Abstract
A substantial array of respiratory, cardiovascular, visceral and somatic afferents are relayed via the nucleus of the solitary tract (NTS) to the brainstem (and forebrain). Despite some degree of overlap within the NTS, specificity is maintained in central respiratory reflexes driven by 2nd order afferent relay neurons in the NTS. While the topographical arrangement of respiratory-related afferents targeting the NTS has been extensively investigated, their higher order brainstem targets beyond the NTS has only rarely been defined with any precision. Nonetheless, the various brainstem circuits serving blood gas homeostasis and airway protective reflexes must clearly receive a differential innervation from the NTS in order to evoke stimulus appropriate behavioral responses.
Accordingly, we have examined the question of which specific NTS nuclei project to particular compartments within the ventral respiratory column (VRC) of the ventrolateral medulla. Our analyses of NTS labeling after retrograde tracer injections in the VRC and the nearby neuronal groups controlling autonomic function indicate a significant distinction between projections to the Bötzinger complex and preBötzinger complex compared to the remainder of the VRC. Specifically, the caudomedial NTS, including caudal portions of the medial solitary nucleus and the commissural division of NTS project relatively densely to the region of the retrotrapezoid nucleus and rostral ventrolateral medullary nucleus as well as to the rostral ventral respiratory group while avoiding the intervening Bötzinger and preBötzinger complexes. Area postrema appears to demonstrate a pattern of projections similar to that of caudal medial and commissural NTS nuclei. Other, less pronounced differential projections of lateral NTS nuclei to the various VRC compartments are additionally noted.
Keywords: Respiratory control, cardiovascular control, retrotrapezoid nucleus, ventral respiratory group, preBötzinger complex, rostral ventrolateral medulla
The nucleus of the solitary tract (NTS) is a complex neuronal aggregate providing a critical interface between afferent information from the viscera, and the central circuits controlling a broad range of functions (Barraco, 1994; Saper, 2004; Bradley, 2007). Respiratory, autonomic, volitional, and emotional behaviors acting in conjunction with central, innate and/or learned sensory associations are all responsive to visceral feedback via the caudal NTS.
Breathing, in particular, is a voluntary and highly motivated somatomotor behavior supported by automatic rhythm and pattern generating circuits located in the rhombencephalon, and particularly within the ventrolateral medulla (Feldman and del Negro, 2006; McCrimmon et al., 2009; Smith et al., 2009). Medullary respiratory circuits include a column of cells in the ventrolateral medulla termed the ventral respiratory column (VRC) that is comprised of at least five serial compartments. Each of these appears to be functionally distinct with respect to the firing pattern evinced by their complement of respiratory neurons, and with respect to their ultimate influence on the control of breathing (Alheid et al., 2008; Smith et al., 2009). Similarly, neurons controlling the sympathetic outflow also occupy a series of compartments that are organized ventrally adjacent to the respiratory network and more-or-less parallel to the VRC (Goodchild and Moon, 2009; Pilowsky et al., 2009).
Caudal NTS efferents strongly and differentially influence the activity of the neurons in ventral medullary cardiorespiratory compartments. Understanding the particular targets of various NTS subdivisions in the brainstem is an important key to understanding the functional organization of both the NTS and ventral medulla. However, the hodology of NTS projections to specific cardiorespiratory brainstem targets remain incompletely characterized. This is particularly true for the targeting of medullary respiratory compartments by various NTS nuclei.
Several studies have addressed the question of NTS efferents to the medulla in the context of efferents to individual respiratory compartments within the VRC (e.g. Ellenberger and Feldman, 1990a, b, 1994; Rosin et al., 2006), as well as with respect to projections from individual subregions of the NTS to the VRC and to the medulla (Otake et al., 1992; Geerling and Loewy, 2006; Rinaman, 2010). Other studies have addressed the overall distinctions between projections to multiple compartments within the VRC, both in the cat (Smith et al., 1989), and in the rat (Núñez-Abades et al., 1993). In the intervening years, however, considerable refinement has occurred both in defining the detailed structure of the NTS (e.g., Herbert et al., 1990; Ciriello et al., 1994; Ruggiero et al., 1996; Paxinos et al., 1999) and in the identification of the compartmental organization of the VRC (Alheid et al., 2002, 2008; Feldman and del Negro, 2006; McCrimmon et al., 2009).
Accordingly, we have reexamined the question of which specific NTS nuclei project to particular VRC compartments. Our analyses of NTS labeling after retrograde tracer injections in the VRC and the nearby autonomic column indicate a significant distinction between projections to the Bötzinger complex (BötC) and preBötzinger complex (preBötC) compared to the remainder of the VRC. Specifically, the caudomedial NTS, including caudal portions of the medial solitary nucleus (SolM; alongside and caudal to area postrema) and the commissural division of NTS (SolC) projects relatively densely to the region of the retrotrapezoid nucleus (RTN) and rostral ventrolateral medullary nucleus (RVL) as well as to the rostral ventral respiratory group (rVRG) while avoiding the intervening BötC and preBötC. Area postrema appears to demonstrate a pattern of projections similar to that of caudal SolM and SolC. Other, less pronounced differential projections of lateral NTS nuclei to the various VRC compartments are additionally noted.
These data have previously been presented in abstract form (Alheid et al., 2010).
2. EXPERIMENTAL PROCEDURES
2.1 Animals
Experiments were performed on 14 male Sprague-Dawley rats (Charles River) weighing 200–400 gms. All surgeries were performed using sterile procedures adapted for small rodents, in accordance with guidelines recommended by the NIH and by the Society for Neuroscience. All procedures were approved by the Northwestern University Animal Care and Use Committee. One, normal rat was prepared for examination of NTS nuclei by injecting FG subcutaneously (25 mg/kg). This directly labels the area postrema, which lies outside of the blood brain barrier. and retrogradely labels motoneurons in the dorsal motor nucleus of the vagus (10N) and hypoglossal nucleus (12N; Leong and Ling, 1990) whose axons project outside of the blood brain barrier. The same brain was subsequently immunolabeled for neuronal specific nuclear protein (NeuN) in order to examine neuronal clusters within the NTS and their disposition relative to the area postrema, 10N and 12N motoneurons. Four animals used in the present analysis also had received a prior injection of the transganglionic tracer DiI (1,1-dioctadecyl-3,3,3,3-tetramethyl-indocarbocyanine perchlorate; Invitrogen #D-282) in the carotid body in order to examine carotid afferents terminating on retrogradely labeled neurons. The results of this experiment will be described elsewhere.
2.2 Surgery
Anesthesia was induced with 5% isoflurane in an induction chamber. Animals were then rapidly switched from the induction chamber to a nose cone attached to a stereotaxic frame where the animal was allowed to freely breathe isoflurane (2.5–3%) for maintenance. The depth of anesthesia was frequently assessed (10–15 min intervals) and judged by the absence of overt retraction responses to a strong noxious paw pinch, and by the absence of changes in heart rate or breathing in response to the noxious stimulation.
Rectal temperature was monitored and maintained at 37.5 ± 1°C by means of a thermistor-controlled heating pad and heat lamp. EMG and ECG activities were continuously monitored using transcutaneous needle electrodes placed on the caudal thorax with a ground wire placed laterally on the abdomen. Oxygen saturation as well as heart rate and respiratory rate were monitored via a pulse oximeter (Mouse Ox, Starr Life Sciences). In a subset of rats a femoral artery was cannulated (PE-50) for monitoring arterial pressure.
For extracellular recording and retrograde tracer injections a dorsal midline incision was made to expose the skull and/or neck. For tracer injections into rostral portions of the VRC (retrotrapezoid nucleus, RTN; Bötzinger complex, BötC; or preBötzinger complex, preBötC) a ~2 × 2 mm opening was made in the skull and a trans-cerebellar approach was used. Stereotaxic coordinates were obtained from the atlas of Paxinos et al. (2009). Nominally, rostral tracer injections (in the Bötzinger, BötC, or preBötzinger, preBötC, complex) were targeted at approximately 1.8 mm lateral to the midline and approximately 3.5 to 4.5 caudal to lambda suture (using the average of this suture as described in the atlas by Paxinos et al., 2009).
Injections into more caudal VRC regions (rostral ventral respiratory group, rVRG) were made after separating the dorsal neck muscles and exposing the dorsal surface of the medulla by opening the cisterna magna. In the latter instance, obex was used as the initial reference point, the skull was mounted with the bregma suture located ~1.5 mm lower than the lambda suture, and the electrode was angled at 16° (dorsocaudal to rostroventral). For caudal portions of the VRC (i.e. the rostral ventral respiratory group; rVRG) nominal targets were at 0.0–1.5 rostral to the most caudal point of the area postrema (obex).
An analgesic, meloxicam (1 mg/kg, s.c.), was administered 30 minutes prior to the end of the experiment. Following the completion of surgery and the recovery of the righting response, the opiate analgesic buprenorphine hydrochloride was administered (0.03 mg/kg, s.c.). Buprenorphine was repeated 12 hour post-operatively and meloxicam at 24 hours. Following survival intervals sufficient for transport of retrograde tracers (≥ 7 days), animals were deeply anesthetized (ketamine-xylazine; 100-20 mg/kg) and perfused transcardially with fixatives. Subsets of animals were subjected to multiple surgeries either for injection of anterograde tracers in peripheral nerves or for single cell recordings in the NTS. These data and the accompanying procedures will be reported separately.
2.3 Retrograde tracer injections
FluoroGold, (FG, Fluorochrome Inc, Denver Co), and/or Fast Blue (FB, #F-5756 Sigma-Aldrich) were injected in the ventral respiratory column (VRC) of the medulla or in ventrally adjacent regions related to control of the sympathetic nervous system at rostral (rostral ventrolateral medullary nucleus; RVL) or mid-levels of the medulla (caudal ventrolateral medullary nucleus; CVL).
In all instances, retrograde tracer injections were targeted by extracellular recording of unit activity in the VRC using the same micropipette used for the tracer injections. Three different recording and injection combinations were used: 1) FG was iontophoresed from a single barrel pipette that was also used for extracellular unit recording; 2) Since the solution containing FB was inappropriate for extracellular recording, FB was injected from a double barrel pipette where one barrel was used for extracellular unit recording and the second barrel for pressure injections of FB, and 3) FG was iontophoresed from one barrel of double barrel pipette that was also used for extracellular recording. The second barrel was used for pressure ejection of the excitatory amino acid DL-homocysteic acid (DLH) as described previously (McCrimmon et al., 1986). DLH injection helped confirm the identity of the cardiorespiratory region injection since the elicited changes in respiratory motor output and arterial pressure are region specific (Monnier et al., 2003). Histologically, the size of the injection sites resulting from iontophoresis of FG were similar to those resulting from the pressure injection of FB. Although FB labeling appeared brighter in photomicrographs than that from FG, the distribution of retrogradely labeled neurons was similar for the two tracers.
Single barrel pipette tips were broken to 2–10 μm o.d. Double barrel pipettes were broken to 4–15 μm o.d. FG was used at 1.5% in 0.1M KAcetate or 0.15M NaCl. FB was used at 1% dissolved in 100% DMSO. DLH (5 mM) was dissolved in 0.15 M NaCl. The recording barrel in the double-barrel pipette used for FB injections was filled with 0.5 M NaCl.
FG was iontophoresed using 1–2 μA X 15 minutes (3sec on and 3sec off). FB or DLH pressure injections were 3–9 nl. The volumes of pressure injections (FB or DLH) were directly monitored by measuring the movement of the meniscus in the injection pipette using a microscope with a calibrated reticle.
Following injections of either FG or FB, a 20–30 minute post-injection waiting period was allowed before removal of the micropipette. Post-injection survival intervals were 7–21 days.
2.4 Extracellular Recording
For extracellular recording and tracer injection the electrode was advanced dorsoventrally and unit and population respiratory unit activity was amplified (2,000 – 10,000 X) and band-pass filtered (300 – 5K Hz). The recorded unit activity was displayed on an oscilloscope and simultaneously recorded and stored in digital format using a computer controlled A/D converter (Cambridge Electronic Devices Ltd.). Once respiratory activity was recorded, its dorsoventral extent was mapped. The electrode was then positioned at the approximate dorsoventral center of the respiratory population and the retrograde tracer injected. If unit activity was weak or absent on an electrode track, additional electrode penetrations were made after moving the electrode in the mediolateral or rostrocaudal planes by 200 μm. In several cases (n=4), two different tracers were injected in the same animal; one tracer (FG) was injected at the region where respiratory neurons were recorded, and using a separate electrode, a second tracer (FB) was injected at the anticipated location of either RVL or CVL. The ventral coordinates for the FB injections were deliberately chosen to place it below the level where respiratory activity was recorded.
