Abstract
The trend toward single-room neonatal intensive care units (NICUs) is increasing; however scientific evidence is, at this point, mostly anecdotal. This is a critical time to assess the impact of the single-room NICU on improving medical and neurobehavioral outcomes of the preterm infant. We have developed a theoretical model that may be useful in studying how the change from an open-bay NICU to a single-room NICU could affect infant medical and neurobehavioral outcome. The model identifies mediating factors that are likely to accompany the change to a single-room NICU. These mediating factors include family centered care, developmental care, parenting and family factors, staff behavior and attitudes, and medical practices. Medical outcomes that plan to be measured are sepsis, length of stay, gestational age at discharge, weight gain, illness severity, gestational age at enteral feeding, and necrotizing enterocolitis (NEC). Neurobehavioral outcomes include the NICU Network Neurobehavioral Scale (NNNS) scores, sleep state organization and sleep physiology, infant mother feeding interaction scores, and pain scores. Preliminary findings on the sample of 150 patients in the open-bay NICU showed a “baseline” of effects of family centered care, developmental care, parent satisfaction, maternal depression, and parenting stress on the neurobehavioral outcomes of the newborn. The single-room NICU has the potential to improve the neurobehavioral status of the infant at discharge. Neurobehavioral assessment can assist with early detection and therefore preventative intervention to maximize developmental outcome. We also present an epigenetic model of the potential effects of maternal care on improving infant neurobehavioral status.
Keywords: preterm, neurobehavior, NNNS, NICU, very-low-birthweight infants, single-room NICU design, epigenetics
The prevalence of preterm birth in the United States is a significant public health problem that has increased in the last decade. Prematurity rates, which have increased steadily since the early 1980s, have shown a slight decrease in the United States to 12.3%. Even so, 1 in 8 infants, or more than 500,000 per year, are premature, and there continues to be a parallel increase in the risk of adverse health outcomes and multiple disabilities.1–4 Advances in perinatal and neonatal care, such as administration of antenatal steroids and surfactant, have improved survival rates for preterm infants. However, the increase in survival is accompanied by a parallel increase in the risk of adverse health outcomes and multiple disabilities.1–3,5 In 1991, Escobar and colleagues6 reported on a meta-analysis of 111 outcome studies of very low birthweight infants (<1500 g at birth), citing a median incidence of disability of 25%.
During the past decade, as survival of infants <1000 g has become more prevalent, investigators continue to report a litany of sequelae related to prematurity and low birthweight, including respiratory, gastrointestinal, immunologic, central nervous system, hearing and vision, and longer-term motor, cognitive, behavioral, and social emotional problems.3,7–12 Work from our group in the National Institute of Child Health and Human Development Neonatal Research Network found that nearly one-half (49%) of infants born at birth weights <1000 g went on to have abnormal neurodevelopmental and/or sensory findings at 18–22 months of age.13 Thirty-seven percent of the study cohort had significant cognitive delays, and 29% had significant motor delays. These infants suffer serious health consequences and their families carry an enormous emotional burden.13,14 In a more recent meta-analysis of very low birthweight infants the focus was on more narrow band behaviors and specific areas of function.15 This analysis showed substantial effect sizes (d statistic) in areas, such as spelling (D = 0.76), attention (D = 0.59), and verbal fluency (D = 0.57). Thus, these infants are also at risk for more subtle deficits that could broaden the impact of very low birthweight on the individual and on society.
Although the range of morbidity in preterm infants is due, in part to the immaturity of their organ systems and their disease states, there is mounting concern that this morbidity may be compounded by an unfavorable environment in the neonatal intensive care unit (NICU). Large variations in outcomes exist among NICUs that cannot be explained by patient mix or other characteristics, such as volume and level of care.16 The determinants of high-quality NICU care need to be better understood to improve the health and development of NICU survivors.
Recommendations for NICU private rooms emerged in the 1990s.17,18 It has been suggested that caring for infants in individual private rooms in a NICU, in contrast to the open-bay environment in which most infants are cared for, may provide the type of high-quality care and physical environment that will lead to improved infant outcome.19,20 Some NICUs have designated a portion of their space for single-room care; however, there is some inconsistency in the types of patients assigned to these spaces. For example, some nurseries use the single-room spaces for their convalescing level II infants, allowing families an opportunity to room-in before discharge. In this model, infants are treated in the open-bay environment until they are physiologically prepared for discharge. Other units assign their more critically ill infants to the single rooms, moving them out to small open-bays when they become more stable. Still others assign most ventilator-dependent infants to the open-bay environment.20 To date, only a modest number of NICUs in the United States have converted all levels of care exclusively to single-family-room care. In the first published report of the benefits of a single-room NICU, Walsh and colleagues20 conducted a retrospective analysis after the move to a single-room NICU and reported a decrease in nosocomial infection rates. In surveys conducted in the same NICU, 6 months before and 6 months after the transition to the private room, nurses reported increased job satisfaction, team member support, and validation of individual opinions. Parents reported improvements in privacy, noise, and lighting in the NICU.21 Clearly there is a paucity of research in this area.
The Single-Room NICU
The NICU is the first extrauterine environment for an increasing number of preterm infants. Critically ill infants require prolonged NICU stays; sometimes as long as 5 months, yet the typical NICU environment could be stressful for infants and their families.22–24 Most NICU patients continue to be cared for in an open-bay environment, in which the sensory impact of the NICU may adversely affect infant outcome.17,25–27 Stimulation in the NICU, such as light and sound, have been related to adverse outcome in preterm infants.28–34 We35 documented sound levels in our open-bay NICU and found that the sound levels rarely dropped to less than 60 dB and often exceeded 70 dB despite the American Academy of Pediatrics recommended maximum level of 45 dB.36 Sound control and other interventions, for example, light reduction, may improve infant medical and neurobehavioral outcome.37–39 The single-room NICU is a strategy that could address environmental concerns and minimize iatrogenic effects by reducing the risk of infection and stress on the preterm infant. Although participation of families in the care of family members receiving critical care has become the accepted standard, and single-room care has long been considered the optimal environment for critically ill adults,40 only in the past decade has this consideration been expanded to include neonatal intensive care.
Our primary interest is the impact of the single-room NICU design on improving the medical and neurobehavioral outcome of the preterm infant. The single-room NICU provides control over several factors that could improve these outcomes, such as those associated with hospital-acquired infections.17,25,41 Rates of nosocomial infection increased significantly when infants were moved to a less-spacious, temporary NICU, and subsequently decreased when infants were moved to a newly constructed facility with an improved sink-to-bed ratio.42–49 Late-onset bacterial sepsis, a serious complication of NICU care, is known to be related to prolonged treatment with central catheters, prolonged time on parenteral nutrition, delayed initiation of enteral feedings and a prolonged period to reach full enteral feeds.17,25,50 Care of high-risk infants in a single family room may promote care practices that impact some of these associations and ultimately impact rates of late onset sepsis. The Vermont-Oxford Neonatal Network has used a quality improvement approach to identify best practices related to nosocomial infection (eg, practices related to hand washing, nutrition, skin care, respiratory care, vascular access, etc) and were able to demonstrate a reduction in late-onset sepsis in participating NICUs.50 Single-family rooms promote better practices for infection control by avoiding overcrowding, providing areas for hand washing within each room, creating an atmosphere that promotes breastfeeding, and providing a more controlled environment for placement of intravascular catheters. In 2 unpublished reports, Oelrich noted an increase in nosocomial infection with the change to single family room care, whereas Rosenblum reported an 11.8% reduction in documented bacterial and fungal infections.51,52 Clearly, there is question of whether single-room NICU design is a significant factor in reducing hospital-acquired infection.
