Abstract
Anabolic-androgenic steroids (AAS) are synthetic derivatives of testosterone originally developed for clinical purposes, but now predominantly taken at suprapharmacological levels as drugs of abuse. To date, nearly 100 different AAS compounds that vary in metabolic fate and physiological effects have been designed and synthesised. While administered for their ability to enhance muscle mass and performance, untoward side effects of AAS use include changes in reproductive and sexual behaviours. Specifically, AAS, depending on the type of compound administered, can delay or advance pubertal onset, lead to irregular oestrous cyclicity, diminished male and female sexual behaviours, and accelerate reproductive senescence. Numerous brains regions and neurotransmitter signalling systems are involved in the generation of these behaviours, and are potential targets for both chronic and acute actions of the AAS. However critical to all of these behaviours is neurotransmission mediated by GABAA receptors within a nexus of interconnected forebrain regions that includes the medial preoptic area (mPOA), the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus of the hypothalamus. Here we review how exposure to AAS alters GABAergic transmission and neural activity within these forebrain regions, taking advantage of in vitro systems and both wild-type and genetically altered mouse strains, in order to better understand how these synthetic steroids affect the neural systems that underlie the regulation of reproduction and the expression of sexual behaviours.
Keywords: adolescence, GABAA receptor, anabolic steroids, GnRH, kisspeptin
I. Chemical Classes and the Patterns of Anabolic Androgenic Steroid Use
Anabolic Androgenic Steroids (AAS) comprise a large class of synthetic androgens that were devised for the treatment of hypogonadal dysfunction in men, initiation of delayed puberty, and growth promotion [1, 2]. While still used in clinical settings to treat wasting syndromes and hypogonadism, these synthetic steroids are predominantly self-administered in an illicit manner to enhance performance or body image [3–6]. Adult men are reported to self-administer AAS at concentrations that reflect 10–100× therapeutic doses of testosterone prescribed to restore circulating levels of testosterone in hypogonadal men [3, 6–10]. Girls and women are reported to take AAS at levels equivalent to or even exceeding those administered by men [7,11]. Thus the same doses self-administered by men may be expected to yield circulating levels of androgens in women and girls, both synthetic and physiological, that are orders of magnitude higher than the normal physiological levels of androgens [7].
While public awareness of steroid abuse is often focused on adult males, in the past several decades, the concern over the use of AAS has expanded to encompass self-administration of AAS by adolescents. Approximately 0.5% of girls and ∼2% of boys are also believed to self-administer AAS [12–14]. The mean age for initiation of abuse is 15 years [15], and it is estimated that a large portion of teenagers take AAS throughout adolescence at a time when the brain is still developing and highly hormone-sensitive [16–18]. Many of these adolescent users are not involved in athletics, but are concerned with body image [12, 13, 19]. Prior reports in human subjects suggest that adolescents may be more sensitive than adults [20] and women may be more sensitive than men [11] to the disruptive effects of AAS. Effects of AAS are thus not monolithic, and will depend not only on the age and sex of the user, but also on the chemical structures of the steroids taken and the complexity of the regime of administration (see below).
Steroid abuse is typified by the concurrent use of multiple AAS (stacking) and a pattern of on-drug and off-drug use (cycling) and pyramiding, the process of administering first escalating then declining doses during the cycle; a process that AAS users think will diminish "catabolic molecules", such as cortisol. AAS users also concurrently self-administer anti-oestrogens and other agents, including 3β-HSD inhibitors [21, for discussion, see 22]. While there are greater than 100 AAS compounds that have been synthesised to date, three major classes of AAS can be described that differ in their chemical structure and metabolic half-lives, and thus their physiological effects. AAS in the first class are derived from esterification of the 17β-hydroxyl group of testosterone and includes testosterone propionate, and testosterone cypionate. Testosterone esters can be hydrolysed into free testosterone, reduced to 5α-dihydrotestosterone or aromatised to oestrogens [3, 23, 24]. Molecules that have been 5α-reduced cannot be metabolised into oestrogens, but may be metabolised into other androgens, such as 3α-androstanediol [3, 24]. The second class of AAS includes the 19-nor-testosterone derivatives such as nandrolone. These AAS are often also esterified, but in conjunction with the long side chain moieties, have a substitution of a hydrogen for the methyl group at C19 [1, 2]. Like the testosterone esters in class I, 19-nortestosterone derivatives can be aromatised to 17β-oestradiol, although not as readily as testosterone [24, 25]. The third class of AAS comprises those compounds that are alkylated at C17, such as 17α-methyltestosterone and stanozolol. Because alkylation retards metabolism by the liver, these AAS, unlike AAS in groups one and two, are orally active [1]. None of the 17α-alkylated steroids can be converted into DHT or aromatised to 17β-oestradiol [24–26], but these compounds may be converted to other androgenic and oestrogenic metabolites [27, 28].
All AAS are biologically active at classical androgen receptors (AR), albeit with significantly different activities [29–31]. The AAS can be aromatised to oestrogenic metabolites [24–26, 31–34] with the capability to interact with both estrogen receptor alpha (ERα) and ERβ and promoting physiological effects that may reflect ER, as well as AR signalling [e.g., see 35–38]. In addition, the AAS can, without metabolic conversion, also signal through ERα and β and the progesterone receptor [39, 40]. While the potency of AAS action at ER is markedly less than 17β-oestradiol, Attardi et al. [40] have shown that the activation of ERE reporter constructs by two individual AAS at concentrations of 10−8 to 10−6 M reached the same level of maximal activation as did physiological concentrations of 17β-oestradiol. Thus, while the EC50 for the two AAS are several orders of magnitude less than is the EC50 for 17β-oestradiol, the activation may be quite relevant to the drug abuse situation where serum levels of AAS have been estimated to reach micromolar concentrations in human subjects who chronically abuse them [7, 41, 42]. This estimation in illicit steroid abusers has been supported by a controlled clinical study of male volunteers who were administered AAS. Data from this study indicated that even moderate, short-term use raised AAS levels in cerebral spinal fluid (CSF) as high as 0.9 µM [8]. Activity of the AAS at ERβ is particularly intriguing given recent data from Handa and colleagues that demonstrate the ability of endogenous androgens to signal through ERβ and to influence neural regulation of the hypothalamic-pituitary-adrenal axis and hormonal control of the stress response [43–46].
In addition to the multiplicity of potential signalling effects through hormone receptors, AAS have also been shown to elicit rapid effects through interactions with a non-AR/ER microsomal binding site, [47], through allosteric modulation of ion channels [48–50], and through allosteric regulation of enzymes involved in the biotransformation of steroids (see below). The complexities in paradigms of AAS administration, the diversity of metabolic fates of the different steroids and the resulting range of signalling capacities of the AAS have important implications for understanding the actions of these steroids, since the distribution of AR and ER vary significantly with sex and age [51–53]. Additionally, it is possible that allosteric modulation of ion channels by AAS may also vary with sex and development, as has been shown for neurosteroid modulation of the GABAA receptor [54–58], although this has yet to be tested for the AAS.
II. AAS Effects on Sexual Behaviours and Reproduction
While self-administered for their ability to enhance athletic performance and body image, AAS use is also associated with untoward behavioural effects [5, 11, 49, 59, 60]. In human abusers and in animal models, one of the most consistent hallmarks of AAS administration is significant and often permanent change in sexual behaviours and reproductive state [11, 61]. Self-administration of high doses of AAS in men and boys has been associated with both increases and decreases in libido and a hypogonadal state characterised by diminished levels of serum LH and FSH, testicular atrophy, and decreased sperm production [61, 62]. In human females, early exposure to high levels of androgens alters the onset of puberty, reproductive competence, libido and sexual arousal, although as with male the direction of elicited changes may vary in different individuals [11, 63, 64].
AAS exposure in animal models replicates many of findings for human subjects, resulting in aberrations in both male and female reproductive and sexual behaviours and accelerated reproductive senescence [49, 60, 65, 66]. Effects of AAS administration vary with the type and regime of the AAS taken and the age of the subjects when drug administration begins [49]. In focusing on the actions of 17α-alkylated AAS in adolescent rodents, it has been reported that treatment of adolescent male rats with 17α-alkylated AAS results in diminished testes weight, decreased ejaculation, scent marking and ultrasonic vocalisations [67–69]. Treatment of adolescent female rodents with moderate or high concentrations of 17α-alkylated AAS has been shown to cause a significant advance in the onset of vaginal opening, as well as a marked delay in first vaginal oestrus and suppression of regular oestrous cyclicity [70, 71]. In adult rats and mice, exposure to moderate to high doses of 17α-alkylated AAS decreases testes weights, mounts, intromissions and ejaculations in males [72, 73] and suppress vaginal and behavioural cyclicity in females [37, 71, 74, 75]. The suppressive effects of AAS on reproductive competence have been attributed to AAS-dependent actions on gonadotrophin secretion [74]. Interestingly, while treatment with a mixture of non-17α-alkylated AAS resulted in increased intromissions and ejaculations in male hamsters treated as adolescents, this combination of AAS did not impair reproductive behaviours in hamsters treated as adults [76]. These studies highlight that the actions of the AAS are complex, varying with the types of steroids administered and the age and sex of the subject.
