Abstract
The Escherichia coli protein DinB is a newly identified error-prone DNA polymerase. Recently, a human homolog of DinB was identified and named DINB1. We report that the DINB1 gene encodes a DNA polymerase (designated polκ), which incorporates mismatched bases on a nondamaged template with a high frequency. Moreover, polκ bypasses an abasic site and N-2–acetylaminofluorene (AAF)-adduct in an error-prone manner but does not bypass a cis–syn or (6-4) thymine–thymine dimer or a cisplatin-adduct. Therefore, our results implicate an important role for polκ in the mutagenic bypass of certain types of DNA lesions.
Keywords: DINB1, polκ, abasic site, AAF, mutation, sequence context
Accurate replication of chromosomal DNAs requires DNA polymerases with high processivity and fidelity (Kornberg and Baker 1992). The high fidelity primarily depends on the proofreading 3′-to-5′ exonuclease activities of replicative DNA polymerases to remove erroneously incorporated nucleotides. Most, if not all, living organisms are endowed with another class of DNA polymerase that lacks this proofreading activity and is able to replicate past damaged bases, but at the increased risk of causing mutations (Friedberg et al. 1995; Friedberg and Gerlach 1999; Johnson et al. 1999d; Woodgate 1999). The Escherichia coli UmuD′2C complex has been recently shown to be one of such enzymes and designated pol V (Reuven et al. 1999; Tang et al. 1999). Saccharomyces cerevisiae has at least three different enzymes for translesion synthesis. Among them, Rev1 incorporates dCMP exclusively opposite an abasic site (Nelson et al. 1996a). Rev3 and Rev7 proteins form DNA polymerase ζ, which is able to bypass a cys–syn thymine–thymine (T-T) dimer (Nelson et al. 1996b). Rad30 is another DNA polymerase (η) that can efficiently bypass cys–syn T-T dimers (Johnson et al. 1999b). Rev1 and Rad30 share some homologies with UmuC, whereas Rev3, the catalytic subunit of pol ζ is similar to replicative DNA polymerases. Recently, a human homolog of Rev3 was identified and shown to be involved in UV-induced mutagenesis (Gibbs et al. 1998). Furthermore, the gene responsible for the human disease xeroderma pigmentosum variant (XP-V) was shown to code for a Rad30 homolog that bypasses a cys–syn T-T dimer, but not a (6-4) T-T dimer (Johnson et al. 1999a; Masutani et al. 1999a,b). The S. cerevisiae Rad30 and the human XPV enzymes are considered to be error-free because they insert two dAMPs opposite a cys–syn T-T dimer in vitro and the absence of their activities leads to enhanced levels of mutagenesis in vivo (Johnson et al. 1999a,b,c; Masutani et al. 1999a,b).
E. coli has another DNA polymerase (DinB, pol IV) (Wagner et al. 1999) with some similarity in amino-acid sequence to UmuC and its homologs (Ohmori et al. 1995; Kim et al. 1997). Overexpression of dinB resulted in a dramatic increase in frameshift mutations in F′lac plasmids without any exogenous DNA-damaging treatment (Kim et al. 1997). Recently, mouse and human homologs of DinB were identified, and the genes were designated Dinb1 and DINB1, respectively (Gerlach et al. 1999; Ogi et al. 1999). Furthermore, it was shown that transient expression of the mouse Dinb1-cDNA in cultured cells caused a nearly 10-fold increase in the incidence of 6-thioguanine resistance mutations, in which one-third were frameshift mutations and most of the rest were base-substitution mutations (Ogi et al. 1999). Therefore, the action of the mammalian DinB homologs seemed to be error-prone, similar to the E. coli DinB. It remained unknown, however, whether mammalian DinB homologs possess any DNA polymerase activity, and if so, whether they could facilitate bypass of damaged DNA. In this paper, we show that a truncated form of the human DINB1 (hDINB1) protein does exhibit DNA polymerase activity that allows for the bypass of certain DNA lesions in an error-prone manner.
