Abstract
From the perspective of neural coding, the considerable trial-to-trial variability in the responses of neurons to sensory stimuli is puzzling. Trial-to-trial response variability is typically interpreted in terms of “noise” (i.e., it represents either intrinsic noise of the system or information unrelated to the stimuli). However, trial-to-trial response variability can be considerably different across stimuli, suggesting that it could also provide an important contribution to the information conveyed by the neural responses about the stimuli. To test this hypothesis, we addressed the problem of discriminating stimulus location from the spike-count responses of neurons recorded in the ventro-postero-medial (VPM) nucleus of the thalamus in anesthetized rats. Using a recently developed information theory approach, we verified that differences between stimuli in the trial-to-trial spike-count variability of the responses provided an important contribution to the overall information carried by the neurons. In addition, we found that the relatively reliable (sub-Poisson) firing regime of our VPM neurons was not only more informative, but also more redundant between neurons compared with a more variable (Poisson) firing regime with the same total number of spikes. The typical increase in trial-to-trial response variability from the periphery to the cortex could therefore serve as a strategy to reduce redundancy between neurons and promote efficient sparse coding distributed in large populations of neurons. Overall, our data suggest that the trial-to-trial response variability plays a critical role in establishing the trade-off between total information and redundancy between neurons in population codes.
A major challenge for system neuroscience is to understand the basic elements of the neural code. Because single neurons typically respond to different stimuli with different average firing rates, possibly the simplest hypothesis is that neurons use a rate-coding scheme to represent sensory information (1, 2). However, average firing rates do not necessarily represent a complete description of the neural responses, partly because neurons can respond with a high degree of variability to different repetitions of the same stimulus (3). Trial-to-trial variability in the neural responses can be due to synaptic noise (4, 5) or can represent information that is not directly related to the stimulus, e.g., information about the state of the system (6–8). However, the relation between trial-to-trial variability in the responses of neurons and the information conveyed by those neurons remains unclear (9).
The rate-coding hypothesis was originally formulated on the basis of classic works in peripheral nerves (10, 11). Indeed, at the first stages of sensory processing, the trial-to-trial variability can be so low that neural responses can be almost deterministic (12–14), i.e., neurons respond with virtually the same number of spikes to different repetitions of the same stimulus. In the ideal deterministic regime, single-trial responses are completely described by average responses (because each single trial is identical to the average). Ascending through sensory systems, neural responses to the same stimuli can become progressively more variable and stochastic (13, 15, 16). When the trial-to-trial variability is such that all spikes emitted by a single neuron are completely independent from each other, that neuron behaves as a Poisson process. In the Poisson regime, distributions of single-trial responses are again fully described by the average firing rates, because a Poisson distribution is completely defined by its mean. Indeed, Poisson distributions have often been used to model neuronal responses (17–19). However, in subcortical structures—and even in cortex—the trial-to-trial variability of the neural responses can be very low, i.e., highly sub-Poisson (13, 20–24). The sub-Poisson regime lies in between the deterministic regime and the Poisson regime, and in general it is not fully described by average firing rates: The trial-to-trial variability of the neural responses needs to be taken into account.
Here we hypothesized that trial-to-trial response variability could provide an important contribution to the information conveyed by the neural responses about the stimuli. This hypothesis is suggested by the fact that trial-to-trial variability can be considerably different across stimuli and could therefore contribute to the discrimination between stimuli. To address the possible contribution of variability to neural coding, we used our recently developed information theory approach—the general Poisson exact breakdown of the mutual information (25)—to quantify the specific contribution of trial-to-trial response variability to the information about stimulus location conveyed by single neurons and pairs of neurons in the ventro-postero-medial (VPM) nucleus of the thalamus of urethane-anesthetized rats.
Results
Variability in the Responses of Single VPM Neurons to Different Stimulus Locations.
We recorded the activity of 40 single VPM neurons responding to electrical stimuli delivered to two locations via whisker pad stimulators (26) separately implanted on the mystacial pad in urethane-anesthetized rats. To be able to robustly quantify the contribution of variability with rigorous information theory measures, we delivered a high number of stimuli per location (800) and we used relatively low stimulus intensities (0.2–1 mA), so that most neurons did not consistently respond to all trials. The average firing rates for each cell in response to the stimuli were measured by the response magnitude (spikes per stimulus) and the spike-count variability was measured by the Fano factor, which is the ratio of the variance to the mean (a Poisson process has a theoretical Fano factor = 1). The basic neurophysiological properties of the observed single-neuron responses, summarized in Table S1, are comparable to the responses of VPM neurons to tactile whisker stimulation (27).