III. Effects of AAS on GABAergic Transmission and Neural Activity in Regions of the Hypothalamus and Forebrain that Regulate the Expression of Sexual and Reproductive Behaviours
The mechanisms by which chronic AAS exposure modifies the expression of sexual behaviours and reproductive competence are only beginning to be uncovered and are likely to reflect both peripheral effects on gonadal tissues and central effects on neuronal circuits. The medial preoptic area (mPOA) is an integral node in a core of reciprocally connected regions of the hypothalamus and forebrain that form the neural networks underlying the regulation of sexual and reproductive behaviours [77]. The neural substrates required for the expression of sexual and reproductive behaviours in rodents have been well documented by lesion studies, anatomical tracer studies, and assessments of c-fos expression associated with mating behaviour. In males and females, the mPOA is a key site for the integration of sensory input and regulation of both sexual performance and motivation [78, 79]. Neurones in the mPOA and the continuum of cells along the rostral periventricular region of the third ventricle (RPV3) also provide critical afferent control over the population of gonadotropin releasing hormone (GnRH) neurones that regulate the hypothalamic-pituitary-gonadal (HPG) axis. Many excellent reviews have recently been published on neural control of GnRH function and will not be recapitulated here except to note that GABAergic control of GnRH pulsatility is essential for pubertal onset and reproductive competence in both sexes [80–82]. The effects of AAS on sexual behaviour and the control of reproduction are likely to reflect the integration of complex signalling and changes in the expression and/or function of many neural substrates [60]. We are highlighting AAS effects on signalling mediated by GABAA receptors both because of the crucial role GABAergic transmission plays in the control of reproduction and because studies demonstrate that the AAS have significant effects on GABAA receptor expression and function [37, 41, 42, 48, 67, 69, 71, 83–87], as well as on the levels of endogenous allosteric modulators of these receptors [36]. Thus, AAS-dependent changes in GABAergic signalling in the forebrain may be a critical conduit by which these steroids impart molecular actions that lead to changes in sexual and reproductive behaviours.
A. Allosteric Modulation of GABAA Receptors by the AAS
The native GABAA receptor is a pentameric ionotropic transmembrane protein for which sixteen different receptor subunit genes (α1–6, β1–3, γ1–3, δ, ε, π, and θ) and numerous alternatively spliced mRNAs have been identified in mammals [88]. Although receptors containing α1, β2 or β3, and γ2 subunits constitute ∼80% of GABAA receptors expressed in the adult brain [88–91], the mPOA and regions of the RPV3 continue throughout adulthood to express high levels of α2-containing receptors (α2 greater than or equal to α1), as well as subunits including ε and γ1, that show limited to negligible expression in other brain regions [37, 67, 69, 71, 84, 85, 92, 93].
Subunit composition imparts significant differences in both the biophysical properties of the receptors and their sensitivity to allosteric modulation, including allosteric modulation by the AAS. For example, the AAS, 17α-methyltestosterone, potentiates responses elicited by brief exposure to millimolar concentrations of GABA at α2β3γ2-containing recombinant receptors expressed in heterologous cells, but has negligible effects at α1β3γ2-containing receptors [86, 87]. Conversely, the AAS significantly potentiate tonic currents mediated by ambient (low µM) concentrations of GABA through α1-containing recombinant receptors, but are without effect on tonic currents mediated by α2-containing receptors [86, 87]. Data from primary murine neurones in which levels of α1 versus α2 subunit mRNAs determined by single cell PCR were correlated with the extent of positive modulation of GABA-dependent currents in indentified neurones supports this finding from receptors expressed in heterologous systems [87]. The relative expression of α1- versus α2-containing receptors may be pertinent to changes in the sensitivity of GnRH neurones as mice make the transition through puberty since it has been reported that there is a late developmental increase at puberty in the numbers of morphologically identified single GnRH neurones in wild type mice that express the α1 subunit [94]. Data from our laboratory from GnRH neurones identified by fluorescence in the GFP-GnRH transgenic line [95] corroborate this finding (Robert McGarr and Carlos Penatti, unpublished).
Neurones within neuroendocrine control regions of rodents, including the mPOA/RPV3, also express two subunits not found at appreciable levels elsewhere in the brain: the γ1 and the ε subunits. While substitution of the γ1 for the γ2 subunit did not appreciably alter acute allosteric modulation by 17α-methyltestosterone for recombinant α2β3x receptors [48], substitution of the ε subunit for the γ2 subunit in recombinant α2β3x receptors dramatically altered the profile of AAS modulation. Specifically, in receptors containing the ε subunit, AAS imposed negative allosteric modulation of both GABA-gated and spontaneous opening of the receptor by a mechanism of allosteric block in which the AAS interacts preferentially with and promotes accumulation in a closed state [83]. Intriguingly, recent studies also suggest that the ε subunit may replace not only the γ, but also the β subunit in the GABAA receptor and that alternative patterns of subunit incorporation may underlie the observed variability in both biophysical and pharmacological properties noted not only in recombinant ε-containing receptors, but also in native neurones in which ε subunits are expressed [96, 97].
The properties of ε-containing receptors are particularly intriguing with respect to AAS effects on the expression of reproductive and sexual behaviours since ε expression is enriched in neuroendocrine control regions including the ventromedial nucleus of the hypothalamus, the mPOA, the septum, and the amygdala [67, 98–101]. Moreover, it has been reported that ε subunits are expressed in virtually all GnRH neurones in the mPOA/anterior hypothalamus [99]. Approximately 70% of GnRH neurones acutely dissociated from neonatal mice display a robust picrotoxin-sensitive current of 70 ± 20 pA which is likely to reflect spontaneous openings of ε-containing receptors, and 17α-methyltestosterone can antagonise this current as it does for both spontaneous and GABA-gated currents in recombinant receptors [83]. Surprisingly, GnRH neurones in intact slices from the mouse do not demonstrate appreciable levels of picrotoxin-sensitive currents that would indicate appreciable expression of ε-containing receptors in these cells [69, 71]. This disparity may reflect a developmental difference, methodological constraints (dissociated versus cells in intact slices) or potential antagonism of ε-containing receptors by lipophilic and endogenous allosteric modulators (e.g., neurosteroids) that may be retained in the slice preparation. Furthermore, while negative modulation by 17α-methyltestosterone of both spontaneous and GABA-gated currents through GABAA receptors in GnRH neurones has been observed [83] and (C.A.A. Penatti, unpublished data), GnRH neurones in the intact slice also exhibit robust and reversible positive modulation to acute application of 17α-methyltestosterone (Figure 1A, B), suggesting notable heterogeneity in receptor composition and expression even within this single identified class of neurone.
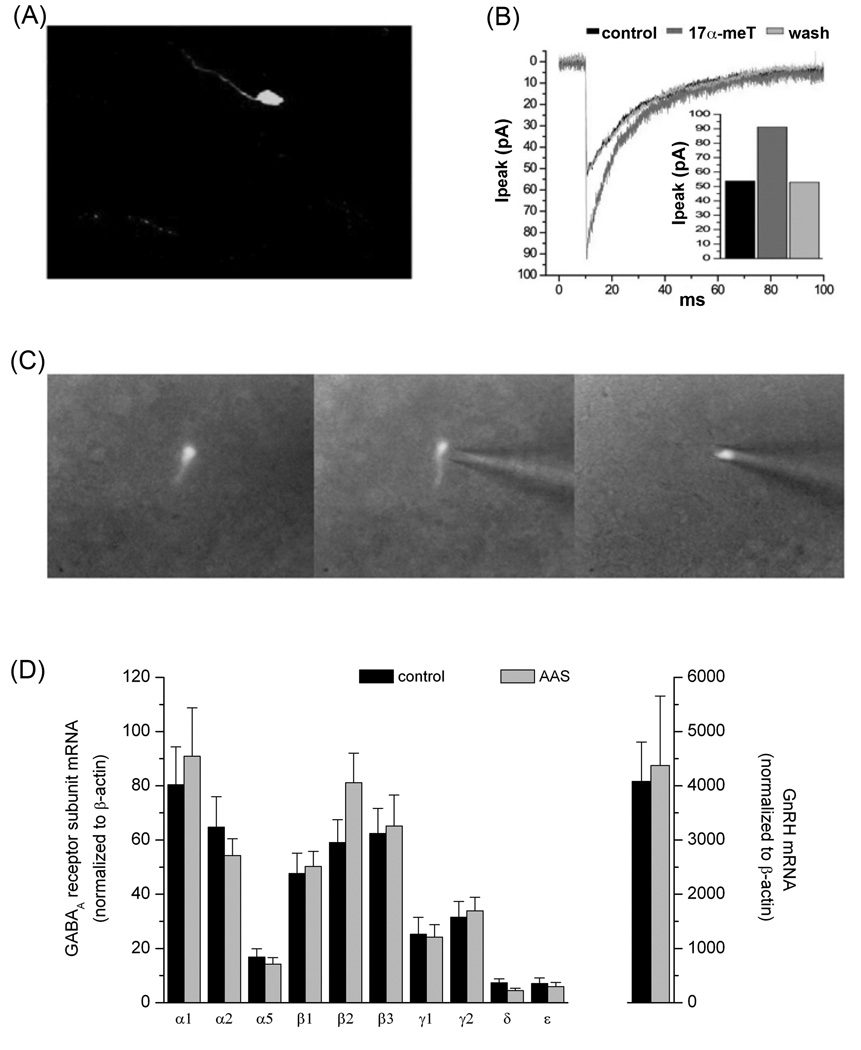
Figure 1.
