Abstract
Objective
Pediatric obsessive-compulsive disorder is characterized by abnormalities of frontal-striatalthalamic circuitry that appear near illness onset and persist over its course. Distinct frontal-striatal-thalamic loops through cortical centers for cognitive control (anterior cingulate cortex) and emotion processing (ventral medial frontal cortex) follow unique maturational trajectories, and altered connectivity within distinct loops may be differentially associated with OCD at specific stages of development.
Method
Altered development of striatal and thalamic connectivity to medial frontal cortex was tested in 60 OCD patients compared to 61 healthy controls at child, adolescent and adult stages of development, using resting state functional connectivity MRI.
Results
OCD in the youngest patients was associated with reduced connectivity of dorsal striatum and medial dorsal thalamus to rostral and dorsal anterior cingulate cortex, respectively. Increased connectivity of dorsal striatum to ventral medial frontal cortex was observed in patients at all developmental stages. In child patients, reduced connectivity between dorsal striatum and rostral anterior cingulate cortex correlated with OCD severity.
Conclusions
Frontal-striatal-thalamic loops involved in cognitive control are hypoconnected in young patients near illness onset, while loops implicated in emotion-processing are hyperconnected throughout the illness.
Keywords: obsessive compulsive disorder, frontal-striatal-thalamic, medial frontal cortex, resting state connectivity, development
Introduction
Obsessive compulsive disorder (OCD) commonly emerges during childhood or adolescence, leading investigators to suggest that altered neurodevelopment1,2 may contribute to the frontal-striatal-thalamic circuitry (FSTC) abnormalities associated with illness3. Indeed, neuroimaging research in pediatric OCD shows frontal-striatal-thalamic involvement from the earliest stages of the disorder4. In adult OCD, positron emission tomography studies show hyperactivity in the caudate, medial dorsal thalamus and medial frontal cortex (MFC) occurs at rest and increases with symptom provocation, leading investigators to posit theoretical models of excessive signaling through frontal-striatal-thalamic circuitry3. A recent resting state functional connectivity study using magnetic resonance imaging found increased connectivity of ventral caudate to MFC in adult patients, consistent with exaggerated synaptic connections between these regions5. Given the childhood origin of FSTC involvement in OCD, mapping the development of striatal and thalamic connectivity to MFC may help to elucidate the brain basis of OCD across the lifespan.
The FSTC system is comprised of parallel, segregated “loops” between distinct portions of the cortex, striatum and thalamus6. Loops of functional relevance for OCD include those passing through dorsal and ventral striatum into the medial dorsal thalamus3 via topographically organized projections from medial frontal cortical centers for cognitive control (e.g., anterior cingulate cortex, ACC)7 and for emotionally driven evaluative functions, including reward-processing and internal mood states (e.g., ventral medial frontal cortex, vMFC)8. These distinct loops are hypothesized to interact at the level of the striatum to tailor goal-directed behaviors in response to internal and external stimuli across cognitive and affective domains8. In OCD, enlarged volume and hyperactive function of the ACC associate with deficits of cognitive control2,3,9, while vMFC alterations – especially hyperactivity in the orbitofrontal cortex – has been linked to excessive valuation of consequences from actions3. These lines of evidence suggest that abnormalities of ACC- and vMFC- loops within FSTC may associate with impaired capacity to flexibly adjust behavior in OCD.
Dramatic development of FSTC occurs in typically developing youth, with somewhat different trajectories in different loops. Earlier development of emotion-processing areas (e.g., vMFC) within this circuitry may interact with more gradual, protracted development of cognitive control centers (e.g., ACC) to support age-related improvements in behavioral flexibility10. For instance, FSTC maturation supports the engagement of cognitive control in response to potential rewards10 and the processing of errors to improve performance on cognitive tasks11. These functional changes are paralleled by age-related decreases in subcortical connectivity to areas of cortex, including the ACC and vMFC12, that could reflect synaptic pruning to support information flow in FSTC12. How developmental decreases in connectivity relate to the maturation of function in specific frontostriatal-thalamic loops remains to be determined. Yet, the unique developmental trajectories of OCD-relevant processes for cognitive control in the ACC and emotional responding in the vMFC10 raise the possibility of loop-specific alterations in the development of FSTC connectivity in OCD.
