Abstract
Background
Heightened immunogenicity, measured one month after the primary series of pneumococcal conjugate vaccine (PCV), in African children was previously hypothesized to be due to increased rates of nasopharyngeal pneumococcal colonization during early infancy.
Methods
We analyzed the effect of selected vaccine-serotype (6B, 19F and 23F) nasopharyngeal colonization prior to the first PCV dose or when colonized for the first time prior to the second or third (2nd/3rd) PCV dose on serotype quantitative and qualitative antibody responses.
Results
Colonization prior to receiving the first PCV was associated with lower geometric mean antibody concentrations (GMCs) one month after the third dose of PCV and six months later to the colonizing-serotype. Colonized infants also had lower geometric mean titers (GMTs) on opsonophagocytosis activity assay (OPA) and a lower proportion had titers ≥8 against the colonizing serotypes (19F and 23F) post vaccination. Colonization occurring only prior to the 2nd/3rd PCV dose was also associated with lower GMCs and OPA GMTs to the colonizing-serotype. The effect of colonization with serotypes 19F and 23F prior to PCV vaccination had a greater effect on a lower proportion of colonized infants having OPA titers ≥8 than the effect of colonization on the lower proportion with antibody ≥0.35 μg/ml.
Conclusion
Infant nasopharyngeal colonization at any stage before completing the primary series of PCV vaccination was associated with inferior quantitative and qualitative antibody responses to the colonizing-serotype.
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccine, HIV, immunogenicity, colonization, hypo-responsiveness
Introduction
Infants in industrializing countries and lower socio-economic groupings become colonized with Streptococcus pneumoniae at an earlier age than children from industrialized countries.(1) Early nasopharyngeal colonization by vaccine-serotypes was previously speculated, through either “priming” or “boosting” of the immune system, as being the reason for higher antibody geometric mean concentrations (GMCs) observed with pneumococcal conjugate vaccine (PCV) (using CRM197 protein) in African children compared to those from Europe and the general USA population. (2-5) This idea was supported by findings that increased social mixing in infancy was associated with enhanced immune response to PCV, attributed to immunological priming from the increased rate of pneumococcal nasopharyngeal colonization.(6) In addition, animal-model studies reported that intranasal colonization by S. pneumoniae resulted in higher IgG responses to subsequent PCV immunization, indicating that colonization elicited immunological memory capable of heightening immune responses to PCV. (7)
More recently, however, studies in Israeli and Phillipino infants have reported that nasopharyngeal colonization of commonly colonizing vaccine-serotypes (i.e. 6B, 19F and 23F) prior to receiving the first dose of two different PCV formulations, was associated with immune hypo-responsiveness to the colonizing serotype following a two or three dose primary series of PCV.(8, 9) This hypo-responsiveness did not, however, affect the immune response to non-colonizing serotypes. To our knowledge, there are no reports on the effect of serotype-specific colonization identified only after the first dose of PCV, i.e. absent prior to the first dose but present prior to receiving either the second or third dose of the primary infant series, on the immune response to PCV. In addition, the effect of early-infant colonization on functional antibody activity, measured by opsonophagocytic activity assay (OPA) is unknown.
The results of a sub-analysis related to the Comprehensive International Program for Research on AIDS-South Africa (CIPRA-SA Project-04),(10) evaluating the effect of early nasopharyngeal colonization on responses to PCV are presented here. The aims of this analysis were: (1). to evaluate the effect of specific vaccine-serotype nasopharyngeal colonization at the time of receiving the first dose of PCV on quantitative and qualitative (OPA) antibody responses one month after the three dose primary series; (2). To evaluate the effect of serotype-specific colonization identified for the first time at either the second and/or third (2nd/3rd) PCV dose on quantitative and qualitative antibody responses one month after the three dose primary series to the colonizing serotype, in comparison to infants in whom colonization was not detected for that serotype at least until the third dose of PCV; (3). Determine the effect of serotype-specific infant colonization whilst receiving the primary series of PCV on antibody concentrations six-months after completion of the primary series of PCV to the colonizing and non-colonizing serotypes.
Methods
Study population
Four groups of children were enrolled in parallel from April 2005 to June 2006 at the Perinatal HIV Research Unit (Johannesburg, South Africa) and the KIDS-Clinical Research Unit (Kid-Cru; Stellenbosch, South Africa). These included HIV-infected infants with CD4+ T-lymphocyte cells ≥25% randomized to either initiate antiretroviral treatment (ART) immediately (HIV+/ART+ group) or defer until clinically (CDC Stage C or investigator-selected severe Stage B) and/or immunologically indicated as per the then prevailing WHO recommendations (HIV+/ART− group).(11) In addition, two groups of HIV-uninfected infants were enrolled: [1]. HIV-exposed uninfected children (HEU), i.e. born to HIV-infected mothers; and [2]. HIV-unexposed uninfected (HUU) infants; i.e. born to HIV-uninfected women. (10) All children were ART naïve at enrolment, except for ART (primarily single-dose nevirapine postpartum) used for the prevention of mother-to-child HIV transmission. Daily trimethoprim-sulfamethoxazole prophylaxis was given to HIV-infected infants throughout infancy and also recommended for HEU infants until approximately six months of age. Details of the study cohort, including quantitative and qualitative antibody responses one-month following the primary series of three doses of 7-valent PCV (i.e. Prevnar®; Wyeth Vaccines, NJ, USA) given at 6, 10 and 14 weeks of age have been published. (10)
Laboratory assays and methods
Nasopharyngeal swabs to detect pneumococcal colonization were taken on the day of vaccination prior to each of the three doses of PCV. Swabs were collected using a Dacron-tipped swab on a flexible aluminum shaft (Cat# 151D, Medical Wire Equipment Co. Ltd.; Wiltshire, England) as described, (2) then inoculated into skim milk tryptone-glucose-glycerin transport media (STGG) and stored at −70 degrees Celsius until processing as recommended at the Respiratory and Meningeal Pathogens Research Unit (RMPRU) laboratory, South Africa.(12) Serotyping was performed using the Quellung method (Statens Serum Institute, Copenhagen, Denmark) at the National Institute for Communicable Diseases (NICD) reference laboratory. Pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F or 23F were categorized as vaccine-serotypes and the rest as non-vaccine serotypes.
