Abstract
Delirium is a frequent form of acute brain dysfunction in patients who are critically ill and is associated with poor clinical outcomes, including a critical illness brain injury that may last for months to years. Despite widespread recognition of significant adverse outcomes, pharmacologic approaches to prevent or treat delirium during critical illness remain unproven. We hypothesize that commonly prescribed statin medications may prevent and treat delirium by targeting molecular pathways of inflammation (peripheral and central) and microglial activation that are central to the pathogenesis of delirium. Systemic inflammation, a principal mechanism of injury, for example, in sepsis, acute respiratory distress syndrome, and other critical illnesses, can cause neuronal apoptosis, blood-brain barrier injury, brain ischemia, and microglial activation. We hypothesize that the known pleiotropic effects of statins, which attenuate such neuroinflammation, may redirect microglial activation and promote an antiinflammatory phenotype, thereby offering the potential to reduce the public health burden of delirium and its associated long-term cognitive injury.
Delirium is a manifestation of acute brain dysfunction that occurs in up to 80% of patients who are critically ill and is associated with higher mortality and long-term cognitive impairment (LTCI), which is akin to a dementia-like cognitive disability.1‐7 This acquired cognitive impairment—critical illness brain injury—has important public health implications for both younger and older patients (the latter an increasingly larger proportion of the population), threatening the functional independence and quality of life of millions of ICU survivors in the coming decades. Given that delirium in the ICU represents early brain dysfunction during critical illness and can be easily assessed using validated bedside instruments,1,8 novel therapies that prevent or treat delirium may prevent its associated immediate and long-term sequelae.
Findings from animal and human studies suggest a neuroinflammatory pathogenesis of delirium and long-term brain dysfunction associated with critical illness. We propose a testable hypothesis, based on existing data, that the pleiotropic effects of statin medications can mitigate the mechanisms of delirium and LTCI associated with critical illness. Specifically, that statins may modify two processes leading to brain injury: neuroinflammation and activation of proinflammatory microglia.
Effects on Neuroinflammation During Critical Illness
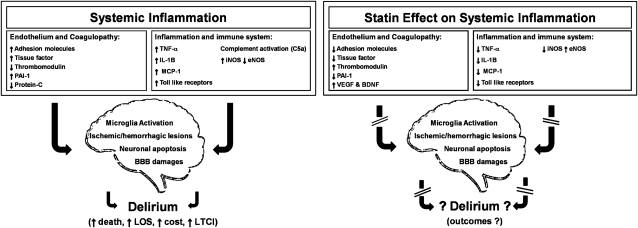
An intense systemic inflammatory response to illness or injury is a key mediator of organ dysfunction during critical illness. Several conditions that often lead to an ICU admission are examples of the deleterious effects of systemic inflammation (eg, severe sepsis, trauma, acute respiratory distress syndrome). Proinflammatory cytokines (eg, tumor necrosis factor [TNF]-α and IL-1β) and chemokines (eg, monocytic chemoattractant protein [MCP]-1) activate leukocytes and endothelial cells (which express leukocyte adhesion molecules), resulting in endothelial damage and tissue factor expression that initiate a procoagulant cascade, ultimately leading to microvascular thrombosis, impaired blood flow, and end-organ damage (Fig 1). In addition, cytokines trigger production of inducible nitric oxide synthase (iNOS), which causes nitric oxide-mediated hypotension, further inflammation, and apoptosis. Ultimately, the characteristic inflammatory state of critical illness causes multiple mechanisms of injury in the brain, including vascular damage, ischemia, breakdown of the blood-brain barrier (BBB), local neuroinflammation, and apoptosis, all of which are observed, for example, in animal models of sepsis and in humans with sepsis-associated delirium.9,10 In addition to directly injuring neurons, this neuroinflammation activates quiescent microglia, the resident macrophages in the brain (Fig 1),10‐12 a process that van Gool and colleagues13 proposed as pivotal to CNS damage from systemic inflammation. Microglia are usually activated to clear apoptotic cells resulting from an injury.14 Their overactivation, however, can be responsible for an exaggerated inflammatory response.14 Van Gool and colleagues,13 in fact, postulated that impaired cholinergic inhibition of microglia is responsible for overactivation of microglia that can persist for months following critical illness, contributing to ongoing neuroinflammation, with resultant neurodegeneration manifesting as severe prolonged delirium and LTCI.