Thus, we sought to determine if altered resting state connectivity between specific FSTC nodes distinguishes stages of development in OCD using resting state functional connectivity MRI. This technique measures correlations of low-frequency blood oxygen level-dependent signal fluctuations between brain regions, and is thought to reflect functional connections that evolve over the course of development12,13. The normally protracted development of immature ACC-based FSTC for cognitive control may set the stage for the release of OCD thoughts and behaviors in at risk youth, leading us to hypothesize alteration of subcortical connectivity to ACC early in the course of OCD. The earlier maturation of FSTC for affective processing in typical youth10, evidence for hyperconnectivity in this loop in adults with OCD5, and the emotional distress associated with symptoms across the lifespan led us to predict increased subcortical connectivity to the vMFC across the stages of development.
Method
Participants
Sixty-seven outpatients with OCD and 68 healthy subjects, ages 8 to 50 years, were interviewed with the Kiddie-Schedule for Affective Disorders-Present and Lifetime Version (youth)14 or the Structured Clinical Interview for DSM Disorders (adults)15. For patients, OCD symptom severity was assessed using the Yale-Brown Obsessive Compulsive Scale, child16 or adult17 versions, as appropriate. Subjects with serious medical/neurological illness, head trauma, and mental retardation were excluded. Healthy controls had no current or past history of psychiatric disorder. Among patients, attention deficit hyperactivity, autistic, psychotic or bipolar disorders were excluded, but comorbid tic, anxiety and depressive disorders - commonly comorbid with OCD - were allowed, provided that OCD was the primary cause of distress and interference. After complete description of the study to the subjects and their parents, written informed consent/assent was obtained.
Of the original sample, 14 patients were excluded: 2 adults due to technical difficulties with image collection (1 OCD, 1 healthy) and 12 youth due to excessive movement (6 OCD, 6 healthy). The remaining 60 patients and 61 healthy control subjects were categorized as children (8 to 12 years), adolescents (13 to 17 years), younger adults (18 to 25 years) or older adults (26 to 40 years), Table 1. Twelve years of age generally marks a transition to pubescence, and is the inflection point at which age-related increases in MFC cortical thickness plateau, and then begin to decline18. Ages 18 – 25 years were chosen to define the young adult group because age-related increases in myelination begin to slow during this time frame, and then decrease19. There were no significant differences in age or gender for any subgroup. However, significantly more adolescent patients were medicated (χ2=13.3, p = .004), and experiencing only subclinical OCD symptoms (YBOCS < 15; χ2=13.2, p = .004) compared to patients in other age groups.
Table 1.
Subject Characteristics
| Controls | n | M:F | Age |
|---|---|---|---|
| Children | 13 | 6:7 | 10.7 (1.7) |
| Adolescents | 16 | 8:8 | 15.3 (1.3) |
| Young Adults | 15 | 7:8 | 21.0 (2.3) |
| Older Adults | 17 | 7:10 | 32.3 (5.9) |
| Patients | Pediatric Onset | Illness Duration | Medsa | (C)YBOCSa | Comorbidities | |||
|---|---|---|---|---|---|---|---|---|
| Children | 11 | 6:5 | 11.0 (1.3) | All | 2.4 (2.0) | SSRI: 3 | 21 (6) | Anxiety D/O: 2 |
| None: 8 | Depressive D/O: 2 | |||||||
| Tic D/O: 3 | ||||||||
| None: 4 | ||||||||
| Adolescents | 18 | 5:13 | 16.0 (1.4) | All | 5.3 (2.8) | SSRI: 10 | 15 (6) | Anxiety D/O: 6 |
| SSRI/atyp: 2 | Depressive D/O: 7 | |||||||
| α-agonist: 1 | Tic D/O: 1 | |||||||
| None: 6 | None: 5 | |||||||
| Young Adults | 18 | 10:8 | 20.0 (1.4) | 89% (16 of 18) | 9.2 (5.1) | SSRI: 3 | 21 (4) | Anxiety D/O: 5 |
| SNRI: 1 | Depressive D/O: 8 | |||||||
| None: 14 | Eating D/O: 3 | |||||||
| Tic D/O: 1 | ||||||||
| Trichotillomania: 1 | ||||||||
| None: 3 | ||||||||
| Older Adults | 13 | 6:7 | 32.0 (6.0) | 70% (9 of 13) | 18 (9.9) | SSRI: 8 | 23 (6) | Anxiety: 4 |
| Buprop: 1 | Depressive D/O: 12 | |||||||
| None: 4 | Eating D/O: 1 |
Note: Mean (standard deviation) reported for continuous measures. Comorbid psychiatric disorders (D/O) included Tic Not Otherwise Specified (NOS), Chronic Motor Tics, and Tourette's Syndrome; Depression NOS, Dysthymia and Major Depression; Separation Anxiety, Generalized Anxiety, Social Phobia, Specific Phobia and Panic; Eating Disorder NOS and Anorexia Nervosa. atyp = atypical antipsychotic; Buprop = bupropion; (C)YBOCS = (Children's) Yale-Brown Obsessive Compulsive Scale; SNRI = serotonin – norepinephrine reuptake inhibitor; SSRI = Selective serotonin reuptake inhibitor.
