Abstract
Introduction
Biologic markers that predict development of invasive breast cancer (IBC) in patients diagnosed with ductal carcinoma in-situ (DCIS) are needed to improve personalized therapy. In this study, we examined the incidence of early IBC in DCIS subgroups defined by immunophenotype.
Methods
Clinical and histologic materials of 143 patients with radiographically suggesting DCIS without obvious evidence of IBC were reviewed. All patients underwent initial biopsy followed by short-term subsequent resection. The presence of IBC, histopathologic features of DCIS and IBC, when present, and their estrogen receptor (ER), progesterone receptor (PR) and HER2 phenotypes were evaluated.
Results
Early IBC was identified on initial biopsy in 6 (4%) and subsequent resection in 24 (17%) patients. HER2 positivity in DCIS was the dominant factor associated with IBC. There was also a significant association between ER/PR/HER2+ DCIS and the presence of IBC. The ER/PR/HER2+ DCIS appeared to be the most unstable precursor, because of the highest invasion rate and frequent association with a discordant phenotype.
Conclusions
HER2 positivity and ER/PR/HER2 phenotype may be used to identify DCIS patients at higher risk of harboring or potentially developing IBC. Strategies targeting HER2 in DCIS may be of potential benefit in preventing IBC in patients with DCIS.
Keywords: breast cancer, ductal carcinoma in-situ (DCIS), HER2, invasive disease, immunohistochemistry
Introduction
The incidence of ductal carcinoma in-situ (DCIS), a precursor of invasive cancer of the breast is increasing, owing largely to the more advanced age of our population and improvements in diagnostic mammography [1, 2]. Both invasive and in-situ mammary carcinomas are highly heterogeneous lesions with variable morphology, clinical presentation and behavior. Even for the low grade DCIS, if left untreated, approximately 40% of patients will develop invasive carcinoma [3]. Biologic markers that predict the presence of occult invasive disease or identify patients at higher risk of developing invasive cancer are needed to more effectively differentiate those patients who will progress to invasive cancer from those with more dormant disease [2].
Recently, gene expression profiling has allowed the classification of invasive breast cancer into subgroups with distinct clinical behaviors. These subgroups include two types of estrogen receptor (ER) positive tumors (luminal A and luminal B subtype) and two types of ER negative tumors (basal-like and HER2 positive subtype) [4, 5]. These different subtypes have been associated with different clinical outcomes [6, 7]. The basal-like and HER2+ subtypes are associated with a poorer prognosis, whereas the luminal A subtype is associated with a better prognosis. Immunohistochemical evaluation of ER and HER2 expression has also been used to reliably categorize tumors as luminal, HER2+ or basal-like subtype [8].
Attempts have been made to categorize DCIS by immunohistochemistry [9, 10]. While subtypes similar to those seen in invasive cancer have been identified in DCIS, there is no consensus regarding the clinical implications of these phenotypic groups in pre-invasive disease.
We have recently reported that HER2 overexpression in DCIS is associated with invasive foci at a relatively higher frequency [11]. Our observation have been recently confirmed by a study based on Chinese population [12]. In this study, we have expanded the study population and also evaluated the other relevant clinico-pathologic features and the subtypes of DCIS as determined by immunohistochemistry. The objective of this study was to assess associations between these features and rate of early invasion, which may ultimately inform decisions on prognosis and management of DCIS.
Materials and Methods
Patients
One hundred ninety-six consecutive patients with mammogram and breast magnetic resonance imaging (MRI) suggesting DCIS without obvious evidence of invasive disease were identified from a group of patients treated by a single surgeon (BJC) being screened for DCIS vaccine study between 2003 and 2009 at the Hospital of the University of Pennsylvania. This study was retrospectively performed on de-identified patients’ information with approval of the internal review board (IRB) at our institution. As part of the retrospective study on archival material, our IRB approved that informed consent was not required from the subjects for this study.
All patients underwent biopsy (core biopsy or needle localization) and subsequent local resection (lumpectomy or simple mastectomy) within a period of 1 to 7 months (median 2 months). Clinical and histologic materials were reviewed in all patients and 53 cases were excluded after initial review because of the lack of appropriate imaging study or histologic material for review and immunohistochemical evaluation, leaving 143 patients for this analysis. In these 143 patients, there was no suspicion of invasive breast cancer by clinical and radiographic evaluation, which was performed before biopsy was done. Typical radiographic findings include heterogeneous or clustered microcalcifications on mammogram and the area of clumped regional enhancement on MRI. The patients with findings suggesting the presence of an infiltrating carcinoma by MRI (mass with speculated margins) were excluded. These were confirmed with histologic MRI guided biopsy. Of note, 25 of 53 patients with HER2 positive DCIS were treated with a HER2 targeted dendritic cell vaccine [13] in the interval between the initial biopsy and subsequent excision.
Histologic and immunohistochemical examination
DCIS was identified in all patients on initial biopsy (39 needle localization and core biopsy for the remaining cases). Histopathological features were assessed on slides of formalin-fixed paraffin-embedded tissues stained with hematoxylin and eosin. A histological grade of low, intermediate or high was assigned according to the nuclear grade for in-situ lesion at time of the diagnosis. Invasive lesion is graded according to Nottingham modification of Bloom-Richardson system. ER, PR and HER2 status was determined with commercially available, FDA approved ER/PR PharmDx tests and HercepTest (DAKO) performed in the Laboratory of the Diagnostic Immunohistochemistry at the Hospital of the University of Pennsylvania at the time of the initial diagnosis following the FDA approved manufacture procedure guidelines. Only nuclear reactivity for ER and PR and membrane reactivity for HER2 were considered significant respectively. The immunoreactivity was interpreted by single pathologist (PJZ) according to the guidelines provided by the manufacturer. Cases were considered positive for ER or PR when the Allred score [14] was 3 or higher. HER2 was scored as positive when HER2 reactivity was 2+ or 3+ in more than 10% of DCIS tumor cells (see Discussion). Based on the result of ER, PR and HER2 test, the DCIS cases were classified in four subgroups; ER and/or PR positive and HER2 negative [ER/PR+/HER2−], ER and/or PR and HER2 positive [ER/PR/HER2+], ER and PR negative and HER2 positive [ER/PR−/HER2+] and ER, PR and HER2 negative [ER/PR/HER2−, triple negative]. The presence of invasive disease, various histopathologic features of DCIS and invasive carcinoma, when present, as well as their ER, PR and HER2 phenotypes were evaluated. HER2 evaluation in invasive lesions was followed by standard guideline [15] and fluorescent in-situ hybridization (FISH) test was performed for the cases with 2+ immunoreactivity.