Results and Discussion
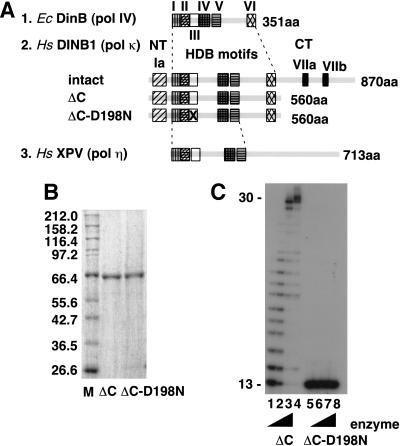
The hDINB1 protein comprises three portions (NT, HDB, and CT regions; see Fig. 1A), among which the NT+HDB and CT regions show 88% and 60% identity, respectively, with the corresponding region of the mouse homolog (Ogi et al. 1999). The HDB region contains all of the multiple motifs conserved among the DinB homologs, suggesting that the HDB region should be essential for a DNA polymerase activity and the CT region might be dispensable for catalytic activity. In support of this idea, the XPV protein (human polη), which contains the common five motifs shared among the UmuC/DinB family proteins in the amino-terminal half, exhibits enzyme activity even in the absence of a portion of its carboxyl terminus (Masutani et al. 1999a,b). Our attempt to overproduce the intact size of hDINB1 with a His–Tag sequence at the carboxyl terminus by using a Baculovirus system resulted in a very low yield, partly attributable to protein degradation (data not shown). We successfully overproduced a truncated form of hDINB1, however, which contained the amino-terminal 1-560 residues (see Fig. 1A) and purified the truncated protein (hereafter designated DINB1ΔC). Also, we purified a mutant form of DINB1ΔC containing a substitution of the well-conserved aspartic acid residue at the 198th position to asparagine (Fig. 1A,B).
Figure 1.
Detection of DNA polymerase activity. (A) A schematic representation of the E. coli DinB, human DINB1, and XPV proteins. Motifs I–V are shared among the UmuC/DinB family proteins, but motif VI is found only in the DinB subgroup and motif Ia is conserved among mammalian and C. elegans DinB homologs (Ogi et al. 1999). Motifs VIIa and VIIb denote Zinc clusters (Gerlach et al. 1999). (B) SDS–PAGE analysis of purified proteins. The purified proteins (350 ng each) were analyzed by electrophoresis on a 10% SDS-PAGE with marker proteins (New England Biolabs) and visualized by CBB staining. (C) DNA polymerase activity. Four different amounts of DINB1ΔC and DINB1ΔC -D198N (0.04, 0.4, 4, and 40 nm from left to right) were added in the reaction mixture. After incubation at 37°C for 15 min, the reaction products were analyzed by electrophoresis on 20% polyacrylamide/7m urea sequencing gel followed by autoradiography.