Properties of GnRH Neurones. (A) Representative photomicrograph of a GnRH neurone from the GFP-GnRH transgenic line generously provided by SM Moenter (University of Michigan; [95]). (B) Acute modulation of GABAA receptor-mediated sPSCs in a GnRH neurone by 17α-methyltestosterone. Average currents (>30 neurones under each condition) recorded in aCSF alone (control), in aCSF supplemented with 1 µM 17α-methyltestosterone (17α-MeT) and following return to aCSF (wash). (C) Photomicrographs illustrating harvesting of the contents of a fluorescent GFP-GnRH neurone for single cell PCR analysis. (D) GABAA receptor subunit mRNA levels in GnRH neurones of gonadally-intact oil-injected (control) and AAS-treated male mice. Data are presented as the 2−ΔCT values, which indicate the average levels (relative to the housekeeping gene β-actin) of subunit mRNAs in GnRH neurones isolated from control (black; n= 10 mice) and AAS-treated (grey; n = 10 mice) for analysis of GABAA receptor subunit mRNA levels and from a separate cohort of 7 control and 7 AAS-treated mice for analysis of GnRH mRNA levels. Data in (D) are from Penatti et al. [69].
While the AAS and the neurosteroids, especially androgenic compounds such as 5α-androstane-3α,17β-diol (3α-diol), have obvious structural similarities, several observations suggest that these two classes of steroids interact with disparate parts of the GABAA receptor and elicit allosteric modulation by separate mechanisms. First, the AAS lack key structural moieties necessary for neurosteroid activity [102, 103]. Second, while neurosteroids at high concentrations can directly gate the GABAA receptors [104], high concentrations of the AAS, 17α-methyltestosterone, do not [50]. Third, as noted above, α subunit composition is critical to the allosteric effects of the AAS. In contrast, the four transmembrane residues in the α1 subunit that are critical for both potentiation and direct activation of the receptor by the positive neurosteroids are conserved among the α and β subunit family members [105] and electrophysiological studies indicate that changes in α or β subunit composition have only a modest effect on positive neurosteroid potentiation [104, 106]. Fourth, modelling studies indicate that the state transitions altered during positive allosteric modulation of γ-containing receptors by the AAS [86, 87] are distinct from those altered by positive neurosteroids [107, 108]. Fifth, substitution of a δ for the γ subunit in α1- and α2-containing receptors confers marked enhancement in modulation elicited by neurosteroids [109, 110] and tracazolate [87, 96]. In contrast, the AAS, 17α-methyltestosterone had no significant effect on responses elicited by GABA from α2β3δ receptors under conditions of either full or partial receptor occupancy [87]. It is likely that the AAS and the neurosteroids may also interact with physically disparate parts of the receptor. For the AAS, an attractive hypothesis supported by data from site directed mutagenesis studies [83] is that these synthetic steroids interact with a promiscuous composite low affinity binding pocket formed by conserved residues in transmembrane segments 2 and 3 of receptor subunits. Such a site has been implicated in the allosteric actions mediated by a number of structurally dissimilar allosteric modulators, including loreclezole, ethanol, specific anesthetics and low affinity benzodiazepine binding [83, 86, 87].
Acute anxiolytic effects of AAS that may possibly reflect allosteric modulation of GABAA receptors have been reported for studies in rodents [111–113]. These acute effects may underlie the initial hypomania and euphoria reported in AAS human abuse [114, 115] that may contribute to the enhancing effects on libido of AAS elicited in some human subjects [11]. However, we note that all of these studies report acute anxiolytic actions of AAS that can be reduced to 3α-diol, not of the 17α-alkylated derivatives. Moreover, we have failed to observe anxiolytic effects in response to acute single doses of testosterone, to 17α-methyltestosterone or to a mixture of testosterone cypionate, methandroestenolone and nandrolone decanoate in either male or female mice as assessed on the elevated plus maze or on the acoustic startle response (A.S. Clark, J.G. Oberlander, M.M. Onakomaiya, and D.M. Porter, preliminary results).
B. Effects of Chronic Exposure to AAS on Regulation of GnRH Cell Function in Adolescent Mice
In animal models, as in human subjects, chronic exposure to AAS at concentrations that reflect high human abuse regimes disrupt reproductive function in both male and female mice [49, 60]. Exposure to these synthetic steroids may have particularly acute repercussions in adolescence, but effects of AAS during adolescence on critical centres that regulate reproduction had not been examined until recently. For adolescent male mice chronic treatment with the AAS, 17α-methyltestosterone, reduced serum levels of LH and FSH and decreased testes’ mass [69]. A comparable treatment of adolescent female mice imposed a prolonged anoestrous state characterised by dioestrous vaginal smears [71]. These changes in peripheral reproductive state were associated with a significant decrease in the frequency of action potentials in GnRH neurones in both sexes. Because of the importance of GABAergic tone in the onset of puberty and control of GnRH secretion following reproductive maturation, the properties of GABAA receptor-mediated responses were examined in detail. Single cell RT-qPCR analysis indicated that GnRH neurones in adolescent male and female mice express a comparable complement of GABAA receptor subunit mRNAs, as do neighboring neurones in the mPOA of adolescent and adult mice [37, 69, 71, 84, 85]. However, unlike non-GnRH neurones in the mPOA, AAS treatment did not induce significant changes in GABAA receptor subunit mRNA levels in these GnRH cells in either male (Figure 1 C, D) [69] or female [71] mice. Moreover, whole-cell patch clamp recording indicated that AAS treatment was without effect on the amplitude or decay kinetics of GABAA receptor-mediated spontaneous postsynaptic currents (sPSCs) miniature sPSCs or tonic currents in GnRH neurones in either sex [69,71]. In contrast, AAS treatment significantly increased the frequency of GABAA receptor-mediated sPSCs in GnRH neurones of both male and female mice [69,71]. Taken together, these data indicate that AAS treatment was without an appreciable effect on the complement or function of postsynaptic GABAA receptors expressed in GnRH neurones and that the likely site of AAS action was on presynaptic afferents to these GnRH cells.
In male mice, the increase in the sPSC frequency in GnRH neurones was correlated with an increase in action potential frequency in neighboring mPOA neurones; neurones that are prime candidates to provide afferent innervation to the GnRH cells [116, 117]. To directly determine if these cells were indeed the source of the enhanced GABAergic inputs to GnRH neurones in AAS-treated male mice, these afferents were both physically (by a surgical cut) and pharmacologically (by GABAA receptor antagonists) disrupted. The physical or pharmacological isolation of lateral GABAergic mPOA afferents abrogated the AAS-induced increase in GABAA receptor-mediated sPSC frequency and the decrease in action potential firing in the GnRH cells of AAS-treated male mice [69].
These data indicate that in adolescent male mice, AAS act predominantly on steroid-sensitive presynaptic neurones within the mPOA, rather than on GnRH neurones themselves, consistent with the paucity of steroid receptor expression in these cells [81, 118, 119]. While GABA may have both excitatory and inhibitory effects on GnRH neurones [120–126], results from this study [69] in adolescent male mice are most consistent with the hypothesis that AAS treatment increases action potential firing in GABAergic mPOA neurones, which in turn provides enhanced inhibitory tone that suppresses the firing rate of GnRH neurones and decreases LH/FSH release (Figure 2). The diminished levels of gonadotrophins are likely to contribute to the decrease in testicular weights observed in AAS-treated animals.