Statistical analysis
Descriptive statistics were generated to examine the distribution of each variable, including median and range for continuous variables and frequency and percentage for categorical variables. Associations between presence of invasive disease and categorical patient and histopathologic characteristics were tested by Fisher’s exact test for nominal variables and a linear trend test for ordinal variables. The nonparametric Wilcoxon rank sum test was used to compare continuous variables between two groups and the Kruskal-Wallis test was used to compare continuous variables among more than two groups. McNemar’s test was employed to test agreement in paired binary response variables. To conduct this test, a 2 × 2 contingency table is constructed. Cells on the diagonal have the same characteristic (i.e., there is agreement) and do not contribute to the test. The test determines whether there is symmetry of counts in cells above the diagonal versus counts in cells below the diagonal. If the two counts differ significantly, this reflects change or disagreement between the paired variables. The McNemar-Bowker test was employed to test agreement in paired multinomial response variables. This test is a generalization of McNemar’s test to variables with more than two responses. Univariate logistic regression models were employed to estimate odds ratios and 95% confidence intervals. The Wald test was used to determine significance of each variable in the model. Multivariable models were employed to estimate odds ratios and assess significance of several variables simultaneously. Both backward elimination and forward selection techniques were used to build models. P < 0.05 was considered statistically significant. All statistical tests were 2-sided. Analyses were conducted using SPSS v17.0 (SPSS Inc, Chicago, IL) or StatXact v8.0 (Cytel, Cambridge, MA).
Results
The study population consisted entirely of women, with a median age of 52 (range 33–87); approximately one-third of patients were 40–49 years (Table 1). Grade of DCIS was low in 12 (8.5%), intermediate in 50 (35.2%) and high in 80 (56.3%) patients. ER was positive in 106 (74.1%) patients and HER2 was positive in 53 (37.1%) patients. Increasing grade was significantly associated with higher rate of HER2 positivity (25% in low, 18% in intermediate and 50% in high grade patients, p=0.001). Patients were divided into four subgroups based on ER, PR and HER2 phenotypes of DCIS. ER/PR+/HER2−(luminal A), ER/PR/HER2+ (luminal B), ER/PR−/HER2+ (HER2), and ER/PR/HER2− (triple negative) phenotypes accounted for 83 (58.0%), 25 (17.5%), 28 (19.6%), and 7 (4.9%) cases, respectively. There was no difference in patient age among the phenotype groups (p=0.39). Median age was: 51 years in ER/PR+/HER2−, 51 years in ER/PR/HER2+, 53 years in ER/PR−/HER2+ and 59 years in triple negative. Histologically, all cases of low grade DCIS had either ER/PR+/HER2− (luminal A) or ER/PR/HER2+ (luminal B) phenotype. High grade DCIS comprised only 41.0% of ER/PR+/HER2− (luminal A) cases but 64.0% of ER/PR/HER2+ (luminal B) cases, 89.9% of ER/PR−/HER2+ cases (HER2), and 85.7% of triple negative cases. Within the ER/PR+/HER2− (luminal A) group, the majority of the cases were ER positive with only two cases being ER negative and PR positive. These two cases were high grade DCIS and no invasive disease was identified. The size of DCIS was not analyzed in this study as precise size measurement is controversial and difficult to assess in DCIS [16].
Table 1.
Patient and DCIS Histopathologic Characteristics
| # | % | |
|---|---|---|
| All patients | 143 | 100.0 |
|
| ||
| Age at diagnosis, | ||
| median | 52 | |
| range | 33 – 87 | |
|
| ||
| Age at diagnosis, decades | ||
| 30 – 39 | 10 | 7.0% |
| 40 – 49 | 49 | 34.3% |
| 50 – 59 | 42 | 29.4% |
| 60 – 69 | 28 | 19.6% |
| 70 – 79 | 9 | 6.3% |
| 80 – 89 | 5 | 3.5% |
|
| ||
| Grade* | ||
| Low | 12 | 8.5% |
| Intermediate | 50 | 35.2% |
| High | 80 | 56.3% |
|
| ||
| ER Status | ||
| ER negative | 37 | 25.9% |
| ER positive | 106 | 74.1% |
|
| ||
| PR Status# | ||
| PRnegative | 47 | 33.3% |
| PRpositive | 94 | 66.7% |
|
| ||
| HER2 Status | ||
| HER2 negative | 90 | 62.9% |
| HER2 positive | 53 | 37.1% |
|
| ||
| Phenotype | ||
| ER/PR+/HER2− | 83 | 58.0% |
| ER/PR/HER2+ | 25 | 17.5% |
| ER/PR−/HER2+ | 28 | 19.6% |
| ER/PR/HER2− | 7 | 4.9% |
one case missing Grade
two cases missing PR status
In addition to DCIS, invasive disease was identified on histologic examination in 30 (21%) of the patients. These cases are summarized in Table 2 (an example shown in Figure 1). In six cases, invasive foci were found at the time of initial biopsy (two needle localization and four core biopsies), all of which were small (maximum 2 mm). No residual or additional invasive disease was identified in the subsequent resection specimen in all six cases that were found to have invasive disease in the initial biopsy. In addition to these six cases with clear invasive lesions in initial biopsy, the initial biopsy specimens of nine more cases showed focal histologic changes deemed to be suspicious but not definitive for invasive lesion at the time of diagnosis without additional immunohistochemical work-up. However, definitive invasive disease was detected in only two of these 9 cases in the subsequent resection. Invasive disease was found in the subsequent resection in 24 cases (3 with needle localization originally and 21 with core biopsies). Twenty-seven of the invasive lesions were of the ductal type, two were of the lobular type and two were of other type (micropapillary and mucinous type). The grade of the foci of invasive non-lobular carcinoma were well-differentiated in 5, moderately differentiated in 6, poorly differentiated in 7 cases and too small to be graded in 10 ( including six cases in which invasive lesion is only found in initial biopsy). The two cases of infiltrating lobular carcinoma were not graded.
