HT, Kim BC, Kim IY, Mamura M, Seong DH, Jang JJ, et al. Raloxifene, a mixed estrogen agonist/antagonist, induces apoptosis through cleavage of BAD in TSU-PR1 human cancer cells. J Biol Chem. 2002;277(36):32510–32515. doi: 10.1074/jbc.M202852200. [DOI] [PubMed] [Google Scholar]
- 212.Kumar NB, Cantor A, Allen K, Riccardi D, Besterman-Dahan K, Seigne J, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59(2):141–147. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 213.Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy GP. Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res. 2002;62(9):2488–2492. [PubMed] [Google Scholar]
- 214.LaVallee TM, Zhan XH, Johnson MS, Herbstritt CJ, Swartz G, Williams MS, et al. 2-methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003;63(2):468–475. [PubMed] [Google Scholar]
- 215.Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132(12):3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 216.Liu Y, Kyle E, Lieberman R, Crowell J, Kellof G, Bergan RC. Focal adhesion kinase (FAK) phosphorylation is not required for genistein-induced FAK-beta-1-integrin complex formation. Clin Exp Metastasis. 2000;18(3):203–212. doi: 10.1023/a:1006729106034. [DOI] [PubMed] [Google Scholar]
- 217.Matsukawa Y, Marui N, Sakai T, Satomi Y, Yoshida M, Matsumoto K, et al. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993;53(6):1328–1331. [PubMed] [Google Scholar]
- 218.Misra RR, Hursting SD, Perkins SN, Sathyamoorthy N, Mirsalis JC, Riccio ES, et al. Genotoxicity and carcinogenicity studies of soy isoflavones. Int J Toxicol. 2002;21(4):277–285. doi: 10.1080/10915810290096441. [DOI] [PubMed] [Google Scholar]
- 219.Mor G, Kohen F, Garcia-Velasco J, Nilsen J, Brown W, Song J, et al. Regulation of fas ligand expression in breast cancer cells by estrogen: functional differences between estradiol and tamoxifen. J Steroid Biochem Mol Biol. 2000;73(5):185–194. doi: 10.1016/s0960-0760(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 220.Neubauer BL, Best KL, Counts DF, Goode RL, Hoover DM, Jones CD, et al. Raloxifene (LY156758) produces antimetastatic responses and extends survival in the PAIII rat prostatic adenocarcinoma model. Prostate. 1995;27(4):220–229. doi: 10.1002/pros.2990270407. [DOI] [PubMed] [Google Scholar]
- 221.Qadan LR, Perez-Stable CM, Anderson C, D’Ippolito G, Herron A, Howard GA, et al. 2-Methoxyestradiol induces G2/M arrest and apoptosis in prostate cancer. Biochem Biophys Res Commun. 2001;285(5):1259–1266. doi: 10.1006/bbrc.2001.5320. [DOI] [PubMed] [Google Scholar]
- 222.Rafi MM, Rosen RT, Vassil A, Ho CT, Zhang H, Ghai G, et al. Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid. Anticancer Res. 2000;20(4):2653–2658. [PubMed] [Google Scholar]
- 223.Schulz P, Bauer HW, Brade WP, Keller A, Fittler F. Evaluation of the cytotoxic activity of diethylstilbestrol and its mono- and diphosphate towards prostatic carcinoma cells. Cancer Res. 1988;48(10):2867–2870. [PubMed] [Google Scholar]
- 224.Shimada K, Nakamura M, Ishida E, Kishi M, Konishi N. Requirement of c-jun for testosterone-induced sensitization to N-(4-hydroxyphenyl)retinamide-induced apoptosis. Mol Carcinog. 2003;36(3):115–122. doi: 10.1002/mc.10107. [DOI] [PubMed] [Google Scholar]
- 225.Yo YT, Shieh GS, Hsu KF, Wu CL, Shiau AL. Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J Agric Food Chem. 2009;57(18):8266–8273. doi: 10.1021/jf901054c. [DOI] [PubMed] [Google Scholar]
- 226.Kumar AP, Garcia GE, Slaga TJ. 2-methoxyestradiol blocks cell-cycle progression at G(2)/M phase and inhibits growth of human prostate cancer cells. Mol Carcinog. 2001;31(3):111–124. doi: 10.1002/mc.1046. [DOI] [PubMed] [Google Scholar]
- 227.Bland LB, Garzotto M, DeLoughery TG, Ryan CW, Schuff KG, Wersinger EM, et al. Phase II study of transdermal estradiol in androgen-independent prostate carcinoma. Cancer. 2005;103(4):717–723. doi: 10.1002/cncr.20857. [DOI] [PubMed] [Google Scholar]
- 228.Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, et al. Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer. BJU Int. 2008;102(4):442–445. doi: 10.1111/j.1464-410X.2008.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD. Transdermal estradiol therapy for advanced prostate cancer--forward to the past? J Urol. 2003;169(5):1735–1737. doi: 10.1097/01.ju.0000061024.75334.40. [DOI] [PubMed] [Google Scholar]