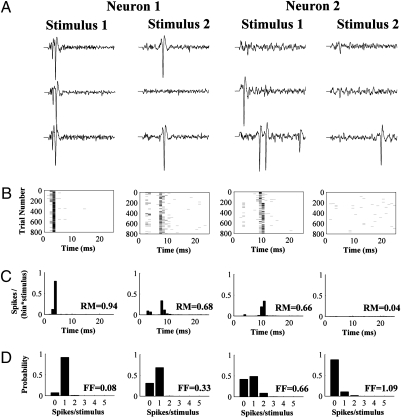
Fig. 1 shows a representative example of two simultaneously recorded cells. The first neuron consistently responded to the stimuli delivered to the first location with a relatively high average firing rate (response magnitude = 0.94 spikes per stimulus) and very low variability (Fano factor = 0.08). The same neuron responded to the stimuli delivered to the second location with lower average firing rate (response magnitude = 0.68 spikes per stimulus) and greater variability (Fano factor = 0.33). Similarly, for the second neuron the larger average firing rate was associated with less variability. This result held for the population as a whole (Table S1): The stimulus location that elicited the larger response magnitude was associated with a smaller Fano factor (paired t test, P < 0.0001). In fact, response magnitudes and Fano factor were negatively correlated (r = −0.65, P < 0.0001; Fig. 2A), consistent with earlier observations in the visual system (13, 23, 28, 29).
Fig. 1.
Discrimination of stimulus location by pairs of VPM neurons. (A) Representative raw single-trial responses (25 ms poststimulus) of a pair of VPM neurons (neuron 1 and neuron 2) to stimulation of two different locations on the whisker pad (stimulus 1 and stimulus 2). (B) Raster plots showing the single-trial responses of the same pair of neurons for all 800 trials per stimulus location. (C) Peri-stimulus time histograms (PSTH) showing the average responses to the stimuli of the same pair of neurons. The response magnitude (RM, average spikes per stimulus) is reported for every PSTH. The x axis (time) is the same for all plots in A–C, with 0 being stimulus onset. (D) Spike-count distributions (probability of occurrence of a particular number of spikes per stimulus in any given trial), corresponding to the responses in A–C. The Fano factor (FF) is reported for every spike-count distribution.
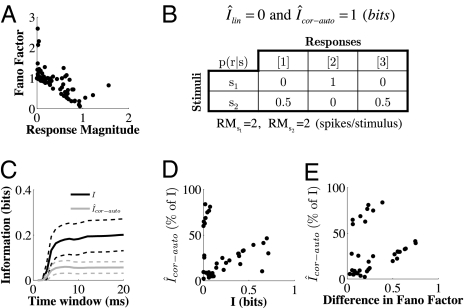
Fig. 2.
Trial-to-trial response variability contributes to the information conveyed by single neurons. (A) Scatter plot of the response magnitude (x axis, average spikes per stimulus) versus the Fano factor (y axis) for all neurons (40) and all stimuli (2). (B) Simple example of a neuron carrying information about two stimuli only by count autocorrelations. The neuron fires always with two spikes in response to the first stimulus and either one spike or three spikes (50% of the trials) in response to the second stimulus. Shown is the conditional probability p(r | s) of the responses to the stimuli and the corresponding information values, assuming that p(s1) = p(s2) = 0.5. (C) Total information (Itot, black line) carried by single neurons about stimulus location and contribution of trial-to-trial response variability, i.e., information due to count autocorrelations (Îcor-auto, gray line). The x axis represents poststimulus time, and information values are calculated for increasing time windows [(0–1 ms), (0–2 ms), … , (0–20 ms)]. The y axis represents information in bits. Solid lines are averages across neurons; dashed lines are 95% confidence intervals. (D) Scatter plot of the information due to count autocorrelations (Îcor-auto, y axis, expressed as percentage of the total information) versus the total information (I, x axis, in bits) considering the entire (0–20 ms) poststimulus window. (E) Scatter plot of the information due to count autocorrelations (Îcor-auto, y axis, expressed as percentage of the total information) versus the difference in Fano factor between stimuli (x axis).
Importantly, in the plane defined by response magnitude (RM) and Fano factor (FF), no neuron fell below the FF = 1 − RM line (Fig. 2A). This line is in fact a theoretical limit, as it represents neurons that fire at most one spike in response to any stimulus, which is a particular case of the sub-Poisson regime: the Bernoulli regime. Many of our VPM neurons were thus operating in a sub-Poisson regime relatively close to Bernoulli. Bernoulli spiking (or binary spiking) has been previously investigated in the auditory cortex (22). On the basis of previous theoretical works (30, 31), DeWeese and colleagues suggested that binary spiking could subserve the stable propagation of packets of spikes from a population of neurons to a downstream population of neurons (22).
Sub-Poisson firing represents a response variability characterized by autocorrelation in the spike count (18): If a spike occurs, the probability of observing a second spike decreases (or increases). Conversely, Poisson firing represents a response variability characterized by absence of autocorrelation in the spike count: If a spike occurs, the probability of observing a second spike is the same as for the first spike, because all spikes are completely independent. To gain insight into the role of variability in neural coding, in the next sections we use our general Poisson exact breakdown of the mutual information (25) to assess the possible informational advantages or disadvantages of sub-Poisson firing compared with Poisson firing for discriminating stimulus location in our VPM neurons.
Trial-to-Trial Response Variability Contributes to the Information Conveyed by Single Neurons.
