Abstract
In species with alternative reproductive tactics, males that sneak copulations often have larger, higher quality ejaculates relative to males that defend females or nest sites. Ejaculate traits can, however, exhibit substantial phenotypic plasticity depending on a male's mating role in sperm competition, which may depend on the tactic of his competitor. We tested whether exposure to males of different tactics affected sperm number and quality in the swordtail Xipophorus nigrensis, a species with small males that sneak copulations and large males that court females. Sperm swimming speed was higher when the perceived competitor was small than when the competitor was large. Plasticity, however, was only exhibited by small males. Sperm number and viability were invariant between social environments. Our results suggest sperm quality is role-dependent and that plastic responses to the social environment can differ between male reproductive tactics.
Keywords: sperm competition, alternative reproductive tactics, phenotypic plasticity
1. Introduction
Parker [1] predicted that, given a trade-off between investment in the ejaculate and other expenditures important in obtaining mates, ‘parasitic’ males that sneak fertilizations should exhibit greater ejaculate investment relative to ‘bourgeois’ males that guard females. As predicted, parasitic males often do have larger testes, ejaculate more sperm of higher quality, and have higher success in sperm competition [2]. Few studies, however, have examined the extent to which ejaculate traits are phenotypically plastic in response to the phenotype of a male's competitor [3,4]. Ecological and demographic factors are known to lead to temporal and spatial fluctuations in the frequency of male reproductive tactics in the wild [5]. Consequently, plasticity in ejaculate traits may be favoured if changes in the phenotype of a male's competitor(s) affects the optimal ejaculate allocation strategy.
Males are predicted to alter ejaculate investment if they consistently experience an advantage (or disadvantage) in sperm competition [6]. Dominant males, for example, are often in the favoured mating ‘role’ in sperm competition because they can exclude subordinates from the best physical and temporal mating positions [7]. Females can also influence mating roles by lengthening or shortening copulation [8], ejecting sperm [9] or biasing sperm use inseminated by preferred males [10], which is typically the bourgeois phenotype in species with alternative tactics [2]. Theory predicts males should increase ejaculate investment when they consistently occupy the disfavoured role and decrease it when in the favoured role [6]. Experimental manipulations of social status in birds and fishes have shown some support for the theory, with sperm quantity and quality quickly declining when males become dominant (the favoured role) [3,4].
We examined whether phenotypic plasticity in ejaculate traits were manifest in the swordtail Xiphophorus nigrensis, an internally fertilized fish with size-dependent alternative male reproductive tactics. Male body size depends on a Y-linked polymorphism that induces sexual maturation and dramatically slows growth [11]. Males that mature at a small size obtain fertilizations by coercively sneaking copulations while males of large and intermediate size court females but rarely sneak [12]. Sperm competition has also played a role in the evolution of the tactics; small males produce more viable sperm and sperm that is longer lived [13]. Large males, however, are superior in male–male competition [14] and preferred by females [15]. Here, we manipulated the tactic of a male's perceived competitor and measured the number and quality of sperm produced. We predicted that small males would elevate ejaculate investment when their competitor was large (sneaker males in the disfavoured role), while large males would reduce investment when their perceived competitor was small (courting males in the favoured role).
2. Material and methods
Xipophorus nigrensis were collected from the Nacimiento Rio Choy, Mexico in May 2008 and housed with visual access to the other sex for at least four weeks prior the study. On the first day of the 14 day trial, sperm were stripped from a large (35.1 ± 1.73 mm standard length (SL), n = 11) or small (25 ± 0.83 mm SL, n = 17) focal male. The male was then placed in the centre of a 23 l aquarium divided evenly into thirds with two translucent, water-permeable barriers. A stimulus female (34.1 ± 1.5 mm SL) was placed in one compartment and either a large (35.4 ± 1.8 mm SL) or small (24.4 ± 1.1 mm SL) stimulus male in the other. The barrier allowed passage of visual and chemical stimuli but prevented physical contact. On day 7, the focal male's ejaculate was stripped for analysis. A stimulus male of the opposite tactic was then swapped into the aquarium after a complete water change while the same female remained in her compartment. On day 14, the focal male's ejaculate was stripped again for the second assay.
