Abstract
Advanced glycation end products (AGEs) play a pivotal role in loss of lens transparency, i.e., cataract. AGEs formation occurs as a result of sequential glycation and oxidation reaction between reducing sugars and protein. AGEs production takes place throughout the normal aging process but its accumulation is found to be more rapid in diabetic patients. In this study, we quantified AGEs and N-(carboxyethyl) lysine (CEL) in human cataractous lenses from non-diabetic (n = 50) and diabetic patients (n = 50) using ELISA. We observed significantly higher (p < 0.001) levels of lens AGEs and CEL in diabetic patients with cataract as compared with their respective controls. The presence of AGEs and CEL was also determined by western blotting and immuno-histochemical analysis. Furthermore, isolated β-crystallin from cataractous lenses of non-diabetic and diabetic patients was incubated with different sugars to evaluate the extent of glycation in a time dependent manner. Our data indicated more pronounced glycation in patients suffering from diabetes as compared to non-diabetics subjects demonstrating the need to focus on developing normoglycemic approaches. Such studies may provide an insight in developing therapeutic strategies and may have clinical implications.
Keywords: AGEs, Cataract, Diabetes, CEL, Non-enzymatic glycation
Introduction
Diabetes mellitus is one of the major health concern all over the world (Pollreisz and Schmidt-Erfurth 2010) characterized by hyperglycemia. Long-term hyperglycemia is involved in the pathogenesis of diabetic micro-vascular and macro-vascular complications affecting the kidneys, nerves, blood vessels, and eyes (Nathan 1993). Prevalence data of WHO has suggested that 366 million people will have diabetes by the year 2030 throughout the world, among which around 120 million patients will be affected in South-East Asian region (http://www.who.int/diabetes/facts/world_figures).
Diabetes is a major risk factor for producing lens opacity called cataract and ultimately loss of vision (Harding 1981; Brian and Taylor 2001). The process of cataractogenesis in diabetic patients is more accelerated as compared with senile subjects with at least 20 years earlier onset. Diabetes is characterized by excessive sugar levels and there is substantial evidence suggesting involvement of these sugars in formation of AGEs through non-enzymatic glycation. The process of glycation is instigated by the formation of Schiff’s base adduct between the carbonyl group of reducing sugars and the free amino group of proteins (Maillard reaction; Stitt 2005). The initial Schiff’s base is then followed by structural Amadori rearrangement or isomerisation producing an amino-ketose derivative (Groenen et al. 1994). These derivatives then undergo series of degradation reactions giving rise to highly reactive dicarbonyl compounds such as glucosone and 3-deoxyglucosone. Dicarbonyls act as protein cross-linking agents and react with free amino group of proteins, producing additional Schiff’s bases. The eventual effect of glycation therefore is the generation of protein aggregates. It has also been observed that production of these AGEs is associated with the generation of chemiluminescence, suggesting the role of reactive oxygen species (Namiki et al. 1993).
AGEs have been observed in normal aging process while excessive levels have been reported in diabetes mellitus (Peppa et al. 2003). Non-enzymatic glycation of lens proteins by reducing sugars (glucose, galactose, etc.) may cause conformational changes resulting in the formation of protein aggregates that precipitate in the lens (Monnier et al. 1979) leading towards lens opacity. AGEs formation is also suggested to be accelerated by oxidative stress as reactive oxygen species enhance cross-linking between carbohydrate moiety and amino group of protein (Peppa et al. 2004). This phenomenon has generated a new term glucoxidation which indicates that the two processes are integrated. Recently, we reported an inverse relationship between levels of antioxidants and glycation (Hashim et al. 2009). There are several AGEs which have been identified structurally (Ahmed et al. 1997). In human lens, numerous AGEs have been discovered including N-(carboxymethyl)-L-lysine (CML), pentosidine, fluorophore LM-1, pyrraline, N-carboxyethyl-lysine (CEL) to name a few (Nagaraj and Monnier 1992; Nagaraj and Sady 1996; Ahmed et al. 1997; Nagaraj et al. 1991; Dunn et al. 1989).
CEL is one of the advanced glycation end product which is formed during the reaction between methylglyoxal and lysine residues. It is a homologue of CML which is formed on reaction of glyoxal (Ahmed et al. 1997). There are several studies which have reported the levels of AGEs in cataract lenses from senile and diabetic patients; but to best of our knowledge, none has so far reported the levels of CEL in senile cataract subjects with diabetes and without diabetes. The objective of the current study was to investigate the extent of glycation and its role in progression of cataract formation by examining AGEs and CEL using enzyme-linked immunosorbent assay (ELISA), immunohistochemical analysis and western blotting. Furthermore, we also conducted studies on β-crystallin to evaluate the ability of being glycated after exposure to sugars in time dependent manner.