More adolescent patients were medicated and had subclinical obsessive compulsive disorder (OCD) symptomatology than patients in other age groups, (p < .005).
Image Acquisition
A 3.0 T GE Signa scanner (General Electric, Milwaukee, Wisconsin) was used to acquire an axial T1-image for alignment; a reverse spiral sequence20 for T2* weighted images (repetition time 2000ms, echo time 30ms, FA = 90, field of view 20 cm, 40 slices, 3.0mm/slice, 64×64 matrix); and, a high resolution T1 scan (spoiled gradient recall, 1.5 mm slices, 0 skip) for anatomic normalization. Time-series data (T2*) were collected over 8 minutes, for a total of 240 volumes. Head movement was minimized through instructions to the participant and packing with foam padding. Subjects were instructed to keep eyes open and fixate on a white crosshair on a black background while “allowing the mind to wander”.
Image Preprocessing
To remove non-neural signal fluctuations, cardiac and respiratory cycles (recorded during the scan) were regressed out of the time-series image-domain data at the subject level in native image space21. Functional data were sinc-interpolated, slice-time corrected, and realigned to the tenth image acquired (“mcflirt”22). Realignment parameters were used to identify excessive movement: up to 3mm, excepting 5 subjects with 3.27 to 3.38 mm (2 healthy: 1 adolescent, 1 older adult; 3 OCD: 2 children and 1 younger adult), since this movement occurred in isolated spikes, was minimally in excess of 3 mm, and there were no group differences in movement at any age. Realignment parameters, mean time-series from the whole brain (global signal), white matter, and cerebral spinal fluid (CSF) were regressed from the data to reduce residual noise effects. To create white-matter and CSF regressors, the high-resolution image for each subject was segmented into grey, white, and CSF using Statistical Parametric Mapping 2 (SPM2), with segments thresholded (> 0.85) and eroded using an image spatial-autocorrelation program to produce subject-specific white-matter and CSF binary masks23. The average time-series in each mask was extracted and regressed from the time-series data. Finally, the time-series data were band-pass filtered (0.01 – 0.10 Hz). To place individual data into a common anatomic reference space for analysis across subjects, functional volumes were warped to the MNI152 template (2mm voxel size). After warping, time series images were spatially smoothed with a 5 mm Gaussian kernel.
Functional Connectivity Analyses
Striatal and thalamic seeds were centered at the ventral striatum, near the nucleus accumbens (x = +/−9, y = 9, z = −8); the dorsal striatum, in the area typically referred to as the head of the caudate (x = +/−13, y = 15, z = 9); and, the medial dorsal thalamus (x = +/− 7.5, y = −13.5, z = 7.5, Figure 1), based on atlas-defined locations shown to exhibit specific patterns of connectivity with MFC5,23.
Figure 1.
Striatal and thalamic seed placement (column 1) are shown next to functional connectivity maps for healthy control (HC, column 2) and obsessive compulsive disorder (OCD, column 3) subjects for each seed: ventral striatum (A), dorsal striatum (B) and medial thalamus (C). Note: Displayed in standard neuroanatomical space (Montreal Neurological Institute) at a threshold of pFDR < .05, whole brain corrected.
Mean time courses were averaged within 5mm radius spheres centered on these coordinates, and correlated with all other voxels of the brain to yield 3-dimensional correlation coefficient images (r images) for each seed. These r images were transformed to z scores using a Fisher r-to-z transformation. The resulting z images were included in two separate 1-way ANOVAS to confirm connectivity of each seed with MFC for each diagnostic group (OCD and healthy). Differences between groups and group × age interactions were tested using 2 (group: OCD, healthy) 4 (age: child, adolescent, younger and older adults) factorial models for each seed. Given our a priori interest in subcortical connectivity with MFC, results were initially evaluated at a threshold of p = 0.005, uncorrected. MFC clusters showing group or group × age effects were tested for significance at p < 0.05, correcting for false discovery rate (FDR)24 within seed-specific MFC search volumes (see Figure S1, available online). Search volumes were defined by combining a midline frontal search region (x = −18 to +19, y = 0 to 70, z = −30 to 50)7 with positive connectivity maps for each seed, thresholded at pFDR < 0.05, corrected for whole-brain comparisons. AlphaSim25 was used to conduct 5,000 Monte Carlo simulations for each seed-specific MFC volume, assuming peak threshold of punc < .005 and smoothness of 10 mm, to determine the number of voxels required per MFC cluster for statistical significance: 29 for left ventral caudate, 20 for right ventral caudate, 35 for left dorsal caudate, 37 for right dorsal caudate, 26 for left medial dorsal thalamus, and 27 for right medial dorsal thalamus. Connectivity measures from significant MFC clusters were extracted and contrasted for OCD and HC at each developmental stage using SPSS (p < 0.05, 2-tailed, corrected). Finally, whole-brain analyses were explored for each seed, using pFDR < 0.05 to correct for multiple comparisons across the brain (see Supplement 1 and Table S1, available online). All analyses were performed in SPM5.