2.5 Fixation and Histology
Following survival intervals for retrograde labeling, animals were deeply anesthetized with Ketamine-Xylazine (80-20 mg/kg respectively) or pentobarbital (100mg/kg) and perfused transcardially with approximately 100 ml of rinse followed by 300–400 ml of fixative solution. The rinse consisted of 0.8% sucrose, 0.8% NaCl, 0.4% D-glucose, 0.25% procaine. The fixative consisted of 4% paraformaldehyde, 0.1M NaPO4 buffer and 15% v/v picric acid (picric acid used as a saturated aqueous solution). The pH of the fixative was adjusted to 7.4 with 0.5M HCl.
Following perfusion-fixation, brains were removed from the calvarium and post-fixed for 24 hours in the same fixative used for perfusion. Subsequently, they were embedded/encapsulated in 10% gelatin. Cooled and trimmed gelatin blocks were then stored overnight in fixative. Brainstems were sectioned at 50 μm and collected in phosphate buffered saline (PBS, in mM: Cl− 140, K+ 42, Na+ 153, PO4− 10). Sections were collected as 6 parallel sets of serial sections. Thus, sections within in a given series were separated by 300μm. At least one set of the parallel series was mounted on glass slides for analysis of retrograde labeling based on the native fluorescence of the tracers. An additional set of sections from the same brain and separated by 150 μm from the first series was usually immunolabeled to convert FG labeling to diaminobenzidine (DAB) as a permanent light-opaque label. In a few instances, the locations of FG and/or FB tracer injections were evaluated relative to immunofluorescent labeling for tyrosine hydroxylase using a set of serial sections intervening between the series analyzed for the retrograde labeling.
2.6 Immunostaining
Immunostaining procedures were identical to those reported in earlier papers (Alheid et al., 2002; Shammah-Lagnado et al., 1999). Briefly, free floating sections were rinsed in PBS (3 × 10 min), pre-treated to reduce background staining, first in NaBH3 (1% in 0.1M NaPO4 buffer) followed by a PBS rinse (3X), then a second pretreatment in 5% methanol-1% H2O2 in PBS, and rinsed in PBS (3X). Sections were then incubated for 30 minutes on a shaker in a carrier buffer consisting of 0.25 % lambda-carrageenan, 1% bovine serum albumin, and 0.05% sodium azide in PBS buffer. The primary antibody was then added to the buffer to make a final working dilution determined by prior pilot immunolabeling tests for each individual antibody. Following a 72 hour incubation at 4°C, sections were again rinsed (3X) in PBS, and incubated in biotinylated secondary antibodies overnight, then rinsed again in PBS. Sections were then incubated in avidin-RITC for 2 hours, rinsed once more in PBS and then mounted on clean, unsubbed slides (for fluorescent sections).
For conversion of FG fluorescent labeling to diaminobenzidine (DAB), sections immunolabeled with the antibody for FG were incubated for 2 hours or overnight in avidin-biotin-horseradish peroxidase reagents (ABC elite kit, Vector labs), and reacted for the oxidation-polymerization of DAB using the glucose-oxidase-peroxidase coupled reaction (Itoh et al., 1979).
Fluorescent labeled sections were mounted on glass slides and covered with a polyvinyl alcohol based coverslipping media (consisting of Mowiol 4–88, glycerol and tris buffer pH 8.5). DAB stained sections mounted on subbed slides, were dried, cleared overnight in xylenes and covered with DPX coverslipping media.
2.7 Antibodies
Mouse anti-neuronal specific nuclear protein (1: 2000; Millipore/Chemicon #MAB377), Rabbit anti-FluoroGold (1: 4000; Millipore/Chemicon #AB153) and mouse anti-tyrosine hydroxylase (1: 3000; Millipore/Chemicon #MAB318) have all been previously characterized in our own lab (anti-tyrosine hydroxylase, anti-FluoroGold; Alheid et al., 2002; Shammah-Lagnado et al., 1996, 1999) and in by others (anti-tyrosine hydroxylase, anti-FluoroGold; anti-NeuN; see Journal of Comparative Neurology antibody database version 5; Wiley Publishing Online: http://onlinelibrary.wiley.com.ezproxy.galter.northwestern.edu/journal/10.1002/(ISSN)1096-9861/homepage/JCN_ANTIDB5.xls).
Relative to FluoroGold immunolabeling, RITC labeling of FG cells was compared to native FG fluorescence in adjacent mounted sections to insure specificity of the antibody. Further, for selected samples, FG neurons immunolabeled with RITC were digitally photographed prior to conversion with ABC reagents and compared with the subsequent DAB labeled FG neurons (Alheid et al., 2002).
2.8 Image capture and analysis
Sections were visually examined using a Zeiss Axioplan Imaging 2 epifluorescence microscope with Zeiss standard band pass fluorescence filter sets for uv, fluorescein, and rhodamine imaging, as well as a triple band pass filter (DAPI, FITC, Texas Red), and a computer controlled liquid crystal RGB slide filter (Cambridge Instruments) for true-color image capture. Digital micrographs were subsequently captured using a QImaging Retiga 4000R CCD camera (2K X 2K pixel) controlled by NIH – ImageJ software (http://rsbweb.nih.gov/ij/). Grayscale and true color images of FG, FB, and RITC labeling images were collected for subsequent analyses. In most cases, images were also collected for the same field of view as the fluorescent labeling using darkfield illumination in order to better characterize the location of retrograde and/or immunolabeling. Individual grayscale images were adjusted for contrast and brightness using Photoshop® CS2 software, and pseudocolor images constructed with RITC, retrograde labeling, and darkfield images copied to the color channels of blank digital (RGB) images. Selected NTS regions containing retrograde labeling were imaged at high magnification (40X) using laser confocal microscopy (Nikon C1 confocal microscope). Image stacks were subsequently analyzed offline to estimate cell sizes of retrogradely labeled NTS neurons.
2.9 Cell sizes
Estimates of relative cell sizes for retrogradely labeled NTS neurons were obtained using ImagePro-Plus software (Mediacybernetics/Roper) on graphic images of the fluorescent neurons created with ImageJ (NIH) and Adobe Photoshop from the original confocal images. Confocal image z-stacks were imported into ImageJ and maximum intensity projections created from the entire stack. Individual neurons were then outlined and filled in Photoshop on a graphical layer which was separate from the digital maximum intensity image. The layer containing the outlined neurons was saved as a separate image and opened in ImagePro-Plus for automated measurement of cell cross sectional areas and maximum and minimum diameters. Numerical data provided by ImagePro Plus was then exported to a Microsoft Excel spread sheet for analysis and generation of descriptive statistics.
3. RESULTS
The following provides an analysis of projections from the various subdivisions of the caudal NTS to the VRC. The caudal NTS for the sake of this analysis is represented by the NTS region containing neurons retrogradely labeled from cardiorespiratory compartments in the ventrolateral medulla. Using this definition, the caudal NTS begins about 300–400 μm rostral to area postrema and includes the portion of the NTS receiving the bulk of peripheral viscerosensory afferents related to cardiorespiratory feedback (e.g. Herbert et al., 1990; Ciriello et al., 1994; Rugierro et al., 1996). To a lesser extent, caudal NTS projections to elements of the cardiovascular regions of the ventrolateral medulla are also considered, insofar as these are sometimes involved by our tracer injections. These regions are generally adjacent and ventral to the VRC practically throughout its entire course (e.g. Goodchild and Moon, 2009; Pilowsky et al., 2009).
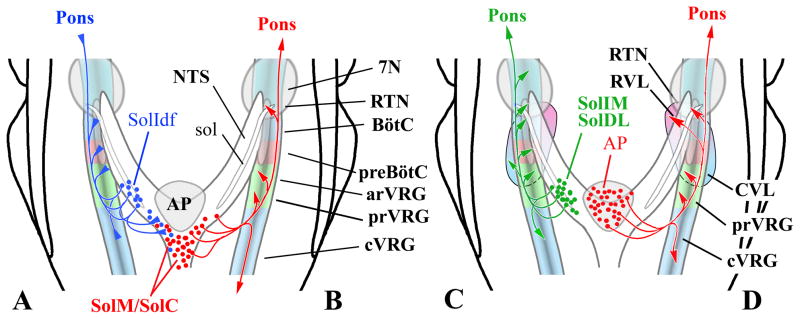
Terminology for the nuclei found within the caudal NTS follows that outlined for the rat by Paxinos et al. (1999, 2009) which, in turn, is based on descriptions by Herbert et al., (1990) and Ruggiero et al., (1996). Subdivisions of the VRC have been recently reviewed (Alheid et al., 2008; McCrimmon et al., 2009) as have autonomic/cardiovascular components of ventrolateral medulla (Goodchild and Moon, 2009; Pilowsky et al., 2009). Respiratory and autonomic compartments of ventrolateral medulla have also been tentatively identified in Paxinos et al. (1999, 2009). Representative coronal sections through the caudal NTS are shown in Fig. 1 and respiratory and autonomic subdivisions of the ventrolateral medulla are schematically depicted in Fig. 2. The respiratory component includes the VRC which is subdivided into the RTN, preBötC and BötC, rostral and caudal ventral respiratory groups (rVRG and cVRG) (Feldman and McCrimmon, 2008). Autonomic regions include the ventrally adjacent rostral and caudal ventral medullary nuclei (RVL and CVL; Pilowsky et al., 2009).
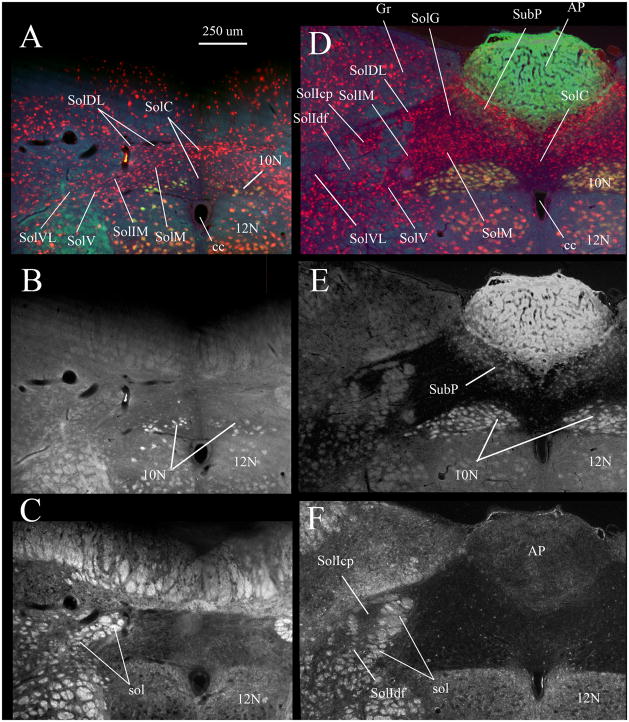
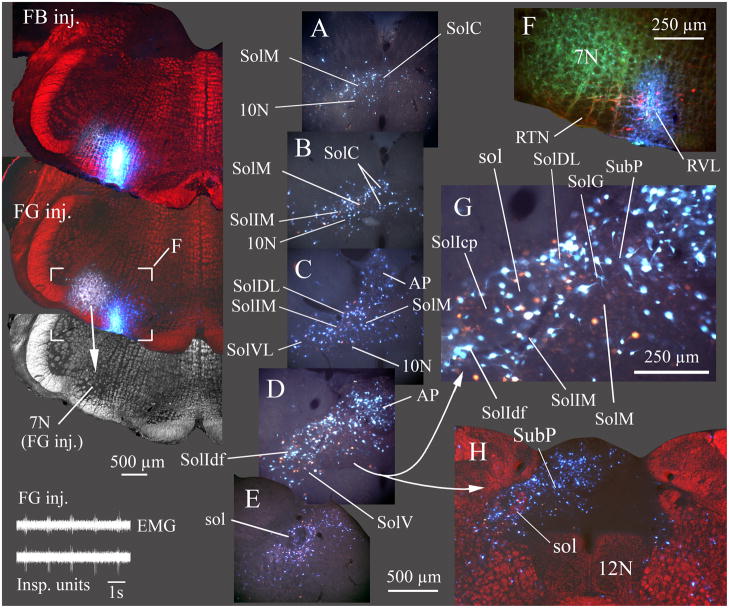
Fig. 1. Caudal NTS subdivisions viewed via NeuN immunostaining (A, D), FluoroGold labeling of vagal motoneurons and area postrema (B, E) and by darkfield illumination of solitary tract axons (C, F).
Nuclei composing the caudal NTS are indicated in A and D. A–C show the same section ~ 300 μm caudal to obex where the commissural nucleus of the solitary tract (SolC) is most prominent. D–F show a single section ~ 400 μm rostral to obex where the area postrema is near its maximal mediolateral extent.
