Abstract
Sleep is not only an essential physiological function, but also serves important roles in promoting growth, maturation, and overall health of children and adolescents. There is increasing interest regarding the impact of sleep and its disorders on the regulation of inflammatory processes and end-organ morbidities, particularly in the context of metabolic and cardiovascular diseases (CVD) and their complications. Obstructive sleep apnea syndrome (OSAS) is an increasingly common health problem in children, and in the last decade, the emergence of increasing obesity rates has further led to remarkable increases in the prevalence of OSAS, along with more prominent neurocognitive, behavioral, cardiovascular and metabolic morbidities. Although the underlying mechanisms leading to OSAS-induced morbidities are likely multi-factorial, and remain to be fully elucidated, activation of inflammatory pathways by OSAS has emerged as an important pathophysiological component of the end-organ injury associated with this disorder. To this effect, it would appear that OSAS could be viewed as a chronic, low-grade inflammatory disorder. Furthermore, the concurrent presence of obesity and OSAS poses a theoretically increased risk of OSAS-related complications. In this review, we will critically review the current state of research regarding the impact of insufficient and disrupted sleep and OSAS on the immune processes and inflammatory pathways that underlie childhood OSAS as a distinctive systemic inflammatory condition in children, and will explore potential interactions between OSAS and obesity.
Keywords: Obstructive Sleep Apnea, Obesity, Insufficient Sleep, Inflammation, Pediatrics
1. Introduction
Sleep is a fundamental process in both mammalian and non-mammalian biology. Although the exact function and purpose of sleep remains somewhat elusive, investigation on the effects of sleep deprivation, disrupted sleep, or curtailed sleep on health and disease states has greatly expanded in the last 3 decades, a great deal of unknowns remain in this field. There is no doubt however, that sleep, similar to physical activity and diet, serves as an important regulator of somatic growth, maturation, and health in children. One obvious corollary of such important role lies in the increased sleep needs that accompany the process of child development since birth till the completion of adolescence.
Since the introduction of modern communications and technological advancements in the last half century, the daily lives and habits of children and their families have dramatically changed, and accordingly, such remarkable changes have markedly impacted innumerable facets of child development. Among other factors, such changes likely contributed to the rising rates of obesity in the pediatric population (Ievers-Landis and Redline, 2007; Must and Parisi, 2009). Indeed, a progressive decline in sleep duration that has been paralleled by a dramatic increase in prevalence of obesity at any age has been the basis for increasing speculation upon their potential interrelationships (Cappuccio et al., 2008; Chen et al., 2008; Flint et al., 2007; Horne, 2008; Nielsen et al., 2011; Patel and Redline, 2004). Considering the proposed role of sleep in hormonal circadian release, insulin release, and end-organ insulin sensitivity, as well as other aspects of cardiovascular function, sleep curtailment or disruption could impose a serious health burden. The impact of sleep loss on metabolic homeostasis has been explored in adults by Van Cauter and her group (Van Cauter et al., 2007; Van Cauter et al., 2004; Van Cauter et al., 2008), but similar studies in children are largely unavailable. Careful studies in healthy young adult volunteers have shown that experimental sleep restriction in a laboratory setting is associated with a dysregulation of the neuroendocrine control of appetite consistent with increased hunger, and also with alterations in certain parameters of glucose tolerance suggestive of an increased risk for diabetes. Community-based data from otherwise healthy children suggest that sleep duration and variability constitute important determinants for occurrence of weight problems, and increased metabolic risk (Spruyt et al., 2011). Lately, increasingly more studies have focused on sleep duration of the child, as well as on the factors determining sleep duration, i.e., media, sleep environment, school start times, parental sleep-wake patterns, and so forth. Parenting style and likely family composition potentially shape household habits and behavioral routines, including sleep (Chassin et al., 2005). For instance, when U.S. preschoolers were exposed to 3 household routines, namely evening meals as a family for more than 5 nights per week, sleeping ≥10.5 hours/night on weekdays, and ≤2 hours/day television, video or other screen-viewing behaviors, the prevalence of obesity in children decreased with 40% when compared to children in whom no such routines were implemented (Anderson and Whitaker, 2010). Therefore, sleep duration and regularity emerge as important determinants of obesogenic behaviors in children, as well as the risk for cardiovascular and metabolic risk.
Inflammation is an essential immune response that enables survival during infection or injury, and maintains tissue homeostasis under a variety of harmful conditions (Medzhitov, 2010). Inflammatory processes come at the cost of transient declines in tissue function, and can contribute to the pathogenesis of disease, since cellular and tissue injury may ensue as a by-product of the inflammatory response. In recent years, evidence has accumulated to implication to activation and propagation of inflammatory pathways in the context of insufficient or disturbed sleep. Since the pioneering studies of Rechtschaffen and colleagues (Benca et al., 1989; Rechtschaffen and Bergmann, 2001), conclusive evidence has shown that sleep deprivation imposes major adverse effects on the ability to overcome infection, on host defense mechanisms, and on the magnitude and characteristics of the inflammatory response (Castanon-Cervantes et al., 2010; Everson and Toth, 2000; Imeri and Opp, 2009; Kapsimalis et al., 2008; Krueger et al., 2011; Lange et al., 2010; Zielinski and Krueger, 2011). Similarly, disruption of sleep integrity, as specifically occurs in obstructive sleep apnea syndrome (OSAS) can also elicit significant inflammatory responses (Bixler, 2009; Bradley and Floras, 2009; Farre et al., 2008; Gozal, 2009a; Gozal et al., 2008a).
OSAS is characterized by episodes of repeated prolonged periods of increased upper airway resistance culminating in partial or complete intermittent obstruction of the upper airway during sleep and accompanied by intermittent snoring, repetitive hypoxemic and hypercapnic events and repeated arousals, the latter leading to sleep fragmentation. Similar to adults with OSAS, children with OSAS will experience substantial alterations in gas exchange and increased arousals, albeit generally to a lesser extent (Goh et al., 2000; Grigg-Damberger et al., 2007; Lopes and Marcus, 2007; Tauman et al., 2004b). These physiological alterations are presumed to lead to increased generation of reactive oxygen species and systemic inflammatory responses that appear to be related with hypoxia-re-oxygenation events (Leuenberger et al., 1995; Suzuki et al., 2006; Wang et al., 2010), and consequently to be mechanistically involved in the acceleration and propagation of end-organ injury, such as atherogenesis (Kheirandish-Gozal et al., 2010a; Lavie and Lavie, 2009; Lavie and Polotsky, 2009; Pack and Gislason, 2009), metabolic (Gozal et al., 2008a; Khalyfa et al., 2011a), and neurocognitive and behavioral disturbances (Ali et al., 1993; Chervin et al., 2002; Chervin et al., 1997; Gozal, 1998; Gozal et al., 2007b; Marcotte et al., 1998; Owens et al., 2000). Furthermore, evidence from our laboratory has suggested that delays in the treatment of pediatric OSAS may lead to irreversible declines in cognitive function, as exemplified by reduced or failing academic performance (Gozal and Pope, 2001). Of equally significant concern is the emerging evidence that cardiovascular morbidity has now been conclusively reported in children with OSAS (Bhattacharjee et al., 2009). Several studies support an association between pediatric OSAS and endothelial dysfunction (Gozal et al., 2007c), as a marker of subclinical cardiovascular disease (Kheirandish-Gozal et al., 2010b; Kheirandish-Gozal et al., 2006; Kim et al., 2009, 2010; Kim et al., 2011), and a potential common pathway linking endothelial dysfunction and cognitive outcomes has also been proposed (Gozal et al., 2010). Furthermore, evidence of systemic hypertension and left ventricular remodeling and dysfunction has now been consistently reported (Amin et al., 2008; Amin et al., 2004; Amin et al., 2005; Guilleminault et al., 2004a; Leung et al., 2006; Marcus et al., 1998; McConnell et al., 2009), along with an increased risk for pulmonary hypertension (Sofer et al., 1988; Tal et al., 1988). While the specific mechanisms attributable to the induction of cardiovascular morbidity in the context of childhood OSAS are not fully understood, several injury-mediated pathways are likely operational (Gozal and Kheirandish-Gozal, 2008; Lavie and Lavie, 2009). Notwithstanding, parallel to the extensive evidence thus far collected in adult patients with OSAS (Arnardottir et al., 2009), it has become apparent that pediatric OSAS mediates the induction of several inflammatory cascades, which are central to the initiation and progression of end-organ morbidity (Calvin and Somers, 2009; Gozal et al., 2008a; Ryan et al., 2009).