Figure 1.
The systemic inflammatory cascade, effects of delirium, and sites of action for statins in critical illness. The systemic inflammatory cascade in critical illness is one of the main drivers of delirium. Different clinical conditions that often lead to an ICU admission could be used as examples of systemic inflammation (eg, severe sepsis, acute respiratory distress syndrome). This figure describes the principal mechanisms responsible for brain injury in critical illness. Consequently, we show the proven pleiotropic effect of statins on the systemic inflammatory cascade, representing the basis by which statins may reduce delirium and its long-term neurologic sequelae in ICU survivors. BBB = blood-brain barrier; BDNF = brain-derived neurotrophic factor; eNOS = endothelial nitric oxide synthase; iNOS = inducible nitric oxide synthase; LOS = length of stay; LTCI = long-term cognitive impairment; MCP = monocytic chemoattractant protein; PAI = plasminogen activator inhibitor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
In vitro and human studies have shown that, in addition to their effect on cholesterol synthesis, statins have complex pleiotropic effects, including antiinflammatory, immunomodulatory, endothelial function-enhancing, and anticoagulant effects.15 These pleiotropic effects may prevent or attenuate delirium during critical illness by acting on causative mechanisms, including neuroinflammation, BBB injury, neuronal apoptosis, ischemia and hemorrhage, and microglial activation (Fig 1).9,10 Specifically, in vitro and animal studies have shown that statins suppress upregulation of toll-like receptors (which trigger inflammation in response to infection) and reduce the release of TNF-α, IL-1β, and MCP-1 as well as leukocyte adhesion molecules involved in the development of endothelial damage and BBB alterations.15,16 Statins also reduce iNOS expression, thereby reducing neuronal apoptosis and increasing BP and cerebral blood flow, and they increase endothelial nitric oxide synthase (eNOS) expression, preserving microcirculatory blood flow via local vasodilation.17 Lastly, statins counteract the procoagulant cascade promoted by inflammation through the following mechanisms: blunting monocytic expression of tissue factor, increasing thrombomodulin availability (important in the activation of protein C), and reducing levels of plasminogen activator inhibitor-1, which impairs the fibrinolytic system.15
Though no studies to date have evaluated the effect of statins on delirium in patients in the ICU, this drug class has been examined in models of traumatic brain injury (TBI),18 which involves pathophysiologic changes (eg, neuronal damage and apoptosis, neuroinflammation, and BBB injury) similar to those observed in other types of critical illness, including sepsis and acute respiratory distress syndrome. The benefits of statins observed in animal studies of TBI include increased hippocampal neuron survival and improved neurologic function.18‐22 In humans, one clinical trial reported a reduction in amnesia and increased orientation in patients with TBI who were treated with rosuvastatin.23 Studies investigating the effect of statins on patients with postoperative delirium, a population with different clinical profiles than patients in the ICU, have yielded inconsistent results.24,25 One retrospective study25 reported an increased risk of postoperative delirium for patients who had elective surgery and were taking statins, whereas a prospective study24 found a significant reduction in postoperative delirium for patients who had cardiac surgery and were taking statins. Well-designed, randomized, placebo-controlled trials are required to determine the true effect of statins on delirium during critical illness.
