Abstract
Improving global yields of important agricultural crops is a complex challenge. Enhancing yield and resource use by engineering improvements to photosynthetic carbon assimilation is one potential solution. During the last 40 million years C4 photosynthesis has evolved multiple times, enabling plants to evade the catalytic inadequacies of the CO2-fixing enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco). Compared with their C3 ancestors, C4 plants combine a faster rubisco with a biochemical CO2-concentrating mechanism, enabling more efficient use of water and nitrogen and enhanced yield. Here we show the versatility of plastome manipulation in tobacco for identifying sequences in C4-rubisco that can be transplanted into C3-rubisco to improve carboxylation rate (VC). Using transplastomic tobacco lines expressing native and mutated rubisco large subunits (L-subunits) from Flaveria pringlei (C3), Flaveria floridana (C3-C4), and Flaveria bidentis (C4), we reveal that Met-309-Ile substitutions in the L-subunit act as a catalytic switch between C4 (309Ile; faster VC, lower CO2 affinity) and C3 (309Met; slower VC, higher CO2 affinity) catalysis. Application of this transplastomic system permits further identification of other structural solutions selected by nature that can increase rubisco VC in C3 crops. Coengineering a catalytically faster C3 rubisco and a CO2-concentrating mechanism within C3 crop species could enhance their efficiency in resource use and yield.
Keywords: CO2 assimilation, rbcL mutagenesis, gas exchange, chloroplast transformation
The future uncertainties of global climate change and estimates of unsustainable population growth have increased the urgency of improving crop yields (1). One possible solution is to “supercharge” photosynthesis by improving the C3 cycle (2, 3). Although a simple idea, this is a complex challenge that involves several possible alternatives. Many of these alternatives focus on enhancing the performance of the CO2-fixing enzyme ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (rubisco), which catalyses the first step in the synthesis of carbohydrates. Despite its pivotal role, rubisco is a slow catalyst, completing only one to four carboxylation reactions per catalytic site per second in plants (4, 5). Moreover CO2 not only is fixed through a complex catalytic process but also must compete with O2. The oxygenation of RuBP produces 2-phosphoglycolate, whose recycling by photorespiration requires energy and results in the futile loss of fixed carbon [∼30% of fixed CO2 in many C3 plants (6)].
To compensate for rubisco's catalytic limitations, plants invest as much as 25% of their leaf nitrogen in rubisco (7). This value is much lower in C4 plants, where a biochemical CO2-concentrating mechanism (CCM) elevates CO2 around rubisco. This optimized microenvironment allows rubisco to operate close to its maximal activity, reducing O2 competition. This CCM has enabled C4 plants to evolve rubiscos with substantially improved carboxylation rates (VC) relative to their C3 ancestors, albeit at the expense of reducing CO2 affinity [i.e., a higher apparent Km for CO2 (KC)] (8, 9). As a consequence, C4 plants require less rubisco, thereby enhancing nitrogen use with improvements in VC correlating with improved efficiency in nitrogen use (10). In addition, the high concentration of CO2 around rubisco allows C4 plants to operate at lower CO2 pressures within their leaf air spaces, thereby reducing their stomatal conductance requirements and the associated H2O loss by transpiration. Indicative of these growth advantages, C4 photosynthesis has evolved independently several times from multiple C3 lineages during the last 20–40 million years (11, 12).
Following nature's example, a number of CO2 transgenic approaches have been designed to emulate CCM strategies in C3 plastids and improve rubisco performance (2). These approaches include elevating the CO2 concentration within chloroplasts using recombinant CO2/HCO3− transporters from cyanobacteria or engineering alternative pathways to bypass photorespiration and release CO2 within the stroma (13, 14). Although each strategy faces continuous challenges in its fine tuning and integration into crops, further improvements in yield and in the efficiency of water and nitrogen use are likely by concurrently “speeding up” rubisco (9).
Identifying the natural changes that result in the faster C4 rubiscos is far from simple, given the complex structure and biogenesis pathway of the hexadecameric rubisco (L8S8) in vascular plants, whose assembly requirements cannot be met by conventional bacterial or in vitro expression systems (15). The catalytic core of L8S8 rubisco comprises four 52-kDa large (L)-subunit pairs which are capped by two sets of 15-kDa small (S)-subunit tetramers that provide structural stability and influence catalysis (16, 17). Although supplementing rice rubisco with S-subunits from the C4 plant sorghum was found to improve VC of the heterologous L8S8 enzyme (16), crosses between C3 and C4 Flaveria and Atriplex species showed C4 catalysis to be maternally inherited (18, 19) and hence defined by the chloroplast-encoded L-subunit gene (rbcL). Therefore, changes in both Rubisco L- and S-subunits can influence catalysis.
Although phylogenetic studies have identified potential L-subunit residues involved in the transition from C3-like to C4-like rubisco, it is uncertain which residues are catalytically determinant (11, 20). Here we undertake a transplastomic approach to identify such residues in vivo. By manipulating the rbcL gene in tobacco to produce hybrid L8FS8t rubiscos (containing variant Flaveria L- and tobacco S-subunits), we demonstrate that Met-309-Ile substitutions in the L-subunit act as a catalytic switch between C4 (309Ile) and C3 (309Met) catalysis in Flaveria rubisco.
Results
Flaveria Rubisco L-Subunit Expression in Tobacco Chloroplasts.