To quantify how much information could be extracted about the discrimination of two stimulus locations from the spike counts in the single-trial responses of VPM neurons, we used Shannon's mutual information between the responses and the stimuli (32). To discriminate two stimulus locations is a binary problem, so the maximum information, i.e., the entropy of the stimuli, is 1 bit. To say that a neuron conveys 1 bit of information means that from any single-trial response we can infer with full certainty which of the two stimuli generated that response.
To estimate the information that is carried by the variability or autocorrelations (Îcor-auto) we first calculated the information that a neuron would convey if it fired according to Poisson distributions (Îlin) with the experimentally measured average firing rates in response to the stimuli. In the notation, the “hat” (^) indicates that the corresponding term uses, at least in part, Poisson equivalent distributions (SI Materials and Methods). We then calculated the information carried by the observed responses of the neurons (I). The difference between the two (Îcor-auto = I − Îlin) defines the information due to count autocorrelations in single neurons and represents the potential informational advantage of the sub-Poisson regime compared with the Poisson regime (25).
In the intuitive case of two stimuli evoking the same response magnitude, all of the information would be due to differences in variability and it would be measured by the count autocorrelations information term (i.e., if Îlin = 0, then Îcor-auto = I). As a simple example, let us consider a neuron that always fires two spikes in response to a stimulus s1 and either one spike (50% of the trials) or three spikes in response to a second stimulus s2 (Fig. 2B). The neural responses perfectly discriminate the two stimuli, but the response magnitude is the same for both stimuli (RM = 2 spikes per stimulus). All of the information is therefore conveyed by count autocorrelations (i.e., Îlin = 0 and Îcor-auto = I = 1 bit).
In our experimental data, single VPM neurons (n = 40) conveyed 0.20 ± 0.22 bits of information about stimulus location (I), ranging from <0.01 bits to as high as 0.71 bits. The information contributed from count autocorrelations (Îcor-auto) accounted for 28.3 ± 25.4% of the total spike-count information carried about stimulus location (Fig. 2C). The relationship between total information and count autocorrelations fell into two informational modes (Fig. 2D). The first mode included neurons that carried a small amount of information (0.03 ± 0.03 bits, n = 8) mostly due to count autocorrelations (70 ± 10%), whereas the second mode included neurons that carried much more information (0.24 ± 0.23 bits, n = 32) with a smaller contribution of count autocorrelations (17 ± 14%). In both modes the percentage of information due to count autocorrelations was higher for neurons conveying more information (r = 0.76, P = 0.0274, n = 8; r = 0.73, P < 0.0001, n = 32; Fig. 2D). The first mode corresponded to neurons that responded to the two locations with similar response magnitudes (<30% difference) and the second mode corresponded to neurons that responded to the two locations with a larger response magnitude difference.
Importantly, in both modes the percentage of information due to count autocorrelations was significantly correlated to the difference in Fano factor between the two stimuli (r = 0.88, P = 0.0035, n = 8; r = 0.82, P < 0.0001, n = 29; three outliers with difference in Fano factor >1 were excluded; Fig. 2E). These results suggest (a) that the relatively reliable (sub-Poisson) firing regime of our VPM neurons is considerably more informative than a more variable (Poisson) regime with the same total number of spikes and (b) that differences in response variability between stimuli contribute to the information about stimulus location conveyed by single VPM neurons.
Trial-to-Trial Response Variability Contributes to the Redundancy Between Neurons.
If variability differences between stimuli contribute to the information carried by single neurons, they could also contribute to the synergy or redundancy between neurons. The first step to address this issue was to establish how much of the information carried by VPM neurons is redundant between neurons (33). We used the fact that we simultaneously recorded from pairs of neurons (n = 40 pairs) and decomposed the total information carried by the pairs into the information carried independently by the individual neurons (Ilin) plus the information gained (synergy) or lost (redundancy) in their joint responses (ΔIsyn). Note that for single neurons I = Ilin, so for pairs of neurons, Ilin is the sum of I (or Ilin) of the individual neurons. As expected, the information about stimulus location was redundant between neurons. The redundancy was 12.5 ± 21.2% of the total spike-count information (Fig. 3A).
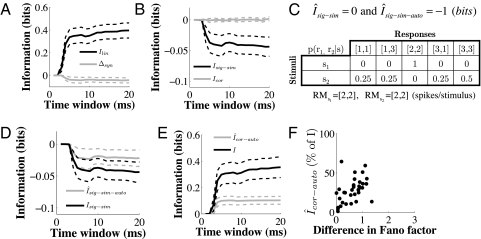
Fig. 3.