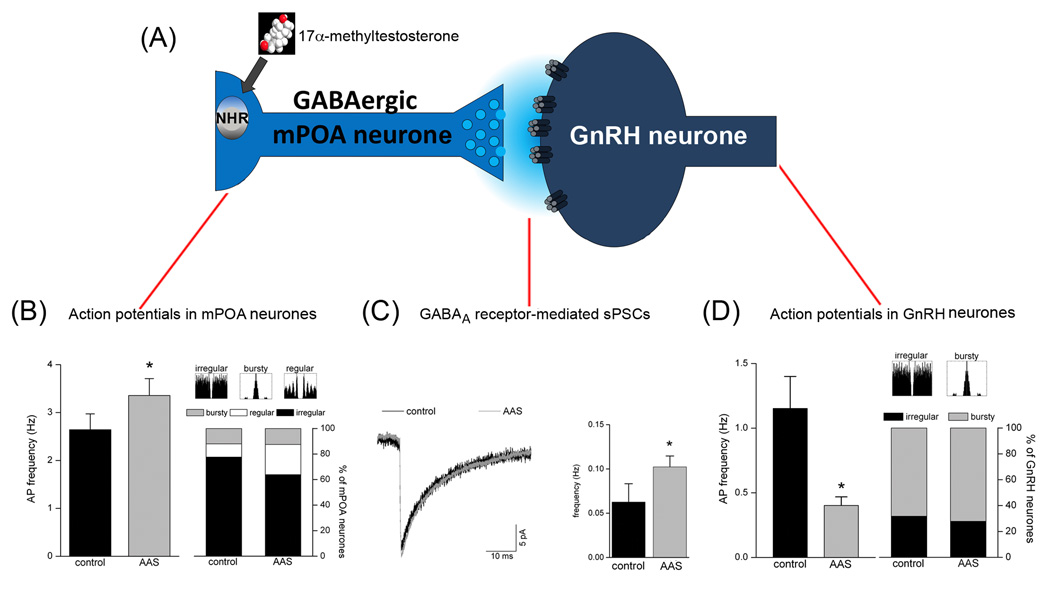
Figure 2.
Model of AAS Action in Adolescent Male Mice Chronically Treated with 17α-Methyltestosterone. Gonadally-intact male mice were treated for 4 weeks beginning at postnatal day (PN) 25–28 with 7.5 mg/kg/day for 6 days/week or with oil (control). (A) Model schematic of GABAergic mPOA inputs to GnRH neurones. Electrophysiological assessments made following the treatment period indicated that AAS treatment (B) significantly (p = 0.015) increased action potential frequency in mPOA neurones of AAS-treated (grey; n = 58 neurones) versus control (black; n = 72 neurones) mice and promoted a trend towards a decrease in the number of neurones that displayed irregular firing patterns; (C) had no effect on the amplitude or kinetics of GABAA receptor-mediated sPSCs recorded from GnRH neurones, as illustrated by averaged currents from neurones from control (grey) and AAS-treated (black) mice, but did significantly (p = 0.014) increase the frequency of these sPSCs (n = 12 neurones for control and n = 13 neurones for AAS-treated); and (D) significantly (p = 2.03 × 10−4) decreased the frequency of action potentials in GnRH neurones from AAS-treated (grey; n = 18 cells) versus control (black; n = 22 cells) without altering action potential patterning in these GnRH neurones. Data are modified from Penatti et al [69].
As with male mice, AAS treatment of female mice was associated with a low frequency of action potential firing in GnRH neurones, with frequencies in AAS-treated animals being comparable to dioestrous littermates and significantly lower than oestrous littermates [71]. As noted, AAS treatment of adolescent females also promoted a higher frequency of GABAA receptor-mediated sPSCs in GnRH neurones. Surprisingly, however, the changes in sPSC frequency and action potential firing in GnRH neurones of female mice did not correlate with action potential firing patterns in the mPOA, suggesting that, unlike males, afferent drive from this region was not critical in mediating AAS effects. In contrast, action potential frequency in the AVPV, as well as expression of kisspeptin, did positively correlate with AAS-dependent changes in GnRH firing [71]. Thus, while chronic AAS treatment may impose the same end-point in adolescent male and female mice (diminished action potential firing in GnRH neruones), the steroid-sensitive afferents that impose these effects are likely to vary between the sexes, with inhibitory GABAergic neurones in the mPOA playing the major role in males and either excitatory kisspeptin and/or inhibitory GABAergic afferents within the AVPV being predominant in females [71].
C. Indirect Effects of AAS on Classical Hormone Receptor Signaling in Neuroendocrine Control Regions
Studies in both adolescent male and female mice chronically exposed to 17α-methyltestosterone support the hypothesis that AAS act to impair GnRH neuronal function and thus reproductive competence through actions on afferent neurones within the mPOA/RPV3. In male mice, pharmacological and physiological manipulations indicate that GABAergic neurones within the central portion of the mPOA play a significant role in imparting AAS-induced changes in GnRH neuronal function. While we conclude that the AAS act on upstream mPOA afferents rather than the GnRH neurones themselves, AAS effects could be mediated by AR signalling, ER signalling and/or nongenomic actions in these mPOA afferents. Neurones within the central portion of the mPOA express high levels of AR [51, 52] and ERα, lower but appreciable levels of ERβ [53, 127, 128], and high levels of aromatase [129]. To assess the importance of classical AR signalling versus other mechanisms in regulating the activity of mPOA afferents, adult male wild type and AR-deficient testicular feminisation (Tfm) mice were treated with a mixture containing equal concentrations of one AAS from each major class of AAS (testosterone cypionate, nandrolone decanoate, and 17α-methyltestosterone) [84]. This treatment regime was used in order to more closely model human use patterns and to optimise the capacity for the AAS to act through not only AR, but also ER and other alternative mechanisms.
In mPOA neurones of wild type male mice, chronic AAS treatment resulted in a significant increase in action potential firing, significant increases in the levels of α5 and β1 subunit mRNAs and a concomitant significant prolongation of GABAA receptor-mediated synaptic current decay that could be attributed to α5-containing receptors [84]. The AAS-dependent increase in α5 subunit expression and prolongation of synaptic currents in the mPOA of adult males mirrored effects of this AAS mixture observed in adult females, where co-treatment with the AR antagonist, flutamide, was shown to inhibit the effect of the AAS [37]. Consistent with the effects of flutamide in females, increases in α5 subunit mRNA expression and prolongation in sIPSC current decay were not evident in AAS-treated male AR-deficient Tfm mice, suggesting that these changes in GABAA receptor subunit expression in the mPOA were mediated through classical AR signaling. Despite the absence of AAS-dependent changes in α5-containing receptor-mediated currents in the Tfm mice, AAS treatment was not without effect in AR-deficient line. Specifically, AAS treatment of Tfm mice elicited a significant decrease in the frequency and amplitude of sIPSCs and a significant decrease in GAD65 mRNA levels in the mPOA. These data are consistent with the interpretation that in the Tfm mutants, AAS treatment results in diminished AP-dependent GABA release from afferents onto central mPOA neurones. While ER-mediated signalling following aromatisation seemed the likely alternative mechanism for mediating these AAS effects in the Tfm mice, surprisingly concomitant treatment of Tfm mice with AAS and tamoxifen (at concentrations that impose ER antagonism) did not block, but rather mimicked, the effects of AAS on GABAergic transmission (Figure 3A). Similarly, AAS and the aromatase inhibitor, formestane, when given individually had comparable effects on GABAA receptor-mediated sIPSCs, but when co-administered in the presence of formestane, the AAS had no further effect (Figure 3A). In contrast to the effects of tamoxifen and formestane with the AAS, concomitant treatment with 17β-oestradiol abrogated the ability of the AAS to decrease sIPSC amplitudes (Figure 3A). Finally, despite dramatically elevated levels of testosterone derived from the injected AAS, there was no parallel conversion to 17β-oestradiol in mPOA tissue of AAS-treated mice (Figure 3B). Taken together, results from this study suggested that the AAS may be acting to inhibit aromatase in the brain and thus antagonise the endogenous ER-mediated signalling that augments GABAergic tone in the mPOA [130]. Support for this hypothesis comes from studies in non-neuronal cell lines in vitro demonstrating that individual AAS inhibit aromatase activity in a dose-dependent fashion [28, 131]. The Ki of 17α-methyltestosterone for inhibition of aromatase was found to be 0.6 µM [132], a concentration likely to be attained with the doses of AAS administered here and relevant to human steroid abuse [49]. Interestingly, chronic, low doses of testosterone propionate have also been shown to decrease the expression of 5α-reductase type I and the endogenous levels of allopregnaolone in cortex and regions of the extended amygdala that are implicated in the expression of sexual behaviors [133]. These studies highlight the fact that the AAS may impart significant effects on neural circuits that regulate reproduction and sexual behaviors not only by steroid receptor mediated-signaling and allosteric modulation of ion channels, but also by direct effects on key steroid biosynthetic enzymes [84,133].
