Abstract
Broadly neutralizing antibodies (bnAbs) are likely to be a key component of protective immunity conferred by an effective HIV-1 vaccine. We and others have reported that putative human germline predecessors of known human bnAbs lack measurable binding to HIV-1 envelope glycoproteins (Env), which could be a new challenge for eliciting human bnAbs. Rhesus macaques have been used as nonhuman primate models for testing vaccine candidates, but little is known about their germline Abs. Here we show the similarities and differences between putative rhesus macaque and human germline predecessors and possible intermediate antibodies of one of the best characterized bnAbs, b12. Similar to the human counterpart, a putative rhesus macaque b12 germline antibody lacks measurable binding to HIV-1 Envs, suggesting that initiation of somatic maturation of rhesus macaque germline b12 predecessor may also be a challenge. However, differences in sequence characteristics and binding properties between macaque and human b12 germline and intermediate antibodies suggest that the two germline predecessors may undergo different maturation pathways in rhesus macaques and in humans. These results indicate that immunogens that could initiate the immune responses and drive somatic mutations leading to elicitation of b12 or b12-like bnAbs in rhesus macaques and in humans are likely to be different. This has important implications for HIV-1 vaccine development.
Keywords: HIV/AIDS, Vaccine, B-cell repertoire, neutralizing antibodies, somatic maturation, rhesus macaque
1. Introduction
HIV-1 has evolved various mechanisms to evade human immune surveillance, including genetic variations, extensive glycosylation, oligomerization of envelope (Env) glycoproteins, and conformational masking [1–3]. Potent broadly neutralizing antibodies (bnAbs) against HIV-1 are rare in natural infections and have not been elicited by any candidate vaccine immunogens. A limited number of broadly HIV-neutralizing human monoclonal antibodies (bnmAbs) isolated from HIV-infected long-term slow or no disease progression individuals allow us to investigate the mechanisms for elicitation of HIV-1-specific bnAbs. We have reported that human bnmAbs were highly divergent from the corresponding germline antibodies, and the putative germline antibody predecessors of known human bnmAbs, including b12, 2G12, 2F5 and 4E10, lack measurable binding to HIV-1 Envs, suggesting that Env structures containing their conserved epitopes may not initiate the humoral immune responses by binding to naïve mature B cells expressing the germline antibodies [4, 5]. This may partially explain why immunogens designed to include the structural determinants of known bnmAbs (i.e. b12 and 4E10) failed to elicit the same or similar bnAbs. Similar findings were reported recently that putative germline antibody predecessors of newly identified human bnmAbs PG9/16 and VRC01 did not bind HIV-1 Envs [6, 7]. These observations indicate that HIV-1 may have evolved a new mechanism for immune evasion by reducing or eliminating immunogenicity of the highly conserved epitopes of bnAbs.
Rhesus macaques have been used as a nonhuman primate model for testing HIV-1 vaccine candidates for prevention of HIV-1 infection [8–13]. Failure in eliciting broadly neutralizing macaque antibodies by any candidate vaccine immunogens prompted us to investigate if rhesus macaques have the same problem as humans in initiating the humoral immune responses that lead to elicitation of bnAbs. For a proof of concept, we used one of the best characterized bnmAbs, b12, as a model antibody in this study. The bnmAb b12 recognizes the CD4 binding site on gp120 [14]. Co-crystal structure of human mature Fab b12 with gp120 core shows that b12 uses its heavy chain only to bind to gp120, and all three heavy chain complementarity determining regions (HCDR1-3) make extensive contacts with gp120 [15]. Importantly, the HCDR2 binds to the phenylalanine cavity on gp120 that overlaps the CD4 binding site, suggesting the importance of somatic mutations in heavy chain V-segment (HV) for affinity maturation of b12 [15]. This was confirmed by site-directed mutagenesis study showing that mutations from “AG” at positions 52 and 53 of HCDR2 in putative human germline b12 to “PY” converted a nonbinding human germline b12 to a binding antibody intermediate with a high affinity (nM) for Envs [5]. We searched rhesus macaque whole genome shotgun sequence, identified a putative rhesus macaque germline b12 predecessor and characterized it for binding activity in comparison with the human counterpart. In an attempt to explore possible maturation pathways of b12 in rhesus macaques and humans, we further isolated possible b12 intermediate heavy chain V-segments (iHVs) from B-cell receptor (BCRs) repertoires of nonimmune rhesus macaques and humans, and compared them in sequence characteristics and binding and neutralization properties. Our results indicate that there are substantial differences between macaque and human germline and intermediate b12 antibodies, suggesting different maturation pathways of b12 in rhesus macaques and humans.
2. Materials and methods
2.1 Cells, proteins and viruses
TZM-bl and 293T were obtained from NIH AIDS Research and Reference Program (ARRP) (Division of AIDS, National Institute of Allergy and Infectious Diseases). Both cell lines were maintained in DMEM containing 10% Fetal Bovine Serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. HIV-1 isolates JRCSF, JRFL and Bal were obtained from the NIH ARRP, GXC44 from Dr. Gerald Quinian (USUHS, Bethesda, MD). Envelope glycoprotein gp120Bal and gp140JRFL was produced in our laboratory using free-style 293 transient transfection system (Invitrogen, Carlsbad, CA) and protein A affinity purification.