Effects on Microglial Activation and Phenotype Switching
The effects of acute systemic inflammation on delirium might be explained through the activation of primed microglia.13,26,27 Perry and colleagues28 described how microglia in the brain can already be activated as a result of an ongoing brain pathology (eg, Alzheimer disease, Parkinson disease, or prion disease) or aging. These microglia are named primed microglia, and their stimulation by central or systemic challenges (eg, an infection) can lead to exaggerated and long-lasting inflammatory responses compared with those of subjects who have unprimed microglia. In fact, van Gool et al13 hypothesized that a cholinergic impairment from systemic inflammation may cause uncontrolled activation of brain microglia that can last for months, especially in patients with primed microglia, and can eventually lead to or worsen neurodegeneration. In a model of prion disease, lipopolysaccharide (LPS) exposure led to activation of microglia, with expression of IL-1β, IL-6, TNF-α, and iNOS, and eventual neuronal death.26
While the hypothesis of impaired cholinergic inhibitory control of the brain microglia still needs to be proven, the cholinergic effects on peripheral macrophages have been well described in animal models using the term “inflammatory reflex.”29,30 It has been reported that the release of acetylcholine through vagal nerve stimulation in response to endotoxin exposure suppresses proinflammatory cytokine release (eg, IL-6, TNF-a, IL-1, IL-18) without affecting the production of the antiinflammatory cytokine IL-10.29 Additionally, in vitro studies have shown that microglia express acetylcholine receptors, in particular the nicotinic receptor α7, supporting the hypothesis that a central antiinflammatory cholinergic pathway may limit the response of microglia in the periphery via release of acetylcholine by neurons.31,32
Systemic inflammation can influence acute and chronic microglial activation, promoting the proinflammatory rather than the antiinflammatory phenotype.26,27 In fact, exposure to LPS has been shown in a rat model of Parkinson disease to shift the primed microglia to a proinflammatory phenotype with increased secretion of IL-1β.27 Additionally, a peripheral infection in animal models of prion disease with primed microglia led to a switching to a proinflammatory phenotype.33 Hughes and colleagues,34 however, raised the question of whether microglia activated by LPS actually led to an enhanced inflammatory state. In this study34 conducted on microglia in animals with prion disease, it was found that microglia engaged in phagocytosis of apoptotic cells remain in an antiinflammatory state, at least with regard to the lack of production of the proinflammatory IL-1B, when exposed to LPS. These data suggest that a phagocytic state does not necessarily imply the production of inflammatory mediators by microglia.
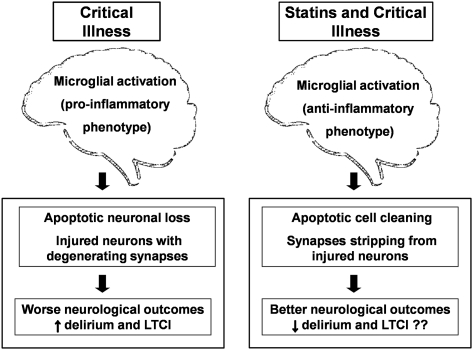
Statins may counteract the inflammation-induced action of proinflammatory-phenotype microglial activation during critical illness. Their actions favor a switch toward antiinflammatory phenotypes that may contribute to neuronal healing rather than damage (Fig 2), a process observed in studies of animal models and cultured mouse microglial cells. Li et al35 reported that mice treated with simvastatin had significantly fewer activated microglia after TBI than mice treated with placebo. Famer and colleagues36 reported a significant reduction in microglia activation in animal models treated with rosuvastatin. Similarly, Townsend et al37 found that lovastatin significantly reduced CD40 expression (a marker of microglial activation) in primary culture microglial cells by indirectly blocking the expression of proinflammatory mediators. In addition, lovastatin significantly increased microglial phagocytic function, an indicator of the antiinflammatory phenotype and a process inhibited by CD40 activation.
Figure 2.
The microglia phenotypes, effects of delirium, and hypothesized mechanism of action of statins in critical illness. A systemic inflammatory cascade caused by infection (eg, sepsis) or by other critical illnesses (eg, acute respiratory distress syndrome) may result in a microglial proinflammatory phenotype potentially leading to worse neurologic outcomes as manifested by delirium and LTCI. The pleiotropic effect of statins might redirect the microglia to an antiinflammatory phenotype, activating mechanisms responsible for brain protection and therefore possibly leading to better immediate and long-term neurologic outcomes for survivors of critical illness. See Figure 1 legend for expansion of the abbreviation.