Results
Functional connectivity patterns for subcortical seed regions showed topographic distinctions as seen in previous work5,23 in both OCD and healthy groups (Figure 1). Seed-specific connectivity patterns were also observed for HC and OCD groups by child, adolescent, and adult stages of development (see Figures S2, S3 and S4, available online). Between group differences and group × age interactions are described below.
Reduced Connectivity in OCD
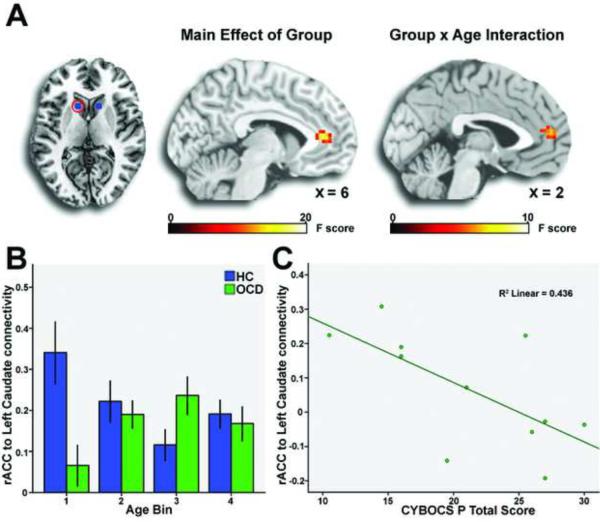
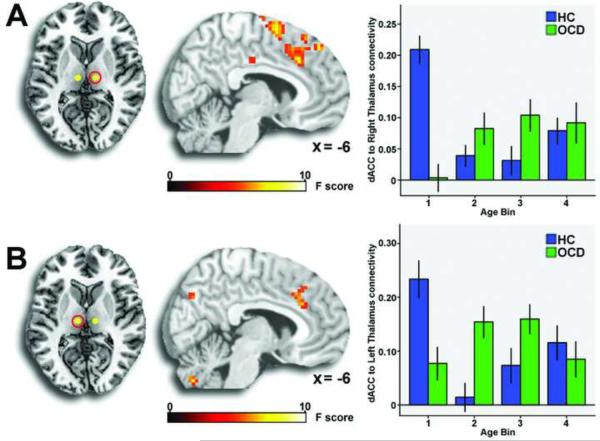
Patients exhibited significantly less connectivity of left dorsal striatum with rostral ACC (Figure 2a, Table 2). A significant group × age interaction was observed for left dorsal striatum connectivity with a nearby region of rostral ACC (Figure 2a, Table 2), driven by less connectivity in the youngest OCD patients compared to similarly aged healthy subjects (Figure 2b). For the right medial dorsal thalamus, a significant group × age interaction was observed on connectivity with the bilateral dorsal ACC, again driven by reduced connectivity in the youngest patients (Figure 3a, Table 2). For the left medial dorsal thalamus, a group × age interaction was observed on connectivity with left dorsal ACC (Figure 3b, Table 2), driven by reduced connectivity in child patients and increased connectivity in adolescent patients compared to healthy controls. There were no main effects of group or group × age interactions for connectivity with left or right ventral striatal seeds.
Figure 2.
A group effect for left dorsal striatum (A, column 1) connectivity with rostral anterior cingulate cortex (rACC; A, column 2) was driven by reduced connectivity in obsessive compulsive disorder patients (OCD) compared to healthy controls (HC). Note: A group × age interaction was also observed for left dorsal striatal connectivity to nearby region of the rostral anterior cingulate cortex (A, column 3), driven by reduced connectivity in the youngest patients to matched controls (B). Left dorsal striatal connectivity to rostral anterior cingulate cortex inversely correlated with symptom severity in child patients (C). Group differences and group × age interactions displayed in standard neuroanatomical space (Montreal Neurological Institute) at a threshold of p < .005, uncorrected. CYBOCS P: Children's Yale-Brown Obsessive Compulsive Scale, Present score.