2.2 Construction of scFv libraries
Rhesus macaque and human scFv libraries were constructed using the pComb3X phagemid as described previously [16]. PBMCs from one uninfected rhesus macaque and 200 human healthy individuals were collected and total RNA and mRNA prepared from the PBMCs using RNeasy Mini kit (Qiagen) and Oligotex mRNA kit (Qiagen). First-strand cDNAs were synthesized using random primer and oligo dT (Invitrogen) according to the instructions from the manufacture. VH1 fragments were amplified from cDNA using VH1-specific primers designed for rhesus macaque [17–19] and human [20] as listed below and PCR cycles: 94 °C for 5min, followed by 10 cycles of 95°C for 15 s, 45°C for 30s and 72°C for 45s, and 20 cycles of 95°C for 15 s, 55°C for 30s and 72°C for 45s, and final extension at 72°C for 10 min. HV-segments were re-amplified from VH1 fragments using the same forward primers and a reverse primer, b12VHger3, and PCR cycles: 94 °C for 5min, followed by 30 cycles of 95°C for 15 s, 55°C for 30s and 72°C for 30s, and final extension at 72°C for 10 min. Human mature b12 DJ-segments linked to mature VL were amplified from mature b12 scFv construct using b12ger3F and sfihis3R as primers. The HV-segments were then assembled by splice overlap extension PCR (SOE-PCR) with PCR-amplified human mature b12 DJ-segments linked to mature VL to generate rhesus macaque and human scFv libraries. All PCR products were gel purified using QIAquick Gel Extraction kit (Qiagen). ScFv fragments were digested with Sfi I and ligated to pComb3X. Ligation products were used to transform TG1 electroporation competent cells, resulting in a macaque scFv library containing 1.1×107 independent clones and a human scFv library containing 5.4×107 independent clones. Phage libraries were prepared using M13KO7 helper phage. Primers used in constructing VH1 rhesus macaque and human scFv libraries are as follow: (W: A or T; R: A or G; S: C or G) Primers for amplification of Rhesus macaque antibody VH1 subfamily:
RMVH17AF: 5′-GCTACCGTGGCCCAGGCGGCCCAGGWGCAGCTGGTGCAGTCTGG-3′
RMVH17BF: 5′-GCTACCGTGGCCCAGGCGGCCGAGGTCCAGCTGGTRCAGTCTGG-3′
RMVH1CF: 5′-GCTACCGTGGCCCAGGCGGCCCAGGTCCAGCTGGTGCARTCCGG-3′
RMJH1R: 5′-GATGGTGCTGGCCGGCCTGGCCTGAGGAGACGGTGACCAGGGCGCC-3′
RMJH2R: 5′-GATGGTGCTGGCCGGCCTGGCCTGAGGAGATGGTGATTGGGGTGCC-3′
RMJH3R: 5′-GATGGTGCTGGCCGGCCTGGCCTGAAGAGACGGTGACCCTGAGCCC-3′
RMJH45R: 5′-GATGGTGCTGGCCGGCCTGGCCTGAGGAGACGGTGACCAGRACTCC-3′
RMJH6R: 5′-GATGGTGCTGGCCGGCCTGGCCTGAGGAGACGGTGACGACGACCCC-3′
Primers for amplification of human antibody VH1 subfamily:
HUVH1B7AF: 5′-GCTACCGTGGCCCAGGCGGCCCAGRTGCAGCTGGTGCARTCTGG-3′
HUVH1CF: 5′-GCTACCGTGGCCCAGGCGGCCSAGGTCCAGCTGGTRCAGTCTGG-3′
HUJH12R: 5′–CGATGGGCCCTTGGTGGAGGCTGAGGAGACGGTGACCAGGGTGCC-3′
HUJH3R: 5′-CGATGGGCCCTTGGTGGAGGCTGAAGAGACGGTGACCATTGTCCC-3′
HUJH45R: 5′-CGATGGGCCCTTGGTGGAGGCTGAGGAGACGGTGACCAGGGTTCC-3′
HUJH6R: 5′-CGATGGGCCCTTGGTGGAGGCTGAGGAGACGGTGACCGTGGTCCC-3′
Primers for amplification of HV-segments from VH1 PCR products:
Sfi5: 5′-GGTTTCGCTACCGTGGCCCAGGCGGCC-3′
b12VHger3: 5′-CTCTCGCACAGTAATACACAG-3′
Primers for amplification of human mature b12 DJ-segments linked to mature VL:
b12VHger3F: 5′-CTGTGTATTACTGTGCGAGAGTGGGGCCATATAG-3′
sfihis3R: 5′-ATGGTGATGGTGATGGTGTCGGCCGGCCTGGCC-3′
Primers for assembly of scFvs: Sfi5 and sfihis3R.