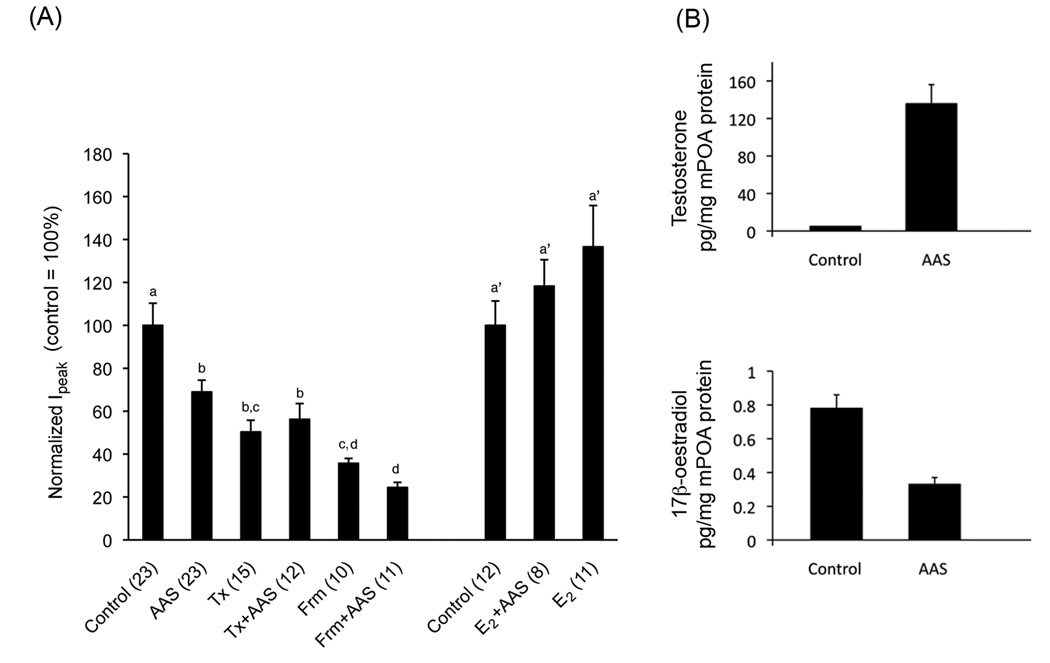
Figure 3.
AAS Antagonism of Endogenous ER Signalling in the AR-deficient Tfm Mouse. (A) Graphical representation of averaged peak sIPSC amplitude from Tfm mice chronically treated with oil alone (Control), the AAS mixture in oil (AAS), tamoxifen (Tx), the AAS cocktail and tamoxifen (Tx+AAS), formestane (Frm) the AAS cocktail and formestane (Frm+AAS), 17β-oestradiol and the AAS cocktail (E2+AAS) or 17β-oestradiol alone (E2). Identical letters indicate means that were not statistically different from one another as assessed by two-way ANOVA followed by the means comparison by least significant means. Numbers in parentheses indicate numbers of cells. (B) Means ± standard errors of the mean levels of testosterone (T) and 17β-oestradiol (E2) in mPOA tissue harvested from control (n = 6 mice for T; n = 8 mice for E2) or AAS-injected (n = 4 mice for T; n = 8 mice for E2) Tfm male mice. Data are modified from Penatti et al. [84].
It is also of interest to note that a recent study indicates that conditional ablation of ERα in kisspeptin neurones of female mice results in two disparate effects: significant advancement of pubertal onset, but subsequent arrest of pubertal maturation [134]. These data are similar to effects of early exposure to the AAS, 17α-methyltestosterone, which significantly advanced pubertal onset while at the same time delaying the maturation of the HPG axis (as indicted by the day of first oestrus; [60]). While the potential effects of AAS inhibition of aromatase activity in female mice has not been assessed, the parallels in these studies suggest that interference of ER signalling via allosteric inhibition of aromatase by the AAS may also play a pivotal role in pubertal disruption in adolescent females.
D. Characteristics of the AR-deficient/GFP-GnRH mouse
To better dissect the roles of AAS through classical AR versus indirect effects on ER signalling through allosteric modulation of aromatase on the function of GnRH neurones themselves, we have generated a new mouse line by backcrossing for greater than 10 generations heterozygous Aw-J/Aw-J EdaTa-6J +/+ArTfm females from Jackson Laboratories with transgenic male GFP-GnRH mice [95]. We refer to these mice as the GT line for GFP-GnRH mice with the Tfm mutation. The resulting strain of mice expresses the same mutation in the AR as does the classical Tfm line, as indicated by conventional PCR genotyping [135] and by a real time PCR-based allelic discrimination utilising a custom mouse androgen receptor (AR) TaqMan®SNP genotyping assay. Added benefits of this new line include that, in our hands, these mice show fewer health problems than do wild type Tabby mice used to generate Tfm offspring, and, as opposed to C57Bl/6J mice that are often used for comparison with Tfm mutants, both wild type and AR-deficient mice in this line are on an identical CBA/C57Bl/6 background [95].
Initial characterisation of this line indicates that the level of action potential activity in key neuroendocrine control regions is significantly altered by the absence of classical AR signalling. Specifically, in on-cell recordings performed according to Penatti et al. [69] at 35°C in artificial cerebral spinal fluid (aCSF), action potential activity was significantly lower in both GnRH neurones (2.2 ± 0.3 vs. 4.2 ± 0.6 Hz; p < 0.01) and mPOA neurones (2.4 ± 0.4 vs. 5.6 ± 0.8 Hz; p < 0.01), but significantly higher in neurones of the arcuate nucleus (6.6 ±0.8 vs. 4.4 ±1.0 Hz; p < 0.01) from GT AR-deficient versus GT wild type male mice (Figure 4A). Levels of mRNA encoding ERβ in the mPOA were not different between AR-mutant and wild type GT mice, while transcripts encoding ERα in the mPOA showed a trend (p < 0.06) towards elevated expression in the mutant line that did not attain significance (Figure 4B). Expression of kisspeptin mRNA in the mPOA and the number kisspeptin immunopositive neurones in the AVPV were significantly (p <0.0001) decreased in the GT AR-mutant mice, but significantly (p <0.0001) increased in the arcuate nucleus (Figure 4C and D).
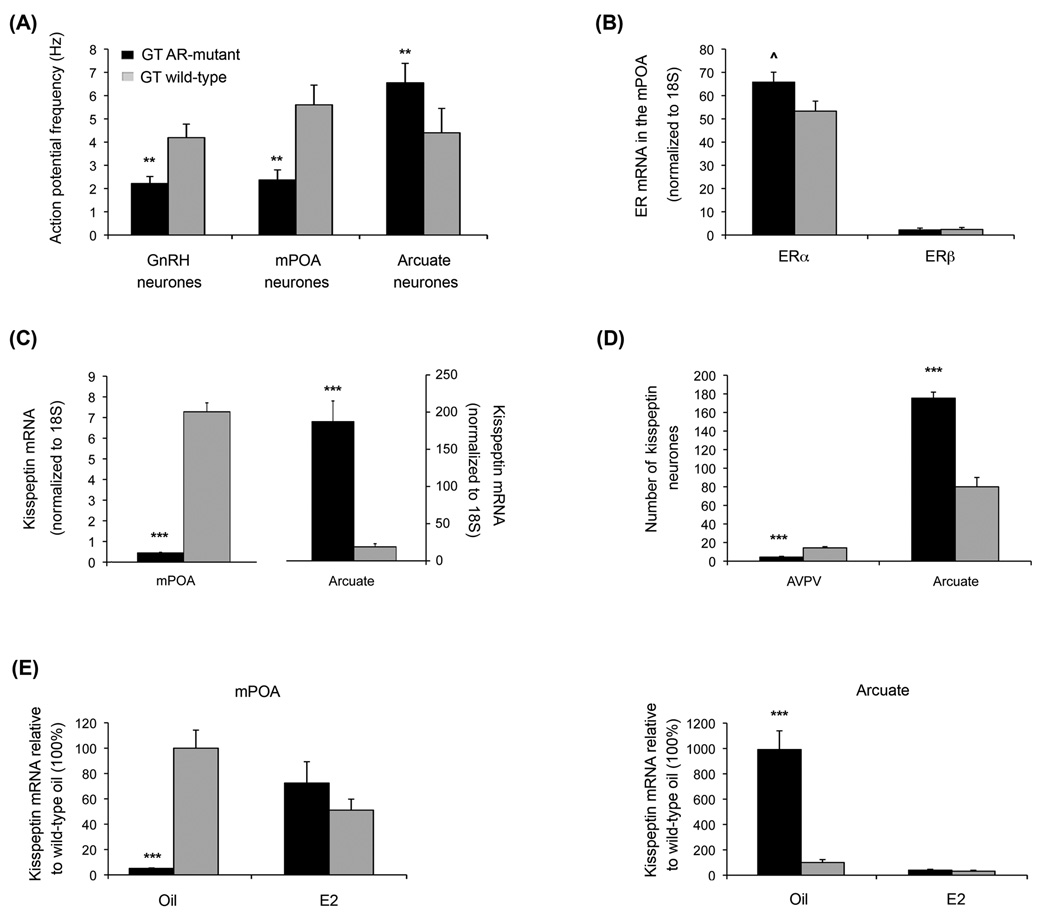
Figure 4.