Table 2.
Group effects and group × age interactions
| Seed |
Connectivity |
Group Difference |
Group × Age Interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Directiond | x, y, z | Z | ke | Directiond | x, y, z | z | ke | ||
| L | rACC | HC > ODC | 6, 39, 6 | 3.82 | 68a | HC1 > OCD1 | −6, 51, 12 | 3.47 | 45 |
| DC | HC2,3,4 = OCD2, 3, 4 | ||||||||
| R | Medial frontal pole | OCD > HC | 15, 69, 9 | 3.47 | 43b | ||||
| DC | |||||||||
| L | L dACC | ------ | ------ | ------ | ------ | HC1 > OCD1 | −9, 27, 27 | 3.42 | 39c |
| MT | OCD2 > HC2 | ||||||||
| OCD3,4 = HC3,4 | |||||||||
| R | Bilateral dACC | ------ | ------ | ------ | ---- | HC1 > OCD1 | −6, 24, 33 | 3.76 | 194 |
| MT | HC2, 3, 4 = OCD2, 3, 4 | 9, 15, 36 | 3.73 | ||||||
Note: dACC = dorsal anterior cingulate cortex, DC = dorsal caudate, HC = healthy control, L = left, R = right, MT = medial-dorsal thalamus, OCD = obsessive compulsive disorder, rACC = rostral anterior cingulate cortex,.
Exclusion of medicated patients in post-hoc analysis revealed a trend towards group × age interaction (HC1 > OCD1; HC2,3,4 = OCD2, 3, 4), but no main effect of group.
Twenty-six of the 43 voxels in this cluster extended laterally from the medial frontal cortext (MFC) search volume.
Exclusion of patients with subclinical obsessive compulsive disorder (OCD) symptoms revealed reduced connectivity in child patients, but no difference between adolescent patients and controls.
Planned contrasts at each developmental stage (1 = Child, 2 = Adolescent, 3 = Young Adult, 4 = Older Adult; p < .05, corrected).
Cluster-level significant at p < .05, corrected for multiple comparisons within medial frontal cortex search volumes.
Figure 3.
For the right medial thalamus (A, column 1), a group × age interaction on connectivity with dorsal anterior cingulate cortex (dACC; A, column 2) was driven by reduced connectivity in the child obsessive compulsive disorder patients (OCD) compared to child healthy controls (HC; A, column 3). Note: For the left medial thalamus (B, column 1) a group × age interaction on connectivity with left dorsal anterior cingulate cortex (B, column 2) was driven by reduced connectivity in child patients, but increased connectivity in adolescent patients compared to matched healthy controls (B, column 3). Group × age interactions displayed in standard neuroanatomical space (Montreal Neurological Institute) at a threshold of p < .005, uncorrected.
Increased Connectivity in OCD
There was a significant effect of group on right dorsal striatum connectivity with medial frontal pole, driven by greater connectivity in OCD patients than healthy subjects (Figure 4, Table 2). Of note, 26 of the 43 voxels in this cluster extended laterally from the MFC search volume. There were no other effects of group, and no group × age interactions driven by increased connectivity in OCD.
Figure 4.
A group effect for right dorsal striatum connectivity to medial frontal pole was driven by increased connectivity for obsessive compulsive disorder patients (OCD) compared to healthy controls (HC). Note: Group differences and group × age interactions displayed in standard neuroanatomical space (Montreal Neurological Institute) at a threshold of p < .005, uncorrected.
Post-hoc analyses
A series of post-hoc ANOVAs tested whether findings from the main analysis remained significant after excluding OCD patients with subclinical symptoms (YBOCs < 15) or taking medication. In general, findings from the primary analysis survived post-hoc testing, including 1) the group effect for right dorsal striatum – ventral MFC hyperconnectivity in patients across all age groups, and 2) the group × age interactions for left dorsal striatum – rostral ACC and right medial dorsal thalamus – dorsal ACC connectivity showing reduced connectivity in child patients.
Post-hoc testing altered primary findings in two instances which, in both cases, reinforced the developmental pattern of reduced subcortical-ACC connectivity in children with OCD. While the primary analysis showed a complex pattern of reduced (children) and increased (adolescent) connectivity of left medial dorsal thalamus with left dorsal ACC in patients compared to controls, only reduced connectivity in child patients remained significant after excluding patients with subclinical symptoms. In addition, while the primary analysis showed increased left dorsal striatum connectivity with the rostral ACC (6, 39, 4) in patients across all age groups, the exclusion of medicated patients revealed a trend-level group × age interaction (F = 2.5, p = .06) instead, and in the same pattern as the group × age interaction for the left DS connectivity to the neighboring rostral ACC cluster (−6, 51, 12; reduced in child patients) from the primary analysis.