There are other medical outcomes that may also improve in a single-room NICU. Necrotizing enterocolitis (NEC) is a serious condition of unknown etiology that is more prevalent among the smallest and most fragile infants. Ischemia and/or reperfusion appear to play a significant role in the development of NEC. Temporal and spatial clustering is often present in an outbreak in the NICU, suggesting the possibility of an infectious etiology. Human milk-fed infants seem to be protected from fulminant NEC.53,54 The single-room NICU aim of reducing infant stress has the potential to improve physiological stability, feeding tolerance and growth. The potential for increased privacy in the single-room NICU alone, offers opportunities for reduced pathogen exposure, increased skin-to-skin holding, breast milk pumping at the bedside, breastfeeding, and the associated behavioral and immunologic benefits for the infant.55,56
Control over environmental stimuli, such as light, sound, and temperature, could reduce stress on infants and caregivers and have other benefits.49,57 Infants, family members and staff will no longer have the same exposure to the sights and sounds of critical care being administered to multiple infants, sources of extraneous noise (ie, staff conversation and multiple alarms) should be reduced,44 and more optimal sleep patterns are likely to be increased.58 The single-room design should be more conducive to the provision of developmental care, including modifying environmental conditions to the needs and tolerances of the individual, for example, cycled lighting.59 Staff will be able to focus on one infant at a time, with fewer distractions and interruptions,19 possibly reducing medication errors.60 The single-room NICU should facilitate family centered care and a more consumer-focused orientation. Additional privacy and confidentiality can be provided for families, in accordance with Health Insurance Portability and Accountability Act guidelines, as well as the enhanced ability to participate in their infants’ care.61 Privacy for pumping of breast milk, skin-to-skin care, putting the infant to breast, parent support counseling, and participation with the medical team on rounds62 should contribute to increased family satisfaction and improved infant medical and neurobehavioral outcome. In addition to the aforementioned considerations, improved thermal regulation, reduced need for sedation, less movement of infants, and reduced overall cost for hospitalization have also been mentioned as advantages of a single-room NICU design.63 Staff may find the physical environment of the single-room design to be more satisfying and report more job satisfaction.20,21,64
However, there are also potential problems with the single-room NICU design. There is concern over infant safety and the potential need for additional nursing staff to effectively observe and monitor infants in single rooms. Isolation of nursing staff who are accustomed to providing care in close proximity with other nurses and reduced availability of collegial support in caring for infants is a possible liability of single-room care. Nurses point out the significant amount of teaching and learning that takes place by observation of other nurses in the open-bay environment. Nurses and house staff also voice concerns regarding the increased presence of family members at the bedside during procedures, including additional infection control concerns. As mentioned previously, there is a report of increased nosocomial infection in the single-room NICU that could be attributable to the increased presence of family members.52 The inherent difficulties of performing (and learning to perform) procedures under the watchful eyes of parents, and a perceived increase in the potential for medical liability, under these circumstances may also be troubling to NICU nurses and house staff. Families also may experience increased isolation and stress as they begin to spend greater amounts of time at the bedside and less time in traditional gathering places, such as waiting areas.20,21,64 Added to these care-related concerns are the logistical difficulties of single-room occupancy by infants and their families. These include increased planning and coordination of services required in preparing, stocking, cleaning, and maintaining individual rooms that will be occupied by the same infant and family members for prolonged periods. There may be greater stress on the nursing staff. Increased staff workload could lead to lower professional satisfaction, and the need for additional staff.20,21,64
Mediating Factors
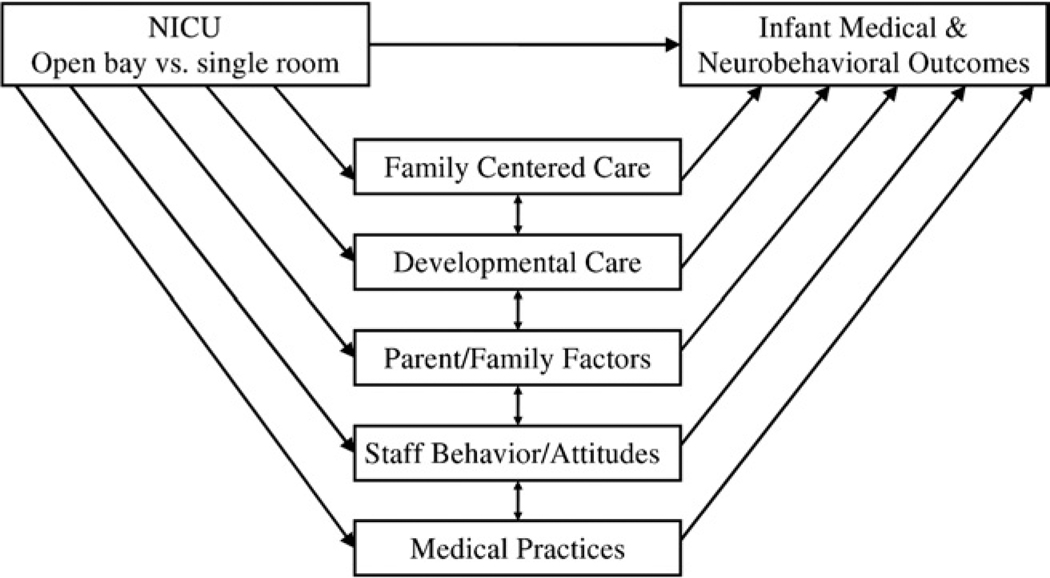
The preceding discussion highlights the many factors that may influence infant outcome in a NICU and that could change with the move to a single-room NICU design. To develop evidence-based practice for optimal standards of care, it is important to analyze the individual contribution of these factors on infant medical and neurobehavioral outcome. Research in the NICU is challenging because the NICU is a highly integrated, interdisciplinary, dynamic system. There are likely to be changes in the NICU correlated with the changeover from open-bay to single-room NICU. These changes could also contribute to medical and neurobehavioral improvements. We have developed a theoretic model (Fig. 1) that may be useful in studying how the changeover from an open-bay NICU to a single-room NICU could affect infant medical and neurobehavioral outcome. The model identifies factors that are likely to accompany the change to a single-room NICU and allows for the inclusion of these factors as potential mediating variables. We have identified 5 categories of possible mediators. These are discussed below and include family centered care, developmental care, parenting and family factors, staff behavior and attitudes, and medical practices.
Figure 1.
Model of NICU design and infant outcome.
Family Centered Care
In general pediatric use, family centered care is a philosophy of care that recognizes and respects the pivotal role of the family in the lives of children with special health needs.62 It is a philosophy that strives to support families in their natural caregiving roles by building upon their unique strengths as individuals and as families. It views parents and professionals as equals in a partnership committed to excellence at all levels of healthcare.65 In neonatal care, family centered care is the active partnership of the parents in the infant’s plan and delivery of care.22 It is a philosophy of care that supports the integrity of the family and individualizing care to promote infant and parent health.66,67 In some cases, family centered care includes developmental care (described below) resulting in terms, such as “developmentally supportive family centered care.”67 Although we recognize that in practice, it may make sense to combine family centered care and developmental care, for heuristic reasons we will keep them separate and treat them as different (albeit related) factors. There are very few studies of infant outcomes related to family centered care. Infants receiving developmentally supportive family centered care showed fewer behavioral stress cues than controls, but there were no differences in any of the physiological or growth variables except respiratory rate.67 A pre-post study of the implementation of potentially better practices found no differences in length of stay and feeding of very-low-birthweight infants.68 In a study in which daily interdisciplinary rounds occurred at the bedside with parents participating in care decisions, significant effects included reduced length of stay, fewer rehospitalizations, reduced use of the emergency department, and reduced parental anxiety.69 Reduced parenting stress, increased comfort level and confidence were also related to family centered care.70
Parental measures of family centered care in the NICU focus on parent satisfaction with the hospitalization experience.67,71 Parents report overall satisfaction but suggest a need for improvement in the following areas: communication with the health care team, more hands-on practice in caring for infants, better relationships with nursing staff, and more room at the infant’s bedside for the family.71 No differences in parental perception of family centered care were found in the above study of infants receiving developmentally supportive care.67 There are also challenges when trying to implement family centered care in an institution where a provider centric model has predominated. Parental satisfaction with family centered care may vary depending on infant medical (eg, birthweight) or parental demographic (eg, education) factors.71 Parents report that poor relationships with the health care staff increase parental stress and that better relationships develop when professionals recognize the mothers’ ability to care for their infant, provide information appropriately and are sensitive to parents’ emotional needs.72–75 Barriers to family centered care reported by nurses include lack of adequate education on implementing elements of family centered care, lack of skills in counseling and family dynamics, and a need for clarification of health professional roles, lack of support and accountability to incorporate family centered care into daily professional practice and no shared understanding of what family-centered care is among professionals and families.65,76,77
Developmental Care
Developmental care is a broad category of interventions designed to reduce the effects of stress on the infant in the NICU.78 The goal is to conserve the infants’ energy for growth, facilitate physiological stability and the infants’ recovery from illness. It has been estimated that 64% of U.S. NICUs have instituted some type of developmental care.79 Descriptions of the components of developmental care differ although there is considerable overlap. According to the National Association of Neonatal Nurses, the principle components of developmental care are flexed positioning, clustering of care, promotion of nonnutritive sucking, kangaroo care, cobedding of multiples, activities to promote self-regulation and state regulation, and collaboration with parents to promote bonding.80 A Cochrane review identified the elements of developmental care as control of external stimuli (vestibular, auditory, visual, tactile), clustering of nursery care activities and positioning or swaddling.78 Developmental care interventions may be used singly (swaddling the infant) or in combinations (eg, control of light, vestibular stimuli and clustering nursing care activities). The Neonatal Individualized Developmental Care and Assessment Program81 includes a combination of developmental care components that are individualized to the needs of each infant. The individualized aspect of developmental care is thought to be especially important.82 There is empiric support to show that when this approach is used, the infant demonstrates improved regulation of motor activity, sleep/wake cycles, heart rate and oxygen saturation levels.83,84 Two recent randomized clinical trials have shown mixed results of Neonatal Individualized Developmental Care and Assessment Program intervention.85,86 Sensitivity training for parents of preterm infants in the NICU was related to reduced infant stress, increased kangaroo care, and increased maturation in brain white matter using diffusion tensor imaging.87 Our own work88 with more of a focus on parental psychological issues and helping them get to “know” their infant showed positive effects of an individualized intervention on both infant and parent behavior.
The Cochrane review examined the best evidence (36 studies) on developmental care to guide nursing practice and research.78 The review served to point out some of the problems in these studies, including the wide variation in the types of interventions included as “developmental care,” the wide range of outcomes, and lack of blinding. Nevertheless, they did conclude that there was evidence of limited benefit of developmental care. Clearly more research in this area is needed. It is noteworthy that developmental care effects were found on medical outcomes (eg, chronic lung disease) as well as neurodevelopment, 2 outcome domains in our model (Fig. 1). Although we acknowledge that skin-to-skin holding/kangaroo care, breastfeeding, and parent visitation and participation in infant care are often considered elements of developmental care, we treat these factors as part of parenting and family factors below.