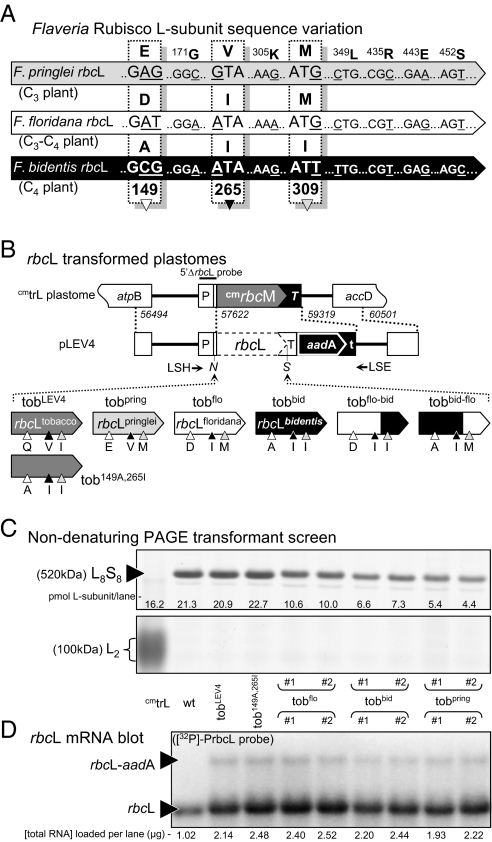
The rbcL genes from Flaveria pringlei (C3), Flaveria floridana (a C3-C4 intermediate), and Flaveria bidentis (C4) were chosen for transformation into tobacco plastids because of their diverse catalytic properties (ref. 21 and below) despite their high sequence similarity (Fig. 1A). As in all Flaveria rbcL genes, nonsilent nucleotide changes occur only at residues 149, 265, and 309 (20). The Flaveria L-subunits show >95% identity with the tobacco L-subunit, with 22–24 amino acid differences in addition to a highly charged TDKDKDKKR extension at the C terminus (Fig S1). The Flaveria rbcL genes were cloned into the plastome-transforming plasmid pLEV4, where the expression of the transgenes is regulated by the native tobacco rbcL gene regulatory sequences [i.e., its promoter, 5′-, and 3′-untranslated sequences (22)]. The transforming plasmids, including the control pLEV4, were introduced biolistically into cmtrL, a tailor-made tobacco master line for integrating rbcL transgenes into the tobacco chloroplast by homologous recombination (Fig. 1B) (23). Two independent transplastomic lines for each rbcL transgene were grown to maturity in soil in air supplemented with 0.5% (vol/vol) CO2. The transformed tobacco lines that incorporated the F. floridana, F. bidentis, and F. pringlei rbcL genes were called “tobflo,” “tobbid,” and “tobpring,” respectively.
Fig. 1.
Flaveria rubisco L-subunit sequence and expression in tobacco chloroplasts. (A) Comparison of Flaveria rbcL sequences that differ only in L-subunit substitutions at amino acids 149, 265, and 309 (20). (B) The transforming plasmid pLEV4 contains a homologous plastome flanking sequence (indicated by dashed lines; numbering indicates region of sequence integration relative to plastome sequence; GenBank ID Z00444) that directed integration of the rbcL transgenes and a promoterless aadA-selectable marker gene into the plastome of the tobacco master line, cmtrL (23). The L-subunit amino acid differences at residues 149 (white triangle), 265 (black triangle), and 309 (gray triangle) in the tobLEV4 (tobacco rbcL control), tobpring (F. pringlei rbcL), tobflo (F. floridana rbcL), tobbid (F. bidentis rbcL), tobflo-bid (chimeric F. floridana–F. bidentis rbcL), tobbid-flo (chimeric F. bidentis–F. floridana rbcL), and tob149A,265I (mutated tobacco rbcL) transplastomic tobacco (tob) lines are shown. Annealing locations of primers LSH, LSE, and the 5′ ΔrbcL probe (24) are shown. N, NheI; S, SalI cloning sites. (C) Nondenaturing PAGE analysis of soluble protein from comparable leaves of independent T0 transplastomic lines, cmtrL, and wild-type tobacco (protein from 1.5 mm2 of leaf was loaded per lane). Homoplasmic transformants produce only L8S8 rubisco (∼520 kDa) and not the ∼100-kDa R. rubrum L2 rubisco produced in cmtrL (23). (D) Detection of rbcL and rbcL-aadA mRNA in total RNA from 3 mm2 (for wild type) or 6 mm2 (other samples) of the leaves sampled in C.
Nondenaturing PAGE (ndPAGE) analysis of the soluble leaf protein was used to confirm the production of the hybrid L8FS8t rubisco (comprising Flaveria L-subunits and tobacco S-subunits) and to assesses the homoplasmicity of the tobpring, tobflo, and tobbid lines (Fig. 1C). Each of the transformed lines examined was deemed to be homoplasmic, because no L2 Rhodospirillum rubrum rubisco from the parental cmtrL line was detected; homoplasmicity was further confirmed by DNA blot analysis (Fig S2).
Differential Expression of the Hybrid L8FS8t Rubiscos.
Differences in the intensities of the L8FS8t bands in ndPAGE indicated that the amounts of rubisco produced in the T0 lines varied (Fig. 1C). This variation was confirmed by quantitative [14C]2-carboxyarabinitol-1,5-bisphosphate (CABP) binding. The amount of L8FS8t produced in the tobflo, tobbid, and tobpring leaves was reduced by approximately 50%, 65%, and 75% relative to wild-type, respectively (Fig. 1C). In contrast, rubisco content in the tobLev4 control transformants matched that in wild-type, indicating that the additional genome changes around rbcL were not the cause of the reduced L8FS8t expression. SDS PAGE-immunoblot analysis showed no unassembled Flaveria L-subunits accumulated as insoluble aggregates.
