Abstract
Previous research has shown that rats can learn matching-to-sample relations with olfactory stimuli; however, the specific characteristics of this relational control are unclear. In Experiment 1, 6 rats were trained to either match or nonmatch to sample in a modified operant chamber using common household spices as olfactory stimuli. After matching or nonmatching training with 10 exemplars, the contingencies were reversed with five new stimuli such that subjects trained on matching were shifted to nonmatching and vice versa. Following these reversed contingencies, the effects of the original training persisted for many trials with new exemplars. In Experiment 2, 9 rats were trained with matching procedures in an arena that provided for 18 different spatial locations for comparison stimuli. Five subjects were trained with differential reinforcement outcomes and 4 with only one type of reinforcer. Differential outcomes and multiple exemplars facilitated learning, and there was strong evidence for generalization to new stimuli for most rats that acquired several conditional discriminations. Performances with novel samples were generally above chance, but rarely reached the high levels obtained during baseline with well-trained stimulus relations. However, taken together, the data from the two experiments extend previous work, show that rats can learn both match and nonmatch relations with different experimental protocols, and demonstrate generalization to novel sample stimuli.
Keywords: matching-to-sample, identity, oddity, reversal learning, rats, abstract concepts
Whether nonhumans can learn abstract concepts such as “same” and “different” has been the focus of a considerable amount of recent research. Identity matching- (MTS) and nonmatching-to-sample (NMTS) procedures have been widely used to operationalize the concepts of “same” and “different”, respectively. These procedures generally require that the subject makes an observing response to a sample stimulus, then two or more comparison stimuli are presented and responses to the stimulus that is physically identical to the sample (in MTS) or different from the sample (in NMTS) are reinforced. High levels of accuracy on MTS or NMTS tasks alone, however, are not sufficient to demonstrate abstract concept learning as such performances can be produced by development of stimulus control by particular sample-comparison configurations or relations (Carter & Werner, 1978). Rather, evidence of generalization (i.e., successful transfer of matching or nonmatching to novel stimuli) is required, and moreover, performance on novel transfer trials should be similar to performance during training to define “full” concept learning (Bodily, Katz & Wright, 2008). Thus, performances which are said to demonstrate abstract concept learning are those in which responding has come under the control of the relation between the sample and the matching (or nonmatching) comparison stimuli in a fashion that is relatively independent of the specific stimuli presented on any given trial (Zentall, Galizio & Critchfield, 2002).
Using such criteria, abstract concept learning (generalized MTS or NMTS) has been demonstrated in a number of species including several new- and old-world primates, sea lions, and birds (see Lazareva & Wasserman, 2008 for a review). It has proven more difficult to demonstrate generalized matching in rodents; however, studies that have failed to obtain such effects have often used visual stimuli (e.g., Iversen, 1993; 1997). When olfactory stimuli have been used, though, there has been more success (e.g., Lu, Slotnick & Silberberg, 1993, Otto & Eichenbaum, 1992), perhaps because olfactory stimuli are more salient to rodents and complex learning is facilitated with this modality (Slotnick, 2001).
Peña, Pitts and Galizio (2006) adapted an olfactory discrimination procedure in which rodents were trained to dig in scented sand to obtain food (Dusek & Eichenbaum, 1997; Mihalick, Langlois, Krienke & Dube, 2000) and found evidence for generalized matching-to-sample in rats. Peña et al. initially trained rats on a single conditional discrimination (two olfactory stimuli) and added novel stimuli as criterion level performances were reached. By the end of the experiment, rats were matching at high levels of accuracy with 20 or more different stimuli and responding to novel stimuli was well above chance levels in 3 of the 4 rats tested.
Because novel stimuli were introduced one or two at a time in the Peña et al. (2006) experiment, it was not possible to identify precisely when generalized matching had occurred. Therefore, one purpose of Experiment 1 was to assess whether multiple exemplar training with 5 or 10 stimuli would be sufficient to result in concept learning. A second purpose was to determine whether rats could learn generalized “oddity” as well as “identity” relations and this feature afforded the opportunity to use a reversal design (e.g., Wilson, Mackintosh & Boakes, 1985; Zentall & Hogan, 1974) to measure transfer. Three rats were trained on an MTS relation with a five-stimulus set, and 3 other rats were trained on an NMTS relation. After reaching above-chance levels of accuracy, each group was switched to a novel five-stimulus set with the same contingencies as the first set. Finally, each group was tested with a third stimulus set under reversed contingencies.
EXPERIMENT 1
Method
Subjects
Seven male Holtzman (Sprague-Dawley) albino rats 90–150 days old at the beginning of the experiment served as subjects (one subject failed to acquire the conditional discrimination and was replaced). Water was available ad lib, but access to food was restricted to maintain approximately 85% of free feeding weight. Subjects were individually housed and maintained on a 12∶12-hr light/dark cycle.
Apparatus
An operant chamber (28 cm long × 26 cm wide × 30 cm high) was modified as described by Peña et al. (2006) for use in the study. The front wall of the chamber was constructed of clear Plexiglas with a 5-cm section removed from the bottom of the front wall of the chamber permitting the insertion of a plastic tray. The tray had three 5-cm (diameter) holes drilled into the top which held 2 oz. plastic cups. The cups were arranged such that when the tray was inserted approximately 12 cm into the apparatus, only the sample cup was accessible to the rat. When the tray was completely inserted, the two comparison cups (8 cm apart) became accessible. Testing was conducted in small room with 70 dB white noise presented through a speaker.
