Abstract
Embryonic patterning in vertebrates is dependent upon the balance of inductive signals and their specific antagonists. We show that Noggin, which encodes a bone morphogenetic protein (BMP) antagonist expressed in the node, notochord, and dorsal somite, is required for normal mouse development. Although Noggin has been implicated in neural induction, examination of null mutants in the mouse indicates that Noggin is not essential for this process. However, Noggin is required for subsequent growth and patterning of the neural tube. Early BMP-dependent dorsal cell fates, the roof plate and neural crest, form in the absence of Noggin. However, there is a progressive loss of early, Sonic hedgehog (Shh)-dependent ventral cell fates despite the normal expression of Shh in the notochord. Further, somite differentiation is deficient in both muscle and sclerotomal precursors. Addition of BMP2 or BMP4 to paraxial mesoderm explants blocks Shh-mediated induction of Pax-1, a sclerotomal marker, whereas addition of Noggin is sufficient to induce Pax-1. Noggin and Shh induce Pax-1 synergistically. Use of protein kinase A stimulators blocks Shh-mediated induction of Pax-1, but not induction by Noggin, suggesting that induction is mediated by different pathways. Together these data demonstrate that inhibition of BMP signaling by axially secreted Noggin is an important requirement for normal patterning of the vertebrate neural tube and somite.
Keywords: Noggin, somite, neural tube, patterning
Patterning of the vertebrate body axes is dependent upon signals produced by discrete organizing centers. Perhaps the best studied of these is Spemann’s organizer, which encompasses the dorsal lip of the blastopore in the gastrulating amphibian embryo. Organizer signaling is implicated in dorsalization of both mesodermal and ectodermal derivatives. Dorsalization of the mesoderm leads to notochord and somite formation, whereas the dorsalized ectoderm forms neural tissue (for review, see Kessler and Melton 1994; De Robertis and Sasai 1996; Harland and Gerhart 1997). Four signals have been described that are expressed within the organizer and that have dorsalizing activity: Noggin (Smith and Harland 1992), Follistatin (Hemmati-Brivanlou et al. 1994), Chordin (Sasai et al. 1994), and Frzb (Leyns et al. 1997; Wang et al. 1997). None of these share identifiable sequence similarity, but there is evidence to suggest that the first three may act by blocking bone morphogenetic protein (BMP) signaling.
Noggin binds several BMPs with very high (picomolar) affinities, with a marked preference for BMP2 and BMP4 over BMP7. By binding tightly to BMPs, Noggin prevents BMPs from binding their receptors (Zimmerman et al. 1996). Chordin also antagonizes BMP signaling by directly binding BMP proteins, thereby preventing receptor activation (Piccolo et al. 1996). Follistatin binds to Activins, thereby preventing Activin signaling (Nakamura et al. 1990). This antagonism extends to the more distantly related BMPs with a preference for BMP7 over BMP4 (Yamashita et al. 1995; Liem et al. 1997). Thus, a key function of these peptides is to antagonize signaling by distinct members of the TGF-β superfamily.
Examination of postgastrulation stage Xenopus embryos indicates that some of these signaling factors are expressed at later stages. For example, Noggin is expressed in the notochord and dorsal neural tube, suggesting a possible role in the central nervous system (CNS) and somite patterning (Smith and Harland 1992); and in the chick, Noggin expression in the dorsal lip of the somite has been implicated in the control of myogenesis (Marcelle et al. 1997; Reshef et al. 1998). We have addressed the role of Noggin in mouse development. Noggin is not essential for neural induction but is required for normal growth and patterning of the neural tube and somite. Thus, inhibition of endogenous BMP signaling by Noggin is essential for elaboration of the vertebrate body plan.
Results
Cloning and expression of Noggin
We isolated a genomic clone that encodes the entire mouse Noggin polypeptide on a single exon (GenBank accession no. U79163). The predicted protein contains 232 amino acids (25 kD) and shares 99% and 80% amino acid identity with the human (Valenzuela et al. 1995) and Xenopus (Smith and Harland 1992) proteins, respectively.
Noggin expression was examined in developing mouse embryos by whole-mount and section in situ hybridization. Embryonic expression was first detected in the node at 7.5 days postcoitum (dpc; arrowed in Fig. 1A). By early somite stages, Noggin expression extended anteriorly along the entire length of the notochord (large arrow in Fig. 1C), a similar pattern to the notochordal marker Brachyury (Fig. 1D). In addition, Noggin was expressed in the dorsal neural tube from the caudal hindbrain to the posterior-most region of the embryo (small arrows in Fig. 1C). By the time cranial neural tube closure was completed (∼9.0 dpc), Noggin expression was continuous along most of the dorsal midline of the neural tube (the roof plate), to its rostral termination at the base of the forebrain (Shimamura et al. 1995; small arrows in Fig. 1E). In contrast to Brachyury (Fig. 1G), expression in the notochord was not uniform but decreased rostrally at this stage (Fig. 1E). Expression in the neural tube and caudal notochord remained essentially unchanged during early organogenesis, from 9.5 dpc (Fig. 1H,L) to 10.5 dpc (data not shown). We also observed weak expression in the dorsal lip of the most rostral somites from 9.5 dpc (arrow in Fig. 1H,J). Expression in the somite contrasts with the chick in which Noggin is strongly expressed even in the most recently formed somites (Marcelle et al. 1997; Reshef et al. 1998). Finally, Noggin was expressed in the rostral sclerotome at 10.5 dpc (data not shown), coincident with the initial stages of cartilage condensation.
Figure 1.
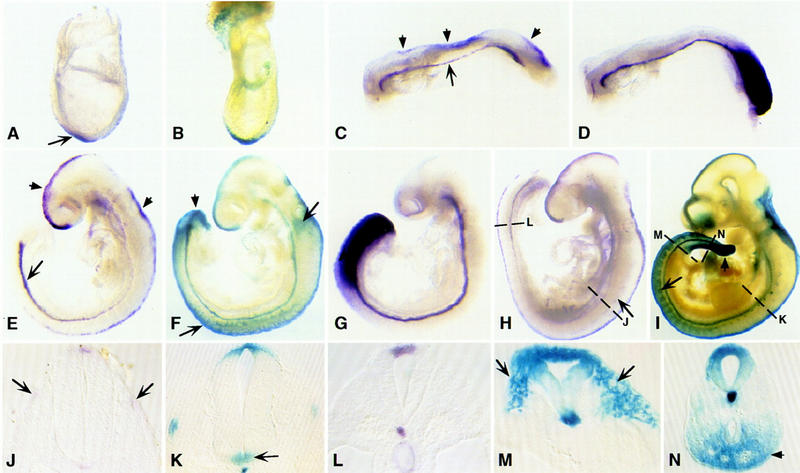
Expression of Noggin during mouse development. (A,C,D,E,G,H,J,L) Whole mount in situ hybridization with Noggin (A,C,E,H,J,L) and brachyury (D,G) probes; (B,F,I,K,M,N) β-galactosidase activity in embryos heterozygous for a targeted allele of Noggin in which Noggin coding sequences were replaced by the lacZ gene. (A) 7.75 dpc; (B) 7.5 dpc; (C,D) 10 somites (8.5 dpc); (E–G) 9.5 dpc; (H,I) 10 dpc—10.5 dpc; (J–N) transverse sections as indicated through embryos in H and I.
Generation of Noggin mutants
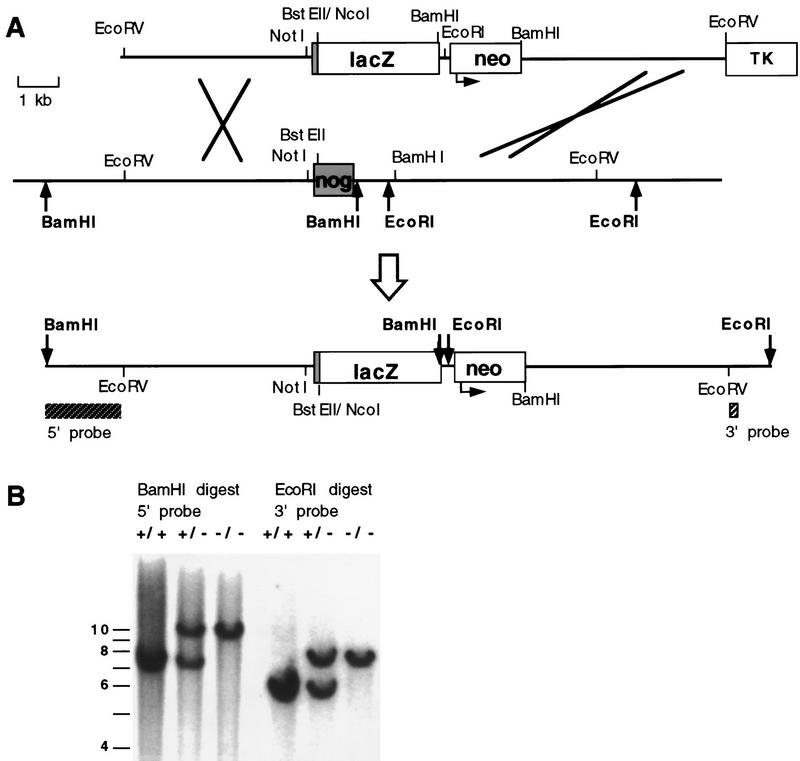
Experiments in a variety of vertebrates have demonstrated the importance of signaling by the node, notochord, and dorsal neural tube in patterning mesodermal and neural tissues. To address the embryonic function of Noggin, we generated a null allele by fusing the first 10 amino acids of the Noggin coding sequence to the lacZ gene of Escherichia coli (Fig. 2A). The remainder of the coding sequence, and some 3′ flanking sequence, were deleted following gene replacement at the Noggin locus (Fig. 2A). A correctly targeted CJ-7 embryonic stem (ES) cell clone was introduced into the mouse germ line and the mutant allele was either outcrossed to the C57BL6/J strain or maintained on an inbred 129/Sv background.
Figure 2.