Blood draws were undertaken immediately prior to receiving the first dose of PCV and at one- and six-months after the third dose of PCV and serum was archived at −70°C until testing. Vaccine-serotype specific capsular IgG antibody was measured by standardized enzyme immunoassay (EIA) at each of the above three time-points. OPA analysis was limited to serotypes 9V, 19F and 23F and undertaken on samples obtained one-month following the third PCV dose. Serotype 6B OPA responses were not undertaken as this exploratory analysis was not envisioned at the time of study design. All immunologic assays were undertaken at RMPRU as described. (10)
Statistical analysis
Data were analyzed using SAS® 9.1 (SAS Institute Inc., Cary, NC, USA.). To minimize confounding, only children who received their first PCV dose within 6-12 weeks of age and subsequent two doses 3-6 weeks after the preceding dose and had the primary immunogenicity blood draw 3-6 weeks following the third PCV dose were included in the analysis. The comparison of effect of nasopharyngeal colonization on immune responses to serotype-specific antibody responses was done for the three most common vaccine serotypes identified prior to the first PCV dose. Due to only a small number of children within any groups being colonized with the specific serotypes at baseline, analysis was controlled for but not stratified by HIV-infection status or use of anti-retroviral treatment.
The effect of early nasopharyngeal colonization on serotype-specific IgG GMCs and OPA geometric mean titers (GMT) was analyzed by analysis of covariance (ANCOVA) with study center, baseline antibody concentration, race, gender and study-group (i.e. HIV-infection and HIV exposure status) as co-variates. Logistic regression with study center, race, gender and study-group as covariates was applied for comparison of effect of nasopharyngeal colonization, prior to or subsequent to the first dose of PCV, on the proportion of children with serotype-specific antibody concentration ≥0.35 μg/ml or OPA titers ≥8. These respective thresholds are suggested as putative measures of community immunity against serotype-specific invasive pneumococcal disease.(13) An α value of ≤0.05 was considered significant.
Ethics Considerations
The study was approved by the Human Subjects Research Committees of the University of the Witwatersrand, Stellenbosch University, the Medicine Control Council of South Africa and Clinical Science Review Committee of the Division of AIDS. Signed informed consent was obtained from the parents of the children. The clinical trials registry reference number for the study is ClinicalTrials.gov NCT00099658.