In A and D red neurons are immunofluorescent for neuron specific nuclear protein (NeuN). In A, B, D and E labeling in the dorsal motor nucleus of the vagus (10N), hypoglossal nucleus (12N), area postrema (AP) and subpostrema nucleus (SubP) resulted from a subcutaneous injection of FluoroGold. In C and F darkfield illumination identifies the myelinated fascicles of the solitary tract (sol).
A and D are true color RGB images captured using a triple band-pass epifluorescence filter (uv/DAPI, FITC, Texas Red) and a RGB liquid crystal filter. Images in B and E were extracted from the green channel of the RGB images in A and D respectively.
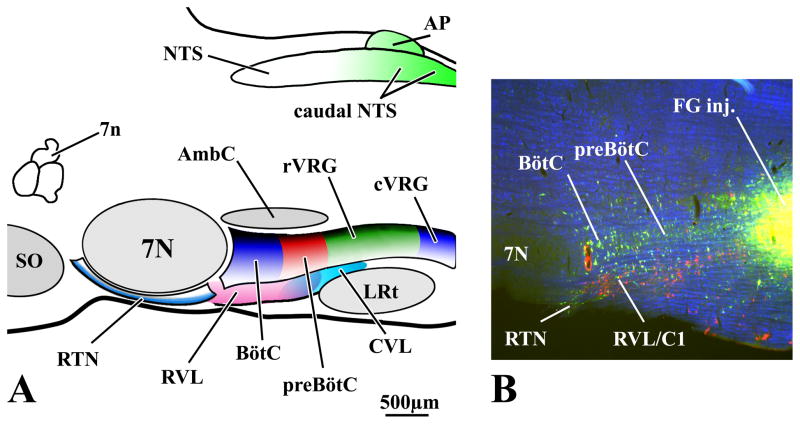
Fig. 2. Compartments of the Ventral Respiratory Column (VRC) of the Medulla.
Compartments of the rat VRC (RTN, BötC, preBötC, rVRG, cVRG) are color-coded and projected onto a sagittal plane in A with local landmarks in gray. Approximate locations of the RVL and CVL are depicted ventral to the VRC. Overlapping aspects of RVL and CVL are depicted by the merged pink and cyan areas located beneath the preBötC. Caudal NTS regions (including the area postrema) providing input to the ventral medullary compartments are also indicated (green). B is a 50 μm thick parasagittal section at about the same scale and mediolateral location as the schematic in A. In B, respiratory-related neurons in the BötC and preBötC are retrogradely labeled from a FG injection in the rVRG. Tyrosine hydroxylase (TH) immunofluorescent neurons (red) ventrally adjacent to rostral compartments of the VRC are a component of the RVL. Note that TH neurons in RVL are not, in general, retrogradely labeled by retrograde tracers injected in the VRC. B is a pseudocolored image with monochrome images of TH neurons (red) and FG neurons (FITC-filter, green) and darkfield images (blue) inserted in the red, green and blue channels (respectively) of a digital image.
We have employed a few exceptions to the terminology used by Paxinos et al. (1999, 2009). The first is that we have retained the abbreviation “NTS” to designate the overall solitary nuclear complex. This is instead of “Sol” as suggested by Paxinos et al. (1999, 2009) and permits us to be consistent with the use of “NTS” as the abbreviation most commonly used in the cardiorespiratory literature. The term NTS also permits a clear distinction between discussions of the entire nuclear complex versus the individual nuclei. Accordingly, we have adopted “Sol” as the prefix, for the individual nuclei within the NTS, consistent with the convention for naming NTS nuclei used in Paxinos et al. (1999, 2009). We have additionally applied two separate terms to identify the interstitial nucleus of the NTS (SolI). SolI designates neurons embedded between the fascicles of the solitary tract (Fig. 1C); we have divided these into compact (SolIcp) and diffuse portions (SolIdf). Paxinos et al. (1999, 2009) apply the term SolI to represent the compact part of the interstitial nucleus; the remainder of the cells scattered within the solitary tract fascicles do not appear to have been assigned an independent designation by these authors.
It is also worth noting that a rather restricted NTS zone is designated as SolC by Paxinos et al (1999, 2009) and this designation is applied within the present report. SolC is restricted to a region that only extends ~200–300 μm lateral to the midline and the area lateral to SolC at levels caudal to area postrema are designated as a caudal extension of SolM (see Herbert et al., 1990; Ruggiero et al., 1996; van der Kooy et al., 1984). These caudal portions of SolM encompass areas that are frequently included within SolC by other authors.
3.1 NTS projections to ventrolateral medulla
Tracer injections were targeted at the respiratory compartments of the ventrolateral medulla (Fig. 2). Regions injected included the dorsal and ventral parafacial areas, the BötC and PreBötC, as well as the anterior and posterior portions of the rVRG. The resulting detailed retrograde labeling in caudal NTS nuclei is depicted for individual representative experiments taken from a larger cohort of cases which are summarized in Fig. 3. For the detailed descriptions of retrograde labeling, the pattern of retrograde labeling is generally described for nuclei occurring in a caudal to rostral order within the NTS.
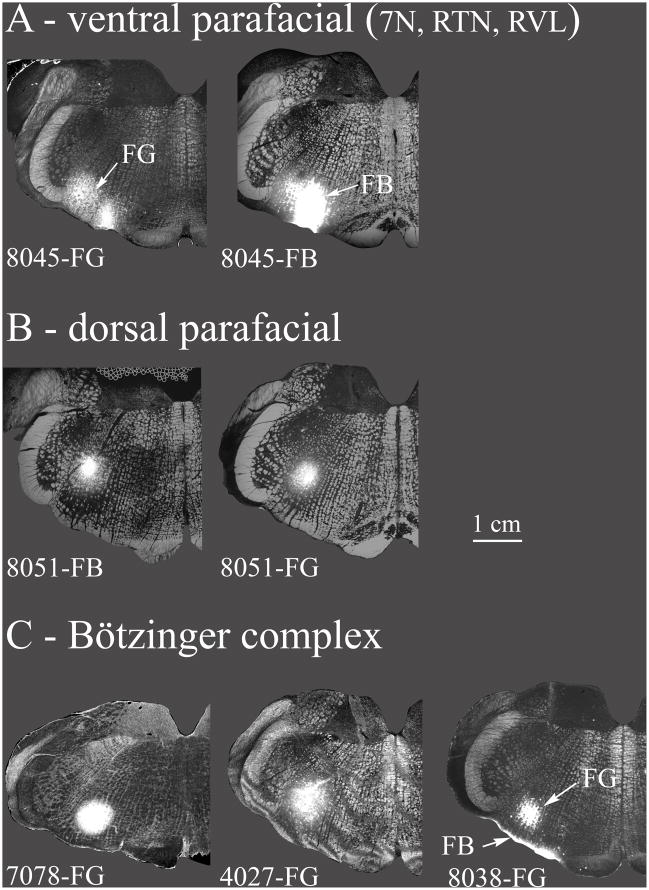
Fig. 3. Retrograde tracer injection sites.
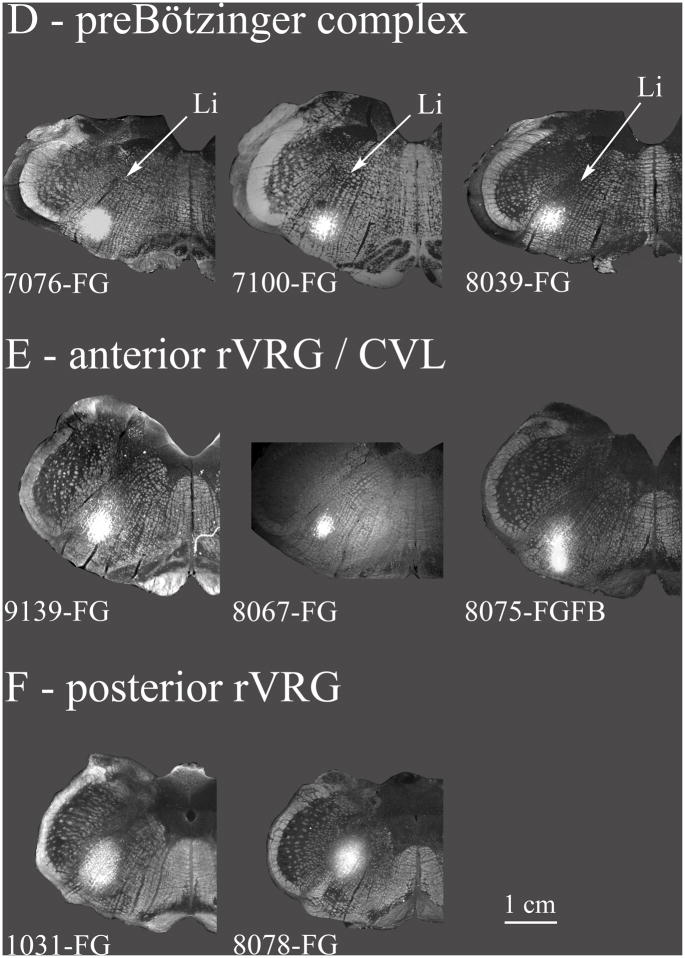
The various retrograde tracer injections in the VRC and nearby areas (7N, RVL, CVL) are summarized. A: Ventral parafacial injections were located ventrally at the caudal end of the facial nucleus. These included the facial nucleus but also the RTN, and a rostral portion of RVL. These injections are depicted in greater detail in Fig. 4. B: Injections are located near the caudal facial nucleus but more dorsal than in A. The dorsal parafacial injections are depicted in greater detail in Fig. 5. C: Injections were classified as within the Bötzinger complex based on their location just caudal to the facial nucleus and by the types of neurons recorded proximal to the injections (see results). In case 8038 the bright band of labeling ventral to the FG injection resulted from a second injection tract where an FB injection was inadvertently made at the ventral surface of the medulla. Very few FB retrogradely labeled neurons were noted in the caudal NTS. Case 7078 is depicted in greater detail in Fig. 6. D: Injections were classified as within the preBötzinger complex on the basis of extracellular recordings near the tracer injection sites (see results) along with the location of the injection at the caudal end of the compact part of n. ambiguus, at approximately the same level as the folded portion of the linear nucleus of the medulla (Li) and just rostral to the lateral reticular formation. Case 7076 is depicted in greater detail in Fig. 7. E: Injections in the anterior part of rVRG (arVRG) were identified by the presence of inspiratory neurons at the injection site, the presence of the anterior portion of the lateral reticular nucleus and the absence of the area postrema; it was distinguished from posterior rVRG by the differential responses to DLH injection, bradypnea in anterior but not posterior rVRG. CVL neurons are generally located ventral to anterior rVRG (e.g. Figs. 2, 10) although functional overlap is indicated where DLH injections aimed at arVRG decrease blood pressure and heart rate (see results and case 9139 depicted in Fig. 8). F: Posterior rVRG injections, as with anterior rVRG, were localized by the presence of inspiratory neurons and the lateral reticular nucleus. In case 8075 FG and FB were differentially targeted at posterior VRG and CVL but overlapped due to the somewhat elongated shape of the FB injection. The presence of the area postrema helps discriminate the posterior rVRG from anterior rVRG as does the absence of significant changes in respiratory rhythm consequent to local DLH injections. Case 1031 is described in Fig. 9.
Injection sites are depicted using a grayscale darkfield image of the relevant section as the base image overlaid by the injection site image using the “lighten” command in Photoshop®. Injections were saved as epifluorescent images for native FG/FB fluorescence, or as inverted brightfield images for DAB stained FG sections.
Overall, a dichotomy is observed in the projections from the caudomedial NTS to the VRC. Specifically, both SolM and SolC are densely populated by neurons that project rostrally in the ventrolateral medulla to parafacial regions, including the RTN and presympathetic neurons in the RVL, as well as to more caudal medullary regions in the vicinity of the rVRG and the adjacent cardiovascular-related cell populations in the CVL. However, few SolM and SolC neurons project to BötC and PreBötC, despite their location interposed between the parafacial regions and the rVRG. In general, the projections of area postrema resemble those of SolM and SolC insofar as they do not appear to project to BötC and PreBötC but do appear to target RTN, RVL, CVL and rVRG.
In contrast to the projections of SolM, SolC and area postrema, lateral nuclei in caudal NTS, including the gelatinous (SolG), dorsolateral (SolDL), interstitial (SolIcp and SolIdf), intermediate (SolIM), ventral (SolV) and ventrolateral (SolVL) nuclei, appear to project more broadly throughout the length of the VRC. Nevertheless, within the broad targeting of the lateral nuclei, some differential targeting of compartments in the ventrolateral medulla by individual NTS nuclei was evident.