Gene replacement at the Noggin locus. (A) Schematic representation of gene replacement strategy in which most of the Noggin coding region was replaced by the lacZ gene of E. coli. (Top) Targeting construct that contains 5.2 kb of 5′ flanking sequence with lacZ fused in-frame to the first 10 amino acids of the Noggin coding exon (shaded), followed by a PGKneo cassette (neo; the arrow indicates the direction of transcription from the PGK promoter) and 5.0 kb of 3′ flanking homology starting at the BamHI site, 1.0 kb downstream of the Noggin stop codon. An MC1–HSVTK (TK) cassette was included for negative selection. (Middle) The wild-type Noggin locus with the single Noggin coding exon (shaded). (Bottom) The map represents the expected targeted allele following gene replacement at the Noggin locus. The positions of diagnostic 5′ and 3′ flanking Southern probes are indicated (hatched boxes) and relevant restriction sites used in genotyping are shown in bold. (B) Analysis of Noggin genotypes. Southern blot analysis demonstrating the expected gene replacement at the Noggin locus and germ line transmission of the targeted allele. Homologous recombinants were identified by hybridizing 5′ and 3′ probes external to the targeting vector sequences to genomic DNA digested with BamHI and EcoRI, respectively. The 5′ probe detects a 7.5-kb wild-type and 10.0-kb targeted band and the 3′ probe a 5.8-kb wild-type and 7.5-kb targeted band.
Diagnostic Southern blot analysis with 5′ and 3′ flanking probes confirmed that the predicted targeted allele was present in Noggin mutants (Fig. 2B). Further, histochemical staining for β-galactosidase activity in heterozygous embryos confirmed that the lacZ gene was expressed in the structures predicted from in situ hybridization studies (Fig. 1B,F,I,K,M,N). We also detected lacZ activity transiently in migrating neural crest cells (large arrows in Fig. 1F,M), in the dorsal root ganglia (a neural crest derivative, arrow in Fig. 1I), in ventral posterior mesoderm (small arrows in Fig. 1 F,I,N), and in the rostral floor plate from 10.5 dpc (large arrows in Fig. 1K). The expression in neural crest cells most likely reflected a perduring of β-galactosidase activity in neural crest cells emerging from Noggin expressing regions of the dorsal neural tube. A similar observation has been made in transgenic embryos which express lacZ under the control of the Wnt-1 enhancer (Echelard et al. 1994). However, expression in the ventral mesoderm and floor plate represent sites where Noggin transcripts are either below the level of detection by in situ hybridization, or where the lacZ gene was ectopically expressed. Ectopic expression could result from either the removal of 3′ flanking regions or from the influence of the PGK promoter.
General Noggin phenotype
Loss of Noggin resulted in a recessive lethal phenotype at birth. Superficial examination revealed multiple defects including a failure of neural tube closure, broad club-shaped limbs, loss of caudal vertebrae, a shortened body axis, and retention of a small vestigial tail (Fig. 3A–C). When examined on an inbred (129/Sv) or F1 hybrid (129/Sv; C57BL6/J) background, there was a pronounced variability in cranial neural tube closure. The brain was almost always open in Noggin mutants on the inbred background (Fig. 3B), but most often closed in mutants on the hybrid background (Fig. 3C).
Figure 3.
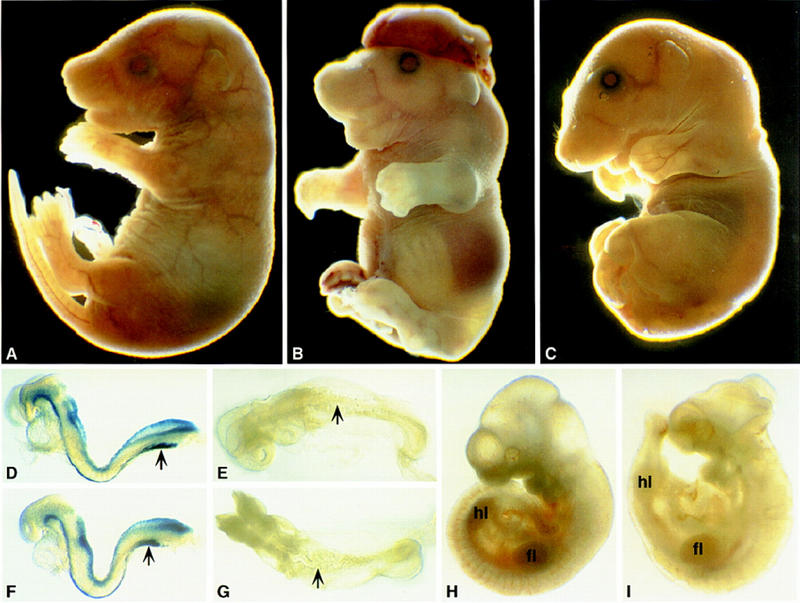
Superficial examination of Noggin mutant phenotype. (A,D,E,H) Wild-type; (B,C,F,G,I) Noggin mutants at 18.5 dpc (A–C), 8.5 dpc (7–8 somites, D–F), 8.75 dpc (12 somites, E–G), and 10.5 dpc (H,I). Noggin mutants in C and F were on a 129/SV;C57BL6/J hybrid background; all others were on an inbred 129/Sv background. Embryos in D and F were stained to visualize lacZ activity from the targeted allele at early somite stages. Forelimb (fl); hindlimb (hl).
To address the role that Noggin may play in events regulated by midline signaling we examined over 400 homozygous mutant embryos collected between 8.0 dpc and 10.5 dpc (from predominantly the 129/Sv background), for morphology, histology, and gene expression. We report elsewhere on the function of Noggin at later stages in the control of cartilage morphogenesis (Brunet et al. 1998).
Staining for β-galactosidase activity at early somite stages revealed no difference in notochord or dorsal neural development between heterozygous and homozygous mutant embryos except for a slight shortening and broadening of the notochordal plate (arrowed in Fig. 3D,F). By the 8–9 somite stage, homozygous embryos on the 129/Sv background could be distinguished from wild-type or heterozygous siblings by a flattening of the elevating neural folds in the mid/hindbrain region (data not shown). Within the next few hours Noggin mutants on both backgrounds developed kinking of the spinal cord (arrows in Fig. 3E,G). A severe neural tube phenotype subsequently emerged. In the brain, the neural tube failed to close between the diencephalon and myelencephalon (129/Sv background) and was kinked along much of its length in presumptive spinal cord regions (both backgrounds; Fig. 3H,I). Occasionally the neural tube was open from the diencephalon to its caudal limit. The open and kinked brain perturbed eye and ear development but otherwise embryos appeared to develop fairly normally anterior to the forelimbs. In contrast, caudal to the forelimb, embryos exhibited a shortened axis, with the hindlimbs closer to the forelimbs; the tail was also short, and the somites and neural tube were considerably reduced in size (Fig. 3H,I).
Histological sections at different axial levels revealed a rostral to caudal increase in the severity of the Noggin phenotype. At the forelimb level, Noggin mutant embryos were essentially normal except for a distended dorsal neural tube (Fig. 4A,B). At lumbar levels, the spinal cord and somites were significantly reduced in size and pockets of neural crest derived cells remained at the dorsal midline (Fig. 4C,D and large arrow in Fig. 5J). Extensive apoptosis was apparent at intermediate and ventral positions within the developing spinal cord (arrows in Fig. 4F). At caudal hindlimb levels, the decrease in neural tissue was more pronounced and the dorsal medial somite, which normally undergoes a mesenchymal transformation in forming the myotome, remained epithelial (arrows in Fig. 4H). A large mass of cells, most likely originating from the neural crest, lay immediately above the epithelial somite (Fig. 4G,H). Considerable cell death was evident in the ventral neural tube (data not shown). Interestingly, at extreme caudal positions, close to the tail bud, the neural tube, notochord, and presomitic mesoderm appeared similar to that of wild-type littermates, although discrete dorsal apoptosis was observed in the dorsal neural tube of mutants (Fig. 4I,J; data not shown). At 9.0 dpc, although the neural tube was already considerably smaller, we could only detect apoptosis localized to the dorsal neural tube in thoracic regions (arrow in Fig. 4L). Thus, whereas Noggin does not appear to be essential for the formation of either mesodermal or neural tissue prior to 10.5 dpc, Noggin is required in caudal regions for normal development of both these tissues.
Figure 4.
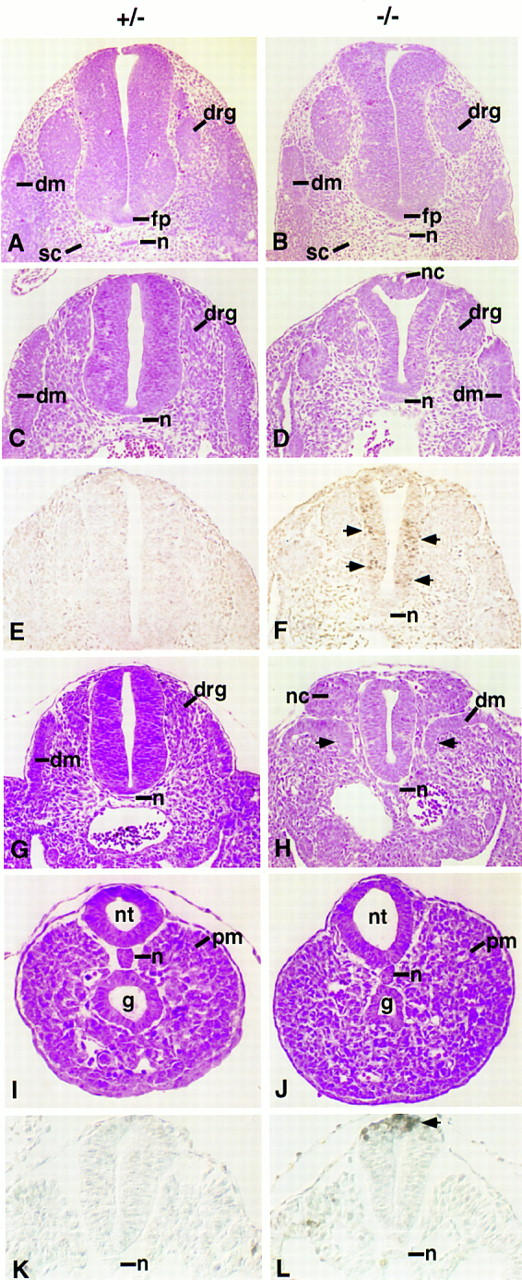
Histological and cell death analysis of spinal cord and somite development in Noggin mutants. Transverse sections through embryos heterozygous (A,C,E,G,I,K) or homozygous (B,D,F,H,J,L) for the targeted Noggin allele at 10.5 dpc (A–J) and 9.0 dpc (K,L). Sections were cut at the level of the forelimb (A,B), between the fore- and hindlimbs (C–F,K,L), at the caudal hindlimb level (G,H), and through the presomitic mesoderm just anterior to the tail bud (I,J). A–D and G–J are hematoxylin and eosin stained; E,F,K, and L underwent the TUNEL reaction to visualize apoptotic cell death. Pairwise comparisons were photographed at the same magnification. (dm) Dermomyotome; (drg) dorsal root ganglia; (fp) floor plate; (g) gut; (n) notochord; (nc) neural crest; (nt) neural tube; (pm) presomitic mesoderm; (sc) sclerotome.