Stimuli
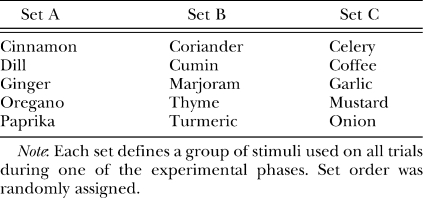
Olfactory stimuli were generated by mixing household spices (Great American Spice Co.) with sterilized play sand at a ratio of 1 g of spice per 100 g of sand (this ratio of spice to sand was shown to be sufficient to mask the scent of the sucrose pellet in Peña et al., 2006). See Table 1 for a list of the odorants used. Stimulus cups were filled to approximately 1 cm below the rim with sand for presentation.
Table 1.
Stimulus sets used in Experiment 1.
Procedure
Pretraining
Initial sessions were used to shape digging in scented sand. First, pairs of stimulus cups containing only 45-mg sucrose pellets were presented. When rats were readily consuming the pellets, pairs of cups filled with sand were presented with a pellet placed on top of the sand. As these were reliably consumed, the sucrose pellets were embedded progressively deeper in the sand until rats were regularly retrieving pellets that were 1 cm below the sand surface.
Match-to-Sample procedure
A trial began with the insertion of the stimulus tray approximately 12 cm into the chamber such that only the sample stimulus cup, which was baited with a pellet on 50% of the trials (variable ratio 2 schedule), was accessible to the rat. The operational definition of response was when the rat's paws or nose made contact with the sand in a cup in such a way that sand was displaced. Immediately after the rat dug in the sample cup, the tray was fully inserted into the chamber (approximately 20 cm), allowing access to the comparison stimulus cups (note that the sample cup remained available as well). One comparison was always the same scent as the sample, and this cup contained a sucrose pellet when the MTS contingency was in effect. The other comparison was a different scent selected from the stimulus set and this cup contained the sucrose pellet when NMTS contingencies were in effect. A correction procedure was used such that when the subject made an incorrect response, access to both comparisons continued until the subject responded to the correct cup. The trial was terminated when the subject responded to the correct cup and consumed the pellet (or when 5 s had elapsed after digging in the correct cup terminated when consumption of the pellet could not be visually confirmed). When a trial terminated, the experimenter removed the tray from the chamber, recorded the rat's response (only the first response of the trial was used to determine percent correct), and replaced the stimulus cups with premixed cups designed for the next trial. The next trial was initiated after a 15-s intertrial interval (ITI). Each session consisted of 24 trials with each stimulus serving as the sample four to five times per session. Sample stimuli were programmed randomly with the constraint that a stimulus could appear as the sample on no more than two consecutive trials. Each stimulus also appeared as a comparison on four to five trials per session.
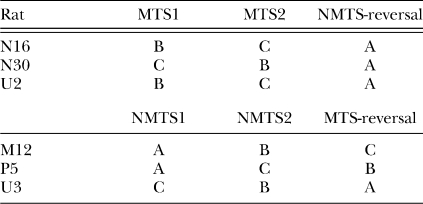
Subjects were randomly assigned to begin training with either MTS or NMTS procedures and each was randomly assigned to one of three sets of five olfactory stimuli (see Tables 1 and 2). When training criteria with these five stimuli in Phase 1 (a minimum of 25 sessions and two consecutive sessions at 90% correct or better) were met, Phase 2 began. In Phase 2 a set of five new stimuli was randomly selected and 15 sessions of training were conducted under the same contingencies in effect in Phase 1 (i.e., MTS subjects continued with a matching contingency and NMTS rats continued with a nonmatching task). Immediately following the completion of Phase 2, the Reversal Phase began. A new stimulus set (whichever set had not yet been presented) was used, but the contingencies with these stimuli were reversed from those in Phases 1 and 2 (i.e., MTS subjects now were exposed to a nonmatching task and NMTS subjects to a matching task) for an additional 15 sessions. The sequence of stimulus sets arranged for each rat is given in Table 2.
Table 2.
Training sequence of sets for each rat in Experiment 1.
Results and Discussion
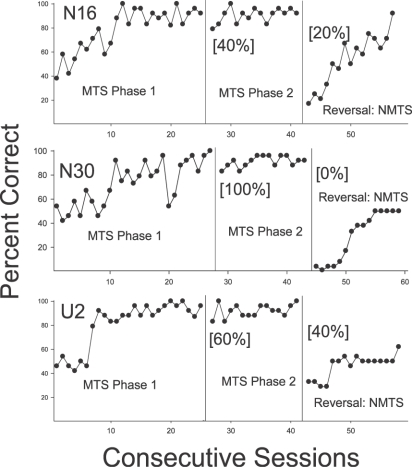
Figure 1 shows the performances of rats trained with a MTS contingency in Phase 1. The 3 rats showed acquisition of matching over a period of 7 to 12 sessions. However, as noted above, 1 rat originally assigned to this training condition developed a strong side preference and after completing 50 sessions in Phase 1 without meeting criterion, this animal was dropped from the study and replaced. The second panel of Figure 1 shows Phase 2 performances and it was noteworthy that all 3 rats showed high levels of accuracy as early as the first session. Trials in which the stimuli served as samples for the first time (“novel-sample” trials) were analyzed separately and percent correct is indicated in brackets in the panel. Rat N30 was correct on all five stimuli the first time they were presented, but the other 2 animals, N16 and U2, were less accurate in their initial response to the samples (40% and 60%, respectively), but by the completion of Phase 2, all 3 rats showed high levels of accuracy.
Fig 1.
Percent correct responding for subjects in Experiment 1 initially trained on MTS, then reversed to NMTS. Bracketed percentages in panels 2 and 3 for each subject represent outcomes on the initial trials in which each novel stimulus served as a sample during that phase.