Results
The analysis included 483 children, including 77 in the HIV+/ART− group, 172 in the HIV+/ART+ group, 120 HEU infants and 114 HU infants. The mean age when the nasopharyngeal swabs were done prior to receipt of each of the primary series of three doses of PCV were 7.3 (Standard deviation; “S.D.” 1.2), 11.4 (S.D. 1.2) and 15.4 (S.D. 1.2) weeks, respectively. Immune responses were measured prior to the first dose of PCV, as well as at 19.5 (S.D. 1.3) and 39.2 (S.D. 1.0) weeks of age. There was no difference in age between groups when swabs were taken or immune responses were measured. The majority (n=438; 90.7%) of study participants were Black African and 223 (46.2%) were males. The median CD4+ percentage prior to receiving the first PCV dose in HIV-infected children was 34.2% (interquartile range: 25.3 to 42.1) and did not differ between HIV+/ART− and HIV+/ART+ infants.
Forty-four (9.1%) of the 483 infants were colonized by vaccine-serotypes prior to receiving the first dose of PCV. The three most common serotypes were 23F (n=11), 6B (n=10) and 19F (n=9). Serotypes 23F, 6B and 19F were identified in a further 13, 13 and 17 children respectively prior to the second and/or third (2nd/3rd) PCV dose and which was absent prior to the first PCV dose.
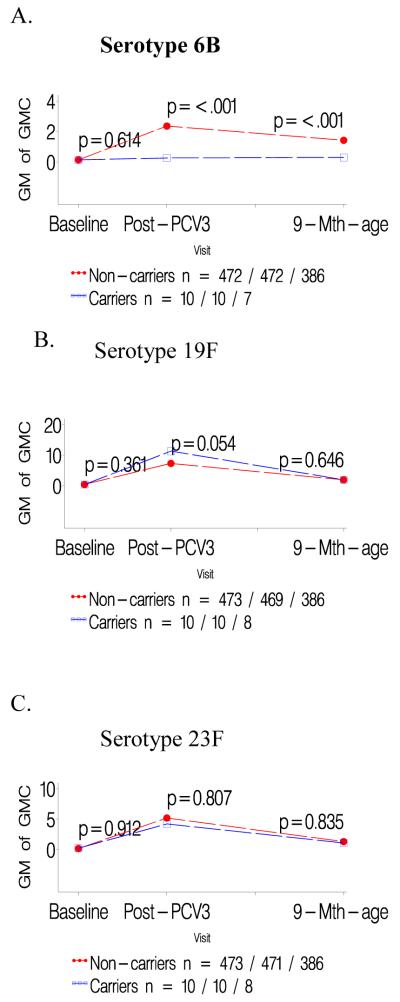
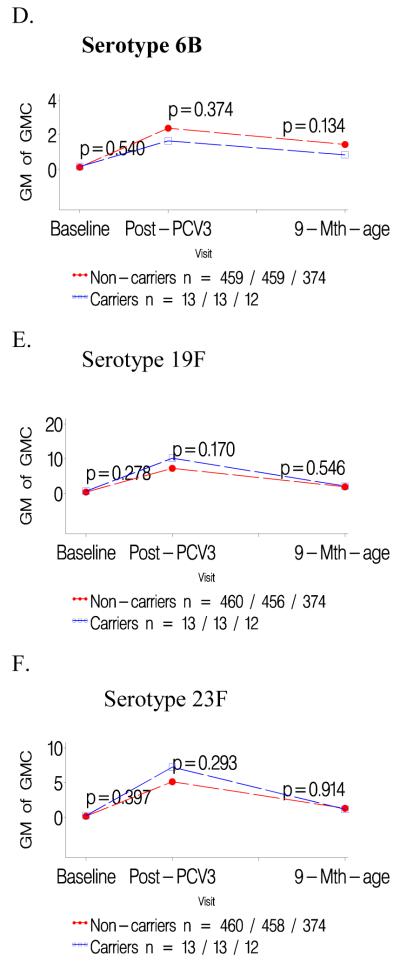
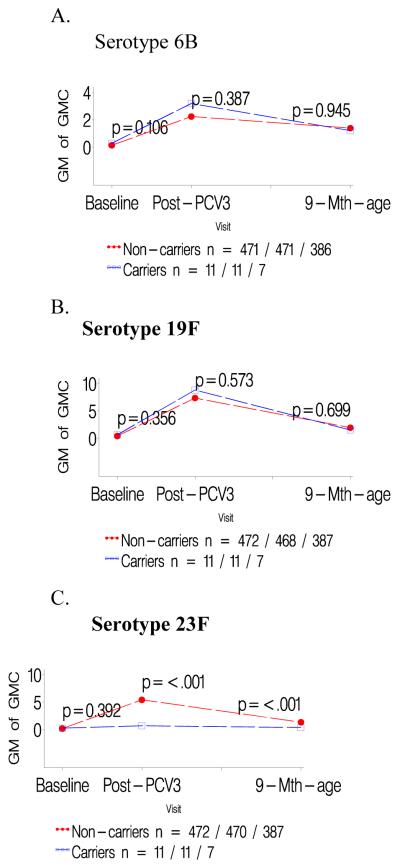
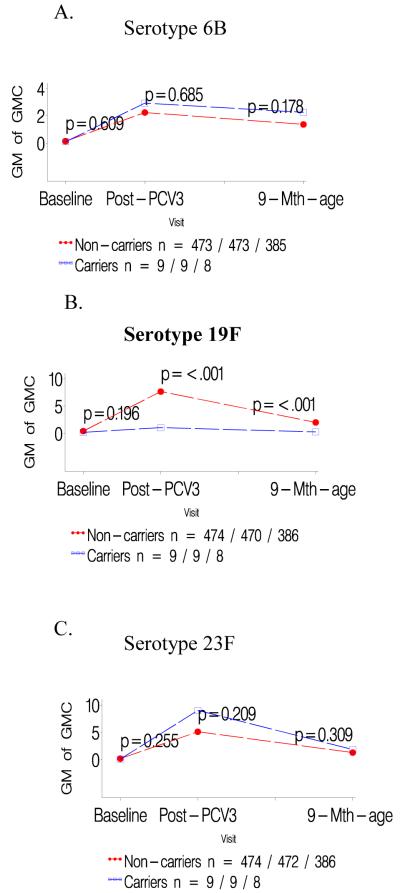
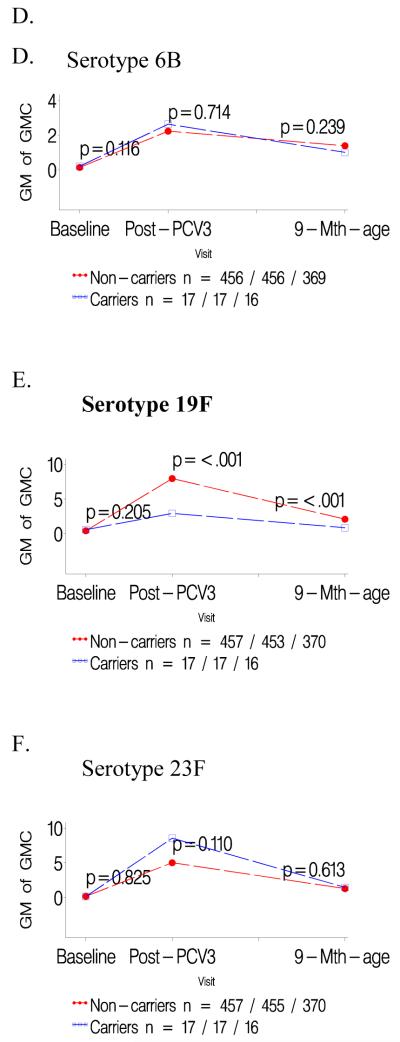
Infants colonized prior to the first PCV dose had consistently lower GMCs to the specific colonizing serotype than children not carrying that serotype prior to the first PCV dose, P<0.001 for all serotypes; table 1. In contrast, GMCs to the other two serotypes analyzed were unaffected when comparing carriers and non-carriers of the third serotype; e.g. GMCs to serotype 19F and 23F were unaffected in infants either colonized or not colonized by 6B; Table 1. The lower serotype-specific GMCs for carriers compared to non-carriers prior to the first PCV dose persisted until six months later; as did the lack of any difference in GMCs for the other two heterotypic serotypes in the related analysis; Figures 1-3 panels A-C.
Table 1.
Post primary series serotype-specific anticapsular IgG geometric mean antibody concentrations (GMCs, μg/ml) 3-6 weeks following the primary three-dose series of pneumococcal conjugate vaccine (PCV), in children who carried S. pneumoniae serotypes 6B, 19F or 23F prior to the first PCV dose, OR only at the 2nd and/or 3rd dose of PCV.