In this paper, we will review our current understanding on the impact of insufficient/disrupted sleep and that of OSAS on immune processes and inflammatory pathways. We will also describe the evidence that supports the current view, whereby childhood OSAS emerges as a distinctive systemic inflammatory condition in children. Finally, we will describe the potential interactions between OSAS and obesity in children.
2. Sleep and Inflammatory Response
A reciprocal relationship between sleep and the immune system appears to be present. Indeed, sleep modulates the immune system function, and conversely activation of the immune system and production of inflammatory cytokines will affect sleep. However, whether or not specific sleep phenomena participate in active and selective immune system regulation is still under scrutiny and debate. In spite of the extensive diversity of inflammatory pathways, inflammation is essentially an adaptive response to noxious conditions to maintain body homeostasis (Iwasaki and Medzhitov, 2010; Medzhitov, 2010). Both beneficial and detrimental aspects of inflammation can be explained from this perspective. A family of critical immunomodulators, i.e., cytokines, has been extensively studied both with respect to their role and effects on host responses to infection, but have also received substantial attention regarding their roles as potential regulators of physiological sleep (Imeri and Opp, 2009; Opp, 2009; Ranjbaran et al., 2007).
The acute phase response refers to rapid and early activation of an immune cascade in response to injury or infection. One of the major components of the innate immune system is represented by the toll-like receptors (TLRs), a class of pattern recognition receptors that recognizes molecular patterns of self and non-self elements, are expressed on tissue-resident macrophages, and induce the production of inflammatory cytokines (e.g. TNF-α, IL-1, IL-6) and chemokines (e.g. CCL2 and CXCL8), as well as prostaglandins (Medzhitov, 2010; Pecchi et al., 2009). These pathways are classically activated by the outer LPS component of bacterial cell membranes (Rosenfeld and Shai, 2006). However, sleep deprivation alone can lead to stimulation of the TLR pathways, and induce cytokine production. A recent study demonstrated that following a night of sleep loss, LPS activation of TLR-4 was greater than following a night of uninterrupted sleep (Irwin et al., 2006).
NF-kB, an important transcription factor underlying activation and regulation of inflammatory pathways, is involved in the transcription of more than 200 genes, including those responsible for the production of cytokines and inflammatory markers (Brasier, 2006; Perkins, 2007). Similarly, NF-kB binding domains have been found in the promoter regions of the genes of many somnogenic substances, such as adenosine A1 receptors, cyclooxygenase-2 (Cox-2), and nitric oxide synthase (NOS) (Krueger et al., 2001). Sleep deprivation, even mild, can induce the activation and translocation of NF-kB in specific brain regions associated with sleep regulation (Basheer et al., 2001; Brandt et al., 2004; Chen et al., 1999; Kuo et al.; Ramesh et al., 2007), which in turn elicits localized inflammatory responses (Irwin et al., 2008). Conversely, the list of cytokines and chemokines that have been studied in laboratory animals or human subjects and demonstrated to affect sleep is quite extensive, and includes IL-1α, IL-1 β, IL-2, IL-4, IL-6, IL-8, I-10, IL-13, IL-15, IL-18, TNF-α, TNF-β, IFN-α, IFN-β, IFN-β and macrophage inhibitory protein 1b (Imeri and Opp, 2009; Vgontzas et al., 2006). Of these, the most extensively studied inflammatory cytokines in sleep regulation are IL-1β and TNF- α (Ranjbaran et al., 2007; Takahashi et al., 1999; Vgontzas et al., 1997). Even though most cytokines were first discovered in peripheral immune system, several cytokines and their receptors have now been shown to be present in the CNS, and to localize to specific cell types or brain regions (Allan and Rothwell, 2001; Eriksson et al., 2000). The CNS detects activation of peripheral immune system through cytokine-induced stimulation of the vagus nerve, through cytokine actions within circumventricular organs, and through active transport of cytokines from the periphery into the CNS (Dantzer et al., 2008). In addition, a role for prostaglandins in the interactions and cross talk regarding inflammatory processes between the periphery and the CNS has emerged (Engblom et al., 2003). However, cytokines are also synthesized de novo and released in the CNS by both neurons (Breder et al., 1988; Ignatowski et al., 1997; Marz et al., 1998) and glia (Garden and Moller, 2006). It has been shown that injection of IL-1 and TNF-α in animal models increases the amount of time spent in NREM sleep. Further, blocking the effect of either of these two substances inhibits both spontaneous sleep and the sleep rebound that normally occurs after sleep deprivation (Krueger, 2008). It is likely that these 2 important cytokines exhibit interdependent effects on sleep, as shown by using a TNF receptor (TNFRF) and a IL-1 receptor fragments (IL-1RF) (Takahashi et al., 1999). Not surprisingly, the systemic levels of TNF-α have been linked to the fatigue and excessive sleepiness seen in patients with rheumatoid arthritis as well as in OSAS patients (Franklin, 1999; Khalyfa et al., 2011b; Vgontzas et al., 1997). Another important cytokine associated with the immune system and sleep behaviors is IL-6. In one study, human volunteers were given IL-6 parenterally, at a dose sufficient to reach levels associated with systemic infection. After 6.5 h, IL-6 elicited significant subjective fatigue and resulted in a marked increase in C-reactive protein (CRP) compared to placebo. There was also a significant suppression of REM sleep, and while total SWS time was similar to the placebo group, SWS time did significantly decrease during the first half of the sleep period and increase during the second half (Spath-Schwalbe et al., 1998). Interestingly, other inflammatory cytokines levels did not increase after injection of IL-6, suggesting that IL-6 is specifically relevant to alterations in sleep patterns (Ranjbaran et al., 2007).
Even though there is increased interest regarding the association between insufficient sleep or disturbed sleep with inflammation, a number of factors including assay sensitivity, individual difference of subject, basal level of inflammation, stress and physical activity prior to sampling should be considered to interpret results. Given this link between pro-inflammatory cytokines, host immune function and the sleep-wake cycle, it is likely that sleep disruption, similar to sleep curtailment, plays a significant role in immune-mediated inflammatory cascades that ultimately contribute to the phenotype of diseases such as obesity and obstructive sleep apnea, which are the focus of this review. These inflammatory response signatures are of particular interest because they may accompany many diseases of modern times as seen in highly industrialized countries, including metabolic syndrome, type 2 diabetes and atherosclerosis.
3. Sleep Loss, Inflammation, and Obesity in Children (Figure 1)
Figure 1.