Characteristics of Neuroendocrine Control Neurones in the GT wild type and GT AR-mutant mouse line. GT AR-mutant and wild type mice were identified by a TaqMan®SNP genotyping assay, which consisted of a common forward and reverse primer and two unique probes based on wild type versus mutant (single base deletion) sequences coupled with different fluorophores (forward primer: 5’-AACTTTCCGCTGGCTCTGT-3’; reverse primer: 5’-CTTGATACGGGCGTGTGGAT-3’; wild type probe: VIC – ACCCCCCGCCCCCT; mutant probe: 6FAM-CACCCCCGCCCCCT). (A) Average AP frequencies recorded in the on-cell configuration from GnRH, mPOA and AVPV neurones. ** Indicates values in wile type male mice were significantly different from AR-mutants with p < 0.01. (B) Relative levels of mRNA encoding ERα and ERβ in mPOA tissue from GT AR mutant and wild type male mice. ^ Indicates a trend that did not attain significance (p = 0.059) towards higher levels of ERα in AR-mutant than wild type male mice. (C) Steady state levels of kisspeptin mRNA were dramatically lower in the mPOA and dramatically elevated in the arcuate of GT AR-mutant versus wild type mice (*** indicates p < 0.001). (D) Immunohistochemical assessment with kisspeptin-10 antibody (Chemicon AB9754, 1:1000) demonstrated significant differences (*** indicates p < 0.001) in the numbers of immunopositive neurones within the 50 µm directly adjacent to the edge of the ventricle in the AVPV and in the arcuate that mirrored differences in mRNA levels for these two regions in GT AR-mutant versus wild type mice. (E) Treatment for two weeks of GT ARmutant male mice with 0.1 mg/kg 17β-oestradiol (E2) restored the pattern of kisspeptin mRNA expression in both the mPOA and the arcuate observed in wild type GT mice.
In gonadally-intact male mice treated for two weeks with 17β-oestradiol, no significant differences in kisspeptin mRNA were evident in either the mPOA or the arcuate between wild type and GT AR-mutant mice (Figure 4E), indicating that signalling via ER can regulate kisspeptin expression in the AR-mutant mice in a manner that recapitulates what has previously been shown for adult mice subject to castration, castrated mice subsequently treated with testosterone or 17β-oestradiol, and mice expressing a hypomorphic allele of the AR [136], as well as for neonatal Wistar rats castrated at birth and either treated or not treated with 17β-oestradiol as adults [137]. Moreover, while LH levels in GT AR-mutant mice were markedly elevated, as has been reported previously for the classical Tfm mutants [138, 139], treatment of GT AR-mutant mice with 17β-oestradiol restored peripheral LH levels to low concentrations (0.17 ± 0.03 ng/mL) comparable to those observed in wild type GT (0.58 ± 0.52 ng/mL) and other gonadally-intact male mice [134, 136, 140]. Taken together these data indicate that ER regulatory mechanisms are retained in this AR-deficient line and that this new mouse strain is a useful model to begin to parse out the role of AAS actions via alternative non-AR-mediated mechanisms that influence the regulation of GnRH neuronal function and the control of the HPG axis.
IV. Conclusions
Anabolic androgenic steroids have significant effects on the function of neurones that control reproductive function. There are both disparities and overlap in the actions of chronic AAS treatment between male and female mice and adolescent and adult mice, highlighting the complexity of actions of these steroids and how their effects may vary with sex, age and a plethora of other environmental and physiological factors yet to be determined. With respect to mechanisms, AAS have significant actions mediated by AR, but their effects are not limited to this classical mechanism. The ability of these compounds to allosterically modulate both GABAA receptors and aromatase suggests a much more expansive repertoire of “alternative” effects. It will be critical in future studies to determine the effects of these synthetic steroids on other ion channels expressed in the brain, as well as other key enzymes that govern steroid synthesis and metabolism in order to more fully understand the actions of these abused drugs on neural function.
Acknowledgments
This work was supported by the NIH (R01-DA18255, R01-DA14137 and T32-DK07508). Funds to purchase the Nikon A1RSi Confocal Workstation utilised in these studies were provided by the NSF (DBI-1039423) and Dartmouth College. The University of Virginia Ligand Assay Core Laboratory, from whom we contracted services, is supported by SCCPIR U54 HD28934 from the NIH.
References
- 1.Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- 2.Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- 3.Kochakian C, Yesalis CE. Anabolic-androgenic steroids: a historical perspective and definition. In: Yesalis CE, editor. Anabolic Steroids in Sport and Exercise. Champaign: Human Kinetics; 2000. pp. 4–33. [Google Scholar]
- 4.Llewellyn W. Body of Science. 6th Edition. Jupiter, FL: 2007. Anabolics; pp. vii–ix. [Google Scholar]
- 5.Trenton AJ, Currier GW. Behavioural manifestations of anabolic steroid use. CNS Drugs. 2005;19:571–595. doi: 10.2165/00023210-200519070-00002. [DOI] [PubMed] [Google Scholar]
- 6.Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98(1–2):1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu FC. Endocrine aspects of anabolic steroids. Clin Chem. 1997;43:1289–1292. [PubMed] [Google Scholar]
- 8.Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172–177. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- 9.Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J Forensic Sci. 2003;48(3):1–6. [PubMed] [Google Scholar]
- 10.Matsumoto AM, Bremner WJ. Serum testosterone assays—accuracy matters. J Clin Endocrinol Metab. 2004;89:520–524. doi: 10.1210/jc.2003-032175. [DOI] [PubMed] [Google Scholar]
- 11.Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem. 1997;43:1262–1279. [PubMed] [Google Scholar]
- 12.Bahrke MS, Yesalis CE, Kopstein AN, Stephens JA. Risk factors associated with anabolic-androgenic steroid use among adolescents. Sports Med. 2000;29:397–405. doi: 10.2165/00007256-200029060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Miller KE, Hoffman JH, Barnes GM, Sado D, Melnick MJ, Farrell MP. Adolescent anabolic steroid use, gender, physical activity, and other problem behaviors. Subst Use Misuse. 2005;40:1637–1657. doi: 10.1080/10826080500222727. [DOI] [PubMed] [Google Scholar]
- 14.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2008. Volume I: Secondary school students (NIH Publication No. 09–7402) Bethesda, MD: National Institute on Drug Abuse; 2009. p. 721. [Google Scholar]
- 15.Bahrke MS, Yesalis CE, Brower KJ. Anabolic-androgenic steroid abuse and performance-enhancing drugs among adolescents. Child Adolesc Psychiatr Clin N Am. 1998;7(4):821–838. [PubMed] [Google Scholar]
- 16.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 17.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 18.Sato SM, Schulz KM, Sisk CL, Wood RI. Adolescents and androgens, receptors and rewards. Horm Behav. 2008;53:647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field AE, Austin SB, Carmargo CA, Taylor CB. Stiegel-Moore RH, Loud KJ, Colditz GA. Exposure to mass media, body shape concerns, and use of supplements to improve weight and shape among male and female adolescents. Pediatrics. 2005;116:214–220. doi: 10.1542/peds.2004-2022. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor LH, Cicero TJ. Anabolic steroids: misuse or abuse? In: Schulkin J, editor. Hormonally Induced Changes in Mind and Brain. Orlando: Academic Press; 1993. pp. 129–156. [Google Scholar]
- 21.Gallaway S. The Steroid Bible. Third edition. Honolulu: Belle International; 1997. [Google Scholar]
- 22.Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS. Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse. Eur Psychiatry. 2006;21:551–562. doi: 10.1016/j.eurpsy.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Martini L. The 5α-reduction of testosterone in the neuroendocrine structures. Biochemical and physiological implications. Endocr Rev. 1982;3:1–25. doi: 10.1210/edrv-3-1-1. [DOI] [PubMed] [Google Scholar]
- 24.Winters SJ. Androgens: endocrine physiology and pharmacology. NIDA Res Monogr. 1990;102:113–130. [PubMed] [Google Scholar]
- 25.Ryan KJ. Biological aromatization of steroids. J Biol Chem. 1959;234:268–272. [PubMed] [Google Scholar]
- 26.Quincey RV, Gray CH. The metabolism of [1,2-3H]17α-methyltestosterone in human subjects. J Endocrinol. 1967;37:37–55. doi: 10.1677/joe.0.0370037. [DOI] [PubMed] [Google Scholar]
- 27.Papaconstantinou AD, Umbreit TH, Goering PL, Brown KM. Effects of 17α-methyltestosterone on uterine morphology and heat shock protein expression are mediated through estrogen and androgen receptors. J Steroid Biochem Mol Biol. 2002;82:305–314. doi: 10.1016/s0960-0760(02)00221-2. [DOI] [PubMed] [Google Scholar]
- 28.de Gooyer ME, Oppers-Tiemissen HM, Leysen D, Verheul HA, Kloosterboer HJ. Tibolone is not converted by human aromatase to 7α-methyl-17α-ethynylestradiol (7α-MEE): Analyses with sensitive bioassays for estrogens and androgens and with LC-MSMS. Steroids. 2003;68:235–243. doi: 10.1016/s0039-128x(02)00184-8. [DOI] [PubMed] [Google Scholar]
- 29.Saartok T, Dahlberg E, Gustafsson JA. Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114:2100–2106. doi: 10.1210/endo-114-6-2100. [DOI] [PubMed] [Google Scholar]