Parenting and Family Factors
Parenting and family factors can affect infant outcome and may also change with the transition to the single-room NICU. These factors include parenting behavior as well as psychological factors. Parenting behavior includes visitation as well as parent’s participation in their infant’s care. The importance of maternal visitation has been well documented and is related to factors, such as length of stay.89 Maternal participation in their infant’s care in the NICU through practices, such as breastfeeding, massage,90 kangaroo care/skin to skin,91–93 and infant care have also been shown to be beneficial. It is reasonable that mothers who have increased privacy as afforded in a single-room NICU will have increased opportunities for provision of skin-to-skin holding, pumping of breast milk and breastfeeding, and will be more likely to continue to provide breast milk for their infants through discharge which in turn may improve infant outcome.94 A recent cost analysis suggested that the impact of breastfeeding on a variety of illnesses could result in a potential cost savings of US$13 billion per year.95
Maternal psychological factors may also affect infant outcome. The typical NICU is stressful for caregivers as well as infants.23,24,96 Maternal depression in the NICU has long been recognized,97–100 and family functioning may also be disrupted by the NICU experience.14,101,102 Our own work examined maternal stress and depression88 and maternal concerns determined by experiences in the NICU that affected mother infant interaction.103 We88 as well as others104 have shown that parenting-based NICU interventions can improve maternal mental health outcomes. Parent satisfaction may also be related to infant outcome. Roman et al105 reported that parent–parent support increased self-esteem and reduced anxiety in a family centered care group. With more privacy for caregiving, such as skin-to-skin holding and breastfeeding and increased participation in infant care, parent satisfaction may also improve.
Staff Behavior and Attitudes
Caring for infants in the NICU is stressful for nurses and the entire staff. Everyday realities in the NICU include unusual sights, painful realities, and life-and-death decisions.106 Factors contributing to this stress include providing highly technological care, regularly confronting ethical-moral dilemmas and interpersonal conflicts among staff and between staff and parents. These conflicts can lead to reduced job satisfaction, high nurse turnover rates and potentially poorer patient out-comes.107,108 These events may lead to burnout shown by emotional exhaustion, depersonalization and a reduced sense of personal accomplishment. Nurses in intensive care environments may experience traumatic stress similar to that of soldiers and rescue workers.109
Stress and trauma may be intensified because neonatal nurses care for the parents as well as the infant. Parents may feel overwhelmed by the NICU environment110–112 and may find it difficult to understand the complex information they receive making it problematic to collaboratively interact with professionals to arrive at treatment decisions.113,114 It is often the NICU nurse who translates medical information, supports parent-professional discussion, and helps parents recognize the infant’s condition and progress. Helping the family work through these emotions to optimally support their infant is part of a team approach, including the NICU nurse, advanced practice nurse, and social worker.88 Most research on staff behavior and attitudes is based on nurse reports, as they represent the largest professional group in a NICU.
Changes in Medical Practice
Changes in medical practice may also occur that could affect infant outcome. Researchers have reported tracking their “in-house” practices in the NICU. Tracking practices in the NICU have been used to measure changes in the incidence of pneumothorax among infants <28 weeks115 and to improve practices for pain management and sedation.116 In other work, a computer-based tracking system led to a reduction in medication errors.117 A comparison of 2 NICUs showed differences in phlebotomy and transfusion practices that were used to identify areas for improvement.118
Studying the Effects of the Single-Room NICU Design
As mentioned previously, there is every reason to hypothesize that the single-room NICU will have positive effects by reducing stress to the infant and parent, improving infant outcome, increasing sensitivity to the developmental needs of the infant, increasing family involvement in care, providing more privacy which will increase breastfeeding and kangaroo care, and increasing staff satisfaction. However, this is still somewhat of an “urban legend.” A retrospective analysis after the move to a single-room NICU showed a decrease in nosocomial infection rates.20 In surveys conducted in the same NICU, 6 months before and 6 months after the transition to the private room, nurses reported increased job satisfaction, team member support, and validation of individual opinions. Parents reported improvements in privacy, noise, and lighting in the NICU.21 In other prospective studies, improvements in the single-room NICU have been reported by the staff in the physical environment, patient care, job, technology and off-the-job quality as improved in the single-room NICU.119 Concerns have also been expressed. Staff and parents perceived the advantages and disadvantages differently.120 These include infant safety, need for more staff, increased isolation of staff and families, complications of having families present during procedures, increased pressure on parents to take care of their infants, and increased stress on staff and logistical complications.20,21,119,120 We are conducting a study to compare infant medical and neurobehavioral outcomes between infants in an open-bay nursery and infants in a single-room NICU by using the model shown in Fig. 1. Our new 70-room single-room NICU opened in the fall of 2009. Our previous open-bay NICU had 60+ beds organized into 5 bays, each housing 10–12 individual bed spaces, falling far short of the recommended minimum guideline of 120 square-feet per bed (Fig. 2A, B). Because we were aware of the dramatic impact that the new NICU could have, we collected data in the “old” open-bay NICU on 150 patients. The demographics of this sample were 22.4% Hispanic, 21.8% black, 49.1% white and 3.6% Asian. The mothers ranged in age from 16 to 42 (mean is 29.26), 49.1% had a high school education or higher, 26.2% had multiple births, and 93% had a partner. Family income was less than US$25,000/year for 45.5% of the sample. Infants birthweight ranged from 490 to 1430 g (mean 1024 g), gestational age ranged from 23 to 33 weeks (mean, 27 weeks). Regarding illness, 17.3% had sepsis, 23.5% had intra-ventricular hemorrhage (grade 1 and 2 only) 26.9% had bron-chopulmonary dysplasia, 11.3% had NEC, and 37.7% had retinopathy of prematurity (30.2% stages 1–2). This sample provides an exciting and unprecedented opportunity to compare infant outcome in the open-bay NICU with the outcome of infants in the new single-room NICU.
Figure 2.
(A) “Old” open-bay NICU. (B) “New” single-room NICU. (Color version of figure is available online.)
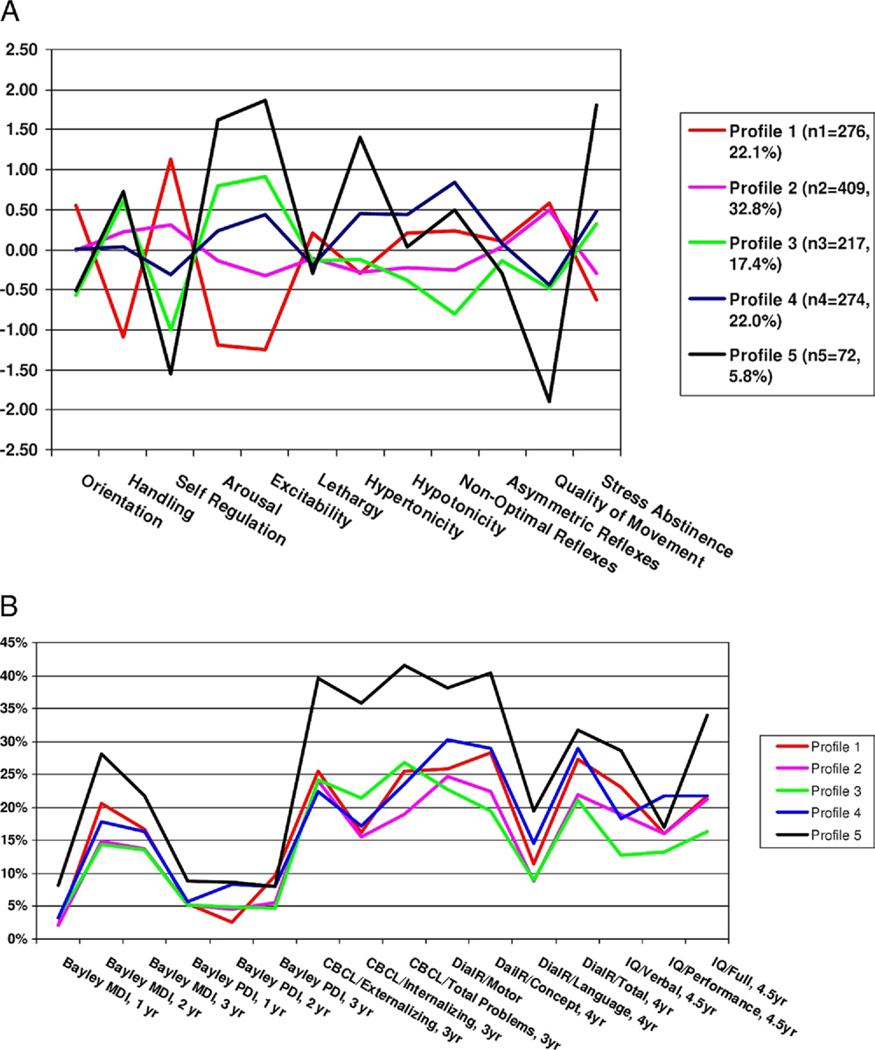
Our primary infant neurobehavioral outcome measure is the NICU Network Neurobehavioral Scale (NNNS).121 The NNNS is a comprehensive assessment of infant neurobehavior that was developed to provide an assessment of both neurological integrity and behavioral function of infants at risk due to factors, such as prenatal exposure to drugs and/or prematurity. The NNNS has been used in studies of cocaine exposed infants,122,123 tobacco-exposed infants,124 infants treated for withdrawal with opiates and phenobarbital versus opiates alone,123,125 withdrawal symptoms in infants of buprenorphine and methadone-treated heroin addicted mothers,126 and infants of depressed mothers.127 Fetal behavior was correlated with scores on the NNNS.128 In a study of preterm infants, NNNS scores were related to brain volumes of white matter, basal ganglia, and total brain tissue129 and impaired neurobehavioral findings on the NNNS was related to decreased regional brain volumes.130,131 In this same sample, NNNS scores predicted motor outcome on the Bayley scales at 24 months. In our work, Miller-Loncar et al132 found that motor scores on the NNNS were related in a path model to motor outcomes at 18 months. The NNNS has also been shown to have long-term predictive validity. Figure 3A shows 5 profiles or “types” of infants on the basis of their NNNS scores from a sample of more than 1200 at risk infants. Infants with profile 5 (Fig. 3A) showed the most abnormal pattern. They had poor attention that required extensive handling, poor regulation, highly aroused and excitable, poor quality of movement and a high number of stress signs. As shown in Fig. 3B, infants with profile 5 were more likely to show abnormalities between 2 and 4½ years on the Bayley Scales, behavior problems on the Child Behavior Checklist, deficits in school readiness (DIAL R), and low IQ. This is important because it suggests that infant neurobehavioral status at time of discharge from the NICU may be related to long-term developmental outcome. Thus, improvements in neurobehavioral status attributable to the single-room NICU could maximize the developmental outcome of NICU graduates. Other neurobehavioral outcomes that we plan to study include sleep state organization and sleep physiology, infant mother feeding interaction scores and pain scores.