3.2 NTS projections to parafacial areas
Figs. 3A, B depict injections at the level of the caudal portion of the facial nucleus. The injections in Fig. 3A include aspects of the RTN, rostral RVL as well as dorsal parafacial regions, caudal facial nucleus and dorsal parafacial regions. These are depicted in detail in Fig. 4 (FB inj, FG inj, F). FG and FB injections in Fig. 3B are centered just dorsal to the facial nucleus and encroach on the dorsal facial nucleus. Unlike the injections in Fig. 3A the more dorsal injections do not include the RVL or RTN and thereby permit a distinction of NTS projections favoring dorsal parafacial vs ventral parafacial (i.e., RVL and RTN).
Fig. 4. Most caudal NTS nuclei and area postrema project to the RTN/RVL region.
In this example (case 8045), two retrograde tracers, FG and Fast Blue (FB) were injected at the caudal end of the facial nucleus. FG was injected where inspiratory unit activity was recorded (inset lower left) and FB was injected slightly caudal and ventral to the FG injection (100 μm caudal, 300 μm medial) but where no respiratory unit activity was evident. The FG injection overlapped the caudal facial nucleus (7N; in grayscale darkfield image from section with FG injection site). In addition to overlap of 7N, some dye extended to the superficial layers of the ventral medulla containing the RTN (F, detail of area marked on section with FG injection). FG and FB injections both overlapped TH immunolabeled neurons in RVL (red neurons in F). Sections A–E progress from caudal to rostral with A ~ 300 μm caudal and E ~ 900 μm rostral to obex. G is an enlarged view of the area in D.
A–E and G are true color images taken with a liquid crystal RGB filter and uv excitation. Retrograde labeling from both the FG and FB injections was widespread, preferentially in ipsilateral caudal NTS and ipsilateral area postrema. H is at about the same level as D and illustrates the preferential ipsilateral retrograde labeling in NTS and AP. Labeled cells were found in every nucleus of the ipsilateral caudal NTS (A–E, and G) except SolCe. In most NTS nuclei, FG and FB labeled cells were coextensive, although not always double labeled (see detail in G). FB labeled cells generally appeared brighter than FG labeled cells in the aqueous media used to coverslip this material; double-labeled cells appeared as an intense blue-white color. EMG in inset is inspiratory motor activity recorded from the chest wall.
Injection sites for FB and FG and the higher power image in H are depicted as pseudo-colored images composed by placing a monochrome darkfield image of the relevant section in the red channel of a blank RGB image, then combining this “red darkfield image” with a true color image of both injected dyes (blue FB and gold FG). The true color image was placed in a layer superficial to the darkfield image and combined with the latter using the “lighten” command in Photoshop CS3® which resulted in replacement of only the darker red areas by the brighter colors of the injection sites or, in H, by the retrograde labeled neurons. EMG in inset shows inspiratory motor activity. ECG activity was manually reduced on the EMG trace to facilitate visualization of the respiratory cycle.
3.2.1 NTS projections to the ventral parafacial areas
Both the FG and FB injection sites in Figs. 3A and 4 (case 8045) incorporated elements of the RTN and RVL regions. Extracellular inspiratory unit activity was recorded at the site where FG was iontophoresed, but few respiratory cells were recorded dorsal or ventral to the injection site. Histologically, the FG injection was located at the caudal end of the facial nucleus and it is possible that the inspiratory recording reflects activity on facial motoneurons. The FG injection site was centered 500 μm deep to the ventral surface of the medulla (Fig. 4F). Ventrally and ventromedially, the fluorescence associated with the injection extended to the medullary ventral surface overlapping the RTN and rostral RVL (Fig. 4F). Involvement by the FG injection of rostral elements of the BötC that are located just caudal to the facial nucleus is also probable, although the expiratory activity typical of the BötC was not recorded anywhere along the dorsoventral extent of the electrode tract. In the same brain, a FB injection (6 nl) was targeted more ventral, 100 μm caudal and 300 μm medial to the FG injection. No respiratory extracellular activity was recorded at the FB injection site. At histology, the FB injection was found to be centered 240 μm deep to the ventral surface. It extended to the base of the brainstem medial and ventral to the facial nucleus at a site approximating medial portions of the RTN and rostral aspects of RVL. Involvement of the RVL in both the FG and FB injection sites is consistent with the observation that both injections overlapped tyrosine hydroxylase positive neurons (Fig. 4F), which at this level of the medulla mainly represent portions of the C1 adrenergic cell group (Phillips et al., 2001). The latter cells, in turn, are a significant component of RVL (e.g., Schreihofer and Guyenet, 1997; Pilowsky et al., 2009).
Each tracer produced a similar pattern of NTS retrograde labeling with substantial numbers of cells observed in the ipsi- but not contralateral NTS and area postrema (Fig. 4AE, G). Overall, although FB retrograde labeling appears more prominent (in part this is because the FB is brighter), FG retrogradely labeled neurons were present in the same NTS nuclei where FB cells were found. Given the overlap in the regions included in the injection sites as well as the similar pattern of the retrograde labeling in the NTS, the results from these two tracer injections are considered together.
For injections ventral to the facial nucleus, SolC was relatively densely labeled (Fig. 4AD) as was the ipsilateral area postrema including the midline region. Contralaterally within the area postrema the ventrolateral aspect was comparatively free of cells (Fig. 4C, H). Significant retrograde labeling was also evident within and around the dorsal motor nucleus of the vagus (10N; Fig. 4A–D). Dense ipsilateral NTS retrograde labeling occurred (Fig. 4B–D, G) in SolDL, SolG, SolM, and the subpostrema area (SubP). Relatively dense retrograde labeling was also found ipsilaterally in SolIM, in both parts of the interstitial nucleus (SolIdf, SolIcp), as well as in SolV and SolVL (Fig. 4B–D, G). An exception to the overlapping distribution of FG and FB neurons occurred in portions of SolM rostral to area postrema (Fig. 4E) where moderate numbers of FB retrogradely labeled neurons were seen but only few cells containing FG were evident.
Within the dorsal reticular formation ventrally adjacent to SolV and SolVL, moderate retrograde labeling was evident bilaterally, but with an ipsilateral predominance (Fig. 4D, H).
3.2.2 NTS projections to dorsal parafacial areas
In the experiment shown in Figs. 3B and 5 (case 8051), FG was iontophoresed at the approximate center of a population of respiratory neurons recorded over a dorsoventral distance of 500 μm. This consisted of background expiratory activity dorsally, changing to inspiratory and expiratory-inspiratory unit activity ventrally. At histology, the injection was centered just dorsal to the facial nucleus at its caudal border (Fig. 5 FG inj). To some extent this injection involved the dorsal part of the facial nucleus and the rostrodorsal part of BötC. More caudally, rostral portions of the compact division of nucleus ambiguus (AmbC) were included within the injection site (not shown). In the same brain, FB was injected (~ 5 nl) 400 μm rostral to the FG injection and at approximately the same dorsoventral level. The FB injection was also centered just dorsal to the facial nucleus. However, it did not involve either BötC or AmbC and no respiratory unit activity was recorded.
Fig. 5. Retrograde labeling of NTS nuclei from tracer injections dorsal to the facial nucleus.
FG and FB were injected immediately dorsal to the facial nucleus with the FG injection located at the caudal end of the nucleus and FB located ~ 400 μm more rostrally (case 8051). Respiratory activity (inspiratory units, see inset) was recorded at the FG injection site but not observed at the FB site. Sections in A–D progress from caudal to rostral with A ~ 400 μm caudal and D ~ 900 μm rostral to obex. In contrast to the pattern of labeling following injections ventral to the facial nucleus (Fig. 4), there was only light retrograde labeling of most caudal NTS nuclei. An exception to this light labeling was the considerable FG labeling of cells in the central NTS nucleus (SolCe in D), particularly due to involvement of the AmbC by the FG injection (Cunningham and Sawchenko, 2000). Additionally, moderately dense retrograde labeling of the intermediate NTS nucleus (SolIM) was seen after the FG, but many fewer neurons after the FB injection. Significant FG and FB labeling of cells in the dorsal reticular formation is also evident in C and D, likely representing premotor neurons to the facial nucleus (Travers and Norgren, 1983). EMG in inset shows inspiratory motor activity. ECG activity was manually reduced on the EMG trace to facilitate visualization of the respiratory cycle.
After the FG injection dorsal to the facial nucleus, no retrograde labeling was evident in the area postrema (Fig. 5C) and only a few cells were retrogradely labeled within SolC (Fig. 5A, B). At levels alongside area postrema, moderately dense FG retrograde labeling was evident in SolIM and labeling extended ventrolaterally to include SolV and SolVL (Fig. 5B, C). Additionally, some cells were retrogradely labeled by FG within SolIcp but relatively few neurons were retrogradely labeled within SolIdf (Fig. 5C), and only scattered FG labeled neurons were seen in SolDL, SolM, SolG, or SubP (Fig. 5BD). The FG injection resulted in prominent retrograde labeling of neurons in the central NTS nucleus (SolCe; Fig. 5D) which is known to specifically target AmbC (Cunningham and Sawchenko, 2000). Finally, a moderate population of FG retrogradely labeled neurons was evident in the dorsal reticular formation just adjacent to the ventrolateral quadrant of the NTS bilaterally, with an ipsilateral preference (Fig. 5B, C). In contrast to the reticular formation, labeling within NTS nuclei alongside the level of the area postrema was essentially ipsilateral (Fig. 5C).
Following the FB injection more rostrally (i.e., dorsal to mid-levels of the facial nucleus), SolC contained only a few retrogradely labeled neurons (Fig. 5A, B). As with the FG injection, no FB retrograde labeling was evident within the area postrema. Compared with the more caudal FG injection in the same brain, even fewer FB retrogradely labeled neurons were located in SolDL, SolM, SolG or in 10N; approximately the same numbers of FG or FB cells were evident in SubP. Modest numbers of FB labeled cells were seen in SolIM, along with larger numbers of FB retrogradely labeled neurons in SolV and SolVL as well as rare labeled cells in SolIcp and SolIdf (Fig. 5AC). Retrogradely labeled NTS cells included a few FB neurons scattered within and around SolCe (Fig. 5D). The FB injection resulted in substantial numbers of retrogradely labeled neurons located bilaterally in the reticular formation ventrolateral to the NTS (Fig. 5C).
3.3 NTS projections to Bötzinger complex and preBötzinger complex
In experiments shown in Figs. 3C, D, 6 and 7, FG was iontophoresed in the BötC and PreBötC respectively. In the experiment in Fig. 6 (case 7078) expiratory activity was recorded over ~ 270 μm dorsoventrally, and FG iontophoresed at the level where the activity was maximal. The FG injection was histologically identified just caudal to the facial nucleus and ventral to AmbC. This rostrocaudal location together with the presence of expiratory unit activity is consistent with this injection site being within the BötC (Ezure et al., 1988; Sun et al., 1998; Schreihofer et al., 1999; Wang et al., 2002; Alheid et al., 2002, 2008; Monnier et al., 2003). In the experiment in Fig. 7 (7076) phase spanning respiratory activity was recorded over approximately 200 μm dorsoventrally at the injection site. This was represented by a mixture of expiratory-inspiratory (E–I) activity, especially dorsally, and inspiratory-expiratory (I-E, or post-inspiratory) activity ventrally. The injection site was histologically identified at the rostrocaudal level of the linear nucleus (Fig. 7C) and rostral to the bifurcation of the lateral reticular nucleus. This location, approximately 700 μm caudal to the facial nucleus, along with the presence of phase-spanning neurons is consistent with the location of the preBötC (Sun et al., 1998; Guyenet and Wang, 2001; Alheid et al., 2002; Wang et al., 2002; Monnier et al., 2003).
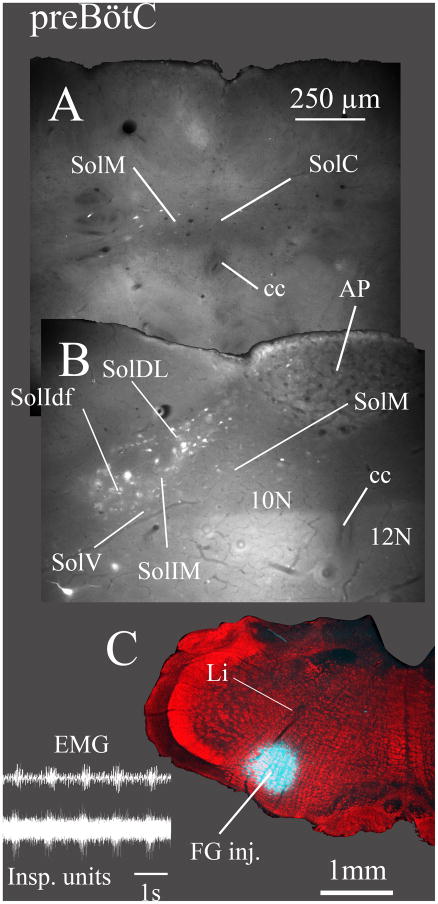
Fig. 6. Lateral and rostral NTS nuclei are retrogradely labeled from the Bötzinger complex (BötC), while few neurons in caudomedial NTS nuclei project to BötC.