- 30.Roselli C. The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Res. 1998;792:271–276. doi: 10.1016/s0006-8993(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 31.Fragkaki AG, Angelis YS, Koupparis M, Tsantili-Kakoulidou A, Kokotos G, Georgakopoulos C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure. Steroid. 2009;74:172–197. doi: 10.1016/j.steroids.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Dimick DF, Heron M, Baulieu EE, Jayle MF. A comparative study of the metabolic fate of testosterone, 17α-methyltestosterone, 19-nor-testosterone, 17α-methyl-19-nor-tesosterone and 17α-methyl-estr-5(10)-ene-17β-ol-3-one in normal males. Clin Chim Acta. 1961;6:63–71. doi: 10.1016/0009-8981(61)90037-7. [DOI] [PubMed] [Google Scholar]
- 33.Schänzer W. Metabolism of anabolic androgenic steroids. Clin Chem. 1996;42(7):1001–1020. [PubMed] [Google Scholar]
- 34.Fragkaki AG, Angelis YS, Tsantili-Kakoulidou AG, Koupparis M, Georgakopoulos C. Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. J Ster Biochem Mol Biol. 2009;115:44–61. doi: 10.1016/j.jsbmb.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Whitney AC, Clark AS. Effects of acute stanozolol treatment on puberty in female rats. Biol Reprod. 2001;64:1460–1465. doi: 10.1095/biolreprod64.5.1460. [DOI] [PubMed] [Google Scholar]
- 36.Pinna G, Costa E, Guidotti Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA. 2005;102(6):2135–2140. doi: 10.1073/pnas.0409643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penatti CAA, Costine BA, Porter DM, Henderson LP. Effects of chronic exposure to an anabolic androgenic steroid cocktail on α5-receptor mediated GABAergic transmission and neural signaling in the forebrain of female mice. Neuroscience. 2009;161:526–537. doi: 10.1016/j.neuroscience.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurling-Kailanto S, Kankaanpää A, Hautaniemi J, Seppälä T. Blockade of androgen or estrogen receptors reduces nandrolone’s ability to modulate acute reward-related neurochemical effects of amphetamine in rat brain. Pharmacol Biochem Behav. 2010;95(4):422–427. doi: 10.1016/j.pbb.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology. 2006;147:3016–3026. doi: 10.1210/en.2005-1524. [DOI] [PubMed] [Google Scholar]
- 40.Attardi BJ, Page ST, Hild SA, Coss CC, Matsumoto AM. Mechanism of action of bolandiol (19-nortestosterone-3β,17β-diol), a unique anabolic steroid with androgenic, estrogenic, and progestational activities. J Steroid Biochem Mol Biol. 2010;118(3):151–161. doi: 10.1016/j.jsbmb.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masonis AET, McCarthy MP. Direct effects of the anabolic/androgenic steroids, stanozolol and 17α-methyltestosterone, on benzodiazepine binding to the GABAA receptor. Neurosci Lett. 1995;189:35–38. doi: 10.1016/0304-3940(95)11445-3. [DOI] [PubMed] [Google Scholar]
- 42.Masonis AET, McCarthy MP. Effects of androgenic/anabolic steroids stanozolol on GABAA receptor function: GABA stimulation of 36Cl− influx and 35S TBPS binding. J Pharmacol Exp Ther. 1996;279:186–193. [PubMed] [Google Scholar]
- 43.Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β,17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pak TR, Chung WCJ, Hinds LR, Handa RJ. Estrogen receptor-β mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148:3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 45.Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternative pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5α-androstane-3β,17β-diol in modulating oestrogen receptor β-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luzardo OP, Machín RP, Díaz-Chico BN, Fernández L. Photoaffinity labeling identification of a specific binding protein for the anabolic steroids stanozolol and danazol: an oligomeric protein regulated by age, pituitary hormones, and ethinyl estradiol. Endocrinology. 2000;141:3377–3387. doi: 10.1210/endo.141.9.7667. [DOI] [PubMed] [Google Scholar]
- 48.Clark AS, Jones BL, Yang P, Henderson LP. Anabolic androgenic steroids and the brain: novel actions at the GABAA receptor and on GABAA receptor mediated-behaviors. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor CRC Press LLC. 2004. pp. 119–141. [Google Scholar]
- 49.Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CAA, Porter DM, Yang P, Henderson LP. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126:122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 50.Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: Effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu S-F, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- 52.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Kudwa AE, Gustafsson J-Å, Rissman EF. Estrogen receptor β modulates estradiol induction of progestin receptor immunoreactivity in male, but not in female, mouse medial preoptic area. Endocrinology. 2004;145:4500–4506. doi: 10.1210/en.2003-1708. [DOI] [PubMed] [Google Scholar]
- 54.Brussaard AB, Wossink J, Lodder JC, Kits KS. Progesterone-metabolite prevents protein kinase C-dependent modulation of γ-aminobutyric acid type A receptors in oxytocin neurons. Proceedings of the National Academy of Science USA. 2000;97:3625–3630. doi: 10.1073/pnas.050424697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasker J. Coregulation of ion channels by neurosteroids and phosphorylation. Science’s STKE. 2000 doi: 10.1126/stke.2000.59.pe1. www.stke.org/cgi/content/full/OC_sigtrans:2000/59/pe1. [DOI] [PubMed]
- 56.Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koksma JJ, van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Lüddens H, Brussaard AB. Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. J Neurosci. 2003;23:788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Widmer H, Ludwig M, Bancel F, Leng G, Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J Physiol (Lond) 2003;48(1):233–244. doi: 10.1113/jphysiol.2002.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruber AJ, Pope HG., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69:19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 60.Clark AS. Henderson LP Behavioral and physiological responses to anabolic androgenic steroids. Neurosci Biobehav Revs. 2003;27:413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 61.Lukas SE. Current perspectives on anabolic-androgenic steroid abuse. Tends Pharmacol Sci. 1993;14:61–68. doi: 10.1016/0165-6147(93)90032-f. [DOI] [PubMed] [Google Scholar]
- 62.Kam PCA, Yarrow M. Anabolic steroid abuse: physiological and anaesthetic considerations. Anaesthesia. 2005;60:685–692. doi: 10.1111/j.1365-2044.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 63.Strauss RH, Liggett MT, Lanese RR. Anabolic steroid use and perceived effects in ten weight-trained women athletes. JAMA. 1985;253:2871–2873. [PubMed] [Google Scholar]
- 64.Elliot DL, Goldberg L. Women and anabolic steroids. In: Yesalis CE, editor. Anabolic Steroids in Sport and Exercise. Champaign: Human Kinetics; 2000. pp. 225–246. [Google Scholar]
- 65.Kraulis I, Naish SJ, Gravenor D, Ruf KB. 5α-androstane-3α,17β diol: inhibitor of sexual maturation in the female rat. Biol. Reprod. 1981;24:445–453. doi: 10.1095/biolreprod24.2.445. [DOI] [PubMed] [Google Scholar]
- 66.Kramer P, Meijs-Roelofs HMA. Retardation of first ovulation in pubertal rats after treatment with 5α-androstane-3α,17β-diol of its 3β-epimer. J Endocrinol. 1982;92:31–35. doi: 10.1677/joe.0.0920031. [DOI] [PubMed] [Google Scholar]
- 67.McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABAA receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43(4):634–645. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 68.Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: A neuroendocrine and behavioral assessment. Pharmacol Biochem Behav. 2006;83(3):410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Penatti CAA, Davis MC, Porter DM, Henderson LP. Altered GABAA receptor-mediated synaptic transmission disrupts the firing of gonadotropin-releasing hormone neurons in male mice under conditions of steroid abuse. J Neurosci. 2010;30(19):6497–6506. doi: 10.1523/JNEUROSCI.5383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark AS, Kelton MC, Whitney AC. Chronic administration of anabolic steroids disrupts pubertal onset and estrous cyclicity in rats. Biol Reprod. 2003;68:465–471. doi: 10.1095/biolreprod.102.008078. [DOI] [PubMed] [Google Scholar]
- 71.Penatti CAA, Oberlander JG, Davis MC, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.05.008. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bronson FH. Effects of prolonged exposure to anabolic steroid on the behavior of male and female mice. Pharmacol Biochem Behav. 1996;53:329–334. doi: 10.1016/0091-3057(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 73.Clark AS, Harrold EV, Fast AS. Anabolic-androgenic steroid effects on the sexual behavior of intact male rats. Horm Behav. 1997;31:35–46. doi: 10.1006/hbeh.1997.1355. [DOI] [PubMed] [Google Scholar]
- 74.Bronson FH, Nguyen KQ, De La Rosa J. Effect of anabolic steroids on behavior and physiological characteristics of female mice. Physiol Behav. 1996;59:49–55. doi: 10.1016/0031-9384(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 75.Blasberg ME, Clark AS. Anabolic-androgenic steroid effects on sexual receptivity in ovariectomized rats. Horm Behav. 1997;32:201–208. doi: 10.1006/hbeh.1997.1422. [DOI] [PubMed] [Google Scholar]
- 76.Salas-Ramirez KY, Montalto PR, Sisk CL. Anabolic androgenic steroids differentially affect social behaviors in adolescent and adult male Syrian hamsters. Horm Behav. 2008;53:378–385. doi: 10.1016/j.yhbeh.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 78.Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Orlando: Academic Press; 2002. pp. 139–213. [Google Scholar]
- 79.Hull EM, Meisel RL, Sachs BD. Male sexual Behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and behavior. Vol. 1. Orlando: Academic Press; 2002. pp. 3–137. [Google Scholar]