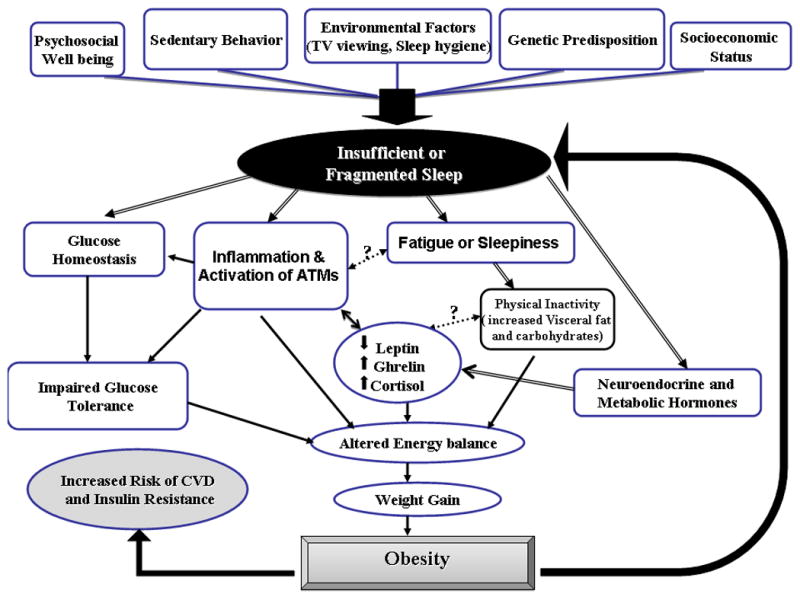
Interaction of insufficient or fragmented sleep and obesity in activating inflammatory pathways leading to increased risk of CVD.
Obesity is becoming a global epidemic in both adults and children and is associated with an increased risk of morbidity and mortality as well as reduced life expectancy (Poirier et al., 2006). Moreover, childhood obesity is a serious and progressively increasing public health problem that has reached epidemic proportions in the United States where it disproportionately affects low-income and minority children (Ogden et al., 2006). It is now well accepted that the physiological disturbances associated with obesity contribute to a chronic state of low-grade systemic inflammation, and that these derangements occur not only in adults but have also been shown to occur in children (Ford, 2003; Ford et al., 2001; Schwarzenberg and Sinaiko, 2006). Extrapolation of data from a large cohort of children using the database from the National Health and Nutrition Examination Survey has shown that BMI was the best predictor of elevated CRP, and that this association did not differ significantly by age, gender or race (Ford, 2003; Ford et al., 2005; Ford et al., 2001).
Obesity leads to a change in an individual's metabolic profile and the accumulation of adipose tissue, which is composed of connective tissue, adipocytes, and the subcutaneous vascular fraction (SVF), the latter containing cells of multiple lineages, such as endothelial cells, adipocyte progenitors, T cell lymphocytes, and macrophages. Obesity provokes structural and metabolic alterations in other organs, including skeletal muscle and liver (Ouchi et al., 2011). Indeed, obesity is closely linked to fat storage in liver, and is nowadays considered as a major risk factor for the development of fatty liver disease. The incidence of nonalcoholic fatty liver disorders (NAFLDs) and obesity are therefore intimately linked (Farrell and Larter, 2006). The amount of fat stored in liver is determined by the balance between fatty acid uptake, endogenous fatty acid synthesis, triglyceride synthesis, fatty acid oxidation, and triglyceride export. Changes in any of these parameters can affect the amount of fat stored in liver. Adipose tissue produces a host of chemokines, also termed adipokines, with well-described effects on metabolism and inflammation. Resistin, adiponectin, leptin, and monocyte chemoattractant protein-1 (MCP-1) are among a group of secreted proteins from adipose tissue which exhibit immunomodulatory functions (Yu and Ginsberg, 2005). The production and secretion of these adipokines is altered during obesity, resulting in a more pro-inflammatory or atherogenic secretion profile. Indeed, whereas secretion of MCP-1, resistin, and other pro-inflammatory cytokines is increased by obesity, the adipose secretion of the anti-inflammatory protein adiponectin is decreased (Kadowaki and Yamauchi, 2005). Although increased visceral fat depots and adipocyte hypertrophy have been linked to a higher degree of adipose inflammation (Jernas et al., 2006; Matsuzawa, 2006), until recently the exact pathways leading to a pro-inflammatory state of adipose tissue in obese individuals remained unidentified. Indeed, in recent years much attention has been diverted to the role of macrophages. Adipocyte-infiltrated macrophages (ATMs), which as mentioned above, are integrally part of the SVF of adipose tissue, are responsible upon activation for the production and release of a wide variety of pro-inflammatory proteins including MCP-1, TNF-α, and IL-6. The development of insulin resistance in adipocytes was closely linked to the infiltration of macrophages (Gustafson, 2010; Suganami and Ogawa, 2010). In addition, alterations in the balance of adipose tissue SVF T cell lymphocyte populations and their effects on inflammatory processes impose unique changes that ultimately lead to adipocyte proliferation and insulin resistance (Feuerer et al., 2009; Matarese et al.; Strissel et al.; Winer et al., 2009; Zeyda et al.).
The effects of sleep on weight regulation, insulin sensitivity, and adipose tissue homeostasis are only now being intensively investigated. Evidence to the important role of circadian genes on metabolism and obesity has recently emerged (Bass and Takahashi, 2010; Marcheva et al.; Turek et al., 2005). In addition, a number of mechanisms, including alterations in hormones that regulate appetite and thus affect caloric intake have also been implicated (Leproult and Van Cauter; Van Cauter et al., 2007; Van Cauter et al., 2008). For example, sleep restriction promotes the release of orexigenic peptides such as ghrelin while reducing leptin plasma levels (Leproult and Van Cauter, 2010; Nedeltcheva et al., 2009). Leptin plays a key role in the modulation of inflammation as well as in the regulation of energy intake and expenditure (Dardeno et al., 2010; Lago et al., 2008). As mentioned above, both sleep loss and sleep disturbances are associated with obesity and contribute to a chronic state of low-grade systemic inflammation. These derangements occur not only in adults but have also been shown to occur in children (Schwarzenberg and Sinaiko, 2006). In a randomized cross-over clinical study in a highly controlled laboratory setting, sleep restriction was associated with decreased leptin and increased ghrelin levels. These changes will lead to a concomitant increase in hunger and appetite, increased insulin resistance, and accumulation of fat and decreased carbohydrate metabolism (Spiegel et al., 2004). In agreement with the physiological responses to reduced sleep duration, several studies have demonstrated an association between sleep duration and the risk to develop childhood overweight and obesity (Chaput et al., 2006) and that short sleep duration is positively associated with obesity in preschool children (Jiang et al., 2009). Taken together, sleep has emerged as a modifiable risk factor for the prevention and treatment of obesity in childhood (Monasta et al., 2010; Must et al., 2009). However, it may be that other factors related to sleep, rather than sleep duration alone may play a role. Indeed, a recent study showed that the presence of high variability in bedtimes and in sleep duration in 4-10 year-old children was a significant and independent risk factor for obesity in addition to short sleep duration (Spruyt et al., 2011). Furthermore, objectively measured sleep duration and variability in these children also revealed significant associations with altered fasting insulin, low-density lipoprotein, and high-sensitivity CRP plasma levels (Spruyt et al., 2011). These findings suggest that educational campaigns, aimed at families and the public in general, and promoting longer and more-regular sleep routines may promote a reduction in obesity rates, as well as improve the risk for metabolic and cardiovascular dysfunction in school-aged children (Spruyt et al., 2011).