Ejaculate traits were assessed as in Smith & Ryan [13]. Briefly, the number of sperm stripped and swimming velocity were determined by activating 4 µl of ejaculate with 12 µl 150 mM KCl, pipetting the solution in a Microcell (Conception Technologies, San Diego, CA, USA) fixed depth (20 µl) slide and analysing the first 2 s of video 1 min post-activation (178 ± 70 sperm tracked, range 44–341). Two subsamples of the stripped ejaculate were measured for each male. Sperm viability was assessed by photographing sperm stained with the Molecular Probes LIVE/DEAD fluorescence assay (3227 ± 1600 sperm, range = 1100–6750) and calculating the proportion alive with ImageJ. An angular transformation was used to normalize the proportions. Data were analysed using repeated-measures ANOVA in Systat v. 11. All statistical tests were two-tailed.
3. Results
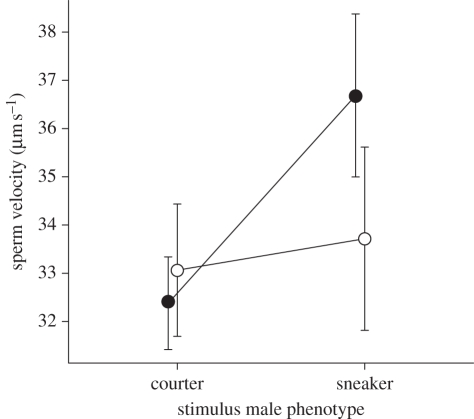
The tactic of the stimulus male had no significant effect on the number of sperm stripped (stimulus male tactic: F1,26 = 2.06, p = 0.16; stimulus × focal male tactic: F1,26 = 1.31, p = 0.26) or sperm viability (stimulus male tactic: F1,26 = 0.96, p = 0.34; stimulus × focal male tactic: F1,26 = 0.49, p = 0.49) of focal males. In contrast, sperm velocity was higher overall in the presence of sneaker males compared with courting males (stimulus male tactic: F1,26 = 4.53, p = 0.043) but a male's response did not depend on his own tactic (stimulus × focal male tactic: F1,26 = 2.46, p = 0.13; figure 1). Our power to detect the interaction, however, was low (1−β = 0.33), so we decomposed the interaction to increase power. We detected a 13 per cent increase in sperm swimming speed when sneakers were exposed to other sneakers compared with when they were exposed to courting males (paired t-test: t16 = 3.04, p = 0.008, Bonferroni-corrected α = 0.025, Cohen's D = 0.76; figure 1). In contrast, the velocity of sperm produced by courting males was not different when the tactic of their competitor was altered (paired t-test: t10 = 0.34, p = 0.74, Cohen's D = 0.12, figure 1). These results suggest the significant overall increase in velocity in the ANOVA, which we had more power to detect (1−β = 0.54), was driven by the response of sneaker males to the treatments.
Figure 1.
Average path velocity of sperm from courting and sneaking males when exposed to stimulus males of each tactic. Depicted are the means ±1 s.e. Filled circles, focal sneakers; open circles, focal courters.
4. Discussion
Several studies have demonstrated male ejaculate traits are phenotypically plastic given variation in the number of male rivals or female mating status [16]. Our study shows that changes in the phenotype of a male's perceived competitor results in rapid alterations in sperm quality, but that plasticity can be tactic-dependent. Only small sneaker males responded to variation in the tactic of their competitor, producing faster swimming sperm in the presence of other small males. Sperm velocity increases sperm competitive ability in swordtails [17] and other internal fertilizers [18], thus plasticity in velocity may be have important consequences for a male's success in sperm competition.
Temporal and spatial heterogeneity in the frequency of alternative male tactics could lead to phenotypic plasticity in ejaculate traits if such shifts also alter the optimal ejaculate allocation strategy. The frequency of sneaker males varies over space and time in swordtails [19] and has well-documented effects on sperm competition risk in other species [20,21]. Mating roles may also fluctuate with tactic frequency by altering male–male dominance interactions and female preferences, both of which can depend on the male phenotypes in competition in the population [22,23].
Ejaculate traits in large male X. nigrensis, however, did not depend on their competitor's phenotype in our study. One explanation is plasticity may not confer a selective advantage for these males. Trade-offs between the ejaculate and other activities that increase reproductive success are at the heart of sperm competition theory, and it is possible that bourgeois males may gain higher fitness returns by allocating energy to resource defence and attracting females rather than investing in sperm competition [20]. Alternatively, competitor phenotype may simply not provide information about the mating roles in X. nigrensis. This is unlikely, however, as male mating tactics can have large effects on mating success [8,15] and small males responded to the treatments in our experiment.