Microglial activation leads to the induction of iNOS, a deleterious component of the inflammatory cascade involved in neuronal damage.38 Statins have been shown to reduce the production of iNOS from activated microglial cells and macrophages.39,40 Additionally, lovastatin was also shown to significantly reduce prostaglandin E2 release from microglia, either under basal conditions or after stimulation by IL-1B, in primary cultures of rat cortical microglia.41 Statins have also been shown in rats and human microglia to reduce the production of the proinflammatory cytokine IL-6.36,42
Circulating cytokines released as the result of an inflammatory response can cross the BBB and activate quiescent microglia or cause an exaggerated inflammatory response in primed microglia. Statins can also counteract the deleterious effects associated with microglial activation through their effects on the peripheral inflammatory status, as described in the first section of this article.
Thus, statins might redirect the pathophysiologic response of the CNS to inflammation during critical illness, promoting an antiinflammatory response, enhancing apoptotic cell cleaning and synapse stripping, and leading to a reduction in delirium and LTCI. Additionally, statins can reduce the immediate increase in neuroinflammation secondary to activation of quiescent and primed microglia. This hypothesis could be tested in animal models of sepsis, correlating the biologic findings of microglial switching with behavioral assessments indicative of delirium.
Effects of Statins and Clinical Outcomes
In clinical trials, statins given late in life have not prevented or delayed the onset of dementia,43 but these results do not preclude a beneficial effect of statins on delirium or LTCI due to critical illness. The use of statins during an immediate inflammatory response, as witnessed in patients who were critically ill, might have different consequences than the use of statins on the low-grade chronic inflammation related to dementia.
Importantly, animal and human studies have also shown that abrupt discontinuation of statins can lead to an acute rebound inflammation and worsening of clinical outcomes.44‐48 Animal studies have demonstrated that short-term withdrawal of statin therapy leads to suppressed eNOS production, elevated oxygen free-radical production, and increased endothelial dysfunction as soon as 2 days after discontinuation.49,50 These changes supersede the beneficial effects of statin therapy on platelet function and neuronal cell protection.46,51
A proinflammatory rebound is reported within 5 days of statins interruption in patients with myocardial infarction.44 The observed proinflammatory state was found to be threefold higher in those patients than in patients not receiving statin therapy before or during hospitalization.44 Demonstrating its importance, inflammation after myocardial infarction has been associated with ventricular dysfunction and sudden death up to 2 years after the initial event.52,53 Heeschen et al47 found an increased cardiac risk in patients who were long-term statin users and who were admitted for acute coronary syndromes in which statins were withdrawn, abrogating the beneficial effect of these drugs on the clinical outcomes. A large case-control study reported that statin withdrawal (within 30 days) led to a twofold increase in the risk of subarachnoid hemorrhage.48 Finally, a randomized clinical trial tested the effects of statin withdrawal during the first 3 days of admission on clinical outcomes in patients admitted for acute stroke.45 Patients for whom statins were withdrawn had a significant 8.67-fold increase in the risk of neurologic deterioration and a 4.66-fold increase in the combined risk of functional dependency and death.45
Observational studies are, therefore, warranted to examine whether the continuation vs discontinuation of statins during critical illness alters inflammatory biomarkers and the course of delirium and, subsequently, the development of LTCI. Additionally, if the results of observational studies are promising, randomized, placebo-controlled trials could investigate the efficacy of statins initiated early during an ICU stay for the prevention or treatment of delirium and the related neurocognitive sequelae coupled with standard clinical outcomes. Because differential effects on neuroinflammation during critical illness might result from treatment with lipophilic vs hydrophilic statins, both types of drugs should be tested in clinical trials. The safety profile of drugs administered during critical illness is always a concern because of alterations in kidney and liver function and other factors predisposing patients to adverse reactions; statins, fortunately, are generally safe, resulting in a very low incidence of myopathy (0.01%) and liver enzyme abnormalities (0.1%) at standard doses.54 Also, an intervention intended to prevent or treat delirium in patients in the ICU needs to work quickly (over hours rather than days or weeks). Animal models of TBI have shown that statins produce pleiotropic effects within a few hours of administration, making them attractive agents for study during critical illness.20 Finally, the effects of statins on the mechanisms of neuronal injury during critical illness can be studied using anatomic and functional neuroimaging to examine brain volumes and functional activation to help understand whether statins promote switching from microglia activation to an antiinflammatory phenotype with reduction in brain atrophy and preservation of brain function.37,55 In conclusion, statins are ideal candidates to investigate in the hope of mitigating the rapidly growing public health problem of ICU delirium and the acquisition of long-term critical illness brain injury affecting thousands of survivors of critical illness annually.