Figure 3.
(A) Five NNNS profiles. (B) Proportion of infants with poor developmental outcomes. (Color version of figure is available online.)
The medical outcomes we plan to measure include sepsis, length of stay, gestational age at discharge, weight, weight gain, illness severity and resource use, gestational age at enteral feeding, and NEC. We expect that the NICU design will affect infant medical and neurobehavioral outcome and that this relationship will be attenuated with the mediators in the model. The mediating factors were described earlier.
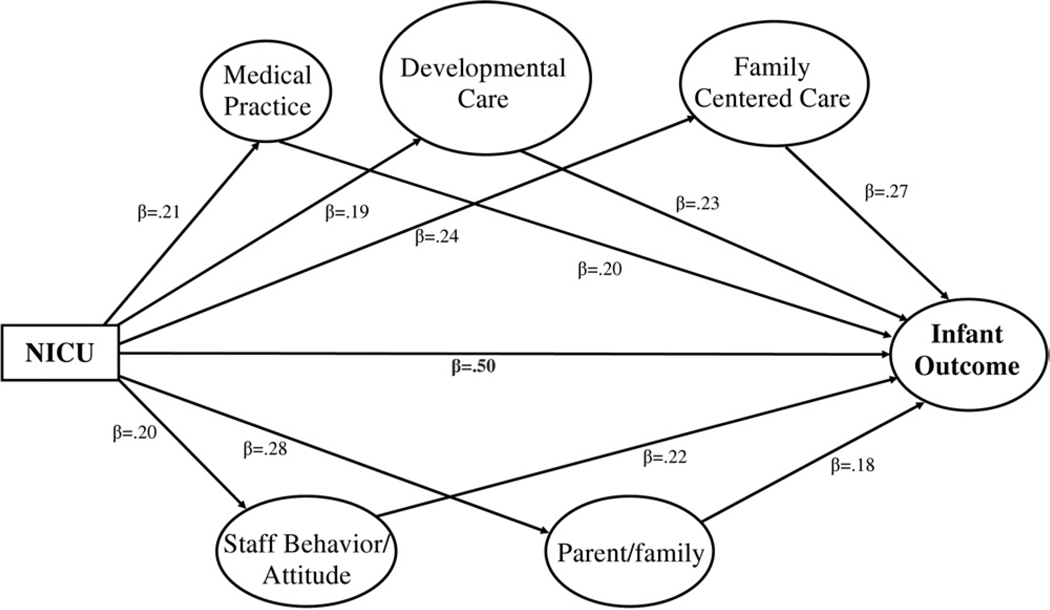
Figure 4 shows an example of a hypothetical outcome that could come from this study design. In the model all the paths represented by beta (β) coefficients are statistically significant. The path from NICU to infant outcome (β = 0.50) indicates that there is improvement in infant outcome in the new NICU with all the other factors in the model controlled. This would be considered a “main effect.” The model also shows that there are also effects of the mediator factors, suggesting that medical practice, developmental care, family centered care, staff behavior/attitudes, and parent/family each make their own independent contribution to improving infant outcome in the new NICU. Other scenarios are also possible. For example, there could be a main effect of the new NICU but that effect is substantially reduced or no longer statistically significant when all the mediating factors are entered into the model.
Figure 4.
Hypothetical outcome effects.
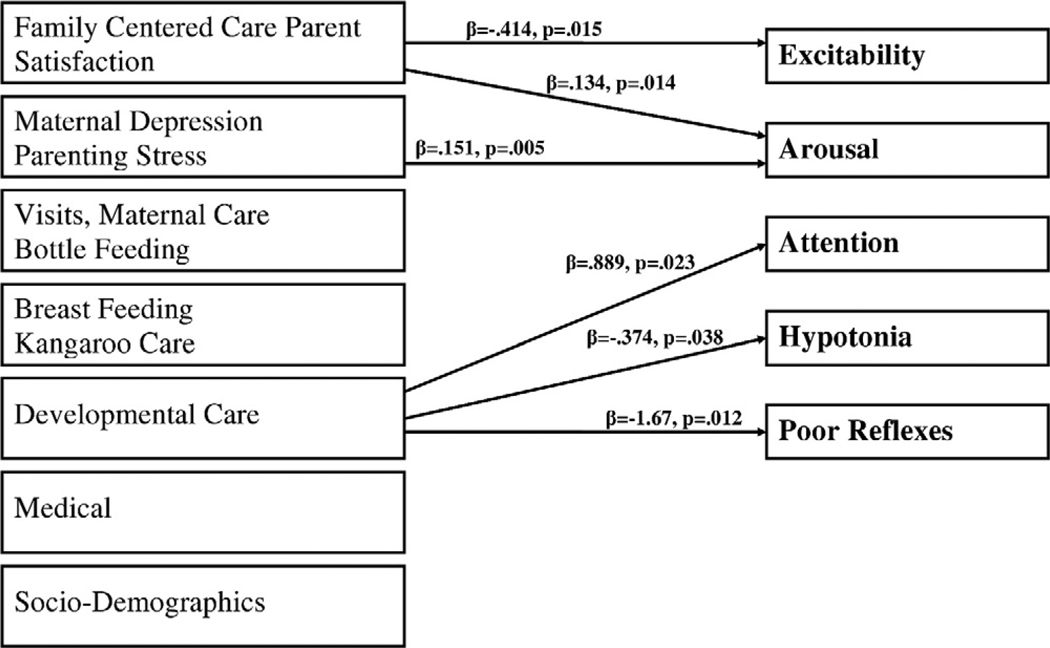
We have already analyzed some of the data from the sample of 150 patients in the open-bay NICU to explore some of the relationships among factors that we expect to change in the single-room NICU. We developed 7 composite scores to measure some of the mediators shown in Fig. 1. We used multivariate analysis to determine the effects of each factor on NNNS summary score with all the other factors controlled. As shown in Figure 5 the composite score indicating more family centered care and higher parent satisfaction was related to lower excitability and lower arousal on the NNNS. The maternal depression and parenting stress score showed that mothers who were less depressed and parents who were less stressed had infants with lower arousal. More developmental care was related to better attention, less hypotonia, and fewer nonoptimal reflexes. These preliminary findings are important because they provide a “baseline” of effects that are present in the open-bay NICU that we expect to change in the single-room NICU. For example, we expect breastfeeding and kangaroo care to increase in the single-room NICU and will also correlate with better NNNS scores. It is also noteworthy that the effects we do find are independent of the other variables in the model. Thus, even in an overcrowded, noisy, open-bay NICU, family centered care, parent satisfaction, maternal depression, parenting stress, and developmental care improve infant neurobehavior. We look forward to comparing these and other findings with data from the single-room NICU using structural equation modeling (a statistical technique) where causal pathways can be measured and we expect the relationships observed to date in the open-bay NICU to become even stronger in the single-room NICU.
Figure 5.
Open-bay factors and infant outcome.
Ideally, randomized trials of the single family room NICU paradigm compared with the bay-style NICU would be conducted. Although there are advantages of a randomized control trial over an observational study, there are also limitations to a randomized trial for this type of study. The infants of parents who agree to let their infants be randomized may not be representative of the NICU population in the same hospital. Infants will already be in an open bay or single room when the parents are approached for consent. Therefore, their decision to participate may be biased in their feelings about the current location of the infant. Because they will be unblinded, their interaction with their infant (which could change infant outcome) could be affected by where the infant is randomized. In addition, our study is also interested in additional factors related to the change from open bay to single-room nursery that may also affect infant outcome. Factors, such as changes in staff attitudes, family centered and developmental care could not be measured if both types of nurseries coexist in the same hospital unless different staff and different care were used in the 2 types of nurseries which are both unethical and impractical.