2.3 Solid-phase panning
Biopanning was performed against gp140JRFL coated on immunotubes (Nunc). Polyclonal phage ELISA with gp140JRFL was carried out and enrichment were observed after 3rd and 4th rounds of panning. Monoclonal phage ELISA was performed to screen the 3rd and 4th round panned libraries for monoclonal phage that bind gp120Bal coated on 96-well microplates. Positive clones were sequenced, and a total of 10 independent clones selected from rhesus macaque and 5 from human scFv libraries.
2.4 Generation of b12 scFv variants
Human germline b12 D(J)-segments liked to VL were PCR-amplified from human germline scFv b12 construct using b12VHger3F and sfihis3R as primers. Replacement of human mature b12 D(J)-segments and VL in isolated scFv clones with human germline b12 D(J)-segments and VL was done by SOE-PCR using Sfi5 and sfihis3R as primers. Mutations from “P(52)Y(53)” to “AG” were done using QuikChange II site-directed mutagenesis kit (Stratagene). All scFv variants were confirmed by DNA sequencing. ScFv-Fc constructs were generated by subcloning scFvs to pEAK10 vector containing genomic Fc gene of human IgG1.
2.5 Expression and purification of scFvs and scFv-Fc fusions
The scFvs were expressed in E. coli HB2151 and purified by IMAC as described previously [21]. ScFv-Fc fusion proteins were produced by transient transfection of free-style 293 cells followed by purification of culture supernatant using protein A affinity column. The size and purity of recombinant antibodies were confirmed by SDS-PAGE.
2.6 ELISA
The gp120Bal was diluted to 1 μg/mL in PBS buffer (pH7.4) and coated on half-well plates by incubation at 4°C overnight. The plates were blocked with 2.5% skim milk in PBS (MPBS) by incubation at 37°C for 1h. Three-fold serially diluted antibodies in MPBS were added to the plates and incubated at 37°C for 1h. Bound scFv antibodies were detected using HRP conjugated to anti-Flag antibodies as 2nd antibody and ABTS or TMB as substrate. Bound scFv-Fc fusions were detected using HRP conjugated to anti-human Fc antibodies as 2nd antibody and TMB as substrate. The optical density at 405nm or 450nm was measured after color development at RT for 20 minutes. EC50s were analyzed using GraphPad prism software.
2.7 Pseudovirus neutralization assay
A standardized TZM-bl cell line-based Env-pseudotyped neutralization assay was used as described [22]. Briefly, pseudotyped viruses were prepared by cotransfection of 70% to 80% confluent HEK 293T cells with pNL4–3.luc.E-R- and HIV-1 Env plasmid using polyethylenimine. 4–6 hrs post transfection, medium was changed to growth medium containing 10% FBS (Sigma, St. Louis, USA). Cells were allowed to grow for additional 40 hrs. The supernatant was harvested, centrifuged at 16,000 rpm for 5 min at 4°C, and filtered through a 0.45 μm pore filter (Millipore). Neutralization assays were carried out in triplicate by preincubation of 50 μL of three-fold serially diluted scFvs with 50 μL of pseudovirus suspension at 37°C for 30 min. Virus-antibody mixtures were then added to TZM-bl cells in 96 wells culture plates (Costar, Corning) and incubated at 37°C with 5% CO2 for 48 hrs. Cells were then washed with PBS and lysed with 60 μL lysis buffer for 30 minutes. 25 μL of luciferin (Promega, Madison, WI) were added to each well and luminescence readings were measured by PE Victor3 luminometer. Antibody concentration that leads to 50% neutralization (IC50) was determined using GraphPad prism software.
3. Results and discussion
3.1 Identification of a putative rhesus macaque b12 germline antibody
Sequence analysis indicates that human mature b12 heavy chain variable region (VH) is most likely derived from a germline antibody predecessor resulting from recombination of IGHV1-3*01 with IGHD3-10*02 and IGHJ6*03, and b12 light chain variable region (VL) from recombination of IGKV3-20*01 and IGKJ2*01 (12). We searched macaque mulatta whole genome shotgun sequence (Mmul_051212) against human IGHV1-3*01 and IGKV3-20*01, and identified two rhesus macaque sequences, NW_001122023.1 on chromosome 7 and NC_007870.1 on chromosome 13, that shared the highest identities with putative human germline b12 heavy and light chain V-segments (HV and LV), respectively. The two germline HV-segments shared 90.6 % identity at DNA level and 89.8 % at amino acid (AA) level, and the two germline LV-segments were 95.8% identical at DNA level and 94.7% at AA level. We also identified putative rhesus macaque germline b12 heavy chain D(J)-segments [HD(J)] and light chain J-segment (LJ) by similarity searching against macaque mulatta whole genome shotgun sequence and all reported rhesus macaque D- and J-segment sequences [17–19]. In agreement with the observation reported by Link, et al. [23], we found that putative rhesus macaque b12 HD(J)- and LJ sequences also differ from the human counterparts (data not shown). To minimize the variations caused by the complexity of HCDR3 and LCDR3, we used human germline b12 HD(J) and LJ in synthesized putative rhesus macaque germline b12 in this study (Fig. 1a, 1b). We focused on the comparison of putative macaque and human germline b12 HV-segments and their possible intermediates that predominantly determine the binding activity of antibodies to Envs.