Contrary to the reduced L8FS8t content in the T0 leaves, there was little or no difference in the Flaveria rbcL mRNA levels in the same leaves relative to wild type (Fig. 1D), indicating that L8FS8t synthesis probably is perturbed posttranscriptionally. As shown previously (23–25), a less abundant rbcL-aadA dicistronic mRNA (∼10% that of the rbcL mRNA) was produced in all transformants as a result of inefficient transcription termination by the tobacco rbcL 3′UTR. The stages that hinder L8FS8t expression during rubisco biogenesis or degradation remain to be identified fully.
Plant Growth and Leaf Photosynthesis.
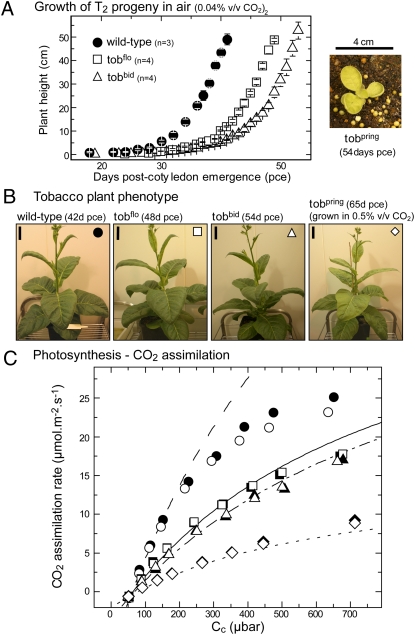
The disparity in L8FS8t levels in leaves of the tobflo, tobbid, and tobpring lines persisted to the T2 progeny and correlated with their relative differences in photosynthesis and growth rates. For the tobflo and tobbid plants, the higher L8FS8t levels produced were sufficient for them to survive through to maturity in air (without CO2 enrichment), although they grew more slowly than wild-type plants (Fig. 2A). In contrast, the tobpring transformants grew poorly in air. As seen previously in tobaccoRst lines producing hybrid L8sS8t rubisco (comprising sunflower L- and tobacco S-subunits) (24), the juvenile tobpring plants displayed a pale green leaf phenotype with marginal curling and dimpling (Fig. 2A Right). This phenotype is likely a consequence of the very low L8FS8t content during early vegetative growth (<3 μmol sites m−2·s−1) and in the young mature leaves (<6 μmol sites m−2·s−1; Fig. 2C) of the tobpring plants. Consistent with their different growth rates and varied L8FS8t contents, the leaf photosynthetic CO2 assimilation rates at varying CO2 partial pressures (pCO2) were slowest for tobpring [but still slightly higher than in tobaccoRst (24)] and were successively better for the tobbid and tobflo lines, albeit still slower than in wild-type tobacco (Fig. 2C). Measurements of the ratio of variable fluorescence to maximal fluorescence (Fv/Fm) in wild-type leaves (0.82 ± 0.01) were identical to those in the three tobFlaveria genotypes (0.83 ± 0.01), indicating no difference in photochemical efficiency under the growth conditions.
Fig. 2.
Measurements of growth and leaf gas exchange in the transformants producing the variant LF8St8 rubiscos. (A) (Left) Comparatively slower growth in air of the tobFlaveria transformants as a function of plant height relative to wild-type. (Right) The tobpring lines grew extremely poorly in air. pce, postcotyledon emergence. (B) Phenotype of the plants from A at age pce as shown. (Scale bars: 4 cm.) Air supplemented with 0.5% (vol/vol) CO2 was used to grow tobpring plants to maturity. (C) Comparative differences in gas-exchange measurements of CO2 assimilation rates at 25 °C under varying chloroplast CO2 pressures (Cc) at growth illumination (400 μmol quanta m−2·s−1). Measurements were made on young mature leaves located at similar canopy positions (fifth leaf from the apical meristem) of physiologically comparable mature plants analogous to those shown in B. Leaf rubisco contents were 25.0 and 30.5; 12.4 and 11.9; 10.1 and 10.8; 4.1 and 4.3 μmol rubisco sites m−2 in the independent wild-type (circles), tobflo (squares; line 1 white, line 2 black), tobbid (triangles; line 1 white, line 2 black), and tobpring (diamonds; line 1 white, line 2 black) plants analyzed, respectively. The lines show the rubisco limited CO2 assimilation rates for wild-type (– –), tobflo (—),tobbid (– · –), and tobpring (····) modeled according to ref. 26 using the catalytic parameters for the respective hybrid LF8St8 rubiscos in Fig. 3 and assuming rubisco was fully activated and a value of 0.3 mol m−2·s−1·bar−1 for mesophyll conductance.
Catalysis by Each L8FS8t Rubisco Matches the Source Flaveria Enzyme.