When the Reversal Phase stimulus set was introduced (note nonmatching was reinforced and designated correct in Figure 1), all 3 rats showed poor performances that persisted for many sessions. Because the rats maintained responding according to the original contingency, this provides strong evidence for the transfer of matching to the set. Percent correct on novel-sample trials is indicated in brackets. Subject N16 obtained 20% correct on his initial exposure to the Reversal stimuli. Thus, on four of these five trials he chose the stimulus that matched the sample despite the reversed contingency. His performance was maintained at well below chance levels through the first three sessions of the Reversal Phase and then began to improve; by the 15th and final session, nonmatching responses were emitted on most trials. Rat N30 provided the strongest evidence for generalized matching on novel-sample trials, displaying matching responses to all five stimuli in Phase 3 (yielding 0% correct NMTS), and also all five in Phase 2 (100% correct MTS). Indeed, Rat N30 continued to show matching behavior consistently for several sessions despite the reversed contingency. After about seven sessions in Phase 3, a position bias developed that continued through the end of the Reversal Phase. Finally, Rat U2 matched on three of the five novel-sample trials, and like the other 2 animals, showed below-chance performance for several sessions following the reversal. Similar to N30, a position bias developed over several sessions in the reversal phase for U2, and nonmatching was not acquired by the end of the Reversal Phase.
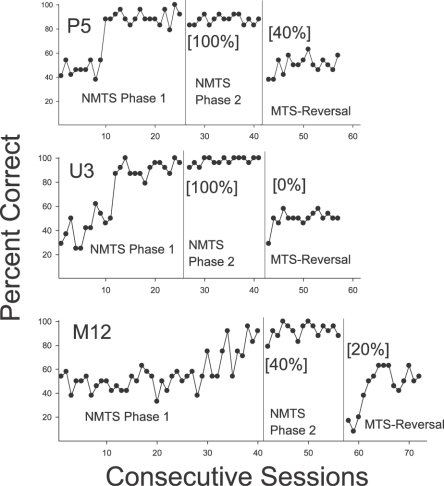
Performances were quite similar for the 3 animals whose Phase 1 training was NMTS (Figure 2). Two rats showed initial acquisition at rates similar to Phase 1 performance of the MTS group, but Rat M12 required more extended training. When Phase 2 was introduced, all 3 rats showed high levels of accuracy as early as the first session; in fact, 2 of the rats (P5 and U3) were correct on each novel-sample trial of the Phase 2 set. Finally, when the Reversal Phase was introduced, all 3 rats showed poor performance initially (i.e., they continued to respond to the nonmatching comparison stimulus) and none exceeded chance levels across the 15 sessions of this phase. All 3 rats also showed evidence of developing a position bias. Overall, there was some evidence of generalized nonmatching on novel-sample trials, particularly in U3, who responded to the nonmatching stimulus on all 10 of the novel sample presentations (achieving 100% correct on Phase 2, and 0% on the Reversal set), and P5, who responded to the nonmatching comparison stimulus on 8 out of 10 novel-sample trials across the two phases.
Fig 2.
Percent correct responding for subjects in Experiment 1 initially trained on NMTS, then reversed to MTS. Bracketed percentages in panels 2 and 3 for each subject represent outcomes on the initial trials in which each novel stimulus served as a sample during that phase.
For statistical analysis purposes, the MTS and NMTS groups were combined. In order to address the question of whether there was a savings or loss in acquisition of the conditional discriminations across training phases, mean percent correct on the first session of each phase was computed. Performances on the first session of Phase 1 were slightly below chance (43.7%), rose to 83.2% on the first session of Phase 2, and declined to 23.0% for the first Reversal Phase session. A one-way ANOVA revealed a significant effect of Phase, F (2, 10) = 47.90, p < .01. Post-hoc t-tests showed that first-session accuracy in Phase 2 was significantly higher than in Phase 1 (p < .01) and was significantly lower for the Reversal Phase than for Phase 1 (p < .05). Because performances were higher on the initial session with the Phase 2 stimuli than they were on the first day of training with a MTS or NMTS procedure, there was a savings due to the transfer of matching (or nonmatching) when rats were exposed to the novel stimuli of Phase 2. Reversal to a contingency different from the one used in training produced a significant performance drop below the initial day of training in Phase 1, again suggesting a generalized transfer of matching or nonmatching to the novel odors of the Reversal Phase. Of course, reinforcement of responding to novel stimuli could have come to influence performances even during a single session and so accuracy on the first trial in which a novel stimulus serves as the sample remains an important criterion for concept learning.
In order to evaluate generalization to novel stimuli, the five novel-sample trials in Phase 2 and the five in the Reversal Phase were combined and scored with respect to how many responses were in accord with the contingency trained in Phase 1. Outcomes of these analyses were clear for Rats N30 and U3 that showed transfer on all 10 novel-sample trials (binomial test p < .01), and perhaps P5 who showed transfer on 8 out of 10 (p < .05). The other 3 rats showed 60–70% transfer (p > .05). Across subjects and phases, transfer was shown on 46/60 novel-sample trials (binomial test, p < .01).
In sum, Experiment 1 supported the findings of Peña et al. (2006) that rats exposed to MTS contingencies with olfactory stimuli develop a pattern of matching that generalizes when novel stimuli and stimulus combinations are introduced. The present study extends these findings to NMTS contingencies which showed patterns very similar to MTS and to a reversal design similar to those that have been used successfully to show transfer of matching in pigeons (Zentall & Hogan, 1974). Further, these results suggest that training with as few as 10 exemplars is sufficient to produce transfer of matching or nonmatching performances.