| Colonization status by serotype prior to first PCV dose | Colonization status by serotype only prior to 2nd and/or 3rd PCV dose |
|||||
|---|---|---|---|---|---|---|
| Serotype 6B carriers vs. non-carriers GMCs |
6B-positive pre- vaccination (n=10) |
6B-negative pre- vaccination* |
P-value | 6B-positive at 2nd or 3rd PCV dose (n=13) |
6B-negative until 3rd PCV dose* |
P-value |
| Anti-6B IgG (Min, Max) | 0.26 (0.08, 0.86) | 2.36 (2.07, 2.70) | <0.001 | 1.66 (0.74; 3.72) | 2.38 (2.08, 2.73) | 0.374 |
| Anti-19F IgG (Min, Max) | 11.35 (5.89, 21.85) | 7.32 (6.73, 7.96) | 0.054 | 10.16 (6.49; 15.90) | 7.25 (6.66, 7.90) | 0.170 |
| Anti-23F IgG (Min, Max) | 4.21 (1.12, 15.75) | 5.19 (4.66, 5.78) | 0.807 | 7.25 (3.63, 14.50) | 5.14 (4.60; 5.74) | 0.293 |
| Serotype 19F carriers vs. non-carriers GMCs |
19F-positive pre- vaccination (n=9) |
19F-negative pre-vaccination |
P-value | 19F-positive at 2nd or 3rd PCV dose (n=17) |
19F-negative until 3rd PCV dose |
P-value |
|---|---|---|---|---|---|---|
| Anti-6B IgG (Min, Max) | 2.93 (1.14, 7.54) | 2.24 (1.96, 2.57) | 0.685 | 2.63 (1.28, 5.40) | 2.23 (1.94, 2.56) | 0.714 |
| Anti-19F IgG (Min, Max) | 1.06 (0.34, 3.26) | 7.67 (7.08, 8.30) | <0.001 | 2.92 (1.33, 6.43) | 7.95 (7.37, 8.57) | <0.0001 |
| Anti-23F IgG (Min, Max) | 9.02(5.27, 15.42) | 5.11 (4.58, 5.71) | 0.209 | 8.56 (5.47, 13.39) | 5.07 (4.48, 5.61) | 0.109 |
| Serotype 23F carriers vs. non-carriers GMCs |
23F-positive pre- vaccination (n=11) |
23F-negative pre-vaccination |
P-value | 23F-positive at 2nd or 3rd PCV dose (n=13) |
23F-negative until 3rd PCV dose |
P-value |
|---|---|---|---|---|---|---|
| Anti-6B IgG (Min, Max) | 3.19 (1.26, 8.10) | 2.24 (1.95, 2.56) | 0.387 | 2.33 (0.75, 7.20) | 2.23 (1.95, 2.56) | 0.941 |
| Anti-19F IgG (Min, Max) | 8.82 (5.00, 15.56) | 7.36 (6.76, 8.00) | 0.573 | 6.97 (3.65, 13.30) | 7.37 (6.76, 8.02) | 0.911 |
| Anti-23F IgG (Min, Max) | 0.68 (0.29, 1.60) | 5.42 (4.87, 6.02) | <0.0001 | 1.06 (0.34, 3.30) | 5.68 (5.12, 6.29) | <0.0001 |
Rows in shaded grey: antibodies against the carried serotype (Min, Max) = Minimum and maximum range.
Note: The number of observations for the non-carriers, based on sample availability, is indicated in Table 2.
Figure 1.
a-c: Comparison of geometric mean antibody concentrations at baseline and one- and six months after three doses of pneumococcal conjugate vaccine (PCV) for colonized versus not colonized with serotype 6B prior to first dose of PCV.
d-f: Comparison of geometric mean antibody concentrations at baseline and one- and six months after three doses of pneumococcal conjugate vaccine (PCV) for infants colonized with serotype 6B only prior to the second and/or third dose of PCV versus those not colonized until the third PCV dose.
Figure 3.
a-c: Comparison of geometric mean antibody concentrations at baseline and one- and six months after three doses of pneumococcal conjugate vaccine (PCV) for colonized versus not colonized with serotype 23F prior to first dose of PCV.
d-f: Comparison of geometric mean antibody concentrations at baseline and one- and six months after three doses of pneumococcal conjugate vaccine (PCV) for infants colonized with serotype 23F only prior to the second and/or third dose of PCV versus those not colonized until the third PCV dose.
Lower GMCs were also observed in children in whom colonization by serotypes 19F (p<0.0001) or 23F (p<0.0001) was first identified only prior to the 2nd/3rd PCV dose, compared to infants never colonized by the specific serotype until the third PCV dose, table 1. A similar, albeit not significant, trend was observed for serotype 6B (p=0.374). Immune responses to the two heterotypic serotypes being analyzed did not differ between colonized and non-colonized children relative to the third serotype, table 1. The lower serotype-specific GMCs among carriers, in relation to first colonization only prior to 2nd/3rd PCV dose, persisted until six months later, albeit only significant for serotype 19F (p<0.001; Figure 1-3 panels D-F).
A lower proportion of serotype 6B carriers prior to the first PCV dose (40.0%) than 6B non-carriers (90.5%; p<0.0001) had anti-6B specific antibody ≥0.35 ug/ml one month after the third PCV dose, whereas the proportions did not differ for the two heterotypic serotypes under study; Table 2. Similar differences were observed for serotype 23F associated colonization at baseline between carriers compared to non-carriers with anti-23F ≥0.35 ug/ml (81.8% vs. 96.8%, respectively; p=0.015); as well as in relation to 23F colonization status only prior to the 2nd/3rd PCV dose (69.2% vs. 97.6%, respectively; p<0.0001). There was, however, no difference in proportion of infants with anti-19F antibody ≥0.35 ug/ml between 19F-carriers and non-colonized infants either in relation to 19F colonization prior to the first PCV dose or only prior to the 2nd/3rd PCV dose; Table 2.
Table 2.
Proportion of children with serotype-specific anticapsular IgG ≥0.35 μg/ml, 3-6 weeks following the primary three-dose series of pneumococcal conjugate vaccine (PCV), in children who carried S. pneumoniae serotypes 6B, 19F or 23F prior to the first PCV dose; or only at the 2nd and/or 3rd dose of PCV.