Association of Connectivity with OCD Symptom Severity
Greater OCD symptom severity was associated with reduced left dorsal striatum-rostral ACC connectivity in child patients (r = .66, p = .03; Figure 2c). There were no other significant associations between symptom severity and connectivity measures for any age group, or for all OCD patients collapsed across age groups.
Discussion
We studied resting state functional connectivity of striatum and thalamus with medial frontal cortex in OCD patients and healthy controls at successive stages of development to test for differences in frontal-striatal-thalamic circuit (FSTC) maturation. The youngest patients exhibited reduced connectivity of subcortical regions with anterior cingulate (ACC) – i.e., dorsal striatum and medial-dorsal thalamus with rostral and dorsal ACC, respectively. In contrast, patients at all stages of development exhibited excessive connectivity of dorsal striatum with the medial frontal pole region of the ventral MFC. These findings suggest that differential patterns of maturation occur within specific FSTC loops over the course of development in patients with OCD.
The maturation of ACC-based FSTC plays a critical role in the development of cognitive control11. The dorsal ACC detects conflict between competing response options7, while the rostral ACC has been shown to activate to errors26 and conflict between emotionally salient stimuli27. Alteration of ACC recruitment by these functions has been repeatedly demonstrated in neuroimaging studies of OCD3, including in child patients9. In healthy youth, subcortical connectivity with ACC decreases from childhood into adolescence12 – a pattern that we also observed and which others have suggested may reflect synaptic pruning to promote information flow through FSTC12. The exact relationship of developmental decreases in ACC-based FSTC connectivity to the normative maturation of cognitive control remains to be determined, however, the earlier pattern of decreasing connectivity in this circuit in OCD may be of relevance to the onset and early course of illness. Given the role that developing ACC-based FSTC for cognitive control plays in capacity to suppress prepotent response sets11, it is possible that premature reduction in its connectivity may contribute to inability to suppress the contextually inappropriate “security concerns” (e.g., contamination/washing, aggression/checking, symmetry/ordering) that occur even in healthy youth1, but are more frequent, distressing and difficult to control in children with OCD. Consistent with this notion, reduced striatal – rostral ACC connectivity was associated with greater symptom severity among the youngest patients in our sample.
Hypoconnectivity of subcortical nodes with dorsal and rostral ACC was not observed past the earliest stage of development, suggesting that reduced connectivity in ACC-based FSTC may represent a developmentally specific pattern exhibited only by young patients within a certain critical period. This interpretation may seem at odds with evidence for altered ACC function in OCD across the age span, including during tasks requiring cognitive control3,9. However, the development of ACC cognitive control function depends not only on the maturation of its connections within FSTC, but also on its role within other brain networks (e.g., cingulopercular network for task control13) implicated in both pediatric9 and adult28 OCD. Additional research combining MR methodologies will be needed to elucidate the relationships between developing ACC connectivity throughout the brain and ACC-based abnormalities of cognitive control in OCD across the lifespan.
It is important to note that our finding of reduced subcortical – ACC connectivity in child, but not adolescent or adult patients, may have been influenced by other factors. For instance, even though the majority of adult patients in our sample reported pediatric onset of OCD, it is still possible that a biologically distinct form of illness in the youngest patients could have influenced our findings. Earlier onset illness may define a unique subtype of OCD associated with higher rates of comorbid tic disorders, male predominance, increased familiality, and particular genetic polymorphisms29. In addition, pediatric onset OCD may remit in up to 40% of cases30, meaning that our youngest group could have included patients with a unique, less persistent form of illness, which might have contributed to the developmental differences in connectivity that we observed.
Our finding of reduced connectivity of dorsal striatum with rostral ACC and medial dorsal thalamus with dorsal ACC in children with OCD stands in partial contrast to recent work showing increased ventral striatum connectivity to the ACC in adult patients5. This apparent discrepancy may stem from different methodologies, since our study was designed to test for interactions between group and developmental stage in OCD patients compared to controls over a wide age range, while prior work tested only for group differences in adults. If decreased subcortical-MFC connectivity specifically characterizes children with OCD, then prior work limited to adult samples could not have detected it. In addition, unique connectivity patterns characterize anatomically distinct elements of FSTC (e.g., medial thalamus, dorsal and ventral striatum31,32), such that the decreased connectivity (dorsal striatum and medial thalamus to ACC) observed in our study may be compatible with increased connectivity (ventral striatum-ACC) observed in prior work5. Alternatively, Type II error may have contributed to our failure to show a pattern of increased dorsal striatum or medial thalamus connectivity with ACC in adult OCD although, theoretically, the larger number of adult patients included in our sample should reduce this possibility.