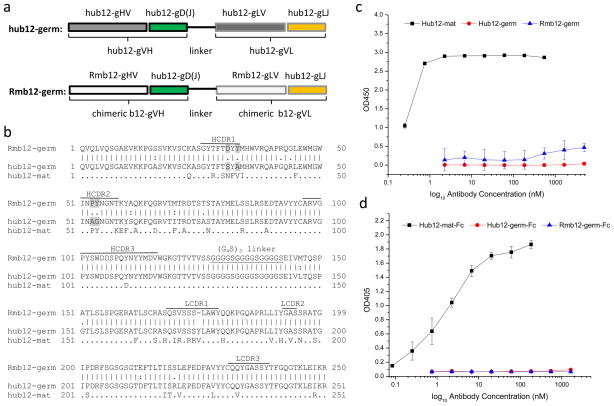
Fig. 1. Lack of binding of putative rhesus macaque germline b12 scFv and scFv-Fc fusion to Envs.
a. Schematic drawing of rhesus macaque and human germline b12 scFvs. b. AA sequence alignment of rhesus macaque germline b12 scFv (Rmb12-germ) with human germline (hub12-germ) and mature (hub12-mat) b12 scFvs. Different residues in HCDR1 and HCDR2 between the two germline scFvs were highlighted in grey. Only somatic mutations that differ from “hub12-germ” were shown in “hub12-mat”. c–d. ELISA of rhesus macaque and human germline b12 scFv (c) or scFv-Fc (d) with recombinant gp120Bal in comparison with human mature b12 scFv (c) or scFv-Fc (d).
3.2 Lack of measurable binding of putative rhesus macaque germline b12 to HIV-1 Envs
Putative rhesus macaque germline b12 VH and VL was covalently linked by a flexible (G4S)3 linker to form a single chain antibody fragment (scFv). We further made a fusion protein, in which putative macaque germline scFv b12 was fused to human Fc of IgG1, in order to test if bivalent macaque germline scFv b12 bound to Envs. Similar to the human counterpart [24], putative macaque germline scFv b12 did not show measurable binding to gp120Bal, neither did the Fc fusion (Fig. 1c, 1d). This result suggests that activation of naïve mature B cells expressing macaque germline b12 leading to elicitation of b12 or b12-like bnAbs in rhesus macaques may not occur if Envs were used as immunogens.
3.3 Unique sequence characteristics of putative rhesus macaque germline b12
Sequence analysis revealed unique sequence characteristics in putative rhesus macaque germline b12 (Fig. 1). Putative macaque germline b12 has pre-existing “PY” at position 52 and 53 of HCDR2. These two AA residues are present only in human mature b12 and are found to be critical for binding of mature b12 to Envs [5, 15]. The fact that putative rhesus macaque germline b12 with the pre-existing “PY” in HCDR2 lacks measurable binding to Envs suggests that the mechanism for lack of binding of macaque germline b12 to Envs may be different from that of human germline b12. This prompted us to investigate if somatic mutations in other regions are more important for macaque germline b12 than human germline b12 to bind to Envs and if macaque germline b12 has advantages over the human counterpart in somatic maturation to become b12 or b12-like bnAbs due to the pre-existing “PY”. We hypothesize that maturation pathways leading to elicitation of b12 or b12-like bnAbs in macaques may be different from those in humans. Somatic mutations in HCDR1 may be critical for maturation of macaque germline b12, while somatic mutations in HCDR2 may be more important than that in HCDR1 for maturation of human germline b12. In an attempt to search for evidence to support our hypothesis, we isolated possible intermediate b12 HV-segments (iHVs) from BCR repertoires of nonimmune rhesus macaques and humans, and compared isolated macaque and human iHVs in sequence characteristics and binding and neutralizing properties when recombined with human mature or germline b12 D(J)-segments and VL.