Research design and methods
Subjects and sample collection
Study population consisted of cataract patients suffering from type 2 diabetes (n = 50) and without diabetes (n = 50). Opaque lenses were collected by extra capsular cataract extraction. Before surgery, all patients underwent complete eye examination in an ophthalmic clinic. The lens opacity was assessed using slit-lamp examination. All experiments were performed as per the guidelines and approval of the institutional ethics committee, and written informed consent was obtained from all participants. All cataract patients with and without diabetes included in the study were the subjects over 60 years of age [non-diabetic subjects: 28 males and 22 females; age (mean ± SD) 61.98 ± 8.71 and 63.26 ± 9.15 years, respectively; diabetic subjects: 26 males and 24 females; age (mean ± SD) 59.11 ± 9.88 and 60.16 ± 10.05 years, respectively]. Diabetic subjects included in the study were using oral hypoglycemic drugs since last 5 years. Subjects who had normal blood glucose level and no history of diabetes were considered as non-diabetic senile cataractous patients. The level of HbA1c of cataract group with diabetes was higher (10.8 ± 0.355%) than non-diabetic cataract group (5.7 ± 0.361%; p < 0.001). Subjects who were smokers or were suffering from any systemic disorder, cardiovascular disease, hepatic disease, renal dysfunction, or anemia or with toxic or traumatic cataract were excluded from the study.
Non-competitive ELISA
Lens sample preparation
Lens samples were homogenized as described earlier (Zarina et al. 2000). Age-matched cataract lenses from diabetic and non-diabetic subjects were weighed and homogenized individually in phosphate-buffered saline (pH 7.4) at 4°C. Homogenate was centrifuged at 10,000×g for 20 min at 4°C. The clear supernatant was used for ELISA and western blotting of AGEs and CEL.
ELISA standards
To measure the level of glycation in lens samples, standard microassay method was adapted as described by Ahmed and Furth (1991). Briefly, all the aliquots of standards (2–40 nmol fructose) were incubated with sodium periodate (NaIO4) and 0.1 M HCl at room temperature. After incubation, standards were cooled on ice for 10 min and pre-chilled 15% ZnSO4 and 0.7 M NaOH were added followed by centrifugation at 9,000×g for 10 min. Aliquots of 100 μl supernatant was added to 200 μl of HCHO detection reagent and read against blank at 405 nm using Heλios Thermo Spectronic UV-Visible spectrophotometer.
AGEs and CEL measurement by ELISA
The amount of AGEs and CEL in crude and incubated β-crystallin was determined by non-competitive ELISA using rabbit polyclonal antibody and mouse monoclonal antibody respectively (Abcam, UK) as described earlier (Zarina et al. 2000). Microtiter plates were read at 650 nm using TECAN micro plate reader. Results are expressed as nM/mg protein.
Immunohistochemical assay
Fresh human lens tissues were immediately fixed in phosphate-buffered formalin (4%) to perform fluorescence immunohistochemical staining as reported earlier (Reed et al. 2001). Two-micrometer thick cryosections were immunostained using specific antibodies for human AGE and CEL (Abcam, UK) and detected with Alexa Flour® goat anti-rabbit and anti-mouse IgG secondary antibodies, respectively. In order to prepare the negative controls, the whole process was same except that the primary antibody was excluded from the incubation mixture.
Western blotting
The clear supernatant was dissolved in sample diluting buffer and resolved on 12% sodium dodecyl sulfate (SDS)–polyacrylamide gel. Protein bands were transferred to PVDF membrane and then blocked using 3% BSA solution for 1 h. The membrane was incubated individually with respective primary antibodies for AGEs and CEL (1:500) overnight. After washing, the blot was incubated with secondary antibodies HRP—conjugated goat anti-rabbit for AGEs and anti-mouse IgG for CEL (1:2,500) that were premixed with Avidin biotinylated antibody (1:3,000). The bands were visualized using ECL Western blotting detection reagents (Millipore, Bedford, MA, USA).