Figure 5.
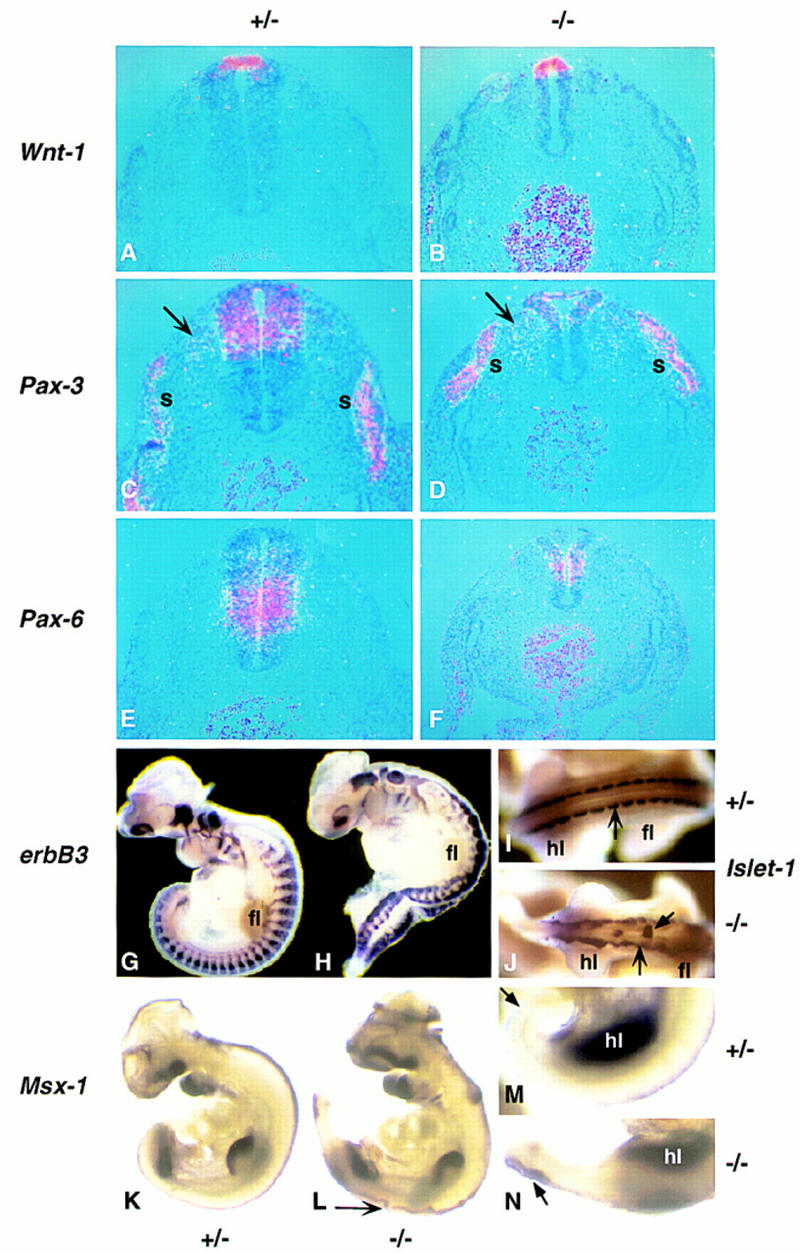
Analysis of dorsal neural tube patterning in Noggin mutants just rostral to the hindlimbs. Embryos heterozygous (A,C,E,G,I,K,M) or homozygous (B,D,F,H,J,L,N) for the targeted Noggin allele were examined at 10.5 dpc by section (A–F) or whole-mount (G–N) in situ hybridization for expression of Wnt-1 (A,B), Pax-3 (C,D), Pax-6 (E, F), erbB3 (G, H), Islet-1 (I, J), and Msx-1 (K–N). The arrow in L indicates open neural tube in the lumbar region. (fl) Forelimb; (hl) hindlimb; (s) somite.
Noggin is required for ventralization of the posterior spinal cord
The expression of Noggin in the roof plate and notochord, both of which are organizing centers responsible for dorsoventral patterning of the vertebrate neural tube (Tanabe and Jessell 1996; Liem et al. 1997), suggests that Noggin may play some role in these events. We therefore examined the expression of a number of molecular markers that define different dorsoventral positions in the early neural tube. To address dorsal development we examined the expression of the Noggin–lacZ fusion, Wnt-1, Wnt-3a, Bmp-6, Lmx-1a, Msx-1, and Follistatin, all of which are expressed in the roof plate; Math1, which is also expressed just lateral to the roof plate; Pax-3, which is restricted to the dorsal half of the spinal cord from the tail to the diencephalon; and Pax-6, which partially overlaps the ventral-most domain of Pax-3 expression, but extends into the ventral half of the neural tube. At 10.5 dpc all of these genes were expressed in their appropriate positions in the neural tube to hindlimb levels in Noggin mutants (Figs. 5A–F,K,L and 8S,T, below; data not shown). Thus, although the presumptive spinal cord was clearly substantially reduced in size in the interlimb region, the earliest features of positional specification in the dorsal half of the neural tube appeared to be unaltered. Further, migrating neural crest cells, which originate from the dorsal neural tube, and one of their derivatives, the dorsal root ganglia, could be identified by expression of the Noggin–lacZ fusion (data not shown), Pax-3 (arrows in Fig. 5C,D), erbB3 (Fig. 5G,H), and Islet-1 (large arrows in Fig. 5I,J). However, migration and differentiation of the neural crest were disrupted, most likely as a result of defective neural tube closure (Fig. 4D,F) and abnormal somite development (see below). Interestingly, Msx-1, unlike other roof plate markers, was ectopically activated in the most caudal neural tube of Noggin mutants (Fig. 5K–N). As Msx-1 is a target of BMP4 signaling in neural tube explants in culture (Liem et al. 1995), this result suggests that Noggin may prevent the premature activation of this BMP target in the developing neural tube. In summary, although we detected increased cell death in undifferentiated regions of the dorsal neural tube, Noggin was not essential for most aspects of the initial steps of dorsal patterning we investigated. In contrast, the initial aspects of ventralization of the posterior neural tube were abnormal in Noggin mutants.
A series of interactions are responsible for the induction of ventral cell fates (reviewed in Ericson et al. 1996, 1997; Tanabe and Jessell 1996). In the first of these, high concentrations of Sonic hedgehog (Shh) produced by the notochord induce the floor plate at the ventral midline. These cells coexpress Shh and the transcriptional regulator Hnf-3β. Lower concentrations of Shh secreted by the notochord and floor plate induce the development of motor neurons ventrolaterally. Motor neurons or their progenitors express c-RET, Islet-1, Islet-2, Sim-1, and Nkx2.2. The exact pattern of marker gene expression and eventual cell fate is thought to depend on the concentration of Shh (Ericson et al. 1997). Analysis of motor neuron deficient Islet-1 mutants suggests a second induction in which motor neurons induce Engrailed-1 (En-1) expressing interneurons (Pfaff et al. 1996); however, more recent evidence argues against a direct role for motor neurons (Ericson et al. 1997).
In wild-type embryos at 10.5 dpc, Islet-1 (small arrows in Fig. 6A,C,E), Nkx2.2 (Fig. 6G), Sim-1 (barbed arrow in Fig. 8Q, below), and c-RET (data not shown) expressing differentiating motor neurons, and En-1 expressing ventral interneurons (arrow in Fig. 6I), extend to the hindlimb level. In contrast, in Noggin mutants motor neuron formation was greatly reduced between the limb buds (arrowhead in Fig. 6D) and was completely absent at hindlimb levels (Figs. 6F,H and 8R, below). Further, no En-1 expressing interneurons were detected caudal to the forelimbs (Fig. 6J). Surprisingly, expression of Shh in the floor plate (large arrow in Fig. 6K,L) and notochord (small arrow in Fig. 6K,L) appeared normal where the absence of ventrolateral neuronal populations was first apparent.
Figure 6.
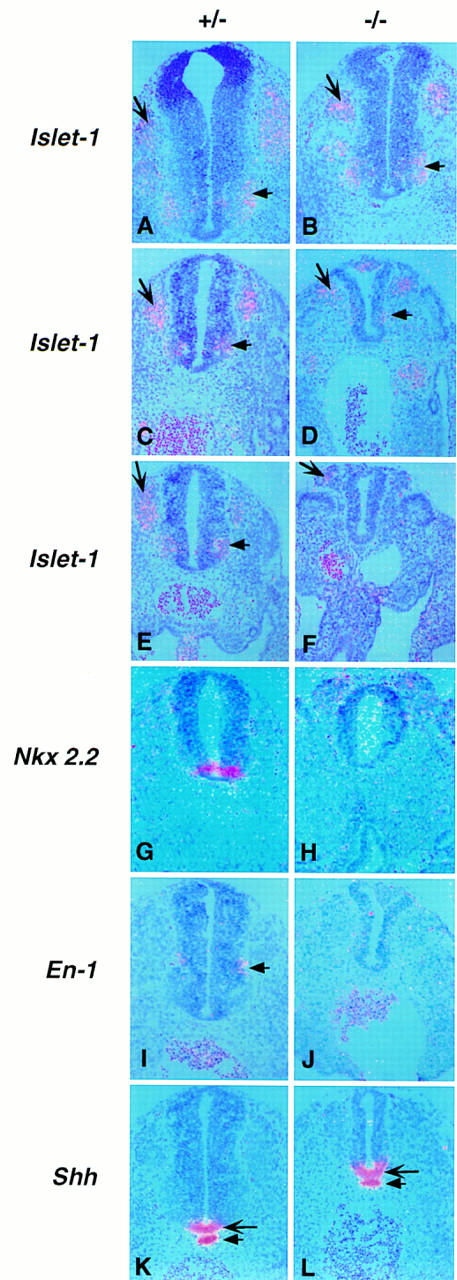
Failure of motor neuron and ventral interneuron development in the absence of Noggin. Section in situ hybridization at the level of the forelimb (A,B), between the limbs (C,D,G,J), and at the hindlimb (E–H) level in 10.5 dpc embryos heterozygous (A,C,E,G,I,K) or homozygous (B,D,F,H,J,L) for the targeted Noggin allele. Differentiating motor neurons or their precursors (short arrow) were detected with an Islet-1 probe (A–F), which also hybridizes to transcripts in dorsal root ganglia (barbed arrow) or with an Nkx2.2 probe (G,H). Differentiating ventral interneurons were detected by En-1 expression (I,J), and notochord and floor plate by Shh (K,L). (For abbreviations, see legend to Fig. 5.) Pairwise comparisons were photographed at the same magnification.