In the context of OSAS, plasma adipokine concentrations were assessed in 130 children undergoing nocturnal polysomnography (Tauman et al., 2007b). In addition to the anticipated association between the degree of obesity and circulating leptin levels, the presence of co-morbid OSAS was independently associated with leptin levels (Tauman et al., 2007b). We should emphasize that although these findings have been subsequently reproduced in other cohorts, whereby there were adverse OSAS-associated effects on the major adipokines, including leptin, ghrelin, and adiponectin (Kelly et al., 2010; Nakra et al., 2008; Tsaoussoglou et al., 2010), not all studies have yielded similar results (Li et al., 2010). Hence, the systemic inflammatory responses associated with obesity that seem to promote metabolic derangements may begin as early as infancy, and the presence of co-morbid OSAS is likely to amplify this risk in children. Further studies will be needed to replicate and expand on these assumptions and findings in the future.
4. Cardiovascular morbidity in the context of pediatric OSAS (Figure 2)
Figure 2.
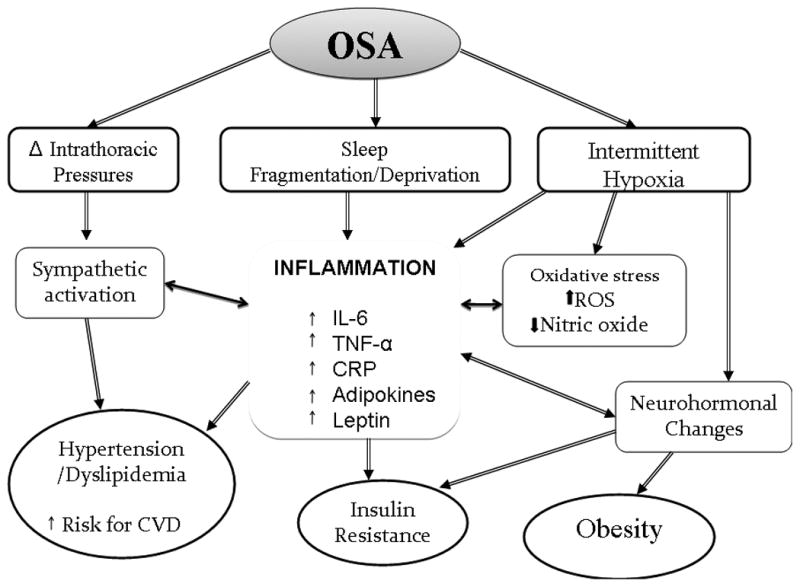
Schematic diagram underlying inflammatory pathways associated with obesity and obstructive sleep apnea syndrome that may lead to increased cardiovascular disease risk in children.
OSAS exposes the cardiovascular system to intermittent hypoxia and increased recurring negative intrathoracic pressures, as well as to episodic arousals, all of which can trigger increased sympathetic hyperactivity, ultimately leading to elevated systemic blood pressure. In addition, OSAS can elicit endothelial dysfunction that will synergistically interact with the increased tonic and reactive sympathetic tone to further exacerbate the burden on the cardiovascular system, and ultimately lead to increased morbidity in both in adults and children (Bhattacharjee et al., 2009). The exact mechanisms behind this cardiovascular dysfunction remain uncertain, but activation of inflammatory pathways and reactive oxygen species (ROS) have been proposed and shown to play critical roles (Gozal, 2009b). Indeed, OSAS has been shown to promote atherosclerosis via the formation and release of cytokines including IL-1, IL-6, TNF-α, and other adipokines from adipose tissues and circulating inflammatory cell (Lavie and Lavie, 2009; Vgontzas, 2008). Similarly, oxidative stress via the increased formation and release of hydrogen peroxide, ROS and reactive nitrogen species, as well as activation of NADPH oxidase will occur, and induce cellular damage in pertinent end-organs such as endothelial cells. Increased inflammation will further the production and release of acute phase reactants such as CRP from the liver, which in turn will induce substantial endothelial dysfunction (Venugopal et al., 2005), possibly via oxidative stress (Devaraj et al., 2009), and either directly or indirectly reduce the bioavailability of nitric oxide (Hein et al., 2009; Valleggi et al., 2010). All these events will promote increased expression of adhesion molecules leading to expansion and propagation of the inflammatory nidus, activation, transformation and trans-vessel migration of monocytes to become foam cells, while inducing increased apoptosis of endothelial cells, and activation of platelets and other pro-coagulant factors (Gozal, 2009b). Support for this model is strongly endorsed by the findings originating from numerous studies in both adult and children. Indeed, raised levels of circulating pro-inflammatory cytokines, chemokines and adhesion molecules have been consistently reported in patients with OSAS when compared with matched controls, with treatment leading to the anticipated improvements in such measures. However, as in many other fields, not all findings are homogeneous and some studies have failed to confirm the changes in this cytokine or another. Indeed, in a recent review by Ryan and colleagues (Ryan et al., 2009) earlier studies that suggested the presence of increased IL-6 levels in adult patients with OSAS (Ciftci et al., 2004; Vgontzas et al., 1997; Yokoe et al., 2003), were not substantiated later on (Ryan et al., 2006). However, the Cleveland Family Study showed an independent association between the soluble IL-6 receptor and the severity of OSAS parameters (Mehra et al., 2006). In addition, Gozal and colleagues showed in a group of children aged 4–9 years who were polysomnographically diagnosed with OSAS that IL-6 plasma levels were higher and IL-10 plasma levels were lower than in age, gender, ethnicity, and BMI matched controls, and that these levels returned to normal values within 4–6 months after adenotonsillectomy (Gozal et al., 2008b).
The available data on the correlation between TNF-α circulating levels and OSAS appear more compelling. It is now well established that this pro-inflammatory cytokine is involved in atherosclerosis by inducing the expression of cellular adhesion molecules that mediate the leukocyte adhesion to the vascular endothelium (Kritchevsky et al., 2005). Several case-controlled studies have demonstrated elevated circulating TNF-α concentration in adult patients with OSAS, independent of obesity, and have also shown a significant fall in this cytokine with effective CPAP therapy (Ciftci et al., 2004; McNicholas, 2009; Minoguchi et al., 2004; Ryan et al., 2006). However, not all adults with OSAS display increased TNF-α plasma concentration, a finding that is accounted for by the variance in specific polymorphisms within the TNF-α gene (Bhushan et al., 2009; Riha, 2009; Riha et al., 2005). Both T cells and monocytes have been suggested as a potential source of TNF-α and other pro-inflammatory cytokines. Gozal and Kheirandish-Gozal (Gozal and Kheirandish-Gozal, 2008) proposed the monocyte as the cardinal cellular target affected by the constitutive alterations in OSAS. In this model, activation of monocytes will initiate a complex biological cascade that will ultimately promote increased proliferation of smooth muscle in the vessel wall, macrophage migration through the disrupted endothelium into the vessel wall, and foam cell formation (Gozal, 2009b; Gozal and Kheirandish-Gozal, 2008). Similarly and likely in parallel to monocyte recruitment, Dyugovskaya and colleagues have elucidated some of the T-cell lymphocyte-dependent inflammatory mechanisms activated by OSAS in adult patients (Dyugovskaya et al., 2005a; Dyugovskaya et al., 2005b). In this context, recurrent hypoxemia will induce specific cytokine secretion through T-cell lymphocyte activation, and the profile of such cytokine network is remarkably similar to those involved in atherogenesis. Phenotypic alterations in CD4+ and CD8+ T cells of OSAS patients were reported to undergo a shift from Th1 to Th2 cytokine production, with subsequent reductions in IL-4 expression. Furthermore, IL-10 expression was negatively correlated with the severity of OSAS, whereas TNF-α correlated positively with OSAS severity measures. In addition, CD8+ T cells of patients with OSAS exhibited marked increases in TNF-α and soluble CD40 ligand, and were particularly cytotoxic against endothelial cells. Interestingly, these inflammatory changes were reversed by treatment with CPAP (Dyugovskaya et al., 2005a; Dyugovskaya et al., 2002, 2003, 2005b). In children, the data implicating TNF-α induction in OSAS are somewhat conflicting. Indeed, the levels of specific pro-inflammatory cytokines have either been normal or elevated across several cohorts (Tam et al., 2006; Waters et al., 2007). However, it is likely that many of these differences pertain to methodological issues in sampling techniques, as well as be due to differences in genetic and environmental factors (Bhattacharjee et al., 2009). A large prospective study from our laboratory which included 298 children, showed that morning levels of TNF-α were increased in the presence of OSAS, particularly in the more severe cases, and that these levels decreased after adenotonsillectomy (Gozal et al, 2010). Furthermore, TNF-α levels were strongly associated with the degree of respiratory-induced sleep fragmentation (Gozal et al., 2010). More recently, Khalyfa and colleagues assessed the genomic variance in the TNF-α, gene in children and found that TNF-α levels were increased in a subset of children with OSAS, particularly among those harboring the TNF-α -308G single nucleotide polymorphism (Khalyfa et al., 2011b).