Type II error seems a more likely explanation for our failure to show increased ventral striatum connectivity with vMFC, since two prior studies have shown hyperconnectivity between these regions in adults with OCD5,33. We observed a sub-threshold increase of ventral striatum connectivity with vMFC (x = 9, y = 69, z = 9; Z = 3.47, k =9) in OCD compared to healthy control subjects that, in post-hoc testing, appeared to be driven by adult patients, raising the possibility that increased ventral striatum – vMFC connectivity may be developmentally specific for adult OCD. To test this possibility, we compared adult patients and controls from our sample, finding a larger, but still sub-threshold increase in connectivity of the ventral striatum with vMFC (x = 9, y = 69, z = 3; Z = 3.83, k = 24). If ventral striatum – vMFC hyperconnectivity in OCD is unique to older patients, then the relatively younger age of the adult patients in our sample (25 +/− 7 years) compared to those in previous work (29 +/− 6 years5; 31 +/− 9 years33) may have reduced our power to detect this effect.
A significant increase in dorsal striatum connectivity with medial frontal pole was observed across child, adolescent and adult stages of development in patients compared to controls, partially replicating prior work showing excessive ventral (rather than dorsal) caudate connectivity with vMFC in adults with OCD5,33. The vMFC is a broadly defined area - including medial frontal pole, subgenual ACC, and medial orbitofrontal cortex. Hyperactivity of medial and lateral orbitofrontal cortex have been among the most consistently reported findings in OCD, and have been linked to altered functional processing of reward and reversal learning, respectively, in adult patients3. A continuum of function has been ascribed to vMFC – from discerning value in orbitofrontal cortex to value-based decision-making in the medial frontal pole34. Although speculative, medial frontal pole involvement in OCD could relate to patients' difficulty suppressing symptoms despite insight that feared outcomes are unrealistic and compulsive behaviors unlikely to achieve outcomes of true value.
The vMFC is typically characterized by projections to the ventral striatum, particularly nucleus accumbens, whereas the dorsal striatum is more often associated with projections to the ACC. However, converging lines of evidence from animal-tracing and human neuroimaging research suggests these regions may interact through overlapping projections in dorsal and ventral striatum8. Given the role of the vMFC in emotion processing, and the ACC-dorsal striatum in cognitive control, these overlapping striatal fibers may provide anatomical substrate for affectively salient information to modulate cognitive control in the service of the flexible behavior8. In OCD, baseline hyperactivity of the dorsal caudate and ventral MFC associates with symptom severity and increases with symptom provocation3, such that excessive connectivity between these regions could underlie failure to suppress the emotionally salient, but contextually inappropriate security concerns (e.g., contamination, safety, order) characteristic of illness.
Alterations of ACC-based FSTC for cognitive control and vMFC-based FSTC for emotion processing during development may increase vulnerability for OCD, but also other forms of psychopathology, including Tourette's syndrome, eating disorders, and attention deficit hyperactivity disorder35. Presumably, certain risk factors (e.g., genetic, environmental) interact with unique FSTC loops at specific maturational stages to impact subsequent FSTC development in association with particular forms of psychopathology. For instance, premature and excessive pruning of ACC-based cognitive control circuitry in children at risk for OCD may interfere with the suppression of prepotent, security-related behaviors, which themselves trigger anxiety1, and could lead to increased signaling in FSTC for emotion-processing, driving excessive connectivity of the striatum to the v MFC. Alternatively, decreased connectivity in cognitive loops may couple with connectivity in emotion processing loops of FSTC to trigger illness onset.