Figure 8.
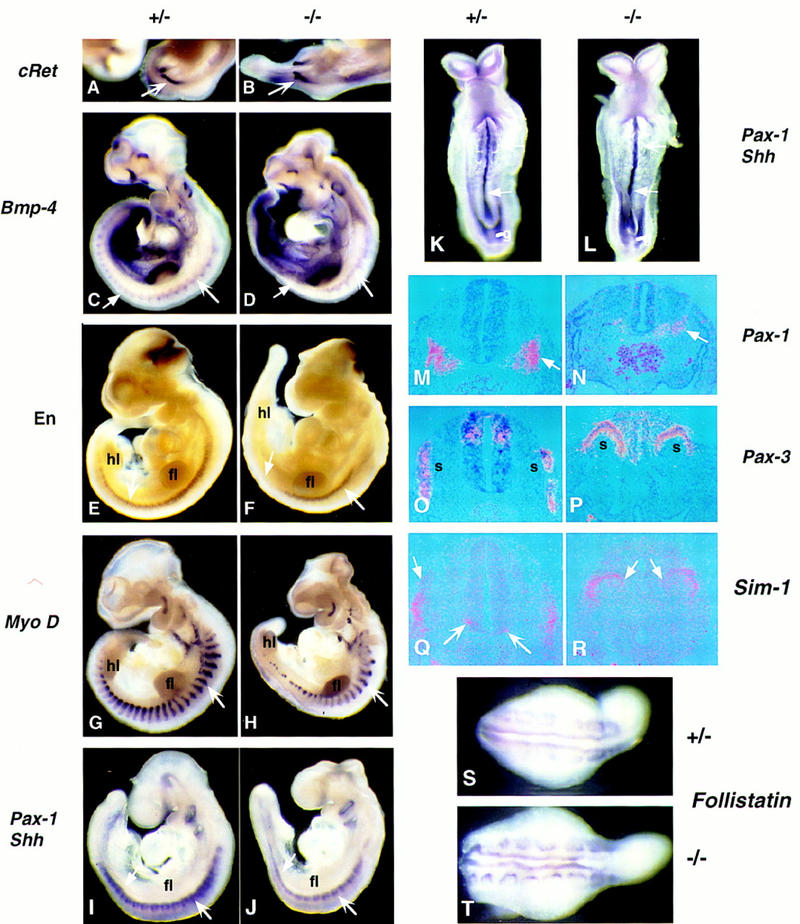
Somite development is disrupted in Noggin mutants. Somite and ventral mesoderm development was analyzed in embryos at 8.5 dpc (K,L), 9.5 dpc (I,J), and 10.5 dpc (A–H,M–T) by whole mount (A–L,S,T) or section (M–P) in situ hybridization using the markers indicated. Sections were probed at, or just rostral to, the level of the hindlimbs. Pairwise comparisons were photographed at the same magnification.
The phenotype was more severe at hindlimb levels where the spinal cord was markedly reduced in size. Here, Pax-3 was expressed throughout most of the spinal cord (data not shown), while Pax-6 expression was lost (Fig. 7A,B). At the ventral midline, only limited floor plate development was apparent despite normal Shh expression in the notochord (small arrow in Fig. 7E,F). Hnf-3β was induced (arrow in Fig. 7C,D) but no Shh expression was detected at the ventral midline of the neural tube (large arrow in Fig. 7E,F). These results indicate that in the absence of Noggin, Shh is not sufficient for normal patterning of the ventral neural tube. However, Shh signaling appeared to be occuring, as Noggin mutants showed up-regulation of the Shh receptor and transcriptional target, Patched, in the ventral neural tube at all axial levels (data not shown).
Figure 7.
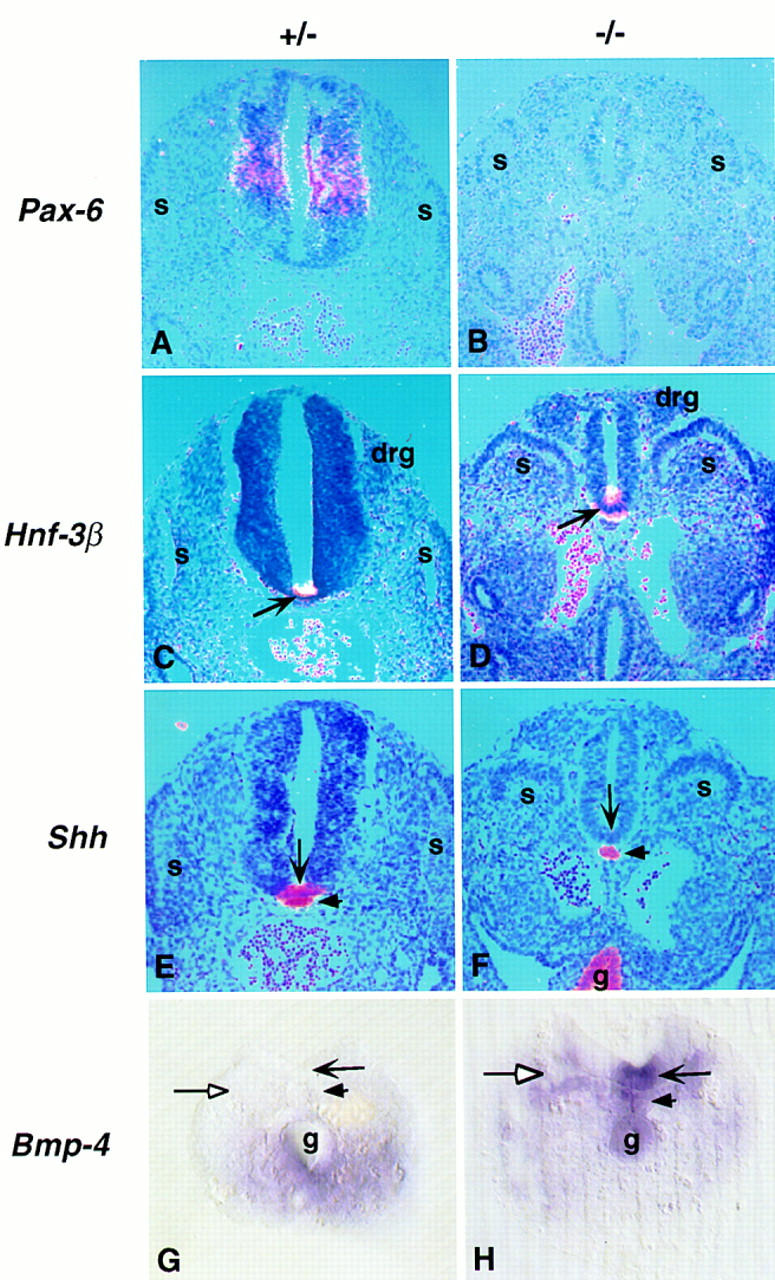
Development of the caudal neural tube in Noggin mutants. Section (A–F) and whole-mount (G,H) in situ hybridization of Pax-6 (A,B), Hnf-3B (C,D), Shh (E,F), and Bmp4 (G,H) probes to 10.5 dpc embryos heterozygous (A,C,E,G) or homozygous (B,D,F,H) for the targeted Noggin allele. (A–F) Hindlimb levels; (G,H) tail region. Pairwise comparisons were photographed at the same magnification. (For abbreviations, see Fig. 5.)
As Noggin is a BMP antagonist (Zimmerman et al. 1996; Liem et al. 1997), and BMPs have been implicated in dorsal neural patterning in spinal cord regions (Liem et al. 1995, 1997), we addressed the relationship between the expression of Bmp2–Bmp7 and caudal neural tube development in Noggin mutants. Unlike the chick, Bmp4 was not expressed in the dorsal neural tube but was expressed in the surface ectoderm (data not shown; Winnier et al. 1995; Dudley and Robertson 1997), coelomic epithelium underlying the neural tube and somites (data not shown), and ventral mesoderm (Fig. 7G) in the tail. Surprisingly, in the absence of Noggin we observed ectopic Bmp4 expression in the notochord (small arrow in Fig. 7H) and at the ventral midline of the neural tube (large arrow in Fig. 7H) together with a dorsal expansion in the unsegmented mesoderm (open arrow in Fig. 7H), but only in the tail region. At more rostral positions no ectopic expression of any of the Bmps investigated was observed (data not shown). This suggests a transient role for Noggin in preventing Bmp4 activation in important ventralizing centers.