Theoretic Models
Theoretic models based on general systems theory133 are relevant for this type of study because they address the failure of linear models to explain variable outcomes for children with serious antecedent conditions, such as prematurity.134 Multilevel, incremental models of developmental change bring biological and social development into one theory. Change creates increases in the individual’s characteristics that expand the scope of their interactions with the environment.135 The neurobiological organization of the preterm infant is seen within the environmental systems of the NICU, including interaction between the child, caregiver and environment.136 There is a strong connection between the medical condition and physiological stability of the infant137 and the infant’s ability to benefit from developmental affordances138 within the environment. During the “in-turning” period, infant resources are primarily focused upon maintenance of physiological stability. Only after the infant is able to tolerate routine care without a loss of physiological stability, can the infant begin to demonstrate organized motor responses and to participate in social interaction with caregivers. The continual interplay between the immature preterm infant and the environment influences infant health, physiological functioning and neurobehavioral organization.81 Mechanisms of brain plasticity show how brain function can be altered through experience.139 Plasticity can be bidirectional and costly to the organism. In preclinical studies, rats with early cortical lesions at birth produced dendritic atrophy, whereas rats with later lesions showed increases in dendritic arborization and better recoveries. However, regeneration of new tissue after injury is not always adaptive and, depending on experience, can limit later proliferation.140 Four developmental courses have been suggested: injury so severe that the child does not recover; development proceeds despite injury; development is impacted early, but the child improves with age; and, developmental dysfunction becomes more evident with age.139,141 Thus, the timing of the injury and extent of plasticity (prenatally, during birth, postnatally), including the postnatal care environment in the NICU impacts later development. However, there may be another model based on developmental plasticity that involves epigenetic mechanisms.
An Epigenetic Model for the Single-Room NICU
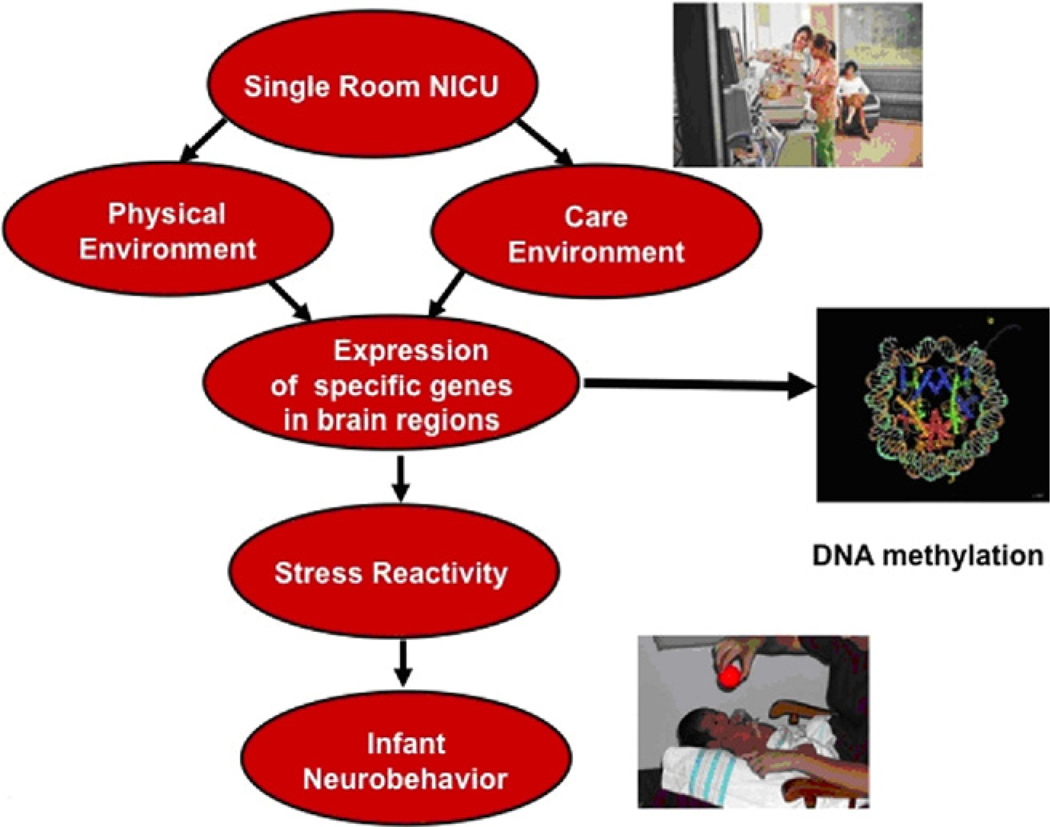
Epigenetic changes that operate either prenatally or postnatally could also explain infant outcome in the NICU. In a word, epigenetics can be viewed as inheritance of information based on gene expression control rather than on gene sequence;142 heritable, environmentally induced changes in chromatin remodeling and gene expression without altering DNA sequences. Here we provide one example of an epigenetic scenario that could occur in a NICU. We have selected this example because it has a history in animal research and because it is likely to be related to the single-room NICU.
Epigenetic changes due to effects of maternal care have been demonstrated in animal models. Rat pups licked and groomed by their mothers were less anxious, handled stress more effectively, and were more nurturing parents of their own offspring. Conversely, pups that were not licked and groomed had early menarche, more sexualized adolescent and adult behavior, greater pregnancy rates, and neglected their young. These maternal behaviors produced an epigenetic effect by altering the methylation status of the glucocorticoid receptor promoter region in the hippocampus of the rat pups.143 The rat pups licked and nurtured at birth had more developed hippocampi and produced less cortisol.143–145 These changes persisted into adulthood as reduced fear and moderated hypothalamic-pituitary-adrenal response to stress.144 Thus, maternal care modifies the expression of genes associated with behavioral and neuroendocrine responses, including response to distress, and fostered more optimal hippocampal synaptic development. The model in Figure 6 shows how a single-room NICU could produce similar effects. The single-room NICU provides the opportunity for enhanced maternal care and more positive mother infant interaction. This includes spending more time with the infant, participating in direct care (as part of family centered care), more breastfeeding, kangaroo care, and in general holding, talking to and interacting with the baby. Recall from our previous discussion that the goal of many of these parenting and developmental interventions were to reduce stress, on the infant and on the parent. These enhanced maternal care behaviors could result in similar epigenetic changes that observed in the rat studies; decreased methylation of the glucocorticoid receptor in the hippocampus in the infants. As a result, the infant would produce less cortisol and show less stress reactivity. On the NNNS these infants would show more modulated, better organized neurobehavior. They should be less reactive, less stressed, less excitable and aroused and show better self regulation and better attention. Thus, at discharge from the single-room NICU, these infants should have the optimal neurodevelopmental profile for long-term positive outcome.
Figure 6.
Model of single-room NICU improving infant outcome through epigenetic changes. (Color version of figure is available online.)
Conclusions
The evolution of the single-room NICU is a major response toward improving the care of preterm infants. It is a credit to the many disciplines that have worked together to bring about this change. The increasing rate of prematurity and associated morbidities in the United States demands a response of this magnitude. The trend toward single-room NICUs is increasing, and, although there is some justification for this change from several perspectives, scientific evidence is, at this point, mostly anecdotal. This is an exciting time with good reason to be optimistic in the development of our NICU “graduates.” The single-room NICU has the potential to improve the neurobehavioral status of the infant at discharge. Our neurobehavioral assessment can help with the early detection of which infants to target for preventive intervention to maximize their developmental outcome. In addition, as we learn more about mechanisms, such as epigenetics that affect infant behavior, we will be able to develop additional interventions to further improve the neurobehavioral status of these infants.