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Girard has received honoraria from Hospira Inc. Dr McAuley has received consultant fees and served on advisory boards for GlaxoSmithKline for acute lung injury and has received lecture fees for organized meetings from AstraZeneca. Dr Ely has received honoraria from GlaxoSmithKline, Pfizer, Lilly, Hospira, Cumberland, and Aspect. Drs Morandi, Hughes, and Pandharipande have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- BBB
blood-brain barrier
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- LTCI
long-term cognitive impairment
- MCP
monocytic chemoattractant protein
- TBI
traumatic brain injury
- TNF
tumor necrosis factor
Footnotes
Funding/Support: Dr Hughes is supported by the Foundation for Anesthesia Education and Research Mentored Research Training Grant. Dr Girard is supported by the National Institutes of Health [Grant AG034257]. Dr McAuley is supported by the Northern Ireland Public Health Agency Research and Development Division Translational Research Group for Critical Care. Dr Ely is supported by the US Department of Veterans Affairs Clinical Science Research and Development Service [Merit Review Award] and the National Institutes of Health [Grant AG027472]. Drs Ely and Girard are both supported by the US Department of Veterans Affairs Tennessee Valley Geriatric Research, Education, and Clinical Center. Dr Pandharipande is supported by the US Department of Veterans Affairs Clinical Science Research and Development Service [Career Development Award].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 5.MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 6.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW, SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) Study Group Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 9.Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33(6):941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- 10.Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119(6):737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 12.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 13.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 14.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7(5):358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 16.Niessner A, Steiner S, Speidl WS, et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. 2006;189(2):408–413. doi: 10.1016/j.atherosclerosis.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 17.McGown CC, Brown NJ, Hellewell PG, Reilly CS, Brookes ZL. Beneficial microvascular and anti-inflammatory effects of pravastatin during sepsis involve nitric oxide synthase III. Br J Anaesth. 2010;104(2):183–190. doi: 10.1093/bja/aep361. [DOI] [PubMed] [Google Scholar]
- 18.Wible EF, Laskowitz DT. Statins in traumatic brain injury. Neurotherapeutics. 2010;7(1):62–73. doi: 10.1016/j.nurt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu D, Goussev A, Chen J, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21(1):21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Lynch JR, Song P, et al. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206(1):59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Mahmood A, Goussev A, et al. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004;101(5):813–821. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Qu C, Goussev A, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24(7):1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapia-Perez JH, Sanchez-Aguilar M, Torres-Corzo JG, et al. Effect of rosuvastatin on amnesia and disorientation after traumatic brain injury (NCT003229758) J Neurotrauma. 2008;25(8):1011–1017. doi: 10.1089/neu.2008.0554. [DOI] [PubMed] [Google Scholar]
- 24.Katznelson R, Djaiani GN, Borger MA, et al. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology. 2009;110(1):67–73. doi: 10.1097/ALN.0b013e318190b4d9. [DOI] [PubMed] [Google Scholar]
- 25.Redelmeier DA, Thiruchelvam D, Daneman N. Delirium after elective surgery among elderly patients taking statins. CMAJ. 2008;179(7):645–652. doi: 10.1503/cmaj.080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain. 2008;131(pt 7):1880–1894. [Google Scholar]