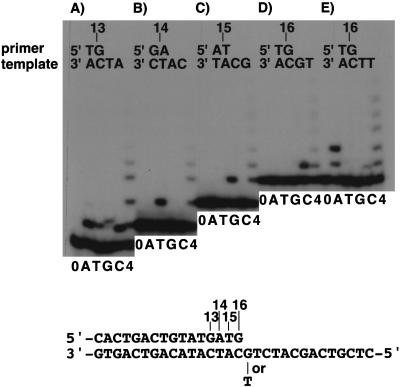
DNA polymerase activity was measured by standard primer extension assays, in which a 5′-labeled 13mer primer was annealed to a 30mer template containing no damage and, after incubation with the purified protein in the presence of four dNTPs, the products were analyzed by electrophoresis on sequencing gels. As shown in Figure 1C, the size of the replication products gradually increased as more amounts of DINB1ΔC were added in the reaction mixture, whereas no elongation was observed with DINB1ΔC–D198N. This result indicates that DINB1ΔC is responsible for the observed nonprocessive DNA polymerase activity. We designated the enzyme DNA polymerase κ (polκ) and the gene POLΚ instead of DINB1 (Polk for the corresponding mouse gene), in accordance with the HUGO Nomenclature Committee. PolκΔC mostly stopped elongation one or two nucleotides before the end of the template (Fig. 1C, lane 3), although full-size product was observed when an equal amount of the enzyme was added to the reaction (40 nm versus 40 nm of the primer-template DNA; Fig. 1C, lane 4). When the 5′-labeled primer with a terminal mismatched base after annealing with the template was incubated with the purified polκΔC in the absence of dNTP, no degradation of the primer was observed, indicating that the preparation was virtually free from intrinsic or contaminating exonuclease (data not shown). For qualitative analysis on the fidelity of polκΔC on a nondamaged DNA template, we measured incorporation of a mismatched base at the end of various primers in the presence of a single dNTP. As shown in Figure 2, B–D, polκΔC selectively incorporated the complementary bases opposite dA, dC, and dG in the template; however, it reproducibly showed high levels of misincorporation opposite T (Fig. 2A,E). Note that the single base change from dG to T is the only difference in the sequence between the templates used in Figure 2, D and E. At the present time, we have no clear explanation for why polκΔC exhibits such high levels of misincorporation, especially of C, opposite nondamaged T.
Figure 2.
Fidelity of polκΔC on nondamaged DNA template. The 30mer with the indicated sequence (A–D) or with the G-to-T substitution (E) was annealed with a 5′-labeled primer of different length. The primer-template (40 nm) was incubated with 0.04 nm of polκ in the absence of dNTP (0) or in the presence of a single dNTP (A, T, G, and C) or all of the four dNTPs (4). The reaction products were analyzed, as described in the legends to Fig. 1.
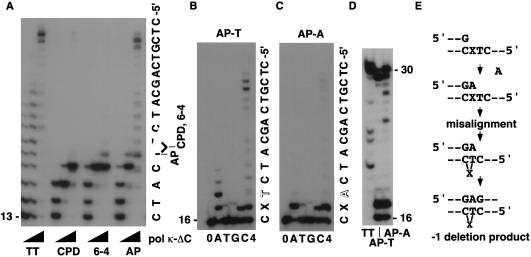
Next, we investigated whether polκΔC could bypass various DNA lesions. As shown in Figure 3A, polκΔC did not bypass a cis–syn or (6-4) T-T dimer. In contrast, polκΔC bypassed a synthetic abasic site that is very similar to, but more stable than, the natural abasic site (Shibutani et al. 1997). Even at an ∼1:10 molar ratio of enzyme versus the primer-template (the rightmost lane in Fig. 3A), polκΔC showed a significant level of bypass past the lesion although it stopped after incorporating one nucleotide opposite the abasic site at an equivalent proportion. To explore which residue could be incorporated opposite abasic site, we employed two different templates containing T (the AP-T template) or dA (the AP-A template) immediately 5′ to the abasic site. In both cases, when incubated with a single dNTP, polκΔC incorporated dAMP most preferentially and to a lesser extent dCMP opposite the abasic site (Fig. 3B,C). Therefore, polκΔC seemed to be similar to many other enzymes preferentially incorporating dAMP opposite abasic sites (Efrati et al. 1997). In the presence of four dNTPs, however, polκΔC was found to generate a −1 deletion during the bypass of the abasic site, which was sequence dependent. The size of the major replication product generated by polκΔC with the AP-T template was shorter by one nucleotide than the product generated with the control nondamaged template (Fig. 3A). The same result was obtained when Klenow fragment was added after the normal reaction by polκΔC, to complete elongation to the end of the template (Fig. 3D). On the other hand, polκΔC generated predominantly the full-size product with the AP-A template. For the reaction, an equal amount of polκΔC (40 nm) was added to the reaction mixture, because the efficiency of elongation past the lesion in the AP-A template was consistently much lower than in the AP-T template (Fig. 3B,C). Taken together, these results suggest that polκΔC preferentially incorporates dAMP opposite the synthetic abasic site, but a re-alignment may occur before the nonprocessive polκΔC enzyme inserts another residue at the dA-abasic mismatched terminus (see Fig. 3E). In the case of the AP-T template, the dA residue incorporated opposite the abasic site can form base-pairing with the T residue 5′ to the abasic lesion in the template, placing the abasic lesion in an extrahelical position. In such a configuration, dGMP can be incorporated opposite the next dC in the template in the presence of four dNTPs, thereby stabilizing the misaligned structure and eventually generating −1 deletion. If dATP is the only available substrate, polκΔC adds dA opposite the T residue 5′ to the abasic site at the dA-abasic mismatched terminus (see lane A in Fig. 3B). With the AP-A template, no slippage is possible and polκΔC has no choice other than to extend the mismatched dA primer. In the template, polκΔC bypassed the abasic site with a reduced frequency and mostly stopped after incorporating one nucleotide opposite the abasic site (Fig. 3C).