Trial-to-trial response variability contributes to the redundancy between neurons. (A) Information carried independently by the individual neurons in each pair (linear term Ilin, black line), and information gained (synergy) or lost (redundancy) in their joint responses (synergy/redundancy term Δsyn; gray line). (B) Redundancy due to signal similarity (Isig-sim, black line) and synergy/redundancy due to noise correlations (Icor, gray line). (C) Simple example of a pair of neurons carrying redundant information in count autocorrelations. Both neurons independently fire always with two spikes in response to the first stimulus and either one spike or three spikes in response to the second stimulus. Shown is the joint conditional probability p(r1, r2 | s) of the responses of the neurons to the stimuli and the corresponding information values, assuming that p(s1) = p(s2) = 0.5. (D) Signal similarity due to count autocorrelations (Îsig-sim-auto, gray line), compared with the total signal similarity (Isig-sim, black line, same as in B). (E) Total information (Itot, black line) carried by pairs of neurons about stimulus location and information due to count autocorrelations (Îcor-auto, gray line). All x axes are as in Fig. 2C; solid lines are averages across neurons; dashed lines are 95% confidence intervals. (F) Scatter plot of the information due to count autocorrelations (Îcor-auto, y axis, expressed as percentage of the total information) versus the difference in Fano factor between stimuli (x axis) for pairs of neurons. The difference in Fano factor between stimuli for pairs of neurons was defined as the Euclidean distance between the vectors of Fano factors corresponding to each stimulus location.
Redundancy between neurons can arise from “signal similarity” (Isig-sim), i.e., similarity between neurons in their responses to the stimuli, and/or from “noise correlations” (Icor), i.e., trial-to-trial correlations between neurons in the variability of their responses (34–37). In our pairs, virtually the entire redundancy between neurons was due to signal similarity (Isig-sim: 12.4 ± 21.2% of the total spike-count information). Noise correlations had a negligible synergic contribution to the total information (Icor: 0.6 ± 3.2% of the total spike-count information). Even though we cannot exclude these small contributions from noise correlations to become relevant in large populations of neurons (38), they did not play a role for the discrimination of stimulus location in our pairs (Fig. 3B).
To determine the contribution of the response variability within neurons to the redundancy between neurons, using the general Poisson exact breakdown of the mutual information we first calculated the redundancy that the neurons would have if they fired in a Poisson regime (Îsig-sim), again maintaining the experimentally measured response magnitudes. We then calculated the redundancy between neurons in the observed responses (Isig-sim). The difference between the two is the redundancy due to count autocorrelations (Îsig-sim-auto = Isig-sim − Îsig-sim), which represents the additional redundancy between neurons in the sub-Poisson regime compared with the Poisson regime. For pairs of neurons, the overall information due to count autocorrelations (Îcor-auto) is the sum of the count autocorrelation information conveyed by the individual neurons (Îlin-auto) corrected for the redundancy due to count autocorrelations (i.e., Îcor-auto = Îlin-auto + Îsig-sim-auto).
In the intuitive case of two stimuli evoking the same response magnitude in both neurons, the redundancy obviously cannot be due to similarity between neurons in their average firing rates, but is instead due to similarity in their variability across stimuli, as measured by the count autocorrelations signal-similarity term (if Îsig-sim = 0, then Îsig-sim-auto = Isig-sim). Let us reconsider the simple example of a neuron that always fires two spikes in response to a stimulus and either one spike (50% of the trials) or three spikes in response to a second stimulus, and let us duplicate this neuron so that we have a pair of identical neurons firing independently (Fig. 3C). The response magnitudes are again the same for both stimuli, so any information and redundancy values are necessarily due to count autocorrelations (Îlin = 0, Îsig-sim = 0). The responses of each neuron perfectly discriminate between the two stimuli (Îlin-auto = 2 bits), so there is maximal redundancy between neurons (Îsig-sim-auto = −1 bit), with count autocorrelations conveying all of the information (Îcor-auto = I = 1 bit).
In our recorded pairs, the redundancy due to count autocorrelations (Îsig-sim-auto) was about half (50.7 ± 96.7%) of the total redundancy between neurons (Isig-sim) (Fig. 3D), with count autocorrelations (Îcor-auto) still accounting for 28.8 ± 27.8% of the total information (I) (Fig. 3E). Therefore, even though the more reliable sub-Poisson regime was more informative compared with the more variable Poisson regime, it was also more redundant.
Finally, for the pairs of neurons the percentage of information carried by count autocorrelations (i.e., the additional information of the sub-Poisson regime compared with the Poisson regime) was significantly correlated to the difference in the population Fano factor between the two stimuli (Pearson's r = 0.48, P = 0.0032; Fig. 3F; pairs with the three outliers were excluded). This result is consistent with what we observed for the single neurons and confirms that this additional information was related to differences between stimuli in the trial-to-trial variability of the responses.
Discussion
Considering the problem of discriminating stimulus location from the spike-count responses of pairs of VPM neurons in anesthetized rats, we obtained the following main results: (i) differences between stimuli in the trial-to-trial spike-count variability of the responses provided an important contribution to the overall information carried by VPM neurons and (ii) the relatively reliable (sub-Poisson) regime of VPM neurons was indeed more informative, but also more redundant between neurons compared with a more variable (Poisson) regime with the same total number of spikes. Response variability, therefore, contributes to the information carried by both single cells and populations of neurons in addition to that carried by average firing rates.