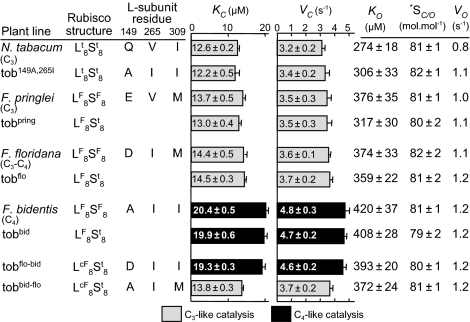
The catalytic properties of the recombinant L8FS8t were compared with the source L8FS8F enzymes from the corresponding Flaveria species (Fig. 3). As seen previously for the L8sS8t rubisco produced in tobaccoRst (24), the L8FS8t and equivalent Flaveria L8FS8F enzymes were catalytically comparable with respect to their carboxylation (VC) and oxygenation (VO) rates, their apparent Michaelis constants (Km) for CO2 (KC) and O2 (KO), and CO2/O2 specificities (SC/O). Of particular interest was the faster carboxylation rate (∼35% higher VC relative to the C3 rubiscos) and lower CO2 affinity (∼50% higher KC) of the hybrid L8FS8t rubisco from tobbid that matched the F. bidentis L8FS8F enzyme (Fig. 3). As expected, when the catalytic properties and content of each L8FS8t hybrid enzyme were used to model CO2 assimilation rates according to Farquhar et al (26), the final values closely matched those measured by whole-leaf gas exchange (Fig. 2C). These results provide confidence both in the accuracy of the measured catalytic properties of the L8FS8t hybrid enzymes and the rubisco-limited CO2-assimilation model.
Fig. 3.
Comparative catalysis at 25 °C of the wild-type and Q149A-V265I mutated tobacco (Lt8St8) rubisco, the source Flaveria (LF8SF8) rubiscos, the hybrid (LF8St8, comprising Flaveria L- and tobacco S-subunits), and chimeric (LcF8St8, comprising chimeric Flaveria L- and tobacco S-subunits) rubisco variants produced in the transplastomic tobacco plants. The L-subunit amino acid residues at codons 149, 265, and 309 in each rubisco type are shown. Values shown are the average ± SD of independent assays (n = 4–24; see Materials and Methods for details). The maximal oxygenation rate (VO) was calculated using the equation SC/O = (VC/KC)/(VO/KO).
Interchanging C3–C4 Catalysis via 309Met-309Ile Substitutions in Flaveria Rubisco.
The catalytic similarity between native F. bidentis rubisco and the hybrid tobbid L8FS8t enzyme indicated that the introduced L-subunit determined the catalytic phenotype. Because the F. bidentis and F. floridana L-subunits differ only at residues 149 and 309 but show significant differences in VC and KC (Fig. 3), domain swapping of their rbcL was used to identify which residue(s) imparted the C4 catalysis of F. bidentis rubisco. The chimeric rbcL gene in the transforming plasmid pLEVflo-bid introduced a Met-309-Ile substitution into the F. floridana rbcL gene, whereas in pLEVbid-flo the chimeric rbcL gene coded an Ile-309-Met substitution in the F. bidentis rbcL gene (Fig. 1B). Both plasmids were transformed into cmtrL, and independent tobflo-bid- and tobbid-flo-transformed tobacco lines were produced that grew to reproductive maturity in air.
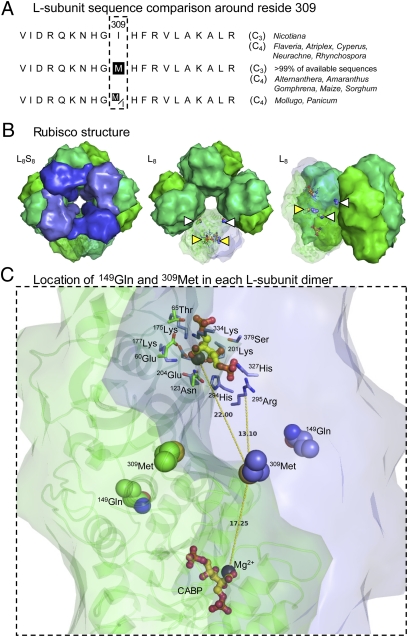
Catalytic analysis of the chimeric (L8cFS8t) rubisco produced in the tobflo-bid T1 progeny showed that the Met-309-Ile substitution increased VC and KC, matching that measured for the F. bidentis and tobbid C4-like rubiscos (Fig. 3). In contrast, introducing an Ile-309-Met mutation into the F. bidentis L-subunit (tobbid-flo lines) reduced VC and KC, resulting in a rubisco with C3-like catalysis. These results demonstrate that 309Ile confers Flaveria rubisco with C4-like catalysis. Although a comparison of higher plant L-subunits shows that 309Met is highly conserved in most C3-plant rubiscos, the tobacco L-subunit encodes 309Ile (Fig. 4A).
Fig. 4.
Conservation and location of the L-subunit residues 149 and 309 in higher plant rubisco. (A) Coding matrix summary of ClustalW-aligned residues 300–319 in L-subunit sequences from data sets with corresponding C3 and C4 speciation data (11, 20, 38). (B) Structure of spinach L8S8 rubisco and L8 core (L-subunits are shown in green; S-subunits are shown in blue) viewed from the top, showing central solvent channel (Left and Center), and from the side (Right). The relative locations of 149Gln (white triangle) and 309Met (yellow triangle) in one L-subunit pair (L2) is shown. The 309Met residues are located at the interface of L-subunits; the 149Gln residues are positioned at the L2–L2 interface toward the surface of the central solvent channel. (C) View of an L-subunit pair (L1 in green showing ribbon structural detail; L2 in blue) showing the positioning of 149Gln and 309Met relative to each Mg2+ (black sphere) and the reaction-intermediate analog 2-CABP (yellow and red ball and stick) bound to the two active sites in the dimer. The conserved active-site residues are shown for one active site. Distances (in Å) from the S atom of 309Met in L2 to each Mg2+ and the Cα atom of the closest conserved active-site residue, 295Arg, are shown. (This figure was prepared with PyMOL using the PDB co-ordinates 8RUC.)