Another prototypic marker of inflammation, CRP, is not only an acute-phase protein produced by the liver in response to IL-6, but its serum concentrations reflect the degree of the inflammatory response, and also provide reliable risk estimates of atherogenesis (Morrow and Ridker, 2000; Ridker, 2001; Ridker et al., 2000). CRP can be found in the atherosclerotic lesions, more specifically in the vascular intima layer, where it co-localizes with monocytes, monocyte-derived macrophages and lipoproteins (Lusis, 2000). This localization makes a direct contribution of CRP to the atherosclerotic process highly likely (Li et al., 2008). Increased levels of CRP have been found in both adults (Can et al., 2006; Chung et al., 2007; Kageyama et al., 2006) and children with OSAS (Larkin et al., 2005; Li et al., 2008; Tauman et al., 2004a; Tauman et al., 2007a), with actual reduction of these levels after treatment (Kageyama et al., 2006; Kheirandish-Gozal et al., 2006; Li et al., 2008). Recently Goldbart and colleagues showed a high CRP level and N-terminal pro-B-type natriuretic peptide, a marker of ventricular strain, in children with OSAS, and these levels returned to normal after treatment with adenotonsillectomy (Goldbart et al., 2010). However, as previously indicated for other biomarkers of inflammation, not all studies in adults (Barcelo et al., 2004; Guilleminault et al., 2004b) or in children (Kaditis et al., 2005; Tam et al., 2006) confirmed the putative association between CRP levels and OSAS severity. It is possible that the strong relationship between CRP levels and obesity (Visser et al., 1999) may have influenced some studies in which the populations being investigated may have not been optimally matched for BMI (McNicholas, 2009; Ryan et al., 2009).
5. Inflammation, and Neurocognitive Morbidity In The Context Of Pediatric OSAS
Another important end-organ adversely affected by OSAS is the neuronal and cognitive system. Since Kales et al (Kales et al., 1985) and Findley et al (Findley et al., 1986) first highlighted the presence of neuropsychological abnormalities in humans with sleep apnea, researchers have attempted to identify mechanisms underlying this morbidity. However, we should remark that the most prominent deleterious effects of OSAS have been described in children (Beebe and Gozal, 2002; Beebe et al., 2003; Gozal and Kheirandish-Gozal, 2007; Key et al., 2009; Kheirandish and Gozal, 2006). Indeed, the cumulative conclusions derived from studies in adults with OSAS have not been as definitive when compared to the overall evidence derived from pediatric studies (Kohler et al., 2010). Of note the differential and enhanced susceptibility of the developing brain to intermittent hypoxia during sleep has been previously demonstrated in a rat model (Gozal E, et al, 2001b). The scope of neurocognitive dysfunction in children with OSAS is rather vast and encompasses attention deficit and hyperactivity disorder (ADHD)-like behaviors, learning problems, memory and intelligence deficits, behavioral disorders and excessive daytime sleepiness (Chervin et al., 2005; Gozal, 2008; Gozal and Kheirandish-Gozal, 2009; Gozal et al., 2001b; O'Brien et al., 2003; O'Brien et al., 2004; Ravid et al., 2009; Visser et al., 1999). Experimental work in rodent spanning the last decade has shed substantial light on some of putative mechanisms of cognitive deficits in OSAS. The 2 major pathophysiological systems implicated in these processes have included activation of inflammatory pathways and the excessive formation and accumulation of free radicals leading to oxidative stress (Gozal, 2009b; Gozal et al., 2002; Ramanathan et al., 2005; Shan et al., 2007; Xu et al., 2004). Indeed, we initially showed that exposures to intermittent hypoxia (IH) in the absence of sleep deprivation during the sleep period induced marked increases in cell apoptosis in the hippocampal and cortical regions along with persistent impaired performance of a cognitive spatial task in rats (Gozal et al., 2001a), and that similar consequences emerged in developing rat pups, albeit with long-term consequences (Kheirandish et al., 2005a; Row et al., 2002). Row and colleagues further showed that administration of the electron spin trapper antioxidant PNU-10133E to rats exposed to intermittent hypoxia was associated with attenuated increases in cortical tissue concentrations of malondialdehyde, a marker of oxidative stress, and isoprostane, a marker of lipid peroxidation, with parallel ameliorations in performance in the water maze (Gozal, 2009b; Row et al., 2002). Subsequent work further implicated additional inflammatory pathways, such as cyclooxygenase 2 (Li et al., 2003), inducible NOS (Li et al., 2004), platelet activating factor (Row et al., 2004), as well as regulators of intracellular reactive oxygen species (Xu et al., 2004). Apolipoprotein E (ApoE) is a lipoprotein synthesized by the liver and brain, which is involved in cholesterol deposition and transport, and has emerged as a major factor for Alzheimer's disease (Laws et al., 2003). Accordingly, Kheirandish and colleagues exposed mice deficient in ApoE to intermittent hypoxia during sleep for 14 days of intermittent hypoxia (Kheirandish et al., 2005b). ApoE null mice were not only markedly susceptible to this OSAS-like paradigm, but also demonstrated significantly greater increases in markers of oxidative stress and inflammatory response (Kheirandish et al., 2005). Of note, an increase in cognitive susceptibility to OSAS has been reported in children harboring the ApoEε4 allele (Gozal et al., 2007a), further buttressing the validity of rodent-based OSAS models in the exploration of mechanistic insights of OSAS morbidity.
As indicated above, mild OSAS in adults is unlikely to be associated with significant cognitive impairments. In contrast, mild sleep apnea in children is associated with cognitive impairments similar to those observed in adult moderate or severe sleep apnea (Lim and Veasey, 2010). Furthermore, substantial variance emerges in the magnitude of cognitive impairments among children with OSAS of similar severity (Fauroux et al., 2008). In this context, the potential importance of the inflammatory response was recently shown by Gozal and colleagues (Gozal et al., 2007b) by comparing 205 snoring children and 73 non-snoring controls. The snoring children were divided into those with and those without OSAS based on their polysomnographic findings. Both CRP levels and platelet counts were significantly higher in OSAS, and more specifically in those with neurocognitive dysfunction. Thus, the level of systemic inflammation, as evidenced by the serum levels of CRP, appears to be associated with the cognitive morbidity that accompanies this condition, and that the magnitude of systemic inflammatory response to the presence of sleep disruption could be a major determinant of end-organ injury.