Methodological Considerations.
We previously showed that temporal information conveyed by spike timing can be more than spike-count information in the entire ventrobasal complex of the thalamus (32). Because temporal codes and rate codes are by no means mutually exclusive (see, e.g., refs. 39 and 40), in the present work we focused on spike count. To investigate the role of trial-to-trial spike-count variability for neural coding, we used both classical neurophysiological measures such as the Fano factor (13, 16) and a rigorous information theory approach recently developed by us: the general Poisson exact breakdown of mutual information (25). This information theory approach represents an integrative generalization of several previous information theory measures, including series expansion (34), exact breakdown (41), synergy/redundancy between neurons (36), and information lost by an optimal decoder that assumes absence of correlations between neurons (35, 41). However, our general Poisson exact breakdown includes two unique measures that are crucial for the present work: (i) the exact contribution of count autocorrelations to the information carried by neurons (Îcor-auto, the series expansion can provide an approximation of this measure) and (ii) the contribution of count autocorrelations to the redundancy between neurons (Îsig-sim-auto). Because count autocorrelations are nothing but deviations from Poisson distributions, the above measures allowed us to quantify, from a rigorous information theory perspective, the advantages and disadvantages of sub-Poisson firing compared with Poisson firing in our VPM neurons. From a more physiological perspective, Îcor-auto depends on the response properties of individual neurons and thus on their “unique biophysical fingerprint” (42). Conversely, Îsig-sim-auto depends on the heterogeneity between neurons (e.g., in the intuitive case of two stimuli evoking the same response magnitude in both neurons, the more the differences in autocorrelations between stimuli are similar for the two neurons, the more the two neurons will be redundant).
An important potential problem with all information theory measures is the bias introduced by limited sampling of the observed neural responses (43). Because the number of different observed responses increases exponentially with the number of neurons and linearly with the number of stimuli, with a high number of neurons and/or stimuli information theory measures rapidly become experimentally unviable. To minimize sampling bias, we adopted several strategies. First, we considered the minimal problem of discriminating between two stimulus locations. Second, we recorded pairs of neurons, which admittedly did not allow us to investigate the possible role of higher-order correlations between more than two neurons (38, 44). Third, we used a number of trials that is one order of magnitude greater compared with previous studies under similar experimental conditions (800 compared with 50–100 trials per stimulus; e.g., refs. 32, 45, and 46). Fourth, we applied proper bias correction procedures (SI Materials and Methods) (47). We can therefore confidently assert that the remarkable contributions of trial-to-trial spike-count variability to the information conveyed by VPM neurons (∼30% of the total information) and to the redundancy between neurons (∼50% of the total redundancy), described here, are not an artifactual consequence of sampling bias in our information measures.
It is important to remark that our experiments were performed in urethane-anesthetized rats, as in our previous studies on thalamic processing (27, 32). On the one hand, these anesthetized conditions are convenient to maintain the animal brain in a reasonably stable state throughout long stimulation protocols (48). On the other hand, however, thalamic responses are highly dependent on the arousal and behavioral state of the animal (48–51). Inferences about sensory processing based on anesthetized data should therefore be cautious: Our experimental conditions simply represent a model to gain general insights about the possible computational roles of trial-to-trial variability in neural responses from a rate-coding perspective.
Finally, variability of neural responses can depend not only on the behavioral/attentive state of the animal (7) but also on the type of stimuli used. Sub-Poisson variability has typically—but not exclusively—been observed in response to punctate stimuli, in different structures and sensory systems (13, 20–24). On the one hand, our results are thus likely to extend beyond the VPM thalamus and beyond the somatosensory system; on the other hand, they prompt for a careful investigation of the relationships between the deterministic/stochastic dynamics of sensory stimuli and the variability of the corresponding neural responses.
Variability in the Responses of Single VPM Neurons to Different Stimulus Locations.
The main neurophysiological substrate for our information theory results is that the trial-to-trial spike-count variability in the neural responses, measured by the classical Fano factor, was smaller for stimuli that elicited responses with a larger average firing rate. This negative correlation between average firing rate and count variability generalizes earlier results obtained in the visual system (12, 13, 23, 28, 29) to the somatosensory system. From a mechanistic perspective, the negative correlation between average firing rate and count variability has been shown to depend on the neurons’ refractory period (12, 13). However, many of our VPM neurons fired in a sub-Poisson regime relatively close to a Bernoulli regime, which is when neurons display binary spiking (i.e., they fire at most one spike per trial). In the Bernoulli regime, which was previously investigated in the rat auditory cortex (22), the negative correlation between average firing rate and count variability is a direct consequence of the relationship between the variance and mean of the Bernoulli distribution [variance = mean(1 − mean), and therefore FF = 1 − mean]. Indeed, binary spiking cannot be due solely to the refractory period (22), but other mechanisms—such as inhibition and short-term synaptic depression (52, 53)—are necessarily involved. Consequently, both intrinsic properties at the cellular level and circuit properties at the network level are likely to contribute to the negative correlation between average firing rate and count variability observed in our VPM neurons.