Amino Acid 149 Is Catalytically Neutral but Can Influence Rubisco Expression.
The matching C4-like catalysis of rubisco from tobflo-bid and tobbid and C3-like catalysis of the tobbid-flo, tobflo, and tobpring rubiscos suggests that changes to amino acid 149 in Flaveria rubisco are catalytically neutral and possibly account for the amino acid heterogeneity at this position (Fig. 1A) (20). Likewise, conservation of 265Ile in F. floridana and F. bidentis rubisco indicates that this residue also is catalytically neutral. The influence of Gln-149-Ala and Val-265-Ile L-subunit substitutions (to match those in F. bidentis rubisco; Fig. 1B) on tobacco rubisco were tested by transforming cmtrL with the pLEV149A,265I. Catalysis by rubisco in the tob149A,265I lines matched that of the wild-type enzyme, demonstrating that both substitutions are catalytically neutral and are not able to impart C4-like catalysis on tobacco rubisco (Fig. 3).
Despite the apparent neutrality of amino acid 149 on catalysis, changes at this position affected the level of the hybrid L8FS8t expression. In young mature leaves of both tobflo-bid and tobflo, whose L-subunits share 149Asp (and 265Ile), the rubisco levels were comparable (10–13 μmol sites m−2·s−1). In contrast, the rubisco content in equivalent leaves from tobbid-flo and tobbid (whose L-subunits code 149Ala and 265Ile) were lower (6–8 μmol sites m−2·s−1) (Fig S3). These results suggest that changes to the amino acid (or its mRNA sequence) at residue 149 might be responsible for the variations in hybrid rubisco expression. However, this did not appear to be the case for tobacco Rubisco as the leaf Rubisco levels in the tob149A,265I lines matched that in the wild-type leaves (Fig. 1C). How changes at residue 149 in the Flaveria L-subunit might differentially influence its translation, folding, and/or assembly with tobacco S-subunits or the stability of L8FS8t complexes remains to be examined.
Discussion
Using transgenic tobacco lines expressing hybrid rubiscos containing Flaveria L- and tobacco S-subunits (L8FS8t), we have identified 309Ile as the key residue that imparts C4-like catalytic properties to Flaveria rubisco. The determinant role of this residue supports observations from prior crossing studies that showed C4 catalysis to be maternally inherited in Flaveria (19). Linkages between catalysis and sequence phylogenies of different Flaveria rubisco L- and S-subunits indicated that C4 catalysis was associated with two positively selected L-subunit amino acid substitutions: Asp-149-Ala and Met-309-Ile (20). Here we show that amino acid differences at position 149 in Flaveria rubisco probably are catalytically silent, because interchanging 149Ala with 149Asp in the L-subunit from either F. floridana or F. bidentis rubisco had no influence on catalysis (Fig. 3). Similarly, tobacco rubisco catalysis was unaffected by Q149A and V265I substitutions (Fig. 3).
A structural/functional explanation for how 309Ile increases VC in Flaveria rubisco is unclear. The similar positioning of conserved catalytic site residues in the crystal structures of catalytically different rubiscos makes it difficult to rationalize how distant changes influence catalysis (4, 20). The structure for spinach L8S8 rubisco (Fig. 4B) shows 309Met located at the L-interface (i.e., between the L-subunits in each L2 dimer) more than 17 Å away from the Mg2+ bound to each catalytic site and at least 13 Å away from the nearest conserved active site residue, 295Arg (Fig. 4C). In contrast, 149Gln is located further away from the active sites and close to the interface of the adjoining L2 dimers that form the L8 core (Fig. 4B). In the absence of a crystal structure for a Flaveria rubisco, it is difficult to explain how insertion of a more hydrophobic 309Ile this far from the active site might influence VC (20).
Despite the improvements in VC imparted by 309Ile in hybrid L8FS8t rubisco, the accompanying reductions in CO2 affinity (i.e., increased KC) precluded gains in carboxylation efficiency. At 25 °C under ambient oxygen levels, the carboxylation efficiency (i.e., VC/KC21%O2) of the C4-like 309Ile-containing rubiscos in tobbid and tobflo-bid (145 mM−1·s−1) were poorer than the C3-like 309Met-L8FS8t enzymes in tobpring and tobflo (150 and 161 mM−1·s−1, respectively). Thus, because of the low CO2 levels within (unstressed) C3 chloroplasts (<10 μM), the faster C4-like enzymes probably provide no advantage to plant growth within a C3 plant (at least at 25 °C), as shown recently in rice (16). As modeled recently, optimal CO2 concentrations required for C4 rubisco are substantially higher (∼80 μM) (8), indicating that taking full advantage of a faster rubisco in a C3 plant will require the combinatorial effect of a suitable CCM, for which a number of strategies are being pursued (2). Some of these approaches already have demonstrated that elevating CO2 pressures within C3 plastids can improve the capacity for CO2 assimilation by reducing the energy costs of photorespiration (13, 14).