Incubation studies
Human lens crystallins were isolated using Sephacryl S-300 HR. Protein (100 mg) was loaded on the column, fractions were collected and read at 280 nm in a UV-visible spectrophotometer. Fractions of α, β, and γ crystallins were separately pooled. Separated peaks were analyzed using 12% SDS-PAGE (Laemmli 1970). Bands were visualized by staining with Coomassie Brilliant Blue R-250.
Incubation of β-crystallin
Fractions of β-crystallin (15 mg) from diabetic and non-diabetic subjects were incubated with 0.1 M glucose, fructose, and galactose, respectively. One milliliter aliquots were collected at different time intervals of 0, 5, 10, and 15 days during incubation. Fractions were dialyzed against deionized water and used for further analyses.
Spectroscopic studies
Absorption spectrum of diabetic and non-diabetic β-crystallin, incubated with sugars (glucose, fructose, and galactose) was recorded from 200 to 700 nm on a Heλios Thermo spectronic UV-Visible spectrophotometer using 1 cm path length quartz cuvett.
Statistical analysis
Data was analyzed using SPSS software (SPSS® for Windows® 10.0) and presented as means ± SD. Statistical significance of the difference between non-diabetic cataractous group and diabetic cataractous group was evaluated by student’s t test. p < 0.001 was accepted as statistically significant.
Results
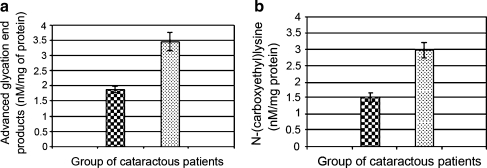
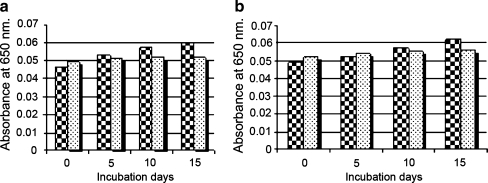
Figure 1 (a, b) represents levels of AGEs and CEL in non-diabetic and diabetic cataractous lenses as determined by ELISA. AGEs and CEL both were found to be significantly (p < 0.001) increased in diabetic samples (3.462 ± 0.22; 2.98 ± 0.27 nM/mg protein, respectively) as compared to non-diabetic samples (1.878 ± 0.13; 1.5 ± 0.14 nM/mg protein, respectively).
Fig. 1.
(a, b) Levels of AGEs and CEL in cataractous lenses from non-diabetic and diabetic patients as determined by ELISA. Each bar represent Mean ± S.D (n = 50) for non-diabetic cataractous subjects  and (n = 50) for diabetic cataractous patients
and (n = 50) for diabetic cataractous patients 
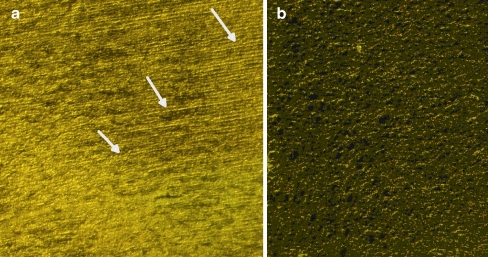
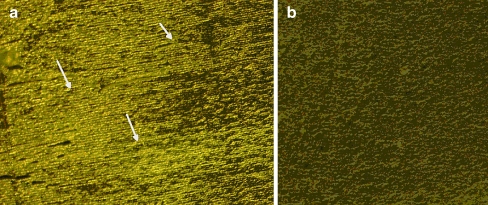
Immunohistochemical analyses were performed using human AGEs and CEL antibodies as shown in Figs. 2 and 3, respectively. The nuclear region of the lens comprises of columnar cells which form lens fibers. Positive immunostaining was observed in the nucleus where antibodies reacted strongly.
Fig. 2.
Immunohistochemical analysis of human lens nuclear region (a) stained for AGE and (b) respective negative control. The arrows indicate positively stained area
Fig. 3.
Immunohistochemical analysis of human lens nuclear region (a) stained for CEL and (b) respective negative control. The arrows indicate positively stained area
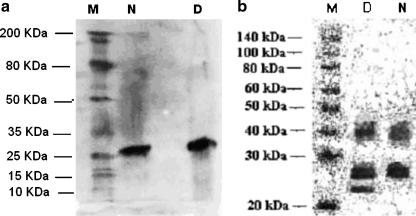
Figure 4 (a, b) illustrates the western blot analysis of human cataractous lenses from diabetic and non-diabetic individuals for AGEs and CEL detection, respectively.
Fig. 4.