3.4 Isolation and characterization of possible human b12 iHVs from BCR repertoires of nonimmune human individuals
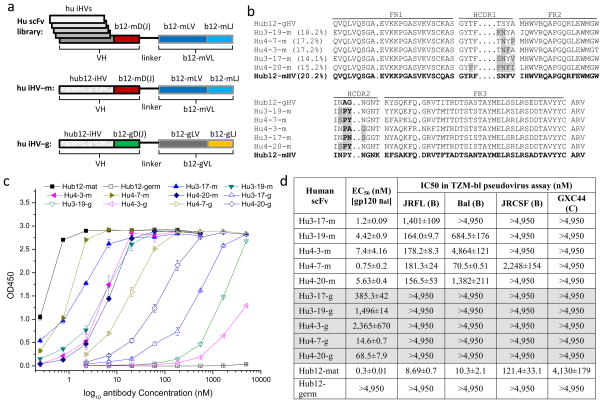
In HIV-1 natural infection, a small percentage of HIV-1 infected human individuals do not progress to AIDS without drug treatment. About one third of such long-term non-progressors develop bnAbs after a few years of chronic infection. We have hypothesized that these individuals may have exposed to certain immunogens prior to HIV-1 infection that initiate somatic maturation of germline bnAbs to generate intermediate antibodies. HIV-1 infection further drives somatic maturation to elicit mature bnAbs. Therefore, in our previous study, we have collected PBMCs from 200 uninfected healthy human individuals and constructed a nonimmune human scFv antibody library using the isolated total RNA and mRNA from the PBMCs. To isolate possible human b12 iHVs, we amplified VH1s from the cDNA that were transcribed from pooled mRNA of 200 samples and re-amplified HV-segments of VH1 fragments. The VH1-HVs were recombined with human mature b12 D(J)-segments that were covalently linked to VL by a flexible (G4S)3 linker (Fig. 2a). The resultant scFv fragments were then cloned to a phagemid vector, pComb3X. Panning phage-displayed human VH1-scFv library against gp140JRFL resulted in isolation of five intermediate scFv antibodies that bound to gp120Bal and neutralized, to various extents, HIV-1 clade B isolates (Fig. 2b, 2c, 2d). All five scFvs had affinities for Balgp120 that were lower than that of mature scFv b12 (EC50 =0.3 nM for Balgp120) (Fig. 2c), but higher than that of the germline HV control, in which germline HV was recombined with mature D(J)-segments and VL, (EC50 =24.1 nM for Balgp120). Their average neutralization activities against four isolates (JRFL, Bal, JRCSF, GXC) were also lower than that of mature b12 (Fig. 2d), but higher than that of the germline HV control (IC50 = 1.4 μM against JRFL, and > 4.95 μM against Bal, JRCSF, GXC), indicating that the isolated five human HV-segments are possible b12 iHVs. We replaced human mature b12 D(J)-segments and VL in the isolated scFvs with human germline b12 counterparts. The resultant five “iHV-g” scFvs showed significantly decreased affinity for gp120Bal and lost neutralization activity against the four isolates tested (Fig. 2c, 2d), indicating that the isolated human b12 iHVs contributed to the binding to Envs, but maturation in b12 D(J)-segments and VL are required for high binding and neutralization activities.
Fig. 2. Characterization of human b12 iHVs.
a. Schematic drawing of human intermediate b12 scFv library. b. AA sequence alignment of the isolated human intermediate b12 HVs (iHVs) with human germline (Hub12-gHV) and mature (Hub12-mHV) HVs. c–d. Binding (c) and neutralization activities (c & d) of human b12 iHVs when recombined with human mature D(J)-segments linked to mature VL (-m) or with human germline D(J)-segments linked to human germline VL (-g). Human mature and germline b12 scFvs were included for comparison.
Sequence analysis revealed relatively high percentage AA changes in the isolated five human iHVs ranging from 14.1 % to 18.2 % compared to the human germline b12 HV-segment, IGHV1-3*01 (Fig. 2b). All five human iHVs have “S” to “N” mutation at position 31 in HCDR1 and “A” to “P” at position 52 in HCDR2. Three of them have “G” to “Y” mutation (hu3-19-m, hu4-7-m and hu4-20-m) at position 53, and the other two either “G” to “N” (hu3-17-m) or “G” to “A” (hu4-3-m) at position 53 (Fig. 2b). This result indicates that somatic mutations in both HCDR1 and HCDR2 are important for human germline b12 to bind to Envs. This is in agreement with the crystal structure of Fab b12 in complex with gp120 core [5, 15].
3.5 Isolation and characterization of possible macaque b12 iHVs from BCR repertoires of nonimmune rhesus macaques
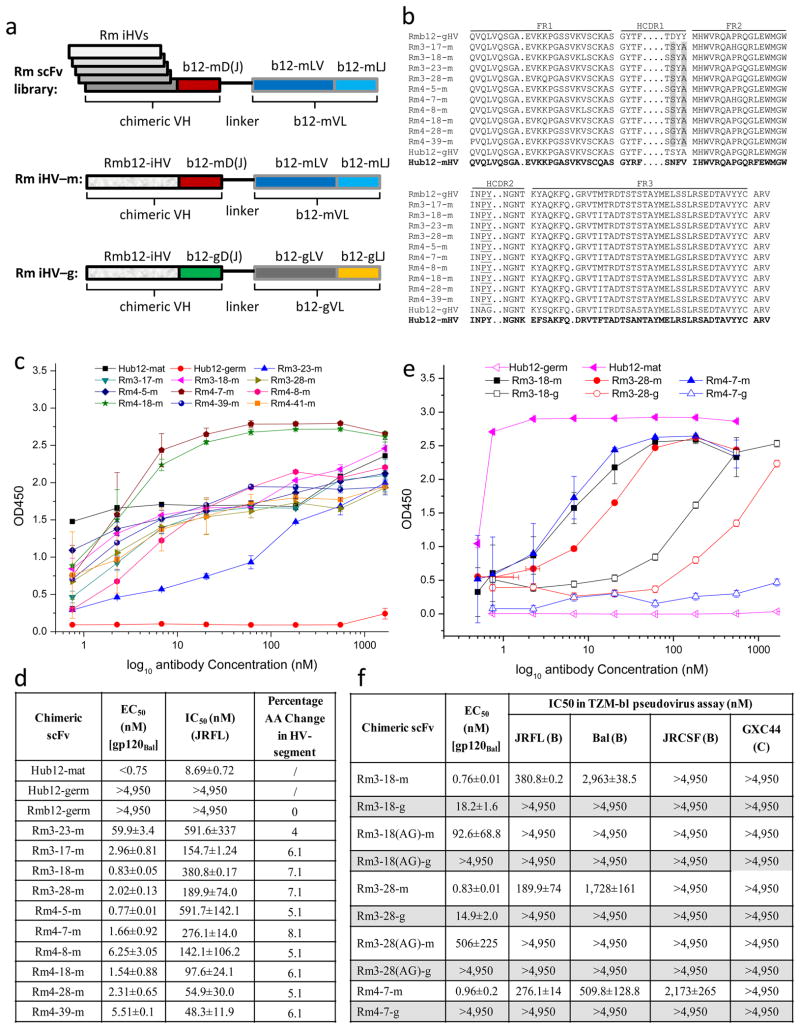