Our results suggest that the carboxylation rate of rubisco in a C3 plant might be increased either by direct replacement with L-subunits sourced from C4 plants (as in the tobbid plants; Fig. 3) or by tailoring appropriate sequence mutations into related C3 rubisco L-subunits (as in the tobflo-bid plants; Fig. 3). Although the first approach suffers from our inability to predict a priori the assembly properties of foreign rubiscos within the chloroplast of the recipient transplastomic line, the second approach would require knowing the catalytic/structural effect of every possible mutation within the context of a particular rubisco enzyme, an understanding that we are still far from achieving. Indeed, the finding that tobacco rubisco encodes 309Ile but shows C3 catalysis highlights the complex natural variation in the sequence–structure–function relationships among plant L-subunits. Even the sequence diversity at position 309 among C4 rubiscos (Fig. 4A) indicates that this residue is not the only one that can impart C4 catalysis. This result is consistent with the polyphyletic evolution of C4 photosynthesis (12) and with predictions that at least eight L-subunit residues (including residue 309 but not residue 149) have been selected for positively by C4 catalysis (11). Experimentally testing these predictions, identifying other catalytically determinant L-subunit residues, and exploring which particular rubiscos are affected by these changes have been hampered by the preferential location of rbcL in the plastome (27) and the small range of species whose plastomes can be transformed stably (28). However, as shown in this and previous studies (24, 29, 30), these experimental limitations may be circumvented by expressing hybrid rubiscos in tobacco plastids. The generality of this system for examining sequence–performance relationships within otherwise inaccessible, catalytically diverse foreign L-subunits remains to be explored fully.
Although this study demonstrates the pervasive role of the L-subunit in shaping catalysis in plant rubisco, the important role of the S-subunits on catalysis cannot be overlooked. The apparent catalytic neutrality of the tobacco S-subunit when assembled with heterologous L-subunits (Fig. 3) (24, 29, 30) contrasts with the recent success in shaping rice rubisco toward C4-like catalysis using heterologous S-subunits from C4 sorghum (16). Similarly, structural changes to the S-subunit have improved Chlamydomonas rubisco catalysis (17). As highlighted recently (20), differences in rubisco S-subunit sequence also may account for the catalytic deviation of Flaveria palmeri rubisco, whose L-subunit sequence matches that of tobflo-bid (coding 309Ile) but shows C3 catalysis.
The similar SC/O values determined for rubisco from F. bidentis, F. floridana, and F. pringlei (Fig. 3) in this study contrast with the slightly varying values determined previously (SC/O = 76 ± 1, 84 ± 1, and 81 ± 1 respectively) (21). The reason for this variation is unknown but may lie in alterations in the catalytic competence as a result of different purification processes (ion exchange chromatography versus ammonium sulfate fractionation), the final enzyme purity, and the length of ultra-cold storage (24). By using fresh rubisco rapidly purified to >95% homogeneity by ion exchange chromatography, our measured SC/O values were highly reproducible between preparations from independent biological replicates.
Here we present an rbcL engineering approach involving hybrid rubisco production in tobacco plastids to unravel the sequence and catalytic diversity of related C3 and C4 rubiscos from Flaveria. Future applications of this experimental system are focused on identifying sequence changes that account for the natural diversity of rubisco performance and testing the feasibility of transplanting these catalytic improvements into the rubisco L-subunits of agriculturally relevant crops. In particular, when coengineered with biotechnological strategies to elevate CO2 around rubisco in C3 plants, a faster rubisco may translate into improved efficiency in water and nitrogen use and the enhanced yields currently enjoyed by C4 plants.
Materials and Methods
RuBP and [14C]2-CABP were synthesized as described (31, 32). Protein content was measured using a dye-binding assay (Pierce) and BSA as a protein standard.
Tobacco Plastome Transformation and Growth.
The transforming plasmid pLEV4 directs the insertion of an rbcL gene and a promoterless aadA gene (coding spectinomycin resistance) into the tobacco plastome in place of the L2 Rhodospirillum rubrum rubisco-coding cmrbcM gene in the plastome of the tobacco master line cmtrL (Fig. 1B) (23). The rbcL gene from F. bidentis, F. pringlei, and F. floridana was PCR amplified from leaf genomic DNA [isolated using the DNeasy Plant Mini Kit (Qiagen)] with the primers 5′ NheIrbcL (5′-AGCTAGCGTTGGATTCAAAGCTGGTGTT-3′ [the NheI site that spans the rbcL codons 9 (Ala) and 10 (Ser) is shown in italics] and 3′ SalIrbcL (5′-TGTCGACTGTTTTTATCTCTTCTTATCCTTATCCT-3′ [the reverse complement of the rbcL stop codon is shown in bold, and the SalI site is shown in italics]. The 1,439-bp NheI–SalI rbcL products were cloned into pLEV4 to give the transforming plasmids pLEVpring, pLEVflo, and pLEVbid. The plasmids pLEVflo-bid and pLEVbid-flo were made by interchanging the 569-bp SphI–SalI fragments of the F. bidentis and F. floridana rbcL genes (Fig. 1B). Mutations coding substitutions Gln-149-Ala and Val-265-Ile in the tobacco rbcL gene in pLEV4 were introduced using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) to produce plasmid pLEVtob149A,265I. All plasmids were sequenced using BigDye terminator sequencing at the Biomolecular Resource Facility, Australian National University (Canberra, Australia).