(a, b) Western blot analysis for the presence of AGE and CEL in human lens water soluble fraction from non-diabetic (N) and diabetic (D) cataractous patients
Incubation studies
Quantitation of AGEs by non-competitive ELISA
Isolated β crystallins from non-diabetic and diabetic cataractous lenses were incubated with carbohydrates (glucose, fructose and galactose 100 mM each) for 0, 5, 10, and 15 days. Aliquots from each sample were taken to analyze for their AGEs content. Results of AGEs determination for fructose are shown in Fig. 5. In case of all sugars, non-diabetic cataractous samples showed more potency to become glycated as compared to diabetic cataractous samples.
Fig. 5.
(a, b) Quantitation of AGEs in β-crystallin fraction incubated with 100 mM glucose and fructose from non-diabetic  and diabetic
and diabetic  cataracous subjects
cataracous subjects
Spectroscopic analysis
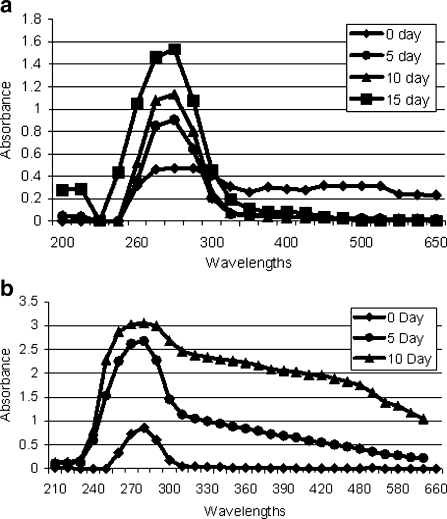
After incubation, samples (from 0 to 15 incubation days) were scanned from 200 to 700 nm. We observed time-dependent increase in structural variation in β-crystallin incubate samples with glucose, fructose, and galactose. However, the variation was more prominent in case of β-crystallin isolated from diabetic samples as shown in Fig. 6 (a, b).
Fig. 6.
(a, b) Spectroscopic analysis of incubated β-crystallin fraction with glucose and fructose from diabetic cataractous lens samples
Discussion
Diabetes mellitus and its complications such as cataract are major health problem of developing countries (Ivancic et al. 2005; Kokiwar et al. 2007). In Pakistan, 6.9 million people are affected by diabetes (Rathur and Boulton, 2007) and approximately 570, 000 adults are blind as a result of cataract (Jadoon et al. 2007). The earlier onset of cataract in diabetic individuals is suggested to be a consequence of non-enzymatic glycation. Glycation of long-lived proteins such as lens crystallins in aging and diabetes has been identified as the most important post-translational modification leading to cross-linking, aggregation, and insolubilization (Monnier and Cerami, 1981; Kumar et al. 2007). Direct correlation between AGEs formation and glycemic level has been reported. The AGEs hypothesis proposes that chemical modification of proteins by glucose during hyperglycemia plays a pivotal role in pathogenesis of diabetic complications (Yamagishi et al. 2007).
We have investigated levels of AGEs and CEL, a derivative of CML in lenses from diabetic and senile lens samples. It is reported in previous studies that level of AGEs is higher in diabetic cataractous lenses than in non-diabetic cataractous lenses (Zarina et al. 2000; Pokupec et al. 2003). As expected, levels of CEL were found to be greater in diabetic lens samples than in non-diabetic samples. Similar results have been observed for Pentosidine (Hashimoto et al. 1997), CML, and immidazolone (Franke et al. 2003).