One concern associated with the apparatus used in the present study was that the odor of the sucrose pellet located in the correct cup, rather than the designated olfactory stimulus, could control choice behavior. In the Peña et al. (2006) study, occasional trials were conducted with no sucrose pellet in either comparison cup and performances were just as accurate on these trials (unbaited) as on standard (baited) trials. Although no such controls were included in the present study, the performances of all 6 animals in the first few sessions of the Reversal Phase provide strong evidence that the odor of the comparison cup spice, not the sucrose pellet, was controlling behavior. In each case, rats persisted in responding according to the sample–comparison relation trained in Phases 1 and 2 even though the sucrose pellet was consistently located in the other comparison cup. After a few sessions, most animals stopped showing relational control of behavior and developed a side bias—again showing that their behavior was not tracking the scent of the food pellet.
One limitation of the reversal design used in Experiment 1 is that it is difficult to assess whether the criteria for “full” concept learning were met. In the MTS literature with pigeons, transfer of matching or nonmatching to novel stimuli has sometimes been above chance levels, but below baseline levels of accuracy. These sub-baseline performances are not considered to meet the criterion to qualify as “full” concept learning, and are instead referred to as “partial” concept learning (see Katz, Wright, & Bodily, 2007; Lazareva & Wasserman, 2010; Wright, 1997 for discussion). Here, in Experiment 1, only 10 novel sample stimuli were available for analysis, limiting comparison with baseline levels. Two rats responded correctly on 10 out 10 novel samples (N30 and U3), and so might be said to have met the criterion for full concept learning, but with so few novel stimuli, outcomes for other animals were inconclusive. Experiment 2 used a design in which rats were trained on a series of stimulus sets in order to address this issue. Additionally, in an effort to reduce the problem of position bias that hampered both acquisition and reversal in Experiment 1, Experiment 2 utilized an arena apparatus in which comparisons could be presented in multiple spatial positions.
EXPERIMENT 2
Experiment 2 involved several modifications to the procedures used in Experiment 1 and Peña et al. (2006). First, an arena apparatus designed to present comparison stimuli in 18 separate locations was used. Second, a new method of stimulus delivery was employed to ensure response definition consistency across experimenters; cups of scented sand were covered with opaque, perforated plastic lids and the criterion response was removal of the lid from the cup, a behavior scored with very high interrater reliability. Additionally, because some rats showed poor acquisition of baseline and reversed conditional discriminations under the reinforcement conditions of Experiment 1, 5 of the 9 rats were trained with a differential outcome procedure (DO; each stimulus was associated with only one of two different reinforcers) as such procedures have been shown to accelerate MTS acquisition (e.g., Nevin, Ward, Jimenez-Gomez, Odum & Shahan, 2009; Urcuioli, 2005). Further, Schmidtke, Katz, and Wright (2009) showed that DO training facilitated same/different concept learning in pigeons. Thus, Experiment 2 was designed to permit a replication in rodents of the Schmidtke et al. study. Finally, in Experiment 2, rats were trained with a single contingency (MTS) with a research design in which meeting training criteria with one stimulus set led to the presentation of a new set of stimuli. A series of such tests made it possible to assess concept learning as a function of progressively increasing the number of exemplars. This also permitted analysis of whether full concept learning (performance on novel-sample trials is equivalent to baseline) or partial concept learning (performance on novel-sample trials is above chance, but below baseline levels) was obtained (Katz, et al., 2007; Lazareva & Wasserman, 2010; Wright, 1997).
Method
Subjects
Subjects were 9 male, Holtzman (Sprague-Dawley) albino rats between 90 and 120 days at the outset of the experiment, housed and maintained as in Experiment 1.
Apparatus
The apparatus was a circular open-field arena (94-cm diameter) with 18 holes (5-cm diameter) arranged in two circular arrays (see Figure 3). Aluminum baffling approximately 30 cm high surrounded the perimeter. A separate holding cage (20 × 30 cm) was located on a table adjacent to the arena and was used to present the sample stimuli and to contain subjects during the ITI. Sample stimuli were placed in the corner of the cage. Once a response to the sample occurred, subjects were placed in the center of the arena apparatus where the comparison stimuli were positioned in a pseudorandom fashion. That is, on any given trial, the comparison stimuli (cups containing scented sand covered by a lid) occupied 2 of the possible 18 holes, with the remaining 16 occupied by empty cups (without lids). The locations of the comparisons varied such that no location was used for more than two consecutive trials.
Fig 3.

Odor arena apparatus showing 18 hole positions designed to hold comparison stimulus cups.
Stimuli
Olfactory stimuli were the same as those used in Experiment 1 with additional stimuli created by mixing liquid oil odorants into the sand base (e.g., peppermint, strawberry, vanilla—one drop per cup), for a total of 50 different odor stimuli. Cups holding the scented sand were loosely covered by a perforated lid which protruded slightly above the arena surface and could easily be displaced by the rat. Cups and lids were used only once each session to prevent scent marking by the rat.
Procedure
Pretraining
Initial training proceeded as in Experiment 1; when rats were able to retrieve buried pellets from stimulus cups, they were then trained to remove lids from the cups. Lids were first placed adjacent to the cup and coverage of the cup was gradually increased until the lid completely covered the cup. Once lid removal became reliable, the MTS procedure began.
MTS procedure
The MTS procedure involved the presentation of a single sample stimulus (covered cup of scented sand baited with a food pellet) in the holding cage. Immediately following sample stimulus lid removal and reinforcer retrieval, the rat was placed into the arena where two comparison stimuli (S+, S−) were located in any 2 of the 18 possible spatial positions (randomly determined on each trial). Response definition included any displacement of the lid from the cup rim using the front paws, snout, or face. A correction procedure was used so that each trial ended with a response to S+, but only the first response was used to determine percent correct. Following the completion of the trial subjects were returned to the holding cage for an ITI of approximately 30 s.