FG was injected caudal to the facial nucleus and below the compact part of nucleus ambiguus (C; case 7078). Section A is ~ 200 μm caudal and B is ~ 300 μm rostral to obex. Expiratory unit activity characteristic of BötC was recorded at the injection site (inset). Moderately dense retrograde labeling was apparent in only a few NTS nuclei, most prominently in the intermediate nucleus (SolIM). Relatively few neurons were labeled in the medial (SolM) or commissural (SolC) nuclei and no retrograde labeling was observed in area postrema (AP). EMG in inset is inspiratory motor activity. ECG activity was manually reduced on the EMG trace to facilitate visualization of the respiratory cycle.
Fig. 7. Lateral and rostral NTS nuclei are retrogradely labeled from the preBötzinger complex (PreBötC) but very few neurons are labeled in caudomedial NTS nuclei or in area postrema (AP).
FG was injected at the level of the caudal end of the compact part of nucleus ambiguus (C; case 7076). Inspiratory unit activity was recorded at the injection site (inset). The FG injection was located at the level at which the dorsal part of the linear nucleus of the medulla (Li) bends ventromedially. Section A is ~ 350 μm caudal and B is ~ 300 μm rostral to obex. Retrograde labeling was moderately dense in the diffuse part of the interstitial (SolIdf), intermediate (SolIM) and dorsolateral (SolDL) nuclei but very sparse in the medial (SolM) and commissural (SolC) nuclei and in the area postrema. EMG in inset is inspiratory motor activity. ECG activity was manually reduced on the EMG trace to facilitate visualization of the respiratory cycle.
Retrograde labeling in the NTS was similar after either BötC or preBötC injections. Practically no retrogradely labeled neurons were seen in SolC (Figs. 6A, 7A). Caudal to (but not at the level of) area postrema, BötC injections gave rise to moderate numbers of retrogradely labeled SolV neurons (Fig. 6A). After either BötC or preBötC injections, the subpostrema region was nearly devoid of retrogradely labeled neurons. In the overlying area postrema, no retrograde labeling was seen after FG injection in the BötC, while only a few scattered neurons were seen in the ipsilateral area postrema following FG injection in the preBötC (Fig. 7B). In SolM only sparse numbers of cells were retrogradely labeled after either BötC or preBötC FG injections (Fig. 6B, 7B) with slightly more SolM neurons evident after the BötC injection. In contrast, moderately dense numbers of labeled cells were seen in lateral portions of the NTS (i.e., in SolIdf, and SolIM; Fig. 6B, 7B) and labeled neurons appeared in SolDL after either the BötC or PreBötC injection. After either injection, SolDL appeared to be more sparsely labeled compared to labeling in nearby portions of SolIM. Few, if any, neurons were retrogradely labeled in SolIcp from the BötC injection (Fig. 6B) while (compared to the BötC injection) preBötC injections resulted in some SolIcp retrograde labeling and somewhat more numerous retrograde labeling in SolIdf (Fig. 7B). As with injections into the BötC, preBötC injections labeled few neurons in SolVL, SolV or in the adjacent dorsolateral reticular formation at the level of area postrema.
Other cases (not shown) analyzed after BötC (Fig. 3C, cases 4027, 8038) or preBötC FG injections (Fig. 3D, cases 7100, 8039) resulted in very similar patterns of labeling to that described above. Specifically, labeling was prominent in lateral NTS nuclei rostral to obex and sparse or absent in from the area postrema or in SolC or caudal SolM at levels caudal to obex. In case 4027 which, in addition to BötC, also impinged upon AmbC and caudal facial nucleus, somewhat greater numbers of cells were evident alongside the area postrema in SolVL, SolM and SubP than occurred after case 7078 (Fig. 6). This additional labeling was similar to that which resulted from the injection at the dorsal caudal facial nucleus (Fig. 5).
Labeling in two additional animals with preBötC FG injections (7100, 8039; Fig. 3C) was similar to that in animal 7076. Both resulted in significant labeling at the level of area postrema in SolIM, SolIdf and SolDL but not in SolVL or SolIcp with only scattered labeling in SolM or SolC at either the level of area postrema or caudal to obex. The only difference was that these cases had slightly more neurons retrogradely labeled in SolV.
3.4 NTS projections to anterior rVRG and CVLM
In the experiment shown in Fig. 8 (case 9139) inspiratory activity was recorded over ~ 500 um, and a small (3 nl) DLH injection made near the midpoint of this range evoked bradypnea accompanied by a decrease in arterial pressure (Fig. 8E). FG injected at the site where respiratory activity was recorded and DLH was injected was histologically located within the VRC caudal to AmbC at mid rostrocaudal levels of the inferior olive and dorsal to the lateral reticular nucleus. The combination of bradypnea and a depression of arterial pressure is characteristic of the anterior but not the adjacent posterior portion of the rVRG or the preBötC (Monnier et al., 2003). The depressor response presumably reflects involvement of the CVLM that, at least in part, lies ventrally adjacent to the VRC at the level of the anterior rVRG (Fig. 2).
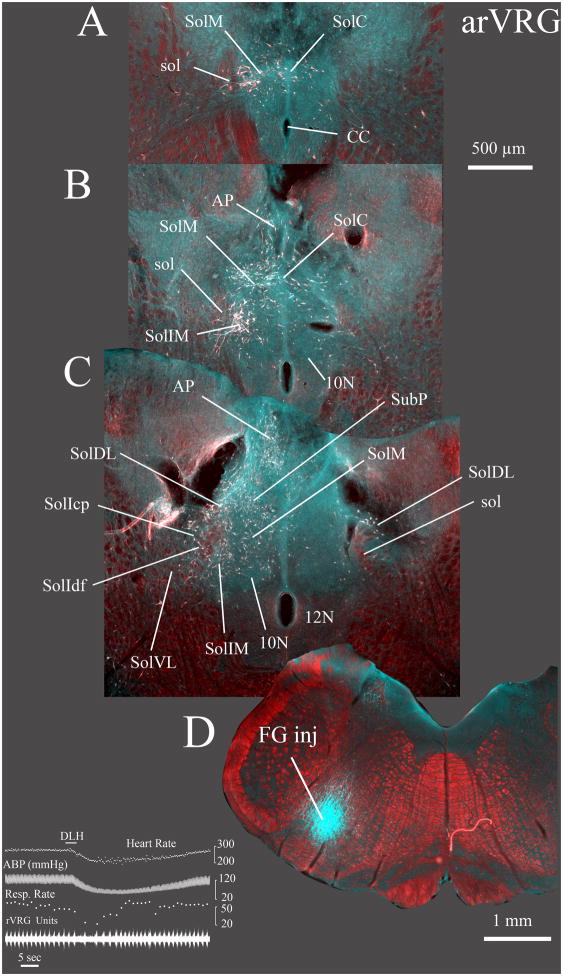
Fig. 8. Widespread labeling is evident in the caudal NTS after injections into a region containing the anterior part of the rostral VRG (arVRG) and the caudal ventrolateral nucleus of the medulla (CVL).
FG was injected into the right arVRG (case 9139) at the site of respiratory unit activity (not shown) while a second electrode was used to record population respiratory activity in the left rVRG. Prior to injecting FG, injection of 3 nl of a 5 mM DLH solution (inset) in the right arVRG evoked a slowing of respiratory rate and decreases in heart rate and arterial pressure. In this instance, the central respiratory rhythm is represented by inspiratory unit activity recorded in the rVRG contralateral to the DLH and FG injections.
Sections A–C progress from caudal to rostral with A ~ 500 μm caudal and C ~ 200 μm rostral to obex. Substantial retrograde labeling was evident throughout the caudal NTS, especially the commissural (SolC) and caudal part of the medial (SolM) nuclei. However, labeling in the ventrolateral NTS (SolVL) was relatively light particularly compared with FG injections more caudally in the posterior part of the rVRG (compare C with Fig. 9B). In the contralateral NTS, a significant number of retrogradely labeled neurons were evident in the dorsolateral nucleus (SolDL in C).
Retrograde labeling is depicted as a pseudo-colored image composed of a monochrome darkfield image placed in the red channel of a blank RGB image, which was combined with inverted brightfield images of the DAB immunolabeled FG neurons. The inverted brightfield image was placed in a layer superficial to the darkfield image and combined with the latter using the lighten” command in Photoshop CS3®. The retrograde labeled neurons in the inverted images appear white on a dark background and therefore replace the (red) darkfield image where they occur. The resulting composite image provides white neurons against a red darkfield image. The FG injection site (D) is a pseudocolored image consisting of a low magnification darkfield image placed in the red channel of a blank RGB image. A grayscale inverted brightfield image of the same section was placed in both the green and blue channels. The FG injection consequently appears cyan against a red darkfield background image.
Injection of FG into the anterior rVRG gave rise to extensive retrograde labeling in the NTS. The density of the NTS labeling was much more prominent in comparison to that arising from the more rostral tracer injections in the PreBötC or BötC. Substantial numbers of neurons in both medial and caudal NTS nuclei were retrogradely labeled from anterior rVRG (Fig. 8AC). In SolC, both at the level of area postrema and caudally, moderate retrograde labeling was evident on the midline and this extended ventrally to include scattered neurons near the central canal. Moderate numbers of retrogradely labeled cells also appeared at the borders of 10N, particularly laterally, dorsally and medially at the level of area postrema (Fig. 8B), Scattered neurons were also retrogradely labeled just ventral to 10N (Fig 8B, C). Moderate numbers of retrograde labeled cells were observed in the ipsilateral area postrema, (Fig. 8C). Only a few labeled cells were evident contralaterally in area postrema, except at its most caudal apex (Fig. 8B). Numerous retrogradely labeled neurons were evident in nearly all ipsilateral NTS nuclei both at the level of the area postrema (i.e., SolDL, SolIM, SolIdf, SolV, SolVL, SolM, SubP, Fig. 8B, C) and more caudally (Fig. 8A). Only light retrograde labeling was seen in SolIcp. In the contralateral NTS, the number of retrogradely labeled neurons was low at rostral levels alongside the area postrema (Fig. 8B) with the largest number of contralateral neurons located in SolDL, and only a few neurons evident in SolM, SolIM, and in SolIdf. Contralateral SolM labeling caudal to obex was more substantial (Fig. 8A) compared to that more rostrally in the same nucleus (Fig. 8B). Modest numbers of retrogradely labeled neurons were evident in the dorsolateral reticular formation ventrolateral to the NTS both ipsi- and contralaterally.
Two additional cases (Fig. 3E, 8067, 8075) with FG and FG/FB injections in arVRG resulted in essentially identical labeling patterns to case 9139. In both these latter instances substantial numbers of retrogradely labeled neurons were evident in at the level of area postrema in SolDL, SolIM, SolIdf, SolM, SubP and SolV, but SolVL was only lightly labeled and practically no labeled cells were evident in SolIcp. Caudal to obex substantial numbers of cells were retrogradely labeled in SolDL, SolC and caudal SolM.
3.5 NTS projections to posterior rVRG
For the injection in Fig. 9 (case 1031), both expiratory and inspiratory activity was recorded over ~ 600um with expiratory activity more prominent dorsally and inspiratory activity more evident ventrally. DLH injections (3, 15 or 30 nl) centered in this region did not modify breathing pattern. FG was iontophoresed at the DLH injection site. Subsequent histological evaluation revealed a moderately large FG injection site centered in the ventrolateral medulla at the rostrocaudal level of area postrema dorsally and the caudal end of the inferior olive ventrally (Fig. 9D). This injection was located within the posterior portion of the rVRG, judged both by its anatomical location, and by the lack of effect of DLH injections, which evoke bradypnea in anterior but not in posterior rVRG (Monnier et al., 2003).