Table 2.
Characteristics of invasive carcinoma found in core biopsy or subsequent resection
| Phenotype (DCIS) | Age | Grade (DCIS) | Diagnosis of invasive lesion | Size (cm) (Invasive) | Phenotype (Invasive) | Differentiation (Invasive) |
|---|---|---|---|---|---|---|
| Invasive disease found in initial core biopsy
| ||||||
| ER/PR/HER2+ | 55 | High | Microinvasion | <0.1 | ER/PR−/HER2+* | N/A |
| ER/PR/HER2+ | 41 | High | Microinvasion | <0.1 | N/A | N/A |
| ER/PR−/HER2+ | 50 | High | IDC, single focus | 0.1 | N/A | N/A |
| ER/PR+/HER2− | 64 | Intermed | IDC | 0.2 | N/A | N/A |
|
| ||||||
| Invasive disease found in initial surgical excision (needle localization)
| ||||||
| ER/PR/HER2+ | 48 | High | Microinvasion | 0.1 | ER/PR−/HER2+* | N/A |
| ER/PR+/HER2− | 51 | High | IDC | 0.2 | N/A | N/A |
|
| ||||||
| Invasive disease found in subsequent resection
| ||||||
| ER/PR+/HER2− | 45 | Low | Microinvasion; mucinous | 0.05 | N/A | N/A |
| ER/PR+/HER2− | 78 | Intermed | Multiple microscopic IDC | Up to 0.1 | N/A | N/A |
| ER/PR+/HER2− | 63 | Intermed | Invasive lobular carcinoma | 0.1 | ER/PR+/HER2− | N/A |
| ER/PR+/HER2− | 36 | High | IDC | 0.1 | N/A | Moderately |
| ER/PR+/HER2− | 43 | High | IDC | 0.5 | ER/PR+/HER2− | Well |
| ER/PR+/HER2− | 50 | High | IDC in distinct area of breast | 0.6 | ER/PR+/HER2− | Moderately |
| ER/PR+/HER2− | 57 | Intermed | IDC | 0.6 | ER/PR+/HER2− | Moderately |
| ER/PR+/HER2− | 56 | Intermed | IDC | 0.9 | ER/PR+/HER2− | Well |
|
| ||||||
| ER/PR/HER2+ | 57 | High | Invasive micro papillary carcinoma | 0.2 | ER/HER2+ (PR−)# | Poorly |
| ER/PR/HER2+ | 65 | High | IDC, 4 foci | 0.2,0.1×2 | Triple –* | Moderately |
| ER/PR/HER2+ | 52 | High | IDC | 0.5 | ER/PR+/HER2−* | Well |
| ER/PR/HER2+ | 43 | High | IDC, multiple foci | 0.1–0.8 | ER/PR−/HER2+* | Moderately |
| ER/PR/HER2+ | 45 | Intermed | IDC | 0.9 | ER/PR+/HER2−* | Well |
| ER/PR/HER2+ | 57 | Intermed | IDC | 1.0 | ER/PR+/HER2−* | Moderately |
| ER/PR/HER2+ | 58 | High | IDC | 1.2 | ER/PR−/HER2+* | Poorly |
|
| ||||||
| ER/PR−/HER2+ | 52 | High | IDC, single focus | 0.1 | ER/PR−/HER2+ | Poorly |
| ER/PR−/HER2+ | 44 | High | Microinvasion | 0.1 | N/A | N/A |
| ER/PR−/HER2+ | 53 | High | IDC | 0.1 | ER/PR−/HER2+ | Poorly |
| ER/PR−/HER2+ | 57 | High | Microinvasion, 3 foci | 0.1 | ER/PR−/HER2+ | Poorly |
| ER/PR−/HER2+ | 55 | High | IDC | 0.3 | ER/PR−/HER2+ | N/A |
| ER/PR−/HER2+ | 44 | High | IDC, discreet from DCIS | 0.3 | ER/PR+/HER2−* | Well |
| ER/PR−/HER2+ | 44 | High | IDC | 0.5 | ER/PR−/HER2+ | Poorly |
| ER/PR−/HER2+ | 40 | N/A | IDC | 0.5 | ER/PR−/HER2+ | Poorly |
|
| ||||||
| Triple – | 67 | High | Invasive lobular carcinoma | 1.5 | ER/PR+/HER2−* | N/A |
IDC: Invasive Ductal Carcinoma
Grade (DCIS): Low, Intermediate, High
Differentiation (invasive): Well: well-differentiated, Moderately: moderately-differentiated, Poorly: poorly-differentiated.
N/A: not applicable, missing data, because they were too small to be characterized.
Signifies phenotype discordance between DCIS and invasive lesion
invasive phenotype is ER+ PR− but overall phenotype is ER/PR+ with concordance between DCIS and invasive lesion
Figure 1.