Trial-to-Trial Response Variability Contributes to the Information Conveyed by Single Neurons.
We observed two informational modes by which trial-to-trial variability in the spike counts of single VPM neurons could convey information about stimulus location, depending on how the neurons responded to the stimuli. The first mode was represented by neurons that responded to the two locations with large differences in the average firing rate: The larger the difference in average firing rate was, the larger the difference in response variability and the larger the contribution of variability to the overall information about stimulus location. This behavior is therefore the direct informational consequence of the negative correlation between firing rate and variability discussed in the previous section. Importantly, the negative correlation between firing rate and variability might erroneously lead one to think that the information conveyed by variability is simply redundant to the information conveyed by average firing rate. This consideration is certainly not the case. In fact, the information conveyed by variability, reported here, is actually the information conveyed by sub-Poisson variability in addition to the information conveyed by the average firing rate alone in the Poisson regime (18, 25). With the notable exceptions of a methodological work applying the series expansion to data from the macaque inferior temporal visual cortex (54) and our methodological work applying the general Poisson exact breakdown to data from the rat whisker cortex (25), the considerable contribution of trial-to-trial spike-count variability to rate coding has been so far largely unexplored.
The second mode we observed in our dataset was represented by neurons that responded to the two stimulus locations with similar average firing rates. In these neurons, even though the overall information was rather small, it was mostly due to differences in response variability between stimuli. Under these conditions, the information conveyed by trial-to-trial spike-count variability parallels the information conveyed by trial-to-trial spike jitters (i.e., latency variability) when the average latencies are similar (32). More generally, if the first moments of spike measures (e.g., average firing rates, average latencies, …) are similar between stimuli, then the additional information due to second or higher moments (e.g., count variability, jitters, …) can fully emerge.
Trial-to-Trial Response Variability Contributes to the Redundancy Between Neurons.
Trial-to-trial variability in the sub-Poisson regime of our VPM neurons was not only more informative compared with the Poisson regime, but also more redundant between neurons. This finding has a general validity: On the one hand a sub-Poisson regime is more informative than a Poisson regime with the same total number of spikes because sub-Poisson distributions are less overlapping than Poisson distributions; on the other hand, a sub-Poisson regime is also more redundant than a Poisson regime with the same total number of spikes because smaller variability within neurons implies higher similarity between neurons.
One might wonder whether the additional information contained in trial-to-trial variability is useful or physiologically relevant. At the very least this information is useful to our understanding of the neural responses even if not used by the brain itself. It is also possible that this information might be more useful for repeated-trials learning than for single-trial encoding. Nonetheless, we speculate that the information contained in the trial-to-trial variability could indeed be decoded by a downstream observer working on a single-trial basis at the population level: If neurons in a population have the same response properties (in absence of noise correlations), then the variability within neurons across trials is the same as the variability across neurons on a single trial. Single-trial variability across similarly tuned neurons (e.g., within the same barreloid in the thalamus) could therefore be used as a population code and be decoded by a downstream observer (e.g., a cortical barrel). From this downstream cortical perspective, excessive redundancy might become a computational disadvantage (55). In fact, at the cortical level, variability seems to have a negligible contribution to the redundancy between neurons (25). The increased trial-to-trial count variability in the neural responses at the cortical level compared with the thalamic level could therefore serve as a strategy to reduce redundancy between neurons and promote efficient sparse coding distributed in large populations of neurons (56–58).
Overall, our data suggest that the trial-to-trial variability of the neural responses plays a critical role in establishing the trade-off between total information and redundancy between neurons in population codes.
Materials and Methods
All experiments were performed following the rules of the International Council for Laboratory Animal Science, European Union regulation 86/609/EEC, and were approved by the Ethical Committee for Animal Research of the Hospital Nacional de Parapléjicos. Data were collected from six male adult Wistar rats (250–350 g) anesthetized with i.p. urethane (1.5 g/kg) at stage III-3/4 (49). Pairs of VPM neurons were recorded simultaneously (band-pass 200 Hz to 7 kHz, sampling rate 20 kHz) by using two high-impedance tungsten electrodes (2–4 MOhm at 1 kHz). Stimuli (50 μs, 0.2–1 mA pulses, interpulse interval >2 s, 800 pulses per location) were delivered through whisker-pad stimulators (26). Trial-to-trial spike-count variability was measured by the Fano factor. The contribution of trial-to-trial spike-count variability to the information conveyed about stimulus location by the responses of single neurons and of pairs of neurons was assessed using the general Poisson exact breakdown of the mutual information (25). Further details can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was partly supported by FundaciĖn para la InvestigaciĖn Sanitaria en Castilla-La Mancha (Gobierno de Castilla-La Mancha) and Fondo de Investigación Sanitaria del Instituto de Salud Carlos III PI08/1852 PI08/1810 (Spain), co-funded by Fondo Europeo de Desarrollo Regional, National Institutes of Science Grant R01 NS05741, and International Foundation for Research in Paraplegia P113.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103168108/-/DCSupplemental.