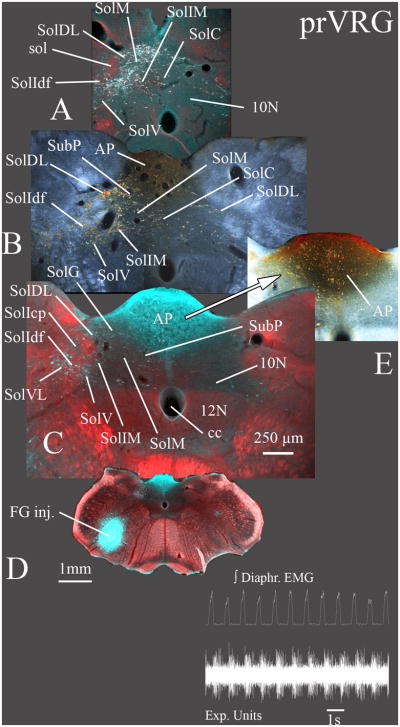
Fig. 9. Widespread labeling is evident in the caudal NTS after injections in the posterior part of the rostral VRG (prVRG).
FG was injected into the rVRG immediately where respiratory neurons were recorded (inset; case 1031). Sections A–C progress from caudal to rostral with A ~ 100 μm caudal and C ~ 300 μm rostral to obex. Retrograde labeling was widespread in the ipsilateral caudal NTS (A – C) and similar to that following injections in the anterior rVRG (arVRG in Fig. 8). However, prVRG provided denser labeling in the ventrolateral nucleus (SolVL; B, C) and sparser labeling in the compact part of the interstitial nucleus (SolIcp; C). Retrograde labeling in the AP (B, E) had an ipsilateral predominance but with a greater bilateral representation compared to more rostral injections in the arVRG (Fig. 8).
Sections B and E are normal darkfield images with the retrograde DAB labeled neurons appearing gold. Sections A, C are pseudo-colored images composed of the DAB immunolabeled FG neurons assembled in Photoshop® as described in the legend for Fig. 8. C is pseudocolored image of the FG injection site constructed similarly to that described for the section with the FG injection site in Fig. 8.
FG injection in the posterior rVRG produced dense retrograde labeling of NTS occurred at the level of obex and caudally, involving almost all ipsilateral nuclei (SolDL, SolIM, SolI, SolM, SolV, and SolVL) as well as midline SolC neurons (Fig. 9A). Scattered neurons were also labeled at the borders of 10N and these were more frequent caudal to obex than at rostral levels alongside the area postrema (Fig. 9A). Relatively dense retrograde labeling was evident in the area postrema that was predominantly ipsilateral, but included relatively dense labeling of cells at the midline (Fig. 9B, E). Retrograde labeling in the NTS at the level of area postrema was moderately dense ipsilaterally in SolV, SolVL, SolIM, SolDL, SolG, and SubP as well as in SolIdf (Fig. 9B). In contrast, SolM was less densely populated by retrogradely labeled neurons at the level of area postrema compared with SolM more caudally (Fig 9A). Significant contralateral labeling occurred at caudal levels near obex and more caudally although there were substantially fewer neurons than on the ipsilateral side (Fig. 9A, B). Contralateral labeled neurons were most evident in SolM and SolIM as well as SolC, while few cells were present in SolDL. Significant numbers of retrogradely labeled neurons were present in the dorsolateral reticular field adjacent to the NTS ipsilaterally, and to a lesser extent contralaterally (Fig. 9B, C).
One additional case (Fig. 3F, 8078) with an injection in prVRG was examined. The injection in 8078 was located slightly more dorsal in prVRG than the injection in (1031). Retrograde labeling for case 8078 was essentially identical to that in case 1031, except that the number of cells labeled in area postrema appeared somewhat less dense.
3.6 NTS projection neuron sizes
Sampled in three animals, NTS neurons projecting to the ventrolateral medulla were generally small (coronal cross sectional area = 111.0 μm2 ± 3.4; max. dia. = 16.7 μ ± 0.3; min. dia. = 8.5 μ ± 0.1; n= 201; mean ± S.E.M.). Within the NTS, retrogradely labeled neurons at the level of area postrema (in SolDL, SolIM, SolM and SolIdf) were slightly smaller (coronal cross sectional area = 93.7 μm2 ± 3.8; max dia. = 14.8 ± 0.4; min. dia.= 8.3 ± 0.2; n = 99) than those caudal to obex, in SolC and SolM (coronal cross sectional area = 127.8 μm2 ± 5.0; max dia. =18.5 ± 0.5, min. dia. = 8.7 ± 0.2; n = 102) (p < 0.05, for area, max. dia.).
4. DISCUSSION
This report is focused on caudal NTS efferents to respiratory related compartments in the VRC (Fig. 10). The most striking observation is the paucity of projections to preBötC and BötC from either SolC or caudal SolM (Fig. 10B). This is despite robust projections from these NTS nuclei to other ventral medullary cardiorespiratory compartments including the area of the RTN/RVL and to the vicinity of the rVRG/CVL. Also notable, is the pattern of ventral medullary projections arising from area postrema which appears similar that of SolC and caudal SolM (Fig. 10B, D) and reinforces the role of the area postrema as a circumventricular sensory area closely integrated functionally and anatomically with adjacent nuclei of the NTS.
Fig. 10. Schematic diagram of differential caudal NTS connections with ventrolateral medulla.
The diagrams provide a synopsis of the differing patterns of innervation of the ventrolateral medulla suggested by the present report in concert with other reports that have examined this question (see Discussion). Arrows at the end of the schematic axons indicate likely excitatory terminations. Triangles at the end of axons indicate inhibitory projections (A).
In A: the output of the lateral, caudal NTS is depicted (blue axons). This projection pattern is typified by 2nd order NTS neurons for slowly adapting pulmonary stretch receptors (Ezure et al., 2002). These neurons are mostly GABAergic, and, in the rat, are mainly located medially within SolIdf (Ezure and Tanaka, 2004; Takakura et al., 2007). B: illustrates the relatively selective projections from SolC and from caudal SolM (red axons) to rVRG and cVRG as well as to RTN, but not to the intervening BötC and preBötC. C: depicts projections from the lateral part of caudal NTS to ventrolateral medulla (green axons). This is typified by the projections from SolDL and from SolIM that project widely throughout the ventrolateral medulla with the exception that SolDL does not project to RVL. D: shows projections from the area postrema (red axons). Overall these are very similar to the projections originating from SolC and caudal SolM. Significant area postrema projections appear to target the RTN/RVL area as well as the region of rVRG/CVL. In contrast, no area postrema projections are seen to BötC and preBötC compartments of the VRC.
4.1 Nomenclature for NTS and VRC nuclei
As outlined in the Results, it is important to note that the terminology we apply for the caudal NTS of the rat generally follows that espoused by Paxinos et al. (1999, 2009) which, in turn, is consistent with the terminology of Herbert et al. (1990), Ciriello et al. (1994) and Ruggiero et al. (1996). A standard NTS nomenclature, has not been applied with any uniformity in the current NTS literature. Consequently, NTS terminology remains a source of confusion when comparing functional-anatomical studies from different labs and from different species. This is particularly problematic where NTS regions under investigation are not depicted in sufficient detail to allow readers to independently assess which NTS areas are being addressed.
The caudal NTS is the focus of the present experiments and represents that portion of the NTS providing efferents to the VRC. The current results are described in terms of the region alongside the area postrema and the region caudal to obex. Obex in the Paxinos et al. (1999, 2009) terminology coincides with the caudalmost tip of the area postrema (often referred to as calamus scriptorius) in animals with a midline area postrema (e.g., rats and mice). In mammals where the area postrema is bilateral, obex generally refers to the point where the central canal opens into the fourth ventricle.
The terminology of the NTS caudal to obex is especially varied in its treatment by different authors. In Paxinos et al. (1999, 2009) as well as in the present report, SolC is applied to a restricted region on, or near, the midline while a caudal extension of SolM is identified laterally adjacent to SolC along its entire rostrocaudal extent, consistent with the earlier suggestion by Van der Kooy et al. (1984). In contrast, it is not uncommon for practically the entire NTS caudal to obex to be designated as SolC.
Despite a genuine attempt to conform to the nomenclature of Paxinos et al. (1999, 2009), we admit to introducing separate terms for what appear to be distinct components of the interstitial nucleus of the NTS. SolIdf and SolIcp are applied to the compact and diffuse parts of the interstitial nucleus (respectively) in order to accommodate the distinctive connections of these two NTS components. In the NTS literature the area identified as the interstitial nucleus is often, but not always, restricted to SolIcp (Mrini and Jean, 1995) leaving the remaining neurons embedded within the fascicles of the solitary tract nameless, or included with other adjacent NTS designations. SolIcp has been identified as a particular focus (although not the exclusive target) of laryngeal afferents to the NTS (e.g. Mrini and Jean, 1995). Ventral portions of SolIdf have been associated with neurons providing NTS relays for pulmonary slowly adapting stretch receptors (Ezure et al., 2002; Takakura et al., 2007) and are, accordingly, particularly relevant to VRC projections from the NTS.
Within the VRC we have also slightly modified the terminology with respect to Paxinos et al. (1999, 2009) by subdividing the rVRG into anterior (arVRG) and posterior parts (prVRG). The justification is presented in earlier analyses (Alheid et al., 2002; Monnier et al., 2003). In the context of the present paper this division is useful in that it accommodates apparent small variations in the NTS projections to these two parts of the rVRG (which are relevant to functional distinctions between these two zones (Monnier et al., 2003; Moreira et al., 2007a). For consistency in comparing findings between the current paper and the discussed literature, we have translated the names applied to the NTS nuclei in some of the earlier studies to conform to the standard terminology of Paxinos et al. (1999, 2009). We have noted where such translations are particularly relevant to the present examination of NTS projections and where the area in question can be clearly identified; most of these are relevant to the designation of SolC or SolI.
Finally, it should be acknowledged that for an area as complex as the NTS with its many distinct afferents and efferents within a compressed CNS location, a static terminology for its constituent nuclei presents its own hazards. We have not, for example, attempted to encompass the complex mosaic presented by the wide variety of neurotransmitter/neurochemical markers present within the NTS (e.g. Mantyh and Hunt, 1984; Geerling and Loewy, 2006; Stornetta et al., 2006; Pickel et al., 2006; Huckstepp et al., 2010; Llewellyn-Smith et al., 2011). The latter prominently include catecholamine neurons found within SolM, which do not appear to project significantly to cardiorespiratory portions of the ventrolateral medulla (Blessing et al., 1987). In contrast, catecholamine neurons within the area postrema are argued to at least target RVL (Blessing et al., 1987; Yamamoto et al., 2003), and possibly also reach the A1 neuron in the ventrolateral medulla (Cunningham et al., 1994). Our focus (within this report) on projections to the VRC were not designed to accommodate examination of the neurochemical nature of the NTS projections to individual compartments of the VRC. These will need to be examined in the future in the context of the differential projections that we describe in the present report.
4.2 Technical considerations
The serially arranged, distinct compartments of the VRC are mainly recognized by their relative content of neurons with specific respiratory-related firing patterns. This generally allows discrimination between the different compartments of the VRC but it should be appreciated that individual compartments consist of multiple neuronal types. Peaks in the distribution of specific classes of neurons are commonly used to define a particular VRC compartment. However, the boundaries are not absolute and individual classes of neurons extend with decreasing frequency into adjacent VRC regions (Alheid and McCrimmon, 2008).
Both the VRC and autonomic regions of the ventrolateral medulla are populated by the motoneurons and preganglionic vagal neurons of the nucleus ambiguus (Bieger and Hopkins, 1987) and nucleus ambiguus neurons are known targets of a variety of inputs from the NTS (e.g. Hayakawa et al., 1998, 2000). In many cases, these afferent pathways to the nucleus ambiguus are undoubtedly also labeled by tracer injections into compartments of the VRC and may complicate the identification of differences amongst the NTS nuclei innervating functional cardiorespiratory compartments. Thus, the heterogeneities we have identified in the NTS projections to the ventrolateral medulla likely underestimate the diversity that exists within these pathways as well as the specificity in projections from discrete NTS nuclei needed to support the mosaic of functional roles expressed in the ventrolateral medulla.
4.3 Selective retrograde labeling of NTS nuclei from the ventrolateral medulla
Our retrograde tracer injections encompassed functionally distinct regions of the ventrolateral medulla including the ventral parafacial region (incorporating RTN and RVL), dorsal parafacial region, the BötC, preBötC, rVRG and CVL. All of these areas receive substantial projections from the NTS, but the pattern of labeling varies with the different injection sites.
4.3.1 Parafacial region injections
The most substantial retrograde labeling in the NTS was observed after injections ventral to the caudal facial nucleus (i.e., ventral parafacial region including RTN and RVL). In particular, SolC, caudal SolM and area postrema provide substantial input to the RTN/RVL region. Further, the input from these NTS nuclei was focused on the ventral parafacial region as they were essentially not labeled by tracer injections into regions adjacent to RTN/RVL (i.e., BötC, facial nucleus, dorsal parafacial region). This projection was also identified by (Rosin et al., 2006; Takakura et al., 2006) although in their descriptions, caudal SolM (present report, and Paxinos et al. (1999, 2009) is included within their depiction of SolC.