Considering their disadvantaged mating role, we expected small males to have higher ejaculate investment in the presence of large, not the small, male competitors. Other studies have found sperm quality is lower in subordinate males when dominance interactions prevent access to females and the energetic costs of aggression are steep, resulting in a ‘wait to mate’ until higher social status can be obtained [24]. We think this explanation is unlikely here because (i) males do not live in stable social groups where females are well-defended, (ii) male growth declines rapidly at sexual maturity preventing predictable transitions in social status with age, and (iii) aggressive interactions between individuals that often mediate these effects were precluded by a physical barrier (although fish could see and smell each other).
Alternatively, small males might be responding to variation in their perception of sperm competitive ability rather than asymmetries in precopulatory sexual selection. A previous study found sneaker male X. nigrensis have more viable and longer lived sperm than courting males [13], both of which are known to contribute to sperm competitive ability in internal fertilizers [25,26]. Differences in sperm competitive ability might have stronger effects on mating roles than male–male competition and female preferences, all of which are known to influence reproductive success in swordtails [15,17,27]. Disentangling the relative importance of precopulatory and post-copulatory interactions on reproductive success is a major aim of research in sexual selection [28]. Our study suggests these episodes of selection might shape plasticity in ejaculate traits, and that the costs and benefits of plasticity differ between male tactics.
Acknowledgements
We thank Hans Hofmann, Ed Theriot and Grace Lee for assistance collecting the data. Three anonymous reviewers provided helpful comments on the article. Permission to collect fish was graciously provided by the Mexican government (DGOPA.11319.031107.-4933).
References
- 1.Parker G. A. 1990. Sperm competition games—sneaks and extra-pair copulations. Proc. R. Soc. Lond. B 242, 127–133 10.1098/rspb.1990.0115 (doi:10.1098/rspb.1990.0115) [DOI] [Google Scholar]
- 2.Taborsky M. 2008. Alternative reproductive tactics in fish. In Alternative reproductive tactics: an integrative approach (eds Olveira R. F., Taborsky M., Brockmann H. J.), pp. 251–299 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Rudolfsen G., Figenschou L., Folstad I., Tveiten H., Figenschou M. 2006. Rapid adjustments of sperm characteristics in relation to social status. Proc. R. Soc. B 273, 325–332 10.1098/rspb.2005.3305 (doi:10.1098/rspb.2005.3305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizzari T., Cornwallis C. K., Froman D. P. 2007. Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc. R. Soc. B 274, 853–860 10.1098/rspb.2006.0080 (doi:10.1098/rspb.2006.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockmann H. J., Taborsky M. 2008. Alternative reproductive tactics and the evolution of alternative allocation phenotypes. In Alternative reproductive tactics: an integrative approach (eds Olveira R. F., Taborsky M., Brockmann H. J.), pp. 25–51 Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Parker G. A. 1990. Sperm competition games—raffles and roles. Proc. R. Soc. Lond. B 242, 120–126 10.1098/rspb.1990.0114 (doi:10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 7.Stoltz J., Neff B. 2006. Male size and mating tactic influence proximity to females during sperm competition in bluegill sunfish. Behav. Ecol. Sociobiol. 59, 811–818 10.1007/s00265-005-0127-3 (doi:10.1007/s00265-005-0127-3) [DOI] [Google Scholar]
- 8.Pilastro A., Mandelli M., Gasparini C., Dadda M., Bisazza A. 2007. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 74, 321–328 10.1016/j.anbehav.2006.09.016 (doi:10.1016/j.anbehav.2006.09.016) [DOI] [Google Scholar]
- 9.Pizzari T., Birkhead T. R. 2000. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789 10.1038/35015558 (doi:10.1038/35015558) [DOI] [PubMed] [Google Scholar]