Noggin is required for somite development
The requirement for Noggin in mesodermal development was addressed by examining embryos between 8.5 dpc and 10.5 dpc of development. Although the notochord extended along the length of the axis to the tail bud at 10.5 dpc, it displayed occasional side branching and buckling in Noggin mutants. Shh, Hnf-3b, and Brachyury were expressed normally (Figs. 6L and 7F; data not shown), but there was a premature loss of lacZ activity in the rostral-most notochord of Noggin mutants suggesting that Noggin may be required for maintenance of its own expression (data not shown). Thus, Noggin is not required for either formation or early development of the notochord. However, after 10.5 dpc, tail development arrested and no new notochord was formed despite continued expression of the tail bud markers Brachyury, Wnt-3a, and Wnt-5a (data not shown). At this time Bmp4 expression was observed to extend into dorsal mesoderm (open arrow in Fig. 7H; data not shown). Whether this change in Bmp4 was responsible for the arrest of tail development is unclear. Expression of c-RET, Bmp7, and Sim-1 in the mesonephric duct (arrows in Fig. 8A,B; data not shown) was similar to wild-type littermates.
In the paraxial mesoderm, Noggin was not essential for segmentation but was required for growth and differentiation of the somite. Recent evidence indicates that somite patterning is governed by a complex network of signals. For example, Shh signaling by the floor plate and notochord induces sclerotome formation (Fan and Tessier-Lavigne 1994; Johnson et al. 1994; Fan et al. 1995; Chiang et al. 1996). In the myotome, Shh and members of the Wnt family, which encode secreted glycoproteins, are implicated in muscle development (Johnson et al. 1994; Munsterberg et al. 1995; Currie and Ingham 1996; Hammerschmidt et al. 1996a). As well as a requirement for certain signals for somite differentiation, recent evidence suggests that inhibition of BMP4 signaling may also play an important role in myotomal development (Reshef et al. 1998). Finally, dermal development is thought to depend on contact, mediated signaling by the ectoderm (Fan and Tessier-Lavigne 1994), for which Wnt family members are strong candidates (Fan et al. 1997).
The development of somites originating rostral to the forelimb at 9.5 and 10.5 dpc appeared grossly normal. In Noggin mutants the expression of Bmp4 (large arrows in Fig. 8 C,D; data not shown), En-1 (large arrows in Fig. 8E,F), Sim-1, and Pax-3 (data not shown) in the dermomyotome; myoD (arrows in Fig. 8G,H), myf5, and myogenin (data not shown) in the myotome; and Pax-1 (small arrows in Fig. 8I,J), scleraxis, and Sox-9 in the sclerotome was similar to wild-type embryos. However, examination of early somite stages (8.5 dpc) indicated that in the absence of Noggin, expression of the sclerotomal marker Pax-1 was delayed by several hours in these rostral-most somites (large arrows in Fig. 8K,L) despite normal Shh expression in the notochord (small arrow in Fig. 8K,L). Between the forelimbs and hindlimbs there was a more dramatic somitic phenotype; a severe reduction in both sclerotomal (small arrow in Fig. 8I,J,M,N) and myotomal, most strikingly dorsal myotomal (Fig. 8G,H), derivatives. Pax-3 expressing limb muscle precursors were present in mutants, even at hindlimb levels (data not shown). We also observed an absence of dermomyotomal expression of Bmp4 (small arrow in Fig. 8D) and En-1 (small arrow in Fig. 8F), indicating that dermomyotomal differentiation was arrested. Interestingly, from the hindlimb caudal, the dorsomedial somite, where dorsal myotomal development normally initiates, remained epithelial and continued to express the predifferentiation marker, Pax-3 (Fig. 8O,P). Furthermore, Sim-1, whose ventrolateral dermomyotomal expression is thought to be regulated by BMP4 signaling (Pourquie et al. 1996), extended into the dorsomedial epithelial somite at the hindlimb level (arrows in Fig. 8R). Finally, Follistatin (which encodes an activin and most likely BMP7 antagonist) appeared to be up-regulated in the dorsomedial somite of Noggin mutants (Fig. 8T). Together, the data indicate that Noggin-mediated antagonism of BMP signaling is essential for normal growth and patterning of the caudal somites.
Noggin is required for Shh-mediated induction of sclerotomal development
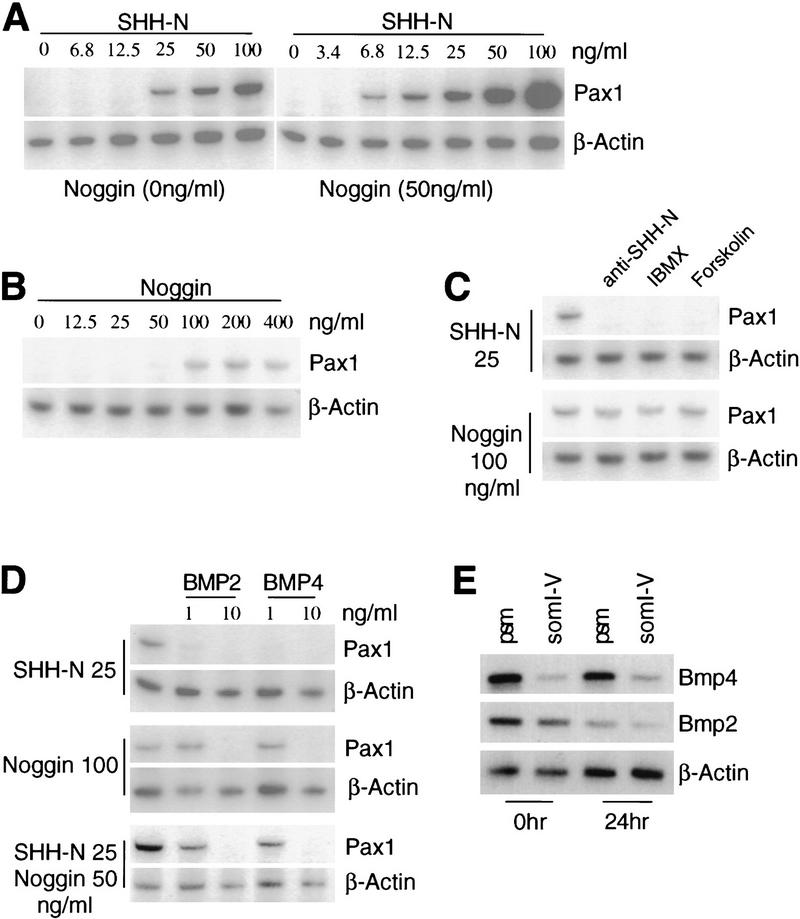
The substantial delay and reduction in sclerotomal and myotomal development suggests that Noggin may cooperate with Shh in patterning the somitic mesoderm. We therefore investigated whether Noggin is able to synergize with Shh in the induction of Pax-1 in presomitic mesoderm (psm) explants (Fan et al. 1995). The presence of Noggin reduced the threshold induction of Pax-1 by SHH-N fourfold (from 25 to 6.8 ng/ml; Fig. 9A), indicating that Noggin synergizes with Shh. Surprisingly, high concentrations of Noggin (>100 ng/ml) were sufficient to activate Pax-1 (Fig. 9B). To determine whether the response to Noggin resulted either from potentiating low levels of Shh present in the psm, or from an early exposure to Shh signaling emanating from the notochord, we added antagonists that are known to abolish SHH-N function in this assay. Addition of a blocking antibody against SHH-N (Ericson et al. 1996), although able to inhibit SHH-N induction of Pax-1, did not block induction by Noggin (Fig. 9C). Furthermore, induction was not blocked by addition of IBMX and forskolin, which antagonize SHH-N signaling by elevating intracellular cAMP (Fig. 9C). Taken together, we conclude that Noggin can act alone to activate Pax-1 expression in the psm explant.
Figure 9.
Noggin and Shh function synergistically to activate the expression of sclerotome marker Pax1 in presomitic mesoderm. Mouse psm explants (9.5 dpc) were cultured in collagen gels for 24 hr in the presence or absence of purified recombinant Noggin, SHH-N (the amino-terminal 19-kD peptide), BMP2, or BMP4. The concentration of each of these is indicated. RNA samples from each culture (two pieces of psm per culture) were extracted and subjected to RT–PCR to assess marker gene expression. Expression of β-actin was assayed as a control for RNA recovery and cDNA synthesis (see Materials and Methods). (A) Psm explants were treated with a series of concentrations of SHH-N (as indicated) with (right) or without (left) 50 ng/ml Noggin. Expression of the sclerotome marker Pax1 and the β-actin control was assessed by RT–PCR. (B) Noggin alone can also activate the sclerotomal marker Pax1. (C) Noggin can induce Pax-1 expression in the presence of a SHH-N blocking antibody, IBMX, and forskolin, whereas all these reagents block SHH-N mediated induction of Pax-1 in psm explants. (D) Purified BMP2 and BMP4 protein can inhibit Pax-1 induction in response to SHH-N, Noggin, or a combination of each protein. (E) The psm and the five caudal-most somites (somI–V) were assayed immediately (0 hr) or after 24 hr of culture (24 hr) for expression of Bmp2 and Bmp4 (30 cycles). RT–PCR products were analyzed by Southern hybridization (see Materials and Methods). β-actin (13 cycles) expression was similar in all samples.
We next tested whether purified recombinant BMP2 or BMP4 can suppress Pax-1 induction. Both these BMPs were potent inhibitors. Ten nanograms/milliliter of either BMP completely abolished Pax-1 induction in response to Noggin, SHH-N, or a combination of the two (Fig. 9D). These results suggest that overcoming inhibitory BMP signaling is necessary to initiate the sclerotomal program. In the embryo, inhibitory BMPs could be supplied by the paraxial mesoderm itself, or by adjacent tissue. In situ hybridization studies have demonstrated that the early somite uniformly expresses Bmp3 (Dudley and Robertson 1997; J. McMahon et al., unpubl.). Moreover, the somite forms adjacent to the notochord that expresses Bmp3 and Bmp7, above the ventral mesoderm that expresses Bmp4, and adjacent to the surface ectoderm that expresses Bmp4 and Bmp7 (Dudley and Robertson 1997; data not shown). In addition, using PCR, we explored the possibility that the psm may express low levels of Bmps not detectable by in situ hybridization. After 30 cycles we detected Bmp2 and Bmp4 (Fig. 9E), but failed to detect Bmp5, Bmp6, and Bmp7 after 35 cycles. Although we cannot rigorously exclude the possible contamination by ventral mesoderm, these results suggest that the psm itself, or neighboring tissues, express those Bmps that are known to interact with Noggin.