Injections into the ventral parafacial region as well as injections into adjacent areas (either immediately dorsal or caudal to the facial nucleus) also retrogradely labeled lateral NTS nuclei including SolDL, SolIM, SolIcp, SolIdf, SolV and SolVL. Many of these nuclei, however, also project to other parafacial regions including the dorsal area adjacent to the facial nucleus (Fig. 5 and Rosin et al., 2006; Rinaman, 2010) as well as to the immediately caudal BötC (Fig. 6 and Núñez-Abades et al, 1993). Among the nuclei in lateral parts of the NTS that were retrogradely labeled by our ventral injections, it appears likely that only SolIM and SolIdf provide afferents to the RTN/RVL region, judging from the retrograde labeling described by Rosin et al. (2006) and Takakura et al. (2007) after small tracer injections in the RTN. While Rosin et al. (2006) do identify SolVL as a potential source of afferents to RTN (their Fig. 9A) this appears more likely to represent SolIdf. Consistent with this evaluation, in a subsequent paper from the same lab (Takakura et al., 2007) the RTN projecting area in question is identified as a part of SolI.
The pattern of retrograde NTS labeling from injections in the RTN/RVL region includes SolIdf, SolIM, SolC and caudal SolM and is consistent with the physiological characterization of a variety of respiratory-related sensory pathways relayed through the NTS to the RTN. These include: peripheral chemoreceptor relay neurons located in SolC and caudal SolM (Takakura et al., 2006), NTS relay neurons (pump cells) for slowly adapting lung stretch receptors located in SolIdf and SolIM (Ezure et al., 2002; Moreira et al., 2007b; Takakura et al., 2007) and likely for rapidly adapting airway/lung stretch receptors (RARs; Otake et al., 2001; Janczewski & Feldman, 2006) located caudally in SolM and SolC (Kubin and Davies, 1988; Lipski et al., 1991; Otake et al., 2001).
Our retrograde labeling of the area postrema is also consistent with work demonstrating projections from the area postrema to the RVL using FG or rhodamine latex beads in the rabbit (Blessing et al., 1987; Polson et al., 1995; Horiuchi et al., 1999) and FG in the rat (Yamamoto et al., 2003). Area postrema innervation of the RTN is also strongly suggested by the retrograde labeling observed after injections into the RTN of cholera toxin B (Rosin et al., 2006). FG, rhodamine beads and cholera toxin B are all arguably resistant to uptake and transport by undamaged axons of passage (Schmued and Fallon, 1986; Colin et al., 1989; Luppi et al., 1990). In contrast to the retrograde labeling experiments including those in the present report, Cunningham et al. (1994) using PHA-L in anterograde tracing of axons from area postrema to the brainstem, argue that area postrema axons pass proximal to, but do not arborize at C1 neurons in RVL, and similarly depict area postrema axons passing ventral to the lateral portions of the facial nucleus without arborizing (see Fig 2 in Cunningham et al., 1994). The discrepancies between these experiments in the presumed targeting of the ventral parafacial region by area postrema are difficult to reconcile without further examination.
While we attribute retrogradely labeled NTS neurons to projections targeting parafacial regions, it should be appreciated that tracer injections into the parafacial regions generally impinge on the facial nucleus itself. However, direct examination of afferents to the facial nucleus indicate that few NTS neurons project to the facial nucleus (Travers and Norgren, 1983; Zerari-Mailly et al., 2005). Consistent with this interpretation, anterogradely labeled NTS terminals appear relatively dense in the regions surrounding, rather than within the facial nucleus (e.g. Rosin et al., 2006; Rinaman, 2010). The largest numbers of facial premotor neurons are located in the reticular formation including the dorsal portion ventrally adjacent to the NTS (Travers and Norgren, 1983). These presumably account for a substantial portion of the retrogradely labeled cells in the dorsal reticular formation after our injections incorporating the facial nucleus, especially its dorsal aspect.
4.3.2 Bötzinger complex and preBötzinger complex injections
The projections of the NTS to the BötC and preBötC differ rather starkly from those that target the RTN/RVL area. This is most evident in the paucity of projections from the caudomedial NTS nuclei, SolC and SolM to BötC and preBötC and an absence of projections from the area postrema. This observation was rather surprising insofar as BötC and preBötC neurons are generally believed to be critically involved in respiratory rhythm generation (Smith et al., 1991; Gray et al., 1999) and a variety of respiratory afferent pathways relayed through SolC and SolM alter respiratory frequency (Kubin et al., 2006). These include peripheral chemoreceptor input from the carotid body (Finley and Katz, 1992; Ciriello et al., 1994), broncho-pulmonary C-fiber afferents (Kubin et al., 1991; Paton, 1998; Moreira et al., 2007a), and pulmonary rapidly adapting stretch receptors (RAR; Kubin and Davies, 1988; Ezure et al., 1991; Otake et al., 2001). Hence, sensory afferent input that is relayed via SolC and caudal SolM must influence inspiratory rhythm generating circuits in the preBötC principally via indirect links. Obvious candidates include long relays such as those arising from peripheral chemoreceptors that have been proposed to alter respiratory rhythm via projections from the caudomedial NTS to the parabrachial and Kölliker-Fuse nuclei of the rostral dorsolateral pons (Song and Poon, 2004; Song et al., 2011). Other alternatives are caudomedial NTS projections to rVRG (Alheid et al., 2002; Monnier et al., 2003; Moreira et al., 2007a) as well as the RTN (Moreira et al., 2007b; Takakura et al., 2006, 2007) that ultimately influence preBötC neurons. For example, it has been argued that the RTN may serve as a convergent focus where neurons responding to central pH/PCO2 also receive afferent input (via the NTS) from a variety of peripheral afferents including peripheral chemoreceptors (Stornetta et al., 2006; Takakura et al., 2006), slowly adapting stretch receptors (Moreira et al., 2007b; Takakura et al., 2007) and C-fibers (Moreira et al., 2007a). Evidence has also suggested that rapidly adapting stretch receptor relays, which are located in the region of SolC and the caudal SolM (Kubin and Davies, 1988; Ezure et al., 1991), likely avoid the BötC and adjacent preBötC but reach the regions of the rVRG, dorsolateral pons and the RTN (Ezure et al., 1991; Otake et al., 1992, 2001). It should be noted that laterally adjacent and partly overlapping the RTN is an area termed the parafacial respiratory group (pFRG; Janczewski et al., 2002; Pagliardini et al., 2011). Neurons in this region are hypothesized to generate an expiratory rhythm and to be excited by inputs from pulmonary rapidly adapting receptors (Janczewski et al., 2002; Pagliardini et al., 2011).
Tracer injections in the BötC result in significant numbers of retrogradely labeled neurons in the lateral nuclei of the NTS adjacent to the solitary tract. These cells are mainly located in SolIM and in SolIdf with some additional contribution from SolV at levels caudal, but not rostral to obex. The NTS projections to the preBötC are similar to those innervating BötC except that labeling of SolI appears slightly more dense and includes neurons within both SolIdf and SolIcp.
The moderate projections from SolIdf to BötC and preBötC are consistent with the demonstrated widespread arborization of the axons arising from slowly adapting pulmonary stretch receptor relay neurons to neurons throughout the ventral respiratory column (Ezure et al., 2002). The slighter higher numbers of retrogradely labeled neurons in SolDL projecting to the preBötC and rVRG (section 4.3.3.2) likely reflects labeling of CVL projections arising from baroreceptor relay neurons in SolDL (Weston et al., 2003).
4.3.3 Retrograde labeling of NTS from rostral VRG and CVL
With respect to the rVRG, we have considered the NTS projections to its anterior (arVRG) and posterior portions (prVRG) separately due to differing functional responses evoked from these regions by chemical stimulation with DLH as well as a somewhat different neuronal composition in which the the arVRG has a greater proportion of propriobulbar neurons and fewer bulbospinal neurons (see Alheid et al., 2002; Monnier et al., 2003). Excitatory amino acid injections into the arVRG produce bradypnea (Monnier et al., 2003) while injections into the caudally adjacent prVRG do not change respiratory rhythm. Moreira et al. (2007a) have argued that the arVRG induced bradypnea may be mediated by activation of rostrally directed inhibitory inputs to the RTN and that this pathway likely mediates at least a portion of the higher order processing of bronchopulmonary C-fiber afferents within the ventrolateral medulla. The latter include projections to the rVRG from a broad region of the caudal NTS that receives bronchopulmonary C-fiber input (Kubin et al., 1991).
4.3.3.1 Retrograde labeling of NTS from anterior rostral VRG and CVL
Respiratory neurons in the arVRG are centered dorsally to CVL neurons (Monnier et al., 2003; Mandel and Shreihofer, 2006). However, arVRG and the underlying CVL are closely apposed regions that are difficult to separate with retrograde tracer injections. Accordingly, the retrograde labeling in NTS we describe after rVRG injections likely reflects afferents to both the rVRG and CVL. Caudal to arVRG, CVL neurons are substantially diminished in numbers (e.g., Weston et al., 2003) so that retrograde tracer injections in prVRG are less liable to be confounded by direct involvement of CVL neurons.
Almost all of the caudal NTS nuclei appear to send projections to the arVRG/CVL region with the exception of SolVL which contained relatively few retrogradely labeled neurons, particularly in comparison with SolVL projections to the prVRG (see 4.3.3.3). In comparison to the BötC and preBötC, the arVRG/CVL region receives dramatically more input from the caudomedial NTS nuclei, SolC and caudal SolM. Additionally, a greater number of neurons were apparently retrogradely labeled in SolDL after injections into the arVRG/CVL than after injections into either the more rostral preBötC or BötC regions, or after injections into the more caudal prVRG. The labeling of SolDL is consistent with the identification of a concentration of baroreceptor relay neurons in this nucleus that provide a substantial excitatory projection to the CVL (Weston et al., 2003).
The widespread nature of NTS afferents to arRVG and CVL reflects the complex nature of this region of the ventrolateral medulla. The rVRG is generally depicted as the locus of inspiratory bulbospinal neurons that provide premotor respiratory inputs to the phrenic nucleus and external intercostal muscles (Alheid et al., 2002; Guyenet et al., 2002). However, its structure is more complex including substantial numbers of vagal motoneurons and brainstem interneurons providing propriobulbar connections (Ellenberger and Feldman, 1990a; Núñez-Abades et al. 1993; Dobbins and Feldman, 1994; Alheid et al., 2002).
This complex meshwork of targets also includes the adjacent A1 catecholamine neurons which appear to be targeted by area postrema axons (Cunningham et al., 1994). According to Cunningham et al. (1994) area postrema axons may also reach non-catecholamine neurons in ventrolateral medulla consistent with our results after arVRG tracer injections as well as the observations of retrograde labeling of area postrema in the in the rat following injections of FB in rVRG (Núñez-Abades et al. 1993) or after rVRG injections of rhodamine beads or H3-aspartate injected (Ellenberger and Feldman, 1990b, 1994).
4.3.3.2 Retrograde labeling of NTS from posterior rostral VRG
Overall, NTS projections to the prVRG resemble those reaching the rostrally adjacent arVRG/CVL region. However, SolVL demonstrates significantly more retrogradely labeled neurons after prVRG injections compared to arVRG/CVL and, as indicated in section 4.3.3.1, many fewer neurons in SolDL. The latter likely reflects the preferential targeting of CVL by SolDL and the location of most CVL neurons somewhat rostral to prVRG, ventral to the arVRG and the preBötC. Other afferent pathways prVRG from SolC and caudal SolM include pulmonary slowly adapting stretch receptors and pulmonary rapidly adapting receptors (Otake et al., 2001; Ezure et al., 2002). Additionally, peripheral chemoreceptor afferent pathways target NTS nuclei (SolC and caudal SolM; Finley and Katz, 1992; Ciriello et al., 1994) that densely project to the prVRG. Since the prVRG contains an enriched population of respiratory premotor neurons the NTS relayed afferent input would be expected to influence the respiratory motor pattern. Effects of these reflexes on respiratory rhythm/frequency would be expected to occur through NTS relays to other brainstem nuclei (see 4.3.2).
While distinctions between arVRG and prVRG were not made by Ellenberger and Feldman (1990b) in depicting the rVRG areas targeted in their retrograde labeling experiments, the pronounced cell labeling depicted in SolVL suggests that prVRG was potentially included within the region of their rhodamine bead injections.