- 230.Ockrim JL, Lalani e, Kakkar AK, Abel PD. Transdermal estradiol therapy for prostate cancer reduces thrombophilic activation and protects against thromboembolism. J Urol. 2005;174(2):527–533. doi: 10.1097/01.ju.0000165567.99142.1f. [DOI] [PubMed] [Google Scholar]
- 231.Gerber GS, Zagaja GP, Ray PS, Rukstalis DB. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology. 2000;55(1):97–101. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]
- 232.Ockrim JL, Lalani EN, Banks LM, Svensson WE, Blomley MJ, Patel S, et al. Transdermal estradiol improves bone density when used as single agent therapy for prostate cancer. J Urol. 2004;172(6 Pt 1):2203–2207. doi: 10.1097/01.ju.0000145511.56476.00. [DOI] [PubMed] [Google Scholar]
- 233.Taneja SS, Smith MR, Dalton JT, Raghow S, Barnette G, Steiner M, et al. Toremifene--a promising therapy for the prevention of prostate cancer and complications of androgen deprivation therapy. Expert Opin Investig Drugs. 2006;15(3):293–305. doi: 10.1517/13543784.15.3.293. [DOI] [PubMed] [Google Scholar]
- 234.Denis L, Morton MS, Griffiths K. Diet and its preventive role in prostatic disease. Eur Urol. 1999;35(5-6):377–387. doi: 10.1159/000019912. [DOI] [PubMed] [Google Scholar]
- 235.Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176(3):965–970. doi: 10.1016/j.juro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 236.Jian L. Soy, isoflavones, and prostate cancer. Mol Nutr Food Res. 2009;53(2):217–226. doi: 10.1002/mnfr.200800167. [DOI] [PubMed] [Google Scholar]
- 237.Lampe JW. Emerging research on equol and cancer. J Nutr. 2010;140(7):1369S–1372S. doi: 10.3945/jn.109.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45(5):399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 239.Milligan SR, Balasubramanian AV, Kalita JC. Relative potency of xenobiotic estrogens in an acute in vivo mammalian assay. Environ Health Perspect. 1998;106(1):23–26. doi: 10.1289/ehp.9810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sonn GA, Aronson W, Litwin MS. Impact of diet on prostate cancer: a review. Prostate Cancer Prostatic Dis. 2005;8(4):304–310. doi: 10.1038/sj.pcan.4500825. [DOI] [PubMed] [Google Scholar]
- 241.Ganry O. Phytoestrogens and prostate cancer risk. Prev Med. 2005;41(1):1–6. doi: 10.1016/j.ypmed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 242.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90(21):1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 243.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47(2):111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 244.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12(7):665–668. [PubMed] [Google Scholar]
- 245.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci. 2004;95(1):65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stattin P, Adlercreutz H, Tenkanen L, Jellum E, Lumme S, Hallmans G, et al. Circulating enterolactone and prostate cancer risk: a Nordic nested case-control study. Int J Cancer. 2002;99(1):124–129. doi: 10.1002/ijc.10313. [DOI] [PubMed] [Google Scholar]
- 247.Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, et al. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer. 1999;33(1):20–25. doi: 10.1080/01635589909514743. [DOI] [PubMed] [Google Scholar]
- 248.Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009;100(11):1817–1823. doi: 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dalais FS, Meliala A, Wattanapenpaiboon N, Frydenberg M, Suter DA, Thomson WK, et al. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64(3):510–515. doi: 10.1016/j.urology.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 250.Jarred RA, Keikha M, Dowling C, McPherson SJ, Clare AM, Husband AJ, et al. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1689–1696. [PubMed] [Google Scholar]
- 251.Miltyk W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, et al. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77(4):875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 252.Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, et al. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1213–1221. [PubMed] [Google Scholar]