- 80.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent A-S, Matagne V, Mungenast AE. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 81.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RPV3) Brain Res Revs. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin releasing hormone surges. Endocrine Revs. 2010;31(4):544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones BL, Whiting PJ, Henderson LP. Mechanisms of anabolic androgenic steroid inhibition of mammalian ε-subunit-containing GABAA receptors. J Physiol (Lond) 2006;573(3):571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penatti CAA, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters neuronal function in the mammalian forebrain via androgen receptor- and estrogen receptor-mediated mechanisms. J Neurosci. 2009;29(40):12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Penatti CAA, Porter DM, Jones BL, Henderson LP. Sex-specific effects of chronic anabolic androgenic steroid treatment on GABAA receptor expression and function in adolescent mice. Neuroscience. 2005;135:533–543. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 86.Yang P, Jones BL, Henderson LP. Mechanisms of anabolic androgenic steroid modulation of α1β3γ2L GABAA receptors. Neuropharmacology. 2002;43:619–633. doi: 10.1016/s0028-3908(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 87.Yang P, Jones BL, Henderson LP. Role of the α subunit in the modulation of GABAA receptors by anabolic androgenic steroids. Neuropharmacolog. 2005;49:300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 88.Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- 89.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fritschy J-M, Möhler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 91.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptor immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 92.Davis AM, Penschuck S, Fritschy J-M, McCarthy MM. Developmental switch in the expression of GABAA receptor subunits α1 and α2 in the hypothalamus and limbic system of the rat. Dev Brain Res. 2000;119:127–138. doi: 10.1016/s0165-3806(99)00150-9. [DOI] [PubMed] [Google Scholar]
- 93.Nett ST, Jorge-Rivera J-C, Myers M, Clark AS, Henderson LP. Properties and sex-specific differences of GABAA receptors in neurons expressing γ1 subunit mRNA in the preoptic area of the rat. J Neurophysiol. 1999;81:192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- 94.Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 95.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- 96.Thompson SA, Bonnert TP, Cagetti E, Whiting PJ, Wafford KA. Overexpression of the GABAA receptor ε results in insensitivity to anaesthetics. Neuropharmacology. 2002;43:662–668. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 97.Jones BL, Henderson LP. Trafficking and potential assembly patterns of ε-containing GABAA receptors. J Neurochem. 2007;103(3):1258–1271. doi: 10.1111/j.1471-4159.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- 98.Moragues N, Ciofi P, Lafon P, Odessa MF, Tramu G, Garret M. cDNA cloning and expression of a γ-aminobutyric acid A receptor ε-subunit in rat brain. Eur J Neurosci. 2000;12:4318–4330. [PubMed] [Google Scholar]
- 99.Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor ε subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967:285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- 100.Moragues N, Cioffi P, Tramu G, Garret M. Localisation of GABAA receptor ε-subunit in cholinergic and aminergic neruones and evidence for co-distribution with the θ-subunit in rat brain. Neuroscience. 2002;111:657–669. doi: 10.1016/s0306-4522(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 101.Sinkkonen ST, Hanna MC, Kirkness EF, Korpi ER. GABAA receptor ε and θ subunits display unusual structural variation between species and are enriched in the rat locus ceruleus. J Neurosci. 2000;20:3588–3595. doi: 10.1523/JNEUROSCI.20-10-03588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 103.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanism of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 105.Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 106.Belleli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Revs. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 107.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. ensitization on fast synaptic transmission. Trends Neurosci 1996; 19: 96–101. [DOI] [PubMed] [Google Scholar]
- 108.Vicini S. THDOC and the GABAA receptor. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor CRC Press LLC. 2004. pp. 63–75. [Google Scholar]
- 109.Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 110.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related Behavior and alters the sensitivity of cortical GABAA receptors in the rat. Hormones and Behavio. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 112.Ågren G, Thiblin I, Tirassa P, Lundeberg T, Stenfors C. Behavioural anxiolytic effects of low-dose anabolic androgenic steroid treatment in rats. Physiol Behav. 1999;66:503–509. doi: 10.1016/s0031-9384(98)00323-0. [DOI] [PubMed] [Google Scholar]
- 113.Aikey JL, Nyby JG, Anmuth, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 114.Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- 115.Bahrke MS, Yesalis CE, Wright JE. Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids. Sports Med. 1996;22:367–390. doi: 10.2165/00007256-199622060-00005. [DOI] [PubMed] [Google Scholar]
- 116.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270(2):209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 117.Hutton LA, Gu G, Simerly RB. Development of a sexually dimorphic projection from the bed nucleus of the stria terminalis to the anteroventral periventricular nucleus in the rat. J Neurosci. 1998;18:3003–3013. doi: 10.1523/JNEUROSCI.18-08-03003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scott CJ, Tilbrook AJ, Rawson JA, Clarke IJ. Gonadal steroid receptors in the regulation of GnRH secretion in farm animals. Anim Reprod Sci. 2000;60–61:313–326. doi: 10.1016/s0378-4320(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 119.Grattan DR, Jasoni CL, Liu X, Anderson GM, Herbison AE. Prolactin regulation of gonadotropin-releasing hormone neurons to suppress luteinizing hormone secretion in mice. Endocrinology. 2007;148(9):4344–4351. doi: 10.1210/en.2007-0403. [DOI] [PubMed] [Google Scholar]
- 120.DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(10):2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 121.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- 122.Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- 123.Moenter SM, DeFazio RA. Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- 124.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M. Activation of A-type γ-aminobutyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol. 2008;20:566–575. doi: 10.1111/j.1365-2826.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 126.Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci. 2009;29:9809–9818. doi: 10.1523/JNEUROSCI.2509-09.2009. 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 128.Nomura M, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor β (ERβ) protein levels in neurons depend on estrogen receptor α (ERα) gene expression and on its ligand in a brain region-specific manner. Brain Res Mol Brain Res. 2003;110:7–14. doi: 10.1016/s0169-328x(02)00544-2. [DOI] [PubMed] [Google Scholar]
- 129.Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 1995;280:561–574. doi: 10.1007/BF00318360. [DOI] [PubMed] [Google Scholar]
- 130.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44:321–326. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 131.Mor G, Eliza M, Song J, Wiita B, Chen S, Naftolin F. 17α-Methyltestosterone is a competitive inhibitor of aromatase activity in Jar choriocarcinoma cells and macrophage-like THP-1 cells in culture. J Steroid Biochem Mol Biol. 2001;79:239–246. doi: 10.1016/s0960-0760(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 132.Hong Y, Cho M, Yuan YC, Chen S. Molecular basis for the interaction of four different classes of substrates and inhibitors with human aromatase. Biochem Pharmacol. 2008;75:1161–1169. doi: 10.1016/j.bcp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:2035–2040. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 134.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107(52):22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor α and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- 136.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 137.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testosterone sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81:1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 138.Murphy L, O’Shaughnessy PJ. Testicular steroidogenesis in the testicular feminized (Tfm) mouse: loss of 17α-hydroxylase activity. J Endocrinol. 1991;131:443–449. doi: 10.1677/joe.0.1310443. [DOI] [PubMed] [Google Scholar]
- 139.Scott IS, Bennett MK, Porter-Goff AE, Harrison CJ, Grocock CA, Scott IS. O'Shaughnessy PJ, Clayton RN, Craven R, Furr BJA, Charlton HM. Effects of the gonadotrophin-releasing hormone agonist 'Zoladex' upon pituitary and gonadal function in hypogonadal (hpg) male mice: a comparison with normal male and testicular feminized (tfm) mice. J Mol Endocrinol. 1992;8(3):249–258. doi: 10.1677/jme.0.0080249. [DOI] [PubMed] [Google Scholar]
- 140.Goulding EH, Hewitt SC, Nakamura N, Hamilton K, Korach KS, Eddy EM. Ex3αERKO male infertility phenotype recapitulates the αERKO male phenotype. J Endocrinol. 2010;207:281–288. doi: 10.1677/JOE-10-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]