References
- 1.Shadlen MN, Newsome WT. Noise, neural codes and cortical organization. Curr Opin Neurobiol. 1994;4:569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 2.London M, Roth A, Beeren L, Häusser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010;466:123–127. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein RB, Gossen ER, Jones KE. Neuronal variability: Noise or part of the signal? Nat Rev Neurosci. 2005;6:389–397. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]
- 4.Calvin WH, Stevens CF. Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol. 1968;31:574–587. doi: 10.1152/jn.1968.31.4.574. [DOI] [PubMed] [Google Scholar]
- 5.Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- 6.Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 2006;26:3697–3712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussar C, Pasternak T. Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task. Proc Natl Acad Sci USA. 2010;107:21842–21847. doi: 10.1073/pnas.1009956107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Averbeck BB. Poisson or not Poisson: Differences in spike train statistics between parietal cortical areas. Neuron. 2009;62:310–311. doi: 10.1016/j.neuron.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Adrian ED, Zotterman Y. The impulses produced by sensory nerve endings: Part 3. Impulses set up by touch and pressure. J Physiol. 1926;61:465–483. doi: 10.1113/jphysiol.1926.sp002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adrian ED, Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a single end-organ. J Physiol. 1926;61:151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci USA. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kara P, Reinagel P, Reid RC. Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron. 2000;27:635–646. doi: 10.1016/s0896-6273(00)00072-6. [DOI] [PubMed] [Google Scholar]
- 14.Jones LM, Depireux DA, Simons DJ, Keller A. Robust temporal coding in the trigeminal system. Science. 2004;304:1986–1989. doi: 10.1126/science.1097779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel A, Hennig RM, Ronacher B. Increase of neuronal response variability at higher processing levels as revealed by simultaneous recordings. J Neurophysiol. 2005;93:3548–3559. doi: 10.1152/jn.01288.2004. [DOI] [PubMed] [Google Scholar]
- 16.Bale MR, Petersen RS. Transformation in the neural code for whisker deflection direction along the lemniscal pathway. J Neurophysiol. 2009;102:2771–2780. doi: 10.1152/jn.00636.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerstein GL, Mandelbrot B. Random walk models for the spike activity of a single neuron. Biophys J. 1964;4:41–68. doi: 10.1016/s0006-3495(64)86768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaglione A, Foffani G, Scannella G, Cerutti S, Moxon KA. Mutual information expansion for studying the role of correlations in population codes: How important are autocorrelations? Neural Comput. 2008;20:2662–2695. doi: 10.1162/neco.2008.08-07-595. [DOI] [PubMed] [Google Scholar]
- 19.Amarasingham A, Chen TL, Geman S, Harrison MT, Sheinberg DL. Spike count reliability and the Poisson hypothesis. J Neurosci. 2006;26:801–809. doi: 10.1523/JNEUROSCI.2948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gur M, Beylin A, Snodderly DM. Response variability of neurons in primary visual cortex (V1) of alert monkeys. J Neurosci. 1997;17:2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller JR, Metha AB, Krauskopf J, Lennie P. Information conveyed by onset transients in responses of striate cortical neurons. J Neurosci. 2001;21:6978–6990. doi: 10.1523/JNEUROSCI.21-17-06978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeWeese MR, Wehr M, Zador AM. Binary spiking in auditory cortex. J Neurosci. 2003;23:7940–7949. doi: 10.1523/JNEUROSCI.23-21-07940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gur M, Snodderly DM. High response reliability of neurons in primary visual cortex (V1) of alert, trained monkeys. Cereb Cortex. 2006;16:888–895. doi: 10.1093/cercor/bhj032. [DOI] [PubMed] [Google Scholar]
- 24.Maimon G, Assad JA. Beyond Poisson: Increased spike-time regularity across primate parietal cortex. Neuron. 2009;62:426–440. doi: 10.1016/j.neuron.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaglione A, Moxon KA, Foffani G. General Poisson exact breakdown of the mutual information to study the role of correlations in populations of neurons. Neural Comput. 2010;22:1445–1467. doi: 10.1162/neco.2010.04-09-989. [DOI] [PubMed] [Google Scholar]
- 26.Moxon KA, Devilbiss DM, Chapin JK, Waterhouse BD. Influence of norepinephrine on somatosensory neuronal responses in the rat thalamus: A combined modeling and in vivo multi-channel, multi-neuron recording study. Brain Res. 2007;1147:105–123. doi: 10.1016/j.brainres.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar J, Morales-Botello ML, Foffani G. Tactile responses of hindpaw, forepaw and whisker neurons in the thalamic ventrobasal complex of anesthetized rats. Eur J Neurosci. 2008;27:378–387. doi: 10.1111/j.1460-9568.2008.06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frishman LJ, Levine MW. Statistics of the maintained discharge of cat retinal ganglion cells. J Physiol. 1983;339:475–494. doi: 10.1113/jphysiol.1983.sp014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mochol G, Wójcik DK, Wypych M, Wróbel A, Waleszczyk WJ. Variability of visual responses of superior colliculus neurons depends on stimulus velocity. J Neurosci. 2010;30:3199–3209. doi: 10.1523/JNEUROSCI.3250-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- 31.Kistler WM, Gerstner W. Stable propagation of activity pulses in populations of spiking neurons. Neural Comput. 2002;14:987–997. doi: 10.1162/089976602753633358. [DOI] [PubMed] [Google Scholar]