Similarly, we isolated a panel of ten possible rhesus macaque b12 iHVs from BCR repertoire of one nonimmune rhesus macaque (Fig. 3a, 3b). All ten “chimeric” scFvs, in which macaque iHVs were recombined with human mature b12 D(J) and VL, showed much higher affinities (nM) for gp120Bal than that of macaque germline HV control, in which macaque germline HV was recombined with human mature b12 D(J)-segments and VL, (EC50=2.5 μM for gp120Bal) (Fig. 3c, 3d). All ten “chimeric” scFvs neutralized, to various extents, HIV-1 clade B isolate, JRFL (Fig. 3d), These results indicate that isolated macaque iHVs can complement with human mature b12 D(J)-segments and VL in binding to Envs and neutralizing HIV-1. To investigate to what extent the isolated macaque iHVs contribute to the binding and neutralization, we replaced human mature b12 D(J)-segments and VL in three “chimeric” scFvs, designated “Rm iHV-m”, with human germline b12 D(J)-segment and VL, designated “Rm iHV-g” (Fig. 3a). The resultant “chimeric” scFvs showed 10–20 fold decrease (i.e. Rm3-18-g and Rm3-28-g) in binding or complete loss (i.e. Rm4-7-g) of binding to gp120Bal (Fig. 3e and 3f), and none of the resultant “chimeric” scFvs neutralized the four HIV-1 isolates tested (Fig. 3f). This demonstrated that isolated macaque b12 iHVs behaved the same as isolated human iHVs in binding to Envs and neutralizing the virus, suggesting their “intermediate” nature in the somatic maturation pathway(s).
Fig. 3. Characterization of rhesus macaque b12 iHVs.
a. Schematic drawing of “chimeric” scFv constructs and libraries. b. AA sequence alignment of isolated macaque b12 iHVs with putative macaque germline b12 HV (Rmb12-gHV), human germline b12 HV (Hub12-gHV) and human mature b12 HV (Hub12-mHV). c–d. Binding (c & d) and neutralization activities (d) of the “chimeric” scFvs, and calculated percentage AA changes in the iHVs (d). e–f. Binding and neutralization activities of macaque b12 iHVs when recombined with human mature D(J)-segments and VL (-m) or with human germline D(J)-segments and VL (-g), and when mutated from “PY” to “AG” at position 52 and 53.
In contrast to isolated human b12 iHVs, isolated macaque iHVs have low levels of somatic maturations ranging from 4.0 % to 8.1%, and somatic mutations occurred mainly in HCDR1 (Fig. 3b, 3d). All ten isolated macaque iHVs have somatic mutations in HCDR1 at position 31, seven from “D” to “S” and three from “D” to “G”, and position 33, all from “Y” to “A”. Notably, pre-existing “PY” in HCDR2 at positions 52 and 53 remained unchanged in all isolated macaque iHVs (Fig. 3b), suggesting the importance of “PY” at positions 52 and 53 for binding of macaque intermediate b12 antibodies to Envs. This was confirmed by our site-directed mutagenesis study showing that mutations from “PY” to “AG” in both versions of two “chimeric” scFvs, designed Rm3-18(AG)-m/g and Rm3-28(AG)-m/g, resulted in significant loss of binding activity and complete loss of neutralization activity (Fig. 3e, 3f), indicating that isolated macaque iHVs may have the same binding pattern as human b12 to Envs.
The existence of b12 iHVs in BCR repertoires of nonimmune rhesus macaques and humans supports our hypothesis that maturation pathways of bnAbs may be initiated by immunogens that are unrelated to HIV-1. Different sequence characteristics of putative macaque and human b12 germline predecessors and their intermediate antibodies suggest that different primary immunogens may be required for initiation of the maturation pathways of b12 in macaques and humans. Different sequence characteristics between the isolated macaque and human iHVs further suggest that Env-based vaccine immunogens for driving somatic maturation of macaque and human b12 intermediate antibodies to b12 or b12-like bnAbs are also likely to be different. In this study, we focused on variations in heavy chain V-segment that plays a critical role in binding of antibodies to Envs. Other segments, including D(J)-segments of heavy chain and V- and J-segments of light chain are also different between rhesus macaque and human based on our sequence similarity search and sequence analysis (data not shown). These regions also play important roles in binding to Envs and neutralizing the virus [25]. Therefore, the differences between macaque and human germline b12 and intermediate antibodies could be substantial. However, because of the largely stochastic nature of the somatic hypermutation, one cannot predict whether the use of the same primary immunogen can initiate somatic maturation of both macaque and human b12 germline predecessors, and lead to elicitation of different intermediate antibodies, yet the same or similar mature antibodies, in this case b12 or b12-like bnAbs. It is possible that even if the maturation pathways are different and different intermediate antibodies are elicited, the mature antibodies could be similar. The final outcome should be strongly dependent also on the nature of additional (secondary) immunogens used in combination with the primary immunogens. Only further experiments in macaques and humans with various combinations of immunogens will show which maturation pathways are most likely.