| Colonization status by serotype prior to first PCV dose |
Colonization status by serotype only prior to 2nd and/or 3rd PCV dose |
|||||
|---|---|---|---|---|---|---|
| Serotype 6B carriers vs. non-carriers |
6B-positive (n=10) |
6B-negative | P-value | 6B-positive (n=13) |
6B-negative until 3rd PCV dose |
P-value |
| Anti-6B: n with ≥0.35 μg/ml (%) | 4 (40.0) | 427/ 472 (90.5) | <0.0001 | 11 (84.6) | 416/ 459 (90.6) | 0.615 |
| Anti-19F: n with ≥0.35 μg/ml (%) | 10 (100.0) | 465/ 469 (99.1) | NE* | 13 (100) | 452/ 456 (99.1) | NE |
| Anti-23F: n with ≥0.35 μg/ml (%) | 9 (90.0) | 455/ 471 (96.6) | 0.436 | 13 (100) | 442/ 458 (96.5) | 0.99 |
| Serotype 19F carriers vs. non-carriers |
19F-positive (n=9) | 19F-negative | P-value | 19F-positive (n=17) |
19F-negative until 3rd PCV dose |
P-value |
|---|---|---|---|---|---|---|
| Anti-6B: n with ≥0.35 μg/ml (%) | 8 (88.9) | 423/473 (89.4) | 0.877 | 15 (88.2) | 408/456 (89.6) | 0.946 |
| Anti-19F: n with ≥0.35 μg/ml (%) | 8 (88.9) | 467/470 (99.4) | NE | 16 (94.1) | 451/453 (99.6) | NE |
| Anti-23F: n with ≥0.35 μg/ml (%) | 9 (100.0) | 455/472 (96.4) | NE | 17 (100.0) | 438/455 (96.3) | NE |
| Serotype 23F carriers vs . non-carriers |
23F-positive (n=11) | 23F-negative | P-value | 23F-positive (n=13) |
23F-negative until 3rd PCV dose |
P-value |
|---|---|---|---|---|---|---|
| Anti-6B: n with ≥0.35 μg/ml (%) | 10 (90.9) | 421/471 (89.4) | 0.960 | 11 (84.6) | 410/458 (89.5) | 0.378 |
| Anti-19F: n with ≥0.35 μg/ml (%) | 11 (100.0) | 464/468 (99.1) | NE | 13 (100) | 451/455 (99.1) | NE |
| Anti-23F: n with ≥0.35 μg/ml (%) | 9 (81.8) | 455/470 (96.8) | 0.015 | 9 (69.2) | 446/457 (97.6) | <0.0001 |
Rows in shaded grey: antibodies against the carried serotype.
NE: The reporting of p-values as NE (not estimable) was due to either quasi-complete separation or complete separation which is a common issue in the Logistic Regression analysis. These p-values would generally have been ≥0.95.
The OPA GMTs were consistently lower in 19F- and 23F-colonized compared to non-colonized children in relation to carrier status either prior to the first PCV dose or when first identified prior to the 2nd/3rd PCV dose, p<0.0001 for all; Table 3. Similar to the antibody IgG GMCs, the difference in GMTs were specific to the serotype associated with colonization and did not affect OPA GMTs for the heterotypic serotypes. In addition, a significantly lower proportion of colonized compared to non-colonized infants had OPA titers ≥ 8 for serotypes 19F and 23F in relation to the pre-vaccination colonization status; and in relation to carrier status when first identified only prior to the 2nd/3rd PCV dose; Table 4. The proportion of colonized compared to non-colonized children with OPA titers ≥ 8 did not differ for the heterotypic serotypes, except for serotype 19F (84.6% vs. 96.8%, p=0.020) in the analysis comparing infants with and without 23F colonization at the 2nd/3rd PCV dose. There was no difference between 6B carriers and non-carriers either in relation to baseline or prior to 2nd/3rd PCV dose colonization-status and OPA GMTs or proportion with OPA ≥ 8 to any of the serotypes analyzed by OPA.
Table 3.
Post primary series opsonophagocytic activity assay (OPA) geometric mean titers (GMT), measured 3-6 weeks after the third pneumococcal conjugate vaccine (PCV) dose, in children who carried S. pneumoniae serotypes 6B, 19F or 23F prior to the first dose; or only detected at the 2nd and/or 3rd vaccine dose.
| Colonization status by serotype prior to first PCV dose |
Colonization status by serotype only prior to 2nd and/or 3rd PCV dose |
|||||
|---|---|---|---|---|---|---|
| Serotype 6B carriers vs . non- carriers |
6B-positive pre- vaccination(n=10) |
6B-negative pre- vaccination (n)* |
P-value | 6B-positive pre- vaccination (n=13) |
6B-negative pre- vaccination |
P-value |
| Anti-9V OPA GMT (Min, Max) | 805 (452, 1435) | 574 (513; 641) | 0.174 | 637 (369, 1099) | 572 (511, 641) | 0.592 |
| Anti-19F OPA GMT (Min, Max) | 423 (183; 976) | 278 (250, 311) | 0.184 | 394 (205, 756) | 276 (247, 309) | 0.261 |
| Anti-23F OPA GMT (Min, Max) | 2584 (867; 7707) | 1519 (1303;1770) | 0.124 | 1524 (606, 3835) | 1519(1300, 1775) | 0.959 |
| Serotype 19F carriers vs . non- carriers |
19F-positive pre- vaccination(n=9) |
19F-negative pre- vaccination |
P-value | 19F-positive pre- vaccination (n=17) |
19F-negative pre- vaccination |
P-value |
|---|---|---|---|---|---|---|
| Anti-9V OPA GMT (Min,Max) | 889 (590 1341) | 573 (513; 640) | 0.430 | 592 (342, 1024) | 573 (511, 641) | 0.965 |
| Anti-19F OPA GMT (Min, Max) | 17 (4, 80) | 296 (267, 328) | <0.0001 | 105 (41, 268) | 308 (279, 340) | <0.0001 |
| Anti-23F OPA GMT (Min, Max) | 4050 (1495,10972) | 1508 (1294, 1757) | 0.100 | 2460 (1391, 4350) | 1481 (1265, 1733) | 0.304 |
| Serotype 23F carriers vs . non- carriers |
23F-positive pre- vaccination (n=11) |
23F-negative pre- vaccination |
P-value | 23F-positive pre- vaccination (n=13) |
23F-negative pre- vaccination |
P-value |
|---|---|---|---|---|---|---|
| Anti-9V OPA GMT (Min, Max) | 964 (497, 1871) | 571 (511, 638) | 0.325 | 404 (195, 838) | 577 (515, 646) | 0.195 |
| Anti-19F OPA GMT (Min, Max) | 484 (295, 794) | 278 (249, 309) | 0.243 | 185 (59, 580) | 281 (252, 313) | 0.187 |
| Anti-23F OPA GMT (Min, Max) | 20 (3, 136) | 1701 (1483, 1950) | <0.0001 | 229 (37, 1411) | 1801 (1582, 2050) | <0.0001 |
Rows in shaded grey: antibodies against the carried serotype. (Min, Max) = Minimum and maximum range.
Note: The number of observations for the non-carriers, based on sample availability, is indicated in Table 4.
Table 4.