Figure 3.
Bypass synthesis by polκΔC past abasic site. (A) Translesion synthesis by polκΔC. The template annealed with its complementary 13mer primer was incubated with polκΔC at three different concentrations (0.04, 0.4, and 4 nm from left to right) in the presence of four dNTPs. TT denotes the control nondamaged templates and the AP-T template was used for abasic site (AP) translesion synthesis. (B,C) The AP-T or AP-A template annealed with the complementary 16mer primer was reacted with 4 nm of polκΔC (D) Following the incubation with polκΔC (4 nm for the AP-T template and 40 nm for the AP-A template) for 15 min in the presence of four dNTPs, Klenow enzyme (exo+ 10 fmoles) was added to complete elongation to the end of the template. After a further 15-min incubation at 37°C, the replication products were analyzed. (E) A plausible model for bypass of abasic site by polκ. X denotes an abasic site.
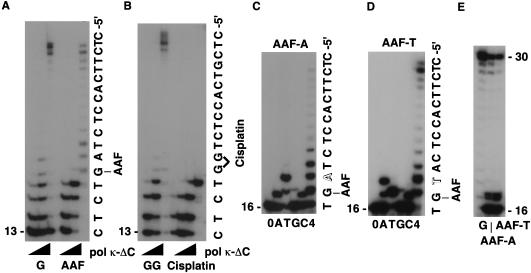
Furthermore, we found that polκΔC was able to bypass an N-2–acetylaminofluorene (AAF)-modified guanine (Fig. 4A), but it did not bypass a cis–diamminedichloroplatinum (cisplatin)-adducted G-G (Fig. 4B). To explore which residue was incorporated opposite dG–AAF, we performed similar experiments as described above, by employing two templates which differed from each other only at the two nucleotides 5′ to the AAF-adduct [3′-(dG-AAF)AT-5′ in the AAF-A template and 3′-(dG-AAF)TA-5′ in the AAF-T template]. When incubated in the presence of a single dNTP, the primer was extended most effectively in the presence of dTTP in both cases (Fig. 4C,D). The size of the major replication products generated with both templates after supplementing with Klenow enzyme was the same length as that generated with the nondamaged template (Fig. 4E). This result indicates that polκΔC most likely bypassed the dG–AAF adduct without skipping over the lesion. Although it remains to be clarified why polκΔC incorporates preferentially T opposite dG–AAF in the two different templates used here, the results shown in Figure 4, C and E, indicate that further elongation past the AAF–adduct was observed with the correct base, that is, T in the AAF-A template or A in the AAF-T template. This result implies that polκΔC retains the ability to select and add the correct base at the mismatched terminus between dG–AAF and T or A.
Figure 4.