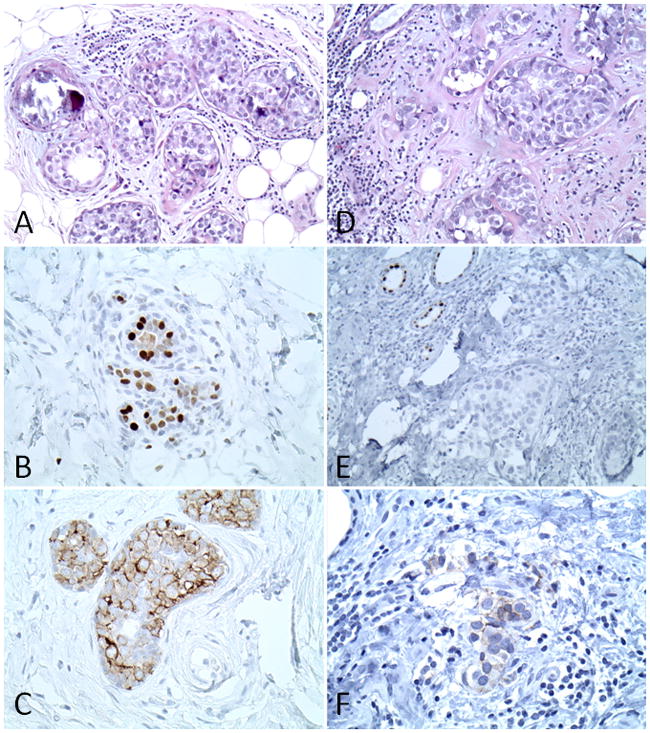
Example of the case that had invasive lesion in subsequent resection. ER/PR/HER2+ DCIS in initial biopsy: A. H&E stain; B. ER PharmDx stain and C. HercepTest stain. DCIS and invasive lesion in subsequent resection: D. H&E stain; E. ER PharmDx stain and F. HercepTest stain. Note the lack of ER and HER2 expression in the invasive element in subsequent resection.
Table 3 displays distributions of patient and DCIS histopathologic variables for cases with and without early invasion. Median age of the 30 patients with early invasive disease was 52 years (range 36–78) and did not differ significantly from those 113 patients with DCIS only, whose median was also 52 years (range 33–87; p=0.53). The majority of these DCIS-associated invasive lesions were small (range 1–15 mm and mean size 4.0 mm). Increasing DCIS grade was significantly associated with higher rate of invasive disease (8.3% in low, 14% in intermediate and 26.30% in high grade patients, p=0.05). Although ER negative and PR negative patients had higher rates of invasion, these associations were not significant (p=0.35 and p=0.20, respectively). HER2 positivity was significantly associated with a higher rate of invasive disease (35.8% in HER2 positive vs. 12.2% vs. HER2 negative patients, p=0.001). These results suggest that HER2 positivity in DCIS is the strongest single marker associated with an occult invasive disease.
Table 3.
Tests of Association between Invasion and Patient and DCIS Histopathologic Characteristics
| Total | Invasion
|
No Invasion
|
Fisher’s exact test p value | Exclude vaccine | Invasion
|
No Invasion
|
Fisher’s exact test p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | |||||
| No. of Patients | 143 | 30 | 21.0 | 113 | 79.0 | 118 | 97 | 82.2 | ||||
| Age at diagnosis | ||||||||||||
| median | 52 | 52 | 0.53* | 52 | 52 | 0.54* | ||||||
| range | 36–78 | 33–87 | 36–78 | 33–87 | ||||||||
|
| ||||||||||||
| Age at diagnosis, decades | 0.45# | 0.56# | ||||||||||
| 30 – 39 | 10 | 1 | 10.0 | 9 | 90.0 | 8 | 1 | 12.5 | 7 | 87.5 | ||
| 40 – 49 | 49 | 10 | 20.4 | 39 | 79.6 | 40 | 6 | 15.0 | 34 | 85.0 | ||
| 50 – 59 | 42 | 14 | 33.3 | 28 | 66.7 | 33 | 10 | 30.3 | 23 | 69.7 | ||
| 60 – 69 | 28 | 4 | 14.3 | 24 | 85.7 | 23 | 3 | 13.0 | 20 | 87.0 | ||
| 70 – 79 | 9 | 1 | 11.1 | 8 | 88.9 | 9 | 1 | 11.1 | 8 | 88.9 | ||
| 80 – 89 | 5 | 0 | 0.0 | 5 | 100.0 | 5 | 0 | 0.0 | 5 | 100.0 | ||
|
| ||||||||||||
| Grade | 0.05# | 0.20# | ||||||||||
| Low | 12 | 1 | 8.3 | 11 | 91.7 | 11 | 1 | 9.1 | 10 | 90.0 | ||
| Intermediate | 50 | 7 | 14.0 | 43 | 86.0 | 45 | 6 | 13.3 | 39 | 86.7 | ||
| High | 80 | 21 | 26.3 | 58 | 73.8 | 61 | 13 | 21.3 | 48 | 78.7 | ||
|
| ||||||||||||
| ER Status | 0.35 | 0.09 | ||||||||||
| ERnegative | 37 | 10 | 27.0 | 27 | 73.0 | 27 | 8 | 29.6 | 19 | 70.4 | ||
| ER positive | 106 | 20 | 18.9 | 86 | 81.1 | 91 | 13 | 14.3 | 78 | 85.7 | ||
|
| ||||||||||||
| PR Status | 0.20 | 0.11 | ||||||||||
| PRnegative | 47 | 13 | 27.7 | 34 | 72.3 | 32 | 9 | 28.1 | 23 | 71.9 | ||
| PR positive | 94 | 17 | 18.1 | 77 | 81.9 | 84 | 12 | 14.3 | 72 | 85.7 | ||
|
| ||||||||||||
| HER2 Status | 0.001 | 0.009 | ||||||||||
| HER2negative | 90 | 11 | 12.2 | 79 | 87.8 | 90 | 11 | 12.2 | 79 | 87.8 | ||
| HER2 positive | 53 | 19 | 35.8 | 34 | 64.2 | 28 | 10 | 35.7 | 18 | 64.3 | ||
|
| ||||||||||||
| Phenotype | 0.009 | 0.04 | ||||||||||
| ER/PR+/HER2− | 83 | 10 | 12.0 | 73 | 88.0 | 83 | 10 | 12.0 | 73 | 88.0 | ||
| ER/PR/HER2+ | 25 | 10 | 40.0 | 15 | 60.0 | 10 | 3 | 30.0 | 7 | 70.0 | ||
| ER/PR−/HER2+ | 28 | 9 | 32.1 | 18 | 67.9 | 18 | 7 | 38.9 | 11 | 61.1 | ||
| ER/PR/HER2− | 7 | 1 | 14.3 | 6 | 85.7 | 7 | 1 | 14.3 | 6 | 85.7 | ||
Wilcoxon rank sum test p value
Linear trend test p value
There was also a significant association between DCIS immunophenotype and the presence of invasive disease (p=0.009) (Table 3). Although invasion rates were higher in HER2 positive DCIS regardless of ER/PR status than HER2 negative DCIS and ER/PR status itself does not contribute significantly to invasion risk, invasive rates were higher in ER/PR/HER2+ (luminal B) (40%) and ER/PR−/HER2+ (HER2) (32.1%) DCIS groups than ER/PR+/HER2− (luminal A) (12%)and ER/PR/HER2− (triple negative) DCIS groups (14.3%).