In closing, we should emphasize that activation of inflammatory pathways most likely reflects the recruitment of multiple lineages of inflammatory cells and is undoubtedly associated with increased cytokine production. Krueger and colleagues have extensively explored the physiologic role of cytokines such as TNF- α, IL-6, IL-β1 and many others, and coined them as “Sleep Regulatory Substances” for their uniquely important roles in the regulation of sleep (Krueger, 2008; Krueger et al., 2001). Furthermore, alterations in circadian clock through forced desynchronization routines (i.e., housing mice in 20-h light/dark cycles), results in cognitive deficits, accelerated weight gain and obesity, as well as in metabolic hormonal changes (Karatsoreos et al., 2011). These findings emphasize the complexity of the pathways linking sleep, sleep disorders, and inflammatory processes.
6. Summary
Considering the close association between sleep, the immune system, and inflammation, it is not surprising that sleep disturbances can initiate or exacerbate inflammatory conditions. While the cause and effect relationship(s) between sleep problems and chronic inflammatory conditions remain to be fully delineated, sleep curtailment or perturbations of sleep need to be viewed as important factors modulating the phenotype of multiple chronic inflammatory diseases, and conversely, that inflammatory process may impose fundamental effects on sleep regulation and integrity. Accordingly, the clear homologies in end-organ morbidities and inflammatory pathway recruitment in the context of obesity and OSAS further suggest that many of such consequences reflect in fact, the critical interactions between sleep and the immune system. Disrupted or insufficient sleep will promote up-regulation of pro-inflammatory signaling pathways, particularly those mediated through TLRs and NF-κB, and also induce metabolically active adipokines, ultimately leading to increased expression of for example pro-atherogenic factors, or of neuronal dysfunction (Gozal et al., 2008a). When such processes begin to develop at a very early stage of life, i.e., during infancy or early childhood, the concern is not only because of their immediate short-term consequences, but rather because of their epigenetic lifelong effects.
Acknowledgments
Funding Sources: LKG is supported by NIH grant K12 HL-090003; DG is supported by National Institutes of Health grants HL-065270 and HL-086662; FH is supported by American Physician Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Archives of disease in childhood. 1993;68:360–366. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, McPhail G, Morgenthal A, Fenchel M, Bean J, Kimball T, Daniels S. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, Bokulic R, Daniels SR. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- Amin RS, Kimball TR, Kalra M, Jeffries JL, Carroll JL, Bean JA, Witt SA, Glascock BJ, Daniels SR. Left ventricular function in children with sleep-disordered breathing. Am J Cardiol. 2005;95:801–804. doi: 10.1016/j.amjcard.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Whitaker RC. Household routines and obesity in US preschool-aged children. Pediatrics. 2010;125:420–428. doi: 10.1542/peds.2009-0417. [DOI] [PubMed] [Google Scholar]
- Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo A, Barbe F, Llompart E, Mayoralas LR, Ladaria A, Bosch M, Agusti AG. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117:118–121. doi: 10.1016/j.amjmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Basheer R, Rainnie DG, Porkka Heiskanen T, Ramesh V, McCarley RW. Adenosine, prolonged wakefulness, and A1-activated NF-kappaB DNA binding in the basal forebrain of the rat. Neuroscience. 2001;104:731–739. doi: 10.1016/s0306-4522(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- Benca RM, Kushida CA, Everson CA, Kalski R, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: VII. Immune function. Sleep. 1989;12:47–52. doi: 10.1093/sleep/12.1.47. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51:416–433. doi: 10.1016/j.pcad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Bhushan B, Guleria R, Misra A, Luthra K, Vikram NK. TNF-alpha gene polymorphism and TNF-alpha levels in obese Asian Indians with obstructive sleep apnea. Respir Med. 2009;103:386–392. doi: 10.1016/j.rmed.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Bixler E. Sleep and society: An epidemiological perspective. Sleep Med. 2009;10 1:S3–6. doi: 10.1016/j.sleep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- Brandt JA, Churchill L, Rehman A, Ellis G, Memet S, Israel A, Krueger JM. Sleep deprivation increases the activation of nuclear factor kappa B in lateral hypothalamic cells. Brain Res. 2004;1004:91–97. doi: 10.1016/j.brainres.2003.11.079. [DOI] [PubMed] [Google Scholar]
- Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Calvin AD, Somers VK. Obstructive sleep apnea and cardiovascular disease. Curr Opin Cardiol. 2009;24:516–20. doi: 10.1097/HCO.0b013e328330c2ed. [DOI] [PubMed] [Google Scholar]
- Can M, Acikgoz S, Mungan G, Bayraktaroglu T, Kocak E, Guven B, Demirtas S. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006;129:233–237. doi: 10.1378/chest.129.2.233. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes (Lond) 2006;30:1080–1085. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose J, Sherman SJ, Davis MJ, Gonzalez JL. Parenting style and smoking-specific parenting practices as predictors of adolescent smoking onset. J Pediatr Psychol. 2005;30:333–344. doi: 10.1093/jpepsy/jsi028. [DOI] [PubMed] [Google Scholar]
- Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gardi J, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276:R1812–1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Archbold KH, Dillon JE, Panahi P, Pituch KJ, Dahl RE, Guilleminault C. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002;109:449–456. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–1192. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28:885–890. doi: 10.1093/sleep/28.7.885. [DOI] [PubMed] [Google Scholar]
- Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Lee T, Choi DJ, Ahn HJ. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Singh U, Rao LV, Jialal I. C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2009;203:67–74. doi: 10.1016/j.atherosclerosis.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyugovskaya L, Lavie P, Hirsh M, Lavie L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur Respir J. 2005a;25:820–828. doi: 10.1183/09031936.05.00103204. [DOI] [PubMed] [Google Scholar]
- Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- Dyugovskaya L, Lavie P, Lavie L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am J Respir Crit Care Med. 2003;168:242–249. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005b;1051:340–350. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- Engblom D, Saha S, Engström L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Nobel S, Winblad B, Schultzberg M. Expression of interleukin 1 alpha and beta, and interleukin 1 receptor antagonist mRNA in the rat central nervous system after peripheral administration of lipopolysaccharides. Cytokine. 2000;12:423–431. doi: 10.1006/cyto.1999.0582. [DOI] [PubMed] [Google Scholar]
- Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R905–916. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- Farre R, Montserrat JM, Navajas D. Morbidity due to obstructive sleep apnea: insights from animal models. Curr Opin Pulm Med. 2008;14:530–536. doi: 10.1097/mcp.0b013e328312ed76. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Fauroux B, Aubertin G, Clement A. What's new in paediatric sleep in 2007? Paediatr Respir Rev. 2008;9:139–143. doi: 10.1016/j.prrv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90:686–690. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- Flint J, Kothare SV, Zihlif M, Suarez E, Adams R, Legido A, De Luca F. Association between inadequate sleep and insulin resistance in obese children. J Pediatr. 2007;150:364–369. doi: 10.1016/j.jpeds.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2003;108:1053–1058. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005;28:878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- Ford ES, Galuska DA, Gillespie C, Will JC, Giles WH, Dietz WH. C-reactive protein and body mass index in children: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. J Pediatr. 2001;138:486–492. doi: 10.1067/mpd.2001.112898. [DOI] [PubMed] [Google Scholar]
- Franklin CM. Clinical experience with soluble TNF p75 receptor in rheumatoid arthritis. Semin Arthritis Rheum. 1999;29:172–181. doi: 10.1016/s0049-0172(99)80028-6. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–686. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- Goldbart AD, Levitas A, Greenberg Dotan S, Ben Shimol S, Broides A, Puterman M, Tal A. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest. 138:528–535. doi: 10.1378/chest.10-0150. [DOI] [PubMed] [Google Scholar]
- Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol. 2008;15:100–106. doi: 10.1016/j.spen.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009b;10 1:S12–16. doi: 10.1016/j.sleep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008a;177:1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007a;69:243–249. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007b;176:188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001a;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics. 2009;123:13–18. doi: 10.1542/peds.2008-0228. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126:e1161–1167. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007c;116:2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- Gozal D, Pope DW., Jr Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107:1394–1399. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- Gozal D, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Khalyfa A, Tauman R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep. 33:319–325. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008b;9:254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Wang M, Pope DW., Jr Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001b;108:693–697. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- Gozal E, Gozal D, Pierce WM, Thongboonkerd V, Scherzer JA, Sachleben LR, Jr, Brittian KR, Guo SZ, Cai J, Klein JB. Proteomic analysis of CA1 and CA3 regions of rat hippocampus and differential susceptibility to intermittent hypoxia. J Neurochem. 2002;83:331–345. doi: 10.1046/j.1471-4159.2002.01134.x. [DOI] [PubMed] [Google Scholar]
- Grigg Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, Wise M, Picchietti DL, Sheldon SH, Iber C. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–240. [PubMed] [Google Scholar]
- Guilleminault C, Khramsov A, Stoohs RA, Kushida C, Pelayo R, Kreutzer ML, Chowdhuri S. Abnormal blood pressure in prepubertal children with sleep-disordered breathing. Pediatr Res. 2004a;55:76–84. doi: 10.1203/01.PDR.0000099791.39621.62. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004b;27:1507–1511. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- Hein TW, Singh U, Vasquez Vivar J, Devaraj S, Kuo L, Jialal I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 2009;206:61–68. doi: 10.1016/j.atherosclerosis.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne J. Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol. 2008;77:266–276. doi: 10.1016/j.biopsycho.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ievers Landis CE, Redline S. Pediatric sleep apnea: implications of the epidemic of childhood overweight. Am J Respir Crit Care Med. 2007;175:436–441. doi: 10.1164/rccm.200606-790PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowski TA, Noble BK, Wright JR, Gorfien JL, Heffner RR, Spengler RN. Neuronal-associated tumor necrosis factor (TNF alpha): its role in noradrenergic functioning and modification of its expression following antidepressant drug administration. J Neuroimmunol. 1997;79:84–90. doi: 10.1016/s0165-5728(97)00107-0. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernas M, Palming J, Sjoholm K, Jennische E, Svensson PA, Gabrielsson BG, Levin M, Sjogren A, Rudemo M, Lystig TC, Carlsson B, Carlsson LM, Lonn M. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- Jiang F, Zhu S, Yan C, Jin X, Bandla H, Shen X. Sleep and obesity in preschool children. J Pediatr. 2009;154:814–818. doi: 10.1016/j.jpeds.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Kaditis AG, Alexopoulos EI, Kalampouka E, Kostadima E, Germenis A, Zintzaras E, Gourgoulianis K. Morning levels of C-reactive protein in children with obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2005;171:282–286. doi: 10.1164/rccm.200407-928OC. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Kageyama N, Nomura M, Nakaya Y, Watanabe T, Ito S. Relationship between adhesion molecules with hs-CRP and changes therein after ARB (Valsartan) administration in patients with obstructive sleep apnea syndrome. J Med Invest. 2006;53:134–139. doi: 10.2152/jmi.53.134. [DOI] [PubMed] [Google Scholar]
- Kales A, Caldwell AB, Cadieux RJ, Vela Bueno A, Ruch LG, Mayes SD. Severe obstructive sleep apnea--II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38:427–434. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–614. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Dougherty S, Cucchiara A, Marcus CL, Brooks LJ. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33:1185–1191. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Molfese DL, O'Brien L, Gozal D. Sleep-disordered breathing affects auditory processing in 5-7-year-old children: evidence from brain recordings. Dev Neuropsychol. 2009;34:615–628. doi: 10.1080/87565640903133608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Gharib SA, Kim J, Capdevila OS, Kheirandish-Gozal L, Bhattacharjee R, Hegazi M, Gozal D. Peripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findings. Sleep. 2011a;34:153–160. doi: 10.1093/sleep/34.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Gozal D. TNF-alpha gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J Pediatr. 2011b;158:81–86. doi: 10.1016/j.jpeds.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Bhattacharjee R, Gozal D. Autonomic alterations and endothelial dysfunction in pediatric obstructive sleep apnea. Sleep Med. 2010a;11:714–720. doi: 10.1016/j.sleep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010b;182:92–97. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med. 2006;2:301–304. [PMC free article] [PubMed] [Google Scholar]
- Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci. 2006;9:388–399. doi: 10.1111/j.1467-7687.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Res. 2005a;58:594–599. doi: 10.1203/01.pdr.0000176915.19287.e2. [DOI] [PubMed] [Google Scholar]
- Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005b;28:1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35:843–850. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- Kim J, Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Gozal D. Circulating microparticles in children with sleep disordered breathing. Chest. 2011 doi: 10.1378/chest.10-2161. In press. [DOI] [PubMed] [Google Scholar]
- Kohler MJ, Lushington K, K DJ. Neurocognitive performance and behavior before and after treatment for sleep-disordered breathing in children. Nature and Science of Sleep. 2010;2:159–185. doi: 10.2147/NSS.S6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Majde JA, Rector DM. Cytokines in immune function and sleep regulation. Handb Clin Neurol. 2011;98:229–240. doi: 10.1016/B978-0-444-52006-7.00015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Kuo TH, Pike DH, Beizaeipour Z, Williams JA. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFkappaB Relish. BMC Neurosci. 11:17. doi: 10.1186/1471-2202-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago R, Gomez R, Lago F, Gomez Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Larkin EK, Rosen CL, Kirchner HL, Storfer Isser A, Emancipator JL, Johnson NL, Zambito AM, Tracy RP, Jenny NS, Redline S. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- Lavie L, Polotsky V. Cardiovascular aspects in obstructive sleep apnea syndrome - molecular issues, hypoxia and cytokine profiles. Respiration. 2009;78:361–370. doi: 10.1159/000243552. [DOI] [PubMed] [Google Scholar]
- Laws SM, Hone E, Gandy S, Martins RN. Expanding the association between the APOE gene and the risk of Alzheimer's disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–588. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- Leung LC, Ng DK, Lau MW, Chan CH, Kwok KL, Chow PY, Cheung JM. Twenty-four-hour ambulatory BP in snoring children with obstructive sleep apnea syndrome. Chest. 2006;130:1009–1017. doi: 10.1378/chest.130.4.1009. [DOI] [PubMed] [Google Scholar]
- Li AM, Chan MH, Yin J, So HK, Ng SK, Chan IH, Lam CW, Wing YK, Ng PC. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatr Pulmonol. 2008;43:34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- Li AM, Ng C, Ng SK, Chan MM, So HK, Chan I, Lam CW, Ng PC, Wing YK. Adipokines in children with obstructive sleep apnea and the effects of treatment. Chest. 2010;137:529–535. doi: 10.1378/chest.09-2153. [DOI] [PubMed] [Google Scholar]
- Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR, Jr, Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med. 2003;168:469–475. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis. 2004;17:44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lim DC, Veasey SC. Neural injury in sleep apnea. Curr Neurol Neurosci Rep. 10:47–52. doi: 10.1007/s11910-009-0078-6. [DOI] [PubMed] [Google Scholar]