Discussion
Noggin and the organizer
Expression of Noggin in the organizer region of the Xenopus embryo (Smith and Harland 1992), and the results of ectopic expression studies in the frog (Smith and Harland 1992; Lamb et al. 1993; Smith et al. 1993) and fish (Hammerschmidt et al. 1996b) are consistent with Noggin playing a central role in specifying dorsal cell fates in both the ectoderm (neural plate) and mesoderm (somite and notochord). The recent demonstration that Noggin is a direct antagonist of ventralizing BMP signals suggests a simple mechanism by which Noggin patterns the embryo, through the graded inhibition of ventral BMP signaling (Zimmerman et al. 1996). However, even though Noggin is expressed in the mouse node, a structure with similar properties to the Xenopus organizer (Beddington 1994), Noggin is not essential for formation of neural tissue, notochord, or somites. Rather, our results demonstrate that Noggin expression in the notochord and roof plate plays a later role in somite and neural tube development.
No phenotype was observed in Noggin mutants until 8.5 dpc, despite the early midline expression of Noggin. There only appears to be a single Noggin gene in vertebrates, so it is unlikely that there is redundancy between Noggin and a second, unidentified family member. However, at least two unrelated secreted polypeptides, Follistatin and Chordin, have similar activities. Both of these are expressed in the early mesoderm and/or node of the gastrulating mouse embryo (Albano et al. 1994; E. De Robertis, pers. comm.). Follistatin null mutants show no defects in organizer function (Matzuk et al. 1995). Chordin mutants have been identified in the zebrafish (Schulte-Merker et al. 1997). One of these, dino, has a ventralized phenotype consistent with some loss in organizer function (Hammerschmidt et al. 1996b). Consequently, it may be necessary to remove Noggin, Chordin, and Follistatin to completely uncover their putative organizer properties.
Noggin and neural tube development
Although Noggin does not play an essential role in the induction of neural tissue, it is required for subsequent development of the neural tube. In Noggin mutants, we observed a failure of neural tube closure in cranial and lumbar regions, a dramatic reduction in the amount of posterior neural tissue, and the progressive failure of ventral development in the posterior neural tube.
Noggin is expressed in the dorsal aspects of the neural plate, the presumptive roof plate, coincident with neural tube closure. Thus, it is possible that Noggin may play a direct role in regulating the cellular processes that lead to the elevation and fusion of the neural folds (for review, see Schoenwolf and Smith 1990). Examination of a large number of molecular markers expressed either in or around the closing neural tube (Wnt-1, Wnt-3a, Bmp6, Follistatin, Lmx-1a, Noggin-lacZ, Math1, Pax-3) failed to reveal any striking difference between Noggin mutants and wild-type siblings suggesting that the failure of neural tube closure does not appear to result from a clear defect in early patterning of the dorsal neural tube. However, we observed premature activation of a BMP4 target, Msx-1, in the caudal neural tube, consistent with a role for Noggin in preventing Msx-1 activation. We also observed enhanced cell death at the dorsal midline of the neural tube in Noggin mutants. As Msx family members have been implicated in the regulation of apoptosis (e.g., Graham et al. 1994) there may be a correlation between these two observations. Furthermore, recent work suggests that regulation of cell death may play an important role in neural tube closure (Weil et al. 1997). Whether there is a link between deregulated cell death and the open neural tube remains to be explored but it seems likely that the Noggin mutants might provide a useful model for studying this important and poorly understood morphological process.
The other obvious CNS phenotype we observed is a reduction in posterior neural development that occurs secondarily to the formation of neural tissue. Caudal to the forelimbs there was a progressive decrease in the diameter of the neural tube that was accompanied by the appearance of extensive cell death, principally in intermediate and ventral regions of the developing spinal cord. Thus, Noggin appears to be required for survival, and possibly proliferation, of neural precursors in the neural tube. A reduction in the size of the neural tube is evident at both dorsal and ventral positions consistent with a role for Noggin in both the dorsal and ventral development. Dorsally, expression of all early regional markers investigated was appropriately positioned and extensive neural crest formation was observed. In vitro studies in the chick have demonstrated that several TGF-β family members, including BMP4 and BMP7, may contribute to the induction of dorsal neurons. Furthermore, they suggest that blocking early BMP activity may be a prerequisite for the emergence of later arising dorsal cell fates (Liem et al. 1997). Whether specific dorsal neuronal populations that emerge later in development are dependent on Noggin function remains to be determined.
Surprisingly, Noggin is clearly essential for establishment of some ventral cell fates in the developing CNS. At posterior lumbar levels, motor neurons and ventral interneurons were depleted or entirely absent. In more posterior positions, floor plate development was initiated but a mature floor plate was not formed. Finally, caudal to the hindlimbs, much of the neural tube appeared to adopt a dorsal, likely neural crest cell fate. How might these results be explained in light of our understanding of growth and patterning of the neural tube?
There is now overwhelming evidence to support the conclusion that Shh signaling plays a key role in induction of two ventral cell fates in the vertebrate CNS, motor neurons and floor plate (for review, see Tanabe and Jessell 1996; Hammerschmidt et al. 1997). In Noggin mutants we observed a failure of motor neuron induction, despite the normal expression of Shh in both the floor plate and notochord, the two signaling centers implicated in motor neuron induction (Yamada et al. 1991; Placzek et al. 1991; Yamada et al. 1993). Thus, in the embryo proper, in the absence of Noggin, Shh is not sufficient for the normal specification of motor neuron fates. As in the Islet-1 mutant (Pfaff et al. 1996), in the absence of motor neuron fates, En-1 expressing ventral interneurons are also missing. The loss of Pax-6 expression in the ventral neural tube is also consistent with these results as motor neuron and interneuron fates arise from Pax-6 expressing neural precursors (Ericson et al. 1996, 1997). In more posterior regions, we also observed that although Shh expression in the notochord was sufficient to activate Hnf-3β at the ventral midline of the neural tube, it was not sufficient to activate Shh itself, a normal feature of the floor plate. Finally, in the most posterior regions, no ventral development occurred even though Shh was expressed in the underlying notochord. Together, these results indicate that Noggin-mediated antagonism of BMP signaling is essential for Shh-mediated ventralization of the mouse neural tube. It is unlikely that Shh signaling is lost as we observed up-regulation of Patched transcription in the ventral neural tube as expected (data not shown). However, Shh signaling does not result in normal ventralization of the spinal cord.
Several lines of evidence support the view that BMP-signaling might prevent Shh action. For example, implantation of Bmp7 expressing cells adjacent to the mouse hindbrain blocks autoinduction of Shh in the floor plate (Arkell and Beddington 1997). Furthermore, addition of BMP4 Dorsalin-1, a TGF-β family member, to neural tube explants strongly inhibits the motor neuron inducing activity (presumably Shh) produced by ventral axial structures (Basler et al. 1993). Thus, the response to ectopic BMPs in these assays resembles the phenotype resulting from loss of Noggin function. A localized requirement for Noggin may also explain why ectopic expression of Shh throughout the neural tube of the Xenopus embryo (Ruiz i Altaba et al. 1995), or at the mid/hindbrain junction in the mouse (Sasaki and Hogan 1994), only results in ectopic floor plate development adjacent to the roof plate where Noggin is expressed.
BMPs, in particular BMP4 and BMP7, have been implicated in the induction of dorsal cell fates in presumptive spinal cord. The surface ectoderm most likely initiates dorsalization of the neural tube (Dickinson et al. 1994; Liem et al. 1995). The ectoderm expresses several BMPs and these appear to play a role in induction of dorsal cell fates (Basler et al. 1993, Liem et al. 1995, 1997; Dudley and Robertson 1997). Thus, in spinal cord regions, BMP signaling has an opposite function to that of Shh. Moreover, there is evidence to suggest that the neural plate is initially dorsalized, presumably in response to early acting BMP signals, and that ventral cell fate specification, which occurs later, requires the suppression of dorsal cell fates (Ericson et al. 1996). Our data show that for Shh to work effectively in the embryo, BMP signaling must be antagonized. The notochord expresses Bmp3 and Bmp7 (Dudley and Robertson 1997), suggesting that Noggin could play a direct role in antagonizing their activities. Although BMP3 binding to Noggin has not been tested, functional studies indicate that it is unlikely to have the same properties as BMP4 (K. Lyons, pers. comm.). However, Noggin does bind BMP7, albeit with considerably weaker affinity than for BMP4. Whether this has physiological significance in the embryo is difficult to assess. Follistatin, which binds BMP7 more strongly, is expressed normally in the roof plate of Noggin mutants. Alternatively, Noggin may prevent the spread of BMP4 from the ventral mesoderm, or the activity of low levels of Bmp2 or Bmp4 in the paraxial mesoderm. In keeping with this hypothesis, we observed the induction of Bmp4 in the notochord and at the ventral midline of the caudal neural tube in Noggin mutants. Thus, in the absence of Noggin, BMP4 in the ventral mesoderm may be able to induce its own expression in more dorsal regions.
The loss of ventral cell fates in Noggin mutants does not appear to be accompanied by a concomitant expansion of dorsal cell fates. Rather, it would appear that many ventral cells die. Intriguingly, this results in a gross reduction of the neural tube that appears to be more pronounced than that of notochord, deficient embryos (van Stratten and Hekking 1991; Yamada et al. 1991). Why should the loss of a single secreted polypeptide generate a more severe phenotype than the complete loss of all notochordal activities? One possible explanation is that in the absence of Noggin, ventral cells would receive conflicting signals since Shh is still produced and cells appear to respond to Shh. These conflicting signals, BMPs (dorsalizing) and Shh (ventralizing), might cause cell death. Interestingly, in the forebrain Shh and BMPs, most likely BMP7, have been proposed to collaborate in the patterning of ventral diencephalic regions (Dale et al. 1997), suggesting that the combination of these signals might lead to the specification of diencephalic neural precurors in the spinal cord. However, we found no evidence for ectopic expression of Nkx2.1 to support such a model (data not shown).
Noggin and somite development
In the absence of Noggin there is marked reduction in both the induction and survival of sclerotomal and myotomal derivatives in the trunk. A large body of evidence indicates that the distinct cell fates are generated within the epithelial somite in response to inductive cues from surrounding tissues (for review, see Brand-Saberi et al. 1996; Cossu et al. 1996). For example, removal of the notochord results in a failure of sclerotomal induction. In vitro, the inductive properties of the notochord are mimicked by Shh (Fan et al. 1995). However, Shh mutants exhibit some Pax-1 expression, indicative of limited sclerotomal development, which is followed by a secondary loss of these cells (Chiang et al. 1996). Thus, Shh is not essential for initiating all sclerotomal development. Our data suggest that Noggin contributes to sclerotomal induction.