Lens crystallins can be divided in to α, β and γ-crystallins. α and β-crystallins are found in lens cortex and both crystallins being rich in lysine are more likely to undergo the process of glycation (Swamy and Abraham 1991). γ-crystallins, on the other hand, is another extremely vulnerable target for glycation and localized in nucleus. Earlier studies indicated that human α-crystallin undergoes post translational modifications with aging and cataract. Glycation of α-crystallin may cause protein unfolding, while in hyperglycemic condition the same process has shown to be increased many folds (Kumar et al. 2007). α and γ-crystallins have been studied extensively with reference to alteration due to glycation. In the current study, we investigated the probability of isolated β-crystallin to be glycated by glucose, fructose, and galactose upon incubation at different time intervals. Initially, increased concentration of AGEs was observed in diabetic samples as compared to non-diabetic subjects and the levels of AGEs increased in time-dependant manner. AGEs formation is a consequence of normal aging process but in case of diabetes, level of glycation is higher (Harding, 2002). Our incubated sample showed the same pattern. Before incubation, the level of glycation was greater in diabetic sample as expected. Incubation with glucose, galactose (data not shown), and fructose resulted in a gradual increase in level of glycation in both non-diabetic and diabetic samples. After 10 days incubation, the level of AGEs became constant in diabetic samples presumably due to the fact that in diabetic samples, all possible glycation sites were already saturated. In aging cells, the level of AGEs and CEL increases gradually but the process of glycation is very slow. The rate of glycation in cataract patients with diabetes is accelerated due to higher sugar levels in hyperglycemic condition (Gul et al. 2009; Duhaiman, 1995). Hence, β-crystallin from diabetic samples being already glycated has less capacity to bind with incubated sugars. It was observed that, among all sugars (glucose, fructose, and galactose) fructose showed a higher affinity to bind with β-crystallin demonstrating that the rate of fructation is faster as compared to glucation and galactation. It has been suggested that fructose, due to its acyclic structure is a more potent glycating agent (Vinson, 2006). Structural variation of the β-crystallins was also analyzed before and after alteration with glucose, galactose, and fructose by measuring absorption spectra. We observed time-dependent structural variation as a consequence of glycation induced by incubated sugars as compared to control samples. The variation was more obvious from 270 to 290 nm. Nagaraj and Sady (1996) reported that incubation of α-crystallin with 3-deoxyglucose causes an alteration at 289 nm which is particular for pyrraline. Our results showed that introduction of high sugar level to lens can alter the structure of lens proteins as well as its function. This model may not reflect true in-vivo picture, however, it may still serve to be an indicator of changes that may occur in cell.
From the above facts and results, it can be proposed that non-enzymatic glycation of β-crystallin by various sugars promotes the alteration in protein structure. Our results also support the hypothesis that AGEs may play essential role in degenerative changes in lens, which occur much earlier in diabetic patients than in non-diabetics.
AGEs formation and cataract progression both are extremely slow processes and both are triggered in presence of reactive oxygen species. Oxidative insult along with AGEs may integrate resulting in acceleration of cataract formation. Approaches towards keeping normal antioxidant levels, utilizing low AGEs-content food and keeping normoglycemia may be beneficial to delay cataract formation.
Acknowledgments
This study was supported by a research grant from the Higher Education Commission, Islamabad, Pakistan. We thank the H.E.C for providing research fellowships to Zehra Hashim.
References
- Ahmed N, Furth AJ. A microassay for protein glycation based on the periodate method. Anal Biochem. 1991;192:109–111. doi: 10.1016/0003-2697(91)90193-W. [DOI] [PubMed] [Google Scholar]
- Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-(carboxyethyl) lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324:565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian G, Taylor H. Cataract blindness: challenges for the 21st century. Bull. WHO. 2001;79:249–256. [PMC free article] [PubMed] [Google Scholar]
- Duhaiman AS. Glycation of human lens proteins from diabetic and (nondiabetic) senile cataract patients. Glycoconj J. 1995;12:618–621. doi: 10.1007/BF00731255. [DOI] [PubMed] [Google Scholar]
- Dunn JA, Patrick JS, Thorpe SR, Baynes JW. Oxidation of glycated proteins: age-dependent accumulation of N-(carboxymethyl) lysine in lens proteins. Biochemistry. 1989;28:9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- Franke S, Dawczynski J, Strobel J, Niwa T, Stahl P, Stein G. Increased levels of advanced glycation end products in human cataractous lenses. J Cataract Refract Surg. 2003;29:998–1004. doi: 10.1016/S0886-3350(02)01841-2. [DOI] [PubMed] [Google Scholar]