Among the 25 vaccine-treated patients, 9 cases (36.0%) were found to have invasive diseases in subsequent excision. The incidence of invasive disease in these vaccine-treated patients was very similar to that of all HER2 positive patients (19/53, 35.8%) and of unvaccinated HER2 positive patients (10/28, 35.7%). Further study with greater numbers of patients is required in order to verify the effect of vaccine treatment as this trial was a feasibility study to assess immune response and not the effect on any invasive disease.
By univariate logistic regression, we found that HER2 positive DCIS were 4 times more likely to have invasion as compared to HER2 negative DCIS (p=0.001, Table 4A). ER negativity and higher grade were also associated with higher risk of invasion but these did not reach statistical significance. By multivariable regression analysis, the odds ratio for invasion was 4.15 for HER2 positive DCIS, after adjusting for both ER status and grade (p=0.004). Neither ER status nor grade was significant predictor in the multivariable model. Phenotype was significantly associated with invasion (p=0.013, Table 4B). As noted previously, the odds ratios were higher for both ER/PR/HER+ (luminal B) and ER/PR−/HER2+ (HER2) DCIS (4.87 and 3.46, respectively). In multivariable regression analysis, the significance of phenotype was to 0.04, after adjusting for grade.
Table 4.
Univariate and Multivariable Models of Invasion
| 4A. | Univariate
|
Multivariable
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total
|
Exclude vaccinated patients
|
Total
|
Exclude vaccinated patients
|
|||||||||
| Unadjusted Odds Ratio | 95% CI | Wald p value | Unadjusted Odds Ratio | 95% CI | Wald p value | Adjusted Odds Ratio | 95% CI | Wald p value | Adjusted Odds Ratio | 95% CI | Wald p value | |
| HER2 Status | ||||||||||||
| HER2+ vs. HER2− | 4.01 | 1.73–9.34 | 0.001 | 3.99 | 1.47–10.82 | 0.007 | 4.15 | 1.56–11.04 | 0.004 | 3.43 | 0.98–12.02 | 0.06 |
|
| ||||||||||||
| ER Status | ||||||||||||
| ER− vs. ER+ | 1.59 | 0.67–3.82 | 0.30 | 2.53 | 0.92–6.96 | 0.07 | 0.54 | 0.19–1.58 | 0.26 | 0.94 | 0.24–3.65 | 0.93 |
|
| ||||||||||||
| Grade | ||||||||||||
| Intermediate vs.Low | 1.79 | 0.20–16.12 | 0.15 | 1.54 | 0.17–14.28 | 0.44 | 2.26 | 0.24–21.61 | 0.36 | 1.84 | 0.19–18.07 | 0.75 |
| High vs. Low | 3.92 | 0.48–32.19 | 2.71 | 0.32–23.14 | 3.80 | 0.42–34.00 | 2.30 | 0.24–21.70 | ||||
| 4B. | Univariate
|
Multivariable
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total
|
Exclude vaccinated patients
|
Total
|
Exclude vaccinated patients
|
|||||||||
| Unadjusted Odds Ratio | 95% CI | Wald p value | Unadjusted Odds Ratio | 95% CI | Wald p value | Adjusted Odds Ratio | 95% CI | Wald p value | Adjusted Odds Ratio | 95% CI | Wald p value | |
| Phenotype | ||||||||||||
| ER/PR+/HER2− | 1.00# | 0.013 | 1.00# | 0.05 | 1.00# | 0.04 | 1.00# | 0.19 | ||||
| ER/PR/HER2+ | 4.87 | 1.72–13.74 | 3.13 | 0.69–14.10 | 4.53 | 1.55–13.22 | 3.17 | 0.67–14.95 | ||||
| ER/PR−/HER2+ | 3.46 | 1.23–9.71 | 4.65 | 1.46–14.75 | 2.35 | 0.76–7.26 | 3.46 | 0.96–12.51 | ||||
| ER/PR/HER2− | 1.22 | 0.13–11.18 | 1.22 | 0.13–11.18 | 0.94 | 0.10–8.93 | 1.06 | 0.11–10.23 | ||||
|
| ||||||||||||
| Grade | ||||||||||||
| Intermediate vs. Low | 1.79 | 0.20–16.12 | 0.15 | 1.54 | 0.17–14.28 | 0.44 | 2.29 | 0.24–22.13 | 0.39 | 1.78 | 0.18–17.64 | 0.78 |
| High vs. Low | 3.92 | 0.48–32.19 | 2.71 | 0.32–23.14 | 3.69 | 0.41 0 33.22 | 2.18 | 0.23–20.52 | ||||
Referent category
In order to minimize any potential effects of vaccine treatment in DCIS natural biology, all the analyses were also performed with exclusion of the vaccinated patients (n=25). With numbers of HER2+ cases and high grade cases being decreased the most from a total of 53 to 28 in HER2+ cases (47%) and 80 to 61 in high grade cases (24%) in the analysis, the statistical differences remained in all but the linear trend test for grade in association with invasion (Table 3) and multivariable analysis (Table 4).