Bypass synthesis by polκΔC past AAF-adduct. (A) The 30mer (AAF-A, 40 nm each) containing the AAF–adduct annealed with its complementary 13mer primer was incubated with three different concentrations of polκΔC (0.04, 0.4, and 4 nm from left to right) in the presence of four dNTPs. G indicates the control nondamaged template. (B) The 30mer containing the cisplatin–adduct annealed with its complementary 13mer primer was treated similarly. GG indicates the control nondamaged template. (C,D) The AAF-A or AAF-T template was annealed with the complementary 16mer primer and the primer-template was reacted with 4 nm of polκΔC. (E) Following the incubation with polκΔC for 15 min in the presence of four dNTPs, Klenow enzyme (exo+ 10 fmoles) was added. After a further 15-min incubation at 37°C, the replication products were analyzed.
As described above, we have shown that polκΔC can bypass two different DNA lesions, a synthetic abasic site and an AAF–adduct, in an error-prone manner depending on the context of the neighboring sequence. PolκΔC was unable, however, to bypass DNA lesions spanning two adjacent nucleotides such as the cis–syn or (6-4) T-T dimer or the cisplatin–adduct. High fidelity of replicative DNA polymerases depends on proofreading activity and strong base-selection, that is, adding only the base complementary to a template base at the perfectly matched primer terminus. Efficient translesion synthesis by a given DNA polymerase becomes possible only when both of the two properties are alleviated to some extent. Consistent with this, polκΔC showed a significant level of misincorporation opposite nondamaged T (Fig. 2A,E), elongation past the abasic site with a probable misalignment (Fig. 3), elongation at the mismatched termini between dG–AAF, and T or A (Fig. 4C,D). Our results demonstrate that a truncated form of polκ deleting the carboxy-terminal 310 amino acids is active as a DNA polymerase capable of continuing DNA synthesis past certain DNA lesions, implying that the carboxy-terminal region is not required for the catalytic activity per se. The carboxy-terminal region containing two copies of Zinc-cluster, which is conserved in eukaryotic DinB homologs (Gerlach et al. 1999; Johnson et al. 1999d), may be involved in increasing processivity through DNA binding by the Zinc-clusters and/or interacting with other protein(s) within the cells. It is intriguing as to how polκ (or any other enzyme for translesion synthesis for that fact) is recruited in vivo to the site where a replicative polymerase is stalled by the presence of a blocking lesion. In any case, because of the low processivity of such enzymes involved in translesion synthesis, error-prone synthesis is probably restricted to a very short region following the lesion, after which a replicative DNA polymerase should take over.
Our results verify the difference between polκ and human polη in some crucial respects regarding translesion synthesis. Whereas polκ carries out error-prone bypass of certain DNA lesions, polη appears to contribute to the general error-free bypass of various DNA lesions. In fact, when the same templates examined in this work were used, polη bypassed not only cis–syn T-T dimer by incorporating two As (Masutani et al. 1999a) but also the AAF-modified G and cisplatin-crosslinked GG, although at lowered efficiencies compared with bypass of cis–syn T-T dimer, by incorporating preferentially dCMP opposite the lesions (Masutani et al. 2000). Furthermore, polη bypassed the abasic site in the AP-A template by incorporating dAMP or dGMP without making −1 deletion (Masutani et al. 2000). XPV patients are predisposed to sunlight-induced skin cancer, probably because an error-prone pathway is used, instead of the polη-dependent error-free pathway, to bypass UV-induced DNA lesions (Cordonnier and Fuchs 1999; Masutani et al. 1999a). It seems unlikely that polκ is involved in the enhanced mutagenesis in XPV cells, since polκΔC was unable to bypass a cis–syn or (6-4) T-T dimer (Fig. 3A). XPV cells are also generally defective in bypassing an AAF–adduct, while still retaining a minor error-prone pathway (Cordonnier et al. 1999). Whether polκ is involved in this minor error-prone pathway needs to be experimentally examined. Our finding that polκΔC incorporated most preferentially T opposite dG–AAF in the two different templates with either A or T 5′ to the lesion is unexpected, but not unprecedented because dG–AAF is known to produce G-to-A transitions, although to a much lesser degree compared with G-to-T transversions or deletion formations, in simian kidney (COS-7) cells (Shibutani et al. 1998).