First, Noggin is required at all axial levels for the normal induction of sclerotome. At rostral levels, induction is delayed but is otherwise normal, at thoracic levels the sclerotomal population is reduced, and at more posterior positions sclerotome is entirely absent. Second, at high concentrations Noggin induces sclerotomal development in the absence of Shh. Third, addition of Noggin to presomitic mesoderm explants lowers the effective dose of Shh which is required to induce sclerotome. Fourth, BMP2 and BMP4 are potent inhibitors of Pax-1 induction by Shh in culture. Moreover, grafts of BMP4-expressing cells inhibit growth and differentiation of the chick sclerotome (Monsoro-Burq et al. 1996). These findings can be explained by a simple model in which BMP signaling generally inhibits early somite differentiation. Countering this inhibition may be essential for sclerotomal induction. Binding of BMP4, and possibly other BMPs, by Noggin would provide a direct antagonism. It is unlikely that BMP signaling inhibits Hedgehog targets by activating PKA (for review, see Hammerschmidt et al. 1997), as Noggin is still able to induce Pax-1 in the presence of PKA agonists.
The notochord and neural tube are also implicated in the induction of muscle derivatives that fail to develop when both tissues are removed (Rong et al. 1992). Shh also appears to play a role in myotome induction, most likely with dorsally expressed members of the Wnt family (Münsterberg et al. 1995; Chiang et al. 1996). The loss of myotomal derivatives in Noggin mutants suggests that suppression of BMP signaling is also necessary to allow effective muscle induction. The somite gives rise to distinct populations of muscle precursors in different positions (Cossu et al. 1996). Myogenic gene expression occurs first in the dorsomedial component that gives rise to the epaxial musculature, then later in the ventrolateral hypaxial precursors. BMP4 is implicated in the regulation of distinct cell fate choices within the myotome. Bmp4 is expressed in the lateral mesoderm adjacent to the hypaxial precursors and application of BMP4 to the dorsomedial component leads to the repression of myogenic gene expression (Pourquie et al. 1995). In contrast, the formation of epaxial muscles from the dorsomedial somite requires an opposing activity, and this appears to arise from the dorsal neural tube during development (Pourquie et al. 1996). In the mouse, Noggin is expressed in the roof plate, then much later in development, Noggin is expressed rather weakly in the dorsal lip of the somite. Dorsomedial expression of Noggin is in general consistent with a role in antagonizing BMP signaling in the regulation of muscle patterning. In the chick, however, Noggin has more pronounced and earlier expression in the dorsal lip of the somite, and here expression in the somite may play a more direct role in controlling myogenesis (Reshef et al. 1998). In Noggin mutants we observed the loss of dorsal myogenic gene expression and a dorsal expansion of Sim-1, a marker of ventrolateral fates, into the dorsomedial dermomyotome at hindlimb levels. In contrast, Noggin is not required for formation of Pax-3-expressing limb muscle precursors at either the fore- or hindlimb levels (data not shown). We also observed an up-regulation of Follistatin in the dorsomedial somite suggesting that its expression may be positively regulated by dorsal BMP signaling. Thus, it is likely that Noggin antagonism of BMP signaling is required for both myotomal and sclerotomal development. However, the failure of dermomyotomal expression of En-1 points to a broader inhibitory role for BMPs in the somite.
Whereas our results establish the principle of Noggin action, we cannot be certain as to the exact identity of the relevant targets. For example, the biochemical characterization of Noggin interactions has been restricted to a subset of BMPs, but there are several other members of the TGF-β family, notably some of the GDFs, which are also coexpressed with Noggin. Furthermore, there is no reason to believe that all family members have been identified. Of those that are known, Bmp3 is expressed in the immature somite and is down-regulated on differentiation (Dudley and Robertson 1997; J.A. McMahon et al., unpubl.). However, BMP3 does not appear to be required for normal somite patterning and has different activities from BMP2 and BMP4, so it is unlikely to be a Noggin target (K. Lyons, pers. comm.). The most plausible candidate is Bmp4, which is strongly expressed in the ventral mesoderm immediately under the paraxial mesoderm and in the coelomic mesoderm underlying the developing somites. In summary, our results demonstrate that elaboration of the vertebrate body plan requires not only the positive action of a number of inductive signals, but also the specific inhibition of others. It is likely that inhibitory molecules will become increasingly important in our understanding of vertebrate development as more are identified and their functions dissected.
Materials and methods
Cloning and targeting of mouse Noggin
Initially, cDNAs partially encoding mouse Noggin were isolated by screening a phage λ gt10 cDNA library (gift of Brigid Hogan) with Xenopus Noggin using standard low stringency conditions. A mouse 129 strain λ genomic library (the generous gift of Rudolf Jaenisch, Whitehead Institute, Cambridge, MA) was screened by hybridization with a mouse Noggin cDNA probe and a single genomic clone was identified (Southern blotting confirmed that there is a single Noggin gene). The Noggin gene replacement construct was generated (see Fig. 2) by blunt end ligation of a 5′, 5.2-kb, EcoRV–BstEII Noggin genomic fragment into an end-filled (T4 polymerase) NcoI site in the lacZ containing plasmid, pSDKlacZ (the gift of Janet Rossant). This resulted in an in-frame fusion of the first 10 amino acids of the Noggin coding sequence with the E. coli lacZ gene containing an SV40 polyadenylation sequence. For positive selection, a PGKneo cassette (Soriano et al. 1991) was cloned downstream of the lacZ gene and a 3′, 5.0-kb, BamHI–EcoRV homology region cloned downstream of this selection cassette. This targeting construct was generated in the plasmid vector pMC1-HSVTK (Mansour and Capecchi 1988) which provides a flanking herpes virus thymidine kinase gene for negative selection against nonhomologous recombinants. The targeting construct was linearized with SalI and electroporated into CJ7 ES cells (Swaitek et al. 1993). A clone with the expected recombination event was identified by Southern analysis using both 5′ and 3′ diagnostic probes (see Fig. 2) and this clone was used to generate germ-line chimeras. The mutated allele was maintained on an inbred 129/Sv background or crossed to C57BL6/J mice to generate embryos on a hybrid background.
Identification of Noggin mutants
Initially, Noggin mutants were identified by Southern blot analysis of yolk sac DNA with a diagnostic 5′ probe (see Fig. 2). Southern hybridization to DNA digested with BamHI (5′ analysis) or EcoRI (3′ analysis) with a coding region probe confirmed that the Noggin coding region was deleted from embryos that displayed a mutant phenotype. Subsequently, a PCR assay was developed in which amplification of the wild-type allele generated a 211-bp product (primers nog1 and nog2) and amplification of the mutant allele generated 160-bp product (primers nog1 and gal1). PCR samples were preheated to 93°C for 90 sec then subjected to 35 cycles of amplification alternating between a 30-sec 93°C denaturation and 45-sec 72°C extension step (McMahon et al. 1992).
PCR primers: nog1, 5′-GCATGGAGCGCTGCCCCAGC-3′; nog2, 5′-GAGCAGCGAGCGCAGCAGCG-3′; gal1, 5′-AAGG-GCGATCGGTGCGGGCC-3′.
Histology, in situ hybridization, and cell death
For routine histological analysis, embryos were fixed in Bouin’s solution, dehydrated, paraffin embedded, sectioned at 6 μm, dewaxed, and either hematoxylin and eosin or toluidine blue counterstained prior to mounting. β-Galactosidase staining and in situ hybridization were essentially as described (Wilkinson et al. 1987; Whiting et al. 1991; Wilkinson 1992). Apoptotic cell death was visualized using the TUNEL procedure with a kit from Boehringer Mannheim.
Explant tissue culture and RT–PCR
Mouse psm was dissected at 9.5 dpc, embedded in collagen gels, and cultured for 24 hr in serum-free medium; OPTI-MEM/F12/DME (50:25:25) supplemented with 5 ng/ml FGF to promote survival in serum-free conditions (Fan and Tessier-Lavigne 1994). Forskolin was added at 90 μm and IBMX at 100 μm (Fan et al. 1995). Anti-SHH-N blocking antibody, 5E1 (Ericson et al. 1996) was added at 3.5 μg/ml. Xenopus Noggin protein was purified from CHOB3 conditioned medium as described (Lamb et al. 1993) and was the gift of José de Jesus. SHH-N protein was the gift of Phil Beachy (Johns Hopkins Medical School, Baltimore, MD) and BMP2 and BMP4 were generously supplied by Genetics Institute. The RNA sample of each explant culture (containing two pieces of psm) was purified and  of each sample was used for RT–PCR reactions in the presence of [32P]dCTP (Amersham) as described previously (Fan et al. 1995). The resulting radioactive PCR products were resolved on 6% polyacrylamide gels, dried, and exposed to X-ray films (Kodak) for 2 hr. The oligonucleotide primers used to detect Pax1 and β-actin were described by Fan et al. (1995). To detect Bmp2 and Bmp4 transcripts in psm and somites, two pieces of psm and two strips of somite I–V (caudal-most five somites) were used for each culture. One half of each RNA sample was used for RT–PCR for 30 cycles (94°C–60°C–72°C cycle). The PCR products were resolved on 2% agarose gels, transferred to Gene Screen filters (NEN), hybridized with [32P]dCTP Bmp2 and Bmp4 cDNA probes, washed under the standard high stringency condition (Sambrook et al. 1989), and exposed to X-ray films for 1 hr. The primers used were 5′-CGGAGACTCTCTCAATGGAC3′ and 5′-GTTCCTCCACGGCTTCTAGT-3′ for Bmp2 which generates a 436-nucleotide product; and 5′-CTCCCAAGAATCATGGACTG-3′ and 5′-AAAGCAGAGCTCTCACTGGT-3′ for Bmp4, which generates a 468-nucleotide product. Bmp2 and Bmp4 gene sequences were described by Feng et al. (1994 and 1995, respectively).
of each sample was used for RT–PCR reactions in the presence of [32P]dCTP (Amersham) as described previously (Fan et al. 1995). The resulting radioactive PCR products were resolved on 6% polyacrylamide gels, dried, and exposed to X-ray films (Kodak) for 2 hr. The oligonucleotide primers used to detect Pax1 and β-actin were described by Fan et al. (1995). To detect Bmp2 and Bmp4 transcripts in psm and somites, two pieces of psm and two strips of somite I–V (caudal-most five somites) were used for each culture. One half of each RNA sample was used for RT–PCR for 30 cycles (94°C–60°C–72°C cycle). The PCR products were resolved on 2% agarose gels, transferred to Gene Screen filters (NEN), hybridized with [32P]dCTP Bmp2 and Bmp4 cDNA probes, washed under the standard high stringency condition (Sambrook et al. 1989), and exposed to X-ray films for 1 hr. The primers used were 5′-CGGAGACTCTCTCAATGGAC3′ and 5′-GTTCCTCCACGGCTTCTAGT-3′ for Bmp2 which generates a 436-nucleotide product; and 5′-CTCCCAAGAATCATGGACTG-3′ and 5′-AAAGCAGAGCTCTCACTGGT-3′ for Bmp4, which generates a 468-nucleotide product. Bmp2 and Bmp4 gene sequences were described by Feng et al. (1994 and 1995, respectively).