Lastly, we examined agreement in immunophenotype between DCIS and invasive lesions. Sufficient invasive tissue was available to allow immunohistochemical staining for ER, PR and HER2 in 22 of 30 DCIS cases with invasive disease. All 6 patients with HER2 negative DCIS also had HER2 negative invasive lesions, while 11 of 16 patients with HER2 positive DCIS also had HER2 positive invasive lesions (Table 5A). In 5 discordant cases, the patients had HER2 positive DCIS and HER2 negative invasive lesions; this lack of symmetry nearly reached statistical significance (p=0.06). Six of 8 patients with ER negative DCIS also had ER negative invasive lesions while 9 of 14 patients with ER positive DCIS also had ER positive invasive lesions (Table 5B). There were 7 discordant cases, 5 patients had ER positive DCIS and ERnegative invasive lesions (Figure 1) and 2 patients had ER negative DCIS and ER positive invasive lesions, but this result was not significant (p=0.45). With regard to phenotype agreement, 12 patients were concordant for phenotype in DCIS and invasion lesions (Table 5C). Interestingly, 8 of the 10 discordant cases had ER/PR/HER2+ (luminal B) DCIS with differing phenotypes in their invasive lesions and the remaining 2 cases had ER/PR+/HER2− (luminal A) invasive lesions with differing phenotypes in their DCIS; this lack of symmetry nearly reached statistical significance (p=0.08). In the 12 patients who were concordant for phenotype in DCIS and invasion lesions, 8 of the 11 (73%) patients had high grade DCIS (Table 2). The grade of the invasive lesion was available in 10 of these 12 patients. Two patients were had well-differentiated (20%), 2 had moderately-differentiated (20%) and 6 had poorly differentiated (60%) disease. Our result suggests that ER/PR/HER2+ (luminal B) DCIS appears to be the most unstable precursor as it was most often associated with invasive disease, and frequently associated with a discordant phenotype between invasive and in-situ components.
Table 5.
Agreement of DCIS and Invasion Phenotypes
| 5A. | Invasive Lesion | McNemar’s test p value | |
|---|---|---|---|
| DCIS Lesion | HER2negative | HER2 positive | |
| HER2 negative | 6 | 0 | 0.06 |
| HER2 positive | 5 | 11 | |
| 5B. | Invasive Lesion | McNemar’s test p value | |
|---|---|---|---|
| DCIS Lesion | ER negative | ERpositive | |
| ERnegative | 6 | 2 | 0.45 |
| ER positive | 5 | 9 | |
| 5C. | Invasive Lesion | McNemar-Bowker test p value | |||
|---|---|---|---|---|---|
| DCIS Lesion | ER/PR+/HER2− | ER/PR/HER2+ | ER/PR−/HER2+ | ER/PR/HER2− | |
| ER/PR+/HER2− | 5 | 0 | 0 | 0 | 0.08 |
| ER/PR/HER2+ | 3 | 1 | 4 | 1 | |
| ER/PR−/HER2+ | 1 | 0 | 6 | 0 | |
| ER/PR/HER2− | 1 | 0 | 0 | 0 | |
Discussion
Small invasive lesions arising in a background of DCIS are frequently undetectable on radiographic evaluation. Such lesions likely represent foci of early progression from the associated precursor lesion. In the presented group of patients with clinical and radiographic findings of DCIS, early (average 0.4 cm) invasive disease was detected by histologic examination in 30 of 143 patients (21.7%). Only a small percentage of these invasive lesions (6 cases; 4% of total cases; 20% of all invasive cases) were detected in the initial biopsy specimen. A majority of cases (24 cases; 80% of all invasive cases) were detected in the subsequent resection specimen. Due to larger tissue sampling size, needle localization biopsy was less likely to miss early invasive disease associated with DCIS than core biopsy (false negative rate 3/37, 8.1% vs. 21/99, 21.2%). For those whose original biopsy showed only DCIS, incidence of invasive disease in subsequent resection was about 17.5% (24/137). This is consistent with other series including one from our early study series comparing the incidence of invasive cancer in patients where core biopsy demonstrates only DCIS [9, 11, 12, 17 and 18]. Our data suggest that significant number of patients, who would have eventually developed invasive lesions after the initial diagnosis of DCIS, were effectively treated by short-term subsequent resection. In addition, the histologic changes showing “suspicious foci but not definitive for invasive disease” in the initial biopsy did not predict confirmatory detection of invasive disease in subsequent resection specimen.
HER2 positivity in DCIS was the dominant factor associated with early invasive disease in this expanded study series (35.8% in HER2 positive vs. 12.2% vs. HER2 negative DCIS, p=0.001), confirming our previous results [11]. A similar observation was recently reported in another study in Chinese patients with similar size of patient cohort, further supporting our current results [12]. HER2 testing is not routinely performed in DCIS specimens as current standard of practice. The strong association between invasive disease and HER2 positive DCIS indicates that HER2 immunostaining might be useful for predicting the presence of occult invasive disease in subsequent resection in patients diagnosed with DCIS in biopsy and help to identify a group of DCIS patients at high risk of developing invasive disease for clinical management.