The experiment was divided into phases determined by the introduction of sets of novel olfactory stimuli. In the initial phase (A), two stimuli were arbitrarily selected from among the 50 available odors. Five rats (F3, H7, G13, G8, J6) were trained with differential outcomes such that a 45-mg grain pellet served as the reinforcer with some stimuli and a 45-mg sucrose served as the reinforcer with others whenever the stimulus was presented—whether as a sample or a matching comparison. For example, in Phase A one stimulus was arbitrarily assigned to grain reinforcement (A1) and the other to sucrose (A2). Differential outcomes were not used for the remaining 4 animals (F6, F5, H8, I30) and sucrose pellets served as reinforcers on all trials for them.
Daily sessions consisting of 24 trials were conducted until a baseline training criterion of two consecutive sessions with 90% correct or better was attained. During the session immediately following criterion, the initial training stimuli were dropped from training and the first set of six stimuli (Phase BCD) was introduced with each stimulus presented four times as a sample and four times as an incorrect comparison in each session. For the rats in the DO conditions, three of the stimuli were associated with grain (B1, C1, D1) and the other three with sucrose reinforcement (B2, C2, D2). Comparison stimulus combinations were balanced so that each sucrose stimulus was paired with each grain stimulus equally often (the same stimulus pairings were used for rats trained with only sucrose reinforcement). The initial exposure to the BCD stimuli constituted the first test of generalized matching and, because correct responses were always reinforced, results from the first trial with each sample stimulus (“novel-sample” trials) were separated for analysis. Training with the BCD stimuli continued until the baseline criterion (two consecutive sessions with 90% correct or better) was met. Following criterion performance, the BCD stimulus set was dropped from training and a new set of six stimuli was introduced for Phase EFG testing and training. The remaining phases of identity MTS training and testing continued in the same fashion, such that a new set of six stimuli was added after the completion of each phase for the remainder of Experiment 2 (see Table 3). The experiment was continued until a generalized matching criterion was met (accuracies on novel-sample trials reached binomial significance, p < .05, across stimulus sets—at least 10/12 or 14/18 correct) or until extended chance level performance was considered as a failure to acquire the baseline conditional discriminations in one of the phases (as evidenced by 25 or more sessions without improvement).
Table 3.
Number of sessions to criterion (or experiment termination) for each subject across training stimulus sets in Experiment 2, arranged by reinforcer condition.
Interobserver reliability sessions were conducted for subjects F6, F3, and G8. During these sessions a rater, blind to which stimulus was S+, was present and independently scored the behavior. Interrater agreement was 100% in each case. Pellet detection trials, trials in which no reinforcers were presented until after a response occurred, were also conducted for subjects F6, F3, G8, H8, H7, and J6 to control for tracking pellet odor. Accuracy during these controls was found to be very similar to baited trials that occurred within the same sessions (M = 87.67% for baseline trials and 89.21% for pellet detection trials, p > .05).
Results and Discussion
Table 3 shows the number of sessions required to meet criterion in each phase. As is evident, 3 of the 4 rats trained with only sucrose reinforcement were unsuccessful in mastering the baseline conditional discriminations. Rats I30 and F5 failed to reach baseline criterion level performance even with the initial two stimuli (Phase A) and the third (H8) later failed with the BCD discriminations. These 3 animals developed a pattern of navigating to the cup nearest to the start location in the arena and then responding to that cup regardless of the odor stimulus it contained.
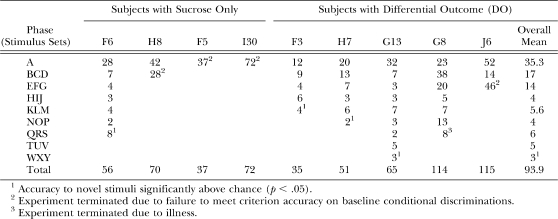
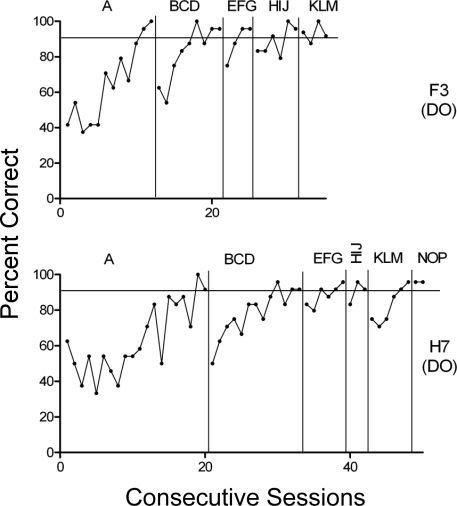
For the 6 remaining animals, Figures 4–6 show performances on a session by session basis. Figure 4 shows performances of Rats F3 and H7 and reveals gradual acquisition of the A and BCD conditional discriminations followed by much more rapid acquisition of EFG and subsequent stimulus sets. Rat F3 reached baseline mastery criteria fairly rapidly (10–12 sessions) during the initial A and BCD phases, and then very rapidly (3–6 sessions) in subsequent phases. Rats H7 (Figure 4), G13, and F6 (Figure 5) developed the initial Phase A conditional discrimination somewhat more slowly than F3, but subsequently showed similar evidence of transfer, with the number of sessions required to reach baseline criterion decreasing across phases for all 3 animals. All 4 of the rats featured in Figures 4 and 5 went on to also meet the generalized matching criterion (significantly above chance performance on novel-sample trials over 12–18 trials). Rats F3, H7 and G13 were all trained using the DO procedure, but it is noteworthy that Rat F6, the only animal to master more than one conditional discrimination using the sucrose-only procedure, also showed performances much like the DO-trained animals across the experiment.
Fig 4.
Session-by-session accuracies across all training sets for subjects F3 and H7 (both trained with differential outcomes, DO). Panel labels depict phases of the study with different stimulus sets and horizontal line shows criterion level performance.