Our findings of reduced dorsal striatum-ACC connectivity in child patients along with increased dorsal striatum-ventral MFC connectivity in OCD across development should be considered in the context of our study's limitations. We have extrapolated from the functional imaging literature to interpret our findings, however, research with converging methods (i.e., fMRI, behavioral) are needed to characterize the relationship between connectivity and function. Similarly, the relationship between resting state connectivity and underlying structure remains unknown, and it is possible that atypical development of FSTC structures in OCD (e.g., ACC2) contributed to our findings. It is also possible that warping to a common adult template could reduce normalization accuracy in younger subjects, since region-specific structural changes occur with development. Localization of striatal hyperconnectivity to medial frontal pole should be viewed with caution, given prior evidence for orbitofrontal pathology in OCD3 and the challenge that mapping orbitofrontal cortex presents for any MR study, given the signal drop-out that can occur in this region20. Small numbers of child subjects represents another limitation of our study; yet, inspection of the data did not suggest reduced connectivity in childhood OCD to be outlier-driven. Compared to patients in other age groups, adolescents with OCD showed lower symptom severity and higher rates of medication usage, however, results withstood post-hoc tests controlling for these variables, and the primary developmental finding – reduced striatum -ACC connectivity in child patients compared to child controls – should not have been influenced by the adolescent sample. Finally, the cross-sectional design of our study raises questions that it cannot answer. Longitudinal work is needed to determine whether alterations of connectivity in any particular FSTC loop influences development in other FSTC components, and to determine how such interactions associate with illness onset, persistence and remission of OCD.
Supplementary Material
Acknowledgments
Funded by the National Institute of Mental Health R01 MH071821 (SFT), K23MH082176 (KDF), and F32 MH082573 (ERS), National Institute of Neurological Disorders and Stroke R01- NS052514 (RCW), National Alliance for Research on Schizophrenia and Depression Young Investigator Awards (KDF, ERS), and Dana Foundation (KDF).
We thank McKenzie Maynor and Keith Newnham of the Universityof Michigan for help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Fitzgerald, Welsh, Stern, Hanna, Abelson, and Taylor, and Mr. Angstadt report no biomedical financial interests or potential conflicts of interest.
References
- 1.Evans DW, Lewis MD, Lobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder. Brain Cogn. 2004 Jun;55(1):220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg DR, Keshavan MS, A.E. Bennett Research Award Toward a neurodevelopmental model of of obsessive--compulsive disorder. Biol Psychiatry. 1998 May 1;43(9):623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- 3.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huyser C, Veltman DJ, de Haan E, Boer F. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev. 2009 Jun;33(6):818–830. doi: 10.1016/j.neubiorev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009 Nov;66(11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 6.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 7.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004 Oct 15;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 8.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010 Jan;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald KD, Stern ER, Angstadt M, et al. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010 Dec 1;68(11):1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somerville LH, Hare T, Casey BJ. Frontostriatal Maturation Predicts Cognitive Control Failure to Appetitive Cues in Adolescents. J Cogn Neurosci. 2010 September 1; doi: 10.1162/jocn.2010.21572. published online ahead of print. doi:10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006 Dec;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009 Jul;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fair DA, Dosenbach NU, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 15.First MB, R.L. S, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician version: User's guide. American Psychiatric Press; Washington, D.C.: 1996. [Google Scholar]
- 16.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997 Jun;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989 Nov;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 18.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006 Mar 30;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 19.Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010 Aug 1;52(1):20–31. doi: 10.1016/j.neuroimage.2010.03.072. [DOI] [PubMed] [Google Scholar]
- 20.Stenger VA, Boada FE, Noll DC. Multishot 3D slice-select tailored RF pulses for MRI. Magn Reson Med. 2002 Jul;48(1):157–165. doi: 10.1002/mrm.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magn Reson Med. 2002 Feb;47(2):344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002 Oct;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 23.Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010 Jul;36(4):713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002 Apr;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 25.Ward BD. Simultaneous inference for fMRI data. 2000 http://homepage.usask.ca/~ges125/fMRI/AFNIdoc/AlphaSim.pdf. Accessed February 1, 2010.
- 26.Taylor SF, Martis B, Fitzgerald KD, et al. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006 Apr 12;26(15):4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006 Sep 21;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Stern ER, Welsh RC, Fitzgerald KD, et al. Hyperactive Error Responses and Altered Connectivity in Ventromedial and Frontoinsular Cortices in Obsessive-Compulsive Disorder. Biol Psychiatry. 2011;69:583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickel DE, Veenstra-VanderWeele J, Cox NJ, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006 Jul;63(7):778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 30.Bloch MH, Craiglow BG, Landeros-Weisenberger A, et al. Predictors of early adult outcomes in pediatric-onset obsessive-compulsive disorder. Pediatrics. 2009 Oct;124(4):1085–1093. doi: 10.1542/peds.2009-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008 Dec;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 32.Fair DA, Bathula D, Mills KL, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai Y, Narumoto J, Nishida S, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry. 2010 November 8; doi: 10.1016/j.eurpsy.2010.09.005. published online ahead of print. doi:10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011 Feb;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009 Jun;166(6):664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.