- 28.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 29.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 30.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 31.De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2(1):4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shytle RD, Mori T, Townsend K, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89(2):337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 33.Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112(1):7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 34.Hughes MM, Field RH, Perry VH, Murray CL, Cunningham C. Microglia in the degenerating brain are capable of phagocytosis of beads and of apoptotic cells, but do not efficiently remove PrPSc, even upon LPS stimulation. Glia. 2010;58(16):2017–2030. doi: 10.1002/glia.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Mahmood A, Lu D, et al. Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin-1beta level after traumatic brain injury. Neurosurgery. 2009;65(1):179–185. doi: 10.1227/01.NEU.0000346272.76537.DC. discussion 185-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Famer D, Wahlund LO, Crisby M. Rosuvastatin reduces microglia in the brain of wild type and ApoE knockout mice on a high cholesterol diet; implications for prevention of stroke and AD. Biochem Biophys Res Commun. 2010;402(2):367–372. doi: 10.1016/j.bbrc.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Townsend KP, Shytle DR, Bai Y, et al. Lovastatin modulation of microglial activation via suppression of functional CD40 expression. J Neurosci Res. 2004;78(2):167–176. doi: 10.1002/jnr.20234. [DOI] [PubMed] [Google Scholar]
- 38.Weldon DT, Rogers SD, Ghilardi JR, et al. Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci. 1998;18(6):2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordle A, Landreth G. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25(2):299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang KC, Chen CW, Chen JC, Lin WW. HMG-CoA reductase inhibitors inhibit inducible nitric oxide synthase gene expression in macrophages. J Biomed Sci. 2003;10(4):396–405. doi: 10.1007/BF02256431. [DOI] [PubMed] [Google Scholar]
- 41.Tringali G, Vairano M, Dello Russo C, Preziosi P, Navarra P. Lovastatin and mevastatin reduce basal and cytokine-stimulated production of prostaglandins from rat microglial cells in vitro: Evidence for a mechanism unrelated to the inhibition of hydroxy-methyl-glutaryl CoA reductase. Neurosci Lett. 2004;354(2):107–110. doi: 10.1016/j.neulet.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 42.Lindberg C, Crisby M, Winblad B, Schultzberg M. Effects of statins on microglia. J Neurosci Res. 2005;82(1):10–19. doi: 10.1002/jnr.20615. [DOI] [PubMed] [Google Scholar]
- 43.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2009;(2):CD003160. doi: 10.1002/14651858.CD003160.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Sposito AC, Carvalho LS, Cintra RM, et al. Brasilia Heart Study Group Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis. 2009;207(1):191–194. doi: 10.1016/j.atherosclerosis.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Blanco M, Nombela F, Castellanos M, et al. Statin treatment withdrawal in ischemic stroke: A controlled randomized study. Neurology. 2007;69(9):904–910. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 46.Gertz K, Laufs U, Lindauer U, et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34(2):551–557. doi: 10.1161/01.str.0000054055.28435.bf. [DOI] [PubMed] [Google Scholar]
- 47.Heeschen C, Hamm CW, Laufs U, Snapinn S, Böhm M, White HD. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Investigators Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105(12):1446–1452. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 48.Risselada R, Straatman H, van Kooten F, et al. Withdrawal of statins and risk of subarachnoid hemorrhage. Stroke. 2009;40(8):2887–2892. doi: 10.1161/STROKEAHA.109.552760. [DOI] [PubMed] [Google Scholar]
- 49.Laufs U, Endres M, Custodis F, et al. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102(25):3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 50.Vecchione C, Brandes RP. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res. 2002;91(2):173–179. doi: 10.1161/01.res.0000028004.76218.b8. [DOI] [PubMed] [Google Scholar]
- 51.Brandes RP, Beer S, Ha T, Busse R. Withdrawal of cerivastatin induces monocyte chemoattractant protein 1 and tissue factor expression in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23(10):1794–1800. doi: 10.1161/01.ATV.0000092126.25380.BC. [DOI] [PubMed] [Google Scholar]
- 52.Anzai T, Yoshikawa T, Shiraki H, et al. C-reactive protein as a predictor of infarct expansion and cardiac rupture after a first Q-wave acute myocardial infarction. Circulation. 1997;96(3):778–784. doi: 10.1161/01.cir.96.3.778. [DOI] [PubMed] [Google Scholar]
- 53.Pietilä KO, Harmoinen AP, Jokiniitty J, Pasternack AI. Serum C-reactive protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur Heart J. 1996;17(9):1345–1349. doi: 10.1093/oxfordjournals.eurheartj.a015068. [DOI] [PubMed] [Google Scholar]
- 54.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 55.Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]