References
- 1.Wilson-Costello D, Friedman H, Minich N, et al. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990’s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 2.Van Baar AL, van Wassenaer AG, Briet JM, et al. Very preterm birth is associated with disabilities in multiple developmental domains. J Pediatr Psychol. 2005;30:247–255. doi: 10.1093/jpepsy/jsi035. [DOI] [PubMed] [Google Scholar]
- 3.Vincer MJ, Allen AC, Joseph KS, et al. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics. 2006;118:1621–1626. doi: 10.1542/peds.2006-1522. [DOI] [PubMed] [Google Scholar]
- 4.Martin JA, Osterman MJ, Sutton PD. Are preterm births on the decline in the United States? Recent Data from the National Vital Statistics System. NCHS Data Brief. 2010:1–8. [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar GH, Littenburg B, Petitti DB. Outcome among surviving very low birthweight infants: a meta-analysis. Arch Dis Child. 1991;66:204–211. doi: 10.1136/adc.66.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battin M, Ling EW, Whitfield MF, et al. Has the outcome for extremely low gestational age (ELGA) infants improved following recent advances in neonatal intensive care? Am J Perinatol. 1998;15:469–477. doi: 10.1055/s-2007-994068. [DOI] [PubMed] [Google Scholar]
- 8.Dahl LB, Kaaresen PI, Tunby J, et al. Emotional, behavioral, social, and academic outcomes in adolescents born with very low birthweight. Pediatrics. 2006;118:449–459. doi: 10.1542/peds.2005-3024. [DOI] [PubMed] [Google Scholar]
- 9.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 10.Hinz SR, Kendrick DE, Vohr BR, et al. Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993–1999. Pediatrics. 2005;115:1645–1651. doi: 10.1542/peds.2004-2215. [DOI] [PubMed] [Google Scholar]
- 11.Indredavick MS, Vik T, Heyerdahl S, et al. Psychiatric symptoms and disorders in adolescents with low birthweight. Arch Dis Child Fetal Neonat Ed. 2004;89:445–450. doi: 10.1136/adc.2003.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoelhorst GM, Rjken M, Martens SE, et al. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age < 32 weeks): the project on preterm and small for gestational age infants 1983 and the Leiden follow-up project on prematurity 1996–97. Pediatrics. 2005;115:396–405. doi: 10.1542/peds.2004-1497. [DOI] [PubMed] [Google Scholar]
- 13.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–94. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 14.Singer LTP, Salvator AM, Guo SP, et al. Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. JAMA. 1999;281:799–805. doi: 10.1001/jama.281.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, et al. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 16.Martin JA, Hamilton BE, Sutton PD, et al. National Vital Statistics Reports 55. Washington, DC: National Center for Health Statistics; 2006. Births: final data for 2004; pp. 1–102. [PubMed] [Google Scholar]
- 17.White R, Whitman T. Design of ICUs. Pediatrics. 1992;89:1267. [PubMed] [Google Scholar]
- 18.White R, Graven S. New concepts, science, experiences drive innovation of designs: the changing face of the newborn ICU. Av Fam Centered Care. 2000;9:7–10. [Google Scholar]
- 19.McGrath JM. Single-room design in the NICU: making it work for you. J Perinat Neonat Nurs. 2005;19:210–211. doi: 10.1097/00005237-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Walsh WF, McCullough KL, White R. Room for improvement: nurses’ perceptions of providing care in a single room newborn intensive care setting. Adv Neonat Care. 2006;6:261–270. doi: 10.1016/j.adnc.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Carlson B, Walsh S, Wergin T, et al. Challenges in design and transition to a private room model in the neonatal intensive care unit. Adv Neonat Care. 2006;6:271–280. doi: 10.1016/j.adnc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Byers JF. Components of developmental care and the evidence for their use in the NICU. Maternal child. Nurs. 2003;28:175–180. doi: 10.1097/00005721-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Bremmer P, Byers JF, Kiehl E. Noise and the premature infant: physiological effects and practice implications. J Obstet Gynecol Neonat Nurs. 2003;32:447–454. doi: 10.1177/0884217503255009. [DOI] [PubMed] [Google Scholar]
- 24.Shaw RJ, Deblois T, Ikuta L, et al. Acute stress disorder among parents of infants in the neonatal intensive care nursery. Psychosomatics. 2006;47:206–212. doi: 10.1176/appi.psy.47.3.206. [DOI] [PubMed] [Google Scholar]
- 25.Long JG, Lucey JF, Philip AG. Noise and hypoxemia in intensive care nursery. Pediatrics. 1980;65:143–145. [PubMed] [Google Scholar]
- 26.Anderson BM, Lindemann R, Bergh K, et al. Spread of methicillink-resistant Staphylococcus aureus in a neonatal intensive unit associated with understaffing, overcrowding and mixing of patients. J Hosp Infect. 2002;50:18–24. doi: 10.1053/jhin.2001.1128. [DOI] [PubMed] [Google Scholar]
- 27.Kibbler C, Quick A, O’Neill AM. The effect of increased bed numbers on MRSA transmission in acute medical wards. J Hosp Infect. 1998;39:213–219. doi: 10.1016/s0195-6701(98)90260-2. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn S. Environmental impact of the NICU on developmental outcomes. J Pediatr Nurs. 1998;13:279–289. doi: 10.1016/S0882-5963(98)80013-4. [DOI] [PubMed] [Google Scholar]
- 29.Glass P, Avery GB, Suramanian KNS, et al. Effects of bright light in the hospital nursery on the incidence of retinopathy of prematurity. N Engl J Med. 1985;313 doi: 10.1056/NEJM198508153130701. 491-440. [DOI] [PubMed] [Google Scholar]
- 30.Lotas MJ. Effect of light and sound in the neonatal intensive care unit environment on the low-birth-weight infants. NAACOGs Clin Issues Perinat Womens Health Nurs. 1992;3:34–44. [PubMed] [Google Scholar]
- 31.American Academy of Pediatrics. Committee on Environmental Health: Noise: a hazard for the fetus and newborn. Pediatrics v100. 1997:724–727. [PubMed] [Google Scholar]
- 32.Graven S. Sound and the developing infant in the NICU: conclusions and recommendations for care. J Perinatol. 2000;20:88–93. doi: 10.1038/sj.jp.7200444. [DOI] [PubMed] [Google Scholar]
- 33.Gray K, Dostal S, Ternullo C, et al. Developmentally supportive care in a Neonatal Intensive Care Unit: a research utilization project. Neonat Netw. 1998;17:33–37. [PubMed] [Google Scholar]
- 34.Kent W, Tank A, Clarke M, et al. Excessive noise levels in the neonatal ICU: potential effects on auditory system development. J Otolaryngol. 2002;31:355. doi: 10.2310/7070.2002.34358. [DOI] [PubMed] [Google Scholar]
- 35.Matook SA, Sullivan MC, Salisbury A, et al. Variations of NICU sound by location and time of day. Neonat Netw. 2010;29:87–95. doi: 10.1891/0730-0832.29.2.87. [DOI] [PubMed] [Google Scholar]
- 36.American Academy of Pediatrics, Committee on Environmental Health: Noise: a hazard for the fetus and newborn. American Academy of Pediatrics 100. Committee on Environmental Health. Pediatrics. 1997;100:724–727. [PubMed] [Google Scholar]
- 37.Miller CL, White R, Whitman TL, et al. The effect of cycled versus non-cycled lighting on growth and development in preterm infants. Infant Behav Dev. 1995;18:87–95. [Google Scholar]
- 38.Rivkees SA, Mayes L, Jacobs H, et al. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 2004;113:833–839. doi: 10.1542/peds.113.4.833. [DOI] [PubMed] [Google Scholar]
- 39.Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks’ gestation have improved growth in cycled light compared to continuous near darkness. J Pediatr. 2002;140:192–199. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhury H, Mahmood A, Valente M. Nurses’ perception of single-occupancy versus multioccupancy rooms in acute care environments: an exploratory comparative assessment. Appl Nurs Res. 2006;19:118–125. doi: 10.1016/j.apnr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava S, Shetty N. Healthcare-associated infections in neonatal units: lessons from contrasting worlds. J Hosp Infect. 2007;65:292–306. doi: 10.1016/j.jhin.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Von D, Brito D, de Almeida Silva H, et al. Effect of neonatal intensive care unit environment on the incidence of hospital-acquired infection in neonates. J Hosp Infect. 2007;65:314–318. doi: 10.1016/j.jhin.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Abraham R, Keller N, Szold O, et al. Do isolation rooms reduce the rate of nosocomial infections in the pediatric intensive care unit? J Crit Care. 2002;17:176–180. doi: 10.1053/jcrc.2002.35809. [DOI] [PubMed] [Google Scholar]
- 44.Chang VT, Nelson K. The role of physical proximity in nosocomial diarrhea. Clin Infect Dis. 2000;31:717–722. doi: 10.1086/314030. [DOI] [PubMed] [Google Scholar]
- 45.Goldmann DA, Durbin WA, Freeman J. Nosocomial infections in a neonatal intensive care unit. J Infect Dis. 1981;144:449–459. doi: 10.1093/infdis/144.5.449. [DOI] [PubMed] [Google Scholar]
- 46.McManus AT, Mason AD, McManus WF, et al. A decade of reduced gram-negative infections and mortality associated with improved isolation of burned patients. Arch Surg. 1994;129:1306–1309. doi: 10.1001/archsurg.1994.01420360096013. [DOI] [PubMed] [Google Scholar]
- 47.Mulin B, Rouget C, Clement C, et al. Association of private isolation rooms with ventilator-associated Acinetobacter baumanii pneumonia in a surgical intensive care unit. Infect Control Hosp Epidemiol. 18:499–503. doi: 10.1086/647655. [DOI] [PubMed] [Google Scholar]