- Lopes MC, Marcus CL. The significance of ASDA arousals in children. Sleep Med. 2007;9:3–8. doi: 10.1016/j.sleep.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte AC, Thacher PV, Butters M, Bortz J, Acebo C, Carskadon MA. Parental report of sleep problems in children with attentional and learning disorders. J Dev Behav Pediatr. 1998;19:178–186. doi: 10.1097/00004703-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- Marz P, Cheng JG, Gadient RA, Patterson PH, Stoyan T, Otten U, Rose John S. Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1998;95:3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med. 16:247–256. doi: 10.1016/j.molmed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- McConnell K, Somers VK, Kimball T, Daniels S, VanDyke R, Fenchel M, Cohen A, Willging P, Shamsuzzaman A, Amin R. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180:42–48. doi: 10.1164/rccm.200808-1324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–399. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Mehra R, Storfer Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, Redline S. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166:1725–1731. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, Adachi M. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, Brug J. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11:695–708. doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Ridker PM. C-reactive protein, inflammation, and coronary risk. Med Clin North Am. 2000;84:149–161. ix. doi: 10.1016/s0025-7125(05)70211-x. [DOI] [PubMed] [Google Scholar]
- Must A, Barish EE, Bandini LG. Modifiable risk factors in relation to changes in BMI and fatness: what have we learned from prospective studies of school-aged children? Int J Obes (Lond) 2009;33:705–715. doi: 10.1038/ijo.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Parisi SM. Sedentary behavior and sleep: paradoxical effects in association with childhood obesity. Int J Obes (Lond) 2009;33 1:S82–86. doi: 10.1038/ijo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakra N, Bhargava S, Dzuira J, Caprio S, Bazzy Asaad A. Sleep-disordered breathing in children with metabolic syndrome: the role of leptin and sympathetic nervous system activity and the effect of continuous positive airway pressure. Pediatrics. 2008;122:e634–642. doi: 10.1542/peds.2008-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12:78–92. doi: 10.1111/j.1467-789X.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Rutherford J, Mehl RC, Wang M, Tuell A, Hume BC, Gozal D. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111:554–563. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, McClimment MC, Gozal D. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13:165–172. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Opp MR. Sleep and psychoneuroimmunology. Immunol Allergy Clin North Am. 2009;29:295–307. doi: 10.1016/j.iac.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Spirito A, Marcotte A, McGuinn M, Berkelhammer L. Neuropsychological and behavioral correlates of obstructive sleep apnea syndrome in children: a preliminary study. Sleep Breath. 2000;4:67–78. doi: 10.1007/BF03045026. [DOI] [PubMed] [Google Scholar]
- Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Patel SR, Redline S. Two epidemics: are we getting fatter as we sleep less? Sleep. 2004;27:602–603. [PubMed] [Google Scholar]
- Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: old story, new insights. Physiol Behav. 2009;97:279–292. doi: 10.1016/j.physbeh.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh V, Thatte HS, McCarley RW, Basheer R. Adenosine and sleep deprivation promote NF-kappaB nuclear translocation in cholinergic basal forebrain. J Neurochem. 2007;100:1351–1363. doi: 10.1111/j.1471-4159.2006.04314.x. [DOI] [PubMed] [Google Scholar]
- Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56:51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Sleep disturbances are associated with reduced school achievements in first-grade pupils. Dev Neuropsychol. 2009;34:574–587. doi: 10.1080/87565640903133533. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation and host defense. Am J Physiol Regul Integr Comp Physiol. 2001;280:R602–603. doi: 10.1152/ajpregu.2001.280.2.R602. [DOI] [PubMed] [Google Scholar]
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Riha RL. Genetic aspects of the obstructive sleep apnoea/hypopnoea syndrome--is there a common link with obesity? Respiration. 2009;78:5–17. doi: 10.1159/000221903. [DOI] [PubMed] [Google Scholar]
- Riha RL, Brander P, Vennelle M, McArdle N, Kerr SM, Anderson NH, Douglas NJ. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- Rosenfeld Y, Shai Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention of sepsis. Biochim Biophys Acta. 2006;1758:1513–1522. doi: 10.1016/j.bbamem.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Li RC, Guo SZ, Brittian KR, Hardy M, Bazan NG, Gozal D. Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem. 2004;89:189–196. doi: 10.1111/j.1471-4159.2004.02352.x. [DOI] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res. 2002;52:449–453. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- Schwarzenberg SJ, Sinaiko AR. Obesity and inflammation in children. Paediatric Respiratory Reviews. 2006;7:239–246. doi: 10.1016/j.prrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Shan X, Chi L, Ke Y, Luo C, Qian S, Gozal D, Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis. 2007;28:206–215. doi: 10.1016/j.nbd.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer S, Weinhouse E, Tal A, Wanderman KL, Margulis G, Leiberman A, Gueron M. Cor pulmonale due to adenoidal or tonsillar hypertrophy or both in children. Noninvasive diagnosis and follow-up. Chest. 1988;93:119–122. doi: 10.1378/chest.93.1.119. [DOI] [PubMed] [Google Scholar]
- Spath Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011 doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Fang J, Krueger JM. Somnogenic relationships between tumor necrosis factor and interleukin-1. Am J Physiol. 1999;276:R1132–1140. doi: 10.1152/ajpregu.1999.276.4.R1132. [DOI] [PubMed] [Google Scholar]
- Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatric pulmonology. 1988;4:139–143. doi: 10.1002/ppul.1950040304. [DOI] [PubMed] [Google Scholar]
- Tam CS, Wong M, McBain R, Bailey S, Waters KA. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006;42:277–282. doi: 10.1111/j.1440-1754.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004a;113:e564–569. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- Tauman R, O'Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007a;11:77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- Tauman R, O'Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 2004b;27:274–278. doi: 10.1093/sleep/27.2.274. [DOI] [PubMed] [Google Scholar]
- Tauman R, Serpero LD, Capdevila OS, O'Brien LM, Goldbart AD, Kheirandish-Gozal L, Gozal D. Adipokines in children with sleep disordered breathing. Sleep. 2007b;30:443–449. doi: 10.1093/sleep/30.4.443. [DOI] [PubMed] [Google Scholar]
- Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas AN. Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:143–150. doi: 10.1210/jc.2009-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valleggi S, Devaraj S, Dasu MR, Jialal I. C-reactive protein adversely alters the protein-protein interaction of the endothelial isoform of nitric oxide synthase. Clin Chem. 2010;56:1345–1348. doi: 10.1373/clinchem.2009.142364. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, Pannain S, Penev P, Tasali E, Spiegel K. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67 1:2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Latta F, Nedeltcheva A, Spiegel K, Leproult R, Vandenbril C, Weiss R, Mockel J, Legros JJ, Copinschi G. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14 A:S10–17. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9 1:S23–28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Jialal I. Effect of C-reactive protein on vascular cells: evidence for a proinflammatory, proatherogenic role. Curr Opin Nephrol Hypertens. 2005;14:33–37. doi: 10.1097/00041552-200501000-00006. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem. 2008;114:211–223. doi: 10.1080/13813450802364627. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083:329–344. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–316. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KA, Mast BT, Vella S, de la Eva R, O'Brien LM, Bailey S, Tam CS, Wong M, Baur LA. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res. 2007;16:388–395. doi: 10.1111/j.1365-2869.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation Correlates With Markers of T-Cell Subsets Including Regulatory T Cells in Adipose Tissue From Obese Patients. Obesity (Silver Spring) doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–642. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]