Each of the pLEV-derived plasmids was introduced biolistically into three leaves of cmtrL1 as described (23), and three to seven independent spectinomycin-resistant plants were obtained for each mutant. Two independent plastome-transformed lines for each introduced rbcL gene were grown to maturity in soil in a growth atmosphere supplemented with 0.5% (vol/vol) CO2 as described (24). At each generation the plants were fertilized artificially with wild-type pollen.
PAGE and Nucleotide Blot Analyses.
The preparation and analysis of soluble leaf protein by SDS/PAGE, nondenaturing PAGE, and immunoblot analysis was performed as described (33). Total leaf genomic DNA was isolated using the DNeasy Plant Mini Kit and used to PCR amplify and sequence the transformed plastome region using primers 5′- CTATGGAATTCGAACCTGAACTT-3′ (LSH) and 5′- GAGGTGTGATACTTGGCTTGATTC-3′ (LSE) (Fig. 1B) (24). DNA blot analysis of the genomic DNA was used to confirm homoplasmicity (Fig S1). Total RNA was extracted from 0.5 cm2 of leaf in 0.8 mL TRIzol (Invitrogen). Six per cent or 12% of the RNA was separated on denaturing formaldehyde gels (34). The RNA was blotted onto Hybond-N nitrocellulose membrane (GE Healthcare) and probed with a 32P-labeled 5′ ΔrbcL probe (Fig. 1B) as described (24).
Rubisco Content and Catalytic Assessments.
Rates of rubisco 14CO2 fixation using soluble leaf protein extract were measured in 7-mL septum-capped scintillation vials in reaction buffer [50 mM Hepes-NaOH (pH 7.8), 10 mM MgCl2, 0.5 mM RuBP] containing varying concentrations of NaH14CO3 (0–67 μM) and O2 (0–25%) (vol/vol), accurately mixed with nitrogen using Wostoff gas-mixing pumps as described (24, 33). Assays (0.5 mL total volume) were started by the addition of activated leaf protein, and the Michaelis constants (Km) for CO2 (KC) and O2 (KO) were determined from the fitted data. Replicate measurements (n = 4–8) were made using protein preparations from two to four different leaves of independently transformed lines. For each sample the maximal rate of carboxylation (VC) was extrapolated from the Michealis–Menten fit and then normalized by dividing the rate by the number of rubisco-active sites quantified by [14C]2-CABP binding (35, 36). Rubisco CO2/O2 specificity (SC/O) was measured as described (37), using freshly extracted rubisco, quickly purified by ion exchange chromatography (24), from at least two separate plants for each independently transformed line.
Growth and Photosynthesis Analysis.
Wild-type (Nicotiana tabacum L. Petit Havana) and transplastomic tobacco lines were grown in growth chambers at 25 °C and 400 ± 50 μmol photons m−2·s−1 as described (24) in air or 0.5% (vol/vol) CO2-enriched air. Plant height from the soil surface to the apical meristem was measured until the first floral apertures emerged. Leaf photosynthesis and dark respiration rates in plants of comparable physiological development (45–50 cm in height; 14 or 15 leaves) were made in the growth chamber using an LI-6400 gas-exchange system (Li-COR) (24). The maximum quantum efficiency of PSII in dark-adapted leaves [variable fluorescence (Fv)/maximum fluorescence (Fm)] was measured in the same leaves using an LI-6400–40 Leaf Chamber Fluorometer.
Supplementary Material
Acknowledgments
This research was supported by Australian Research Council Discovery Grant FT0991407 (to S.M.W.) and by project AGL2009-07999 (Plan Nacional, Spain) (to J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109503108/-/DCSupplemental.
References
- 1.Edgerton MD. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 2009;149:7–13. doi: 10.1104/pp.108.130195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raines CA. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: Current and future strategies. Plant Physiol. 2011;155:36–42. doi: 10.1104/pp.110.168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Caemmerer S, Evans JR. Enhancing C3 photosynthesis. Plant Physiol. 2010;154:589–592. doi: 10.1104/pp.110.160952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson I, Backlund A. Structure and function of Rubisco. Plant Physiol Biochem. 2008;46:275–291. doi: 10.1016/j.plaphy.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Parry M, Madgwick P, Carvalho J, Andralojc P. Prospects for increasing photosynthesis by overcoming the limitations of Rubisco. J Agric Sci. 2007;145:31–43. [Google Scholar]
- 6.Zhu X-G, Portis AR, Long SP. Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant Cell Environ. 2004;27:155–165. [Google Scholar]
- 7.Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- 8.Savir Y, Noor E, Milo R, Tlusty T. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proc Natl Acad Sci USA. 2010;107:3475–3480. doi: 10.1073/pnas.0911663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitney SM, Houtz RL, Alonso H. Advancing our understanding and capacity to engineer nature's CO2-sequestering enzyme, Rubisco. Plant Physiol. 2011;155:27–35. doi: 10.1104/pp.110.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum O, et al. Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol. 2005;137:638–650. doi: 10.1104/pp.104.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christin P-A, et al. Evolutionary switch and genetic convergence on rbcL following the evolution of C4 photosynthesis. Mol Biol Evol. 2008;25:2361–2368. doi: 10.1093/molbev/msn178. [DOI] [PubMed] [Google Scholar]
- 12.Sage RF, Christin PA, Edwards EJ. The C4 plant lineages of planet Earth. J Exp Bot. 2011;62:3155–3169. doi: 10.1093/jxb/err048. [DOI] [PubMed] [Google Scholar]