- 48.Rao KKM. Structural requirements for infection control in hospitals. J Acad Hosp Admin. 2004;16:7–12. [Google Scholar]
- 49.Ulrich R, Quan X, Zimring C, et al. The Role of the Physical Environment in the Hospital of the 21st Century: a One-In-a-Lifetime Opportunity: Report to the Center for Health Design for the Designing the 21st Century Hospital Project. Robert Wood Johnson Foundation. 2004 [Google Scholar]
- 50.Horbar JD, Rogowski J, Plsek PE, et al. Collaborative quality improvement for neonatal intensive care. Pediatrics. 2001;107:14–22. doi: 10.1542/peds.107.1.14. [DOI] [PubMed] [Google Scholar]
- 51.Rosenblum D. Single family room care: before and after data. Clearwater, FL: The Physical and Developmental Environment of the High-Risk Infant Conference; 2005. [Google Scholar]
- 52.Oelrich T. Single room NICU: fad or future. Denver, CO: AIA Academy of Architecture for Health Conference; 2003. [Google Scholar]
- 53.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotizing enterocolitis. Semin Fetal Neonat Med. 2006;11:369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Yeo SL. NICU update: state of the science of NEC. J Perinat Neonat Nurs. 2006;20:46–50. doi: 10.1097/00005237-200601000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Hill PD, Aldag JC, Demirtas H, et al. Mood states and milk output in lactating mothers of preterm and term infants. J Hum Lact. 2006;22:305–314. doi: 10.1177/0890334406290003. [DOI] [PubMed] [Google Scholar]
- 56.Spatz DL. State of the science: use of human milk and breast-feeding for vulnerable infants. J Perinat Neonat Nurs. 2006;20:51–55. doi: 10.1097/00005237-200601000-00017. [DOI] [PubMed] [Google Scholar]
- 57.Philbin MK. Planning the acoustic environment of a neonatal intensive care unit. Clin Perinatol. 2004;31:331–352. doi: 10.1016/j.clp.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Johnson BH, Abraham MR, Parrish RN. Designing the neonatal intensive care unit for optimal family involvement. Clin Perinatol. 2004;31:353–382. doi: 10.1016/j.clp.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Blackburn S, Patteson D. Effects of cycled light on activity state and cardiorespiratory function in preterm infants. J Perinat Neonat Nurs. 1991;4:47–54. doi: 10.1097/00005237-199103000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Flynn EA, Barker KN, Gibson JT, et al. Impact of interruptions and distractions on dispensing errors in an ambulatory care pharmacy. Am J Health Syst Pharm. 1999;56:1319–1325. doi: 10.1093/ajhp/56.13.1319. [DOI] [PubMed] [Google Scholar]
- 61.Griffin T. Family-centered care in the NICU. J Perinat Neonat Nurs. 2006;20:98–102. doi: 10.1097/00005237-200601000-00029. [DOI] [PubMed] [Google Scholar]
- 62.Shelton TL, Jeppson ES, Johnson BH. Family-Centered Care for Children With Special Health Care Needs. Washington, DC: Association for the Care of Children’s Health; 1987. [Google Scholar]
- 63.Smith J, Bajo K, Hager J. Planning a developmentally appropriate neonatal intensive care unit. Clin Perinatol. 2004;31:313–322. doi: 10.1016/j.clp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Harris DD, Shepley MM, White RD, et al. The impact of single family room design on patients and caregivers: executive summary. J Perinatol. 2006;26:S38–S48. [Google Scholar]
- 65.Bruce B, Ritchie J. Nurses’ practices and perceptions of family centered care. J Pediatr Nurs. 1997;12:214–222. doi: 10.1016/S0882-5963(97)80004-8. [DOI] [PubMed] [Google Scholar]
- 66.Malusky SK. A concept analysis of family-centered care in the NICU. Neonat Netw. 2005;24:25–32. doi: 10.1891/0730-0832.24.6.25. [DOI] [PubMed] [Google Scholar]
- 67.Byers JF, Lowman LB, Francis J, et al. A quasi-experimental trial on individualized, developmentally supportive family-centered care. J Obstet Gynecol Neonat Nurs. 2006;35:105–115. doi: 10.1111/j.1552-6909.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 68.Saunders RP, Abraham MR, Crosby MJ, et al. Evaluation and development of potentially better practices for improving family-centered-care in neonatal intensive care units. Pediatrics. 2003;111:437–449. [PubMed] [Google Scholar]
- 69.Forsythe P. New practices in the transitional care center improve outcomes for babies and their families. J Perinatol. 1998;18:S13–S17. [PubMed] [Google Scholar]
- 70.Cooper LG, Gooding JS, Gallagher J, et al. Impact of a family-centered care initiative on NICU care, staff and families. J Perinatol. 2007;27 suppl 2:S32–S37. doi: 10.1038/sj.jp.7211840. [DOI] [PubMed] [Google Scholar]
- 71.Bruns DA, Klein S. An evaluation of family-centered care in a level III NICU. Infants Young Child. 2005;18:222–233. [Google Scholar]
- 72.Van Riper M. Family-provider relationships and well-being in families with preterm infants in the NICU. Heart Lung. 2001;30:74–84. doi: 10.1067/mhl.2001.110625. [DOI] [PubMed] [Google Scholar]
- 73.Bruns DA, McCollum JA. Partnerships between mothers and professionals in the NICU: caregiving, information exchange, and relationships. Neonat Netw. 2002;21:12–23. doi: 10.1891/0730-0832.21.7.15. [DOI] [PubMed] [Google Scholar]
- 74.Fenwick J, Barclay L, Schmied V. Struggling to mother: a consequence of inhibitive nursing interaction in the neonatal nursery. J Perinat Neonat Nurs. 2001;15:49–64. doi: 10.1097/00005237-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Holditch-Davis D, Miles MS. Mothers’ stories about their experiences in the neonatal intensive care unit. Neonat Netw. 2000;19:13–21. doi: 10.1891/0730-0832.19.3.13. [DOI] [PubMed] [Google Scholar]
- 76.Petersen MF, Cohen J, Parsons V. Family-centered care: do we practice what we preach? J Obstet Gynegol Neonatal Nursing. 2004;33:421–427. doi: 10.1177/0884217504266772. [DOI] [PubMed] [Google Scholar]
- 77.Heerman JA, Wilson ME. Nurses’ experiences working with families in a NICU during implementation of family focused developmental care. Neonat Netw. 2000;19:23–29. doi: 10.1891/0730-0832.19.4.23. [DOI] [PubMed] [Google Scholar]
- 78.Symington A, Pinelli JM. Distilling the evidence on developmental care: a systematic review. Adv Neonat Care. 2002;2:198–221. doi: 10.1053/adnc.2002.34546. [DOI] [PubMed] [Google Scholar]
- 79.Aspaugh A, Leick-Rude JK. Developmental care teams in the neonatal intensive care unit: survey on current status. J Perinatol. 1999;19:48–52. doi: 10.1038/sj.jp.7200101. [DOI] [PubMed] [Google Scholar]
- 80.National Association of Neonatal Nurses (NANN): Infant and Family-Centered Care: Guidelines for Practice. Des Moines, IA: NANN; 2000. [Google Scholar]
- 81.Als H. A synactive model of neonatal behavioral organization: framework for the assessment and support of the neurobehavioral development of the premature infant and his parents in the environment of the neonatal intensive care unit. Phys Occup Ther Pediatr. 1986;6:3–55. [Google Scholar]
- 82.McGrath JM. Developmentally supportive caregiving and technology in the NICU: isolation or merger of intervention strategies? J Perinat Neonat Nurs. 2000;14:78–91. doi: 10.1097/00005237-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Beal JS. Evidence for best practices in the neonatal period. Maternal child. Nurs. 2005;30:397–403. doi: 10.1097/00005721-200511000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Als H. Individualized developmental care for the very low birthweight preterm infant: medical and neurofunctional effects. JAMA. 1994;272:853–858. [PubMed] [Google Scholar]
- 85.Maguire CM, Walther FJ, Sprij AJ, et al. Effects of individualized developmental care in a randomized trial of preterm infants <32 weeks. Pediatrics. 2009;124:1021–1030. doi: 10.1542/peds.2008-1881. [DOI] [PubMed] [Google Scholar]
- 86.Peters KL, Rosychuk RJ, Hendson L, et al. Improvement of short- and long-term outcomes for very low birthweight infants: Edmonton NID-CAP trial. Pediatrics. 2009;124:1009–1020. doi: 10.1542/peds.2008-3808. [DOI] [PubMed] [Google Scholar]
- 87.Milgron J, Newnham C, Anderson P, et al. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res. 2010;67:330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]
- 88.Meyer EC, Garcia Coll CT, Lester BM, et al. Family-based intervention improves maternal psychological well-being and feeding interaction of preterm infants. Pediatrics. 1994;93:241–246. [PubMed] [Google Scholar]
- 89.Zeskind PS, Iacino R. Effects of maternal visitation of preterm infants in the neonatal intensive care unit. Child Dev. 1984;55:1887–1893. [PubMed] [Google Scholar]
- 90.Field T, Diego M, Hernandez-Reif M. Preterm infant massage therapy research: a review. Infant Behav Dev. 2010;33:115–124. doi: 10.1016/j.infbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feldman R, Eidelman AI. Intervention methods for premature infants: how and do they affect development. Clin Perinatol. 1998;25:613–626. [PubMed] [Google Scholar]
- 92.Ludington SM. Kangaroo Care GSK: The Best You Can Do for Your Preterm Infant. New York: Bantum Publishing Group; 1993. [Google Scholar]
- 93.Ludington-Hoe SM, Johnson MW, Morgan K, et al. Neurophysiologic assessment of neonatal sleep organization: preliminary results of a randomized, controlled trial of skin contact with preterm infants. Pediatrics. 2006;117:e909–e923. doi: 10.1542/peds.2004-1422. [DOI] [PubMed] [Google Scholar]