- Groenen PJ, Merck KB, Jong WW, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- Gul A, Rahman MA, Salim A, Simjee SU. Advanced glycation end products in senile diabetic and nondiabetic patients with cataract. J Diab Comp. 2009;23:343–348. doi: 10.1016/j.jdiacomp.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Harding JJ. Changes in lens proteins in cataract. In: Bloemendal H, editor. Molecular and cellular biology of the eye lens. New York: Wiley; 1981. pp. 327–365. [Google Scholar]
- Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1:465–479. doi: 10.1016/S1568-1637(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Hashim Z, Ilyas A, Saleem A, Salim A, Zarina S. Expression and activity of paraoxonase 1 in human cataractous lens tissue. Free Rad Biol Med. 2009;46:1089–1095. doi: 10.1016/j.freeradbiomed.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Arai K, Yoshida S, Chikuda M, Obara Y. Pentosidine and autofluorescence in lenses of diabetic patients. Jpn J Ophthalmol. 1997;41:274–277. doi: 10.1016/S0021-5155(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Ivancic D, Mandic Z, Barac J, Kopic M. Cataract surgery and post operative complication in diabetic patients. Coll Antropol. 2005;29:55–58. [PubMed] [Google Scholar]
- Jadoon Z, Shah SP, Bourne R, Dineen B, Khan MA, Gilbert CE, Foster A, Khan MD. Cataract prevalence, cataract surgical coverage and barriers to uptake of cataract surgical services in Pakistan: the Pakistan national blindness and visual impairment survey. Br J Ophthalmol. 2007;91:1269–1273. doi: 10.1136/bjo.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokiwar PR, Gupta S, Durge PM. Prevalence of diabetes in a rural area of central India. Int J Diab Dev Ctries. 2007;27:8–10. doi: 10.4103/0973-3930.34750. [DOI] [Google Scholar]
- Kumar PA, Kumar MS, Reddy GB. Effect of glycation on rat alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408:251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211:491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Stevens VJ, Cerami A. Nonenzymatic glycosylation, sulfhydryl oxidation and aggregation of lens proteins in experimental sugar cataracts. J Exp Med. 1979;150:1098–1107. doi: 10.1084/jem.150.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Monnier VM. Isolation and characterization of a blue fluorophore from human eye lens crystallins: in vitro formation from Maillard reaction with ascorbate and ribose. Biochim Biophys Acta. 1992;1116:34–42. doi: 10.1016/0304-4165(92)90125-e. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sady C. The presence of a glucose-derived Maillard reaction product in the human lens. FEBS Lett. 1996;382:234–238. doi: 10.1016/0014-5793(96)00142-1. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci USA. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M, Oka M, Otsuka M, Miyazawa T, Fujimoto K, Namiki K. Weak Chemiluminescence at an early stage of Maillard reaction. J Agric Food Chem. 1993;41:1704–1709. doi: 10.1021/jf00034a035. [DOI] [Google Scholar]
- Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- Peppa M, Uribarri J, Vlassara H. Glucose, advanced glycation end products, and diabetes complications: What is new and what works. Clin Diab. 2003;21:186–187. doi: 10.2337/diaclin.21.4.186. [DOI] [Google Scholar]
- Peppa M, Uribarri J, Vlassara H. The role of advanced glycation end products in the development of atherosclerosis. Curr Diabetes Rep. 2004;4:31–36. doi: 10.1007/s11892-004-0008-6. [DOI] [PubMed] [Google Scholar]
- Pokupec R, Kalauz M, Turk N, Turk Z. Advanced glycation endproducts in human diabetic and non-diabetic cataractous lenses. Graefe Arch Clin Exp Ophthalmol. 2003;241:378–384. doi: 10.1007/s00417-002-0616-2. [DOI] [PubMed] [Google Scholar]
- Pollreisz A, Schmidt-Erfurth U. Diabetic cataract pathogenesis, epidemiology and treatment. J Ophthalmol. 2010 doi: 10.1155/2010/608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathur HM, Boulton AJ. The diabetic foot. Clin Dermatol. 2007;25:109–120. doi: 10.1016/j.clindermatol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Reed NA, Oh D-J, Czymmek KJ, Duncan MK. An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods. 2001;253:243–252. doi: 10.1016/S0022-1759(01)00374-X. [DOI] [PubMed] [Google Scholar]
- Stitt AW. The Maillard reaction in eye diseases. Ann NY Acad Sci. 2005;1043:582–597. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- Swamy MS, Abraham EC. Differential glycation of rat alpha, beta and gamma crystallins. Exp Eye Res. 1991;52:439–444. doi: 10.1016/0014-4835(91)90040-L. [DOI] [PubMed] [Google Scholar]
- Vinson JA. Oxidative stress in cataracts. Pathophysiology. 2006;13:151–162. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Matsui T, Ueda S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and cardiovascular disease (CVD) in diabetes. Cardiovasc Hematol Agents Med Chem. 2007;5:236–240. doi: 10.2174/187152507781058681. [DOI] [PubMed] [Google Scholar]
- Zarina S, Zhao HR, Abraham EC. Advanced glycation end products in human senile and diabetic cataractous lenses. Mol Cell Biochem. 2000;210:29–34. doi: 10.1023/A:1007015416572. [DOI] [PubMed] [Google Scholar]