In the current study, we chose to utilize the original HercepTest scoring criteria (2+ or 3+ in more than 10% of DCIS tumor cells) to determine HER2 over-expression in DCIS instead of the more stringent criteria currently used for invasive disease as a predictor of response to conventional anti-HER2 therapy [14]. The reason for this approach is that our study is aimed to evaluate HER2 as a marker for a group of patients who have high risk of developing invasive lesions, but not as a predictor for response to anti-HER2 therapy. Setting a low threshold would lower the false negative rate and maximize the value as a screening marker. In fact, 11 cases out of 53 HER2 positive DCIS were scored as 2+ HER2 positive and four of these 11 cases were associated with invasive lesions, suggesting this group falls into a category that has a similar behavior to 3+ HER2 positive cases. This approach is further supported by the fact that recent studies have shown that even low and moderate HER2 (1+ and 2+) expression is associated with poor clinical outcome in DCIS [19, 20].
The significance of the distinct molecular phenotypes is well established in IBC but is relatively unknown in DCIS. In addition to HER2 and other clinic-pathologic factors, we also evaluated the incidence of early IBC in various DCIS categorized as Luminal A, Luminal B, HER2+ and Triple Negative groups according to their ER, PR and HER2 immunophenotype. Although there was no significant association between ER/PR expression and early invasion, HER2 positive subgroups, ER/PR+/HER2+ (Luminal B) and ER/PR−/HER2+ (HER2+) were also associated with higher rate of early invasion than HER2− subgroups( ER/PR+/HER2− (Luminal A) and ER/PR/HER2− (Triple negative) DCIS groups).
The prevalence of the distinct molecular phenotypes has been known to differ significantly in DCIS compared to invasive breast cancer [21]. Recently, several attempts to categorize DCIS by immunohistochemistry have been made [9, 10]. The luminal A (ER/PR+/HER2−) and the basal-like (triple negative) phenotypes (both HER2 negative) have been reported to be more frequent in invasive cancers (73.4% and 10.9%, respectively) than in DCIS lesions (62.5 % and 7.7%, respectively) [10]. Conversely, luminal B and HER2+ phenotypes are more frequent in DCIS (13.2% and 13.5%, respectively) than in invasive lesions (5.2% and 5.7%, respectively) [10]. The results of our study using immunohistochemical methods largely concurred with these previous findings demonstrating higher incidence of HER2 expression in DCIS than invasive ductal carcinoma [10, 21]. The slightly higher incidence of HER2 positivity in DCIS in our study (53/143, 37.1%) than the previous studies may have resulted, in part, from the different criteria used to establish HER2 positivity in DCIS in contrast to the current more stringent criteria requiring 3+ intensity and at least 30% positive tumor cells to be considered for positive results for invasive carcinoma [15] (see later discussion). However, a study by Park et al. using FISH method showed a significantly higher incidence of HER2 gene amplification in DCIS (50% of pure DCIS cases) than invasive ductal carcinoma (29%) in their series [18] corroborating that the higher incidence of HER2 overexpression in DCIS likely represents a naturally occurring phenomenon.
Because of the different incidence of HER2 expression in invasive and in-situ lesions, one would expect that not all the invasive lesions would have the same ER/PR/HER2 immunophenotype as the associated background DCIS. In the 22 cases of the DCIS with invasive disease in which sufficient invasive disease was available for immunohistochemical testing (Table 5), immunophenotype was discordant between the invasive and in-situ disease in 5 of the 16 HER2 positive DCIS cases. Of the 11 cases with HER2 negative invasive lesions, 6 had HER2negative DCIS and 5 had HER2 positive DCIS lesions. In addition, 8 of 9 invasive carcinomas arising from ER/PR/HER2+ (luminal B) DCIS demonstrated loss of at least one of ER, PR or HER2 when compared to their associated DCIS. In contrast, ER, PR and HER2 phenotype of the invasive disease remained unchanged in all 5 cases of ER/PR+/HER2− (luminal A) DCIS.
It has previously been argued that the lower rate of HER2 expression in invasive disease compared to in situ disease indicates that HER2 expression is not essential for disease progression. Conversely, the strong association between HER2 positive DCIS with early invasive disease in this study suggests a role for HER2 expression in early cancer progression or at least as a biomarker for DCIS patients with higher rate of development of the invasive disease at the same site of the DCIS. Also noteworthy is the finding that HER2 positive DCIS may be associated with HER2 negative invasive disease. In some cases, the down regulation of HER2 seen in patients treated with HER2 targeted vaccination in our series questions the role of this treatment in the discordant HER2 expression in subsequent invasive disease. However, HER2 was down regulated in both vaccinated and unvaccinated DCIS, and interestingly, more frequently in unvaccinated cases (3/7, 42.9%) than vaccinated ones (2/9, 22.2%), although these rates are imprecise due to the small numbers of cases. This observation, although preliminary, does not support the notion that down-regulation of HER2 in invasive disease arising in HER2 positive DCIS is due to HER2 targeted vaccine therapy.
The conversion of intraductal to invasive disease is a complex process and our findings suggest that during this process, certain biologic pathways may be activated transiently, reflected by the discordance between ER/PR and HER2 immunoexpression in invasive and in situ components in some cases. This phenomenon may also reflect the variable degree of pathway instability in cancer cells as it appears that ER/PR/HER2+ (luminal B) DCIS is a particularly unstable phenotype with a high malignant or invasive potential. Moreover, the high frequency of phenotypic shift in patients with ER/PR/HER2+ (luminal B) DCIS and early invasive disease, whether vaccines were administered against HER2 or not, highlights the role of natural or induced immunity in shaping tumor phenotype during disease progression.