- 94.Vohr BR. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118:115–123. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 95.Bartick M, Stuebe A, Shealy KR, et al. Closing the quality gap: promoting evidence-based breastfeeding care in the hospital. Pediatrics. 2009;124:e793–e802. doi: 10.1542/peds.2009-0430. [DOI] [PubMed] [Google Scholar]
- 96.Miles MS, Funk SG, Kasper MA. The neonatal intensive care unit environment: sources of stress for parents. AACN Clin Issues Crit Care Nurs. 1991;2:346–354. doi: 10.4037/15597768-1991-2022. [DOI] [PubMed] [Google Scholar]
- 97.Brooten D, Gennaro S, Brown L, et al. Anxiety, depression, and hostility in mothers of preterm infants. Nurs Res. 1988;37:213–216. [PubMed] [Google Scholar]
- 98.Corter C, Minde K. Impact of infant prematurity on family systems. In: Wolraich M, editor. Advances in Developmental Behavioral Pediatrics. Greenwich, CT: JAI Press; 1987. [Google Scholar]
- 99.Crnic KA, Ragozin AS, Greenberg MT, et al. Social interaction and developmental competence of preterm and full-term infants during the first year of life. Child Dev. 1983;54:1199–1210. [PubMed] [Google Scholar]
- 100.Jeffcoate JA, Humphrey ME, Lloyd JK. Disturbance in parent-child relationship following preterm delivery. Dev Med Child Neurol. 1979;21:344–352. doi: 10.1111/j.1469-8749.1979.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 101.Macey TJ, Harmon RJ, Easterbrooks MA. Impact of premature birth on the development of the infant in the family. J Consult Clin Psychol. 1987;55:846–852. doi: 10.1037//0022-006x.55.6.846. [DOI] [PubMed] [Google Scholar]
- 102.McCormick MC, Stemmler MM, Bernbaum JC, et al. The very low birth weight transport goes home: impact on the family. J Dev Behav Pediatr. 1986;7:217–223. doi: 10.1097/00004703-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Keren M, Feldman R, Eidelman AI, et al. Clinical interview for high-risk parents of premature infants (CLIP) as a predictor of early disruptions in the mother-infant relationship at the nursery. Infant Ment Health J. 2003;24:93–110. [Google Scholar]
- 104.Melnyk B, Feinstein NF, Alpert-Gillis L, et al. Reducing premature infants’ length of stay and improving parents’ mental health outcomes with the creating opportunities for parent Empowerment (COPE) Neonatal Intensive Care Unit Program: a randomized, controlled trial. Pediatrics. 2006;118:1414–1427. doi: 10.1542/peds.2005-2580. [DOI] [PubMed] [Google Scholar]
- 105.Roman LA, Lindsay JK, Boger RP, et al. Parent-to-parent support initiated in the neonatal intensive care unit. Res Nurs Health. 1995;18:385–394. doi: 10.1002/nur.4770180504. [DOI] [PubMed] [Google Scholar]
- 106.Swanson SC. Have you seen what I’ve seen? The Health Care Workers’s Surviv Man. 2002;2:229–231. [Google Scholar]
- 107.Hinshaw AS, Atwood JR. Nursing staff turnover, stress, and satisfaction: models, measures, and management. In: Werley H, Fitzpatrick J, editors. Annual Review of Nursing Research. New York: Springer; 1983. pp. 133–153. [DOI] [PubMed] [Google Scholar]
- 108.Blegen M. Nurses’ job satisfaction: a meta-analysis of related variables. Nurs Res. 1993;42:36–41. [PubMed] [Google Scholar]
- 109.The Advisory Board Company: The Journey Begins, Retaining Nursing Talent, Reforming Nursing Costs in An Era of Lasting Shortage. Washington, DC: Advisory Board Company; 1999. [Google Scholar]
- 110.Goldson E. The neonatal intensive care unit: premature infants and parents. Infants Young Child. 1992;4:31–42. [Google Scholar]
- 111.Lawhon G. Facilitation of parenting the premature infant in the newborn intensive care nursery. J Perinat Neonat Nurs. 2002;16:71–82. doi: 10.1097/00005237-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 112.Meyer EC, Coll CTG, Seifer R, et al. Psychological distress in mothers of preterm infants. J Dev Behav Pediatr. 1995;16:412–417. [PubMed] [Google Scholar]
- 113.Harrison H. Making lemonade: a parent’s view of “quality of life” studies. J Clin Ethics. 2001;12:239–250. [PubMed] [Google Scholar]
- 114.Hurst I. Vigilant watching over: mothers’ actions to safeguard their premature babies in the newborn intensive care nursery. J Perinat Neonat Nurs. 2001;15:39–57. doi: 10.1097/00005237-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Walker MW, Shoemaker M, Riddle K, et al. Clinical process improvement: reduction of pneumothorax and mortality in high-risk preterm infants. J Perinatol. 2002;22:641–645. doi: 10.1038/sj.jp.7210786. [DOI] [PubMed] [Google Scholar]
- 116.Dunbar AE, Sharek PJ, Mickas NA, et al. Implementation and case study results of potentially better practices to improve pain management of neonates. Pediatrics. 2006;118 suppl 2:S87–S94. doi: 10.1542/peds.2006-0913E. [DOI] [PubMed] [Google Scholar]
- 117.Myers TF, Venable HH, Hansen JA. Computer-enhanced neonatology practice evolution in an academic medical center. NICU Clinical Effectiveness Task Force. J Perinatol. 1998;18:S38–S44. [PubMed] [Google Scholar]
- 118.Ringer SA, Richardson DK, Sacher RA, et al. Variations in transfusion practice in neonatal intensive care. Pediatrics. 1998;101:194–200. doi: 10.1542/peds.101.2.194. [DOI] [PubMed] [Google Scholar]
- 119.Smith TJ, Schoenbeck K, Clayton S. Staff perceptions of work quality of a neonatal intensive care unit before and after transition from an open bay to a private room design. Works. 2009;33:211–227. doi: 10.3233/WOR-2009-0868. [DOI] [PubMed] [Google Scholar]
- 120.Domanico R, Davis D, Coleman F, et al. Documenting the NICU design dilemma: parent and staff perceptions of open Ward versus single family room units. J Perinatol. 2010;30:343–351. doi: 10.1038/jp.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lester BM, Tronick EZ. The NICU network neurobehavioral scale (NNNS) Pediatrics. 2004;113 (suppl):631–699. [Google Scholar]
- 122.Napiorkowski B, Lester BM, Freier MC, et al. Effects of in utero substance exposure on infant neurobehavior. Pediatrics. 1996;98:71–75. [PubMed] [Google Scholar]
- 123.Coyle MG, Ferguson A, LaGasse L, et al. Diluted tincture of opium (DTO) and phenobarbital vs. DTO alone for neonatal opiate withdrawal. J Pediatr. 2002;140:561–564. doi: 10.1067/mpd.2002.123099. [DOI] [PubMed] [Google Scholar]
- 124.Law KL, Stroud LR, LaGasse LL, et al. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 125.Coyle MG, Ferguson A, LaGasse L, et al. Neurobehavioral effects of treatment for opiate withdrawal. Arch Dis Child Fetal Neonat Ed. 2005;90:F73–F74. doi: 10.1136/adc.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson RE, Jones HE, Jasinski DR, et al. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 127.Salisbury AL, Lester B, Seifer R, et al. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29:331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salisbury A, Duncan-Fallone M, Lester BM. Neurobehavioral assessment from fetus to infant: the NICU Network Neurobehavioral scale and the fetal neurobehavior Coding Scale. Ment Retard Dev Disabilities Res Rev. 2005;11:14–20. doi: 10.1002/mrdd.20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brown N, Doyle LW, Bear MJ, et al. Altered regional cerebral volumes predict patterns of impaired neurobehavior in the very preterm infant at term. Pediatr Acad Soc Abstr. 2005;58:2666. [Google Scholar]
- 130.Brown N, Anderson P, Howard K, et al. Clinical validity of early neurobehavioral assessments of very preterm infants [abstract] Pediatr Acad Soc Abstr. 2004;57:3302. [Google Scholar]
- 131.Brown N, Doyle LW, Bear MJ, et al. Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics. 2006;118:2461–2471. doi: 10.1542/peds.2006-0880. [DOI] [PubMed] [Google Scholar]
- 132.Miller-Loncar C, Lester BM, Seifer R, et al. Predictors of motor development in children prenatally exposed to cocaine. Neurotoxicol Teratol. 2005;27:213–220. doi: 10.1016/j.ntt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 133.von Bertalanffy L. General Systems Theory: Foundations, Development, Applications. New York: Braziller; 1968. [Google Scholar]
- 134.Sameroff A. Developmental systems: contexts and evolution. In: Mussen P, editor. Handbook of Child Psychology. New York: Wiley; 1983. pp. 237–294. [Google Scholar]
- 135.Ford DH, Lerner RM. Developmental System Theory: an Integrative Approach. Newbury Park, CA: Sage; 1992. [Google Scholar]
- 136.Barnard KE, Eyres SJ. Part 2: The First Year of Life. Vol. 2. Washington, DC: US Government Printing Office; 1979. Child Health Assessment. [Google Scholar]
- 137.Gorski P, Davison MF, Brazelton TB. Stages of behavioral organization in the high-risk neonate: theoretical and clinical considerations. Semin Perinatol. 1979;3:61–72. [PubMed] [Google Scholar]
- 138.Gibson JJ. The Ecological Approach to Visual Perception. Boston, MA: Houghton Mifflin; 1979. [Google Scholar]
- 139.Kolb B. Brain Plasticity and Behavior. Mahwah, NJ: Erlbaum; 1995. [Google Scholar]
- 140.Kolb B, Gibb R. Early Brain Injury, Plasticity and Behavior. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 141.Luciana M. Cognitive development in children born preterm: implications for theory of brain plasticity following early injury. Dev Psychopathol. 2003;15:1017–1047. doi: 10.1017/s095457940300049x. [DOI] [PubMed] [Google Scholar]
- 142.Gallou-Kabani C, Junien C. Nutritional epigenomics of metabolic syndrome: new perspective against the epidemic. Diabetes. 2005;54:1899–1906. doi: 10.2337/diabetes.54.7.1899. [DOI] [PubMed] [Google Scholar]
- 143.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 145.Liu D, Caldji C, Sharma S, et al. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]