While not injected in the present study cVRG injections of FB also resulted in retrograde labeling of caudomedial NTS and area postrema in the experiments reported by Núñez-Abades et al. (1993).
4.4 Conclusions
The NTS is the primary site of termination of cardiopulmonary afferents with a rough topographic distribution of termination sites to NTS nuclei associated with the different afferent modalities (Kubin et al., 2006). In turn, the differential NTS nuclear targeting of brainstem and forebrain sites provide the mechanisms for the afferent specific patterns of cardiorespiratory reflexes as well as the more general alterations in arousal and orientation that accompany deviations from cardiorespiratory homeostasis. Our data highlights differences in the projections from the caudal NTS nuclei targeting cardiorespiratory cell groups in the ventrolateral medulla as well as a broad pattern of differences in the projections to respiratory rhythm generating (i.e., preBötC, BötC) versus motor pattern forming elements of the VRC (i.e., posterior rVRG). While a differential pattern of innervation of the VRC by NTS nuclei has been suggested in earlier literature (e.g., Ezure et al., 1991; Otake et al., 1992, 2001; Núñez-Abades et al., 1993) the extent of the differences was unanticipated. The findings suggest that at least some of the reflex pathways that have prominent effects on both respiratory rhythm and pattern via relays through caudal NTS nuclei do so by direct projections to VRC nuclei normally associated with changes in respiratory pattern but only indirectly engage rhythmogenic regions of the VRC. Examples of this indirect action on respiratory rhythm have been espoused in several papers. These include, for example, the argument that full expression of the peripheral chemoreceptor reflex influence on rhythm demand participation of long ascending projections from the NTS to the dorsolateral pons (Song et al., 2011). Additionally, Moriera et al. (2007a) suggested that a significant component of the bradypnea that results from activation of bronchopulmonary C-fibers is an indirect effect of NTS C-fiber relays targeting both the arVRG and the RTN rather than a direct input to inspiratory rhythm forming elements in the preBötC.
Interestingly, the pattern of projections arising from the area postrema at least superficially mirrors the outputs of SolC and caudal SolM (Fig. 10B, D) and the area postrema has been functionally linked to cardiovascular regulation and possibly respiratory control (Srinivasan et al., 1993; Polson et al., 1995; Horiuchi et al., 1999). While the strength of the area postrema projections to the ventrolateral medulla have been questioned (Cunningham et al., 1994) the substantial retrograde labeling of the area postrema in the current experiments is reinforced by the earlier observations of area postrema projections to the RTN (Rosin et al., 2006) and RVL (Blessing et al., 1987; Polson et al., 1995; Yamamoto et al., 2003) as well as area postrema projections to rostral and caudal VRG (Ellenberger and Feldman, 1990b, 1994; Núñez-Abades et al., 1993).
Acknowledgments
We thank E.W. Ballou for helpful comments and careful reading of the manuscript. This work was supported by NHLBI grants HL088580 and HL095731.
Abbreviations
- 7n
facial nerve
- 7N
facial nucleus
- 10N
dorsal motor nucleus of the vagus
- 12N
hypoglossal nucleus
- AmbC
nucleus ambiguus – compact part
- AP
area postrema
- BötC
Bötzinger complex
- arVRG
anterior part of the rostral ventral respiratory group
- C1
adrenergic neurons in rostral ventrolateral medulla
- cc
central canal
- CVL
caudal ventrolateral nucleus of the medulla
- cVRG
caudal division of the ventral respiratory group
- DLH
DL-homocysteic acid
- FB
Fast Blue
- FG
FluoroGold
- Gr
gracile nucleus
- Li
linear nucleus of the medulla
- LRt
lateral reticular nucleus
- NeuN
neuronal specific nuclear protein (NeuN
- NTS
nucleus of the solitary tract
- pFRG
parafacial respiratory group
- preBötC
preBötzinger complex
- prVRG
posterior rostral ventral respiratory group, RTN, retrotrapezoid nucleus
- RVL
rostral ventrolateral nucleus of the medulla
- rVRG
rostral division of the ventral respiratory group
- SO
superior olive
- sol
solitary tract
- SolC
commissural nucleus
- SolCe
central nucleus
- SolDL
dorsolateral nucleus
- SolG
gelatinous nucleus
- SolIcp
interstitial nucleus compact part
- SolIdf
interstitial nucleus diffuse part
- SolIM
intermediate nucleus
- SolM
medial nucleus
- SolV
ventral nucleus
- SolVL
ventrolateral nucleus
- SubP
subpostrema nucleus
- VRC
ventral respiratory column
- VRG
ventral respiratory group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF. Efferents from medial and commissural nuclei (SolM, SolC) of the rat nucleus of the solitary tract (NTS) target the ventral respiratory group (VRG) and regions adjacent to the facial nucleus while avoiding the Bötzinger and preBötzinger complex. Society for Neuroscience. 2010 Abstracts #388. [Google Scholar]
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraco IRA. Nucleus of the Solitary Tract. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Hedger SC, Joh TH, Willoughby JO. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Res. 1987;419:336–340. doi: 10.1016/0006-8993(87)90604-4. [DOI] [PubMed] [Google Scholar]
- Bradley RM. The role of the nucleus of the solitary tract in gustatory processing. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- Ciriello J, Hochstenbach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Barraco IRA, editor. The Nucleus of the Solitary Tract. Boca Raton, FL: CRC Press; 1994. pp. 35–50. [Google Scholar]
- Colin W, Donoff RB, Foote WE. Fluorescent latex microspheres as a retrograde tracer in the peripheral nervous system. Brain Res. 1989;486:334–339. doi: 10.1016/0006-8993(89)90520-9. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58:635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol. 2000;417:448–466. [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990a;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I: Nucleus ambiguus and ventral respiratory group. J Comp Neurol. 1990b;294:202–211. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Origins of excitatory drive within the respiratory network: anatomical localization. NeuroReport. 1994;5:1933–1936. doi: 10.1097/00001756-199410000-00023. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M, Yamada H. Distribution of medullary respiratory neurons in the rat. Brain Res. 1988;455:262–270. doi: 10.1016/0006-8993(88)90085-6. [DOI] [PubMed] [Google Scholar]
- Ezure K, Otake K, Lipski J, She RB. Efferent projections of pulmonary rapidly adapting receptor relay neurons in the cat. Brain Res. 1991;564:268–278. doi: 10.1016/0006-8993(91)91463-b. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y, Otake K. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J Comp Neurol. 2002;446:81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, McCrimmon DR. Neural Control of Breathing. In: Squire LR, et al., editors. Fundamental Neuroscience. San Diego: Elsevier-Academic Press; 2008. pp. 855–872. [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Moon EA. Maps of cardiovascular and respiratory regions of rat ventral medulla: focus on the caudal medulla. J Chem Neuroanat. 2009;38:209–221. doi: 10.1016/j.jchemneu.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Wang H. Pre-Bötzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Ito H, Seki M. Monosynaptic inputs from the nucleus tractus solitarii to the laryngeal motoneurons in the nucleus ambiguus of the rat. Anat Embryol (Berl) 2000;202:411–420. doi: 10.1007/s004290000120. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zheng JQ, Seki M, Yajima Y. Synaptology of the direct projections from the nucleus of the solitary tract to pharyngeal motoneurons in the nucleus ambiguus of the rat. J Comp Neurol. 1998;393:391–401. [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Potts PD, Polson JW, Dampney RAL. Distribution of neurons projecting to the rostral ventrolateral medullary pressor region that are activated by sustained hypotension. Neuroscience. 1999;89:1319–1329. doi: 10.1016/s0306-4522(98)00399-6. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sugimoto T. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 1979;175:341–346. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske I, Morrison SF, Cravo SL, Reis DJ. Identification of baroreceptor reflex interneurons in the caudal ventrolateral medulla. Am J Physiol. 1993;264:R169–178. doi: 10.1152/ajpregu.1993.264.1.R169. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Sites of termination and relay of pulmonary rapidly adapting receptors as studied by spike-triggered averaging. Brain Res. 1988;443:215–221. doi: 10.1016/0006-8993(88)91615-0. [DOI] [PubMed] [Google Scholar]
- Kubin L, Kimura H, Davies RO. The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J Physiol. 1991;435:207–228. doi: 10.1113/jphysiol.1991.sp018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SK, Ling EA. Labeling neurons with fluorescent dyes administered via intravenous, subcutaneous or intraperitoneal route. J Neurosci Methods. 1990;32:15–23. doi: 10.1016/0165-0270(90)90067-p. [DOI] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon Neurons Project Widely to Autonomic Control Areas in the Mouse Brain. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Fort P, Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 1990;534:209–224. doi: 10.1016/0006-8993(90)90131-t. [DOI] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP. Neuropeptides are present in projection neurones at all levels in visceral and taste pathways: from periphery to sensory cortex. Brain Res. 1984;299:297–312. doi: 10.1016/0006-8993(84)90711-x. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Feldman JL, Speck DF. Respiratory motoneuronal activity is altered by injections of picomoles of glutamate into cat brain stem. J Neurosci. 1986;6:2384–2392. doi: 10.1523/JNEUROSCI.06-08-02384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Mitchell GS, Feldman JL, Alheid GF. Respiration: Network control. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 6. Oxford: Elsevier, Academic Press; 2009. pp. 79–89. [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. J Neurophysiol. 2007a;98:3627–3637. doi: 10.1152/jn.00675.2007. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol. 2007b;580:285–300. doi: 10.1113/jphysiol.2006.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrini A, Jean A. Synaptic organization of the interstitial subdivision of the nucleus tractus solitarii and of its laryngeal afferents in the rat. J Comp Neurol. 1995;355:221–236. doi: 10.1002/cne.903550206. [DOI] [PubMed] [Google Scholar]
- Núñez-Abades PA, Morillo AM, Pasaro R. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst. 1993;42:99–118. doi: 10.1016/0165-1838(93)90042-s. [DOI] [PubMed] [Google Scholar]
- Otake K, Ezure K, Lipski J, Wong She RB. Projections from the commissural subnucleus of the nucleus of the solitary tract: an anterograde tracing study in the cat. J Comp Neurol. 1992;324:365–378. doi: 10.1002/cne.903240307. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y, Tanaka I, Ezure K. Morphology of pulmonary rapidly adapting receptor relay neurons in the rat. J Comp Neurol. 2001;430:458–470. doi: 10.1002/1096-9861(20010219)430:4<458::aid-cne1043>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol. 1998;79:2365–2373. doi: 10.1152/jn.1998.79.5.2365. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Carrive P, Wang H, Wang P-Y. Chemoarchitectonic atlas of the rat brainstem. Academic Press; San Diego: 1999. [Google Scholar]
- Paxinos G, Watson C, Carrive P, Kirkcaldie M, Ashwell KWS. Chemoarchitectonic atlas of the rat brainstem. Academic Press; San Diego: 2009. [Google Scholar]
- Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J Comp Neurol. 2001;432:20–34. doi: 10.1002/cne.1086. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Lung MS, Spirovski D, McMullan S. Differential regulation of the central neural cardiorespiratory system by metabotropic neurotransmitters. Philos Trans R Soc Lond B Biol Sci. 2009;364:2537–2552. doi: 10.1098/rstb.2009.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson JW, Potts PD, Li YW, Dampney RA. Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla after sustained hypertension in conscious rabbits. Neuroscience. 1995;67:107–123. doi: 10.1016/0306-4522(95)00034-g. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mtui EP, Otake K, Anwar M. Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. Journal of Comparative Neurology. 1996;364:51–67. doi: 10.1002/(SICI)1096-9861(19960101)364:1<51::AID-CNE5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central Autonomic System. In: Paxinos G, editor. The Rat Nervous System. San Diego: Elsevier Academic Press; 2004. pp. 761–796. [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Efferent connections of the caudal part of the globus pallidus in the rat. J Comp Neurol. 1996;376:489–507. doi: 10.1002/(SICI)1096-9861(19961216)376:3<489::AID-CNE10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94:1097–1123. doi: 10.1016/s0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience. 2011;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Bongianni F, Fontana GA, Pantaleo T. Respiratory responses to electrical and chemical stimulation of the area postrema in the rabbit. J Physiol. 1993;463:409–420. doi: 10.1113/jphysiol.1993.sp019601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J Neurophysiol. 2007;98:374–381. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci. 2002;22:3755–3764. doi: 10.1523/JNEUROSCI.22-09-03755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol. 2003;460:525–541. doi: 10.1002/cne.10663. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerari-Mailly F, Buisseret P, Buisseret-Delmas C, Nosjean A. Trigemino-solitarii-facial pathway in rats. J Comp Neurol. 2005;487:176–189. doi: 10.1002/cne.20554. [DOI] [PubMed] [Google Scholar]