Proportion of children with serotype-specific opsonophagocytic activity (OPA) geometric mean titer (GMT) ≥8, measured 3-6 weeks after the third pneumococcal conjugate vaccine (PCV) dose, in children who carried S. pneumoniae serotypes 6B, 19F or 23F prior to the first dose; or only detected at the 2nd and/or 3rd vaccine dose.
| Colonization status by serotype prior to first PCV dose |
Colonization status by serotype only prior to 2ndand/or 3rd PCV dose |
|||||
|---|---|---|---|---|---|---|
| Serotype 6B carriers vs. non-carriers |
6B-positive pre- vaccination (n=10) |
6B-negative pre-vaccination |
P-value | 6B-positive pre- vaccination (n=13) |
6B-negative pre- vaccination |
P-value |
| Anti-9V: n with OPA titre ≥8 (%) | 10 (100) | 461/472 (97.7) | NE* | 13 (100) | 448/459 (97.6) | NE |
| Anti-19F:n with OPA titre ≥8 (%) | 10 (100) | 455/473 (96.2) | NE | 13 (100) | 442/460 (96.1) | NE |
| Anti-23F: n with OPA titre ≥8 (%) | 10 (100) | 450/471 (95.5) | NE | 13 (100) | 437/458 (95.4) | NE |
| Serotype 19F carriers vs. non-carriers |
19F-positive pre- vaccination (n=9) |
19F-negative pre vaccination |
P-value | 19F-positive pre- vaccination (n=17) |
19F-negative pre-vaccination |
P-value |
|---|---|---|---|---|---|---|
| Anti-9V: n with OPA titre ≥8(%) | 9 (100) | 462/473 (97.7) | NE | 17 (100) | 445/456 (97.6) | NE |
| Anti-19F:n with OPA titre ≥8 (%) | 4 (44.4) | 461/474 (97.3) | <0.0001 | 14 (82.4) | 447/457 (97.8) | 0.001 |
| Anti-23F: n with OPA titre ≥8 (%) | 9 (100) | 451/472 (95.6) | NE | 17 (100) | 434/455 (95.4) | NE |
| Serotype 23F carriers vs. non-carriers |
23F-positive pre- vaccination (n=11) |
23F-negative pre-vaccination |
P-value | 23F-positive pre- vaccination (n=13) |
23F-negative pre-vaccination |
P-value |
|---|---|---|---|---|---|---|
| Anti-9V: n with OPA titre ≥8 (%) | 11 (100) | 460/471 (97.7) | NE | 13 (100) | 447/458 (97.6) | NE |
| Anti-19F:n with OPA titre ≥8 (%) | 11 (100) | 454/472 (96.2) | NE | 11 (84.6) | 443/459 (96.5) | 0.021 |
| Anti-23F: n with OPA titre ≥8 (%) | 3 (27.3) | 457/470 (97.2) | <0.0001 | 9 (69.2) | 448/457 (98.0) | <0.0001 |
Rows in shaded grey: antibodies against the carried serotype.
NE: The reporting of p-values as NE (not estimable) was due to either quasi-complete separation or complete separation which is a common issue in the Logistic Regression analysis. These p-values would generally have been ≥0.95. not estimable
Discussion
Our study corroborates the findings of two recently published studies reporting that nasopharyngeal colonization prior to the first PCV dose in young infants was associated with hypo-responsiveness to the specific colonizing serotypes. (8, 9) Our study further extends the findings to African populations that include HIV-infected and HIV-exposed uninfected children. In addition, our analysis expanded on the previous findings in two ways. Firstly we showed that a similar hypo-responsiveness was evident in children colonized with that serotype prior to receiving any PCV dose and for those who became colonized after the initial PCV dose but prior to receipt of the 2nd/3rd PCV dose for at least two (19F and 23F) of the three serotypes. However, the persistence of this effect on GMCs six months after completion of the primary series was less evident (only significant for 19F) in children only identified to be colonized at the 2nd/3rd PCV dose compared to colonization prior to the first PCV dose, where the lower serotype-specific GMCs persisted for all three serotypes.
Nevertheless, our analysis does not validate the previous speculation that serotype-specific pneumococcal colonization, either prior to or during the time of completing the primary series of PCV, contributes toward the higher serotype-specific GMCs observed in immunogenicity studies in children from settings with a high rate of pneumococcal colonization during early infancy. (2-5, 14, 15) Our study was not powered to explore whether there was any independent association of HIV-infection status in the interaction of early infant colonization on immune responses to PCV, hence, controlling for both HIV-infection status and antiretroviral treatment in the analysis. It is possible that infants born to HIV-infected mothers may be at greater risk of acquisition of serotypes explored for in our study from their mothers. Gill et al reported that HIV-infected African women were 1.9 fold more likely to be colonized by pneumococcus - especially serogroups 6, 19 and 23- than HIV-uninfected women. (16)
Our study also examined the effect of vaccine-serotype colonization pre- or during receipt of PCV on the functionality of serotype-specific antibody as measured by OPA. Lower serotype specific OPA GMTs were identified in colonized compared to non-colonized infants for both serotypes 19F and 23F, in relation to colonization prior to the first or 2nd/3rd -PCV dose. However, the difference in proportion of serotype-specific colonized infants achieving OPA titers ≥8 was less affected when colonization first occurred prior to the 2nd/3rd PCV dose than when identified prior to the first PCV dose for serotype 19 F (82.4% vs. 44.4%, respectively) and 23F (69.2% vs. 27.3%, respectively). The importance of considering both IgG concentration and OPA titers in evaluating the differential effect of colonization on the immune response was evident in that the proportion of subjects with OPA ≥8 tended to be lower than the proportion with IgG concentrations ≥0.35 ug/ml for serotypes 19F (44.4% vs. 88.9%, respectively; p=0.17) and serotype 23F (27.3% vs. 81.8%, respectively; p=0.015). Furthermore, although there was no difference observed in the proportion of 19F colonized compared to non-colonized infants with anti-19F IgG ≥0.35 ug/ml, the proportion with anti-19F OPA ≥8 titers was lower in colonized infants. The significance of these findings include that OPA measures complement-mediated phagocytic killing and may be considered a preferable outcome measure than serotype-specific antibody concentration of ≥0.35 ug/ml, because opsonophagocytosis is thought to be the main protective response in vivo. (13, 17)
Although our study focused on the impact of colonization whilst receiving the primary series of PCV on quantitative and qualitative immune measurements, these findings need to be further examined with regard to the effect of early colonization upon subsequent anamnestic responses. Dagan et al. measured quantitative antibody levels one month after a booster dose of PCV and reported that lower serotype-specific antibody concentrations persisted, although to a lesser extent, in the group who had been colonized with the corresponding serotype prior to the first PCV dose.(8) These data suggest that early pneumococcal colonization may affect anamnestic responses to the specific colonizing serotype, which, may be a more important in providing long term protection against invasive pneumococcal disease.