Finally, we should emphasize our finding that polκΔC is able to bypass abasic sites with variable efficiencies and in an error-prone manner that is heavily dependent on the sequence context. Very recently, Johnson et al. (2000) reported that the human DINB1 gene product (they designated it polθ instead of polκ) was unable to bypass an abasic site under the conditions they used. The difference between their result and ours may be attributable to the fact that they examined a single species of the template with a low concentration of the enzyme (0.5 nm against 10 nm of DNA substrate). As shown in Figure 3A, polκΔC did not bypass the abasic site in the AP-T template at the enzyme/template ratio of 1:100, although the enzyme showed a substantial extent of bypass at the ratio of 1:10. Abasic sites are the most common lesions in DNA within cells, and are generated by spontaneous hydrolysis of the N-glycoside bond (estimated 2,000–10,000 per cell per day) as well as intermediates during the course of repairing base-damage generated by carcinogenic agents and ionizing radiation (Lindahl 1993). The mRNAs coding for polκ are most highly expressed in testes in both humans and mice, although they are also expressed at lower levels in many other tissues (Gerlach et al. 1999; Ogi et al. 1999). Taken together, the results described above implicate polκ in carcinogenesis in somatic cells and causing higher mutation rates in males than in females (Crow 1997).
Materials and methods
Protein purification
A truncated form of hDINB1 (DINB1ΔC) and a corresponding mutant containing an amino acid change of aspartic acid to asparagine at residue 198, both with a 6xHis–tag attached at the carboxyl terminus, were overproduced using the BAC-to-BAC Baculovirus Expression System kit (GIBCO BRL). Briefly, derivatives of the vector plasmid FASTBAC1 (GIBCO BRL) containing the corresponding DNA sequence were constructed via several steps of PCR amplification and subcloning from a plasmid that contained the full-length DINB1 cDNA sequence. The plasmids were used to transform the E. coli strain DH10BAC to spontaneously generate recombinant bacmids carrying the respective protein-coding region in a Baculovirus sequence. High-molecular-weight DNAs containing the recombinant bacmid were prepared and used to transfect the Sf9 cells.
The Sf9 cells infected with the recombinant Baculovirus were lysed in Lysis buffer [20 mm sodium phosphate (pH 7.4), 0.3 m NaCl, 1% Nonidet-P 40] on ice with occasional stirring. The lysate was centrifuged at 50,000 rpm in Beckman 70Ti rotor for 1 hr and the supernatant containing the truncated DINB1 protein was supplemented with imidazole at the final concentration of 50 mm, and applied to 1 ml of a Ni-NTA silica resin (Qiagen) packed in an HR5/5 connected to an FPLC system. The column was washed with A buffer [20 mm sodium phosphate (pH7.4), 0.3 m NaCl, 10% glycerol] containing 50 mm imidazole and eluted in a gradient of 50–500 mm imidazole in A buffer. The truncated DINB1 protein was eluted at ∼180 mm imidazole.