- 13.Kebeish R, et al. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat Biotechnol. 2007;25:593–599. doi: 10.1038/nbt1299. [DOI] [PubMed] [Google Scholar]
- 14.Lieman-Hurwitz J, Rachmilevitch S, Mittler R, Marcus Y, Kaplan A. Enhanced photosynthesis and growth of transgenic plants that express ictB, a gene involved in HCO3- accumulation in cyanobacteria. Plant Biotechnol J. 2003;1:43–50. doi: 10.1046/j.1467-7652.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 15.Mueller-Cajar O, Whitney SM. Directing the evolution of Rubisco and Rubisco activase: First impressions of a new tool for photosynthesis research. Photosynth Res. 2008;98:667–675. doi: 10.1007/s11120-008-9324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa C, Hatanaka T, Misoo S, Miyake C, Fukayama H. Functional incorporation of sorghum small subunit increases the catalytic turnover rate of rubisco in transgenic rice. Plant Physiol. 2011;156:1603–1611. doi: 10.1104/pp.111.177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spreitzer RJ, Peddi SR, Satagopan S. Phylogenetic engineering at an interface between large and small subunits imparts land-plant kinetic properties to algal Rubisco. Proc Natl Acad Sci USA. 2005;102:17225–17230. doi: 10.1073/pnas.0508042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry JA. Genetic control of the kinetic parameters of RuP2 carboxylase: Studies of a C3 and a C4 species and their F hybrid. Carnegie Institution of Washington Annual Report. 1983;82:96–99. [Google Scholar]
- 19.Hudson GS, et al. Comparisons of rbcL genes for the large subunit of ribulose-bisphosphate carboxylase from closely related C3 and C4 plant species. J Biol Chem. 1990;265:808–814. [PubMed] [Google Scholar]
- 20.Kapralov MV, Kubien DS, Andersson I, Filatov DA. Changes in Rubisco kinetics during the evolution of C4 photosynthesis in Flaveria (Asteraceae) are associated with positive selection on genes encoding the enzyme. Mol Biol Evol. 2011;28:1491–1503. doi: 10.1093/molbev/msq335. [DOI] [PubMed] [Google Scholar]
- 21.Kubien DS, Whitney SM, Moore PV, Jesson LK. The biochemistry of Rubisco in Flaveria. J Exp Bot. 2008;59:1767–1777. doi: 10.1093/jxb/erm283. [DOI] [PubMed] [Google Scholar]
- 22.Alonso H, Blayney MJ, Beck JL, Whitney SM. Substrate-induced assembly of Methanococcoides burtonii D-ribulose-1,5-bisphosphate carboxylase/oxygenase dimers into decamers. J Biol Chem. 2009;284:33876–33882. doi: 10.1074/jbc.M109.050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitney SM, Sharwood RE. Construction of a tobacco master line to improve Rubisco engineering in chloroplasts. J Exp Bot. 2008;59:1909–1921. doi: 10.1093/jxb/erm311. [DOI] [PubMed] [Google Scholar]
- 24.Sharwood RE, von Caemmerer S, Maliga P, Whitney SM. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiol. 2008;146:83–96. doi: 10.1104/pp.107.109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney SM, Andrews TJ. Photosynthesis and growth of tobacco with a substituted bacterial Rubisco mirror the properties of the introduced enzyme. Plant Physiol. 2003;133:287–294. doi: 10.1104/pp.103.026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farquhar G, vonCaemmerer S, Berry J. A biochemical-model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 27.Kanevski I, Maliga P. Relocation of the plastid rbcL gene to the nucleus yields functional ribulose-1,5-bisphosphate carboxylase in tobacco chloroplasts. Proc Natl Acad Sci USA. 1994;91:1969–1973. doi: 10.1073/pnas.91.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maliga P, Bock R. Plastid biotechnology: Food, fuel, and medicine for the 21st century. Plant Physiol. 2011;155:1501–1510. doi: 10.1104/pp.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharwood R, Whitney S. In: The Chloroplast: Basics and Applications. Ca R, editor. Dordrecht, The Netherlands: Springer Science; 2010. pp. 285–306. [Google Scholar]
- 30.Zhang XH, et al. Hybrid Rubisco of tomato large subunits and tobacco small subunits is functional in tobacco plants. Plant Sci. 2011;180:480–488. doi: 10.1016/j.plantsci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Kane HJ, Wilkin JM, Portis AR, Andrews TJ. Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate. Plant Physiol. 1998;117:1059–1069. doi: 10.1104/pp.117.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce J, Tolbert NE, Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980;19:934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- 33.Whitney SM, Sharwood RE. Linked Rubisco subunits can assemble into functional oligomers without impeding catalytic performance. J Biol Chem. 2007;282:3809–3818. doi: 10.1074/jbc.M610479200. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2000. [Google Scholar]
- 35.Ruuska S, et al. The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Aust J Plant Physiol. 1998;25:859–870. [Google Scholar]
- 36.Whitney SM, Andrews TJ. Plastome-encoded bacterial ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) supports photosynthesis and growth in tobacco. Proc Natl Acad Sci USA. 2001;98:14738–14743. doi: 10.1073/pnas.261417298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane HJ, et al. An improved method for measuring the CO2/O2 specificity of ribulosebisphosphate carboxylase-oxygenase. Aust J Plant Physiol. 1994;21:449–461. [Google Scholar]
- 38.Christin P-A, et al. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution. 2011;65:643–660. doi: 10.1111/j.1558-5646.2010.01168.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.