Fig 5.
Session-by-session accuracies across all training sets for subjects G13 (DO) and F6 (sucrose only). Panel labels depict phases of the study with different stimulus sets and horizontal line shows criterion level performance.
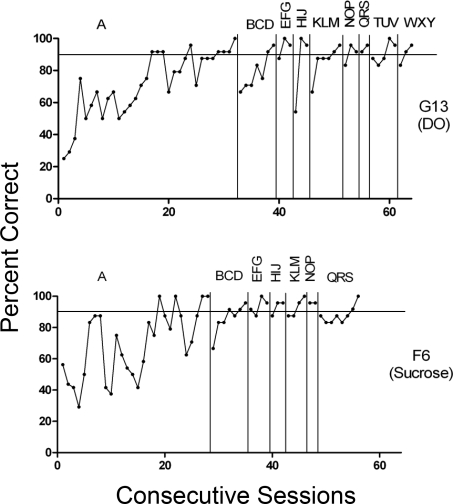
Fig 6.
Session-by-session accuracies across all training sets for subjects G8 and J6 (DO). Panel labels depict phases of the study with different stimulus sets and horizontal line shows criterion level performance.
Figure 6 shows performances of the remaining 2 DO animals; both failed to reach the generalized matching criterion. Rat G8 did develop accurate matching across several phases of the study, but was slow to meet mastery criterion and was dropped from the study due to illness after more than 100 sessions without meeting the criterion for generalized matching. Rat J6 met baseline criteria for Phases A and BCD after fairly extended training, but then failed to meet criterion in Phase EFG and was dropped from the study after 46 sessions in this phase. These data illustrate that although DO training was more likely to produce successful matching in the present study, it was not sufficient to guarantee successful outcomes in all cases.
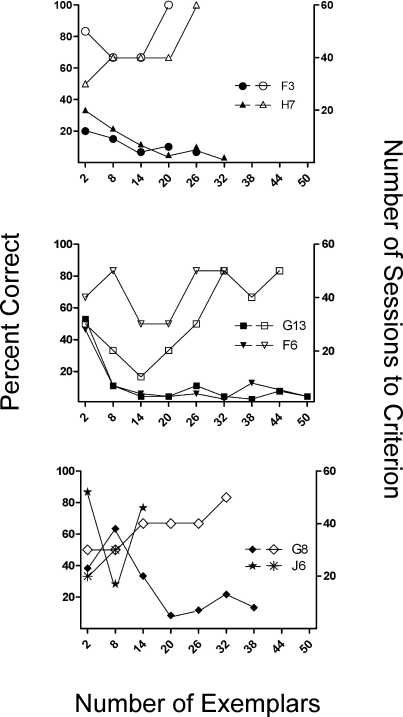
Critical to the evaluation of concept learning is performance with novel stimuli. In order to examine the emergence of generalized matching, Figure 7 shows percent correct for trials on which a stimulus served as a sample for the first time (white symbols) as well as the number of sessions required to meet baseline mastery criterion (black symbols) plotted as a function of the increasing number of exemplars as training continued through the experiment. The 4 rats that met the generalized matching criteria are shown in the top two panels and reveal similar patterns. Rats F3 and H7 (top panel) showed a gradual decline in the number of sessions required to meet training criterion as the number of exemplars increased. Performance on novel-sample trials similarly improved: Rat H7 met the generalized matching criterion (10/12 correct, p < .05) after training with 26 exemplars, and F3 met it (10/12 correct, p < .05) after exposure to 20 exemplars. Rats G13 and F6 (middle panel) showed a steep decline in number of sessions required to meet the training criterion, but required relatively more training to meet the generalized matching criterion. Rat G13 performed at or below chance levels on novel-sample trials for several phases, but after exposure to 38–50 exemplars, generalized matching appeared to emerge with 14 correct on his final 18 novel-sample trials (p < .05). Rat F6 generally showed above chance performances with novel samples, and improved to meet the generalized matching criterion after training with 38 exemplars (10/12 correct, p < .05).
Fig 7.
Percent correct on novel-sample trials (open symbols, left vertical axis) and number of sessions to criterion (closed symbols, right vertical axis) are plotted as a function of the number of exemplars as training progressed in Experiment 2. The top panel shows subjects with approximately 40 total sessions (F3 and H7), the middle panel shows the same function for subjects with approximately 60 total sessions (G13 and F6), and the lower panel for subjects with approximately 100 total sessions (G8 and J6).
The bottom panel of Figure 7 shows performances of the two rats that failed to meet the generalized matching criterion. In the case of Rat J6, this failure appears related to poor baseline acquisition as he advanced through only 14 exemplars. Rat G8 was exposed to 38 exemplars and failed to meet the generalized matching criterion; however, it should be noted that some improvement in accuracy on trials with novel sample stimuli was apparent as the number of exemplars increased. Thus, 4 of the 5 rats that advanced beyond Phase BCD in training went on to meet our criterion for generalized identity matching.