The results from our study might explain in part, the higher number of vaccine failures and consequently lower vaccine efficacy estimates against invasive pneumococcal disease, albeit with overlapping 95% confidence intervals, observed in African and Navajo Indian children than in the general USA population;(3, 18-20) and the higher serotype specific estimates of the correlate of protection in African children.(21) The suggestion that in settings with a high acquisition rate of nasopharyngeal colonization by vaccine serotypes a greater proportion of children may be sub-optimally protected against invasive pneumococcal disease may, however, become less important following widespread use of PCVs. Specifically, introduction of PCV in the USA was associated with a rapid reduction in prevalence of vaccine-serotype colonization in vaccinated and –unvaccinated individuals,(22) including in communities with a high rate of pneumococcal colonization during early infancy.(23) In addition, there has been virtual disappearance of vaccine-serotype invasive disease among vaccinated and unvaccinated populations in a diversity of settings.(24-27) This effect was attributed to a critical proportion of young children, who generally are the important source of transmission of pneumococci in developed countries, having become protected from acquiring new vaccine serotypes following vaccination. Consequently, transmission of these vaccine serotypes was probably interrupted in the community leading to the subsequent elimination of these serotypes in the community. The dynamics of pneumococcal transmission in developing countries, including rural settings in Africa have not yet been fully established. This may differ compared to developed countries as the prevalence of nasopharyngeal colonization in older children and adults in Africa remains high (>40%) compared to developed countries (<15%).(28) Consequently, there may be ongoing transmission of vaccine-serotypes from older individuals to very young infants prior to the latter receiving PCV, which may potentially continue affecting their immune responses to colonizing vaccine-serotypes.
Dagan et al. have previously discussed the likely mechanism for serotype-specific colonization interfering with the immune response to that specific serotype in the first PCV immunization. They suggested that polysaccharide from nasopharyngeal colonization could be systemically absorbed and prevent serotype-specific B lymphocyte differentiation in the marginal zone of the spleen and lymph nodes; thereby inducing B cell fatigue. (8) They also described that the effect of early infant colonization on immune responses to the specific serotypes persisted even after a booster PCV dose. A question posed by Dagan et al, was whether the effect of early nasopharyngeal colonization on immune responses could be modified by alternate dosing schedules, for example a first PCV dose at 4 weeks of age. Our observation of hypo-responsiveness even after the first dose of PCV, albeit to a lesser extent compared to colonization prior to the first PCV dose, suggests that vaccinating at an early age will have only limited effect on circumventing the effect of early infant colonization. The study by Dagan et al. included children who received three doses of PCV at 2, 4 and 6 months of age. Despite a later start with immunization and increased interval between the PCV doses in Israeli children, (8) the GMCs one month after three doses of PCV was higher among infants in our study for the colonized and more so for those not colonized for serotypes 19F and 23F prior to the first PCV dose. This indicates that there are other factors, excluding exposure to serotypes from colonization prior to PCV vaccination, which result in enhanced immunogenicity of PCV in African children.
In conclusion, our findings raise questions as to whether the immunogenicity of PCV can be improved in settings with a high rate of early nasopharyngeal colonization by modulating risk factors associated with early-infant colonization. Identifiable risk factors associated with increased susceptibility such as overcrowding, in-door pollution and viral infections are, however, inherently difficult or unlikely to be modifiable. Whether interventions such as antibiotic prophylaxis, attempting to reduce pneumococcal nasopharyngeal colonization by at receipt of the primary infants’ series are warranted is uncertain and may rather contribute to emergence of bacterial antibiotic resistance. Ongoing monitoring of the effect of widespread PCV implementation in low resource settings, with a high acquisition rate of early infant colonization, is warranted to determine whether any targeted strategies to reduce early acquisition of pneumococcal colonization will be required.
Figure 2.
a-c: Comparison of geometric mean antibody concentrations at baseline and one- and six months after three doses of pneumococcal conjugate vaccine (PCV) for colonized versus not colonized with serotype 19F prior to first dose of PCV.
d-f: Comparison of geometric mean antibody concentrations at baseline and one- and six months after three doses of pneumococcal conjugate vaccine (PCV) for infants colonized with serotype 19F only prior to the second and/or third dose of PCV versus those not colonized until the third PCV dose.
Acknowledgements
Collaborators and Centers for study: South Africa: Avy Violari, Ronelle van Niekerk, James McIntyre, Wilma Pelser, Aneesa Naeem Sheik, Melissa Budge, Munira Saleh, Afaaf Liberty, Erica Lazarus, Sindile Mashinini, Sibongile Dlamini, Valerie Kemese, Jean Bolton (Perinatal HIV Research Unit); Mark F Cotton, Helena Rabie, Anita Janse van Rensburg, Els Dobbels, George Fourie, Marietjie Bester, Wilma Orange, Ronelle Arendze, Catherine Andrea, Marlize Smuts, Kurt Smith, Theresa Louw, Alec Abrahams, Kenny Kelly, Amelia Bohle, Irene Mong, Jodie Howard, Tanya Cyster, Genevieve Solomon, Galroy Benjamin, Jennifer Mkalipi, Edward Barnes (Children’s Infectious Diseases Clinical Research Unit); Glenda Gray, Ian Sanne, ‘Ravindre Panchia, Christie Davies, Morna Cornell (CIPRA-SA); Peter Adrian; Shabir A Madhi; Nadia van Niekerk (Respiratory and Meningeal Pathogens Research Unit)
United States of America: Karen Reese, Jeff Nadler (DAIDS/NIAID/NIH), Patrick Jean-Philippe (HJF-DAIDS), Jim McNamara (DAIT/NIAID/NIH), Rod Hoff (REDI Centre), Sandi Lehrman (Merck), Chuck Oster (Walter Reed), Sharon Nachman (New York City University). United Kingdom: Abdel G Babiker, Diana M Gibb, (Medical Research Council Clinical Trials Unit, London) Finland: Helena Kayhty
National Institute for Communicable Diseases (for serotyping of isolates): Linda De Gouveia,
Funding sources:
Support for this study was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes for Health (NIH), through the Comprehensive International Program of Research on AIDS (CIPRA) network, grant number U19 AI53217. Additional support for this work was provided with Federal funds from the National Institute of Allergies & Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200800014C; and Gates Grand Challenge PneumoCarr Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
The content of this publication does not necessarily reflect the views or policies of NIAID, nor does mention of trade names, commercial projects, or organizations imply endorsement by the US Government.