The pre-existing “PY” in the HCDR2 of the putative rhesus macaque b12 germline predecessor may lead to shortening of possible maturation pathways in rhesus macaques compared to humans. The fact that ten macaque b12 iHVs with low percentage AA changes were isolated from the BCR repertoire of one rhesus macaque and five human b12 iHVs with high percentage AA changes from the BCR repertoires of 200 uninfected human individuals supports this notion. One can expect that in rhesus macaques, elicitation of b12 or b12-like bnAbs is more likely than in humans although such attempts have not succeeded in humans, neither in macaques so far.
Putative rhesus macaque germline antibodies for other known human bnmAbs may also be different from their human counterparts. The human bnmAb VRC01 neutralized 90% of the circulating HIV-1 isolates tested and is the most potent bnmAb reported so far [6]. VRC01 is also highly mutated (32% in VH) compared to the closest germline antibody gene, IGHV1-2*02. Similar to b12, VRC01 uses its HCDR2 to bind to the CD4 binding site on gp120 [26]. We identified a putative rhesus macaque germline VRC01 heavy chain V-segment (GenBank: AANU01128648.1) that is the closest to the human counterpart. The two germline VRC01 HVs share only 89.58% at AA level and the main difference is also located in HCDR2. The V-segment of originally paired human light chain of VRC01 shares 92% identity at AA level with putative rhesus macaque germline VRC01 LV. These results raise a question if rhesus macaque models are appropriate models for testing efficacy of HIV-1 vaccine immunogens designed to elicit bnAbs, such as b12 and VRC01. Therefore, development of alternative animal models for testing HIV-1 vaccine immunogens may be considered. When we did similarity search against all available genome sequence databases, we found that sequences from Gorilla gorilla and Pan troglodytes were the closest ones to human germline b12 HV-segment. Putative gorilla and Pan troglodytes germline b12 HV-segments shares 98% and 99% identities with the human counterpart, respectively. Nevertheless, using gorilla and troglodytes as models for testing HIV-1 vaccine candidates would be unrealistic. Humanized mouse models may be appropriate models for this purpose, but more experimental data are required to show the appropriateness.
4. Conclusion
Similar to putative human germline b12, putative rhesus macaque germline b12 lacks measurable binding to HIV-1 Envs, suggesting that initiation of the humoral immune responses leading to elicitation of bnAbs in rhesus macaque may also be a challenge. Nevertheless, different sequence characteristics between human and macaque germline b12 predecessors suggest that primary immunogens required for initiation of maturation pathways resulting in b12 or b12-like bnAbs in rhesus macaques and in humans are likely to be different. Different sequence characteristics between the two panels of isolated human and macaque b12 iHVs further suggest that human and macaque germline b12 predecessors may undergo different maturation pathways in vivo upon activation, and macaque germline b12 predecessor may have advantages over the human counterpart in maturing to b12 or b12-like bnAbs due to the pre-existing “PY” in the macaque germline b12 HCDR2. These findings may have implications for the design and testing of vaccine immunogens as components of an efficacious AIDS vaccine.
Putative macaque and human b12 germline predecessors and possible macaque and human germline b12 intermediate antibodies are different.
Different primary immunogens may be required to initiate somatic maturation and different envelope-based immunogens to drive the maturations.
Animals with human germline antibodies, e.g., humanized mice, could be better models than monkeys with monkey germline antibodies.
Acknowledgments
We thank Jeffrey Lifson, Nancy Longo, Ruth Ruprecht, Peter Kwong, Tongqing Zhou, Xiaodong Xiao, Prabakaran Ponraj and Zhiwei Chen for helpful discussions, Jeffrey Lifson for providing rhesus macaque PBMCs, Gerald Quinnan and Dennis Burton for providing reagents. This work was supported by the Intramural Research Program of the University of Hong Kong, and by the General Research Fund (GRF) of Hong Kong to M-Y.Z., and by the Intramural Research Program of the CCR, NCI, NIH, and the Bill and Melinda Gates Foundation to D.S.D.