- 32.Foffani G, Morales-Botello ML, Aguilar J. Spike timing, spike count, and temporal information for the discrimination of tactile stimuli in the rat ventrobasal complex. J Neurosci. 2009;29:5964–5973. doi: 10.1523/JNEUROSCI.4416-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghazanfar AA, Stambaugh CR, Nicolelis MA. Encoding of tactile stimulus location by somatosensory thalamocortical ensembles. J Neurosci. 2000;20:3761–3775. doi: 10.1523/JNEUROSCI.20-10-03761.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panzeri S, Schultz SR, Treves A, Rolls ET. Correlations and the encoding of information in the nervous system. Proc Biol Sci. 1999;266:1001–1012. doi: 10.1098/rspb.1999.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nirenberg S, Latham PE. Decoding neuronal spike trains: How important are correlations? Proc Natl Acad Sci USA. 2003;100:7348–7353. doi: 10.1073/pnas.1131895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneidman E, Bialek W, Berry MJ., 2nd Synergy, redundancy, and independence in population codes. J Neurosci. 2003;23:11539–11553. doi: 10.1523/JNEUROSCI.23-37-11539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latham PE, Nirenberg S. Synergy, redundancy, and independence in population codes, revisited. J Neurosci. 2005;25:5195–5206. doi: 10.1523/JNEUROSCI.5319-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneidman E, Berry MJ, 2nd, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature. 2006;440:1007–1012. doi: 10.1038/nature04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci. 2004;7:170–177. doi: 10.1038/nn1177. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Rotter S, Aertsen A. Spiking activity propagation in neuronal networks: Reconciling different perspectives on neural coding. Nat Rev Neurosci. 2010;11:615–627. doi: 10.1038/nrn2886. [DOI] [PubMed] [Google Scholar]
- 41.Pola G, Thiele A, Hoffmann KP, Panzeri S. An exact method to quantify the information transmitted by different mechanisms of correlational coding. Network. 2003;14:35–60. doi: 10.1088/0954-898x/14/1/303. [DOI] [PubMed] [Google Scholar]
- 42.Padmanabhan K, Urban NN. Intrinsic biophysical diversity decorrelates neuronal firing while increasing information content. Nat Neurosci. 2010;13:1276–1282. doi: 10.1038/nn.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panzeri S, Senatore R, Montemurro MA, Petersen RS. Correcting for the sampling bias problem in spike train information measures. J Neurophysiol. 2007;98:1064–1072. doi: 10.1152/jn.00559.2007. [DOI] [PubMed] [Google Scholar]
- 44.Montani F, et al. The impact of high-order interactions on the rate of synchronous discharge and information transmission in somatosensory cortex. Philos Trans A Math Phys Eng Sci. 2009;367(1901):3297–3310. doi: 10.1098/rsta.2009.0082. [DOI] [PubMed] [Google Scholar]
- 45.Panzeri S, Petersen RS, Schultz SR, Lebedev M, Diamond ME. The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron. 2001;29:769–777. doi: 10.1016/s0896-6273(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 46.Foffani G, Chapin JK, Moxon KA. Computational role of large receptive fields in the primary somatosensory cortex. J Neurophysiol. 2008;100:268–280. doi: 10.1152/jn.01015.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magri C, Whittingstall K, Singh V, Logothetis NK, Panzeri S. A toolbox for the fast information analysis of multiple-site LFP, EEG and spike train recordings. BMC Neurosci. 10:81. doi: 10.1186/1471-2202-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci. 2005;25:10990–11002. doi: 10.1523/JNEUROSCI.3229-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- 50.Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartings JA, Simons DJ. Inhibition suppresses transmission of tonic vibrissa-evoked activity in the rat ventrobasal thalamus. J Neurosci. 2000;20:RC100. doi: 10.1523/JNEUROSCI.20-19-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Rolls ET, Franco L, Aggelopoulos NC, Reece S. An information theoretic approach to the contributions of the firing rates and the correlations between the firing of neurons. J Neurophysiol. 2003;89:2810–2822. doi: 10.1152/jn.01070.2002. [DOI] [PubMed] [Google Scholar]
- 55.Barlow H. Redundancy reduction revisited. Network. 2001;12:241–253. [PubMed] [Google Scholar]
- 56.Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science. 2000;287:1273–1276. doi: 10.1126/science.287.5456.1273. [DOI] [PubMed] [Google Scholar]
- 57.Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Chechik G, et al. Reduction of information redundancy in the ascending auditory pathway. Neuron. 2006;51:359–368. doi: 10.1016/j.neuron.2006.06.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.