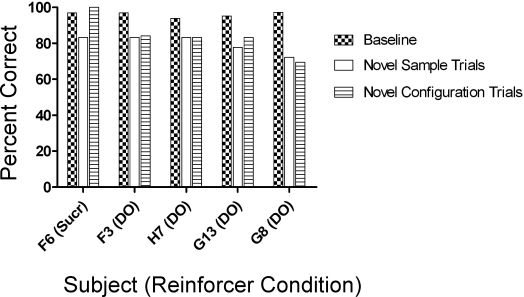
In order to address the question of whether performances in the present study met the criteria for full concept learning, Figure 8 summarizes performances across the experiment for the 5 rats that advanced beyond the BCD training phase. Accuracy on the criterion baseline sessions defines performance for well learned conditional discriminations (checkered bars), and is compared to performances on novel-sample trials (white bars). Full concept learning requires that accuracies on novel-sample trials to be equivalent to these well learned baseline levels. In addition, Figure 8 shows performances on trials in which the sample stimuli appeared for the first time in a particular configuration (novel configurations—striped bars). Accuracy on such trials should also be equivalent to baseline levels given the development of full concept learning. All 5 rats showed novel-sample performances that were consistently below the very high levels of accuracy obtained during baseline. With the exception of Rat F6, accuracies on trials involving novel configurations were also below baseline levels. Within-subject ANOVA confirmed the reliability of these differences, F (2, 8) = 9.83, p = .007. Baseline performance (M = 95.97% correct) was higher than performance on novel-sample trials (M = 79.99% correct, p < .01) and novel-configuration trials (M = 84.06% correct, p < .05). Performances on novel-sample and novel-configuration trials were similar (p > .05). These results indicate less than full concept learning.
Fig 8.
Mean percent correct is plotted for the criterion baseline sessions (two phases for F6, F3, H7 and three phases for G13 and G8) alongside the criterion probe and novel combination accuracies (i.e., generalized matching performance).
GENERAL DISCUSSION
Studies of MTS learning and generalized matching in rats have generally been more successful with olfactory stimuli (Lu, et al., 1993; Otto & Eichenbaum, 1992; Peña et al., 2006) than with other modalities (e.g. Iversen, 1993; 1997). The present studies replicate and extend the findings of Peña et al. with olfactory stimuli, but also illustrate some limitations. For example, in Experiment 1, 6 animals showed fairly rapid acquisition of MTS and NMTS contingencies using the digging procedure employed by Peña et al., but 1 animal failed to acquire olfactory stimulus control entirely. Further, following a reversal from matching- to nonmatching-to-sample contingencies or vice versa, 5 of 6 subjects developed position preferences and ultimately failed to show reversal.
Experiment 2 involved the use of a large arena apparatus in which the stimulus could be placed in any of 18 positions in order to reduce the likelihood of spatial position as a competing source of stimulus control, yet 2 of 3 animals trained without DO contingencies failed to acquire even a single conditional discrimination under these conditions. The addition of DO contingencies seemed to promote acquisition, though, as all 5 rats trained under the DO conditions acquired at least A and BCD discriminations. For the 3 rats that showed acquisition failures, a variation of the position preference problem may have emerged. Each developed a pattern of moving in a particular heading and then responding to the first cup encountered rather than responding under olfactory stimulus control. One feature of Experiment 2 that may have contributed to the acquisition difficulties was the inherent delay between the presentation of the sample in the holding cage and the actual encounter with the comparison stimuli which depended upon how rapidly the animal navigated the apparatus and the placement of the stimuli on each trial. In any case, the fact that these acquisition problems were largely overcome through the use of DO contingencies illustrates that, despite these difficulties, the arena apparatus may have some utility in stimulus control research (see MacQueen, Bullard & Galizio, 2011).
In both experiments, once rats had mastered several conditional discriminations, evidence of transfer to novel stimuli was obtained. In Experiment 1, there were substantial savings on MTS or NMTS acquisition on the second stimulus set following mastery of the first. Even more striking was the interference with acquisition on the third set of stimuli under reversed contingencies. Performance on the first exposure to stimuli in Phase 2 and the Reversal Phase also provided evidence of transfer. Although only 10 novel stimulus tests were provided in Experiment 1, 3 of the 6 individual subjects showed levels of accuracy that were significantly above chance (using the binomial test) and overall performance for the 6 animals combined was also significantly above chance.
Experiment 2 provided training with more exemplars and thus more opportunity for exposure to novel stimuli. On this most critical criterion for the assessment of concept learning (Katz, et al., 2007; Lazareva & Wasserman, 2008), there was strong evidence for transfer. Four of the 5 animals advancing beyond the BCD Phase were able to meet a generalized matching criterion of at least 10/12 or 14/18 correct responses on novel-sample trials. Across all subjects, comparison of accuracies on novel-sample trials to criterion level baseline performance revealed reliable differences with novel-sample responding averaging approximately 80% correct as compared to baseline accuracies which averaged well above 90%. This difference is of some importance, as it has been argued that equivalent performances on baseline and novel stimuli are necessary in order to make the claim of full concept learning (Katz, et al., 2007; Lazareva & Wasserman, 2010; Wright, 1997). The results of Experiment 2 appear to provide an example of partial concept learning in rats.
Partial transfer is not an uncommon outcome in the pigeon literature, as Katz, et al. (2007) note, but the basis for it in those studies is not clear. Indeed, the notion of partial learning of a same–different concept seems counterintuitive from a cognitive perspective. The all-or-none aspect of the concept begs the question of what could result in the less than complete transfer that is often seen. However, a more behavioral account of the phenomenon may clarify the analysis by recognizing that MTS training contingencies can result in different stimulus control topographies (McIlvane & Dube, 2003). Early in training these might include position biases and idiosyncratic stimulus preferences which presumably become weaker sources of stimulus control with more extended exposure to the training contingencies. As training progresses, a mixture of control by stimulus configurations, item-specific sample–comparison relations, and more general sample–comparison relations may emerge (Katz, Bodily, & Wright, 2008; Wright, 1997). When training criteria have been met, control by the sample–comparison relation may have become the dominant stimulus control topography occasioned by stimuli in the training set, but when novel stimuli are presented to assess transfer, older stimulus control topographies may compete with relational control, resulting in performance that, while above chance, is below baseline levels. Thus, it could be argued that competing stimulus control topographies such as those described may provide an account for partial concept learning in the present study and in previous literature with pigeons. Another possible source of transfer is based on stimulus generalization from similar odors trained in earlier sets (Mackintosh, 2000). Interestingly, partial concept learning in pigeons tends to emerge following training with 32–64 stimuli or more, whereas full concept learning requires many more exemplars (Schmidtke et al., 2009). In contrast, Experiment 2 of the present study showed partial concept learning after training with 26–50 different odor stimuli and Experiment 1 after as few as 10 stimuli. Thus, rats trained with multiple odor stimuli may require somewhat fewer exemplars than pigeons to attain partial concept learning, and it certainly seems plausible to predict that full concept learning would have emerged with additional odor exemplars.