- 10.Edvardsson M., Göran A. 2000. Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc. R. Soc. Lond. B 267, 559–563 10.1098/rspb.2000.1037 (doi:10.1098/rspb.2000.1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampert K. P., Schmidt C., Fischer P., Volff J.-N., Hoffmann C., Muck J., Lohse M. J., Ryan M. J., Schartl M. 2010. Determination of onset of sexual maturation and mating behavior by melanocortin receptor 4 polymorphisms. Curr. Biol. 20, 1729–1734 10.1016/j.cub.2010.08.029 (doi:10.1016/j.cub.2010.08.029) [DOI] [PubMed] [Google Scholar]
- 12.Ryan M. J., Causey B. A. 1989. Alternative mating behavior in the swordtails Xiphophorus nigrensis and Xiphophorus pygmaeus (Pisces, Poeciliidae). Behav. Ecol. Sociobiol. 24, 341–348 10.1007/BF00293262 (doi:10.1007/BF00293262) [DOI] [Google Scholar]
- 13.Smith C. C., Ryan M. J. 2010. Evolution of sperm quality but not quantity in the internally fertilized fish Xiphophorus nigrensis. J. Evol. Biol. 17, 1759–1771 [DOI] [PubMed] [Google Scholar]
- 14.Morris M. R., Batra P., Ryan M. J. 1992. Male–male competition and access to females in the swordtail Xiphophorus nigrensis. J. Evol. Biol. 4, 980–986 [Google Scholar]
- 15.Ryan M. J., Hews D. K., Wagner W. E. 1990. Sexual selection on alleles that determine body size in the swordtail Xiphophorus nigrensis. Behav. Ecol. Sociobiol. 26, 231–237 10.1007/BF00178316 (doi:10.1007/BF00178316) [DOI] [Google Scholar]
- 16.Wedell N., Gage M. J. G., Parker G. A. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 17.Gasparini C., Simmons L. W., Beveridge M., Evans J. P. 2010. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS ONE 5, 1–5 10.1371/journal.pone.0012146 (doi:10.1371/journal.pone.0012146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denk A. G., Holzmann A., Peters A., Vermeirssen E. L., Kempenaers B. 2005. Paternity in mallards: effects of sperm quality and female sperm selection for inbreeding avoidance. Behav. Ecol. 16, 825–833 10.1093/beheco/ari065 (doi:10.1093/beheco/ari065) [DOI] [Google Scholar]
- 19.Rios-Cardenas O., Tudor M. S., Morris M. R. 2007. Female preference variation has implications for the maintenance of an alternative mating strategy in a swordtail fish. Anim. Behav. 74, 633–640 10.1016/j.anbehav.2007.01.002 (doi:10.1016/j.anbehav.2007.01.002) [DOI] [Google Scholar]
- 20.Alonzo S. H., Warner R. R. 2000. Allocation to mate guarding or increased sperm production in a Mediterranean wrasse. Am. Nat. 156, 266–275 10.1086/303391 (doi:10.1086/303391) [DOI] [PubMed] [Google Scholar]
- 21.Zamudio K., Sinervo B. 2000. Polygyny, mate-guarding, and posthumous fertilization as alternative male mating strategies. Proc. Natl Acad. Sci. USA 97, 14 427–14 432 10.1073/pnas.011544998 (doi:10.1073/pnas.011544998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farr J. A., Travis J., Trexler J. C. 1986. Behavioural allometry and interdemic variation in sexual behaviour of the sailfin molly, Poecilia latipinna (Pisces: Poeciliidae). Anim. Behav. 34, 497–509 10.1016/S0003-3472(86)80118-X (doi:10.1016/S0003-3472(86)80118-X) [DOI] [Google Scholar]
- 23.Gibson R. M., Langen T. A. 1996. How do animals choose their mates? Trends Ecol. Evol. 11, 468–470 10.1016/0169-5347(96)10050-1 (doi:10.1016/0169-5347(96)10050-1) [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick J. L., Desjardins J. K., Milligan N., Montgomerie R., Balshine S. 2007. Reproductive tactic-specific variation in sperm swimming speeds in a shell-brooding cichlid. Biol. Reprod. 77, 280–284 10.1095/biolreprod.106.059550 (doi:10.1095/biolreprod.106.059550) [DOI] [PubMed] [Google Scholar]
- 25.García-González F., Simmons L. W. 2005. Sperm viability matters in insect sperm competition. Curr. Biol. 15, 271–275 10.1016/j.cub.2005.01.032 (doi:10.1016/j.cub.2005.01.032) [DOI] [PubMed] [Google Scholar]
- 26.Pizzari T., Worley K., Burke T., Froman D. 2008. Sperm competition dynamics: ejaculate fertilising efficiency changes differentially with time. BMC Evol. Biol. 8, 332. 10.1186/1471-2148-8-332 (doi:10.1186/1471-2148-8-332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerer E. J., Kallman K. D. 1989. Genetic basis for alternative reproductive tactics in the pygmy swordtail, Xiphophorus nigrensis. Evolution 43, 1298–1307 10.2307/2409364 (doi:10.2307/2409364) [DOI] [PubMed] [Google Scholar]
- 28.Hunt J., Breuker C. J., Sadowski J. A., Moore A. J. 2009. Male–male competition, female mate choice and their interaction: determining total sexual selection. J. Evol. Biol. 22, 13–26 10.1111/j.1420-9101.2008.01633.x (doi:10.1111/j.1420-9101.2008.01633.x) [DOI] [PubMed] [Google Scholar]