Abbreviations
- Env
envelope glycoproteins
- CDRs
complementarity determining regions
- BCR
B-cell receptor
- VH
heavy chain variable region
- VL
light chain variable region
- scFv
single chain antibody fragment
- FBS
fetal bovine serum
- SOE-PCR
splice overlap extension PCR
- IMAC
immobilized metal chelating chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwong PD, Wyatt R, Majeed S, Robinson J, Sweet RW, Sodroski J, et al. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure. 2000 Dec 15;8(12):1329–39. doi: 10.1016/s0969-2126(00)00547-5. [DOI] [PubMed] [Google Scholar]
- 2.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002 Dec 12;420(6916):678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Kwon YD, Zhou T, Wu X, O’Dell S, Cavacini L, et al. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009 Nov 20;326(5956):1123–7. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: Implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun. 2009 Sep 11;390(3):404–9. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao X, Chen W, Feng Y, Dimitrov DS. Maturation Pathways of Cross-Reactive HIV-1 Neutralizing Antibodies. Viruses. 2009;I:801–17. doi: 10.3390/v1030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational Design of Envelope Identifies Broadly Neutralizing Human Monoclonal Antibodies to HIV-1. Science. 2010 Jul 8;329(5993):856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pancera M, McLellan JS, Wu X, Zhu J, Changela A, Schmidt SD, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010 Aug;84(16):8098–110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. Aids. 2008 Jan 30;22(3):339–48. doi: 10.1097/QAD.0b013e3282f3ca57. [DOI] [PubMed] [Google Scholar]
- 9.Cristillo AD, Wang S, Caskey MS, Unangst T, Hocker L, He L, et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology. 2006 Mar 1;346(1):151–68. doi: 10.1016/j.virol.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Derby NR, Kraft Z, Kan E, Crooks ET, Barnett SW, Srivastava IK, et al. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J Virol. 2006 Sep;80(17):8745–62. doi: 10.1128/JVI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Zhou F, Greer CE, Legg H, Tang T, Luciw P, et al. Antibody responses against HIV in rhesus macaques following combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles. AIDS Res Hum Retroviruses. 2006 Oct;22(10):993–7. doi: 10.1089/aid.2006.22.993. [DOI] [PubMed] [Google Scholar]
- 12.Kraft Z, Derby NR, McCaffrey RA, Niec R, Blay WM, Haigwood NL, et al. Macaques infected with a CCR5-tropic simian/human immunodeficiency virus (SHIV) develop broadly reactive anti-HIV neutralizing antibodies. J Virol. 2007 Jun;81(12):6402–11. doi: 10.1128/JVI.00424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008 Jun;14(6):617–21. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994 Nov 11;266(5187):1024–7. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 15.Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007 Feb 15;445(7129):732–7. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang MY, Shu Y, Phogat S, Xiao X, Cham F, Bouma P, et al. Broadly cross-reactive HIV neutralizing human monoclonal antibody Fab selected by sequential antigen panning of a phage display library. J Immunol Methods. 2003 Dec;283(1–2):17–25. doi: 10.1016/j.jim.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Helmuth EF, Letvin NL, Margolin DH. Germline repertoire of the immunoglobulin V(H)3 family in rhesus monkeys. Immunogenetics. 2000 Jun;51(7):519–27. doi: 10.1007/s002510000170. [DOI] [PubMed] [Google Scholar]
- 18.Bible JM, Howard W, Robbins H, Dunn-Walters DK. IGHV1, IGHV5 and IGHV7 subgroup genes in the rhesus macaque. Immunogenetics. 2003 Mar;54(12):867–73. doi: 10.1007/s00251-003-0536-2. [DOI] [PubMed] [Google Scholar]
- 19.Andris JS, Miller AB, Abraham SR, Cunningham S, Roubinet F, Blancher A, et al. Variable region gene segment utilization in rhesus monkey hybridomas producing human red blood cell-specific antibodies: predominance of the VH4 family but not VH4-21 (V4-34) Mol Immunol. 1997 Feb;34(3):237–53. doi: 10.1016/s0161-5890(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 20.Barbas CF, Burton DR, Scott JK, Silverman GJ. Phage Display: A Laboratory Mannual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 21.Zhang MY, Shu Y, Rudolph D, Prabakaran P, Labrijn AF, Zwick MB, et al. Improved breadth and potency of an HIV-1-neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J Mol Biol. 2004 Jan 2;335(1):209–19. doi: 10.1016/j.jmb.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005 Aug;79(16):10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Link JM, Larson JE, Schroeder HW. Despite extensive similarity in germline DH and JH sequence, the adult Rhesus macaque CDR-H3 repertoire differs from human. Mol Immunol. 2005 May;42(8):943–55. doi: 10.1016/j.molimm.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Wu-Baer F, Sigman D, Gaynor RB. Specific binding of RNA polymerase II to the human immunodeficiency virus trans-activating region RNA is regulated by cellular cofactors and Tat. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7153–7. doi: 10.1073/pnas.92.16.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan T, Li J, Zhang MY. A single mutation turns a non-binding germline-like predecessor of broadly neutralizing antibody into a binding antibody to HIV-1 envelope glycoproteins. MAbs. 2011 Jul 1;3(4) doi: 10.4161/mabs.3.4.15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural Basis for Broad and Potent Neutralization of HIV-1 by Antibody VRC01. Science. 2010 Jul 8;329(5993):811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]