Acknowledgments
We thank the staff in our animal facility, Audrey Huang for assistance in isolating the Noggin genomic clone, Marty Shea for initial in situ hybridization analysis of Noggin expression, and Scott Lee for generously jumping in at the last minute, Lisa Brunet for the Southern analysis in Fig. 2B, José de Jesus for Noggin protein, Phil Beachy for Shh protein, Genetics Institute for BMP2 and BMP4, and Bianca Klumpar for histology. We thank the following people for gifts of probes: B. Hermann, J. Johnson, M. Goulding, T. Jessell, A. Dudley, B. Hogan, V. Pachnis, A. Joyner, H. Weintraub, E. Olson, C. Wright, P. Koopman, and G. Fischbach. Work in A.P.M’s laboratory was supported by grants from the American Cancer Society (DB 88) and National Institutes of Health (NIH). Work in R.M.H.’s laboratory was supported by NIH grant GM49346. S.T. was supported by a long-term fellowship from the Human Frontier Science Program (HFSP) and L.B.Z. by a National Research Service Award (NRSA) fellowship from NIH. C.M.F. is supported by the Arnold and Mabel Beckman Foundation and the Alfred P. Sloan Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL amcmahon@biosun.harvard.edu; FAX (617) 496-3763.
References
- Albano RM, Arkell R, Beddington RSP, Smith JC. Expression of inhibin subunits and follistatin during postimplantation mouse development: Decidual expression of activin and expression of follistatin in primitive streak, somites, and hindbrain. Development. 1994;120:803–813. doi: 10.1242/dev.120.4.803. [DOI] [PubMed] [Google Scholar]
- Arkell R, Beddington RSP. BMP-7 influences pattern and growth of the developing hindbrain of mouse embryos. Development. 1997;124:1–12. doi: 10.1242/dev.124.1.1. [DOI] [PubMed] [Google Scholar]
- Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: Regulation of cell differentiation by dorsalin-1 a novel TGFβ family member. Cell. 1993;73:687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Beddington RSP. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Wilting J, Ebensperger C, Christ B. The formation of somite compartments in the avian embryo. Int J Dev Biol. 1996;40:411–420. [PubMed] [Google Scholar]
- Brunet, L.J., J. McMahon, A.P. McMahon, and R.M. Harland. 1998. Noggin, cartilage morphogenesis and joint formation in the mammalian skeleton. Science (in press). [DOI] [PubMed]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cossu G, Tajbakhsh S, Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–455. doi: 10.1038/382452a0. [DOI] [PubMed] [Google Scholar]
- Dale JK, Vesque C, Lints TJ, Sampath TK, Furley A, Dodd J, Placzek M. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by the prechordal plate. Cell. 1997;90:257–269. doi: 10.1016/s0092-8674(00)80334-7. [DOI] [PubMed] [Google Scholar]
- DeRobertis EM, Sasai Y. A common plan for dorso-ventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Dickenson ME, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP-7 deficient embryos. Develop Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of sonic hedgehog signaling required for specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Fan C-M, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: Evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Fan C-M, Porter JA, Chiang C, Chang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: Direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- Fan C-M, Lee CS, Tessier-Lavigne M. A role for WNT proteins in induction of the dermomyotome. Devel Biol. 1997;191:160–165. doi: 10.1006/dbio.1997.8713. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Harris MA, Ghosh-Choudhury N, Feng M, Mundy GR, Harris SE. Structure and sequence of mouse bone morphogenetic protein-2 gene (BMP-2): Comparison of the structures and promoter regions of BMP-2 and BMP-4 genes. Biochim Biophys Acta. 1994;1218:221–224. doi: 10.1016/0167-4781(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Chen D, Cooney AJ, Tsai MJ, Harris MA, Tsai SY, Feng M, Mundy GR, Harris SE. The mouse bone morphogenetic protein-4 gene: Analysis of promoter utilization in fetal rat calvarial osteoblasts and regulation by COUP-TFI orphan receptor. J Biol Chem. 1995;270:28364–28373. doi: 10.1074/jbc.270.47.28364. [DOI] [PubMed] [Google Scholar]
- Graham A, Francis-West P, Brickell P, Lumsden A. The signaling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372:684–686. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes & Dev. 1996a;10:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija G, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: Requirement of a Bmp-4 like ventralizing activity and its dorsal repressor. Genes & Dev. 1996b;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet. 1997;13:14–21. doi: 10.1016/s0168-9525(96)10051-2. [DOI] [PubMed] [Google Scholar]
- Harland RM, Gerhart JC. Formation and function of Spemann’s organizer. Annu Rev Cell Devel Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Laufer E, Riddle RD, Tabin CJ. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1166–1174. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Hogan BLM. Involvement of bone morphogenetic protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;121:1433–1442. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The atlas of mouse development. London, UK: Academic Press; 1992. [Google Scholar]
- Kessler DS, Melton DA. Vertebrate embryonic induction—mesodermal and neural patterning. Science. 1994;266:596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancapoulos GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeister T, Kim S-H, Piccolo S, De Robertis EM. Frz-b is a secreted antagonist of wnt signaling in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Jessell TM. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Mansour SLR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryonic-derived stem cells: A general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Marcelle C, Stark MR, Bronner-Fraser M. Coordinate action of BMPs, Wnts, Shh, and Noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–3963. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1 −/Wnt-1− mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A-H, Duprez D, Watanabe Y, Bontoux M, Vincent C, Brickell P, LeDouarin N. The role of bone morphogenetic protein in vertebrate development. Development. 1996;122:3607–3616. doi: 10.1242/dev.122.11.3607. [DOI] [PubMed] [Google Scholar]
- Münsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes & Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- Placzek, M., M. Tessier-Lavigne, T.M. Jessell, and J. Dodd. 1991. Control of dorso-ventral pattern in vertebrate neural development: Induction and polarizing properties of the floor plate. Development (Suppl. 2) 113: 105–122. [PubMed]
- Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993;117:205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl-1 in motor neuron generation reveals a motor-neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, DeRobertis EM. Dorso-ventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Breant C, LeDouarin NM. Control of somite patterning by signals from the lateral plate. Proc Natl Acad Sci. 1995;92:3219–3223. doi: 10.1073/pnas.92.8.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O, Fan D-M, Coltey M, Hirsinger E, Watanabe Y, Breant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin N. Lateral and axial signals involved in avian somite patterning: A role for BMP-4. Cell. 1996;84:461–471. doi: 10.1016/s0092-8674(00)81291-x. [DOI] [PubMed] [Google Scholar]
- Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenesis. Genes & Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong PM, Teillet M-A, Ziller C, LeDouarin NM. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992;115:657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Jessell TM, Roelink H. Restriction to floor plate induction by hedgehog and winged helix genes in the neural tube of frog embryos. Mol Cell Neurosci. 1995;6:106–121. doi: 10.1006/mcne.1995.1011. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sasaki H, Hogan BLM. HNF-3β as a regulator of floor plate development. Cell. 1994;76:103–115. doi: 10.1016/0092-8674(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, DeRobertis EM. Xenopus chordin: A novel dorsalizing factor activated by organizer specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanics of neuralation: Traditional viewpoint and recent advances. Development. 1990;109:243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JBE, Lassar AB. A role for bone morphogenetic protein in the induction of cardiac myogenesis. Genes & Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JLR. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the C-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Swaitek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes & Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Teillet M-A, Le Douarin NM. Consequences of neural tube and notochord excision on the development of the peripheral nervous system in the chick embryo. Dev Biol. 1983;98:192–211. doi: 10.1016/0012-1606(83)90349-4. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Economides AN, Rojas E, Lamb TM, Nuñez L, Jones P, Ip NY, Espinosa R, Brannan CI, Gilbert DJ, Copeland NG, Jenkins NA, LeBeau MM, Harland RM, Yancopoulos GD. Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci. 1995;15:6077–6084. doi: 10.1523/JNEUROSCI.15-09-06077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Straaten HWM, Hekking JWM. Development of the floor plate, neurons, and axonal outgrowth pattern in the early spinal cord of the notochord deficient chick embryo. Anat Embryol. 1991;184:55–63. doi: 10.1007/BF01744261. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Weil M, Jacobson MD, Raff MC. Is programmed cell death required for neural tube closure? Curr Biol. 1997;7:281–284. doi: 10.1016/s0960-9822(06)00125-4. [DOI] [PubMed] [Google Scholar]
- Whiting J, Marshall H, Cook M, Krumlauf R, Rigby PWJ, Stott D, Allemann RK. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox 2.6 gene expression. Genes & Dev. 1991;4:180–189. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In situ hybridization: A practical approach. Oxford, UK: IRL Press; 1992. pp. 75–83. [Google Scholar]
- Wilkinson DG, Baile JA, McMahon AP. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987;50:79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BLM. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes & Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: Polarizing activity of the floor plate and notochord. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: Motor induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- Yamashita H, tenDijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin C-H, Miyazono K. Osteogenic Protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]