Systemic hormonal therapy has been shown to decrease the risk of invasive cancer in ER positive DCIS [22] and adjuvant therapy with tamoxifen is now widely accepted for treating ER positive DCIS. However, there is no consensus regarding the role for systemic therapy in patients with ER negative DCIS. Currently, neoadjuvant therapy using trastuzumab or other HER2 targeting therapies are under investigation as a treatment option for patients with HER2 positive DCIS [23]. Indeed, the National Surgical Adjuvant Breast and Bowel Project (NSABP) is conducting a phase III randomized trial of trastuzumab for patients with HER2 overexpressing DCIS treated with breast-conserving surgery yielding negative margins [22, 23]. Other novel therapies targeting HER2 are also recently under investigation for breast cancer prevention in patients with HER2 positive DCIS. Although there is a general concern that anti-HER2 treatment may play a role in HER2 negative conversion in invasive disease, our finding did not indicate such risk. In addition, our data showed that a lower incidence of invasive disease in anti-HER2 vaccine treated patients than untreated patients, which seems to suggest a potential cancer preventive effect of this anti-HER2 vaccine treatment. However, whether or not such HER2 targeted therapy could result in more phenotypic instability in DCIS and the subsequent arising of HER2 negative invasive disease, or has a cancer preventive effect needs further investigation.
Conclusions
In summary, our study demonstrates that invasive disease is identified in 21% of the patients who had DCIS with no radiographic evidence of invasive disease. The majority of these invasive lesions were small. Immunophenotypical classification of DCIS, especially HER2 reactivity, might be useful in identifying a group of DCIS patients with higher risk for the presence or subsequent development of early invasive disease. However, the long term implication of such prediction needs to be validated. Furthermore, the significantly greater rate of invasive disease in HER2 positive DCIS in this study suggests that effective targeting of HER2 in DCIS may be of particular benefit in preventing the development of invasive breast cancer and is certainly worthy of further investigation.
Acknowledgments
This study is supported in part by the University of Pennsylvania Abramson Cancer Center Support Grant P30-CA016520 and NIH Grant 5 R01-CA096997-06.
A subset of this work was presented at the United States and Canadian Academy of Pathology (USCAP) 2009 Annual Meeting in Boston, MA.
References
- 1.Valenzuela M, Julian TB. Ductal carcinoma in situ: biology, diagnosis, and new therapies. Clin Breast Cancer. 2007;7:676–681. [PubMed] [Google Scholar]
- 2.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22–24, 2009. J Natl Cancer Inst. 2010;102:161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 3.Page DL, Dupont WD, Rogers LW, et al. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76:1197–2000. doi: 10.1002/1097-0142(19951001)76:7<1197::aid-cncr2820760715>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA. 1999;96:9212–9217. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makretsov NA, Huntsman DG, Nielsen TO, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makretsov NA, Huntsman DG, Nielsen TO, et al. Hierarchial clustering analysis of tissue microarray immunostaining data identifies prognostically significant group of breast carcinoma. Clin Cancer Res. 2004;10:6143–6151. doi: 10.1158/1078-0432.CCR-04-0429. [DOI] [PubMed] [Google Scholar]
- 9.Meijnen P, Peterse JL, Antonini N, et al. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer. 2008;98:137–142. doi: 10.1038/sj.bjc.6604112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotype of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Research. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roses RE, Paulson EC, Sharma A, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18 :OF1–4. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao N, Zhang GC, Liu YH, et al. HER2-positive status is an independent predictor for coexisting invasion of ductal carcinoma in situ of the breast presenting extensive DCIS component. Pathol Res Pract. doi: 10.1016/j.prp.2010.08.005. in press. [DOI] [PubMed] [Google Scholar]
- 13.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67 :1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 14.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. American Society of Clinical Oncology; College of American Pathologists. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 16.Lester SC, Connolly JL, Amin MB. College of American Pathologists protocol for the reporting of ductal carcinoma in situ. Arch Pathol & Lab Med. 2009;133:13–14. doi: 10.5858/133.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Horimoto Y, Tokuda E, Arakawa A, et al. Significance of HER2 Protein Examination in Ductal CarcinomaIn Situ. Surg Res. doi: 10.1016/j.jss.2009.07.030. in press. [DOI] [PubMed] [Google Scholar]
- 18.Hoorntje LE, Schipper ME, Peeters PH, et al. The finding of invasive cancer after a preoperative diagnosis of ductal carcinoma-in-situ: causes of ductal carcinoma-in-situ underestimates with stereotactic 14-gauge needle biopsy. Ann Surg Oncol. 2003;10:748–753. doi: 10.1245/aso.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Camp RL, Dolled-Filhart M, King BL, Rimm DL. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 2003;63:1445–1448. [PubMed] [Google Scholar]
- 20.Gilcrease MZ, Woodward WA, Nicolas MM, et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol. 2009;33:759–767. doi: 10.1097/PAS.0b013e31819437f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park K, Han S, Kim HJ, Shin E. HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemicstry. Histopathology. 2006;48:702–707. doi: 10.1111/j.1365-2559.2006.02403.x. [DOI] [PubMed] [Google Scholar]
- 22.Boughey JC, Gonzalez RJ, Bonner E, Kuerer HM. Current treatment and clinical trial developments for ductal carcinoma in situ of the breast. The Oncologist. 2007;12:1276–1287. doi: 10.1634/theoncologist.12-11-1276. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez RJ, Buzdar AU, Fraser Symmans W, et al. Novel clinical trial designs for treatment of ductal carcinoma in situ of the breast with trastuzumab (Herceptin) Breast J. 2007;13:72–75. doi: 10.1111/j.1524-4741.2006.00366.x. [DOI] [PubMed] [Google Scholar]