DNA polymerase assay
The 30mer with the sequence 5′-CTCGTCAGCATCTTCATCATACAGTCAGTG-3′ was used as a nondamagd template. The same 30mer containing a cys–syn dimer (CPD) or (6-4) photoproduct at the underlined site was chemically synthesized as described previously (Murata et al. 1990; Iwai et al. 1996). The two 30mers containing tetrahydrofuran instead of natural abasic site, AP-A (5′-CTCGTCAGCATCAXCATCATACAGTCAGTG-3′) and AP-T (5′-CTCGTCAGCATCTXCATCATACAGTCAGTG-3′), in which X denotes abasic site, were synthesized as described (Fujiwara et al. 1999). The two AAF-modified templates, AAF-A (5′-CTCTTCACCTCTAGTCTCCTACACACTCAATC-3′) and AAF-T (5′-CTCTTCACCTCATGTCTCCTACACACTCAATC-3′) were prepared by treating the intact 30mers with N-acetoxy–AAF as described (van Vuuren et al. 1993) and the cisplatin-modified template (5′-CTCGTCACCTCGGTCTCCTACAGTCAGTG-3′ with the interlinked GG at the underlined site) was prepared as described (Fujiwara et al. 1999). Purity of the templates containing a lesion was checked in that no bypass product was observed by Klenow enzyme. Primers of different lengths and sequences were labeled at the 5′-end using T4 polynuclotide kinase and [γ-32P] ATP and annealed with template at a molar ratio of 1:1. Standard reactions (10 μl) contained 40 mm Tris-HCl (pH8.0), 5 mm MgCl2, 100 μm each of four dNTPs, 10 mm DTT, 250 μg/ml BSA, 60 mm KCl, 2.5% glycerol, 40 nm primer-template, and an indicated amount of enzyme. After incubation at 37°C for 15 min, reactions were terminated by the addition of 10 μl of formamide followed by boiling. The products were subjected to 20% polyacrylamide/7m urea gel electrophoresis followed by autoradiography.
Acknowledgments
We thank Dr. R. Woodgate at The National Institutes of Health for greatly improving the manuscript and Dr. E. Bruford at the HUGO Nomenclature Committee for advice in naming the hDINB1 protein. We also thank the two anonymous referees for careful reading of the manuscript and constructive advice. This work was supported in part by Grants-In-Aid for Scientific Research of priority area from the Ministry of Education, Culture, Sports, and Science of Japan.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL hohmori@virus.kyoto-u.ac.jp; FAX 81-75-751-3977.
References
- Cordonnier AM, Fuchs RPP. Replication of damaged DNA: Molecular defect in xeroderma pigmentosum variant cells. Mutat Res. 1999;435:111–119. doi: 10.1016/s0921-8777(99)00047-6. [DOI] [PubMed] [Google Scholar]
- Cordonnier AM, Lehman AR, Fuchs RPP. Impaired translesion synthesis in xeroderma pigmentosum variant extracts. Mol Cell Biol. 1999;19:2206–2211. doi: 10.1128/mcb.19.3.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF. The high spontaneous mutation rate: Is it a health risk? Proc Natl Acad Sci. 1997;94:8380–8386. doi: 10.1073/pnas.94.16.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrati E, Tocco G, Eritja R, Wilson SH, Goodman MF. Abasic translesion synthesis by DNA polymerase β violates the “A-rule.” Novel types of nucleotide incorporation by human DNA polymerase β at an abasic lesion in different sequence contexts. J Biol Chem. 1997;272:2559–2569. doi: 10.1074/jbc.272.4.2559. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Gerlach VL. Novel DNA polymerases offer clues to the molecular basis of mutagenesis. Cell. 1999;98:413–416. doi: 10.1016/s0092-8674(00)81970-4. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W. DNA repair and mutagenesis. Washington, D.C.: ASM press; 1995. [Google Scholar]
- Fujiwara Y, Masutani C, Mizukoshi T, Kondo J, Hanaoka F, Iwai S. Characterization of DNA recognition by the human UV-damaged DNA-binding protein. J Biol Chem. 1999;274:20027–20033. doi: 10.1074/jbc.274.28.20027. [DOI] [PubMed] [Google Scholar]
- Gerlach VL, Aravind L, Gotway G, Schultz RA, Koonin EV, Friedberg EC. Human and mouse homologs of Escherichia coli DinB (DNA polymerase IV), members of the UmuC/DinB superfamily. Proc Natl Acad Sci. 1999;96:11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs PEM, McGregor WG, Maher VM, Nisson P, Lawrence CW. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase ζ. Proc Natl Acad Sci. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai S, Shimizu M, Kamiya H, Ohtsuka E. Synthesis of a phosphoramidite coupling unit of the pyrimidine (6-4) pyrimidone photoproduct and its incorporation into oligodeoxynucleotides. J Am Chem Soc. 1996;118:7642–7643. [Google Scholar]
- Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999a;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine–thymine dimer by yeast DNA polymerase, Polη. Science. 1999b;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- ————— Requirement of DNA polymerase activity of yeast Rad30 protein for its biological function. J Biol Chem. 1999c;274:15975–15977. doi: 10.1074/jbc.274.23.15975. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Washington MT, Prakash S, Prakash L. Bridging the gap: A family of novel DNA polymerases that replicate faulty DNA. Proc Natl Acad Sci. 1999d;96:12224–12226. doi: 10.1073/pnas.96.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Prakash S, Prakash L. The human DINB1 gene encodes the DNA polymerase Polθ. Proc Natl Acad Sci. 2000;97:3838–3843. doi: 10.1073/pnas.97.8.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-R, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: An overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc Natl Acad Sci. 1997;94:13792–13797. doi: 10.1073/pnas.94.25.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A, Baker TA. DNA replication. 2nd Ed. New York, NY: W. H. Freeman & Co.; 1992. [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999a;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature. 1999b;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- Masutani, C., Kusumoto, R., Iwai, S., and Hanaoka, F. 2000. Mechanisms of accurate translesion synthesis by human DNA polymerase η. EMBO J. (in press). [DOI] [PMC free article] [PubMed]
- Murata T, Iwai S, Ohtsuka E. Synthesis and characterization of a substrate for T4 endonuclease V containing a phosphorodithioate linkage at the thymine dimer site. Nucleic Acids Res. 1990;18:7279–7286. doi: 10.1093/nar/18.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996a;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Lawrence CW, Hinkle DC. Thymine–thymine dimer bypass by yeast DNA polymerase ζ. Science. 1996b;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- Ogi T, Kato T, Jr, Kato T, Ohmori H. Mutation enhancement by DINB1, a mammalian homologue of the Escherichia coli mutagenesis protein DinB. Genes Cells. 1999;4:607–618. doi: 10.1046/j.1365-2443.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Hatada E, Qaio Y, Tsuji M, Fukuda R. dinP, a new gene in Escherichia coli, whose product shows similarities to UmuC and its homologues. Mutat Res. 1995;347:1–7. doi: 10.1016/0165-7992(95)90024-1. [DOI] [PubMed] [Google Scholar]
- Reuven NB, Arad G, Maor-Shoshani A, Livneh Z. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA, and SSB and is specialized for translesion replication. J Biol Chem. 1999;274:31763–31766. doi: 10.1074/jbc.274.45.31763. [DOI] [PubMed] [Google Scholar]
- Shibutani S, Takeshita M, Grollman AP. Translesional synthesis on DNA templates containing a single abasic site. A mechanistic study of the “A rule”. J Biol Chem. 1997;272:13916–13922. doi: 10.1074/jbc.272.21.13916. [DOI] [PubMed] [Google Scholar]
- Shibutani S, Suzuki N, Grollman AP. Mutagenic specificity of (Acetylamino)fluorene-derived DNA adducts in mammalian cells. Biochemistry. 1998;37:12034–12041. doi: 10.1021/bi981059+. [DOI] [PubMed] [Google Scholar]
- Tang M, Shen X, Frank EG, O'Donnell M, Woodgate R, Goodman MF. UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren AJ, Appeldoorn E, Odijk H, Yasui A, Jaspers NG, Bootsma D, Hoeijmakers JH. Evidence for a repair enzyme complex involving ERCC1 and complementing activities of ERCC4, ERCC11 and xeroderma pigmentosum group F. EMBO J. 1993;12:3693–3701. doi: 10.1002/j.1460-2075.1993.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Gruz P, Kim S-R, Yamada M, Matsui K, Fuchs RPP, Nohmi T. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- Woodgate R. A plethora of lesion-replicating DNA polymerases. Genes & Dev. 1999;13:2191–2195. doi: 10.1101/gad.13.17.2191. [DOI] [PubMed] [Google Scholar]