Conflict of interest: Receipt of research grants, consultancies and honoraria from GSK and Pfizer (SAM, KPK, AvG). Prevenar® was donated by Wyeth.
References
- 1.O’Brien KL, Dagan R. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine. 2003 May 16;21(17-18):1815–25. doi: 10.1016/s0264-410x(02)00807-1. [DOI] [PubMed] [Google Scholar]
- 2.Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180(4):1171–6. doi: 10.1086/315009. 1999. [DOI] [PubMed] [Google Scholar]
- 3.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Northern California Kaiser Permanente Vaccine Study Center Group Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000 Mar;19(3):187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Nurkka A, Ahman H, Korkeila M, Jantti V, Kayhty H, Eskola J. Serum and salivary anti-capsular antibodies in infants and children immunized with the heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2001;20(1):25–33. doi: 10.1097/00006454-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Obaro SK, Adegbola RA, Chang I, Banya WA, Jaffar S, Mcadam KW, et al. Safety and immunogenicity of a nonavalent pneumococcal vaccine conjugated to CRM197 administered simultaneously but in a separate syringe with diphtheria, tetanus and pertussis vaccines in Gambian infants. Pediatr Infect Dis J. 2000;19(5):463–9. doi: 10.1097/00006454-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Salt P, Banner C, Oh S, Yu LM, Lewis S, Pan D, et al. Social mixing with other children during infancy enhances antibody response to a pneumococcal conjugate vaccine in early childhood. Clin Vaccine Immunol. 2007 May;14(5):593–9. doi: 10.1128/CVI.00344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabquer B, Shriner AK, Smithson SL, Westerink MA. B cell mediated priming following pneumococcal colonization. Vaccine. 2007 Mar 1;25(11):2036–42. doi: 10.1016/j.vaccine.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis. 2010 May 15;201(10):1570–9. doi: 10.1086/652006. [DOI] [PubMed] [Google Scholar]
- 9.Vakevainen M, Soininen A, Lucero M, Nohynek H, Auranen K, Makela PH, et al. Serotype-specific hyporesponsiveness to pneumococcal conjugate vaccine in infants carrying pneumococcus at the time of vaccination. J Pediatr. 2010 Nov;157(5):778–83. e1. doi: 10.1016/j.jpeds.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 10.Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010 Aug 15;202(3):355–61. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early Antiretroviral Therapy and Mortality among HIV-Infected Infants. N Engl J Med. 2008 Nov 20;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien KL, Bronsdon MA, Dagan R, Yagupsky P, Janco J, Elliott J, et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J Clin Microbiol. 2001 Mar;39(3):1021–4. doi: 10.1128/JCM.39.3.1021-1024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jodar L, Butler J, Carlone G, Dagan R, Goldblatt D, Kayhty H, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003 Jul 4;21(23):3265–72. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 14.Millar EV, O’Brien KL, Zell ER, Bronsdon MA, Reid R, Santosham M. Nasopharyngeal carriage of Streptococcus pneumoniae in Navajo and White Mountain Apache children before the introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2009 Aug;28(8):711–6. doi: 10.1097/INF.0b013e3181a06303. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien KL, Moisi J, Moulton LH, Madore D, Eick A, Reid R, et al. Predictors of pneumococcal conjugate vaccine immunogenicity among infants and toddlers in an American Indian PnCRM7 efficacy trial. J Infect Dis. 2007 Jul 1;196(1):104–14. doi: 10.1086/518438. [DOI] [PubMed] [Google Scholar]
- 16.Gill CJ, Mwanakasale V, Fox MP, Chilengi R, Tembo M, Nsofwa M, et al. Impact of human immunodeficiency virus infection on Streptococcus pneumoniae colonization and seroepidemiology among Zambian women. J Infect Dis. 2008 Apr 1;197(7):1000–5. doi: 10.1086/528806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieninger DM, Kueper K, Steul K, Juergens C, Ahlers N, Baker S, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010 Jun 7;28(25):4192–203. doi: 10.1016/j.vaccine.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005 Apr;365(9465):1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 19.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003 Oct 2;349(14):1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003 Aug 2;362(9381):355–61. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 21.Siber GR, Chang I, Baker S, Fernsten P, O’brien KL, Santosham M, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007 May 10;25(19):3816–26. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 22.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005 Sep;116(3):e408–13. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien KL, Millar EV, Zell ER, Bronsdon M, Weatherholtz R, Reid R, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007 Oct 15;196(8):1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 24.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May 1;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 25.Van Effelterre T, Moore MR, Fierens F, Whitney CG, White L, Pelton SI, et al. A dynamic model of pneumococcal infection in the United States: implications for prevention through vaccination. Vaccine. 2010 May 7;28(21):3650–60. doi: 10.1016/j.vaccine.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Lacapa R, Bliss SJ, Larzelere-Hinton F, Eagle KJ, McGinty DJ, Parkinson AJ, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008 Aug 15;47(4):476–84. doi: 10.1086/590001. [DOI] [PubMed] [Google Scholar]
- 27.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007 Apr 25;297(16):1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 28.Hill PC, Townend J, Antonio M, Akisanya B, Ebruke C, Lahai G, et al. Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis. 2010 Jun 1;50(11):1468–76. doi: 10.1086/652443. [DOI] [PubMed] [Google Scholar]