In sum, the data presented in these two experiments highlight the opportunity to study abstract concept learning in rats with a variety of training procedures including using olfactory stimuli, multiple exemplars, a nonautomated response, and DO. Acquisition in many subjects was quite rapid and transfer to new stimuli apparent. Nevertheless, relational control was not seen in all subjects under all conditions, underscoring the need to test concept formation in a variety of settings and using multiple procedural variations.
Acknowledgments
The authors would like to thank Laurence Miller, Tracy Peña and Rhiannon Thomas who assisted in data collection.
REFERENCES
- Bodily K.D, Katz J.S, Wright A.A. Matching-to-sample abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34(1):178–184. doi: 10.1037/0097-7403.34.1.178. [DOI] [PubMed] [Google Scholar]
- Carter D.E, Werner T.J. Complex learning and information processing by pigeons: a critical analysis. Journal of the Experimental Analysis of Behavior. 1978;29:565–601. doi: 10.1901/jeab.1978.29-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek J.A, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen I.H. Acquisition of matching-to-sample performance in rats using visual stimuli on nose keys. Journal of the Experimental Analysis of Behavior. 1993;59(3):471–482. doi: 10.1901/jeab.1993.59-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen I.H. Matching-to-sample performance in rats: A case of mistaken identity. Journal of the Experimental Analysis of Behavior. 1997;68(1):27–45. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.S, Wright A.A, Bodily K.D. Issues in the comparative cognition of abstract-concept learning. Comparative Cognition & Behavior Reviews. 2007;2:79–92. doi: 10.3819/ccbr.2008.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.S, Bodily K.D, Wright A.A. Learning strategies in matching to sample: If–then and configural learning by pigeons. Behavioural Processes. 2008;77:223–230. doi: 10.1016/j.beproc.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareva O.F, Wasserman E.A. Categories and concepts in animals. In: Menzel R, et al., editors. Learning and memory: A comprehensive reference. Vol. II: Behavioral approaches. Amsterdam: Elsevier; 2008. pp. 197–226. (Eds.) [Google Scholar]
- Lazareva O.F, Wasserman E.A. Category learning and concept learning in birds. In: Mareschal D, Quinn P.C, Lea S.E.G, editors. The making of human concepts. New York: Oxford University Press; 2010. pp. 151–172. (Eds.) [Google Scholar]
- Lu X.M, Slotnick B.M, Silberberg A.M. Odor matching and odor memory in the rat. Physiology & Behavior. 1993;53(4):795–804. doi: 10.1016/0031-9384(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Mackintosh N.J. Abstraction and discrimination. In: Heyes C, Huber L, editors. The evolution of cognition. Cambridge, MA: MIT Press; 2000. pp. 123–142. (Eds.) [Google Scholar]
- MacQueen D.A, Bullard L, Galizio M. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiology of Learning and Memory. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvane W.J, Dube W.V. Stimulus control topography coherence theory: Foundations and extensions. The Behavior Analyst. 2003;26:195–213. doi: 10.1007/BF03392076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalick S.M, Langlois J.C, Krienke J.D, Dube W.V. An olfactory discrimination procedure for mice. Journal of the Experimental Analysis of Behavior. 2000;73(3):305–318. doi: 10.1901/jeab.2000.73-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin J.A, Ward R.D, Jimenez-Gomez C, Odum A.L, Shahan T.A. Differential outcomes enhance accuracy of delayed matching to sample but not resistance to change. Journal of Experimental Psychology: Animal Behavior Processes. 2009;32(1):74–91. doi: 10.1037/a0012926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Olfactory learning and memory in the rat: A “model system” for studies of the neurobiology of memory. In: Chobor K, Serby M, editors. The Science of Olfaction. New York: Springer-Verlag; 1992. pp. 213–244. (Eds.) [Google Scholar]
- Peña T, Pitts R.C, Galizio M. Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior. 2006;85(2):203–221. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K.A, Katz J.S, Wright A.A. Differential outcomes facilitate same/different concept learning. Animal Cognition. 2009;13:583–589. doi: 10.1007/s10071-009-0292-2. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends in Cognitive Sciences. 2001;5(5):216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J. Behavioral and associative effects of differential outcomes in discrimination learning. Learning and Behavior. 2005;33:1–21. doi: 10.3758/bf03196047. [DOI] [PubMed] [Google Scholar]
- Wilson B, Mackintosh N.J, Boakes R.A. Transfer of relational rules in matching and oddity learning by pigeons and corvids. Quarterly Journal of Experimental Psychology. 1985;37B:313–332. [Google Scholar]
- Wright A.A. Concept learning and learning strategies. Psychological Science. 1997;8:119–123. [Google Scholar]
- Zentall T, Hogan D. Abstract concept learning in the pigeon. Journal of Experimental Psychology. 1974;102(3):393–398. [Google Scholar]
- Zentall T, Galizio M, Critchfield T.S. Categorization, concept learning, and behavior analysis: An introduction. Journal of the Experimental Analysis of Behavior. 2002;78:237–248. doi: 10.1901/jeab.2002.78-237. [DOI] [PMC free article] [PubMed] [Google Scholar]